Abstract
Several types of pesticides, including organochlorines, are known to suppress or modulate immune responses. The present study evaluated the immunotoxicity of the organochlorine pesticide methoxychlor (MXC) in female BALB/c, C3H/He, and ICR mice. Mice were given oral MXC doses of 0, 30, 100, and 300 mg/kg each day for 7 consecutive days. On day 4, the mice also received an intravenous injection of sheep red blood cells (SRBC). The splenic plaque-forming cell (PFC) IgM response and the serum anti-SRBC IgM antibody titer were evaluated while splenic lymphocytes were counted by flow cytometry and the spleen underwent histopathological analysis. Significant decreases in IgM PFC responses were seen in BALB/c, C3H/He, and ICR mice that received MXC doses of 100 and 300 mg/kg. Similar changes in serum anti-SRBC IgM antibody titers occurred in three strain mice. Flow cytometric analysis revealed significantly decreased splenic T-cell (CD3+) populations in a dose dependent manner in BALB/c mice, and in the 300 mg/kg of MXC-treated group of C3H/He mice. Germinal center (GC) B-cell (CD19+PNA+) populations were significantly decreased in the 300 mg/kg of MXC-treated groups of all three mouse strains and in the 30 and 100 mg/kg of MXC-treated groups of BALB/c and C3H/He strain mice. Histopathological analysis revealed decreased cellularity of the periarteriolar lymphoid sheath (PALS; T-cell area) and decreased GC development in all three strains of mice treated with 300 mg/kg MXC. These results suggest that MXC has an immune-suppressive effect in mice, and that our protocol may be useful for rapidly detecting immunosuppression induced by environmental chemicals.
Introduction
Several types of pesticides, including organochlorine, organophosphate, and organotin compounds, are known to play roles in dysregulating immune functions (Kunimatsu et al., Citation1996; Crittenden, Citation1998; Gennari et al., Citation2002; Neishabouri et al., Citation2004; Suke et al., Citation2006; Dutta et al., Citation2008; Keil et al., Citation2009). Some studies have reported that organochlorine pesticides such as dichlorodiphenyltrichloroethane (DDT) and lindane, as well as organophosphate pesticides such as parathion, malathion, and daiazinon, suppress humoral immune response to T-dependent antigens (Pruett et al., Citation1992; Banerjee et al., Citation1996, Citation1998; Banerjee, Citation1999; Galloway and Handy, Citation2003). Methoxychlor (MXC), an organochlorine pesticide, is a p,p′-methoxy derivative of DDT that has relatively low toxicity in mammals and lower bioaccumulation and higher degradability than DDT in the environment (Kapoor et al., Citation1970; Bal, Citation1984). Organochlorines such as DDT and MXC were widely used in agriculture and pest control after being introduced in the 1940s. Because of their persistence in the environment, bioaccumulation in the food chain, and possible health effects, the US Environmental Protection Agency (EPA) restricted and banned the use of most organochlorine pesticides during the 1970s and 1980s (EPA, 2004). However, organochlorine pesticides continue to be used for applications such as mosquito and malaria control in developing countries (WHO, Citation2011). Moreover, measurable amounts of MXC and its metabolites can still be found in human tissues, even where the chemical is not used (OSPAR Commission, Citation2002). Thus, the human health effects of MXC exposure remain an important public health concern.
In our previous studies, we demonstrated that MXC affects the immune system, leading to atrophy of CD4+CD8+ double-positive T-cells in the thymus (Takeuchi et al., Citation2002; Fukuyama et al., Citation2010a). Chapin et al. (Citation1997) reported that perinatal and juvenile exposure to MXC resulted in markedly decreased thymus weights in rats. Furthermore, in vitro MXC exposure caused elevations in T-cell apoptosis and apoptosis-related factors in both human and mouse T lymphocytes (Fukuyama et al., Citation2010b). These reports suggest that MXC has immunosuppressive potential mediated by induction of thymic T-cell apoptosis.
