Abstract
This study was designed to evaluate some immunological responses of male White Leghorn (WL) chicks kept on an ochratoxin A (OTA)-contaminated diet. For this purpose, 350 1-day-old male WL chicks were divided into five groups (A–E). Group A was kept as control, while Groups B, C, D, and E were fed OTA-contaminated feed at 0.1, 0.5, 1.0, and 1.5 mg/Kg diet, respectively, for 21 days, and then basal ration for the remaining period. At 14- and 16-days of age, random chicks (n = 10) from each group were used for analyses of phagocytic function of the reticuloendothelial system or for measuring the lymphoproliferative responses to intradermally-administered T-cell mitogen, phytohemagglutinin-P (PHA-P), respectively. At 30-days of age, abdominal macrophages were collected from 15 chicks/group and utilized for determination of their phagocytic potential and for nitrite production. Antibody (Ab) titers (i.e., total antibodies, IgM, and IgG) against sheep red blood cells (SRBC) were determined at 7 and 14 days after a primary (at 7 days of age) and a booster (given 14 days after primary [at 21-days of age]) dose (intravenous) of the antigen. Data from the present study showed that the relative weight of the bursa of Fabricius of chicks fed OTA for 14 and 21 days and the spleen of chicks fed OTA for 21 days were significantly lower than their control counterpart. Phagocytic function of reticuloendothelial system evaluated by carbon clearance, and lymphoproliferative response to PHA-P, of chicks kept on OTA-contaminated diet were significantly lowered. The percentage of abdominal macrophages displaying phagocytosis of SRBC, the number of SRBC/macrophage, and nitrite production were each significantly lower in cells from chicks in the OTA-fed groups. Total Ab (at days 7 and 14 post-booster SRBC injection) and IgG (at day 14 post-primary and day 7 post-booster SRBC injection) titers against SRBC showed significant reductions in the groups fed OTA-contaminated diet. The findings of this study are in line with the previous work suggesting the immunosuppressive effect of OTA in male WL chicks regarding functional impairment in some of the components of the immune system.
Introduction
Mycotoxins, the secondary metabolites of some toxigenic fungi, are unavoidable contaminants of human and animal food and feeds (Wood, Citation1992; Choudhary and Kumari, Citation2010). The CAST report of 2003 estimated that 25–30% of the world crop may be contaminated with mycotoxins, and the estimated losses due to mycotoxins in animal industries have been estimated to be as much as several hundred million dollars per annum. In the long list of >300 known mycotoxins, e.g., aflatoxins, ochratoxins, zearalenone, tricothecene, T-2 toxins, fumonisin, and deoxynivalenol, each are well known for their toxicities (Brera et al., Citation1998; Dewegowda et al., Citation1998; Binder et al., Citation2007).
Ochratoxin A (OTA), among the different classes of mycotoxins, is an important contaminant of cereals intended for use in animal and poultry feed (Agag Citation2004; Baydar et al., Citation2005; Saha et al., Citation2007; Saleemi et al., Citation2009), and is produced by seven species of Aspergillus and six species of Penicillium. Aspergillus ochraceus, from which the toxins acquired their name, appears to be the predominant ochratoxin producer (Trenk et al., Citation1971; Varga et al., Citation1996). OTA is characterized by numerous toxicities. Various studies have illustrated its nephrotoxic, hepatotoxic, immunotoxic, teratogenic, genotoxic, mutagenic, and/or neurotoxic effects, as well as its capacity to impart growth suppressive effects in different avian and mammalian species (Gilani et al., Citation1978; Kumar et al., Citation2004; Robbiano et al., Citation2004; Wangikar et al., Citation2005; ul-Hassan et al., Citation2010, Citation2011).
In general, the toxicological effects of mycotoxins are attributed to a decrease in feed intake, reduced nutrient absorption (along with concomitant altered metabolism), endocrine disturbances, and immunosuppression (Whitlow and Hagler, Citation2002). Though the specific cellular and molecular mechanisms underlying the immunotoxic effects of OTA are not known, inhibition of protein, DNA, and RNA synthesis, as well as degenerative/apoptotic changes in key immune system cells due to ochratoxicosis provide significant clues to determining how OTA mediates its effects (Sharma, Citation1993; Santin et al., Citation2002; Rocha et al., Citation2005; Al-Anati and Petzinger, Citation2006). In mice, feeding OTA-contaminated diet at 2.6 mg/Kg feed for 90 days resulted in the significant depression in the levels of CD4+ or CD8+ cell types and in the mitogenic responses to Concanavalin A (Thuvander et al., Citation1995). In an experiment, feeding OTA at 2 mg/Kg diet to broiler chicks for 42 days, Santin et al. (Citation2002) reported significantly lower humoral immune responses (as demonstrated by decreased titers to Newcastle disease virus). Depressed humoral and cell-mediated immune responses were also noted by Sawale et al. (Citation2009) in laying hens that had been maintained on an OTA-contaminated diet (at 1 ppm) for 60 days.
