Abstract
The present study sought to verify the utility of the non-radioactive endpoints LLNA BrdU (5-bromo-2′-deoxyuridine) ex vivo incorporation and cytokine release using auricular lymph node cells isolated from BALB/c mice topically treated with a strong (formaldehyde or p-phenylene-diamine [PPD]), moderate sensitizer (cinnamal), or weak sensitizer (eugenol). Stimulation index (SI) and EC3 values were calculated for each agent. Based on the results of ex vivo LLNA-BrdU assays, EC3 values were calculated to be 0.29, 0.09, 1.91, and 16.60% for formaldehyde, PPD, cinnamal, and eugenol, respectively. These results were in good agreement with data from previous standard radioactive LLNA. Cytokine analyses indicated TH1 and TH2 cytokine involvement in the regulation of murine contact allergy and these could be utilized as endpoints in assessments of contact allergy in mice. In conclusion, the current study provided evidence that the non-radioactive endpoint LLNA BrdU ex vivo incorporation could be of use as a viable alternative approach to assess the skin sensitization potential of test compound with respect to improving animal welfare. This is of particular importance in the case of any laboratory where it might be difficult to handle and/or readily employ radioisotopes. Further studies will be required to confirm—across test agents—the reproducibility as well as the limits of utility of this new ex vivo BrdU method.
Introduction
Over the past 20+ years, the Murine Local Lymph Node Assay (LLNA)—itself an alternative to traditional animal skin sensitization models (e.g., guinea pig maximization test, Buehler test) for assessment of the skin sensitizing ability of drugs, cosmetic materials, pesticides, or industrial chemicals—has been increasingly used in hazard assessment and has undergone rigorous evaluation and validation (Kimber and Weisenberger, Citation1989; CitationICCVAM, 1999; ECVAM, Citation2000). The use of this method is difficult in any laboratory because such a radioisotope (RI)-based method requires special facilities and handling procedures. Several authors have conducted investigations into the development of alternative non-RI methods for performing LLNA (Takeyoshi et al., Citation2001, Citation2005, Citation2006; Lee et al., Citation2002; Suda et al., Citation2002; Ehling et al., Citation2005a, Citationb; Idehara et al., Citation2008).
The LLNA:BrdU (5-bromo-2′-deoxyuridine)-ELISA (enzyme-linked immunosorbent assay) method—a modification of the original LLNA—involves assessing lymphocyte proliferation using ELISA to measure BrdU incorporation instead of measuring radioactivity due to [3H]- thymidine incorporation (Takeyoshi et al., Citation2001). This modification has recently been peer-reviewed by the National Toxicology Program (NTP) Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM) and was approved by the Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) (CitationICCVAM 2010).
We recently investigated the following non-radioactive endpoints of LLNA in response to dinitrochlorobenzene (DNCB): BrdU incorporation ex vivo and in vivo, as well as formation/release of interleukins (IL)-2, -4, and -5, and interferon (IFN)-γ from isolated lymph node cells (Ulker et al., Citation2011). While the results of the in and ex vivo assays were compatible both with one another and with standard radioactive LLNA results for DNCB, cytokine levels were affected by BrdU injection into the mice. We therefore concluded that the ex vivo BrdU incorporation method would provide a more accurate (in terms of avoiding artefactual cytokine induction/related effects on immune system cells) reflection of the allergic or irritant potential of any given test compound.
The studies reported here sought to more fully verify the utility of two non-radioactive endpoints, i.e., ex vivo LLNA:BrdU-ELISA and cytokine release from auricular lymph node cells, for predicting allergic and irritant potentials of test chemicals. In these studies, cells from mice treated topically with a strong (formaldehyde or p-phenylenediamine [PPD]), moderate sensitizer (cinnamal), or weak sensitizer (eugenol) were utilized.
Materials and methods
Mice
Female Balb/c mice were obtained from Gulhane Military Medical Academy (Ankara, Turkey) for use in these studies. All mice were housed in animal facilities maintained at 23°C with a relative humidity of 55% and with a 12 h light/dark cycle, and provided mousechow (Optima, Kırklareli, Turkey) and water ad libitum. The mice were maintained in these facilities until they reached 8–12-weeks-old, at which time the treatment phase(s) of the study were initiated. All animal procedures were conducted in an AAALAC accredited facility under an animal protocol approved by the Ankara University Animal Experiments Ethics Board.
