Abstract
Skin diseases including dermatitis constitute ≈ 30% of all occupational illnesses, with a high incidence in the printing industry. An outbreak of contact dermatitis among employees at an ink ribbon manufacturing plant was investigated by scientists from the National Institute for Occupational Safety and Health (NIOSH). Employees in the process areas of the plant were exposed to numerous chemicals and many had experienced skin rashes, especially after the introduction of a new ink ribbon product. To identify the causative agent(s) of the occupational dermatitis, the murine local lymph node assay (LLNA) was used to identify the potential of the chemicals used in the manufacture of the ink ribbon to induce allergic contact dermatitis. Follow-up patch testing with the suspected allergens was conducted on exposed employees. Polyvinyl butyral, a chemical component used in the manufacture of the ink ribbon in question and other products, tested positive in the LLNA, with an EC3 of 3.6%, which identifies it as a potential sensitizer; however, no employees tested positive to this chemical during skin patch testing. This finding has implications beyond those described in this report because of occupational exposure to polyvinyl butyral outside of the printing industry.
Introduction
More than 13 million employees in the United States (US) are potentially exposed to chemicals that can be absorbed through the skin. Chemical exposure can lead to contact dermatitis, the most common occupational skin disorder; responsible for up to 30% of all cases of occupational disease in industrialized nations. Epidemiologic data suggest that contact dermatitis accounts for ≈ 95% of all cases of occupational skin disease, imposing considerable social and economic implications (Burnett et al., Citation1998; Clark and Zirwas, Citation2009). Time off work, loss of workplace productivity, reduced quality-of-life, and medical and worker compensation costs are several factors accounting for the loss of billions of dollars.
Printing is one of the larger manufacturing industries in the US; the Bureau of Labor Statistics estimated it employed 594,100 individuals in 2008, with 54% of those employed contributing to the production aspect (BLS, Citation2009). Employment in the printing industry has been associated with a high risk for contact dermatitis, with the estimated annual average incidence rate of ≈ 86 cases per 100,000 employees (Nethercott, Citation1988; Livesley et al., Citation2002). There have been many case reports of sensitizing effects of chemicals used in the printing industry (Garabrant, Citation1985; Nethercott and Nosal, Citation1986; Shapiro et al., Citation2001). As examples, a silk-screen printer who presented with dermatitis on wrist, arms, and eventually face, had a positive patch test for several acrylics and select chemicals contained within the epoxy and ink components used in the printing process (Jolanki et al., Citation1994). Another study investigated several printing employees who developed contact dermatitis after exposure to ultraviolet printing inks (Nethercott et al., Citation1983). Patch and laboratory testing confirmed urethane acrylate, a chemical used in the offset printing process, as a responsible chemical. Additional chemicals/reagents used within the printing industry suspected to induce dermatitis include alcohols, alkalis, developers, etching solutions, greases, waxes, inks, potassium dichromate, formaldehyde, hydroquinone, glues, and gums.
Scientists from the National Institute for Occupational Safety and Health (NIOSH) investigated several reports of dermatitis among employees at an ink ribbon manufacturing plant that manufactures, packages, and ships wax, wax-resin, and resin-based ink ribbons throughout the world. The ink ribbons consist of a sturdy plastic film on which single or multiple coatings of ink mixtures are applied to one or both sides. Three ink coatings are used in the manufacture of this ink ribbon, and each coating is composed of numerous chemicals. Upon examination of 18 individuals who reported rash at the ink ribbon manufacturing plant, 17 employees had dermatitis on their hands, wrists, and/or forearms, with two of the 17 also having dermatitis on the face and/or lower extremities. One employee had dermatitis on the legs only. Characteristics of the employee dermatitis included: erythematosus; slightly indurated xerotic patches; and some with scale and/or fissures, and some with small (< 3 mm) erythematous papules and/or papulo-vesicles. Thirteen of these employees had consulted a physician because of their rash. A total of 291 out of 349 (83%) employees who worked at the facility participated in a health questionnaire; 60 employees (21%) reported developing dermatitis on their hands, wrists, or forearms since they began working at the ink manufacturing plant. Among these 60 employees, 35 reported that the dermatitis improved during time away from work either usually or always.
A combined murine local lymph node assay (LLNA) with follow-up employee patch testing was used to evaluate the irritancy and sensitization potential of chemicals used in the manufacturing process to identify the causative agents of the reported dermatitis.
