Abstract
The aim of the study was to assess the activity of AP-1 family proteins, e.g. Fra-1, Fra-2, JunB, JunD, and FosB, engaged in the regulation of inducible nitric oxide synthase (iNOS) expression and the production of NO by neutrophils (PMN) exposed to N-nitrosodimethylamine (NDMA) xenobiotic. Isolated human PMN were incubated in the presence of NDMA. iNOS mRNA expression was then analyzed using Northern blot and the expression of other proteins in the cytoplasmic and nuclear fractions were assessed using Western blot. The obtained results indicate that NDMA increased iNOS mRNA and protein expression in human PMN. Furthermore, it increased the expression of Fra-1, Fra-2, JunB, and JunD in the cytoplasmic fraction, and FosB expression in the fractions of analyzed cells. As a consequence of inhibiting p38 pathway and JNK, reduced iNOS expression and NO production was noted in PMN exposed to NDMA. Inhibition of the p38 pathway resulted in reduced expression of all analyzed proteins in the cytoplasmic fraction of PMN exposed to NDMA. Furthermore, increased Fra-2 expression and reduced FosB expression were found in the nuclear fraction of those cells. Inhibiting ERK5 pathway resulted in increased JunB expression in both fractions of the analyzed cells. Therefore, no changes in the expression of analyzed proteins in the presence of NDMA were observed in PMN pre-incubated with JNK pathway inhibitor. In conclusion, the results here indicate a role of Fra-1, Fra-2, JunB, JunD, and FosB transcription factors in the regulation of iNOS expression and NO production by human neutrophils exposed to NDMA.
Introduction
The AP-1 transcription factor is a dimer composed of Jun family proteins (e.g. c-Jun, JunB, JunD) and Jun family proteins bound to Fos family proteins (e.g. c-Fos, FosB, Fra-1, Fra-2). The binding of Fos and Jun proteins results from the presence of structural domains that allow for formation of the ‘leucine zipper’ (Foletta et al., Citation1998). The expression of three jun genes and four fos genes might lead to the creation of 18 possible basic variations of AP-1 factor, and a much larger number of variations with different degrees of phosphorylation (Karin et al., Citation1997).
AP-1 regulates the transcription of genes containing the sequences of the tetradecanoate-13-acetate (TPA)-response element (TRE) or cAMP-response element (CRE) in the promoters (Trelease et al., Citation1999). Changes in the composition of AP-1 may impact its affinity to specific bindings and initiate the expression of a different set of genes and, as a consequence, regulate cell proliferation, differentiation, or apoptosis (Shaulian and Karin, Citation2002). Activation of the AP-1 factor by de novo synthesis of its components, as well as by phosphorylation of the existing components, may occur as a result of activity of various signaling pathways, i.e. MAP kinases (Whitmarsh and Davis, Citation1996).
The MAP kinase family includes c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinases (ERK1/2), p38 kinase, and mitogen-activated protein kinase MAPK (ERK5/BMK1) (Treisman, Citation1996). MAP kinases may be activated in a cell in response to numerous endo- and exogenous factors (Zhang and Dong, Citation2007). The latter include xenobiotics, such as N-nitrosamines, including N-nitrosodimethylamine (NDMA). This particular compound is most frequently found in food, tobacco smoke, and industrial pollution (Tricker and Preussmann, Citation1991).
NDMA can also be formed endogenously in an organism from various precursors occurring naturally in food or added artificially (Hebels et al., Citation2011). Available data indicate that binding with oxygen plays a significant role in the biosynthesis of nitro compounds. In vivo, the reaction occurs with the participation of nitric oxide (NO) which, in the presence of oxygen, takes on nitrating properties (Hebels et al., Citation2011). It has also been suggested that, among the immune system’s cells, the neutrophils (PMN), through NO production, may induce intra-organic NDMA synthesis (Jablonski, Citation2006).
It has been reported that the effect of this compound on the organism, even in small doses, causes severe organ damage and neoplastic transformation of cells (Tricker and Preussmann, Citation1991; Hebels et al., Citation2011). Previous studies also indicated that this xenobiotic, widespread in the environment, impacts the activity of immune system cells. The effects of NDMA on the functions and lifespan of PMN via the apoptosis pathway have been described (Jablonski et al., Citation2011). It has been determined that NDMA stimulates PMN to produce reactive oxygen and nitrogen species, including superoxide anion radical and nitric oxide. It is believed that these agents are, to a large extent, responsible for the cytotoxic activity of NDMA (Ratajczak-Wrona et al., Citation2011).
The physiological and pathological role of NO in an organism is closely related to its concentration at a reaction site. NO synthesized in picomolar volumes is sufficient to induce signals in cells. However, synthesized in nano- or micromolar volumes, NO acts as a defensive cytotoxin with a wide impact on the activity of various cells in situ. Aside from the direct impact, NO can also create biologically-active binding events in a host (Wink and Mitchell, Citation1998).