Chemicals
Formaldehyde (Fluka, Germany) at concentrations of 0.1, 0.25, 0.50, or 1% (w/v), p-phenylenediamine (PPD; Fluka) at concentrations of 0.025, 0.05, 0.10, or 0.25% (w/v), cinnamal (Sigma, St. Louis, MO) at concentrations of 0.5, 1, 5, 10% (w/v), and/or eugenol (Sigma) at concentrations of 2.5, 10, 20, 50% (w/v) were applied to the animals in these studies. Each agent was prepared in acetone:olive oil (4:1 v/v; AOO); a control set of mice was treated with AOO in all studies. The applied doses were selected according to previous standard LLNA studies (Kimber et al., Citation2003; Basketter et al., Citation2007) and OECD guideline LLNA test procedures (OECD, Citation2002).
Ex vivo BrdU incorporation
Five groups of mice (n = 4/group) were exposed topically (on dorsum of both ears) for 3 consecutive days to 25 µl of different doses of known sensitizers or vehicle (AOO) alone daily. All mice were rested on Day 4 and then euthanized by cervical dislocation on Day 5 to permit collection of their auricular lymph nodes. The excised right and left lymph nodes from each mouse were pooled and homogenized, and the released cells suspended in 15 ml physiological saline. After counting, some cells from the suspension were seeded into 96-well culture plates (at 105 cells in 90 µl volume per well) in RPMI 1640 medium supplemented with 10% fetal bovine serum [FBS] and 1% penicillin–streptomycin [all Biochrom, Berlin, Germany]). After culture had occurred for 48 h at 37°C, BrdU (10 µl of a 10 µM BrdU solution; final concentration = 1 µM BrdU) was then added to the wells for a 24 h labeling period. The cells in the wells were then recovered by aspiration and the extent of BrdU incorporation measured by ELISA (Roche, Penzberg, Germany), according to manufacturer instructions. The absorbance was measured at a wavelength of 450 nm (OD450) with a reference wavelength of 620 nm, using an ELISA reader (SpectraMAX, Molecular Devices Inc., Sunnyvale, CA) microplate reader; these values were used to define the BrdU labeling index.
Calculations of stimulation indices (SI)
Stimulation Index (SI) was calculated as the ratio of the mean of ex vivo BrdU incorporation (labeling index) for each treatment group vs that of the vehicle control group.
Calculation of EC3 value
The EC3—a measure of relative skin sensitizing potency of a substance—is expressed as the estimated concentration of chemical necessary to produce a 3-fold increase in proliferation in draining lymph nodes compared with the spontaneous proliferation that occurs with cells from vehicle-treated control hosts. The method represents a simple linear interpolation of the points in the dose–response curve which lie immediately above and below the classification threshold, i.e., a stimulation index of 3. If the data-points lying immediately above and below the SI value of 3 have the co-ordinates (a, b) and (c, d), respectively, then the EC3 value may be calculated using the following equation (Basketter et al., 1999b; 2007):
Culture of lymph node cells and cytokine determinations
Harvested lymph node cells from the ex vivo protocols outlined above were seeded in a 24-well culture plate (at 5 × 106 cells/well) in 1 ml of RPMI 1640 medium supplemented with 10% FBS and 1% penicillin-streptomycin. In the case of the ex vivo method, the cells used here were obtained from the pooled cells from Day 4-rested hosts prior to removal of aliquots for use in BrdU staining/analyses (see above). Following the seeding steps, the wells were then supplemented with 5 µg/ml of phytohemagglutinin-L (PHA-L; Biochrom). PHA-L was selected as the mitogen here (as opposed to Concanavalin A) in that it has been widely used for mitotic stimulation of T-lymphocytes. After 72 h of culture in a 37°C incubator containing 5% CO2, supernatants were collected and stored at −80°C until analyzed for levels of IL-2, -4, and -5 and IFNγ. The levels of each cytokine in the culture supernatants were measured using commercially-available ELISA kits (BenderMed Systems, Burlingame, CA), according to the manufacturer’s instructions. The Stimulation Index (SI) was calculated for each cytokine based on the ratio of the mean cytokine level for the given treatment group vs that of the vehicle control group.