Materials and methods
Test articles
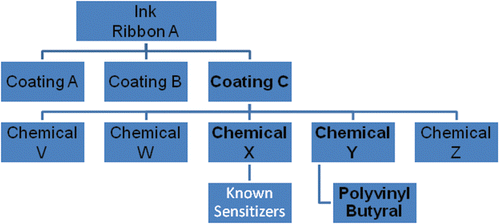
The manufacturer revealed the chemical composition analysis to the investigators for the purpose of health evaluation of the employees. However, this information is proprietary; for that reason, the coatings will be referred to hereafter as Coatings A, B, and C (). Coating C is composed of five chemicals (Chemicals V–Z). After extensive literature searches, Chemical Y was selected for testing based on the lack of information about the individual ingredients. Although proprietary, more information was known about the sensitization potential of the other four components (Chemical mixtures V, W, X, and Z) of Coating C (). Polyvinyl butyral was selected for further testing because it was the main and suspect ingredient in Chemical Y (). Coatings A, B, C, and Chemical Y were provided by the ink ribbon manufacturer for the animal studies. Polyvinyl butyral (product #182567, 80% purity, CAS# 63148-65-2) and the positive controls; α-hexylcinnamaldehyde (HCA, CAS# 101-86-0), 2,4-dinitrofluorobenzene (DNFB, CAS # 70-34-8), and toluene 2,4-diisocyanate (TDI, CAS# 584-84-9) were purchased from Aldrich Chemical Company, Inc. (Milwaukee, WI).
Animals
Female BALB/c mice were used in this study (Woolhiser et al., Citation2000; Klink and Meade, Citation2003). The mice were purchased from Taconic (Germantown, NY) at 6–8 weeks-of-age. Upon arrival, the animals were allowed to acclimate for a minimum of 5 days. Each animal was randomly assigned to treatment group, weighed, and individually identified via tail marking using a permanent marker. A preliminary analysis of variance on body weights was performed to ensure homogeneous distribution of animals across treatment groups. A maximum of five mice per cage were housed in ventilated plastic shoebox cages with hardwood chip bedding, NIH-31 modified 6% irradiated rodent diet (Harlan Teklad, Frederick, MD), and tap water from bottles ad libitum. The temperature in the animal facility was maintained between 68–72°F and the relative humidity between 36–57%. The light/dark cycle was maintained on 12-h intervals. All animal experiments were performed in the Association for Assessment and Accreditation of Laboratory Animal Care accredited NIOSH animal facility in accordance with an animal protocol approved by the Institutional Animal Care and Use Committee.
Concentration range finding and toxicological studies
Range finding studies were performed to select the concentration of each ink coating and/or chemical to be used for dermal exposures. Maximum concentrations that were soluble in the vehicle and did not cause toxicity were selected for the subsequent studies. Overt clinical toxicity was evaluated, although visual monitoring for appearance (ruffled fur, discharge from eye, nose and anus). Briefly, mice were topically treated with acetone vehicle and increasing concentrations of test article(s) on the dorsal surface of each ear (25 µl per ear) for 3 consecutive days. For these studies, Coating A (25–50%), Coating B (25–50%), and Coating C (50–100%) were tested at the concentrations indicated due to solubility limitations. Chemical Y (1.25–7%) and polyvinyl butyral (1.5–6.0%) were tested at the indicted concentrations based on the results from the initial analysis of the Ink Coatings. Animals were allowed to rest for 2 days following the last exposure and then weighed and examined for signs of toxicity including loss of body weight and ruffled fur. On the 6th day, the mice were euthanized by CO2 asphyxiation, weighed, and examined for gross pathology.
Combined local lymph node and irritancy assays
To determine irritancy and sensitization potential, a combined LLNA was conducted. The LLNA was performed according to the method described in the Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) Peer Review Panel report with minor modifications (NIEHS, Citation1999). Briefly, mice (five per group) were topically treated with acetone vehicle, increasing concentrations of test article, or positive control (30% HCA for LLNA and 0.3% DNFB for irritancy) on the dorsal surface of each ear (25 µl per ear) for three consecutive days. Irritancy measurements were then performed as previously described (Woolhiser et al., Citation1999). In brief, the thickness of the right and left ear pinnae of each mouse was measured using a modified engineer’s micrometer (Mitutoyo Co., Japan) before the first chemical administration and 24 h following the final exposure. The mean percentage of ear swelling was calculated based on the following equation: [(mean post-challenge ear thickness − mean pre-challenge ear thickness)/mean pre-challenge thickness] × 100.