While information is available in the literature about the immunotoxic effects of OTA (at high doses of 2–5 mg/Kg diet) in various animal species (Santin et al., Citation2002; Al-Anati and Petzinger, Citation2006) including the WL chicken (adults as well as chicks), little is known about the immunological effects of this toxin in male WL chicks. It is important to note that earlier studies reported sex-specific responses to OTA intoxication in rats (Castegnaro et al., Citation1998) and chicks (Stoev Citation2010), where males were more sensitive to OTA when compared to females. This difference in response—at least in rats—was attributed to the differences in expression and/or activity of select drug-metabolizing enzymes (i.e., cytochrome P450s [CYPs] isoforms) that convert OTA into reactive intermediates that form DNA-adducts and lead to carcinogenic outcomes in the hosts (Pfohl-Leszkowicz et al., Citation1998). Thus, keeping in mind all the above-mentioned factors, the present study was designed to evaluate some immunological responses of male (being presumptively more sensitive than female counterparts) WL chicks that are maintained (for 3 weeks) on a diet contaminated with different levels of OTA.
Materials and methods
Ochratoxin and feed
The lyopholized spores of A. ochraceus (CECT 2948, Culture Collection Center, University De Valencia, Valencia, Spain) were rehydrated in nutrient broth and inoculated on sterile potatoes dextrose agar (PDA) plates, and the plates were incubated at 28°C for 5 days. The colony characteristics of the A. ochraceus were observed; for microscopic studies, slide cultures were stained with lactophenol cotton blue. Spores of A. ochraceus were re-cultured on PDA slants and incubated at 28°C for 2 weeks.
For the production of OTA, wheat grains were used as a substrate, as described by Trenk et al. (Citation1971) and ul-Hassan et al. (Citation2011). For this purpose, 80 g of wheat grains were soaked in 80 ml distilled water in a 1 L Erlenmeyer flask for 2 h and then autoclaved for 20 min at 121°C. An inoculum prepared from a 2-week-old slant culture of A. ochraceus in 3 ml sterile water (having 0.005% Triton 100X) was then mixed with the autoclaved wheat in the flask. The flask was then incubated for 14 days at 28°C (at 39% relative humidity) in the dark and shaken once daily to break the mycelial growth. On day 15, the flask was autoclaved at 121°C for 3 min to destroy the mycelial growth. Ochratoxin A was then extracted from the fermented wheat in acetonitrile-water and quantified using high-performance liquid chromatography (HPLC) and fluorescent detection methods (Bayman et al., Citation2002).
Basal diet (21% total protein and 2900 Kcal/Kg metabolizable energy) was prepared without addition of toxin binder, vitamins, minerals, and antibiotics. Prior to use, each batch of the basal diet was analyzed for ochratoxin, aflatoxin, and zearalenone to ensure that the levels of each were <1.0 µg/Kg. OTA-contaminated feeds were prepared by incorporation of known quantities of OTA. For this purpose, fermented wheat grains were extracted by soaking in a 3-fold quantity of chloroform (1:3) overnight and then filtered through cotton cloth. All the chloroform was then evaporated and the concentrated residues re-suspended into polyethylene glycol (PEG). This suspension was then evenly mixed in the required quantity of basal feed to prepare the experimental feeds containing each desired concentration of OTA. Prior to being used for feeding, the OTA concentration in each experimental diet was verified by HPLC.
Induction of ochratoxicosis in white leghorn chicks
All animal experiments were conducted according to the rules and regulations of the Animal Ethics Committee, Faculty of Veterinary Science, at the University of Agriculture, Faisalabad (Pakistan). White Leghorn (WL) chicks (350 total, each 1-day-old) were procured from a local hatchery and then kept in a poultry house at the Department of Pathology in the University of Agriculture Faisalabad, Pakistan, under standard environmental conditions. Animal rooms were kept at 33°C during the first week of age (and reduced by ≈ 2.5°C per week thereafter), a 60% relative humidity, and with a 12-h/12-h light-dark cycle; all chicks had access to fresh water ad libitum.
