Abstract
Associations between painting, sensitization, and respiratory disease have received little attention, despite the extensive use of paint and paint removal products. The objectives of this study were to investigate the possible immunotoxicity and pulmonary toxicity induced by paints in Egyptian painter workers. This study was carried out on 60 adult males. Subjects were designated as controls (n = 30 healthy persons) or paint-exposed workers (n = 30). The controls and workers were then divided into four equal groups (15 individuals/group): Group I, Control group—never smoked; Group II, Smoker controls; Groups III, paint-exposed non-smoking workers; and Group IV, paint-exposed smoker workers. A complete physical examination, chest radiograph, and pulmonary function test (PFT) were performed with each subject. Serum levels of immunoglobulin (Ig) E and interleukin (IL)-4, -6, and -10, WBC sub-set counts, total numbers of WBC, and leukocyte differentials were also assessed. The pulmonary toxicity due to the paint exposures appeared in the form of allergic manifestations in the respiratory tract, significant reductions in FVC, FEV1, FEV1/FVC ratio and PEF parameters, and a reticular pattern in both lung fields. Immunotoxicity was evidenced by increases in total leukocyte levels, total lymphocytes, CD8+ T-lymphocytes, B (CD19+)-lymphocytes, NK (CD3+CD16+CD56+) cells, and eosinophils, as well as a significant decrease in CD4+ T-lymphocyte; there were also significant elevations in serum IgE, IL-4, and IL-6, and a significant reduction in IL-10, levels in these hosts. Based on these results, we assert that repeated paint exposure is associated with pulmonary and immune system toxicities that may lead to an augmentation of allergic diseases.
Keywords::
Introduction
Exposure to paints/paint removal products is a common cause of lung disease. Although both the respiratory tract and skin are potential routes for these types of exposures, it has been assumed that the primary means of host sensitization is via inhalation of these agents. Relatively little is known about the toxicology of paints/paint removal products and the mechanisms by which these inhaled products cause lung injury are not clear. Paint chemistry has changed over time. Paint is a generic name for a number of different products, and its potential toxicity depends on their types and solvents used in its manufacture. One of the two major groups of paints is latex paints (wherein the resin is acrylic-, vinyl-, or styrene-based and the solvent is water); the other is oil-based resin paints and the solvent is petroleum-based/organic (like toluene or xylene) (Scélo et al., Citation2009). These (and other commonly used) solvents are volatile at room temperature and have considerable potential for toxicity (during occupational exposure) by inhalation (Zaidi et al., Citation2007).
In addition to organic solvents, many commonly used paints contain polyurethane. The latter is from a family of polymers that are generally derived from di-isocyanates, poly-ols, or similar compounds. Toluene di-isocyanate (TDI), 4,4′-methylenediphenyl di-isocyanate (MDI), and 1,6′-hexamethylene di-isocyanate (HDI) are often used for creating polyurethane foams, plastic products, and resins. Inhalation of isocyanates results in inflammation and decrements in lung function and can lead to isocyanate-induced asthma (Vandenplas et al., Citation1993; Wisnewski and Redlich, Citation2001; Nakashima et al., Citation2002), lung function impairment (Tornling et al., Citation1990), and pneumonitis (Simpson et al., Citation1996) in exposed individuals (primarily workers without proper personal protection devices [proper face masks, etc.]).
In a population study of 12 industrialized countries (Kogevinas et al., Citation1999), increased risk for developing asthma were noted in many occupations whose workers were potentially exposed to isocyanates, including house painters, cleaners, and spray painters. In a study of occupational asthma in Sao Paulo, Brazil during 1995–2000 (Mendoca et al., Citation2003), men who were ill with asthma reported increased previous exposures to isocyanates, often in conjunction with airborne metal dusts/fumes, oil mists, wood dusts, and anhydrides. Similarly, Kanaji et al. (Citation2007) reported that inhalation of paint fumes/associated products induced chronic exogenous lipoid pneumonia; CT scans of subjects in that study revealed multiple nodules in both lungs.