We attempted to evaluate the immunosuppressive effects of MXC in mice by evaluating the T-cell-dependent antibody response to sheep red blood cells (SRBC) with plaque-forming cell (PFC) assay and enzyme-linked immunosorbent assay (ELISA) methods and by conducting phenotype analysis of splenic lymphocytes (Luster et al., Citation1992, 1993) and histopathological analysis of the spleen.
Materials and methods
Chemicals
Methoxychlor (lot no. 87H1099, > 95% purity) was obtained from Sigma-Aldrich (St. Louis, MO). MXC was dissolved in corn oil (Hayashi Chemicals, Tokyo) so that the desired dose was delivered at a dose volume of 10 ml/kg. All dosing solutions were stored at 4°C.
Animals and housing conditions
Female BALB/c, C3H/He, and ICR (Crlj:CD1) mice were purchased from Charles River Japan (Kanagawa, Japan) at 6 weeks-of-age. The mice were housed under controlled lighting (lights on from 07:00 to 19:00), temperature (22 ± 3°C), humidity (55 ± 15%), and ventilation (at least 10 complete fresh-air changes hourly). Food (Certified Pellet Diet MF, Oriental Yeast Co., Tokyo) and water were available ad libitum. This study was conducted in accordance with the Guidelines for Animal Experimentation of the Japanese Association for Laboratory Animal Science (Japanese Association for Laboratory Animal Science, Citation1987).
Treatment protocol
After a 1-week acclimatization period, mice (7-week-old) were randomly allocated to treatment and control groups (n = 7–8 per group). Mice were given MXC at oral gavage doses of 0 (vehicle control), 30, 100, and 300 mg/kg body weight each day for 7 days (Days 1–7). The dose levels used in this study followed our previous study (Fukuyama et al., Citation2010a). On Day 4, each mouse was given an intravenous injection of 6 × 107 SRBC (sheep red blood cells; Nippon Bio-Supply Center, Tokyo) in 0.2 ml saline. All mice were anesthetized and sacrificed the day after the final MXC dose was administered (Day 8). Blood samples were taken from the inferior vena cava, and serum samples were stored at −80°C until use. The spleen was removed from each mouse and divided in half. One half was placed in modified Eagle medium (MEM; Invitrogen, Carlsbad, CA) prior to preparation of single-cell suspensions, and the remaining half was fixed with 10% buffered formalin for histopathological examination.
Splenic cell preparation
Single-cell suspensions of the spleen in 5 ml of MEM supplemented with 5% heat- inactivated fetal bovine serum (FBS; Gibco, Tokyo) were prepared by pressing spleen halves through stainless-steel screens and sterile 70-µm nylon cell strainers (Falcon, Tokyo). The number of spleen cells was determined with a Coulter counter Z2 (Beckman Coulter, Tokyo).
IgM PFC response to SRBC
The Cunningham modification of the antibody-producing PFC assay was used (Jerne and Nordin, Citation1963; Cunningham and Szenberg, Citation1968) to determined IgM production by spleen cells. Briefly, an aliquot of the spleen cell suspension, packed SRBC, and guinea pig complement (Denka Seiken Co., Tokyo) were combined to make a 1:10 dilution of spleen cells (1 × 106–2 × 106 cells), 1% (v/v) SRBC, and a 1:30 dilution of guinea pig complement in MEM containing 5% FBS. This sample suspension was mixed, poured into a Cunningham chamber (Takahashi Giken Glass Co., Tokyo), and incubated for 1.5 h at 37°C in a controlled atmosphere of 5% CO2. The number of IgM-producing PFC/spleen cells was determined by counting the number of plaques for each sample.
