Abstract
Monochloramine has been used to provide a disinfecting residual in water distribution systems where it is difficult to maintain an adequate free-chlorine residual or where disinfection by-product formation is of concern. The goal of this study was to characterize the immunotoxic effects of chloramine in female B6C3F1 mice when administered via the drinking water. Mice were exposed to chloramine-containing deionized tap water at 2, 10, 20, 100, or 200 ppm for 28 days. No statistically significant differences in drinking water consumption, body weight, body weight gain, organ weights, or hematological parameters between the exposed and control animals were noted during the experimental period. There were no changes in the percentages and numbers of total B-lymphocytes, T-lymphocytes, CD4+ and CD8+ T-lymphocytes, natural killer (NK) cells, and macrophages in the spleen. Exposure to chloramine did not affect the IgM antibody-forming cell response to sheep red blood cells (SRBC) or anti-SRBC IgM antibody production. Minimal effects, judged to be biologically insignificant, were observed in the mixed-leukocyte response and NK activity. In conclusion, chloramine produced no toxicological and immunotoxic effects in female B6C3F1 mice when administered for 28 days in the drinking water at concentrations ranging from 2–200 ppm.
Introduction
Often referred to as monochloramine by water utilities, chloramine (CHL) has long been used to provide a disinfecting residual in water distribution systems where it is difficult to maintain an adequate free-chlorine residual or where disinfection by-product formation is of concern (Eaton et al., Citation1973). Some water systems use (ammonium chloride, NH4Cl) as a secondary disinfectant to maintain a disinfectant residual throughout the distribution system, so that drinking water remains safe as it travels from the treatment facility to the consumers. Moreover, it provides better control against bacterial re-growth in systems with large storage tanks and dead-end water mains (EPA, Citation2010a). Although CHL is less effective than chlorine as a disinfectant, it is more stable and extends disinfectant benefits throughout a water utility’s distribution system (EPA, Citation1978). Because CHL is not as reactive as chlorine, it forms fewer disinfection by-products.
CHL is a respiratory irritant (Lévesque et al., Citation2006; Bowen et al., Citation2007; Jacobs et al., Citation2007), and some of the water disinfection by-products of CHL treatment (e.g., iodoacids) are more toxic than those of chlorine (Plewa et al., Citation2004). CHL vapors and its disinfection by-products can accumulate in indoor air and concentrate in an enclosed area such as a shower stall, small bathroom, kitchen, or apartment. In a study by Zierler et al. (Citation1986) it was found that there was an increase in deaths from influenza and pneumonia in communities that used CHL. In addition to inhalation, dermal contact is another important route of exposure to CHL (Lindstrom et al., Citation1997; Bowen et al., Citation2007; Weaver et al., Citation2009). Further, there have been claims that CHL might be responsible for both dermal and respiratory illnesses (Vermont Department of Health, Citation2011).
The immune system plays an important role in homeostasis and host resistance to pathogens. Based on the number of people potentially exposed to CHL, the present study was conducted to assess the immune system of female B6C3F1 mice that drank water spiked with CHL for 28 days. US Environmental Protection Agency (EPA) regulations limit the CHL concentration in drinking water to 4 ppm (EPA, Citation2010b). In this study, five CHL concentrations (2, 10, 20, 100, and 200 ppm) were utilized. Immune function testing was conducted to investigate effects on innate, cell-mediated, and humoral immunity. The assays chosen for immunotoxicity evaluation have been proven to be robust in detecting possibly adverse effects under conditions that are relevant to actual human exposure to chemicals (Luster et al., Citation1988; Krzystyniak et al., Citation1995).
Materials and methods
Animal handling and chemicals
All studies were conducted in accordance with the VCU Institutional Animal Care and Use Committee at an AAALAC-accredited facility. Female B6C3F1 mice were obtained from Taconic Farms (Germantown, NY) at 4–6 weeks-of-age. After 2 weeks in quarantine, the mice were housed in exposure rooms maintained at ≈21–24°C and 40–60% relative humidity, with automatic 12-h light/dark cycles until the beginning of the study. Mice were group-housed (4/cage) in shoebox cages covered with plastic filter tops. Each experimental group contained eight mice. Harlan Taklad Laboratory Diet (NIH 07) and drinking water (either vehicle or appropriate CHL solution) were provided ad libitum. Mice were 8–10-weeks-of-age at the beginning of the studies.
