Abstract
Histamine, involved in many inflammatory reactions and immune responses, is reported to suppress—via H4R stimulation—injury concomitant with the late phase of warm hepatic ischemia/re-perfusion (I/R). The current study investigated the possible effects of histamine on the acute phase of hepatic I/R injury, and the possible underlying mechanisms like oxidative stress and release of inflammatory cytokines (e.g., tumor necrosis factor (TNF)-α nd interleukin [IL]-12). Rats were divided into naïve, sham-operated, and I/R groups. The I/R group was divided into sub-groups and pre-treated with histaminergic ligands before induction of ischemia. Anesthetized rats were subjected to warm ischemia for 30 min by occlusion of the portal vein and hepatic artery, then re-perfused for 90 min. Rats in the control I/R group showed significant increases in hepatic malondialdehyde (MDA), TNFα, and IL-12 contents, and in plasma alanine transaminase (ALT) and aspartate transaminase (AST) levels, along with significant decreases in hepatic reduced glutathione (GSH) content and marked diffuse histopathologic damage. Pre-treatment with histamine resulted in significant mitigation of each of these end-points. The protective effect of histamine was not antagonized by pre-treatment with mepyramine (H1R antagonist) or ranitidine (H2R antagonist) and completely reversed by pre-treatment with thioperamide (H3R and H4R antagonist). In addition, the histamine protective effect was mimicked by pre-treatment of rats with clozapine (H4R agonist). These observations strongly suggested that histamine has a protective effect against hepatic I/R-mediated tissue injury during the acute phase, and this effect was mediated through an H4R stimulation that led to a decrease in IL-12 and TNFα production—outcomes that consequently decreased localized oxidative stress and afforded hepatic protection in general.
Introduction
Hepatic ischemia re-perfusion (I/R) injury is a phenomenon whereby cellular damage in a hypoxic liver is accentuated following restoration of oxygen delivery (Pereyra et al., 1975). Hepatic I/R injury can be categorized into warm I/R injury and cold-storage re-perfusion injury. Warm I/R injury is relevant clinically in cases of hepatic surgery, liver transplantation, hypo-volemic shock, some types of toxic liver injury, veno-occlusive disease, and Budd–Chiari syndrome (Teoh and Farrell, Citation2003).
Several studies showed that there is a biphasic mode of hepatocellular injury following I/R. This process consists of both an acute phase occurring within the first 6 h following ischemia and a sub-acute phase that occurs 6–24 h post-ischemia (Jaeschke, Citation1998) (Martinez-Mier et al 2000). Hepatic injury during the acute phase of I/R is mainly mediated by activated Kupffer cells and occurs in conjunction with increased oxidative stress in the tissue and enhanced production of a number of pro-inflammatory mediators—including cytokines and chemokines (Jaeschke et al., Citation1991;) (Hisama et al., Citation1996). In contrast, liver injury during the late phase is mainly mediated by neutrophils (Jaeschke et al., Citation1990).
Histamine receptors participate in different physiological and pathophysiological conditions. For example, H2R stimulation depresses immunoreactivity by reducing chemotactic responsiveness of inflammatory cells, whereas H1R action provokes anaphylactic reactions and bronchial asthma (Hirasawa et al., Citation1987; Packard and Khan, Citation2003; Dy and Schneider, Citation2004). H4R also mediates immune responses and inflammatory cell recruitment (Hofstra et al., Citation2003; Ling et al., Citation2004) and suppresses production of interleukin (IL)-12, a key inducer of cell mediated immunity (Hilkens et al., Citation1997; Gutzmer et al., Citation2005) and essential for development of hepatic I/R injury (Lentsch et al., Citation1999). Histamine release is also enhanced during ischemia and might contribute to (neuro)protection against ischemic damage (Adachi et al., Citation2001; Adachi, Citation2002). On the other hand, impairment of histaminergic transmission or suppression of histaminergic activity appears to aggravate ischemic neuronal damage (Sugimoto et al., Citation1994; Adachi et al., Citation2001).
Histamine has been found to be effective in the suppression of hepatic I/R injury concomitant with the late phase, apparently via the stimulation of H4R (Adachi et al., Citation2006). To date, the exact mechanism of protective action of histamine remains unclear. Therefore, this study was undertaken to investigate possible effects of histamine on hepatic I/R-mediated tissue injury during the acute phase. In addition, possible mechanisms that may be involved in any protective outcomes were also investigated.
Materials and methods
Animals
Adult male albino rats (14-weeks-old, 150–200 g) were obtained from the animal facilities of the National Research Center (NRC, Cairo, Egypt) for use in these studies. Rats were acclimated for 1 week prior to the experiment. All rats were housed under specific pathogen-free conditions with a 12-h light/12-h dark cycle, constant temperature (25 ± 2°C) and relative humidity (55 ± 5%), and had ad libitum access to standard rodent chow and filtered water. All animal studies were approved by the Institutional Animal Care and Use Committee and were conducted in accordance with guidelines established by the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
After acclimatization, the rats were weighed and then randomly allocated into several experimental groups: Naïve (no surgery was carried out; n = 8); Sham-operated (hepatic artery and portal vein were exposed but not clamped; n = 8); and, I/R wherein a total of 56 rats were randomly distributed into seven equal sub-groups (n = 8/group) and injected with different drugs (or control vehicle) three times (i.e., at 24, 12, and 0 h) prior to the induction of ischemia. Specifically, the drug regimens were: (a) 20 mg histamine/kg (in saline [Sigma Aldrich, St. Louis, MO] subcutaneously [SC]); (b) histamine + 3 mg mepyramine/kg (in saline [Sigma]) SC 15 min before histamine); (c) histamine + 10 mg ranitidine/kg (in saline [Medical Union Pharmaceuticals Co., Ismailia, Egypt] SC 15 min before histamine); (d) histamine + 5 mg thioperamide/kg (in saline [Sigma] SC 15 min before histamine); (e) 15 mg clozapine/kg (in dilute acetic acid vehicle [Sigma] SC); or (F) saline vehicle SC; or (G) buffered dilute acetic acid vehicle SC at same volume as given to rats in the other groups. Each of these regimens was based on the work performed in studies by Adachi et al. (Citation2006). In all cases, a period of 1 week was allowed to elapse between randomization and when surgeries were performed; this would allow time for each rat to adapt to new cage mates and re-establish relationships and hierarchy, and thereby reduce stresses to the animals that could potentially have an impact on the study outcomes.