These chicks were divided into five groups (A–E), each consisting of 70 birds. Chicks in Group A were kept on basal chick starter ration (verified not to contain OTA or aflatoxin-B1 (AFB1) contents at a level > 1.0 µg/Kg diet), while those in Groups B, C, D, and E, were provided OTA-contaminated feed at 0.1, 0.5, 1.0, or 1.5 mg OTA/Kg diet, respectively, for a period of up to 21 days. Thereafter, all chicks were switched over to a basal ration for the remaining period of the study, i.e., until they reached a maximum of 35 days-of-age, or until they were euthanized to provide biologic materials for analyses at intervening timepoints post-exposure. The average feed intake along the course of the experiment was ≈ 19.63 g/chick/day. This meant that each bird consumed from 1.96–29.44 µg OTA daily (at the corresponding range from lowest to highest OTA level, respectively) during the 21-day period. The degree of mortality that occurred among each experimental group over the course of the 3-week feeding period was monitored to determine if there were any overt lethality associated with any given exposure regimen.
Necropsy and immunological organs weight
At 14- and 21-days of age, 10 chicks from each group were euthanized by cervical dislocation and their bursa of Fabricius, thymus, and spleen were harvested. These organs were weighed and their relative weight (as percentage of total body weight) was calculated.
Assessment of phagocytic activity of reticuloendothelial cells in the peripheral blood (via measures of carbon clearance activity)
The phagocytic activity of the reticuloendothelial system was determined by measuring clearance of colloidal carbon from the peripheral blood, as described previously by Sugiura et al. (Citation2001), with minor modifications adapted to chicks. Briefly, Black Indian Ink (Pelikan, 4001) was centrifuged at 3000 x g for 30 min and the supernatant fraction was collected and then injected into the right wing vein (at 1 ml/Kg body weight [BW]) of 10 14-day-old chicks/group. Blood samples (200 µl/chick) were collected from the left wing vein at 3 and 15 min post-ink injection. The collected blood samples were immediately transferred into 4 ml aqueous sodium citrate (1%) solution and then centrifuged for 4 min at 50 x g. Optical density of the supernatant was measured at 675 nm in a Spectro 20D + Rs-232 spectrophotometer (LaboMed, Inc. Culver City, CA). The phagocytic index was calculated according to the following equation:
where, t3 = 3 min, t15 = 15 min, ODt3 = optical density at 3 min after the injection of carbon solution, and ODt15 = optical density at 15 min after the injection of carbon solution.
Preparation of peritoneal macrophages
Macrophage functions were assessed in 30-day-old chicks (n = 15/group) according to the methods of Qureshi and Havenstein (Citation1994). Briefly, a pre-soaked (overnight) 3% suspension of Sephadex G-50 (Sigma, St. Louis, MO) in normal saline was injected intraperitoneally into each chick (at dose rate = 1 ml/100 g BW). At 42-h post-injection, each chick was euthanized by cervical dislocation and its abdominal cavity flushed with 30 ml hepranized saline (sterile heparin 0.5 U/ml normal saline) to permit collection of abdominal exudate cells (AEC). The AEC sample was centrifuged at 285 x g for 10 min, and the resultant supernatant discarded. The cell pellet was re-suspended in 4 ml RPMI-1640 complete growth medium supplemented with 5% heat-inactivated fetal calf serum and antibiotic-antimycotic solution (all from PPA Laboratories GmbH, Dartmouth, MA). The total number of cells was then counted on a hemo-cytometer and adjusted to the levels needed for use in phagocytosis and nitrite production assays (see below).
Macrophage phagocytic ability
A 1% suspension of sheep red blood cells (SRBC, in RPMI growth medium, prepared from freshly-collected sheep blood at the Department of Microbiology in the University of Agriculture Faisalabad) was prepared for use as particulate antigen for the AEC. Macrophage monolayers were established by the addition of 1 × 106 AEC/ml from each sample into sterile petri dishes (each dish containing three glass coverslips). After 1 h of incubation in a 5% CO2/95% air chamber, the coverslips were washed to remove non-adherent cells. The remaining macrophage monolayers were then co-incubated at 41°C in a humidified 5% CO2 atmosphere for 60 min with a 1.0 ml (3%) suspension of SRBC that was added to each dish. Any non-internalized SRBC were washed away prior to fixing the coverslips in methanol. The coverslips were then stained with Wright-Giemsa stain and mounted onto microscopic slides with DPX mountant (Merck-BDH, Lutterworth, UK). A total of 200 macrophages were then scored for phagocytosis and number of SRBC engulfed/macrophage. For this, coverslips were examined under a M7000DB light microscope (Swift, Tokyo, Japan) at 1000× magnification.