Five concentrations of CHL were utilized: 2, 10, 20, 100, and 200 ppm; these doses were based on the concentrations of CHL detected in the drinking water: 0–3.5 ppm from July 1999 to August 2000 at 19 sites in the Raleigh, NC, water distribution system (DiGiano et al., Citation2002) and the EPA public guideline value for CHL in drinking water (i.e., 4 ppm). CHL exposure levels were chosen to include a concentration known to occur in the finished drinking water (i.e., 2 ppm) and increasing concentrations based on 1/3 log increases to a maximum of 200 ppm, or 50-times greater than the EPA’s mandated maximum residual disinfectant level.
CHL solutions at each level administered were obtained from the Midwest Research Institute (MRI, Kansas City, MO) in weekly shipments. CHL solutions were prepared under conditions of alkaline pH (> 8.0) by adding 0.01 M sodium hypochlorite to 0.1 M ammonium chloride mixed in 0.01 M sodium hydroxide-sodium chloride. The final CHL solution was diluted in deionized tap water to desired concentrations. The CHL solutions, found to be stable for at least 1 week when stored at ambient conditions while protected from light, were prepared fresh every week in deionized tap water at MRI and shipped to VCU (under those optimal standards). CHL was administered in polycarbonate screw-top red bottles with teflon/silicone septa (Anacare Corp., Bellmore, NY). Water bottles were changed daily. For each concentration, there was an additional bottle prepared and placed in an empty cage in order to control for drip losses. Deionized tap water, also obtained from MRI, was used as the vehicle control, and the background CHL concentrations in the deionized tap water were determined to be between 0–0.4 ppm.
Cyclophosphamide (CPS; Sigma Chemical Co., St. Louis, MO) was given as the positive control for analyses of hematological parameters, spleen IgM antibody-forming cell (AFC) response, and the mixed-leukocyte response. The positive control animals received 50 mg/kg of CPS on each of the last 4 days of the exposure period by intraperitoneal (IP) injection (0.1 ml/10 g of body weight [BW]). Rabbit anti-asialo GM1 antibody (AAGM1, Wako BioProducts, Richmond, VA) was administered intravenously (IV) in a volume of 0.2 ml at a 1:10 dilution 24 h prior to sacrifice as the positive control in the natural killer (NK) cell assay.
Water consumption
Water bottles were changed daily during the 28-day exposure period. The amount of water consumed was based on the weight loss (Δg) of the drinking water during each 1-day period. Each bottle was filled with deionized tap water or the appropriate CHL solution and weighed (initial weight) prior to being placed on the cage. Bottles were removed from the cages and re-weighed (post-weight) after 24 h. On each rack, a control drip bottle was established to correct for handling of cages and inadvertent rack movement. The control drip bottle was placed in an identical cage used in the study; however, no mice were present in the cage. The post-weight value was subtracted from the initial weight of the bottle, and then the Δg of the control drip bottle for the same period was subtracted to yield the corrected Δg. To establish the consumption total (i.e., g/day/mouse), the final Δg was divided by the number of animals per cage (n = 4). Since each dose group consisted of eight animals (two cages of four mice each), the average of the drinking water consumed from the two cages was calculated.
Toxicological studies
Animal body weights were obtained weekly as an index of overt toxicity. On Day 29 of the study (the first day of dosing was designated as Day 1), mice were anesthetized by CO2 inhalation and blood was collected from the retro-orbital sinus into EDTA tubes for hematological analysis. Mice were then euthanized by excess CO2 followed by cervical dislocation. Animals were examined for gross pathology, and the following organs were removed and weighed: thymus, lungs, liver, spleen, and kidneys with adrenals. Selected hematological parameters were assessed, including the number of erythrocytes and leukocytes, hemoglobin (Hb), hematocrit, mean corpuscular volume (MCV), mean cell Hb (MCH), and mean cell Hb concentration (MCHC). The percentage of reticulocytes was evaluated using the RNA stain thiazole orange (Becton Dickinson, San Jose, CA) by flow cytometric analysis. Differential white blood cell counts were determined from blood smears stained with Wright-Giemsa (Fisher Scientific, Pittsburgh, PA).