Induction of hepatic I/R
After overnight fasting, rats were anesthetized via an intraperitoneal injection of 1.3 g urethane/kg body weight (Squadrito et al., Citation2000). The abdomen was cleansed with 70% ethanol and then swabbed with Betadine®. A midline abdominal incision was performed beginning at the mid-abdomen and ending at the xyphoid process. Body temperature was maintained at 37°C by use of a heating lamp. The abdomen was explored and hepatic ischemia induced by clamping the portal vein and hepatic artery for 30 min using a non-traumatic clamp (Su et al., Citation2003). Re-perfusion was then performed for 90 min following the removal of the clamp.
Sample collection
Immediately following the I/R procedure, blood collection was performed by cardiac puncture to permit analysis of plasma alanine (ALT) and aspartate aminotransferase (AST) levels/activities. Thereafter, the rats were euthanized by dissection of the portal vein and exsanguination. At necropsy, the liver was carefully removed and rinsed thoroughly with saline; the tissue was weighed and portions placed in 10% neutral buffered formalin (pH 7.4) for subsequent histopathological examination. Remaining tissue was placed at −20°C for later biochemical study.
Determination of hepatic TNFα and IL-12 p70 levels
Hepatic tissues (≈ 0.25 g/rat) were homogenized in ice-cold phosphate-buffered saline (PBS, pH 7.4) containing protease inhibitor cocktail and 0.05% (v/v) Tween-20. Samples were then centrifuged at 3000 rpm for 10 min and the resultant supernatant collected and analyzed for TNFα in an ELISA assay as part of an Assaymax TNFα kit (Gentaur, Dublin, Ireland). Samples were also assessed for IL-12 p70 in an ELISA assay as part of a Cusabio Biotech IL-12 (p70) kit (Wuhan, China). Each rat sample was analyzed in duplicate and values were determined by extrapolation from standard curves prepared in parallel using kit-provided TNFα or IL-12 (p70) standards. Sensitivities of the TNFα and IL-12 (p70) kits were < 10 and 0.8 pg/ml, respectively.
Determination of hepatic reduced glutathione (GSH) contents
Hepatic GSH contents were determined via the method of Ellman (Citation1959). Briefly, hepatic tissues (≈ 0.25 g/rat) were first homogenized in ice-cold 0.1 M phosphate buffer (pH 7.4), and then sulfosalicylic acid (4%) was added to precipitate proteins. After centrifugation at 3000 rpm for 10 min, the supernatant was recovered. To 0.5 ml of supernatant, 4.5 ml of bis-(3-carboxy-4-nitrophenyl) disulfide reagent was added, and the absorbance was measured at 412 nm in a double-beam UV-VIS spectrophotometer (Model UV-1601 PC, Shimadzu, Kyoto, Japan).
Determination of hepatic lipid peroxide levels
Hepatic malondialdehyde (MDA) content was assayed as an indirect indicator of in situ lipid peroxidation (Yoshioka et al., Citation1979). Briefly, hepatic tissues (≈ 0.25 g/rat) were homogenized in 10 ml ice-cold 1.15% (w/v) potassium chloride solution. To 0.5 ml of homogenate, 3 ml of 0.5% (w/v) trichloroacetic acid and 1 ml 0.6% (w/v) thiobarbituric acid were added; the entire solution was then mixed and heated for 45 min in a boiling waterbath. After cooling, 4 ml n-butanol was added and the sample vigorously shaken. After allowing for phase separation, the butanol layer was isolated and the absorbance of the pink colored product in the layer measured at 535 nm in the double-beam spectrophotometer.
Determination of plasma ALT and AST levels
Plasma levels of ALT and AST were determined colorimetrically based on the method of Reitman and Frankel (Citation1957) using ALT and AST kits purchased from Biodiagnostic (Cairo, Egypt). According to the manufacturer’s instructions, ALT and AST levels were estimated via measures of pyruvate and oxaloacetate formation, respectively, using kit-provided enzyme substrates. After completion of the reaction, each sample was then analyzed at 546 nm and values were extrapolated from a kit-provided reference table. Samples with activities > 80 IU/L were diluted with 0.9 % NaCl and re-assayed. Each rat sample was analyzed in duplicate. Sensitivities of the ALT and AST kits were 4 and 7 IU/L, respectively.
Histopathological examination of hepatic sections
At the end of the re-perfusion period, the liver was removed, washed with saline, and prepared for histopathological examination. The liver was immediately fixed in 10% buffered formalin solution (pH 7.4) for 24 h, and then routinely processed in ascending grades of alcohol, then xylene. The tissues were then embedded in paraffin wax, serially-sectioned to ≈ 4 µm thickness, and stained with Hematoxylin and Eosin (H&E Sigma). Ultimately, each stained tissue section was examined using a light microscope (Olympus BX 51, Olympus America, Melville, NY) and photographed with a digital camera (Olympus DP11) connected to the microscope. Observations were recorded in representative photomicrographs taken at 250×.