Statistical analyses
Means and standard errors were calculated for the lymph node weights and for the optical density (OD) values obtained by ELISA for each treatment group. All data were initially analyzed via one-way ANOVA; if the analyses indicated significant difference, the difference(s) between the vehicle control and each treatment group were then analyzed using a Dunnett t-test.
Results
Lymph node-related parameters outcomes
In these studies, five groups of Balb/c mice for ex vivo studies were exposed topically (on the dorsum of both ears) to 0.10, 0.25, 0.50, or 1.00% formaldehyde, 0.025, 0.050, 0.100, or 0.250% PPD, 0.5%, 1%, 5%, or 10% cinnamal, or 2.5%, 10.0%, 20.0%, or 50.0% eugenol—or to AOO vehicle alone—on three consecutive days (i.e., Days 1–3). In the ex vivo LLNA-BrdU studies, mice received no BrdU injection but were instead rested on Day 4. One day later (i.e., 2 days after final application, Day 5), all mice were sacrificed. The auricular lymph node weights and lymph node cell count values associated with each mouse on Day 5 are shown in . The mean lymph node weights and cell counts of animals given 0.5% formaldehyde, 0.1% PPD, 1.0% cinnamal, or 10.0% eugenol were significantly increased compared with those associated with the vehicle-treated counterparts.
Table 1. Lymph node weight, and cell count after treatment of mice with different concentrations of formaldehyde, PPD, cinnamal, and eugenol in AOO. Each value shown is the mean ± SD (n = 5/group).
When EC3 values derived from the ex vivo BrdU labeling studies were calculated for each chemical, values for formaldehyde, PPD, cinnamal, and eugenol were seen to be 0.29, 0.09, 1.91, and 16.60%, respectively. The data for all OD450, SI, and EC3 values from the current ex vivo BrdU labeling studies are presented in . EC3 values for these four agents when using a standard LLNA method (Kimber et al., Citation2003; Basketter et al., Citation2007) and the ex-vivo LLNA-BrdU method are shown in .
Table 2. OD450. SI. and EC3 values from ex vivo BrdU labeling studies.
Table 3. EC3 values for the four test chemicals using standard LLNA and ex-vivo LLNA-BrdU methods.*
Cytokine determinations
Levels of IL-2, -4, and -5, and of IFNγ in the lymph node cell culture supernatants are provided in ; SI values are provided in . When formaldehyde was applied at a concentration of 0.5%, levels of IFNγ and the two TH2 cytokines IL-4 and -5 in the cultures was significantly increased (p < 0.01). A significant (p < 0.05) induction of IL-2 was detected at as low a concentration of 0.1%.
Figure 1. Cytokine levels in cultures of auricular lymph node cells from mice treated daily (3 days) with formaldehyde. †p < 0.05, *p < 0.01; Significant differences from vehicle control (Dunnett’s test).
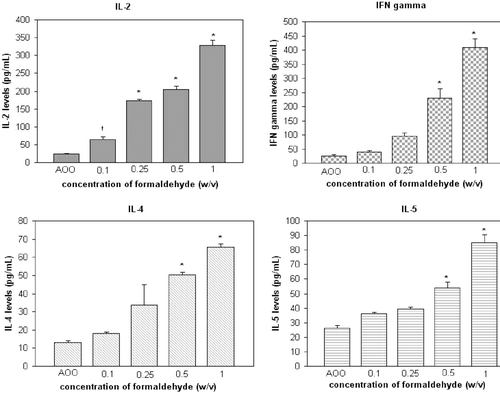
Figure 2. Cytokine levels in cultures of auricular lymph node cells from mice treated daily (3 days) with PPD. †p < 0.05, *p < 0.01; Significant differences from vehicle control (Dunnett’s test).