There are numerous clear examples in the literature reporting how occupational exposure to chemicals induce effects that affect either/both host sensitization or responses upon re-challenge to a given antigen. In light of the above-noted increased associations between paint/isocyanate exposures and asthma, it is somewhat surprising that associations between painting, sensitization, and respiratory disease have received little attention. Thus, the objectives of this study were to generate initial data regarding possible immunologic/pulmonary toxicities induced in Egyptian house painters (roller and brush painters). This information will be used to guide future studies to better determine which component(s) in the paints/removal products were most likely causative of any particular set(s) of adverse outcomes.
Materials and methods
Subjects
All participants were volunteer subjects. This study was conducted in accordance with the Helsinki Declaration of 1964 as revised in 2009. All participants signed an informed consent form before commencement of the study.
All potential study enrollees first had to fill out a standardized Tuohilampi questionnaire that contained questions on work/exposure history, respiratory symptoms, respiratory diseases, atopy, smoking habits, and overall health history (Kilpelainen et al., 2001). Atopy was registered if the participant had had clinical symptoms of atopic disease at some time in life (i.e., asthma, allergic rhinitis, or atopic dermatitis).
In addition, none of the participants could have a current infection, current/past alcohol consumption, or be taking any medication affecting immune response. All ex-smokers were excluded from the study; participants had to declare if they ‘never’ smoked or were ‘current’ smokers; exposure to second-hand smoke was a confounder that could not be used for screening purposes as there was no way to control for degree of this type of exposure off the job, etc. With respect to selection of controls, additional criteria used were that a subject had to declare that they had not been exposed (within 12 months) to paint/other chemicals (e.g., hydrocarbons, metals, pesticides) that cause respiratory symptoms, ventilatory disorders, or abnormal radiographic changes in their lungs.
Ultimately, after excluding applicants based on the various criteria cited above, the study was then performed with 60 adult males (25–47 years-of-age) from Tanta, Egypt. Controls (n = 30) were persons with no known occupational exposure to fresh house paint(s), and were age- and lifestyle (diet, smoking, residence, hygiene)-matched to the exposed house-painter subjects (n = 30). Once enrolled, occupational histories (paint/non-paint working times, shifts, environs) were prepared for each subject, and then they underwent a complete physical examination, postero-anterior chest radiograph, and pulmonary function testing (PFT). Thereafter, controls and painters were then sub-divided into two groups each (n = 15 /group) as: Group I: Controls that never smoked; Group II: Controls that still smoked; Group III: Painters that never smoked; and Group IV: Painters that still smoked.
For purposes of population/exposure scenario definition, all the painters studied here; had used a roller or brush on the job; had not used gloves or personal protective [respiratory] equipment; had used solvent-based paints for painting wood and window frames; switched to water-based paints for doing walls and ceilings; and used thinner, benzene, or gasoline to clean the (oil-based) paints from their hands and clothes. Based on this wide array of factors, the present studies were deemed reflective of a ‘mixed-type’ exposure for the painter subjects.
Chest radiography
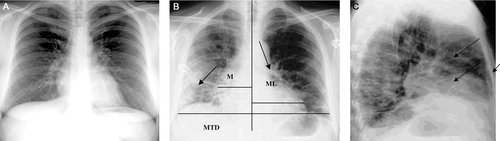
Subjects underwent Posterior-Anterior (PA) chest X-ray, using a Siemens instrument (Mobilett II, Alberta, Canada). Standard PA chest X-rays were read in a blinded manner by a certified radiologist. The size of the film (Agfa Company, Mortsel, Belgium) was 35 × 35 cm, the distance of the subject from the X-ray tube was ≈ 6 ft, the electrical voltage was 100 kv, and the intensity was ≈ 500 mA. In order to calculate the cardiothoracic ratio (CTR), we measure the two longest distances (ML and MR) from the central vertical line to the left and right heart boundaries, respectively. Again we measure the longest horizontal distance (MTD) from the left to the right boundary of the lung. Then, we can calculate the CTR as follows: CTR = MR + ML/MTD.