Statistical analysis
Results were expressed as the mean (± SD). Comparisons between different groups were carried out by one-way analysis of variance (ANOVA) followed by Tukey-Kramer test using Minitab computer software (Version 15; Minitab Inc., State College, PA). Statistical significance was accepted at p < 0.05.
Results
Effects of histaminergic ligands on hepatic lipid peroxides contents (measured as MDA)
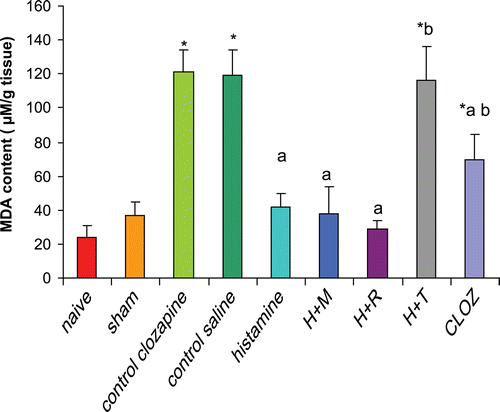
Rats subjected to hepatic I/R displayed a significant increase (398% and 233%) in hepatic MDA contents as compared to that in the organs from naïve and sham-operated rats, respectively. Pre-treatment of rats with 20 mg histamine/kg led to a significant reduction (65%) in hepatic MDA contents as compared to levels in the tissues from the control I/R saline rats. Treatment with 3 mg mepyramine/kg or 10 mg ranitidine/kg did not affect histamine-induced reduction in hepatic MDA contents, while treatment with 5 mg thioperamide/kg blocked any histamine-induced reduction in hepatic MDA contents. Treatment with 15 mg clozapine/kg led to a significant reduction (42%) in hepatic MDA contents as compared to levels in the tissues from the control I/R acetic acid rats, mimicking the effect of histamine ).
Figure 1. Effects of histaminergic ligands on hepatic lipid peroxides contents (measured as MDA). Rats were subjected to either sham operation or hepatic ischemia followed by re-perfusion. I/R-operated rats were pre-treated three times (at 24, 12, and 0 h) before induction of ischemia with control vehicle(s), histamine (20 mg/kg, SC), mepyramine (3 mg/kg, SC 15 min prior to histamine), ranitidine (10 mg/kg, SC 15 min prior to histamine), thioperamide (5 mg/kg, SC 15 min prior to histamine), or clozapine (15 mg/kg, SC). Hepatic lipid peroxides contents (i.e., MDA) were determined at the end of the re-perfusion period. Data are presented as mean (± SD; n = 8/group) MDA content (µM/g tissue). Value is significantly different from the * sham-operated, a I/R, or b histamine group at p < 0.05. H, histamine; M, mepyramine; R, ranitidine; T, thioperamide; Cloz, clozapine.
Effects of histaminergic ligands on hepatic GSH contents
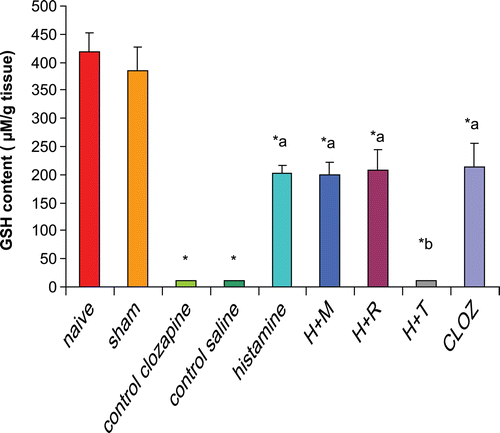
Rats subjected to hepatic I/R displayed a significant decrease (97%) in hepatic GSH contents as compared to that in the organs from naïve and sham-operated rats. Pre-treatment of rats with 20 mg histamine/kg led to a significant increase (1924%) in hepatic GSH content as compared to levels in tissues from control I/R saline rats. Treatment with 3 mg mepyramine/kg or 10 mg ranitidine/kg did not affect histamine-induced increases in hepatic GSH contents; treatment with 5 mg thioperamide/kg blocked any histamine-induced increase in hepatic GSH. Treatment with 15 mg clozapine/kg led to a very significant increase (1964%) in hepatic GSH contents as compared to levels in the tissues from the control I/R acetic acid rats mimicking the effect of histamine ().
Figure 2. Effects of histaminergic ligands on hepatic GSH contents. Rats were subjected to either sham operation or hepatic ischemia followed by re-perfusion. I/R-operated rats were pre-treated three times (at 24, 12, and 0 h) before induction of ischemia with control vehicles, histamine, mepyramine, ranitidine, thioperamide, or clozapine. For doses/timing of doses, please see legend to . Hepatic GSH contents were determined at the end of the re-perfusion period. Data are presented as mean (± SD; n = 8/group) GSH content (µM/g tissue). Value is significantly different from the * sham-operated, a I/R, or b histamine group at p < 0.05. H, histamine; M, mepyramine; R, ranitidine; T, thioperamide; Cloz, clozapine.
Effects of histaminergic ligands on plasma ALT level
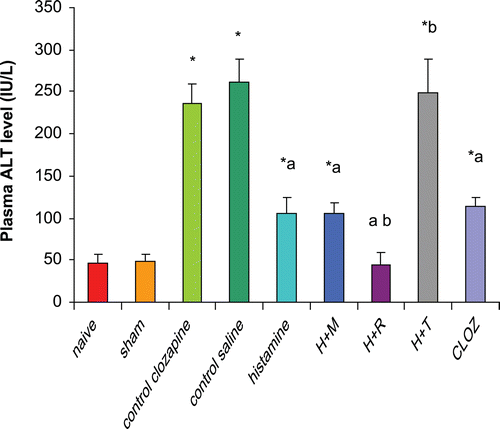
Rats subjected to hepatic I/R displayed significant increases (470% and 440%) in plasma ALT levels as compared to those in organs from naïve and sham-operated rats, respectively. Pre-treatment of rats with 20 mg histamine/kg led to a significant reduction (60%) in plasma ALT as compared to levels in the plasma from control I/R saline rats. Treatment with 3 mg mepyramine/kg did not affect histamine-induced reduction in plasma ALT; conversely, 10 mg ranitidine/kg treatment enhanced the histamine-induced reductions in plasma ALT. Treatment with 5 mg thioperamide/kg blocked any histamine-induced reduction in plasma ALT. Treatment with 15 mg clozapine/kg led to a significant reduction (52%) in plasma ALT level as compared to levels in plasma of control I/R acetic acid rats (mimicking the effect of histamine) ().