One of the enzymes responsible for NO synthesis in PMN is inducible nitric oxide synthase (iNOS). This synthase synthesizes NO as a result of metabolic conversion of L-arginine into L-citrulline (Alderton et al., Citation2001). In the promoter sequence of the iNOS gene are a number of sites that bind various transcription factors, including AP-1 factor (Marks-Konczalik et al., Citation1998). In a previous study in our laboratory, it was shown that one impact of NDMA on human PMN was an induced activation of iNOS and production of NO via a pathway dependent on the c-Jun transcription factor and activated by MAP kinases p38 and JNK (Ratajczak-Wrona et al., unpublished results). Taking into consideration that NDMA can exert toxic effects as a matrix or through active metabolites, we also found that NDMA acts on PMN as a parent compound (Ratajczak-Wrona et al., unpublished results). An assessment of the participation of other AP-1 family proteins will also allow for determining the true role of this factor in iNOS regulation and NO production in neutrophils exposed to NDMA.
Accordingly, the aim of the study was to assess the activity of AP-1 family proteins, e.g. Fra-1, Fra-2, JunB, JunD, and FosB in the regulation of iNOS expression and the production of NO by human neutrophils exposed to NDMA. Analyses of the activation of MAP kinases p38, ERK5, and JNK at the same time would allow for a determination of their role in the induction of selected transcription factors in those cells. Ultimately, researching the expression of AP-1 transcription factors induced by MAP kinases in neutrophils exposed to NDMA will lead to a better understanding of the molecular foundations underpinning the mechanisms of this xenobiotic’s activity on this select leukocyte population.
Materials and methods
Isolation and incubation of PMN
The study involved a group of 20 healthy people, aged from 25–50 years, volunteer blood donors from Regional Centre for Transfusion Medicine, Bialystok, Poland. The study was approved by the Ethics Committee of the Medical University of Bialystok (R-I-002/98/2011) and all persons gave written informed consent.
PMN were isolated from heparinized (10 U/ml, Heparin, Polfa, Lodz, Poland) whole blood by density centrifugation using Gradisol G gradient 1.115 g/ml (Polfa) (Zeman et al., Citation1988). Sera were obtained from blood samples collected without anti-coagulant agents. The PMN were further purified by positive selection using a Midi-MACS magnetic separation system (Miltenyi Biotec, Bergisch Gladbach, Germany) that employed MicroBeads conjugated to monoclonal anti-human CD16 antibodies.
PMN were suspended at a concentration of 5 × 106 cells/ml in Hanks’ Balanced Salt Solution 1X (Invitrogen™, Carlsbad, CA) containing the subject’s own serum (7.4%, 20/270 µl), 100 U penicillin/ml, and 50 ng streptomycin (Polfa Trachomin SA, Warsaw, Poland). The cells (200 µl aliquots) were then placed into wells of microplates (Microtest III-Falcon, BD Biosciences, Bedford, MA) and incubated for 2 h at 37°C in a 5% CO2 incubator (Nuaire™ US Autoflow, Plymouth, MN). PMN in the wells were then treated with 20 µl NDMA (Sigma, Steinheim, Germany) to attain a final concentration of 0.74 µg/µl in the well; control wells received vehicle only. The PMN were then cultured a further 2 h before supernatants were collected. Assessments of viability (via trypan blue exclusion) showed that PMN were > 93% viable after the treatment.
In order to determine the role of MAP kinase in the activation of AP-1 family proteins engaged in regulation of iNOS expression in PMN exposed to NDMA, selective inhibitors of particular pathways were used. In these studies, cells were pre-incubated with: 40 µM SB203580 (Calbiochem, San Diego, CA)—a selective p38 pathway inhibitor (Lee et al., Citation1994); 30 µM BIX02189—a selective MEK5/ERK5 inhibitor (Tatake et al., Citation2008) (Selleck Chemicals, Houston, TX); or with 40 µM SP600125—a selective JNK pathway inhibitor (Bennett et al., Citation2001) for 1 h before the addition of NDMA. Preliminary studies showed that the presence of any of the inhibitors did not affect cell viability.
RNA isolation and Northern blotting
iNOS mRNA expression in human neutrophils was measured by Northern Blotting analysis. Total RNA was extracted from PMN using a single-step Trizol–phenol–chloroform extraction process (TriPure Isolation Reagent, Rosche Diagnostics, Indianapolis, IN; chloroform Sigma) (Chomczynski and Sacchi, Citation1987). Total RNA that was precipitated was then dissolved in RNAsecure (Applied Biosystems, Carlsbad, CA). The RNA concentration of the extracts was determined from the absorbances at 260 and 280 nm in an Pharmacia Gene Quant spectrophotometer (Pharmacia, Hayward, CA); all samples were expected to (and did) have an 260/280 absorbance ratio of ≈ 1.75.