Immunological studies
Natural killer cell activity
The activity of NK cells was assayed in CHL-treated mice, as described by Reynolds and Herberman (Citation1981) with modifications (Auttachoat et al., Citation2009; Smith et al., Citation2010). Single cell suspensions of splenocytes (effector cells) were aseptically prepared from the mice that received CHL, vehicle, or the AAGM1 positive control. Serial dilutions at six concentrations (2 × 107, 1 × 107, 5 × 106, 2.5 × 106, 1.25 × 106, and 0.625 × 106 cells/ml) of cells from each mouse were made (in duplicate) in a volume of 100 μl within 96-well round-bottom microtiter plates. Target [51Cr]-labeled YAC-1 cells (labeled using Na51CrO4 [NEN Life Science Products, Inc., Boston, MA] and protocols previously described in Smith et al. (Citation2010)), in a volume of 0.1 ml at 1 × 105 cells/ml, were then added to each well to yield effector:target (E:T) ratios of 200:1, 100:1, 50:1, 25:1, 12.5:1, and 6.25:1. Spontaneous release and maximum [51Cr] release were each determined by adding 0.1 ml of, respectively, medium or Triton X-100 (0.1%) to each of 12 replicate wells containing target cells only. Following a 4-h incubation at 37°C, the plates were centrifuged and an aliquot of 0.1 ml of the supernatant was removed from each well and counted for [51Cr] released using a γ-counter (1480 Wizard Gamma-well Counter, Perkin-Elmer, Waltham, MA) to obtain counts per minute (cpm). The percent cytotoxicity at each effector concentration was then calculated as (experimental release CPM − spontaneous release CPM)/(maximum release CPM − spontaneous release CPM).
Hemolytic plaque assay for detecting IgM antibody-forming cells (AFC assay)
The primary IgM AFC response to sheep red blood cells (SRBC) was assessed using a modification of the hemolytic plaque assay of Jerne and Nordin (Citation1963), as described by White et al. (Citation2010). Mice were immunized (IV) with 7.5 × 107 SRBC (Lampire Biological Laboratories, Pipersville, PA) 4 days prior to sacrifice. Spleen cell suspensions were prepared and an aliquot of cells was added to a test tube containing guinea pig complement, SRBC, and warm agar (46–48°C). The mixture was plated in a petri dish, covered with a microscope coverslip, and incubated at 37°C for 3 h. Plaques that developed were counted using a plaque viewer (Bellco Glass, Inc., Vineland, NJ) at 6.5X magnification. All data were then expressed as AFC/106 spleen cells and AFC (×103)/spleen.
Quantitation of serum IgM antibody titers to the T-dependent antigen SRBC
An ELISA assay was performed as described by Temple et al. (Citation1993, with modification) to determine serum titers of primary IgM antibodies to SRBC in the same hosts evaluated in the AFC assay. Briefly, high-salt release SRBC antigens were diluted 1:100 in PBS (phosphate-buffered saline, pH 7.4) and applied to Immulon 2 flat-bottom microtiter plates (Dynex Labs, Chantilly, VA). Plates were incubated at 4°C for ≈ 24 h and then washed with PBS containing 0.05% Tween 20 to prevent non-specific binding. Serum was serially diluted in washing buffer (starting at 1:16 dilution) in the plates, and the plates then incubated for 60 min at room temperature. After washing, secondary antibody (1:500 dilution of affinity purified horseradish peroxidase-conjugated goat anti-mouse IgM antibody; Southern Biotechnology Associates, Inc., Birmingham, AL) was added and allowed to incubate for 60 min. Unbound antibodies were then washed off and peroxidase substrate (2,29-azino-bis-[3-ethyl-benzthiazoline-6-sulfonic acid], Sigma) was added. The plates were incubated for 45 min and then analyzed on an ELISA plate reader (Molecular Devices Thermomax Plate Reader, Rockville, MD). Antibody titers were calculated using SoftMax software 2.32.
One-way mixed-leukocyte response (MLR)
The response to allogeneic leukocytes from DBA/2 mice was assessed as previously described (Holsapple et al., Citation1983) using splenocytes from mice exposed to CHL. One hundred microliters of responder splenocytes (1 × 106 cells/ml) were co-cultured with stimulator cells (DBA/2 spleen cells at a ratio of 4:1) in a U-bottom microtiter plate (Costar 3799, Cambridge, MA). Stimulator cells were treated with mitomycin C (50 μg/ml) to inhibit cell proliferation. The cells were cultured for 5 days, during the last 18 h in the presence of 1 µCi [3H]-thymidine (ICN Biomedicals, Inc., Costa Mesa, CA). The cells were collected with a cell harvester and counted in a LKB liquid scintillation counter. The incorporation of [3H]-thymidine into the DNA was used as a measure of cell proliferation, and the data were expressed as cpm/1 × 105 cells.