Pulmonary function tests
Pulmonary function tests (PFT), including those to measure mean percentage predicted values of forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), peak expiratory flow (PEF), and forced mid-expiratory flow between 25–75% of FVC (FEF25–75%), followed American Thoracic Society guidelines (1979). All PFT were measured using a portable Vitalograph-COM-PACT calibrated spirometer (Vitalograph Ltd, Buckingham, UK) interfaced with a Toshiba Portable Personal Computer (T2110CS), using Spirotrac software (Vitalograph). Subjects were encouraged to produce their greatest possible effort, and further encouragement was provided by the software’s incentive display. Judgment about the technical quality of forced maneuvers was made using the following standard criteria; the FEV1 and FVC were taken as the highest values from the first three technically satisfactory forced expirations and the FVC value chosen did not exceed the next highest by more than 0.3 L (Cotes et al., Citation1983).
The spirometer was calibrated twice daily with a 1 L syringe in accordance with manufacturer protocols. The mean percentage predicted value was based on subject age, weight, standing height, gender, and ethnic group, as calculated and adjusted using the spirometer. Subject standing height and weight were measured in their normal working clothes. Subjects were requested not to shower or smoke for at least 2 h prior to the test, and were trained to become familiar with the maneuvers. Before the actual test, each subject rested in a sitting position for ≈ 5 min. They were then asked to stand in front of the spirometer, as comfortably as possible, and a nose clip was applied. All PFT were carried out by one operator between 10:00 AM and 5:00 PM. At least three acceptable measures were performed for each subject. If the subject showed great variability among the various FVC volumes, up to eight measures were taken. The largest volumes (as percentage predicted lung function) were selected for analysis. The percentage predicted lung values were calculated as: % Predicted lung value = (observed capacities/expected capacities) × 100.
Biochemical analyses
Collection of blood samples
Blood samples were collected into plastic syringes and transferred into two Vacutainer tubes (one heparin-coated for whole blood examination and one EDTA-coated for serum; Becton-Dickinson, Franklin Lakes, NJ). Blood samples in heparinized tubes were maintained at room temperature for no longer than 4 h before the assays for WBC sub-set counts, total WBC numbers, and leukocyte differentials were performed. Serum samples were centrifuged at 1400 × g for 10 min within 30 min of collection. Resultant fluids were recovered and stored at −20°C until used for analyses of the end-points reported below.
Determination of WBC sub-set counts, total numbers of WBC, and leukocyte differentials
All WBC sub-set counts, total numbers of WBC, and leukocyte differentials were determined with a Coulter counter (Beckman Coulter, Inc., Fullerton, CA). Lymphocyte sub-sets were also analyzed by flow cytometry (EPICS XL, Beckman Coulter, Inc.) according to standard methods. Enumeration by flow cytometry included the following cells: T-cells (CD3/fluorescein isothiocyanate [FITC]), B-cells (CD19/phycoerythrin [PE]), two types of T-cell sub-sets (CD4/FITC, CD8/PE), and natural killer (NK) cells (CD3/phycoerythrin-Texas red [ECD], CD16/FITC, and CD56/PE). All antibodies were purchased from Beckman Coulter. The cut-off point was visually set at a level above background positivity using isotype controls. A total of 10,000 events per sample were collected for analyses. All flow data were analyzed using BD FACSDiva software (BD, Franklin Lakes, NJ) and are expressed in terms of numbers of given cell type within the total of 10,000 cells analyzed.
Determination of immunoglobulin E
Serum immunoglobulin (Ig) E (Dade Behring BN ProSpec, Marburg, Germany) levels were measured by nephelometry. Manufacturer reference values were used for levels below 2 SD from the mean to define low immunoglobulin levels. Nephelometric analyses were made with a BN ProSpec nephelometer (Dade Behring).