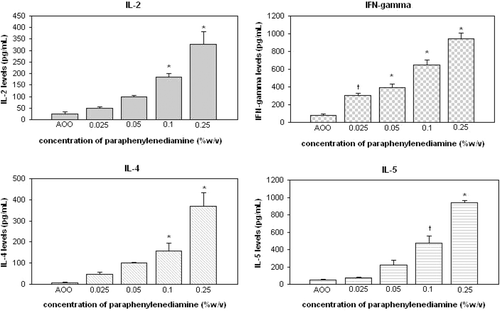
Figure 3. Cytokine levels in cultures of auricular lymph node cells from mice treated daily (3 days) with cinnamal. †p < 0.05, *p < 0.01; Significant differences from vehicle control (Dunnett’s test).
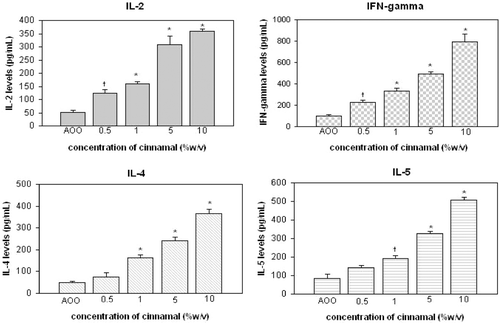
Figure 4. Cytokine levels in cultures of auricular lymph node cells from mice treated daily (3 days) with eugenol. †p < 0.05, *p < 0.01; Significant differences from vehicle control (Dunnett’s test).
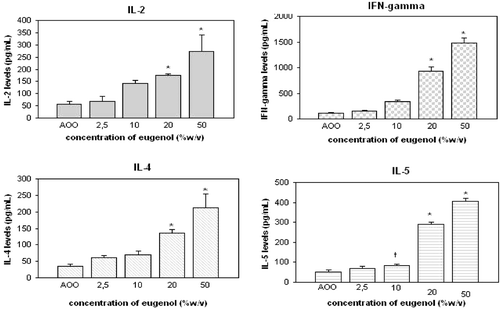
Table 4. Cytokine SI values for auricular lymph node cells from mice treated daily (3 days) with formaldehyde, PPD, cinnamal, or eugenol.
With respect to PPD, IL-2, -4, -5, and IFNγ formation/release was strongly elevated in a manner reflecting a clear dose-related trend. This strong elevation was also reflected in the SI values. Specifically, IL-2, -4, and -5 levels in the cultures were significantly increased when PPD was applied to the mice at a concentration of 0.1% (p < 0.01, p < 0.01, p < 0.05, respectively, for the indicated interleukins). Significant (p < 0.05) induction of IFNγ was noted at a 0.025% level. All elevations were correspondingly reflected in the calculated SI values.
Results for cinnamal indicated that IL-2 and IFNγ formation started to significantly increase at an application level of 0.5% (p < 0.05); levels of IL-4 and IL-5 started to increase significantly at the 1% level (p < 0.05). For eugenol, the levels of each of the four cytokines were first significantly increased at the 10% application level.
Discussion
Takeyoshi et al. (Citation2001) investigated BrdU incorporation into lymph node cells using a cell proliferation ELISA kit; this has been deemed the LLNA:BrdU-ELISA method. Although the principle of the LLNA:BrdU-ELISA method is close to that of the original radio-isotopic LLNA assay, this non-isotopic method appears to be less sensitive, i.e., the non-isotope LLNA tended to yield lower EC3 values than the standard LLNA (Takeyoshi et al., Citation2006). In spite of this, the Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) recently approved use of LLNA:BrdU-ELISA to evaluate sensitization potentials of test agents (CitationICCVAM, 2011). However, ICCVAM also recommended further studies to characterize the limitations of the LLNA:BrdU-ELISA method. This is due, in part, to an additional finding of its potential for generating false positive results when borderline ‘positive’ responses (at an SI of 1.6–1.9) are obtained. As a result, while the ICCVAM approved use of this non-isotopic method for evaluations of test agents, they also stated that an SI ≥ 3 must be achieved to reliably define chemicals as sensitizers.