Enumeration of splenocyte subsets
Flow cytometric analysis to quantify total T-lymphocytes, T-lymphocyte subsets, total B-lymphocytes, NK cells, and monocytes in mice that received CHL treatment was conducted using a previously described method (Guo et al., Citation2000). After being washed with staining buffer (1% bovine serum albumin [w/v] and 0.1% sodium azide in PBS), splenocytes were incubated with monoclonal antibody (mAb) labeled with phycoerythrin (PE) or fluorescein isothiocyanate (FITC) diluted 1:80 in staining buffer for 30 min at 4°C. After incubation, the cells were washed twice and propidium iodide (PI) was added to label dead cells. Antibodies utilized included: goat anti-mouse Ig-FITC, rat anti-mouse CD4-PE, rat anti-mouse CD8a-FITC, Armenian Hamster anti-mouse CD3-FITC, mouse anti-mouse NK1.1, and rat anti-mouse Mac3-FITC, all obtained from BD Pharmingen (San Diego, CA). Isotype matched antibodies to irrelevant epitopes were used as the controls. The samples were analyzed with a FACScan (Becton Dickinson, San Jose, CA) flow cytometer with CellQuest software. The majority of the red cell population and debris were eliminated electronically by increasing the threshold setting on the dot plot of forward scatter (FSC). A minimum of 5000 events/sample was acquired.
Statistical analysis
Data were analyzed for homogeneity using the Bartlett’s Test. Homogeneous data were subsequently evaluated using a one-way analysis of variance (ANOVA); a Dunnett’s test was used to compare differences between the treatment and vehicle control groups. Non-homogenous data were evaluated using a non-parametric ANOVA. Significant differences between the experimental and control groups were subsequently evaluated using a Wilcoxon Rank Test. Positive controls were compared to vehicle animals using a Student’s t-test. A treatment was considered statistically significant from the control group at p-values ≤ 0.05.
Results and discussion
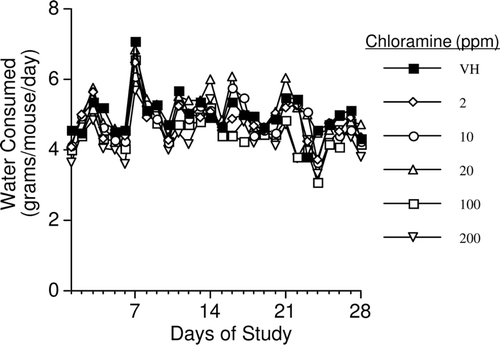
Mice showed no overt signs of toxicity, as evidenced by no observed changes in gait, fur status, drainage from orifices, or general grooming. When mean water consumption was evaluated after combining the data from five studies, no significant differences were observed between any of the CHL-exposed animals and the vehicle control group ().
Figure 1. Average drinking water consumption in female B6C3F1 mice exposed to chloramine for 28 days in their drinking water. Values represent mean (± SE) consumption (n = 40).
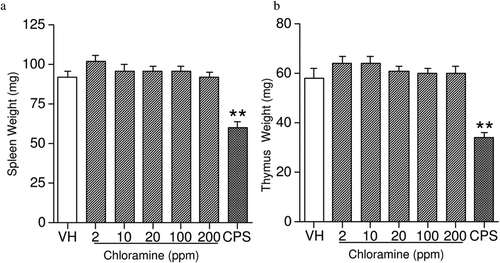
The combined mean starting body weights (BW) of the vehicle-exposed mice was 20.9 (± 0.2) g and the mean ending BW for vehicle-exposed mice was 24.1 (± 0.3) g. Over the course of the studies, there were no significant differences in either BW () or BW gain () between CHL-exposed mice and vehicle controls. No significant alterations in organ weights (including liver, spleen, thymus, lungs, and kidneys [with adrenals]) were noted in CHL-exposed mice when the data were expressed as either absolute organ weight () or percent of total BW (data not shown). These observations were consistent with reports of the effects of CHL exposure in male rats, except that a reduced relative spleen weight was reported in the rats treated with 38 ppm CHL in their water from weaning to 12 weeks-of-age (Exon et al., Citation1987). The sex, species, age, and length of exposure may be the contributing factors for this discrepancy.