Total RNA (10 µg) from each sample was denatured at 65°C in a solution containing 12.5 µl formamide, 2.5 µl 10X MOPS buffer (200 mM 3-{N-morpholino}-propanesulfonic acid [Fluka Analytical, Steinheim, Germany]), 50 mM sodium acetate (Sigma), 10 mM EDTA, pH 8.0 (Promega Corporation, Madison, Wl), and 4 µl formaldehyde (Sigma), and then fractionated by electrophoresis over 1% agarose gels containing MOPS buffer. The size-separated RNA was transferred onto nylon membranes (0.45 µm; Biodyne B, Pre-Cut Modified, Thermo Scientific, Pierce, Rockford, IL) by capillary blotting in 20X SCC buffer (Invitrogen™). After baking at 80°C for 2 h and a 45-min pre-hybridization process, membranes were hybridized with biotin-labeled oligonucleotide probes (for iNOS and β-actin [for housekeeping gene purposes]) for 18 h at 45°C. The oligonucleotide probes for inducible NO synthase (iNOS) and β-actin mRNA were, respectively, 5′-TCTCTCGGCCACCTTTGATGAG-3′ and 5′-AAATCGTGCGTGACA-TTAAGG-3′ (Integrated DNA Technologies, Coralville, IA). Both the iNOS and β-actin sites were then visualized via a reaction using a chemiluminescent substrate (North2South Chemiluminescent Hybridization and Detection Kit, Thermo Scientific) and blot exposure to Kodak X-Omat XAR-film Hyperfilm (Amersham, Arlington Heights, IL).
Protein isolation and Western blotting
Cytoplasmic and nuclear extracts from PMN (3 × 106 cells total/sample) were prepared using a NucBuster™ Protein Extraction Kit (Calbiochem). Step-wise extraction delivered two distinct cellular protein fractions: cytoplasmic and nuclear. The concentration of protein in each was determined with a Qubit™ Protein Assay Kit (Invitrogen). An antibody against PARP-1 (1:5000; Calbiochem) and against Hsp90α (1:2000; Calbiochem) was used as an internal control within the nuclear and cytoplasmic fractions, respectively.
The extracts were suspended in Laemli’s buffer (Bio-Rad Laboratories, Herkules, CA), loaded at 20 µg/well, and then electrophoresed over a 4% stacking and 10% separating SDS-PAGE gel. The resolved proteins were electrotransferred onto 0.45-µm pore-size nitrocellulose membranes (Bio-Rad). These were then blocked with Tris-Buffered Saline (TBS)/Caseine buffer (Bio-Rad), washed with TBS-T (TBS containing 0.05% Tween-20), and incubated with Qentix™ Western Blot Signal Enhancer (Thermo Scientific). The membranes were then incubated for 10 min at room temperature in SNAP (Protein Detection System; Millipore, Billerica, MA) with 1:100 dilutions of primary monoclonal antibodies (all Santa Cruz Biotechnology, Heidelberg, Germany) against iNOS, Fra-1, Fra-2, JunB, JunD, or FosB. After washing with 0.1% TBS-T, the membranes were incubated for 10 min at room temperature with alkaline phosphatase anti-mouse IgG Ab (1:200; Vector Laboratories, Burlingame, CA). Immunoreactive protein bands were then visualized using a BCIP/NBT Liquid substrate system (Sigma), and intensities determined using ImageJ software (NIMH, Bethesda, MD) and reported as arbitrary units (AU).
Assay for nitrite production
Synthesis of NO was determined by an assay of the culture supernatant for nitrite, a stable reaction product of NO with molecular oxygen. Total NO concentration is commonly determined as a sum of nitrite and nitrate concentrations. NO production by PMN was determined using an indirect method based on measurement of nitrite concentration in culture supernatants according to Griess’s reaction (Tsikas, Citation2007). In the samples analyzed, nitrates were reduced to nitrite in the presence of cadmium (Sigma), and then converted to nitric acid that yielded a color reaction with Griess reagent (Sigma). Nitrite concentrations were determined by spectrophotometric analysis at 540 nm with extrapolation from a standard curve prepared in parallel. Nitric oxide products were expressed as µM/106 cells in 270 µl supernatant.
Statistics
Results were analyzed using Statistica version 9.1. (StatSoft, Inc., Tulsa, OK). Data distribution normality was determined using a Kolmogorov-Smirnov test. Since data were not normally distributed, for comparison of variations between assayed groups, Mann-Whitney U non-parametric tests were applied to un-related results. For analysis of correlation between parameters tested, a Pearson’s linear correlation was used and its significance assessed using a Student’s t-test for correlation coefficient. A p-value ≤0.05 was accepted as statistically significant. All data are presented as mean ± SE.