In a previous study, we made some modifications to the LLNA:BrdU-ELISA to make the assay more sensitive (Ulker et al., Citation2011). In our modified method, we used an SI ≥ 3 as the cut-off for classification of chemicals as sensitizers; EC3 values were also calculated for use in comparisons against studies that utilized the standard radioactive LLNA. Using the in vivo and ex vivo BrdU incorporation methods, EC3 values of 0.059% and 0.054% were calculated for dinitrochlorobenzene (DNCB). With both the in vivo and ex vivo incorporation methods, results with lymph node cells tested right after harvest were comparable to those obtained in a standard radioactive LLNA.
The study here also aimed to improve the welfare of the test animals (Reduction, Refinement, and Replacement)—albeit slightly (i.e., they still had to be treated and ultimately euthanized)—by having isolated lymphocytes labeled with BrdU ex vivo (by adding BrdU at the 48-h point in lymphocyte cell culture) instead of having this done in vivo (in situ) (i.e., labeling by intraperitoneal BrdU injection). This method would allow for the avoidance of the additional pain and distress to a host that would occur due to the intraperitoneal BrdU injection step.
In this study, we sought to verify the utility of two non-radioactive endpoints of LLNA, i.e., BrdU ex vivo incorporation (ex vivo LLNA-BrdU ELISA) and cytokine release from the auricular lymph node cells isolated after topical treatment of murine hosts with a strong (formaldehyde or PPD), moderate (cinnamal), or a weak (eugenol) sensitizer. According to our EC3 results, formaldehyde and PPD can be classified as strong allergens, cinnamal a moderate sensitizer, and eugenol a weak sensitizer. Such results were in good agreement with previous classifications obtained by Kimber et al. (Citation2003) and Basketter et al. (Citation2007) using the standard radioactive LLNA.
Many studies have tried to characterize the chemical sensitizers according to the cytokine profiles they provoke (Dearman et al., Citation1996a, Citationb, Citation2003; Dearman and Kimber, Citation1999; Manetz and Meade, Citation1999; Hayashi et al., Citation2001; Manetz et al., Citation2001; Plitnick et al., Citation2002; van Och et al., Citation2002; Vandebriel et al., Citation2003). Changes (i.e., increases) in cytokine levels are likely related to T-helper cell proliferation that plays an important role in the induction and elicitation of contact sensitivity. In our study, we assessed the release of IL-2 and IFNγ (TH1 cytokines) and of IL-4 and IL-5 (TH2 cytokines) from the isolated lymph node cells. We found that for formaldehyde and PPD, there was a dose-related (trend) increase in formation/release of IL-2 and IFNγ by the treated animals’ lymph node cells. On the other hand, there was an enhancement in the SI values for IL-4 and IL-5 that was related to the doses of chemicals employed (for cells generated in either protocol); however, the increases were not as dramatic as those noted in the cases of IL-2 and IFNγ. This dose-related trend was observed in the cells from mice that had been treated with each of the four test agents, with different cytokine release profiles resulting. These findings demonstrate not only that TH1 cytokines (such as IL-2 and IFNγ) but also TH2 cytokines (such as IL-4 and IL-5) can play important roles in elicitation of contact sensitivity. The cytokine analysis results also indicated that these cytokines might be considered as useful endpoints in determinations of contact allergy in mice.
In conclusion, although negative chemical data would be indispensable for evaluating the assay performance for this method, the non-radioisotopic LLNA measuring ex vivo BrdU incorporation into lymph node cells might be a promising modification of the BrdU-based LLNA as a sensitive method with respect to improving animal welfare as far as when it is used to assess strong, moderate, and weak sensitizers. Additional replicates and inclusion of more chemicals will be for to verify the sensitivity, reproducibility, and overall utility of this new method.
Acknowledgments
This study has been supported by the Scientific and Technological Research on Council of Turkey (TUBITAK) (Project number: 107S365).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Basketter, D. A., Gerberick, F. Kimber, I. 2007. The local lymph node assay and the assessment of relative potency: Status of validation. Contact Derm. 57:70–75.