Serum anti-SRBC IgM antibody titer
The serum anti-SRBC-specific IgM antibody titer was determined by ELISA as described by Temple et al. (Citation1993). Briefly, each flat-bottomed microplate well (Nalge Nunc International K.K., Tokyo) was coated with SRBC-membrane antigen, which had been extracted with 0.1% sodium dodecyl sulfate (100 µl, 2 µg/ml), in coating buffer (BD Pharmingen, San Diego, CA) and incubated overnight at 4°C. The content of each well was then removed, and the plate was washed 3-times with wash buffer (BD Pharmingen). Non-specific binding was blocked by incubation with 200 µl of assay diluent (BD Opt EIA reagent set, BD Pharmingen) for 2 h at room temperature (RT). Mouse serum was diluted in the assay diluent (from 1:4 to 1:16,384), 100 µl of the diluted serum was added to each well, and the plates were incubated for 2 h at RT. Next, 100 µl of peroxidase-conjugated affinity-purified anti-mouse IgM (Rockland, Inc., Gilbertsville, PA; dilution 1:15,000) was added to each well, and the plates were incubated for a further 2 h at RT. Each plate was then developed using tetramethylbenzidine (100 µl/well) for 30 min at RT. Optical density (OD) was read at a wavelength of 450 nm with a microplate reader (Molecular Devices, Osaka, Japan). All data were calculated as serum IgM antibody titer (log values of serum dilution to reach 0.5 OD).
Cell staining and flow cytometric analysis
Flow cytometric analysis was performed using splenocytes stained with fluorescein isothiocyanate (FITC)-labeled anti-CD3 antibody (clone 145-2C11), peridinin chlorophyll protein (PerCP)-Cy5.5-labeled anti-CD19 antibody (1D3) (BD Pharmingen), and FITC-labeled peanut agglutinin (PNA, Vector, Burlingame, CA). To avoid non-specific binding, 1 × 106 cells were incubated with 20% normal goat serum for 10 min at 4°C; this was followed by incubation with the FITC-, PE-, and PerCP-Cy5.5-conjugated monoclonal antibodies for 30 min at 4°C in the dark. The cells were washed twice with phosphate-buffered saline (PBS; Gibco) supplemented with 5% FBS, re-suspended at 1 × 106 cells per tube in 1 ml of PBS, and then analyzed with a FACSCaliber flow cytometer (BD Pharmingen) using the Cell Quest program (BD Pharmingen). For each sample, 10,000 events were collected and analyzed for expression of antigens.
Histopathology
Each spleen was processed, embedded in paraffin, sectioned at a thickness of 5 µm, and then stained with hematoxylin and eosin. Tissue sections were then examined by microscopy using an optical microscope (Olympus, Tokyo).
Statistical analyses
Data are presented as the mean ± SD. PFC response, IgM antibody titer, and flow cytometry results were analyzed by analysis of variance (ANOVA) followed by a Dunnett’s multiple comparison test. Histopathology data were analyzed using a Fisher’s exact probability test (one-tail analysis). A p-value < 0.05 was considered to be significant.
Results
Anti-SRBC IgM responses
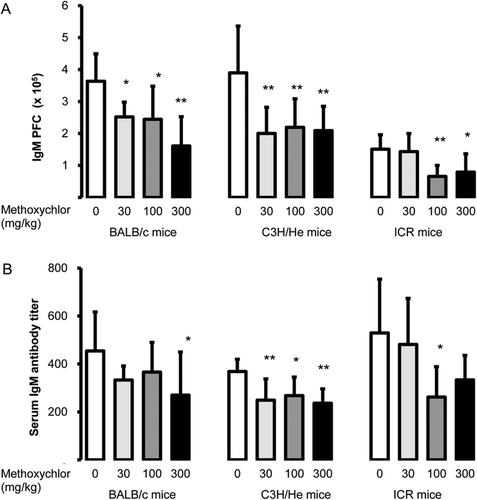
The IgM PFC responses to 30, 100, and 300 mg MXC/kg in BALB/c and C3H/He mice, and at 100 and 300 mg/kg in ICR mice, were significantly decreased compared to those seen in the vehicle controls (). The responses in the 300 mg/kg groups of each strain were suppressed to ≈ 50% of the control values. ELISA analyses revealed that the serum anti-SRBC IgM antibody titres were significantly decreased at all MXC doses in C3H/He mice. Decreases in this parameter were significant only at 300 mg/kg in BALB/c mice and only at 100 mg/kg in ICR mice ().