Results
mRNA iNOS expression using Northern blot
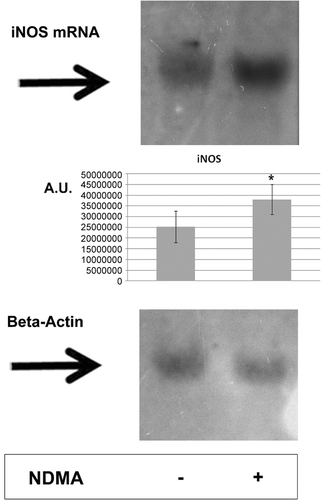
The assessment of mRNA iNOS expression confirmed the previously observed increase in the expression of this synthase at the protein level () (unpublished results).
Figure 1. Northern blot analysis for the expression of iNOS mRNA in human PMN. Cells were treated with 0.74 µg NDMA/µl for 2 h. Total RNA (10 µg/sample from 107 total cells) were then size-fractioned by agarose gel electrophoresis and transferred onto a positively-charged nylon membrane. Detection of iNOS mRNA or β-actin mRNA was performed using oligonucleotide probes labeled with biotin. Hybridization was followed by chemiluminescent detection of hybrids and analyzed using ImageJ software. Similar results were obtained from 10 independent experiments; one representative blot is shown.
Protein expression using Western blot
Both in the cytoplasmic and the nuclear fraction of PMN, the presence of the following proteins was observed: Fra-1 (42 kDa), Fra-2 (40 kDa), JunB (39 kDa), JunD (40 kDa), and FosB (45 kDa) (). Incubation of the PMN with NDMA triggered an increase of the expression of all five of these proteins in the cytoplasmic fraction. In the nuclear fraction of those cells, only an increase in FosB expression was noted. No readily noticeable changes were observed for the other four proteins of interest here.
Figure 2. Expression of iNOS, Fra-1, Fra-2, JunB, JunD, and FosB in PMN. (A) PMN were treated with or without SB203580 (40 µM), BIX02189 (30 µM), or SP600125 (40 µM) for 1 h before addition of NDMA (0.74 µg/µl). The cytoplasmic and nuclear fractions obtained from the cells were used to detect iNOS, Fra-1, Fra-2, JunB, JunD, and FosB protein levels by Western blot. The results shown are representative of five independent experiments. (B) Band intensity was quantified using ImageJ software and expressed in arbitrary units (AU). Data shown are mean (± SE) of five independent experiments. * Value significantly different between cells without and with NDMA (p < 0.05); #value significantly different between cells treated with NDMA but pre-incubated without or with the inhibitor (p < 0.05).
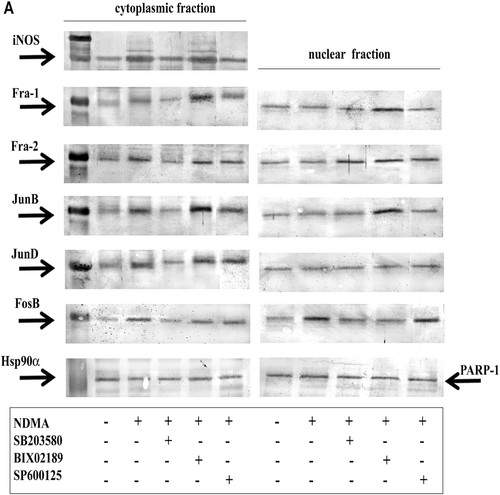
In order to learn the role of MAP kinases: p38, ERK5, and JNK in the induction of the analyzed AP-1 family proteins, experiments with inhibitors of specific kinases were performed. As a result of incubation with the p38 pathway inhibitor SB203580, reduced iNOS expression in the cytoplasmic fraction of neutrophils exposed to NDMA was seen as compared to that in cells incubated without the inhibitor (unpublished results). At the same time, significantly lower expression of all the analyzed proteins (e.g., Fra-1, Fra-2, JunB, JunD, and FosB) was seen in the cytoplasmic fraction. In the nuclear fraction, there were no changes in Fra-1, JunB, and JunD expression; however, an increased Fra-2 expression and reduced FosB expression was detected ().
After applying the ERK5 pathway inhibitor (BIX02189) no changes of iNOS expression in the cytoplasmic fraction of PMN exposed to NDMA were shown, as compared to cells incubated without the inhibitor. An increased Fra-1 and JunB expression was observed in both fractions of analyzed cells, as compared to cells incubated without the inhibitor. After inhibiting the ERK5 pathway no changes in Fra-2 and FosB expression in the cytoplasmic fraction were observed. An increased Fra-2 expression and reduced FosB expression were shown in the nuclear fraction. No changes in JunD expression in both PMN fractions were observed ().
In the presence of the JNK pathway inhibitor (SP600125) a reduced iNOS expression in the cytoplasmic fraction of PMN exposed to NDMA was confirmed (unpublished results). No changes of expression of all analyzed transcription factors were observed in the cytoplasmic fraction of those cells, as compared to the expression in cells without the inhibitor. Also, no changes in the expression of those factors in the nuclear fraction were observed, with the exception of Fra-2, which exhibited increased expression ().