Animals were allowed to rest for 2 days following the last exposure. On Day 6, mice were injected intravenously via the lateral tail vein with 20 µCi [3H]-thymidine (2 Ci/mmol; Dupont NEN, Boston, MA). Five hours after [3H]-thymidine injection, animals were euthanized via CO2 inhalation, and the left and right superficial parotid cervical draining lymph nodes (DLN) located at the bifurcation of the jugular vein were excised and pooled for each animal. Single cell suspensions were made and incubated overnight in 5% trichloroacetic acid (TCA), and samples were counted using a Packard Tri-Carb 2500TR liquid scintillation analyzer (Packard Instrument Co., Meriden, CT). Stimulation indices (SI) were calculated by dividing the mean disintegrations per minute (DPM) per test group by the mean DPM for the vehicle control group. EC3 values (concentration of chemical required to induce a 3-fold increase over the vehicle control) were calculated based on the equation from Basketter et al. (Citation1999).
Total serum IgE
To further characterize the hypersensitivity response (IgE- vs T-cell-mediated), total serum IgE was evaluated. Mice were treated with acetone or increasing concentrations of ink coating topically on the dorsal surface of each ear (25 µl per ear) for four consecutive days. Animals were allowed to rest for 6 days after the final exposure and then euthanized on Day 10 by CO2 inhalation. Animals were weighed, and examined for gross pathology at the end of the experiment. The following organs were removed, cleaned of connective tissue, and weighed: liver, spleen, kidneys, and thymus. DLN were collected (two nodes/animal/tube) in 4 ml PBS (phosphate-buffered saline, pH 7.4) for subsequent immune phenotyping analysis. Blood samples were collected via cardiac puncture. Sera were separated by centrifugation and frozen at –20°C for next-day analysis of IgE by ELISA. A standard colorimetric sandwich ELISA was performed as previously described (Butler, Citation2000). All antibodies and isotype controls were purchased from BD Pharmingen (San Jose, CA).
In brief, 96-well flat bottom plates (Dynatec Immulon-2; Fisher Scientific Co., Pittsburgh, PA) were coated with (2 µg/ml in PBS) purified monoclonal rat anti-mouse IgE antibody (clone R35–72), sealed with plate sealers, and incubated overnight at 4°C. The plates were washed three times with PBS/Tween-20 and then blocked for 1 h with 2% newborn calf serum (NCS; Thermo Scientific Hyclone, Logan, UT) and 0.05% [w/v] sodium azide at room temperature (RT). Initial dilutions (1:10) were made from the serum samples, and IgE control standards were prepared at 500 ng/ml. All dilutions were made in 2% NCS and 0.05% sodium azide. Serum samples and IgE control standard (mouse IgE anti-TNP, clone C38-2) were serially diluted (1:2), added to the coated plates in a 100 µl volume and incubated at RT for 1 h. The plates were washed 3-times with PBS/Tween-20. Biotin-conjugated rat anti-mouse IgE (clone R35–92) was added in a 100 µl volume and plates were incubated at RT for 1 h. The plates were washed three times with PBS/Tween 20. Streptavidin-alkaline phosphatase (BD Pharmingen, San Jose, CA) was added (100 µl of a 1:400 dilution), and plates were incubated for 1 h at RT. p-Nitrophenyl phosphate (Sigma, St. Louis, MO) was used as the alkaline phosphatase substrate and added to the plates in a 100 µl volume. The plates were allowed to develop for up to 30 min at RT or until the OD reading of the highest standard reached 3.0. Absorbance was determined using a Spectramax Vmax plate reader (Molecular Devices, Sunnyvale, CA) at 405–605 nm. Data analysis was performed using the IBM Softmax Pro 3.1 (Molecular Devices), and the IgE concentrations for each sample were interpolated from a standard curve using multi-point analysis.