Nitrite production (as index for oxidative killing)
Lipopolysaccharide (LPS, Escherichia coli O55:B5, Sigma)-induced nitrite production by the isolated abdominal macrophages was quantified as described by Green et al. (Citation1982). Macrophages (calculated number) from each of the chicks were cultured in 24-well plates at 41°C in a 5% CO2 atmosphere. After 1 h incubation, non-adherent cells were removed by washing with complete medium; the remaining cells were then exposed to LPS (1 µg/well) for 24 h. After this incubation period, an aliquot (200 µl) of culture supernatant was collected from each well and each mixed with 200 µl Griess reagent (0.1% naphathylenediamine dihydrochloride and 1% sulfanilamide in 5% phosphoric acid) in 96-well microtitre plates. The plate was incubated for 15 min at 37°C and optical density (OD) in each well then measured at 540 nm using a Biochrom microplate reader (Holliston, MA). A standard curve for the assay was generated using various dilutions of sodium nitrite solution in RPMI-1640 complete medium. Nitrite levels in the supernatant samples were then extrapolated from a standard curve that was generated in parallel from known concentration sodium nitrite solutions.
Lymphoproliferative response to phytohemagglutinin
At 16-days of age, 10 birds/group were injected with phytohemagglutinin (PHA-P) (MP Biomedicals, Inc., Cleveland, OH) to study the lymphoproliferative response described by Corrier (Citation1990) and ul-Hassan et al. (Citation2011). For this purpose, 100 µg PHA-P (dissolved in 0.1 ml normal saline) was injected into the intra-digital space between digits 3 and 4 of the right foot. At the same time, 0.1 ml normal saline was injected into a similar site in the left foot. Skin thickness was then measured 24 and 48 h post-injection using a constant tension micrometer (Global Sources, Shanghai, China). The thickness response was then calculated and expressed (mm) as: cutaneous basophilic hypersensitivity response = (right foot skin thickness − left foot skin thickness) at each timepoint.
Antibody response to SRBC
A 3% suspension of sheep red blood cells (SRBC, in saline solution, prepared from freshly-collected sheep blood at the Department of Microbiology in the University of Agriculture, Faisalabad) was injected intravenously into 7-day-old chicks/group (n = 10/group); a booster dose was injected 14 days later (at 21-days of age). Blood was collected 7 and 14 days after the primary and booster doses, and the corresponding serum was separated and stored at −20°C until analyzed for antibodies using methods described by Delhanty and Solomon (Citation1966), Yamamoto and Glick (Citation1982), and Qureshi and Havenstein (Citation1994). In this process, the serum samples were heat-inactivated at 56°C for 30 min and then analyzed for total, mercaptoethanol-2 (ME)-sensitive (IgM), and ME-resistant (IgG) anti-SRBC antibodies. The results were expressed as log2 of the reciprocal of the highest dilution exhibiting agglutination.
Statistical analysis
A completely-randomized design was used in the experiment. Means of groups were compared using Duncan’s Multiple Range test (Duncan, Citation1955), as described by Steel et al. (Citation1997). The data were analyzed using an MSTATC statistical package (Department of Crop and Soil Sciences, Michigan State University, East Lansing, MI). Data were considered significantly different from one another at p-values ≤ 0.05.
Results
Overt lethality from the exposures
The results indicate that, among the various treatment groups, the incidence of death (mortality) was ‘highest’ in chicks that were provided the highest OTA dose tested, i.e., 1.5 mg OTA/Kg diet (). By the end of the 3-week regimen, there was a 5% death incidence among the chicks in this particular group; chicks provided the 1.0 mg OTA/Kg diet also succumbed (i.e., 3.33% mortality) by this timepoint. Exposures to the 0.1 and 0.5 mg OTA/Kg diets led to no overt lethality over the 3-week period.
Table 1. Mortality rates (percentages) among male white leghorn chicks provided OTA-contaminated feed for 3 weeks.
Relative weights of immunological organs
Relative weight of bursa of Fabricius of chicks fed OTA for 14 and 21 days were significantly lower as compared to the values seen in the chicks from the control group (). There was no significant difference in the relative weight of thymus (as compared to control) in the chicks fed an OTA-contaminated diet except in the chicks from group E, which were kept on the OTA-contaminated diet for 14 days. Finally, no significant different was observed (as compared to control) in the relative weight of spleen of the chicks fed OTA for 14 days. In contrast, the chicks fed OTA for 21 days showed significantly lower values in the relative spleen weight as compared to the values shown by the chicks from the control group.
Table 2. Relative organs weights (% of body weight) of male white leghorn chicks maintained on OTA-contaminated feed.