Assessment of the total concentration of NO in PMN supernatants
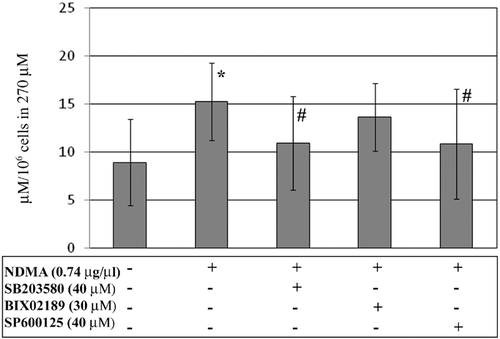
The exposure of PMN to NDMA resulted in an increased release rate of nitric oxide as compared to that in vehicle-treated cells (). To determine the participation of the MAP kinases p38, ERK5, and JNK in the production of NO via iNOS in PMN exposed to NDMA, the concentration of NO was assessed in the presence of selective inhibitors of each specific kinase. Application of the p38 pathway inhibitor or the JNK pathway inhibitor reduced the total NO concentration in the supernatants of PMN exposed to NDMA, as compared to production by cells without the inhibitor. In the presence of NDMA, no changes were observed in the release of NO by PMN pre-incubated with the ERK5 pathway inhibitor as compared to that by cells without the inhibitor.
Figure 3. Concentrations of total NO from PMN. PMN were treated with or without SB203580 (40 µM), BIX02189 (30 µM), or SP600125 (40 µM) for 1 h before addition of NDMA (0.74 µg/µl). Two hours after addition of NDMA, the nitrite concentrations were measured as a marker of NO production. *Value significantly different between cells without and with NDMA (p < 0.05); #value significantly different between cells treated with NDMA but pre-incubated without or with the inhibitor (p < 0.05). Data are expressed as µM/106 cells (in 270 µl supernatant) and are shown as mean (± SE) of 20 experiments.
Discussion
The results of the study have shown for the first time that N-nitrosodimethylamine treatments of human PMN induces activation of iNOS and NO production via a pathway dependent on AP-1 transcription factors from the Fos (Fra-1, Fra-2, FosB) and Jun (JunB and JunD) families. The observed increases in Fra-1, Fra-2, JunB, JunD, and FosB expression in the cytoplasmic fraction, in the concurrent absence of changes in the nuclear fraction (except for FosB), indicated that this factor may be activated by a different mechanism.
In view of these observations showing that the impact of NDMA on human PMN induces iNOS activation via a pathway depending on c-Jun, it appears particularly significant that among the analyzed proteins, NDMA only activated the FosB transcription factor at the nucleus level. It has been suggested that FosB, as compared to Fra-1 and Fra-2, exhibits an increased ability to bind c-Jun protein, which in turn might lead to an increased transcriptional activity of the AP-1 complex (Wisdom and Verma, Citation1993). High transcription capability of this dimer arises from the presence of FosB trans-activation motifs in C- and N-terminal domains. This sequence is directly engaged in the transcriptional activation through the stabilization of the AP-1 complex (Tkach et al., Citation2003).
The observed differences in the expression of analyzed Fos and Jun family proteins between the cytoplasmic and the nuclear fraction might first of all arise from the different activity of kinases inducing the transcription factors of those proteins (Karin, Citation1995). The activated MAP kinases have the ability of transferring the cytoplasm to the nucleus, where they induce the expression of appropriate genes, thus triggering the synthesis of proteins in the cytoplasm. The synthesized proteins are able to move from the cytoplasm to the nucleus, where they form the AP-1 dimer and bind to DNA (Symons et al., Citation2006; Zhang and Dong, Citation2007).
Available data indicate that p38 kinase does not directly activate AP-1 proteins (Reddy and Mossmann, Citation2002). Rather, it is able to regulate the transcription of Jun/Fos proteins by way of phosphorylation of other transcription factors (i.e. ATF-2, ELK-2, SAP-1) which, by binding to jun and fos gene promoters, increase their expression (Reddy and Mossmann, Citation2002). The results of our own studies indicate, however, that p38 kinase participates directly in the activation of Fra-1, JunB, JunD, and FosB factors in human PMN exposed to NDMA. On the other hand, increased Fra-1, Fra-2, and JunB expression in NDMA-exposed PMN, as seen after inhibiting the ERK5 pathway, suggests that this kinase is not engaged in the activation of those factors.