Phenotypic analysis of draining lymph node cells
Following euthanasia of animals used in the IgE analysis assays, the IgE+B220+ cell populations in the DLN were analyzed for groups treated with vehicle, test article, or positive control (2.5% TDI). Lymph node cell phenotypes were analyzed using flow cytometry, as described by Manetz and Meade (Citation1999). DLN were dissociated using the frosted ends of two microscope slides. Cell counts were performed using a Coulter Counter (Z2 model, Beckman Coulter, Fullerton, CA), and 1 × 106 cells per sample were added to the wells of a 96-well plate. Cells were washed using staining buffer (1% bovine serum albumin/0.1% sodium azide in PBS) and then incubated with Fc block (clone 2.4G2). The cells were then incubated with anti-CD45RA/B220 (phycoerythrin [PE]-conjugated, clone RA3-6B2) and anti-IgE antibodies (fluorescein isothiocyanate [FITC]-conjugated, clone R-35–72) or appropriate isotype controls, diluted in staining buffer, washed, and incubated with propidium iodine (PI; 5 µg/ml). All antibodies and isotype controls were purchased from BD Pharmingen. After a final wash, cells were re-suspended in staining buffer and analyzed with a Becton Dickinson FACSVantage flow cytometer using a PI viability gate.
Statistical analyses
For analysis, mean DPM per group were first tested for homogeneity using the Bartlett’s Chi-Square Test. Homogenous data were analyzed using a one-way analysis of variance (ANOVA). If the ANOVA showed significance at p < 0.05 or less, the Dunnett’s Multiple Range t-test was used to compare treatment groups with the control group. Linear trend analysis was performed to determine test article exposure concentration-related effects for the specified endpoints. Differences were considered significant at p < 0.05 as compared to vehicle control.
Patch testing of employees
Skin patch testing was performed on employees who completed an initial NIOSH health questionnaire and answered ‘yes’ to either ‘Have you had dermatitis on your hands, wrists, or forearms (excluding fronts of elbows) in the past 4 weeks?’ or ‘Did you have dermatitis on your hands, wrists, or forearms (excluding fronts of elbows) in the month before [the most recent NIOSH visit]’. Chemotechnique Diagnostics® laboratory (Vellinge, Sweden) obtained Chemical Y and polyvinyl butyral, performed feasibility studies, and prepared skin patch test dilutions of non-standard workplace substances. The ink ribbon manufacturer provided Coating C for the patch testing. Employees were patch tested with three non-irritating concentrations (i.e., 1.75%, 3.50%, and 7.00%) of Chemical Y and polyvinyl butyral. Acetone was used as the vehicle for preparing Chemical Y and polyvinyl butyral. Chemotechnique® North American Standard Series July-06 (NA-1000) patch test allergens were used to identify common skin allergies in participants with a history of dermatitis. IQ Ultra Patch Chambers were used. Seven employees, who answered ‘no’ to ‘Have you had dermatitis on your hands, wrists, or forearms (excluding fronts of elbows) since you began working at the ink manufacturing plant?’ were used as comparison participants and were patch tested to the non-standard workplace substances to confirm that the vehicle and chemicals used were non-irritating at the concentrations used. Patch test results were read and interpreted by a NIOSH physician with assistance from the contract dermatologist using standard clinical practice methods (Li et al., Citation2003). Patches were removed 48 h after placement, at which time an initial reading was performed. A second reading took place 96 h after placement and a final reading and interpretation was performed at 168 h after placement. The NIOSH Human Subjects Review Board approved the skin patch testing protocol.
Results
In vivo studies identified Coating C to be an irritant and sensitizer
There were no deaths in mice related to exposure for the in vivo studies. All mice appeared clinically normal throughout the course of these studies, with no overt clinical toxicity observed (data not shown). A significant increase in ear swelling was observed following dermal exposure to Coating C reaching statistical significance at the 75% dose 24 h post-final exposure ( and ). No increases in ear swelling were observed after treatment with Coatings A and B ( and ). DNFB (0.3%) was used as a positive control for irritancy studies and resulted in an average significant increase of 60% ear swelling post-treatment for all studies. Coating C was also the only ink coating that tested positive in the LLNA, with an EC3 value of 44.80% (). A dose responsive (Linear Trend test; p < 0.01) increase in DLN proliferation was identified following dermal treatment with Coating C, with counts from the animals in the high dose group (75%) significantly elevated over the vehicle control animals. SI values of 1.2, 2.0, and 5.5 were identified for the 18.75%, 37.50%, and 75.00% treatment groups, respectively. HCA (30%) was used as a positive control for these experiments and resulted in an average SI value of 12.8 ().
Table 1. Llna phenotypic, and ige analysis after exposure to coating C.
Figure 2 Ear swelling as a result of ink coating treatment. Analysis of irritation after topical application of (A) Coating A, (B) Coating B, and (C) Coating C. Bars represent means (± SE) of five mice (i.e., 10 ears)/group. Levels of statistical significance denoted as ** p ≤ 0.01 compared to acetone vehicle.