- Dearman, R. J., Basketter, D. A. Kimber, I. 1996a. Characterization of chemical allergens as a function of divergent cytokine secretion profiles induced in mice. Toxicol. Appl. Pharmacol. 138:308–316.
- Dearman, R. J., Betts, C. J., Humphreys, N., Flanagan, B. F., Gilmour, N. J., Basketter, D. A. Kimber, I. 2003. Chemical allergy: Considerations for the practical application of cyto-kine profiling. Toxicol. Sci. 71:137–145.
- Dearman, R. J. Kimber, I. 1999. Cytokine fingerprinting: Characterization of chemical allergens. Methods 19:56–63.
- Dearman, R. J., Moussavi, A., Kemeny, D. M. Kimber, I. 1996b. Contribution of CD4+ and CD8+ T-lymphocyte subsets to the cytokine secretion patterns induced in mice during sensiti-zation to contact and respiratory chemical allergens. Immunology 89:502–510.
- Ehling, G., Hecht, M., Heusener, A., Huesler, J., Gamer, A. O., van Loveren, H., Maurer, T., Riecke, K., Ullmann, L., Ulrich, P., Vandebriel, R. Vohr, H. W. 2005a. An European inter-laboratory validation of alternative endpoints of the murine local lymph node assay: First round.Toxicology 212:60–68.
- Ehling, G., Hecht, M., Heusener, A., Huesler, J., Gamer, A. O., van Loveren, H., Maurer, T., Riecke, K., Ullmann, L., Ulrich, P., Vandebriel, R. Vohr, H. W. 2005b. An European inter-laboratory validation of alternative endpoints of the murine local lymph node assay: Second round.Toxicology 212:69–79.
- European Centre for the Validation of Alternative Methods (ECVAM). 2000. Statement on the validity of the local lymph node assay for skin sensitization testing. ATLA 28:366–367.
- Hatao, M., Harýya, T., Katsumura, Y. Kato, S. 1995. A modification of the local lymph node assay for contact allergenicity screening: Measurement of IL-2 as an alternative to radioisotope-dependent proliferation assay. Toxicology 98:15–22.
- Hayashi, M., Higashi, K., Kato, H. Kaneko, H. 2001. Assessment of preferential TH1 or TH2 induction by low-molecular-weight compounds using a reverse transcription-polymerase chain reaction method: Comparison of two mouse strains C57BL/6 and BALB/c. Toxicol. Appl.Pharmacol. 177:38–45.
- Idehara, K., Yamagishi, G., Yamashita, K. Ito, M. 2008. Characterization and evaluation of a modified local lymph node assay using ATP content as a non-radio isotopic endpoint.J.Pharmacol. Toxicol. Meth. 58:1–10.
- Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) Report. 1999. The Murine Local Lymph Node Assay: a test method for assessing the allergic contact dermatitis potential of chemicals/compounds. The Results of an Independent Peer Review Evaluation Coordinated by ICCVAM and NICEATM. NIH Publication No. 99-4494. Research Triangle Park, NC: National Institute of Environmental Health Sciences. Available online at: http://iccvam.niehs.nih.gov/methods/immunotox/llna_PeerPanel98.htm.
- Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) Report March 2010. ICCVAM test method evaluation report on the murine local lymph node assay: BrdU-ELISA A nonradioactive alternative test method to assess the allergic contact dermatitis potential of chemicals and products. NIH Publication No. 10-7552. Research Triangle Park, NC: National Institute of Environmental Health Sciences. Availableonline at: http://iccvam.niehs.nih.gov/methods/immunotox/llna-ELISA/TMER.htm.
- Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) Test Method Evaluation Report 2011. Usefulness and limitations of the murine local lymph node assay for potency categorization of chemicals causing allergic contact dermatitis in humans. NIH Publication No. 11–7709. Research Triangle Park, NC: National Institute of Environmental Health Sciences. Available online at: http://iccvam.niehs.nih.gov/methods/immunotox/llna-ELISA/LLNA-pot/TMER.htm.