Our own observations also indicate that JNK kinase is not related to the induction of Fra-1, JunB, JunD, or FosB factors in PMN exposed to NDMA. Different results were obtained by other investigators who claimed that JNK kinases phosphorylate AP-1 proteins (McDonald, Citation2004; Zhang et al., Citation2004). Yazgan and Pfarr (Citation2002) showed in Chinese hamster ovary cells (CHO) that JNK kinase can phosphorylate JunD and thus increase its transcriptional activity, despite the lower (as compared to c-Jun) binding capacity of this factor.
The participation of p38 kinase and the absence of an impact from ERK5 and JNK kinases on induction of the analyzed transcription factor, as demonstrated in the applied research model, might have been caused by different activation times of those kinases in PMN exposed to NDMA. In our own earlier studies, it was seen that NDMA increases iNOS expression and NO production in PMN by simultaneous activation of two MAP kinases, JNK and p38; there, in shorter exposures, the p38 pathway exhibited more effective activation. Longer-term exposures to this xenobiotic led to stronger activation of the JNK rather than the p38 pathway in PMN (Ratajczak-Wrona et al., Citation2011).
Okada et al. (Citation2003) confirmed the impact of time of activity of the stimulating factors on transcription factor expression. Those authors have shown, in RAW264 macrophage-like cells, that fosB gene expression was higher already within 60 min of stimulation with interferon-γ and/or lipopolysaccharide (LPS). Increased expression of junB, junD, fra-1, and fra-2 genes was noted in those cells even outwards to the 8th hour of culture. Mollinedo et al. (Citation1991), in a study of human PMN incubated 1 h with phorbol myristate acetate (PMA), showed that there was an increased expression of genes encoding JunB and JunD transcription factors.
Another reason for the differences in the cellular location of the analyzed transcription factors in PMN exposed to NDMA may be the ineffective translation of Jun and Fos family proteins, which can cause slow movement of those proteins in the cell (Cloutier, Citation2003). It has also been suggested that the activation of neutrophils does not trigger changes of the total volume of Jun/Fos proteins in those cells (Cloutier et al., Citation2003). Those authors achieved results similar to our own indicating the absence of changes in the expression of JunD transcription factor in the nuclear fractions in neutrophils. However, those earlier studies did not report differences between the expression of JunD protein in the nuclear fraction of PMN stimulated with tumor necrosis factor (TNF)-α and phytohemagglutinin (PHA), and in non-stimulated PMN. Kanai et al. (Citation2004) have shown the activation of Fra-1 and JunB factors in the nuclear fraction of human neutrophils stimulated with LPS. The differences between the results shown by the aforementioned authors and our own might be the result of the 2-h longer incubation time.
As a general opinion, Jun, c-Jun, JunB, and JunD family proteins exhibit the same properties related to gene activation (Deng and Karin, Citation1993). It has been determined, however, that JunB has a reduced homodimerization capacity and a 10-times lower capacity to bind with DNA than c-Jun. It has been suggested that JunB, due to its competition against c-Jun, might suppress the transcriptional activity of c-Jun and, as a consequence, take the role of the stronger and more effectively phosphorylating transcription factor, leading to the maximum functional activity (Pastore et al., 2000). On the other hand, JunD transcription factor can be phosphorylated by heterodimerization with JunB or c-Jun. Furthermore, JunD, contrary to c-Jun, cannot undergo ubiquitination and degradation which, in consequence, leads to a prolonged half-life time of this factor (Zwacka et al., Citation1998).
There have been a few reports suggesting that the mechanism of the toxic activity of NDMA might be related to stimulation of the generation of reactive oxygen species (ROS) and the impact on the anti-oxidant status of a host (Jablonski et al., Citation2001). Available data suggest that the activation of AP-1 transcription factor is also impacted by the intracellular redox status. The cysteine present in Jun/Fos proteins can undergo oxidation, which leads to intermolecular disulfide formation and thus to inhibition of binding to DNA. Paradoxically, the ROS may trigger activation of AP-1 proto-oncogenes (Reddy and Mossmann, Citation2002). Thus, the observed changes in the expression of transcription factors in PMN might arise from the activity of not only the xenobiotic, but also its induced ROS. Despite the fact that previous observations have not indicated the participation of c-Fos in iNOS induction in PMN exposed to NDMA, the currently observed changes in the expression of Fra-1, Fra-2, and FosB proteins in the analyzed cells suggest a significant role of the Fos family in this process. Further studies on this subject will yield a more precise explanation of the mechanism behind the observed changes.
In conclusion, the obtained results indicate a role of Fra-1, Fra-2, JunB, JunD, and FosB transcription factors in the regulation of iNOS expression and NO production by human neutrophils exposed to NDMA. Furthermore, the results confirm the complexity of the iNOS regulation mechanism in those cells, which appears to be resultant of the activity of various simultaneously activated proteins.