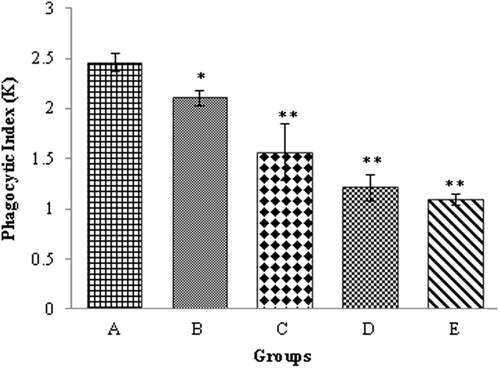
Phagocytic index of the blood reticuloendothelial system
The data of phagocytic index (K) of the reticuloendothelial system of the chicks fed OTA-contaminated diet are shown in . The K value of this system was significantly (p ≤ 0.05) lower in all chicks kept on the OTA-contaminated diets as compared to among control chicks in Group A.
Macrophage functions assays
The abdominal macrophages of the chicks kept on an OTA-contaminated diet displayed significantly lower percentages experiencing phagocytic activity against SRBC as compared to among the cells obtained from control Group A chicks (). Macrophages from the chicks in Group E revealed the lowest percentage of phagocytosis (15.890 ± 0.506; mean ± SE), while the chicks in Group A had the highest values (27.050 ± 0.669). The numbers of SRBC that were engulfed/macrophage were also significantly lower in cells harvested from the abdominal cavity of chicks fed OTA-contaminated diets as compared to by cells obtained from control chicks. The lowest value (1.500 ± 0.018) was seen in cells from Group E chicks and again the highest value (2.550 ± 0.026) was with Group A chick cells. Nitrite production by macrophages treated with LPS was significantly lower from cells from chicks in each of the OTA-fed groups when compared with cells from the Group A hosts. The highest concentration (23.890 ± 0.499) was produced by Group A (control) chick cells, whereas the concentration (11.940 ± 0.256) was produced by cells from chicks in Group E.
Table 3. Macrophage functional assays using cells from chicks provided OTA-contaminated feed for 3 weeks.
Lymphoproliferative response to phytohemagglutinin
Lymphoproliferative responses to intradermal administration of PHA-P into the toe web of the chicks were assessed (). The skin thickness response measured at 24 and 48 h post-PHA-P injection revealed the degree of immunosuppression, with significantly lower values in all the groups maintained on OTA-contaminated diets compared to those of their counterpart chicks in Group A. The highest value of skin thickness (mm) at 24 h post-PHA-P injection was found in Group A chicks, i.e., 0.410 ± 0.005, while the lowest value (0.026 ± 0.006) was noted in Group E animals. At 48 h post-injection, again the highest value was with Group A (0.240 ± 0.003) as compared to the lowest value (0.170 ± 0.006) with chicks in Group E, i.e., those who were maintained on the highest OTA dose (1.5 mg OTA/Kg diet).
Table 4. Lymphoproliferative response against PHA-P in male white leghorn chicks provided OTA-contaminated feed for 3 weeks.
Antibody response to SRBC
The antibody (Ab) titers against SRBC in chicks fed OTA are shown in . At day 7 post-primary SRBC injection, titers of total Ab, IgM, and IgG did not significantly differ among any of the experimental groups. In contrast, at day 14 post-primary injection, the IgG titer in Group C, D, and E chicks was significantly lower compared to that in chicks in Group A (control). Total Ab titers, at day 7 post-booster SRBC injection, were significantly lower in the groups that had been maintained on OTA-contaminated diets as compared to control chick titers. Compared to in controls, the IgG titer was also significantly lowered in all OTA-fed groups (except group B). Chicks in Groups C, D, and E had significantly lower titers of total Ab at day 14 of post-booster injection of OTA as compared to values seen in chicks in group A. Anti-sheep IgM antibody response was not affected in chicks treated with OTA.
Table 5. Anti-SRBC antibody titers in chicks provided OTA-contaminated feed for 3 weeks.
Discussion
A significant decrease in the relative weight of lymphoid organs, particularly the bursa of Fabricius, was noted in male WL chicks maintained on OTA-contaminated feed. A similar effect on broiler chick immune system organ weights—due to ochratoxicosis—was reported by CitationDwivedi and Burns (1984), Hanif et al. (Citation2008), and Awaad et al. (Citation2011). Feeding an OTA-contaminated (0.4 and 0.8 mg/Kg diet) diet to male broiler chicks from the day after hatching to 5 weeks-of-age resulted in the significant decrease in thymus weight (Elaroussi et al., Citation2006). This effect of OTA may be attributed to cytotoxic and degenerative changes in the lymphoid follicles (Al-Anati and Petzinger, Citation2006).