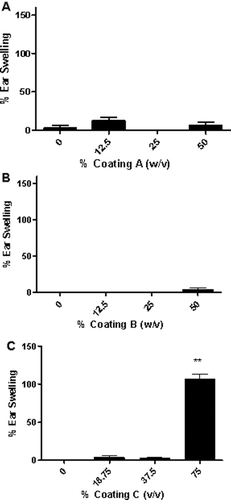
Figure 3. Sensitization potential following dermal treatment with ink coatings. Analysis of the sensitization potential of (A) Coating A, (B) Coating B, and (C) Coating C using the LLNA. [3H]-thymidine incorporation into draining lymph node cells of BALB/c mice following exposure to vehicle or concentrations of ink mixture shown above. Numbers appearing above bars represent stimulation indices for each concentration tested. Bars represent means (± SE) of five mice/group. Levels of statistical significance denoted as * p ≤ 0.05 compared to acetone vehicle.
![Figure 3. Sensitization potential following dermal treatment with ink coatings. Analysis of the sensitization potential of (A) Coating A, (B) Coating B, and (C) Coating C using the LLNA. [3H]-thymidine incorporation into draining lymph node cells of BALB/c mice following exposure to vehicle or concentrations of ink mixture shown above. Numbers appearing above bars represent stimulation indices for each concentration tested. Bars represent means (± SE) of five mice/group. Levels of statistical significance denoted as * p ≤ 0.05 compared to acetone vehicle.](/cms/asset/d68415ef-94fc-4a2c-b713-5a7d19c44b7b/iimt_a_654364_f0003_b.gif)
Exposure to Coating C did not induce an increase in local or systemic IgE levels
Treatment with Coating C (18.75–75.00%) did not produce an elevation in total serum IgE levels (); 2.5% TDI was used as a positive control for these experiments and resulted in a significant elevation of total IgE (~ 1500 ng/ml) when compared to vehicle. Phenotypic analysis of the DLN of mice treated with Coating C showed dose responsive (Linear Trend test; p < 0.01) increases in the B220+andIgE+B220+ cell populations. Consistent with the LLNA results, a statistically significant increase in percent B220+ cells (27.9 ± 0.2, % counts) was noted following treatment with 75% Coating C. A significant increase in percent and absolute IgE+B220+-expressing cells (10.5 [± 2.7] % counts; 1.5 [± 0.3] × 106 cells) was also identified, reaching significance at 75% (). Although the number of IgE+B220+-expressing cells was mildly increased after treatment, this number was within the historical control range, and the ratio compared to B220+-expressing cells was similar to that previously described for T-cell-mediated sensitizers (Manetz and Meade, Citation1999). TDI (2.5%) was used as a positive control for these experiments and resulted in significant elevations of IgE+B220+ (28.03 [± 1.49] % counts) and B220+ (31.18 [± 1.60] % counts) cell populations (). No changes in body or organ weight (spleen, liver, kidney, and thymus) were observed after treatment with any concentration.
Identification of polyvinyl butyral as a sensitizing component in Coating C
Chemical Y tested positive in the LLNA with a calculated EC3 value of 5.8% (). A dose responsive increase (Linear Trend test; p < 0.05) in DLN proliferation was identified following dermal treatment with polyvinyl butyral, with counts from the animals in the high dose group (7.5%) significantly elevated over the vehicle control animals. SI values of 1.2, 2.0, and 5.5 were identified for the 1.5%, 3.0%, and 6.0% treatment groups, respectively. An EC3 value of 3.6% was calculated (); 30% HCA was used as a positive control for these experiments and resulted in an average SI value of 17.1 for all experiments.
Figure 4. Sensitization potential following dermal treatment with Chemical Y. Analysis of the sensitization potential of Chemical Y using the LLNA. [3H]-thymidine incorporation into draining lymph node cells of BALB/c mice following exposure to vehicle or concentrations of Chemical Y shown above. Numbers appearing above bars represent stimulation indices for each concentration tested. Bars represent means (± SE) of five mice per group. Levels of statistical significance denoted as * p ≤ 0.05 compared to acetone vehicle.