Determination of interleukins-4, -6, and -10
Serum interleukin (IL)-4, -6, and -10 levels were measured by ELISA, using reagents purchased from eBioscience (Ready-SET-Go, ELISA reagents; San Diego, CA). Briefly, each MaxiSorp ELISA 96-well flat bottom plate (Nunc-Immuno Plate, Roskilde, Denmark) well was coated with 100 µl of capture antibody in coating buffer, and then the plate incubated overnight at 4°C. Wells were aspirated; washed five times (each) with 250 µl wash buffer/well, and then received 100 µl of each test sample: 100 µl kit-provided standards were added to appropriate wells. The plate was covered and incubated at room temperature (RT) for 2 h (or overnight at 4°C for maximal sensitivity). Five aspirates/washes (as above) were then done before 100 µl of detection antibody (diluted in 1× assay diluent) solution was added to each well and the plate incubated at RT for 1 h. Five aspirates/washes (as above) were then done before 100 µl strept-avidin-horseradish peroxidase (diluted in 1× assay diluent) solution was added to each well and the plate incubated again at RT for 30 min. Five aspirates/washes (as above) were then done; here, the wells were soaked in wash buffer for 1–2 min prior to aspiration. After the final wash, 100 µl Substrate Solution was added to each well and the plate was incubated at RT for 15 min before 50 µl Stop Solution was added to each well. The absorbance in each well was then monitored at 450 nm in a BioTek EL340 microplate reader (BioTek Instruments, Winooski, VT). Each sample was tested in duplicate in the same assay, and thawed only once.
Statistics
All data were analyzed by analysis of variance (ANOVA) and a Student’s t-test to determine if there were significant differences (for each given endpoint) among the various population groups studied. Pearson correlation coefficients were used to determine the relationships between the various immune and respiratory parameters, and the length of exposure. Relationships were considered statistically significant at p < 0.05. The SPSS Version 17.0 (SPSS Inc., Chicago, IL) package was used for all statistical analyses. Results are expressed as means (± SD) and are considered statistically significant at p-values < 0.05.
Results
shows the demographic details of the painter and non-painter groups. The average age, number of cigarettes per day, and duration of smoking for painter and non-painter groups were suitably matched. The frequency distributions of lifestyle confounding factors showed no significant differences between the two groups. The average period of exposure (8 h workday) to paint-bearing atmosphere was 5.73 (± 2.31; SD) and 5.13 (± 1.68) years for workers in Group III and Group IV, respectively (). Due to widely varying sets of exposures, it was not possible for ‘dose’ levels over the periods of worker exposure to be calculated for the purposes of this study. As such, the results herein should be seen as reflective of either ‘YES’ exposure to paint or ‘NO’ exposure to paint status only. Causative agents within the paints will need to be determined in follow-on studies with better-controlled exposure scenarios.
Table 1. Demographic details of control and exposed subjects.
The results of lung function tests () indicate that paint exposure (by both smokers and non-smokers) was associated with significant reductions in PEF, FVC, FEV1, FEV1/FVC ratios, and FEF25–75% values as compared with those for non-smoker/non-paintersubjects. Only the PEF values of the non-smoker/painter showed significant reductions compared to smoker/non-painters. Smoker/painters evidenced significant reductions in all PFT parameters compared with values for their smoker/non-painter counterparts. Lastly, 80% of the painters (both non-smoker and smoker) showed reticular patterns in both lung fields. In comparison, 40% of the non-smoker/non-painters and 60% of the smoker/non-painters displayed reticular patterns in both lungs. These painters (both types) also evidenced increased bilateral bronchovascular marking (with bilateral hilar congestion) and cardiothoracic ratios (; ratio = 56.80 [± 5.51]% for smoker/painters and 52.75 [± 3.97]% for non-smoker/painters); ratios were 44.05 [± 3.52]% for smoker/non-painters and 42.70 [± 3.29]% for non-smoker/non-painters.
Table 2. Percentage predicted lung function among exposed and control subjects.
Figure 1. X-ray films in paint exposure workers. (a) Normal chest radiography. (b) Reticular pattern of both lung fields is noted, as well as an increased cardiothorathic ratio and bilateral increased bronchovascular markings with bilateral hilar congestion. (c) Lateral view showing increased bronchovascular marking with hilar congestion. MTD, Maximum Transverse Diameter of the heart; ML + MR, Maximum Cardiac Diameter.