Figure 1. Anti-SRBC IgM response. Data reported as (A) PFC or (B) serum antibody titer in female BALB/c, C3H/He, and ICR mice following MXC treatment. Values are expressed as mean ± SD (n = 7–8 per group). Value significantly different from the control (Dunnett’s multiple comparison test) at *p < 0.05 and **p < 0.01.
Flow cytometric analysis
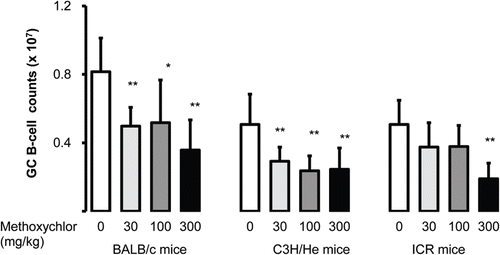
In experiments to assess whether MXC alters the phenotype of splenocytes or activates B-cells, lymphocytes were stained with anti-CD3, -CD19, and -PNA antibodies in order to analyze populations of T-cells (CD3+, ), B-cells (CD19+, ), and germinal center (GC) B-cells (PNA+CD19+, ). Both T- and B-cell populations were decreased by MXC in a dose-dependent manner relative to control levels in BALB/c mice; the changes were statistically significant in the 30, 100, and 300 mg/kg groups. The T-cell population in the 300 mg/kg C3H/He group was significantly decreased. Levels of GC B-cells in BALB/c and C3H/He mice—in response to MXC doses of 30, 100, and 300 mg/kg—were also significantly decreased compared to levels in the control hosts. In ICR mice, GC B-cell levels decreased significantly relative to the controls only in the 300 mg/kg group.
Figure 2. Splenic lymphocyte levels. Level (i.e. counts) of (A) T-cells (CD3+) and (B) B-cells (CD19+) in spleens of female BALB/c, C3H/He, and ICR mice following MXC treatment. Values are expressed as mean ± SD (n = 7–8 per group). Value significantly different from the control (Dunnett’s multiple comparison test) at *p < 0.05 and **p < 0.01.
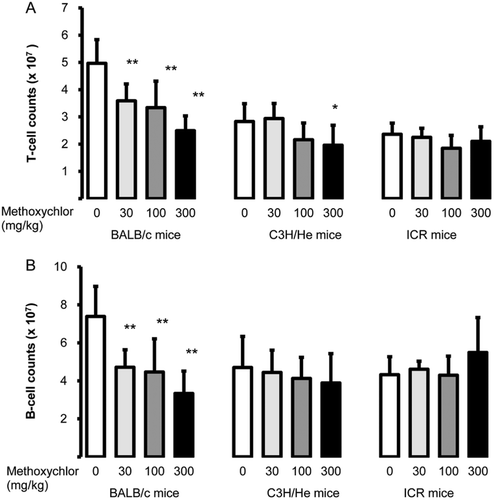
Figure 3. Splenic germinal center (GC) lymphocyte levels. Levels (i.e. counts) of germinal center (GC) B-cells (PNA+CD19+) in spleens of female BALB/c, C3H/He, and ICR mice following MXC treatment. Values are expressed as mean ± SD (n = 7–8 per group). Value significantly different from the control (Dunnett’s multiple comparison test) at *p < 0.05 and **p < 0.01.
Histopathology
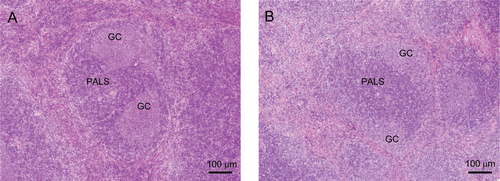
In BALB/c, C3H/He, and ICR mice immunized with SRBC and treated with 300 mg MXC/kg, the periarteriolar lymphocyte sheath (PALS, T-cell area) was often decreased in cellularity (i.e. in three or four of the eight mice in each group) (). Similarly, GC development was decreased in four or five of the eight hosts in each group. In the 100 mg/kg BALB/c and ICR groups, these same lesions were apparent in two or three of the eight mice in those dosage groups. In control mice, a large cluster of GC development was seen in the white pulp (). A decreased incidence of the cluster of extracellular foci (PALS-associated foci) was detected only in C3H/He strain mice treated with 300 mg MXC/kg (four of the eight; ).