Figure 3. Effects of histaminergic ligands on plasma ALT level. Rats were subjected to either sham operation or hepatic ischemia followed by re-perfusion. I/R-operated rats were pre-treated three times (at 24, 12, and 0 h) before induction of ischemia with control vehicles, histamine, mepyramine, ranitidine, thioperamide, or clozapine. For doses/timing of doses, please see legend to . Plasma ALT levels were determined at the end of the re-perfusion period. Data are presented as the mean (± SD; n = 8/group) ALT levels (IU/L). Value is significantly different from the * sham-operated, a I/R, or b histamine group at p < 0.05. H, histamine; M, mepyramine; R, ranitidine; T, thioperamide; Cloz, clozapine.
Effects of histaminergic ligands on plasma AST level
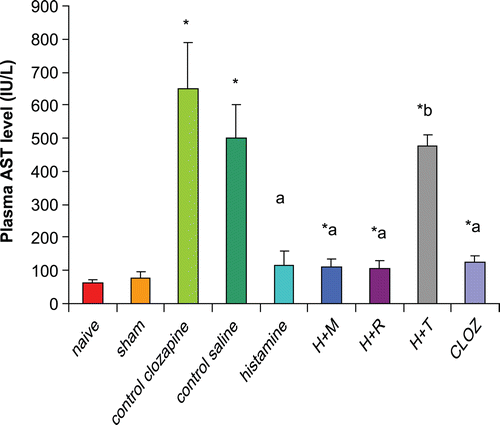
Rats subjected to hepatic I/R displayed significant increases (972% and 720%) in plasma AST levels as compared to those in organs from naïve and sham-operated rats, respectively. Pre-treatment of rats with 20 mg histamine/kg led to a significant reduction (77%) in plasma AST as compared to levels in the plasma from control I/R saline rats. Treatment with 3 mg mepyramine/kg or 10 mg ranitidine/kg did not affect histamine-induced reductions in plasma AST; 5 mg thioperamide/kg treatment blocked any histamine-induced reduction in plasma AST. Treatment with 15 mg clozapine/kg led to a significant reduction (81%) in plasma AST level as compared to levels in plasma of control I/R acetic acid rats ().
Figure 4. Effects of histaminergic ligands on plasma AST level. Rats were subjected to either sham operation or hepatic ischemia followed by re-perfusion. I/R-operated rats were pre-treated 3-times (at 24, 12, and 0 h) before induction of ischemia with control vehicles, histamine, mepyramine, ranitidine, thioperamide, or clozapine. For doses/timing of doses, please see legend to . Plasma AST levels were determined at the end of the re-perfusion period. Data are presented as the mean (± SD; n = 8/group) AST levels (IU/L). Value is significantly different from the * sham-operated, a I/R, or b histamine group at p < 0.05. H, histamine; M, mepyramine; R, ranitidine; T, thioperamide; Cloz, clozapine.
Effects of histaminergic ligands on TNFα contents
Rats subjected to hepatic I/R displayed significant increases (36% and 27%) in hepatic TNFα content as compared to that in organs from naïve and sham-operated rats, respectively. Pre-treatment of rats with 20 mg histamine/kg led to a significant reduction (18%) in hepatic TNFα content as compared to levels in tissues from control I/R saline rats. Treatment with 3 mg mepyramine/kg or 10 mg ranitidine/kg did not affect the histamine-induced reductions in hepatic TNFα content; 5 mg thioperamide/kg treatment blocked any histamine-induced reduction in hepatic TNFα. Treatment with 15 mg clozapine/kg led to a significant reduction (15%) in TNFα content as compared to levels in tissues from control I/R acetic acid rats ().
Figure 5. Effects of histaminergic ligands on TNFα contents. Rats were subjected to either sham operation or hepatic ischemia followed by re-perfusion. I/R-operated rats were pre-treated 3-times (at 24, 12, and 0 h) before induction of ischemia with control vehicles, histamine, mepyramine, ranitidine, thioperamide, or clozapine. For doses/timing of doses, please see legend to . Hepatic TNFα contents were determined at the end of the re-perfusion period. Data are presented as the mean (± SD; n = 8/group) TNFα content (pg/g tissue). Value is significantly different from the * sham-operated, a I/R, or b histamine group at p < 0.05. H, histamine; M, mepyramine; R, ranitidine; T, thioperamide; Cloz, clozapine.