The in vivo phagocytic index of the circulating reticuloendothelial system, determined here by measures of the clearance of colloidal carbon from peripheral blood, was significantly lower in chicks reared on OTA-contaminated feed (as compared to values seen among chicks kept on basal ration). Unfortunately, there is no parallel study in the published literature regarding the in situ phagocytic potential of the reticuloendothelial system of OTA-fed birds; nonetheless, the decrease in phagocytic index seen here may be attributed, at least in part, to impairment of the reticuloendothelial system as a result of the induced ochratoxicosis.
Abdominal macrophages harvested from the chicks fed OTA-contaminated rations showed significant depression (as compared with the cells from the control group) in phagocytic potential against SRBC and nitrate production in response to a lipopolysaccharide stimulus. Alvarez et al. (Citation2004) reported significant lower bactericidal capabilities of macrophages collected from Wistar rats administered 50 and 450 µg OTA/Kg BW. A similar decrease in phagocytic potential by heterophils was reported during ochratoxicosis in broiler chicks (Chang and Hamilton, Citation1980). This decrease in macrophage phagocytic potential may be due to an increased production of free radicals and/or a decrease in the production of cytokine due to OTA, as reported by Muller et al. (Citation1999) and Girish and Smith (Citation2008).
Intra-dermal administration of PHA-P primarily involves stimulation of T-lymphocyte proliferation, with minimal effect on B-lymphocytes (Tizard, Citation1995). In the present study, the lymphoproliferative response to PHA-P was significantly lower (as compared to Group A) in the chicks kept on OTA-contaminated diets. The finding of our study is in line with results shown by Elaroussi et al. (Citation2006) that reported significant decreases in the skin thickness response to PHA-P in male broiler chicks kept on an OTA-contaminated ration (i.e., at 0.4 and 0.8 mg/Kg diet) for 5 weeks. The significant decrease in the thymus weight and depletion of lymphoid cells noted here—and also reported earlier by Elauorssi et al. (2006), Al-Anati and Petzinger (Citation2006), and Dwividi and Burns (Citation1985)—provide significant clues to allow us to better understand the depression in T-lymphocyte functions in OTA-treated birds.
Antibody titers (total) against intravenous administration of SRBC of the chicks kept on the OTA-contaminated diets were significantly lower as compared to those in the chicks not fed OTA. There are many reports demonstrating the decrease in antibody titer in avian and mammalian species, to different types of antigens, as a result of OTA exposure. In an OTA feeding experiment, Singh et al. (Citation1990) reported significant decreases in antibody titers against SRBC in broiler chicks in response to chronic ochratoxicosis. In a similar study conducted by Santin et al. (Citation2002), broiler chicks were fed 2 mg OTA/Kg diet for 42 days and experienced a significant decrease in antibody titers against New Castle disease virus. This decrease in antibody response to SRBC may be due to a decrease in the number of immunoglobulin(s)-producing cells; this outcome was suggested by an experiment by Moura et al. (Citation2004) in which broiler chicks injected (intraperitoneally) with 0.04 mg OTA/Kg BW evinced significant depression in their numbers of lymphocytes.
The present study here investigated the effects of different doses of OTA on some cellular and humoral parameters pertinent to the immune function of male WL chicks. Based upon the current results, it can be concluded that feeding an OTA-contaminated diet to male WL chicks results in functional impairment in some of the components of the immune system. This clearly illustrates that the consumption of dietary OTA may give rise to an increased risk of infection by opportunistic pathogens. Thus, care must be taken while monitoring the OTA levels in all the diet ingredients used in the poultry (and potentially livestock as well) industries.
Acknowledgments
The authors highly acknowledge the financial support of the Higher Education Commission of Pakistan during the study period.
Declaration of interest
The authors report no conflicts of interest. The authors are alone responsible for the content and writing of the paper.
References
- Agag B. I. 2004. Mycotoxins in foods and feeds, 1–aflatoxins. Ass. Univ. Bull. Environ. Res. 7:173–205.
- Al-Anati L., and Petzinger E. 2006. Immunotoxic activity of ochratoxin A. J. Vet. Pharmacol. Ther. 29:79–90.
- Alvarez L., Gil A. G., Ezpeleta O., Garciajalo J. A., and Cerain A. L. 2004. Immunotoxic effects of ochratoxin A in the Wistar rats after oral administration. Food Chem. Toxicol. 42:825–834.
- Awaad M. H., Atta A. M.,Abd El-Ghany W. A., Elmenawey M., Ahmed K., Hassan A. A., Nada A. A., and Abdelaleem G. A. 2011. Effect of a specific combination of mannan-oligosaccharides and β-glucans extracted from yeast cell wall on the health status and growth performance of ochratoxicated broiler chickens. J. Anim. Sci. 7:82–96.