Figure 2. (a) Body weight and (b) change in body weight in female B6C3F1 mice exposed to chloramine for 28 days in their drinking water. Values represent mean (± SE) body weight and change in body weight (n = 40).
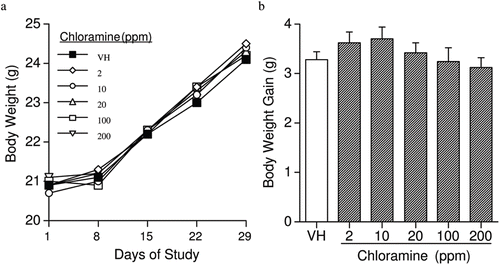
Figure 3. Absolute weights of the (a) spleen and (b) thymus in female B6C3F1 mice exposed to chloramine for 28 days in their drinking water. Values represent mean (± SE) organ weights derived from eight mice/group. ** p ≤ 0.01 when compared to vehicle.
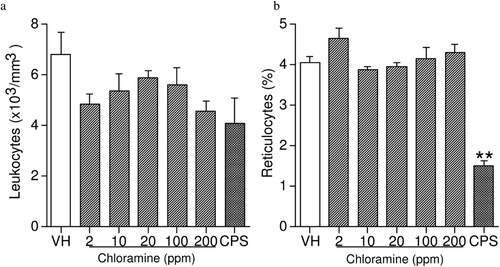
Exposure to CHL had no effects on either leukocyte counts or percent reticulocytes (). In addition, hemoglobin, hematocrit, MCV, MCH, MCHC, platelets, and leukocyte differentials were unaffected by CHL (data not shown). Treatment with the positive control CPS produced a 63% decrease in reticulocytes, in addition to the anticipated changes in the other hematological parameters. It has been reported that hemodialysis fluids containing 3–4 ppm CHL produce severe hemolytic anemia in patients following repeated treatment (Kjellstrand et al., Citation1974; Lockhart, Citation1998; Fenves et al., Citation2000). Lack of effect on these hematological parameters in female B6C3F1 mice following 28 days of CHL exposure in the drinking water suggests the route of exposure may determine CHL toxicity. However, Abdel-Rahman et al. (Citation1984) reported that the glutathione content in rat blood was significantly decreased after 4 months of CHL treatment at 1 and 100 ppm, and significant decreases were observed in all treatment groups after 6 months of CHL treatment (1–100 ppm). In that study, red blood cell counts and hematocrit were significantly decreased after 3 months, and both hemoglobin concentration and MCHC were also decreased by 10 months of treatment. Thus, exposure of mice to CHL for longer periods may be necessary to insure that CHL does not adversely affect the hematological system.
Figure 4. Leukocyte counts and percentage of reticulocytes in female B6C3F1 mice exposed to chloramine for 28 days in their drinking water. Values represent mean (± SE) counts and percentages derived from eight mice/group. ** p ≤ 0.01 when compared to vehicle.
NK cells are a first line of defense deployed by the innate immune system and play an important role in killing certain types of tumor cells. We examined NK activity by measuring NK cytotoxicity toward YAC-1 tumor cells (endpoint = percent cytotoxicity at each E:T ratio). Exposure to CHL did not significantly affect activity of NK from treated animals as compared to that by cells from the vehicle controls (). The positive control, AAGM1, significantly decreased NK cell activity as anticipated (). A lack of effect on NK cells has also been reported in male rats that have been exposed to CHL (9–38 ppm) via the drinking water from weaning to 12 weeks-of-age (Exon et al., Citation1987).
Table 1. Natural killer cell activity in female B6C3F1 mice exposed to chloramine in the drinking water for 28 days.
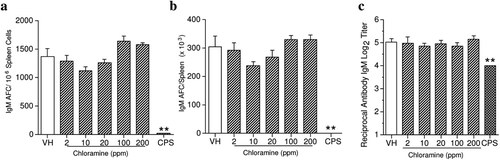
In these studies there were no CHL-associated effects on humoral immunity, as evaluated by the spleen IgM AFC response to SRBC when presented as AFC/106 spleen cells (specific activity; ) or as AFC (×103)/spleen (total activity; ). Furthermore, serum titers of IgM anti-SRBC antibodies were similar in treated and control groups (). The positive control CPS significantly decreased the IgM AFC response and serum IgM anti-SRBC antibodies. In contrast, anti-KLH IgG antibody levels were significantly suppressed in a rat study (Exon et al., Citation1987) where animals were treated with either 9 or 19 ppm CHL in their water. A possible explanation for the discrepancy is that the secondary immune response was measured in the rat study, while the primary immune response (IgM) was evaluated in our studies. Thus, further studies to examine the effects of CHL on the memory immune response, including the levels of antigen-specific IgG and IgE, and the expression of memory cell phenotypic markers by T-lymphocytes (e.g., CD44, CD45, CD62L, and chemokine receptors) and B-lymphocytes (e.g., CD80, CD35) may be useful for risk assessment purposes.