![Figure 4. Sensitization potential following dermal treatment with Chemical Y. Analysis of the sensitization potential of Chemical Y using the LLNA. [3H]-thymidine incorporation into draining lymph node cells of BALB/c mice following exposure to vehicle or concentrations of Chemical Y shown above. Numbers appearing above bars represent stimulation indices for each concentration tested. Bars represent means (± SE) of five mice per group. Levels of statistical significance denoted as * p ≤ 0.05 compared to acetone vehicle.](/cms/asset/7fa12128-423b-408c-a034-cd57759eb6ac/iimt_a_654364_f0004_b.gif)
Figure 5. Sensitization potential following dermal treatment with polyvinyl butyral. Analysis of the sensitization potential of polyvinyl butyral using the LLNA. [3H]-Thymidine incorporation into draining lymph node cells of BALB/c mice following exposure to vehicle or polyvinyl butyral. Numbers appearing above bars represent stimulation indices for each concentration tested. Bars represent means (± SE) of five mice/group. Levels of statistical significance denoted as * p ≤ 0.05 compared to acetone vehicle.
![Figure 5. Sensitization potential following dermal treatment with polyvinyl butyral. Analysis of the sensitization potential of polyvinyl butyral using the LLNA. [3H]-Thymidine incorporation into draining lymph node cells of BALB/c mice following exposure to vehicle or polyvinyl butyral. Numbers appearing above bars represent stimulation indices for each concentration tested. Bars represent means (± SE) of five mice/group. Levels of statistical significance denoted as * p ≤ 0.05 compared to acetone vehicle.](/cms/asset/e35f237a-834c-418e-a268-cbd6da83b461/iimt_a_654364_f0005_b.gif)
Patch test results
Thirteen of 40 eligible employees were patch tested. The remaining 27 employees could not be contacted, no longer worked for the company, or refused to be tested. Several employees with more severe cases of dermatitis declined to be patch tested. One study participant was unable to continue the testing due to skin discomfort and could not be evaluated. None of the other employees who agreed to be patch tested had positive reactions to Coating C, Chemical Y, or polyvinyl butyral; however, some employees with severe dermatitis did not participate. Seven study participants had positive patch test results to one or more of the 50 common North American allergens including thiuram mix and mixed dialkyl thiourea, 4-tert-butylphenolformal-dehyde resin, potassium dichromate, nickel, Amerchol L101, bacitracin and neomycin sulfate, 4-phenylenediamine base, cinnamic aldehyde, balsam of Peru, fragrance mix, disperse blue mix 106/124, and composite mix. These positive results were considered to be unrelated to their current work exposures. Eight of the 13 study participants were diagnosed with irritant contact dermatitis; five of the eight had additional skin diagnoses (i.e., dyshidrotic dermatitis, lichen simplex chronicus, seborrheic dermatitis, and psoriasiform dermatitis). An irritant reaction was presumed to account for the dermatitis not proven to be cutaneous allergy by patch testing.
Discussion
This report describes the use of the LLNA, which has been accepted as a standalone assay for hazard identification of skin sensitizers, to identify a sensitizing component used in the manufacturing of an ink ribbon. The standard LLNA was not originally evaluated for the testing of formulations. However, the Interagency Coordinating Committee on the Validation of Alternative Methods (ICVAAM) recently recommended, due to a nomination by the US Consumer Product Safety Commission, to re-evaluate the LLNA applicability domain, allowing the LLNA to be used to test any chemical or product, including pesticide formulations, metals, substances in aqueous solutions, and other products such as natural complex substances and dyes, unless the chemical or product to be tested has properties that may interfere with the ability of the LLNA to detect skin-sensitizing substances (ICCVAM, Citation2010).
Dermal exposure to Coating C (proprietary ingredients) was identified as an irritant and tested positive for sensitization in the LLNA. This data, coupled with results from immune cell phenotyping and IgE analysis, suggests that at least one of the chemicals contained within this ink coating is a T-cell-mediated contact sensitizer. One component of Coating C, Chemical Y (EC3 = 5.8%), and its main constituent polyvinyl butyral (EC3 = 3.6%), were both positive in the LLNA when tested at working concentrations. Based on the chemistry of the reactions used to produce these chemicals and their purity, these studies do not definitively identify the reactive components. For example, sensitization potential is associated with polyvinyl butyral that may contain monomers and by-products of its reaction chemistry. The resin polyvinyl butyral is synthesized through reactions of polyvinyl acetate with butyraldehyde (CH3CH2CH2CHO) (CAS# 123-72-8; possible sensitizer) and formaldehyde (CH2O) (CAS# 50-00-0; known irritant and sensitizer). The polyvinyl butyral tested in these studies was documented to be 80% polymer; with the remaining 20% most likely a mixture of the free monomer (vinyl butyral and vinyl acetate) and the compounds vinyl alcohol, vinyl acetate, butyraldehyde, and formaldehyde. The calculated EC3 value for formaldehyde is 0.96% (de Jong et al., Citation2007); therefore, depending on the concentration of formaldehyde, or possibly butyraldehyde, in polyvinyl butyral, this could account for some portion of the sensitization potential observed.