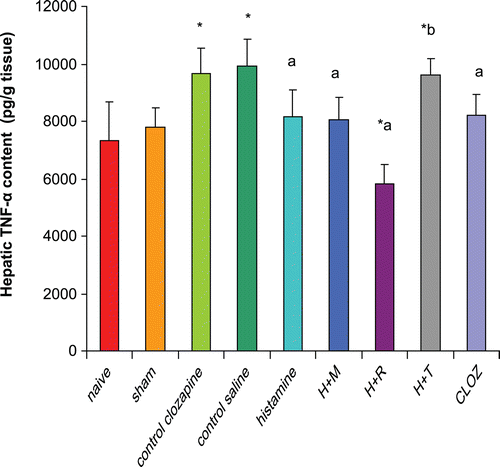
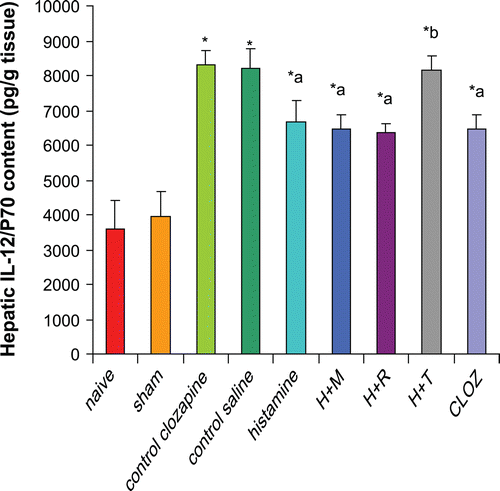
Effects of histaminergic ligands on IL-12 (p70) contents
Rats subjected to hepatic I/R displayed significant increases (131% and 110%) in hepatic IL-12 (p70) contents compared to those in organs from, respectively, naïve and sham-operated rats. Pre-treatment of rats with 20 mg histamine/kg led to a significant reduction (19%) in hepatic IL-12 (p70) content compared to levels in tissues from control I/R saline rats. Treatment with 3 mg mepyramine/kg or 10 mg ranitidine/kg did not affect histamine-induced reductions in hepatic IL-12 (p70); 5 mg thioperamide/kg treatment blocked any induced reduction in IL-12 (p70). Treatment with 15 mg clozapine/kg led to a significant reduction (22%) in hepatic IL-12 (p70) content as compared to levels in tissues from control I/R acetic acid rats ().
Figure 6. Effects of histaminergic ligands on IL-12 (p70). Rats were subjected to either sham operation or hepatic ischemia followed by re-perfusion. I/R-operated rats were pre-treated 3-times (at 24, 12, and 0 h) before induction of ischemia with control vehicles, histamine (20 mg/kg, SC), mepyramine (3 mg/kg, SC) 15 min prior to histamine, ranitidine (10 mg/kg, SC) 15 min prior to histamine, thioperamide (5 mg/kg, SC) 15 min prior to histamine or clozapine (15 mg/kg, SC). Hepatic IL-12 (p70) contents were determined at the end of the re-perfusion period. Data are presented as the mean (± SD; n = 8/group) Hepatic IL-12 (p70) contents (pg/g tissue). Value is significantly different from the * sham-operated, a I/R, or b histamine group at p < 0.05. H, histamine; M, mepyramine; R, ranitidine; T, thioperamide; Cloz, clozapine.
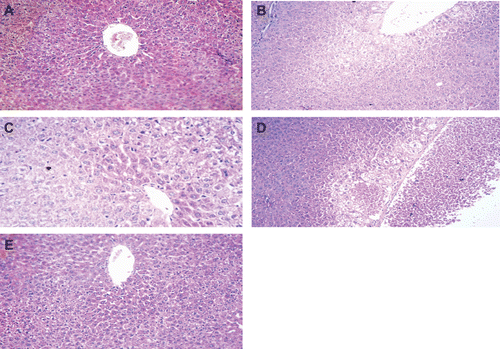
Effect of histaminergic ligands on liver histology
Histopathological examination of liver tissues from naïve rats revealed completely normal livers with regard to lobular architecture, blood sinusoids, and central veins (data not shown). The tissues of sham-operated rats were apparently normal except for some mild central venous dilatation and congestion ). In contrast, liver sections from I/R-operated rats revealed disturbed architecture with necrosis and vacuolar degeneration around a dilated congested central vein (). In the liver sections of rats pre-treated with 20 mg histamine/kg, the liver showed minimal vacuolar degeneration peripherally ). Rats that received mepyramine or ranitidine prior to histamine administration did not abolish the protective effect of histamine (data not shown). On the other hand, rats pre-treated with thioperamide prior to histamine showed massive central zonal necrosis around a dilated engorged central vein with variable degrees of vacuolar degeneration ). Lastly, rats pre-treated with 15 mg clozapine/kg showed mild vacuolar degeneration in their hepatocytes and dilated central vein ).
Figure 7. Histology of liver sections recovered from rats in the various treatment groups. All images are 250× magnifications of H&E-stained tissues and are representative of samples from rats in each group. Section from: (a) sham-operated rat showing apparently normal liver except for mild central venous dilatation and congestion; (b) I/R-operated rat (control) showing disturbed architecture with necrosis and vacuolar degeneration around a dilated congested central vein; (c) histamine pre-treated rat showing minimal vacuolar degeneration peripherally; (d) rat that received thioperamide prior to histamine, showing massive central zonal necrosis around a dilated, engorged central vein with variable degrees of vacuolar degeneration; and (e) clozapine pre-treated rat showing mild vacuolar degeneration in hepatocytes and a dilated central vein.
Discussion
The present study was conducted in the more clinically relevant model of warm hepatic I/R injury through occlusion of the portal vein and hepatic artery for 30 min, then allowing re-perfusion for 90 min. I/R injury was confirmed biochemically by increases in plasma levels of the liver enzymes AST and ALT and histopathologically by a presence of diffuse vacuolar degeneration, dilatation of the central vein, and central zonal necrosis. These results were in agreement with previous studies of different (Adachi et al., Citation2006) and similar (Su et al., Citation2003) models of hepatic I/R injury.