Figure 5. Chloramine exposure had no effect on the spleen IgM antibody-forming cell (AFC) response presented as (a) IgM AFC/106 spleen cells (specific activity), (b) IgM AFC/spleen (total activity), and (c) serum IgM antibody titer. The mice were exposed to deionized tap water (vehicle) or chloramine in their drinking water for 28 days. Cyclophosphamide (CPS) was given as a positive control by IP injection at 50 mg/kg in the last 4 days of the exposure period. Values represent mean (± SE) specific activity, total activity, and/or titers from eight mice/group. ** p ≤ 0.01 when compared to vehicle.
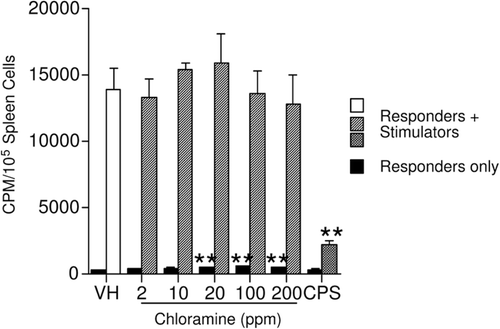
The effect of CHL on cell-mediated immunity was assessed here using a one-way MLR. There were no significant changes in the proliferative response of the responders to allogeneic leukocytes following CHL exposure (). The small but statistically significant increases in basal (responders only) cell proliferation at the three highest dose levels were not considered biologically significant in the absence of an altered T-lymphocyte-mediated response. CPS (positive control) significantly decreased the proliferative response of the splenocytes in the presence of stimulator cells, as expected. A lack of effect on T-lymphocytes has also been reported in male rats in which the delayed-type hypersensitivity reaction to bovine serum albumin was not altered following exposure to CHL (9–38 ppm)-containing water (Exon et al., Citation1987).
Figure 6. Effect of chloramine on the mixed-leukocyte response. The mice were exposed to tap water (vehicle) or chloramine in drinking water for 28 days. Values represent mean (± SE) response derived from eight mice/group. CPS, cyclophosphamide. ** p ≤ 0.01 when compared to vehicle.
To enumerate leukocyte populations in the spleen, cells were labeled with appropriate monoclonal antibody and absolute and percent values of spleen CD3+, CD4+CD8−, CD4−CD8+, and immature T-lymphocytes (CD4+CD8+), as well as of B-lymphocytes (Ig+), NK cells (NK1.1+CD3−), and monocytes (Mac-3+) were determined. It has been our experience that the absolute values are the more appropriate representation of this type of data. These values incorporate changes in spleen cell number and are more representative of what is occurring in vivo. No significant effects were observed on any of the parameters, when evaluated either as absolute values or percent values (). The positive controls significantly decreased both the absolute numbers and percent values (with the exception of CD4+CD8+ cells) of each cell type.
Table 2. Percent and absolute values of differential splenocytes in female B6C3F1 mice exposed to chloramine in the drinking water for 28 days.
An increase in asthma due to exposure from CHL in indoor swimming pool areas has been reported (Bernard et al., Citation2003), which may be due to an irritant effect of CHL or other non-specific mechanisms. Bowen et al. (Citation2007) have suggested that contact with CHL or inhalation of CHL-contaminated air is associated with respiratory illness—as is expected with a mucosal irritant—and that dermal absorption or ingestion may not be necessary to produce symptoms. Therefore, further studies to examine the effects of inhalational exposure to CHL on the immune cell populations within the lungs of mice and to determine if there exists an exacerbation of asthma symptoms following challenge of these mice with respiratory antigens are warranted.