Table 1. Histopathology—incidence of microscopic lesions in spleens of mice immunized with SRBC.
Figure 4. Histopathology of the spleen. Representative spleen tissue from (A) control and (B) 300 mg/kg MXC-treated mouse. Note that spleens from animals immunized with SRBC had decreased cellularity of periarteriolar lymphocyte sheath (PALS), and germinal center (GC) development with MXC treatment. Hematoxylin and eosin staining. Scale bar = 100 µm.
Discussion
The T-dependent antibody response is used as an immunotoxicity index because it depends on the functional capacity and co-operation of numerous cell types, including B-cells, T-cells, and macrophages (Rock et al., Citation1984; Lanzavecchia, Citation1985; Reiner et al., Citation1994; Niiro and Clark, Citation2002; Rahim et al., Citation2005). In the current study, IgM PFC responses decreased in all three strains of mice treated with MXC (see ). Suppression of the anti-SRBC IgM antibody titer was also detected in the serum (see ). A decrease in GC B-cell (CD19+PNA+) populations (see ) and decreased GC development () were found in MXC-treated mice through FACS and histopathology analysis, respectively. GC development is considered to be a T-cell-dependent antigen response because it requires functional stimulation from T-cells (Vieira and Rajewsky, Citation1990; Takahashi et al., Citation1998); these results suggest that the organochlorine pesticide MXC potentially suppresses humoral immune response in mice. Other organochlorine pesticides, such as DDT and lindane, are known to induce humoral immune suppression following immunization of mice with SRBC, tetanus toxoid, or both (Banerjee et al., Citation1996; Banerjee, Citation1999). The evidence from this study strongly suggests that MXC has an immunosuppressive effect in mice; a suppressed immune response has been considered doubtful in rats. The latter is due in part to the fact that IgM PFC responses were decreased in young male rats exposed to MXC in utero (Chapin et al., Citation1997), but increased in male rats with perinatal MXC exposure (White et al., Citation2005).
Following 7 days of treatment with MXC, flow cytometric analysis demonstrated T-cell populations were decreased in a significantly and dose-dependent manner in BALB/c mice, and in the C3H/He mice in the 300 mg/kg dosage group decreased significantly (see ). Similarly, we detected a decreased cellularity in the PALS (T-cell area) in all three strains of mice in the 300 mg/kg dosage group. Thus, a relationship between a suppressive change in IgM PFC responses and decreases in the splenic T-cell (CD3+) population and the T-cell area of PALS is implied as a result of short-term repeated MXC exposure. T-cell function, especially for helper T-cells, has also been suggested as a target of immunotoxicity via the suppression of antibody production (Tomar and Kerkvliet, Citation1991; Lundberg et al., Citation1992; Kruman et al., Citation1996). Therefore, these decreases in T-cell function may suppress the humoral immune response in mice. We previously demonstrated that MXC affects the immune system in rats, leading to atrophy of CD4+CD8+ double-positive T-cells in the thymus (Takeuchi et al., Citation2002) and decreases of CD3+ T-cells in the spleen (Takeuchi et al., Citation2004). Additionally, we showed that MXC is associated with an elevation in T-cell apoptosis and apoptosis-related factors in both human and mouse T-lymphocytes following in vitro exposure (Fukuyama et al., Citation2010a). The cumulative evidence suggests that the T-cells are a possible target for MXC-induced immunotoxicity.