- Baydar T., Engin A. B., Girgin G., Aydin S., and Sahin G. 2005. Aflatoxin and ochratoxin in various types of commonly consumed retail ground samples in Ankara, Turkey. Ann. Agric. Environ. Med. 12:193–197.
- Bayman P., Baker J. L., Doster M. A., Michailides T. J., and Mahoney N. E. 2002. Ochratoxin production by Aspergillus ochraceus Group and Aspergillus Alliaceus. Appl. Environ. Microbiol. 68:2326–2329.
- Binder E. M., Tan L. M., Chin L. J., Handle J., and Richard J. 2007. Worldwide occurrence of mycotoxins in commodities, feeds, and feed ingredients. Anim. Feed Sci. Tech. 137:265–282.
- Brera C., Marina M., and Marta C. 1998. Evaluation of impact of mycotoxins on human health: Sources of errors. Microchem. J. 59:45–49.
- Castegnaro M., Mohr U., Pfohl-Leszkowicz A., Esteve J., Steinmann J., Tillmann T., Michelon J., and Bartsch H. 1998. Sex- and strain-specific induction of renal tumors by ochratoxin A in rats correlates with DNA adduction. Int. J. Cancer 77:70–75.
- Chang C. F., and Hamilton P. B. 1980. Impairment of phagocytosis by heterophils from chickens during ochratoxicosis. Appl. Environ. Microbiol. 39:572–575.
- Choudhary A. K., and Kumari P. 2010. Management of mycotoxins contamination in preharvest and post harvest crops: Present status and future prospects. J. Phytol. 2:37–52.
- Corrier D. E. 1990. Comparison of Phytohemagglutinin-induced cutaneous hypersensitivity reactions in the interdigital skin of broiler and layer chicks. Avian Dis. 34:369–373.
- Council for Agricultural Science and Technology (CAST). 2003. Mycotoxins: Risks in Plant, Animal, and Human Systems. Ames, IA: Council for Agricultural Science and Technology.
- Delhanty J. J., and Solomon J. B. 1966. The nature of antibodies to goat erythrocytes in the developing chicken. Immunology 11:103–113.
- Devegowda G., Raju M. V., and Swamy M. V. 1998. Mycotoxins: Novel solution in their counteraction. Foodstuffs 7:12–15.
- Duncan D. B. 1955. Multiple range and multiple F-tests. Biometrics 11:1–42.
- Dwivedi P., and Burns R. B. 1984. Effect of ochratoxin A on immunoglobulins in broiler chicks. Res. Vet. Sci. 36:117–121.
- Dwivedi P., and Burns R. B. 1985. Immunosuppressive effects of ochratoxin A in young Turkeys. Avian Pathol. 14:213–225.
- Elaroussi M. A., Mohamed F. R., El-Barkouky E. M., Atta A. M., Abdou A. M., and Hatab M. H. 2006. Experimental ochratoxicosis in broiler chickens. Avian Pathol. 35:263–269.
- Gilani S. H., Bancroft J., and Reily M. 1978. Teratogenicity of ochratoxin A in chick embryos. Toxicol. Appl. Pharmacol. 46:543–546.
- Girish C. K., and Smith T. K. 2008. Impact of feed-borne mycotoxins on avian cell-mediated and humoral immune responses. World Mycotoxin J. 1:105–121.
- Green L. C., Wagner D. A., Glogowski J., Skipper P. L., Wishnok P. S., and Tannebaum S. R. 1982. Analysis of nitrate, nitrite and (15N) nitrite in biological fluid. Anal. Biochem. 126:131–138.
- Hanif N. Q., Muhammad G., Siddique M., Khanum A., Ahmed T., Gadahai J. A., and Kaukab G. 2008. Clinico-pathomorphological, serum biochemical and histological studies in broilers fed ochratoxin A and a toxin deactivator (Mycofix® Plus). Br. Poult. Sci. 49:632–642.
- Kumar A., Jindal N., Shukla C. L., Asrani R. K., Ledoux D. R., and Rottinghaus G. E. 2004. Pathological changes in broiler chickens fed ochratoxin A and inoculated with Escherichia coli. Avian Pathol. 33:413–417.
- Moura M. A., Machado C. H., Porfirio L. C., and Freire R. B. 2004. Effects of ochratoxin a on broiler leukocytes. Braz. J. Poult. Sci. 3:187–190.
- Muller G., Kielstein P., Rosner H., Berndt A., Heller H., and Kohler H. 1999. Studies of the influence of ochratoxin A on immune and defense reactions in weaners. Mycoses 42:495–505.
- Pfohl-Leszkowicz A., Pinelli E., Bartsch H., Mohr U., and Castegnaro M. 1998. Sex and strain differences in Ochratoxin A metabolism and DNA adduction in two strains of rats. Mol. Carcinogen. 23:76–83.