In conclusion, CHL, when administered for 28 days in the drinking water at doses from 2–200 ppm produced minimal toxicological and immunotoxic effects in female B6C3F1 mice. The highest exposure level of CHL evaluated here was 200 ppm (or 200 mg/L). Assuming a density of 1 g/ml for the deionized tap water vehicle, this is equivalent to 40 mg/kg per day for a 25 g mouse consuming 5 g water/day, which, on an equivalent surface area basis, is ≈ 3.2 mg/kg for humans. The US EPA regulations limit the CHL concentration in drinking water to 4 ppm (EPA, Citation2010b). A 60 kg person with a daily consumption of 2 L of water containing this maximum allowable level of CHL would be exposed to ≈ 133 μg CHL/kg, a value 24-times less than the exposure calculated above to occur at the highest concentration of CHL evaluated in this study. The absence of immunotoxic effects in these studies suggests that there is little concern for immunotoxicity due to exposure to CHL in the drinking water for 28 days.
Acknowledgment
This work was supported in part by EPA Interagency Agreement DW75937992 and the Division of Intramural Research at the NIEHS through Contract ES55538. The authors thank D. L. Musgrove and R. D. Brown for their technical assistance, and Dr Michelle Hooth and Dr Marsha Ward for their comments and review of the manuscript.
Declaration of interest
Dr Kimber L. White, Jr. is the owner of a company, ImmunoTox®, Inc., that conducts immunotoxicological studies under Good Laboratory Practices (GLP). This work was supported by the National Institute of Environmental Health Sciences [ES55538]. This article may be the work product of an employee or group of employees of the National Institute of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH), however, the statements, opinions, or conclusions contained therein do not necessarily represent the statements, opinions, or conclusions of NIEHS, NIH, or the US government. This report has been reviewed by the Environmental Protection Agency’s Office of Research and Development and approved for publication. Approval does not signify that the contents necessarily reflect the views of the Agency nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
References
- Abdel-Rahman M. S., Suh D. H., and Bull R. J. 1984. Toxicity of monochloramine in rat: An alternative drinking water disinfectant. J. Toxicol. Environ. Health 13:825–834.
- Auttachoat W., Germolec D. R., Collins B. J., Luebke R. W., White K. L., and Guo T. L. 2009. Immunotoxicological profile of chloroform in female B6C3F1 mice when administered in drinking water. Drug Chem. Toxicol. 32:77–87.
- Bernard A., Carbonnelle S., Michel O., Higuet S., De Burbure C., Buchet J. P., Hermans C., Dumont X., and Doyle I. 2003. Lung hyper-permeability and asthma prevalence in schoolchildren: Unexpected associations with the attendance at indoor chlorinated swimming pools. Occup. Environ. Med. 60:385–394.
- Bowen A. B., Kile J. C., Otto C., Kazerouni N., Austin C., Blount B. C., Wong H. N., Beach M. J., and Fry A. M. 2007. Outbreaks of short-incubation ocular and respiratory illness following exposure to indoor swimming pools. Environ. Health Perspect. 115:267–271.
- DiGiano F. A., Zhang W. D., Travaglia A., Francisco D. E., and Wood M. 2002. Occurrence of Bacterial Regrowth and Nitrification in the Raleigh Distribution System and Development of an EPANET Model for Future Assessments. Report No. 338 April 2002. Available online at: http://www.ncsu.edu/wrri/reports/summaries/338.html
- Eaton J. W., Kolpin C. F., Swofford H. S., Kjellstrand C. M., and Jacob H. S. 1973. Chlorinated urban water: A cause of dialysis-induced hemolytic anemia. Science 181:463–464.
- EPA (Environmental Protection Agency). 1978. Interim primary drinking water regulations. “Control of Organic Chemical Contaminant in Drinking Water.” Fed. Reg. Part II:5756–5780.
- EPA (Environmental Protection Agency). 2010a. Information about chloramines in drinking water. Available online at: http://www.epa.gov/safewater/disinfection/chloramine/pdfs/chloramine2.pdf
- EPA (Environmental Protection Agency). 2010b. Website: basic information about disinfec-tants in drinking water: Chloramine, chlorine and chlorine dioxide. In: Basic Information about Regulated Drinking Water Contaminants. Available online at: http://water.epa.gov/drink/contaminants/basicinformation/disinfectants.cfm
- Exon J. H., Koller L. D., O’Reilly C. A., and Bercz J. P. 1987. Immunotoxicologic evaluation of chlorine-based drinking water disinfectants, sodium hypochlorite, and monochloramine. Toxicology 44:257–269.
- Fenves A. Z., Gipson J. S., and Pancorvo C. 2000. Chloramine-induced methemoglobinemia in a hemodialysis patient. Semin. Dial. 13:327–329.