Pre-treatment of rats with histamine protected against the acute phase of hepatic I/R injury as evidenced by the significant reduction in the plasma level of ALT and AST and confirmed by the histopathological findings. This protective effect of histamine is not blocked by mepyramine (specific H1R antagonist), or ranitidine (specific H2R antagonist) but completely blocked by thioperamide (an H3R and H4R antagonist) and mimicked by clozapine (anti-psychotic with H4R agonist activity). To our knowledge, no other study has investigated the effect of histamine during the acute phase of hepatic I/R injury; nonetheless, these results appear to be in agreement with a previous study by Adachi et al. (Citation2006), who investigated the effect of histamine during the late phase of hepatic I/R injury. Based on the current findings and those of the Adachi group and Motoki et al. (Citation2008), it is now clear that H4R is responsible for the action of histamine during the acute phase and the late phase.
The protective effect of histamine may be due to its effect on IL-12 cytokine production. IL-12 is a potent heterodimeric cytokine which was found to be essential for development of hepatic I/R injury (Lentsch et al., Citation1999). IL-12 expression was increased in the liver during endotoxemia (Zisman et al., Citation1997). In addition, exogenous administration of IL-12 in normal mice induces Kupffer cell activation, up-regulation of hepatic vascular cell adhesion molecules, and leukocyte accumulation in the liver (Myers et al., Citation1998). Furthermore, mice treated with anti-IL-12 or in IL-12 p40−/− mice, there is a greatly reduced hepatic neutrophil accumulation and hepatocellular injury following hepatic I/R injury induction (Lentsch et al., Citation1999).
In the present study, hepatic IL-12 (p70) content was significantly elevated in the I/R group as compared to the sham-operated and naïve groups. Pre-treatment of rats with histamine significantly reduced hepatic IL-12 (p70) content through stimulation of H4R. In support of these results, a previous study by Gutzmer et al. (Citation2005) showed that H4R stimulation suppressed IL-12 (p70) production in human monocyte-derived dendritic cells. Recently, it has been demonstrated that H4R activation on human 6-sulfo-LacNAc (slan) dendritic cells produced lower levels of TNFα and IL-12 (Gschwandtner et al., Citation2011). Also in agreement with the results of the current study, Motoki et al. (Citation2008) found that dimaprit (H2R and H4R agonist) resulted in a marked decrease in the plasma concentration of IL-12 at 4–24 h from the start of the re-perfusion. In contrast to those results, Elenkov et al. (Citation1998) reported that histamine inhibited the secretion of human IL-12 (p70) through H2R, but not H1R or H3R stimulation. Unfortunately, that study was performed prior to identification of H4R by Nakamura et al. (Citation2000). Also, the fact that H2R stimulation decreases IL-12 is not a surprise. Gutzmer et al. (Citation2005) reported that both H2R and H4R stimulation suppressed IL-12 (p70) production in human monocyte-derived dendritic cells (MoDC), but using different signaling mechanisms.
According to the results of the current study, H4R stimulation only was responsible for suppression of IL-12 production and mediation of any liver protection against I/R injury. The relationships between IL-12 secretion and TNFα production (Lentsch et al., Citation1999; Matsushita et al., Citation1999; Köken et al., Citation2004) as well as that between TNFα formation and the acute (Rudiger and Clavien, Citation2002) or late phases (Colletti et al., Citation1996, Citation1998) of hepatic I/R injury have been well defined. As a result, the effect of histamine and H4R stimulation on TNFα production was investigated in the current study.
In the present study, hepatic TNFα content was increased significantly in the I/R group compared to sham-operated and naïve groups. These results were in agreement with previous studies. Colletti et al. (Citation1990) found that the plasma TNFα level was increased significantly as early as 30 min following re-perfusion. Scales et al. (Citation1994) also showed that levels of biologically-active TNFα were increased early post-ischemia. Pre-treatment of rats with histamine resulted in a significant reduction of hepatic TNFα content through H4R stimulation. The effect of histamine on TNFα production may be due to direct H4R stimulation or secondary to the decrease of hepatic IL-12 content or both, this point was not covered in the current study. These results were in agreement with a recent study by Gschwandtner et al. (Citation2011) where they demonstrated that H4R activation on human slan dendritic cells produced lower levels of TNFα and IL-12.
Formation of reactive oxygen species (ROS) is an important mechanism of injury, especially during the acute phase of hepatic I/R injury (Jaeschke and Farhood, Citation1991; Togashi et al., Citation2000; Nardo et al., Citation2001). Data obtained from studies that utilized Kupffer cell depletion and antioxidants suggest that Kupffer cell-derived oxidant formation was a primary mediator of the early phase of tissue injury and that NADPH-oxidase could be a key factor underlying this outcome (Bremer et al., Citation1994; Shiratori et al., Citation1994; Bailey and Reinke, Citation2000). In the current study, hepatic MDA and GSH contents were used as indices of oxidative stress. Hepatic MDA content was increased significantly in the I/R groups; this indicated increased liver oxidative stress and lipid peroxidation post-ischemia and was in agreement with previous studies (Nardo et al., Citation2001; Seo and Lee, Citation2002; Su et al., Citation2003). Pre-treatment of rats with histamine significantly reduced hepatic MDA content. Further, hepatic GSH content was depleted from the livers of rats in the I/R group while rats pre-treated with histamine had a significant increase in hepatic GSH. Effects of histamine on both were mediated through H4R stimulation. The H4R mediated effect of histamine on ROS may be related to its effects on TNFα production, as shown by a previous study by Scales et al. (Citation1994) where they found that TNFα neutralizing antibody significantly decreased both ROI and AST. This may also be related to the effect of histamine on production of IL-12—a potent activator of Kupffer cells (Myers et al., Citation1998)—and represent a primary source of ROS during the acute phase (Bailey and Reinke, Citation2000).