Numerous toxic environmental chemicals such as pesticides, organotins, and heavy metals play roles in the dysregulation of immune functions and/or have a potential to evoke immunosuppressive effects. While immunosuppression might be expected to increase the risk of infectious and neoplastic diseases, such dysregulation of immune functions and/or immunosuppression may predispose a host to a highly increased risk of allergies and auto- immune disease. Ample evidence of these immunotoxic outcomes exists in the literature. Treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) can produce dose-dependent immunosuppression (Smialowicz et al., Citation2004) and increased risk of autoimmunity (Mustafa et al., Citation2008) in mice. Additionally, MXC exposure can induce immunosuppression, as shown in our study, and the development of autoimmune disease in (NZB × NZW) F1 female mice (Sobel et al., Citation2005). Therefore, focusing on immunosuppression might be an important first step in immunotoxicity screening.
We attempted to develop a short-term method to detect immunosuppression. According to our results, immunosuppressive effects occurred in mice treated with MXC. These effects were apparent in decreased T-cell-dependent antibody response determined by measuring PFCs and serum antibody titers, a decreased T-cell population revealed by phenotype analysis of the splenic lymphocytes, and reduced GC development in the spleen shown by histopathology. In conclusion, we induced immunosuppressive reactions by using MXC as a representative organochlorine pesticide, and we suggest that our protocol may be useful for rapidly detecting immunosuppression induced by environmental chemicals within a short period of time.
Acknowledgments
The authors thank Drs Y. Shuto, H. Ueda, A. Motomura, Y. Komatsu, M. Kumagaya, T. Kazami, S. Akema, Y. Jibiki, and Y. Chiba at the Institute of Environmental Toxicology (IET) for their useful discussions, suggestions, and technical assistance.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bal, H S. 1984. Effect of methoxychlor on reproductive system of the rat. Proc. Soc. Exp. Biol. Med. 176:187–196.
- Banerjee, B D. 1999. The influence of various factors on immune toxicity assessment of pesticide chemicals. Toxicol. Lett. 107:21–31.
- Banerjee, B. D., Pasha, S. T., Hussain, Q. Z., Koner B. C.Ray, A. 1998. A comparative evaluation of immunotoxicity of malathion after subchronic exposure in experimental animals. Indian J. Exp. Biol. 36:273–382.
- Banerjee, B. D., Koner, B. C., Ray, A.Pasha, S. T. 1996. Influence of subchronic exposure to lindane on humoral immunity in mice. Indian J. Exp. Biol. 34:1109–1113.
- Chapin, R. E., Harris, M. W., Davis, B. J., Ward, S. M., Wilson, R. E., Mauney, M. A., Lockhart, A. C., Smialowicz, R. J., Moser, V. C., Burka, L. T.Collins, B. 1997. The effects of perinatal/juvenile methoxychlor exposure on adult rat nervous, immune, and reproductive system function. Fundam. Appl. Toxicol. 40:138–157.
- Crittenden, P. L. 1998. Immunotoxicological assessment of methyl parathion in female B6C3F1 mice. J. Toxicol. Environ. Health 54:1–20.
- Cunningham, A. J.. Szenberg, A. 1968. Further improvements in the plaque technique for detecting single antibody-forming cells. Immunology 14:599–601.
- Dutta, R., Mondal, A. M., Arora, V., Nag, T. C.Das, N. 2008. Immunomodulatory effect of DDT (bis[4-chlorophenyl]-1,1,1-trichloroethane) on complement system and macrophages. Toxicology 252:78–85.
- [EPA] United States Environmental Protection Agency. 2004. Methoxychlor Re-registration Eligibility Decision (RED). EPA Publication No. EPA 738-R-04-010 Available online at: http://www.epa.gov/oppsrrd1/REDs/methoxychlor_red.htm.
- Fukuyama, T., Kosaka, T., Tajima, Y., Hayashi, K., Shutoh, Y.Harada, T. 2010a. Detection of thymocytes apoptosis in mice induced by organochlorine pesticides methoxychlor. Immunopharmacol. Immunotoxicol. 33:193–200.
- Fukuyama, T., Tajima, U., Ueda, H., Hayashi, K., Shutoh, Y., Harada, H.,and Kosaka, T. 2010b. Apoptosis in immunocytes induced by several type of pesticides. J. Immunotoxicol. 7:39–56.