Note to Readers
Similar to what had been observed in previous studies that utilized PMN isolated only via density gradient separations, the results of the study here that utilized the MACS CD16 method confirmed that there was influence of NDMA on iNOS expression in human neutrophils. There are, however, some reports that point out that the binding of the CD16 receptor on PMN leads to the changes in the activation status of these cells, resulting in events such as degranulation and phagocytosis (Reibman et al., Citation1991; Barabé et al., Citation1998). As binding of CD16 is a key to the MACS selection process, whether or not this may have impacted upon the results here remains open to question. It should be assumed that the selection would affect both ‘control’ and ‘treated’ cells; however, the impact of the treatment (i.e. NDMA) on the magnitude of this induced artifactual effect cannot be surmised at this point (i.e. the NDMA could affect how the cells respond to CD16 binding itself). Future studies are absolutely warranted to clarify if there were any artifactual influences from the MACS method, as this would have an impact on investigations well beyond those of our own research group.
Acknowledgments
The authors thank Mrs Malgorzata Walko-Lachowicz for expert technical assistance.
Declaration of interest
This scientific work was supported from the budget for science in the years 2010–2011 by the Ministry of Science and Higher Education under the grant IP2010 0346.70 Iuventus Plus. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Alderton, W.K., Cooper, C.E.Knowles, R.G. 2001. Nitric oxide synthases: Structure, function and inhibition. Biochem. J. 357:593–615.
- Barabé, F., Gilbert, C., Liao, N., Bourgoin, S.G.Naccache, P.H. 1998. Crystal-induced neutrophil activation VI. Involvment of Fc γ RIIIB (CD16) and CD11b in response to inflammatory microcrystals. FASEB J. 12:209–220.
- Bennett, B.L., Sasaki, D.T., Murray, B.W., O’Leary, E.C., Sakata, S.T., Xu, W., Leisten, J.C., Motiwala, A., Pierce, S., Satoh, Y., Bhagwat, S.S., Manning, A.M.Anderson, D.W. 2001. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. USA 98:13681–13686.
- Chomczynski, P., and Sacchi, N. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156–159.
- Cloutier, A., Ear, T., Borissevitch, O., Larivée, P.McDonald, P.P. 2003. Inflammatory cytokine expression is independent of the c-Jun N-terminal kinase/AP-1 signaling cascade in human neutrophils. J. Immunol. 171:3751–3761.
- Deng, T.Karin, M. 1993. JunB differs from c-Jun in its DNA-binding and dimerization domains, and represses c-Jun by formation of inactive heterodimers. Genes. Dev. 7:479–490.
- Foletta, V.C., Segal, D.H.Cohen, D.R. 1998. Transcriptional regulation in the immune system: all roads lead to AP-1. J. Leukoc. Biol. 63:139–152.
- Hebels, D.G., Jennen, D.G., van Herwijnen, M.H., Moonen, E.J., Pedersen, M., Knudsen, L.E., Kleinjans, J.C.de Kok, T.M. 2011. Whole-genome gene expression modifications associated with nitrosamine exposure and micronucleus frequency in human blood cells. Mutagenesis 26:753–761.
- Jablonski, J., Jablonska, E.Chojnowski, M. 2001. The influence of very low doses of N-nitrosodimethylamine (NDMA) on the apoptosis of rat Neutrophils in vivo. The role of reactive oxygen species. Toxicology 165:65–74.
- Jablonski, J., Jablonska, E., Iwanowska, J., Marcinczyk, M., and Moniuszko-Jakoniuk, J. 2006. The influence of human neutrophils on N-nitrosodimethylamine (NDMA) synthesis. Immunopharmacol. Immunotoxicol. 28:93–102.
- Jablonski, J., Jablonska, E., and Leonik, A. 2011. The effect of N-nitrosodimethylamine (NDMA) on Bax and Mcl-1 expression in human neutrophils. Bull. Environ. Contam. Toxicol. 87:638–642.
- Kanai, K., Asano, K., Hisamitsu, T.Suzaki, H. 2004. Suppression of matrix metalloproteinase-9 production from neutrophils by a macrolide antibiotic, roxithromycin, in vitro. Mediators Inflamm. 13:313–319.
- Karin, M. 1995. The regulation of AP-1 activity by mitogen-activated protein kinases. J. Biol. Chem. 270:16483–16486.
- Karin, M., Liu, Z.Zandi, E. 1997. AP-1 function and regulation. Curr. Opin. Cell Biol. 9:240–246.
- Lee, J.C., Laydon, J.T., McDonnell, P.C., Gallagher, T.F., Kumar, S., Green, D., McNulty, D., Blumenthal, M.J., Heys, J.R.Landvatter, S.W. 1994. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature 372:739–746.
- Marks-Konczalik, J., Chu, S.C., Moss, J. 1998. Cytokine-mediated transcriptional induction of the human inducible nitric oxide synthase gene requires both activator protein 1 and nuclear factor кB-binding sites. J. Biol. Chem. 273:22201–22208.
- McDonald, P.P. 2004. Transcriptional regulation in neutrophils: teaching old cells new tricks. Adv. Immunol. 82:1–48.