- Guo T. L., McCay J. A., Brown R. D., Musgrove D. L., Butterworth L., Munson A. E., Germolec D. R., and White K. L. 2000. Glycidol modulation of the immune responses in female B6C3F1 mice. Drug Chem. Toxicol. 23:433–457.
- Holsapple M. P., Munson A. E., Munson J. A., and Bick P. H. 1983. Suppression of cell-mediated immunocompletence after subchronic exposure to diethylstilbestrol in female B6C3F1 mice. J. Pharmacol. Exp. Ther. 227:130–138.
- Jacobs J. H., Spaan S., van Rooy G. B., Meliefste C., Zaat V. A., Rooyackers J. M., and Heederik D. 2007. Exposure to trichloramine and respiratory symptoms in indoor swimming pool workers. Eur. Respir. J. 29:690–698.
- Jerne N. K., and Nordin A. A. 1963. Plaque formation in agar by single antibody-producing cells. Science 140:405–406.
- Kjellstrand C. M., Eaton J. W., Yawata Y., Swofford H., Kolpin C. F., Buselmeier T. J., von Hartitzsch B., and Jacob H. S. 1974. Hemolysis in dialyzed patients caused by chloramines. Nephron 13:427–433.
- Krzystyniak K., Tryphonas H., and Fournier M. 1995. Approaches to the evaluation of chemical-induced immunotoxicology. Environ. Health. Perspect. 103 (Suppl 9):17–22.
- Lévesque B., Duchesne J. F., Gingras S., Lavoie R., Prud’Homme D., Bernard E., Boulet L. P., and Ernst P. 2006. The determinants of prevalence of health complaints among young competitive swimmers. Int. Arch. Occup. Environ. Health 80:32–39.
- Lindstrom A. B., Pleil J. D., and Berkoff D. C. 1997. Alveolar breath sampling and analysis to assess trihalomethane exposures during competitive swimming training. Environ. Health Perspect. 105:636–642.
- Lockhart A. C. 1998. A hemodialysis patient with chloramine-induced hemolysis. A discussion of the mechanism. N. C. Med. J. 59:248–250.
- Luster M. I., Munson A. E., Thomas P. T., Holsapple M. P., Fenters J. D., White K. L. Lauer L. D., Germolec D. R., Rosenthal G. J., and Dean J. H. 1988. Development of a testing battery to assess chemical-induced immunotoxicity: National Toxicology Program’s guidelines for immunotoxicity evaluation in mice. Fundam. Appl. Toxicol. 10:2–19.
- Plewa M. J., Wagner E. D., Richardson S. D., Thruston A. D., Woo Y. T., and McKague A. B. 2004. Chemical and biological characterization of newly discovered iodoacid drinking water disinfection byproducts. Environ. Sci. Technol. 38:4713–4722.
- Reynolds C. W., and Herberman R. B. 1981. In vitro augmentation of rat natural killer (NK) cells activity. J. Immunol. 126:1581–1585.
- Smith M. J., Germolec D. R., Luebke R. W., Sheth C. M., Auttachoat W., Guo T. L., and White K. L. Jr. 2010. Immunotoxicity of dibromoacetic acid administered via drinking water to female B6C3F1 mice. J. Immunotoxicol. 7:333–433.
- Temple L., Kawabata T. T., Munson A. E., and White K. L. Jr. 1993. Comparision of ELISA and Plaque-Forming Cell assay for measuring the humoral immune response to SRBC in animal treated with benzo(a)pyrene or cyclophosphamide. Fund. Appl. Toxicol. 21:412–419.
- Vermont Department of Health. 2011. Monochloramine as a Drinking Water Disinfectant. Available online at: http://healthvermont.gov/enviro/water/chloramine.aspx
- Weaver W. A., Li J., Wen Y., Johnston J., Blatchley M. R., and Blatchley E. R. 3rd. 2009. Volatile disinfection by-product analysis from chlorinated indoor swimming pools. Water Res. 43:3308–3318.
- White K. L., Musgrove D. L., and Brown R. D. 2010. The sheep erythrocyte T-dependent antibody response (TDAR). Meth. Mol. Biol. 598:173–184.
- Zierler S., Danley R. A., and Feingold L. 1986. Type of disinfectant in drinking water and patterns of mortality in Massachusetts. Environ. Health Perspect. 69:275–279.