- Galloway, T.Handy, R. 2003. Immunotoxicity of organophosphorous pesticides. Ecotoxicology 12:345–363.
- Gennari, A., Bol, M., Seinen, W., Penninks, A.Pieters, R. 2002. Organotin-induced apoptosis occurs in small CD4+CD8+ thymocytes and is accompanied by an increase in RNA synthesis. Toxicology 175:191–200.
- Japanese Association for Laboratory Animal Science. 1987. Guidelines for animal experimentation. Exp. Anim. 36:285–288.
- Jerne, N. K.Nordin, A. A. 1963. Plaque formation in agar by single antibody-producing cells. Science 140:450.
- Kapoor, I. P., Metcalf, R. L., Nystrom, R. F.Sangha, G. K. 1970. Comparative metabolism of methoxychlor, methiochlor, and DDT in mice, insects, and in a model ecosystem. J. Agric. Food Chem. 18:1145–1152.
- Keil, D. E., McGuinn, W. D., Dudley, A. C., EuDaly, J. G., Gikeson, G. S.Peden-Adams, M. M. 2009. N, N-diethyl-m-toluamide (DEET) suppresses humoral immunological function in B6C3F1 mice. Toxicol. Sci. 108:110–123.
- Kruman, I. I., Ramiya, V.Bondada, S. 1996. A role for T-cell CD4 in contact-mediated T-dependent B-cell activation. Cell Immunol. 173:236–245.
- Kunimatsu, T., Kamita, Y., Isobe, N.Kawasaki, H. 1996. Immunotoxicological insignificance of fenitorothion in mice and rats. Fundam. Appl. Toxicol. 33:246–253.
- Lanzavecchia, A. 1985. Antigen-specific interaction between T- and B-cells. Nature 314:537–539.
- Lundberg, K., Dencker, L., and Grinvik, K. O. 1992. 2,3,7,8-tetrachloro- dibenzo-p-dioxin inhibits the activation of antigen-specific T-cells in mice. Int. J. Immunopharmacol. 14:699–705.
- Luster, I. M., Portier, C., Pait, D. G., Rosenthal, G. J., Germolec, D. R., Corsine, E., Blaylock, B. L., Pollock, P., Kouchi, Y., Craig, W., White, K. L., Munson, A. E.Comment, C. E. 1993. Risk assessment in immunotoxicology II. Relationships between immune and host resistance tests. Fundam. Appl. Toxicol. 21:71–82.
- Luster, M. I., Portier, C., Pait, D. G., White, K. L., Gennings, C., Munson, A. E.Rosenthal, G. J. 1992. Risk assessment in immunotoxicology I. Sensitivity and predictability of immune tests. Fundam. Appl. Toxicol. 18:200–210.
- Mustafa, A., Holladay, S. D., Goff, M., Witonsky, S. G., Kerr, R., Reilly, C. M., Sponenberg, D. P.Gogal, R. M. Jr. 2008. An enhanced postnatal autoimmune profile in 24-wk-old C57BL/6 mice developmentally exposed to TCDD. Toxicol. Appl. Pharm. 232:51–59.
- Neishabouri, E. Z., Hassan, Z. M., Azizi, E.Ostad, S. N. 2004. Evaluation of immunotoxicity induced by diazinon in C57Bl/6 mice. Toxicology 196:173–179.
- Niiro, H.Clark, E. A. 2002. Regulation of B-cell fate by antigen-receptor signals. Nat. Rev. Immunol. 2:945–956.
- OSLO PARIS Convention (OSPAR) Commission. 2002. OSPAR Background Document on Methoxychlor (2004 update). pp. 1–22. Available online at: http://www.ospar.org/documents/dbase/publications/p00147_background%20document%20on%20methoxychlor.pdf.
- Pruett, S. B., Han, Y., Munson, A. E.Fuchs, B. A. 1992. Assessment of cholinergic influences on primary humoral immune response. Immunology 77:428–435.