- Mollinedo, F., Vaquerizo, M.J., Naranjo, J.R. 1991. Expression of c-jun, jun B, and jun D proto-oncogenes in human peripheral-blood granulocytes. Biochem. J. 273(Pt 2):477–479.
- Okada, S., Obata, S., Hatano, M., Tokuhisa, T. 2003. Dominant-negative effect of the c-fos family gene products on inducible NO synthase expression in macrophages. Int. Immunol. 15:1275–1282.
- Ratajczak-Wrona, W., Jablonska, E., Garley, M., Jablonski, J.Radziwon, P. 2011. Effect of N-Nitrosodimethylamine on inducible nitric oxide synthase expression and production of nitric oxide by neutrophils and mononuclear cells: the role of JNK signalling pathway. APMIS 119:431–441.
- Ratajczak-Wrona, W., Jablonska, E., Garley, M., Jablonski, J., Radziwon, P.Iwaniuk, A. The role of MAP kinases in the activation of AP-1 and NF-ĸB transcription factors engaged in the regulation of iNOS expression in neutrophils exposed to N-nitrosodimehtylamine (unpublished results).
- Reddy, S.P.Mossman, B.T. 2002. Role and regulation of activator protein-1 in toxicant-induced responses of the lung. Am. J. Physiol. Lung Cell Mol. Physiol. 283:L1161–L1178.
- Reibman, J., Haines, K.A., Gude, D.Weissmann, G. 1991. Differences in signal transduction between Fc γ receptors (Fc γ RII, Fc γ RIII) and FMLP receptors in neutrophils. Effects of colchicine on pertussis toxin sensitivity and diacylglycerol formation. J. Immunol. 146:988–996.
- Shaulian, E.Karin, M. 2002. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 4:E131–E136.
- Symons, A., Beinke, S.Ley, S.C. 2006. MAP kinase kinase kinases and innate immunity. Trends Immunol. 27:40–48.
- Tatake, R.J., O’Neill, M.M., Kennedy, C.A., Wayne, A.L., Jakes, S., Wu, D., Kugler, S.Z. Jr, Kashem, M.A., Kaplita, P.Snow, RJ. 2008. Identification of pharmacological inhibitors of the MEK5/ERK5 pathway. Biochem. Biophys. Res. Commun. 377:120–125.
- Tkach, V., Tulchinsky, E., Lukanidin, E., Vinson, C., Bock, E.Berezin, V. 2003. Role of the Fos family members, c-Fos, Fra-1 and Fra-2, in the regulation of cell motility. Oncogene 22:5045–5054.
- Treisman, R. 1996. Regulation of transcription by MAP kinase cascades. Curr. Opin. Cell Biol. 8:205–215.
- Trelease, R.B., Henderson, R.A.Park, J.B. 1999. A qualitative process system for modeling NF-кB and AP-1 gene regulation in immune cell biology research. Artif. Intell. Med. 17:303–321.
- Tricker, A.R.Preussmann, R. 1991. Carcinogenic N-nitrosamines in the diet: Occurrence, formation, mechanisms and carcinogenic potential. Mutat. Res. 259:277–289.
- Tsikas, D. 2007. Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: appraisal of the Griess reaction in the L-arginine/nitric oxide area of research. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 851:51–70.
- Whitmarsh, A.J., and Davis, R.J. 1996. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J. Mol. Med. 74:589–607.
- Wink, D.A.Mitchell, J.B. 1998. Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic. Biol. Med. 25:434–456.
- Wisdom, R.Verma, I.M. 1993. Proto-oncogene FosB: the amino terminus encodes a regulatory function required for transformation. Mol. Cell Biol. 13:2635–2643.
- Yazgan, O.Pfarr, CM. 2002. Regulation of two JunD isoforms by Jun N-terminal kinases. J. Biol. Chem. 277:29710–29718.
- Zeman, K., Tchorzewski, H.Majewska, E. 1988. Simple and fast method of simultaneous isolation of lymphocytes and the polymorphonuclear cells from peripheral blood. Immunol. Pol. 13:217–220.
- Zhang, Q., Kleeberger, S.R.Reddy, S.P. 2004. DEP-induced fra-1 expression correlates with a distinct activation of AP-1-dependent gene transcription in the lung. Am. J. Physiol. Lung Cell Mol. Physiol. 286:L427–L436.
- Zhang, Y.Dong, C. 2007. Regulatory mechanisms of mitogen-activated kinase signaling. Cell Mol. Life Sci. 64:2771–2789.
- Zwacka, R.M., Zhang, Y., Zhou, W., Halldorson, J.Engelhardt, J.F. 1998. Ischemia/reperfusion injury in the liver of BALB/c mice activates AP-1 and nuclear factor kappaB independently of IкB degradation. Hepatology 28:1022–1030.