In conclusion, this study has demonstrated the protective effect of histamine against the acute phase of hepatic I/R injury. This protective effect of histamine was mediated by H4R stimulation and may be explained by a H4R stimulation-induced decrease in pro-inflammatory cytokines (IL-12 and TNFα) that consequently decrease ROS.
Acknowledgments
The authors acknowledge the support of the Faculty of Pharmacy, Tanta University, Egypt grants to perform this research.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Adachi, N. 2002. Cerebral ischemia and histamine. Nihon Yakurigaku Zasshi 120:215–221.
- Adachi, N., Liu, K., Motoki, A., Nishibori, M. Arai, T. 2006. Suppression of ischemia/re-perfusion liver injury by histamine H4 receptor stimulation in rats. Eur. J. Pharmacol. 544:181–187.
- Adachi, N., Seyfried, F. J. Arai, T. 2001. Blockade of central histaminergic H2 receptors aggravates ischemic neuronal damage in gebril hippocampus. Crit. Care Med. 29:1189–1194.
- Bailey, S. M. Reinke, L. A. 2000. Antioxidants and gadolinium chloride attenuate hepatic parenchymal and endothelial cell injury induced by low flow ischemia and re-perfusion in perfused rat livers. Free Rad. Res. 32:497–506.
- Bremer, C., Bradford, B. U., Hunt, K. J., Knecht, K. T., Connor, H. D., Mason, R. P. Thurman, R. G. 1994. Role of Kupffer cells in the pathogenesis of hepatic re-perfusion injury. Am. J. Physiol. 267:G630–636.
- Colletti, L. M., Cortis, A., Lukacs, N., Kunkel, S. L., Green, M. Strieter, R. M. 1998. Tumor necrosis factor up-regulates inracellular adhesion molecule-1, which is important in neutrophil-dependent lung and liver injury associated with hepatic ischemia and re-perfusion in the rat. Shock 10:182–191.
- Colletti, L. M., Kunkel, S. L., Walz, A., Burdick, M. D., Kunkel, R. G., Wilke, C. A. Strieter, R. M. 1996. The role of cytokine networks in the local liver injury following hepatic ischemia/re-perfusion in the rat. Hepatology 23:506–514.
- Colletti, L. M., Remick, D. G., Burtch, G. D., Kunkel, S. L., Strieter, R. M. Campbell, D. A. Jr. 1990. Role of tumor necrosis factor-α in the pathophysiologic alterations after hepatic ischemia re-perfusion injury in the rat. J. Clin. Invest. 85:1936–1943.
- Dy, M. Schneider, E. 2004. Histamine-cytokine connection in immunity and hematopoiesis. Cytokine Growth Factor Rev. 15:393–410.
- Elenkov, I. J., Webster, E., Papanicolaou, D. A., Fleisher, T. A., Chrousos, G. P. Wilder, R. L. 1998. Histamine potently suppresses human IL-12 and stimulates IL-10 production via H2 receptors. J. Immunol. 161:2586–2593.
- Ellman, G. L. 1959. Tissue sulfahydryl groups.. Arch. Biochem. Biophys 82:70–77.
- Gschwandtner, M., Schäkel, K., Werfel, T. Gutzmer, R. 2011. Histamine H4 receptor activation on human slan-dendritic cells down-regulates their pro-inflammatory capacity. Immunology 132:49–56
- Gutzmer, R., Diestel, C., Mommert, S., Köther, B., Stark, H., Wittmann, M. Werfel, T. 2005. Histamine H4 receptor stimulation suppresses IL-12p70 production and mediates chemotaxis in human monocyte-derived dendritic cells. J. Immunol. 174:5224–5232.
- Hilkens, C. M., Kalinski, P., de Boer, M. Kapsenberg, M. L. 1997. Human dendritic cells require exogenous IL-12-inducing factors to direct the development of naive T-helper cells toward the TH1 phenotype. Blood 90:1920–1926.
- Hirasawa, N., Ohuchi, K., Watanabe, M. Tsurufuji, S. 1987. Role of endogenous histamine in post-anaphylactic phase of allergic inflammation in rats. J. Pharmacol. Exp. Ther. 241:967–973.
- Hisama, N., Yamaguchi, Y., Ishiko, T., Miyanari, N., Ichiguchi, O., Goto, M., Mori, K., Watanabe, K., Kawamura, K., Tsurufuji, S., and Ogawa, M. 1996. Kupffer cell production of cytokine-induced. neutrophil chemoattractant following ischemia/reperfusion injury in rats. Hepatology 24:1193–1198.
- Hofstra, C. L., Desai, P. J., Thurmond, R. L. Fung-Leung, W. P. 2003. Histamine H4 receptor mediates chemotaxis and calcium mobilization of mast cells. J. Pharmacol. Exp. Ther. 305:1212–1221.
- Jaeschke, H. Farhood, A. and Smith, C. W. 1990. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. Faseb J 4:3355–3359.
- Jaeschke, H. Farhood, A. 1991. Neutrophil and Kupffer cell-induced oxidant stress and ischemia/re-perfusion injury in rat liver. Am. J. Physiol. 260:G355–362.
- Jaeschke, H., Bautista, A. P., Spolarics, Z., and Spitzer, J. J. 1991. Superoxide generation by Kupffer cells and priming of neutrophils during reperfusion after hepatic ischemia. Free Radic. Res. commun 15:277–284.
- Jaeschke, H. 1998. Mechanisms of reperfusion injury after warm ischemia of the liver. J. Hepatobiliary Pancreat. Surg 5:402–408.
- Köken, T., Serteser, M., Kahraman, A., Akbulut, G. Dilek, O. N. 2004. Which is more effective in the prevention of renal ischemia-re-perfusion-induced oxidative injury in the early period in mice: Interleukin (IL)-10 or anti-IL-12? Clin. Biochem. 37:50–55.