In this study, no skin patch test participants reacted to any of the workplace substances. It is unclear if this result was due to factors such as lack of employee participation or the low concentration of test allergen selected for patch testing. In addition to the limited number of employees tested, there is also a possibility that decreased/lack of recent exposure to the chemical components used in the manufacture of this ink ribbon may have influenced the patch test results. There was ≈ 2 years between the initiation of the study and the employee patch testing. During this time the company implemented engineering and administrative controls that were recommended by NIOSH scientists, and employees were educated on skin health and methods to prevent dermatitis. Personal protective equipment, such as gloves suitable for workplace exposures that NIOSH recommended, were also made available to the employees. The final report (NIOSH, Citation2011) including recommendations is at: http://www.cdc.gov/niosh/hhe/reports/pdfs/2007-0261–3122.pdf. Although sensitization to contact allergens is usually believed to persist throughout life in humans, there is a possibility that the employees became desensitized or tolerant over this timeframe due to lack of ongoing exposure (Keczkes, Citation1984; Lee and Maibach, Citation2001; Nielsen et al., Citation2001). Although this topic is still under debate, studies have suggested that, if the offensive agent is removed, the hypersensitivity response may subside or disappear (Keczkes, Citation1984).
There is potential for occupational exposure to polyvinyl butyral outside of the printing industry. It is currently manufactured and marketed by a number of companies worldwide, including DuPont (Wilmington, DE) (‘Butacite’-brand PVB, introduced in 1938), Solutia (St. Louis, MO) (Saflex-brand PVB, introduced in 1940), Kururay Specialties Europe (Frankfurt, Germany) (‘Trosifol’-brand PVB), and Sekisui (Kyoto, Japan). It is used in many industries, and the major uses of this resin include: a coating for lumber and metals, priming paint for metals, concrete coating, waterproof coating, protective coating for gloss surfaces, coating for leather, and metal foil. Due to the unique properties such as outstanding binding efficiency, optical clarity, adhesion to a large number of surfaces, toughness combined with flexibility, it is used as an adhesive for metals, glass, transfer printing, and heat sealing, and in the manufacturing of glass fiber reinforced plastic and laminated materials.
Conclusions
The data described in this study has implications beyond those described in this report because of occupational exposure to polyvinyl butyral outside of the printing industry. Despite the negative human patch test results, the data generated from the animal studies stresses the importance for taking precautions in handling substances identified as potential allergens, because prolonged exposure could result in future skin sensitization. Emphasis on the use of engineering and administrative controls along with appropriate personal protective equipment is needed to protect workers from occupational exposure to potentially sensitizing chemicals.
In summary, polyvinyl butyral and coatings containing polyvinyl butyral resulted in irritant and T-cell-mediated hypersensitivity responses when tested at working concentrations in a murine model.
Acknowledgements
Statistical support was provided by Charles Mueller; medical support was provided by Elena Page and Ayodele Adebayo; all work at the Hazard Evaluations and Technical Assistance Branch of the National Institute for Occupational Safety and Health. This work was performed and financially supported by the National Institute for Occupational Safety and Health. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content of this manuscript.
References
- Basketter D. A., Lea L. J., Dickens A., Briggs D., Pate I., Dearman R. J., and Kimber I. 1999. A comparison of statistical approaches to the derivation of EC3 values from local lymph node assay dose responses. J Appl Toxicol. 19:261–266.
- 2009. BLS (Bureau of Labor Statistics). U.S. Department of Labor, Career Guide to Industries, 2008-09 Edition, Printing. Available online at: http://www.bls.gov/oco/cg/cgs050.htm, accessed 21 October 2009.
- Burnett C. A., Lushniak B. D., McCarthy W,. and Kaufman J. 1998. Occupational dermatitis causing days away from work in U.S. private industry, 1993. Am J Ind Med. 34:568–573.
- Butler J. E. 2000. Enzyme-linked immunosorbent assay. J Immunoassay. 21:165–209.