- Rahim, R. T., Meissler, J. J., Adler, M. W.Eisenstein, T. K. 2005. Splenic macrophages and B-cells mediate immunosuppression following abrupt withdrawal from morphine. J. Leukocyte Biol. 78:1185–1191.
- Reiner, S. L., Zheng, S., Wang, Z.E., Stowing, L.Loksley, R. M. 1994. Leishmania promastigotes evade interleukin-12 interleukin-12 (IL-12) induction by macrophages and stimulate a broad range of cytokines from CD4+ T-cells during initiation of infection. J. Exp. Med. 179:447–456.
- Rock, K. L., Benacerraf, B.Abbas, A. K. 1984. Antigen presentation by hapten-specific B-lymphocytes. J. Exp. Med. 160:1102–1113.
- Smialowicz, R. J., Burgin, D. E., Williams, W. C., Diliberto, J. J., Setzer, R. W.Birnbaum, L. S. 2004. CYP1A2 is not required for 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced immuno- suppression. Toxicology 197:15–22.
- Sobel, E. S., Gianini, J., Butfiloski, E. J., Croker, B. P., Schiffenbauer, J.Roberts, S. M. 2005. Acceleration of autoimmunity by organochlorine pesticides in (NZB × NZW) F1 mice. Environ. Health Perspect. 113:323–328.
- Suke, S. G., Kumar, A., Ahmed, R. S., Chakraborti, A., Tripathi, A. K., Mediratta, P. K.Banerjee, B. D. 2006. Protective effect of melatonin against propoxur-induced oxidative stress and suppression of humoral immune response in rats. Indian J. Exp. Biol. 44:312–315.
- Takahashi, Y., Dutta, P. R., Cerasoli, D. M.Kelsoe, G. 1998. In situ studies of the primary immune response to (4-hydroxy-3-nitrophenyl)-acetyl. V. Affinity mutation develops in two stages of clonal selection. J. Exp. Med. 187:885–895.
- Takeuchi, Y., Kosaka, T., Hayashi, K., Ishimine, S., Ohtuka, R., Kuwahara, M., Yoshida, T., Takahashi, N., Chiba, Y., Takeda, M., Maita, K., and Harada, T. 2004. Alterations in the developing immune system of the rat after perinatal exposure to methoxychlor. J. Toxicol. Pathol. 17:165–170.
- Takeuchi, Y., Kosaka, T., Hayashi, K.Takeda, M., Yoshida, T., Fujisawa, H., Teramoto, S., Maita, K.Harada, T. 2002. Thymic atrophy induced by methoxychlor in rat pups. Toxicol. Lett. 135:199–207.
- Temple, L., Kawabata, T. T., Munson, A. E.White, K. L. Jr. 1993. Comparison of ELISA and plaque-forming cell assays for measuring the humoral immune response to SRBC in rats and mice treated with benzo[a]pyrene or cyclophosphamide. Fundam. Appl. Toxicol. 21:412–419.
- Tomar, R. S.Kerkvliet, N. I. 1991. Reduced T-helper cell function in mice exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Toxicol. Lett. 57:55–64.
- Vieira, P.Rajewsky, K. 1990. Persistence of memory B-cells in mice deprived of T-cell help. Int. Immunol. 2:487–494.
- White, K. L. Jr, Germolec, D. R., Booker, C. D., Hernendez, D. M., McCay, J. A., Delclos, K. B., Newbold, R. R., Weis, C.Guo, T. L. 2005. Dietary methoxychlor exposure modulates splenic natural killer cell activity, antibody-forming cell response and phenotypic marker expression in F0 and F1 generations of Sprague Dawley rats. Toxicology 207:271–281.
- WHO (World Health Organization). 2011. Global Incecticide Use for Vector-borne Disease Control: A 10-year Assessment, 2000–2009. 5th ed. WHO/HTM/NTD/VEM/WHOPES/2011. Available online at: http://whqlibdoc.who.int/publications/2011/9789241502153_eng.pdf, Accessed 10 January 2012.