- Lentsch, A. B., Yoshidome, H., Kato, A., Warner, R. L., Cheadle, W. G., Ward, P. A. Edwards, M. J. 1999. Requirement for IL-12 in pathogenesis of warm hepatic ischemia/re-perfusion injury in mice. Hepatology 30:1448–1453.
- Ling, P., Ngo, K., Nguyen, S., Thurmond, R. L., Edwards, J. P., Karlsson, L. Fung-Leung, W. P. 2004. Histamine H4 receptor mediates eosinophil chemotaxis with cell shape change and adhesion molecule upregulation. Br. J. Pharmacol. 142:161–171.
- Martinez-Mier, G., Toledo-Pereyra, L. H., and Ward, P. A. 2000. Adhesion molecules in liver ischemia and reperfusion. J. Surg. Res 94:185–194.
- Matsushita, T., Ando, K., Kimura, K., Ohnishi, H., Imawari, M., Muto, Y. Moriwaki, H. 1999. IL-12 induces specific cytotoxicity against regenerating hepatocytes in vivo. Int. Immunol. 11:657–665.
- Motoki, A., Adachi, N., Liu, K., Takahashi, H. K., Nishibori, M., Yorozuya, T., Arai, T. Nagaro, T. 2008. Suppression of ischemia-induced cytokine release by dimaprit and amelioration of liver injury in rats. Basic Clin. Pharmacol. Toxicol. 102:394–398.
- Myers, K. J., Eppihimer, M. J., Hall, L. Wolitzky, B. 1998. IL-12-induced adhesion molecule expression in murine liver. Am. J. Pathol. 152:457–468.
- Nakamura, T., Itadani, H., Hidaka, Y., Ohta, M. Tanaka, K. 2000. Molecular cloning and characterization of a new human histamine receptor, HH4R. Biochem. Biophys. Res. Commun. 279:615–620.
- Nardo, B., Caraceni, P., Pasini, P., Domenicali, M., Catena, F., Cavallari, G., Santoni, B., Maiolini, E., Grattagliano, I., Vendemiale, G., Trevisani, F., Roda, A., Bernardi, M. Cavallari, A. 2001. Increased generation of reactive oxygen species in isolated rat fatty liver during postischemic reoxygenation. Transplantation 71:1816–1820.
- Packard, K. A. Khan, M. M. 2003. Effects of histamine on TH1/TH2 cytokine balance. Int. Immunopharmacol. 3:909–920.
- Reitman, S. and, Frankel, S. A. 1957. The determination of serum glutamic oxaloacetic and glutamic pyruvic transaminases. Am. J. Clin. Path 28:56–63.
- Rudiger, H. A. Clavien, P. A. 2002. Tumor necrosis factor-α, but not Fas, mediates hepatocellular apoptosis in the murine ischemic liver. Gastroenterology 122:202–210.
- Scales, W. E., Campbell, D. A., Green, M. E. Jr., and Remick, D. G. 1994. Hepatic ischemia/re-perfusion injury: Importance of oxidant/tumor necrosis factor interactions. Am. J. Physiol. 267:G1122–1127.
- Seo, M. Y. Lee, S. M. 2002. Protective effect of low dose of ascorbic acid on hepatobiliary function in hepatic ischemia/re-perfusion in rats. J. Hepatol. 36:72–77.
- Shiratori, Y., Kiriyama, H., Fukushi, Y., Nagura, T., Takada, H., Hai, K. Kamii, K. 1994. Modulation of ischemia/re-perfusion-induced hepatic injury by Kupffer cells. Dig. Dis. Sci. 39:1265–1272.
- Squadrito, F., Altavilla, D., Squadrito, G., Ferlito, M., Deodato, B., Arlotta, M., Minutoli, L., Campo, G. M., Bova, A., Quartarone, C., Urna, G., Sardella, A., Saitta, A. Caputi, A. P. 2000. Protective effects of cyclosporine-A in splanchnic artery occlusion shock. Br. J. Pharmacol. 130:339–344.
- Su, J. F., Guo, C. J., Wei, J. Y., Yang, J. J., Jiang, Y. G. Li, Y. F. 2003. Protection against hepatic ischemia-re-perfusion injury in rats by oral pre-treatment with quercetin. Biomed. Environ. Sci. 16:1–8.
- Sugimoto, K., Abe, K., Lee, T. H., Sakurai, E., Yanai, K., Kogure, K., Itoyama, Y. Watanabe, T. 1994. Histamine depletion in brain caused by treatment with (S) α-fluoro-methylhistidine enhances ischemic damage of gerbil hippocampal CA2 neurons. Brain Res. 66:279–283.
- Teoh, N. C., and Farrell, G. C. 2003Hepatic ischemia reperfusion injury: Pathogenic mechanisms and basis for hepatoprotection. J. Gastroenterol. Hepatol 18:891–902.
- Togashi, H., Shinzawa, H., Matsuo, T., Takeda, Y., Takahashi, T., Aoyama, M., Oikawa, K. Kamada, H. 2000. Analysis of hepatic oxidative stress status by electron spin resonance spectroscopy and imaging. Free Rad. Biol. Med. 28:846–853.
- Yoshioka, T., Kawada, K., Shimada, T. Mori, M. 1979. Lipid peroxidation in maternal and cord blood and protective mechanism against activated oxygen-toxicity in blood. Am. J. Obstet. Gynecol. 135:372–376.
- Zisman, D. A., Kunkel, S. L., Strieter, R. M., Gauldie, J., Tsai, W. C., Bramson, J., Wilkowski, J. M., Bucknell, K. A. Standiford, T. J. 1997. Anti-IL-12 therapy protects mice in lethal endotoxemia but impairs bacterial clearance in murine Escherichia coli peritoneal sepsis. Shock 8:349–356.