- Clark S. C., and Zirwas M. J. 2009. Management of occupational dermatitis. Dermatol Clin. 27:365–83, vii.
- De Jong W. H., De Klerk A., Beek M. T., Veenman C, and Van Loveren H. 2007. Effect of Prolonged Repeated Exposure to Formaldehyde Donors with Doses Below the EC3 Value on Draining Lymph Node Responses. J Immunotoxicol. 4:239–246.
- Garabrant D. H. 1985. Dermatitis from aziridine hardener in printing ink. Contact Derm. 12:209–212.
- 2010. ICCVAM (Interagency Coordinating Committee on the Validation of Alternative Methods). ICCVAM Test Method Evulation Report on Using the Local Lymph Node Assay for Testing Pesticide Formulations, Metals, Substances in Aqueous Solutions, and Other Products. The results of an independent peer review evaluation coordinated by the ICCVAM and the NICEATM. NIH PublicationNo. 10–7512. National Institute of Environmental Health Sciences. 2010. Available online at: http://www.iccvam.niehs.nih.gov, accessed 13 June 2011.
- Jolanki R., Kanerva L., Estlander T., and Tarvainen K. 1994. Concomitant sensitization to triglycidyl isocyanurate, diaminodiphenylmethane and 2-hydroxyethyl methacrylate from silk-screen printing coatings in the manufacture of circuit boards. Contact Derm. 30:12–15.
- Keczkes K. 1984. Does contact sensitivity last? Int J Dermatol. 23:108–109.
- Klink K. J., and Meade B. J. 2003. Dermal exposure to 3-amino-5-mercapto-1,2,4-triazole (AMT) induces sensitization and airway hyperreactivity in BALB/c mice. Toxicol Sci. 75:89–98.
- Lee E. E., and Maibach H. I. 2001. Is contact allergy in man lifelong? An overview of patch test follow-ups. Contact Derm. 44:137–139.
- Li L. F., Sujan S. A., and Wang J. 2003. Detection of occupational allergic contact dermatitis by patch testing. Contact Derm. 49:189–193.
- Livesley E. J., Rushton L., English J. S., and Williams H. C. 2002. The prevalence of occupational dermatitis in the UK printing industry. Occup Environ Med. 59:487–492.
- Manetz T. S., and Meade B. J. 1999. Development of a flow cytometry assay for the identification and differentiation of chemicals with the potential to elicit irritation, IgE-mediated, or T cell-mediated hypersensitivity responses. Toxicol Sci. 48:206–217.
- Nethercott J. R. 1988. Dermatitis in the printing industry. Dermatol Clin. 6:61–66.
- Nethercott J. R., and Nosal R. 1986. Contact dermatitis in printing tradesmen. Contact Derm. 14:280–287.
- Nethercott J. R., Jakubovic H. R., Pilger C., and Smith J. W. 1983. Allergic contact dermatitis due to urethane acrylate in ultraviolet cured inks. Br J Ind Med. 40:241–250.
- NIEHS (National Institute of Environmental Health Sciences). 1999. The murine local lymph node assay: A test method for assessing the allergic contact dermatitis potential of chemicals/compounds. Fed Regist. 64:14006–14007.
- Nielsen N. H., Linneberg A., Menné T., Madsen F., Frølund L., Dirksen A., and Jørgensen T. 2001. Persistence of contact allergy among Danish adults: An 8-year follow-up study. Contact Derm. 45:350–353.
- NIOSH (National Institute for Occupational Safety and Health). 2011. Tapp, L. C., Durgam, S., and Mueller, C. Centers for Disease Control and Prevention, and National Institute for Occupational Safety and Health. Evaluation of Contact Dermatitis Among Ink Ribbon Manufacturing Employees - New York. Health Hazard Evaluation Report HETA 2007-0261–3122. 2011.
- Shapiro M., Mowad C., and James W. D. 2001. Contact dermatitis due to printer’s ink in a milk industry employee: Case report and review of the allergen paraphenylenediamine. Am J Contact Dermatitis. 12:109–112.
- Woolhiser M. R., Munson A. E., and Meade B. J. 1999. Role of sensitization routes in the development of Type I hypersensitivity to natural rubber latex in mice. Am J Ind Med. Suppl 1:139–141.
- Woolhiser M. R., Munson A. E., and Meade B. J. 2000. Comparison of mouse strains using the local lymph node assay. Toxicology. 146:221–227.