- Kimber, I. Weisenberger, C. 1989. A murine local lymph node assay for the identification of contact allergens. Assay development and result of an initial validation study. Arch. Toxicol. 63:274–282.
- Kimber, I., Basketter, D. A., Butler, M., Gamer, A., Garrigue, J. L., Gerberick, G. F., Newsome, C., Steiling, W. Vohr, H. W. 2003. Classification of contact allergens according to potency: Proposals. Food Chem. Toxicol. 41:1799–1809.
- Lee, J. K., Par, J. H., Park, S. H., Kim, H. S. Oh, H. Y. 2002. A non-radioisotopic endpoint for measurement of lymph node cell proliferation in a murine allergic contact dermatitis model, using bromodeoxyuridine immunohistochemistry.J. Pharmacol. Toxicol. Methods 48;53–61.
- Manetz, T. S. Meade, B. J. 1999. Development of flow cytometry assay for the identification and differentiation of chemicals with the potential to elicit irritation, IgE-mediated, or T-cell-mediated hypersensitivity responses. Toxicol. Sci. 48:206–217.
- Manetz, T. S., Pettit, D. A. Meade, B. J. 2001. The determination of draining lymph node cell cytokine mRNA levels in BALB/c mice following dermal sodium lauryl sulfate, dinitro-fluorobenzene, and toluene diisocyanate exposure. Toxicol. Appl. Pharmacol. 171:174–183.
- Organization for Economic Co-operation and Development (OECD). 2002. OECD Test Guideline No: 429. Guideline for the Testing of Chemicals. Skin Sensitization: Local Lymph Node Assay. Available online at: http://iccvam.niehs.nih.gov/methods/immunotox/llnadocs/OECD429.pdf
- Plitnick, L. M., Loveless, S. E., Ladics, G. S., Holsapple, M. P., Selgrade, M. J., Sailstad, D. M. Smialowicz, R. J. 2002. Cytokine profiling for chemical sensitizers: Application of the ribonuclease protection assay and effect of dose. Toxicol. Appl. Pharmacol. 179:145–154.
- Suda, A., Yamashita, M., Tabei, M., Taguchi, K., Vohr, H.W., Tsutsui, N., Suzuki, R., Kikuchi, K., Sakaguchi, K., Mochizuki, K. Nakamura, K. 2002. Local lymph node assay with non-radioisotope alternative endpoints. J. Toxicol. Sci. 27:205–218.
- Takeyoshi, M., Ilda, K., Shiraishi, K. Hoshuyama, S. 2005. Novel approach for classifying chemicals according to skin sensitizing potency by non-radioisotopic modification of the local lymph node assay. J. Appl. Toxicol. 25:129–134.
- Takeyoshi, M., Noda, S., Yamasaki, K. Kimber, I. 2006. Advantage of using CBA/N strain mice in a non-radioisotopic modification of the local lymph node assay. J. Appl. Toxicol. 26:5–9.
- Takeyoshi, M., Yamasaki, K., Yakabe, Y., Takatsuki, M. Kimber, I. 2001. Development of non-radioisotopic endpoint of murine local lymph node assay based on 5-bromo-2'-deoxyuridine (BrdU) incorporation. Toxicol. Lett. 119:203–208.
- Ulker, O. C., Atak, A., Ates, I. Karakaya, A. 2011. Evaluation of auricular lymph node cell lymphocyte proliferation and cytokine production as non-radioactive endpoints during murine contact allergy. J. Immunotoxicol. 8:131–139.
- van Och, F. M., van Loveren, H., de Jong, W. H. Vandebriel, R. J. 2002. Cytokine production induced by low-molecular-weight chemicals as a function of the stimulation index in a modified local lymph node assay: An approach to discriminate contact sensitizers from respiratory sensitizers. Toxicol. Appl. Pharmacol. 184:46–56.
- Vandebriel, R. J., de Jong, W. H., Hendriks, J. J. van Loveren, H. 2003. Impact of exposure duration by low molecular weight compounds on IFNγ and IL-4 mRNA expression and production in the draining lymph nodes of mice. Toxicology 188:1–13.