- Qureshi M. A., and Havenstein G. B. 1994. A comparison of immune performance of a 1991 commercial broiler with a 1957 random-bred strain when fed “typical” 1957 and 1991 broiler diets. Poult. Sci. 73:1805–1812.
- Robbiano L., Baroni D., Carrozzino R., Mereto E., and Brambilla G. 2004. DNA damage and micronuclei induced in rat and human kidney cells by six chemicals carcinogenic to the rat kidney. Toxicology 204:187–195.
- Rocha O., Ansari K., and Doohan F. M. 2005. Effects of trichothecene mycotoxins on eukaryotic cells: A review. Food Addit. Contam. 22:369–378.
- Saha D., Acharya D., Roy D., Shrestha D., and Dhar T. K. 2007. Simultaneous enzyme immunoassay for the screening of aflatoxin B1 and ochratoxin A in chili samples. Anal. Chim. Acta 584:343–349.
- Saleemi M. K., Khan M. Z., Khan A., and Hasan I. J. 2009. Mycoflora of poultry feeds and mycotoxins producing potential of Aspergillus species. Pak. J. Botany 42:427–434.
- Santin E., Paulillo A. C., Maiorka P. C., Alessi A. C., Krabbe E. L., and Maiorka A. 2002. The effects of ochratoxin/aluminosilicate interaction on the tissues and humoral immune response of broilers. Avian Pathol. 31:73–79.
- Sawale G. K., Gosh R. C., Ravikanth K., Maini S., and Rekhe D. S. 2009. Experimental mycotoxicosis in layer induced by ochratoxin A and its amelioration with herbomineral toxin binder ‘Toxiroak’. Int. J. Poult. Sci. 8:798–803.
- Sharma R. P. 1993. Immunotoxicity of mycotoxins. J. Dairy Sci. 76:892–897.
- Singh G. S., Chauhan H. V. S., Jha G. I., and Singh K. K. 1990. Immunosuppression due to chronic ochratoxicosis in broiler chicken. J. Comp. Pathol. 103:399–410.
- Steel R. G., Torrie J. H., and Dickey D. A. (Eds.) 1997. Principles and Procedures of Statistics: A Biometrical Approach. 3rd Edition. Boston, MA: WCB McGraw Hill Boston, pp. 194–195.
- Stoev S. D. 2010. Studies on carcinogenic and toxic effects of ochratoxin A in chicks. Toxins 2:649–664.
- Sugiura H., Sugiura H., Nishida H., Inaba R., Mirbod S. M., and Iwata H. 2001. Effects of different durations of exercise on macrophage functions in mice. J. Appl. Physiol. 90:789–794.
- Thuvander A., Breitholtz-Emanuelsson A., and Olsen M. 1995. Effects of ochratoxin A on the mouse immune system after subchronic exposure. Food Chem. Toxicol. 33:1005–1011.
- Tizard I. R. (Ed.) 1995. Immunology: An Introduction. Philadelphia, PA: Saunders College Publishing.
- Trenk H. L., Butz M. E., and Chu F. S. 1971. Production of ochratoxins in different cereal products by Aspergillus ochraceus. Appl. Microbiol. 21:1032–1035.
- ul-Hassan Z., Khan M. Z., Khan A., and Javed I. 2010. Pathological responses of White Leghorn breeder hens kept on ochratoxin A-contaminated feed. Pak. Vet. J. 30:118–123.
- ul-Hassan Z., Khan M. Z., Khan A., Javed I., and Saleemi M. K. 2011. Immunological status of progeny of breeder hens kept on ochratoxin A (OTA)-contaminated feed. J. Immunotoxicol. 8:122–130.
- Varga J., Kevei E., Rinyu E., Teren J., and Kozakiewicz Z. 1996. Ochratoxin production by Aspergillus species. Appl. Environ. Microbiol. 62:4461–4464.
- Wangikar P. B., Dwivedi P., Sinha N., Sharma A. K., and Telang A. G. 2005. Teratogenic effects in rabbits of simultaneous exposure to ochratoxin A and aflatoxin B1 with special reference to microscopic effects. Toxicology 215:37–47.
- Whitlow L. W., and Hagler W. M. Jr,. 2002. Mycotoxins in feeds. Feedstuffs 74:1–10.
- Wood G. E. 1992. Mycotoxins in foods and feeds in the United States. J. Anim. Sci. 70:3941–3949.
- Yamamoto Y., and Glick B. 1982. A comparison of the immune response between two lines of chickens selected for differences in the weight of the bursa of Fabricius. Poult. Sci. 61:2129–2132.