Among the control subjects, smoker/non-painters displayed significant increases in total leukocytes (WBC) and lymphocytes (), total CD4+ T-lymphocyte levels (), and CD4+:CD8+ ratios (data not shown in ) compared with values associated with non-smoker /non-paintercounterparts. In the controls, smoking appeared to have had no effect on levels of ‘all’ T-lymphocytes (i.e., all CD3+ cells), B-lymphocytes (CD19+), or NK cells (CD3+CD16+CD56+), nor on levels of neutrophils, monocytes, or eosinophils.
Figure 2. Total leukocyte, neutrophil, lymphocyte, monocyte, and eosinophil (each reported as cells/L [× 109]), as well as basophil (cells/L [× 108]), counts in whole blood samples. Data shown are mean [± SD] values from 15 workers per group. Data significantly different from (a) non-smoker/non-painter and (b) smoker/non-painter control values at p < 0.05 are indicated.
![Figure 2. Total leukocyte, neutrophil, lymphocyte, monocyte, and eosinophil (each reported as cells/L [× 109]), as well as basophil (cells/L [× 108]), counts in whole blood samples. Data shown are mean [± SD] values from 15 workers per group. Data significantly different from (a) non-smoker/non-painter and (b) smoker/non-painter control values at p < 0.05 are indicated.](/cms/asset/e5c0087b-13b2-4c6a-b3c2-c7a835914aad/iimt_a_714005_f0002_b.gif)
Figure 3. Lymphocyte sub-population levels of CD3+ cells (= all T (CD3+)-lymphocytes), CD4+ cells (CD4+ T-lymphocytes), CD8+ cells (CD8+ T-lymphocytes), CD3+CD16+CD56+ cells (NK cells), and CD19+ cells = B-lymphocytes in whole blood samples. Data shown are mean [± SD] number of cells (of given type)/out of 10,000 cells analyzed from 15 workers per group. Data significantly different from (a) non-smoker/non-painter and (b) smoker/non-painter control values at p < 0.05 are indicated.
![Figure 3. Lymphocyte sub-population levels of CD3+ cells (= all T (CD3+)-lymphocytes), CD4+ cells (CD4+ T-lymphocytes), CD8+ cells (CD8+ T-lymphocytes), CD3+CD16+CD56+ cells (NK cells), and CD19+ cells = B-lymphocytes in whole blood samples. Data shown are mean [± SD] number of cells (of given type)/out of 10,000 cells analyzed from 15 workers per group. Data significantly different from (a) non-smoker/non-painter and (b) smoker/non-painter control values at p < 0.05 are indicated.](/cms/asset/464e438b-7308-4148-8ad4-26716a343c4e/iimt_a_714005_f0003_b.gif)
With respect to the workers, non-smoker/painters evidenced significant increases in total WBC and lymphocytes (), and in CD8+ T-lymphocytes, B-lymphocytes, NK cells, and eosinophil levels as compared with non-smoker /non-paintercounterparts ( and ). In contrast, these workers displayed a significant decrease in CD4+ T-lymphocytes and CD4+:CD8+ ratios (data not shown in ). The values for total neutrophils, monocytes, basophils, and ‘all’ T-lymphocytes revealed no significant job-related effect. Interestingly, in comparison with the controls, non-smoker/painters displayed significant increases in eosinophils, basophils, CD8+ lymphocytes, B-lymphocytes, and NK cells, and a significant decrease in CD4+ T-lymphocytes. Moreover, counts of total leukocytes, neutrophils, and monocytes, total lymphocyte count, and levels of ‘all’ T- lymphocytes showed no significant changes.
With the smoker/painters, total WBC, lymphocytes, eosinphils, and, as well as all ‘all’ T (CD3+)-, CD8+-, and B-lymphocytes, and NK cell levels were each significantly increased in comparison to levels in non-smoker/non-painters. Conversely, these same workers displayed a significant relative decrease in CD4+-lymphocyte and basophils levels; neutrophil and monocyte levels appeared unaffected. In comparison to smoker/non-painters, these particular workers had a significant increase in total WBC, lymphocytes, and eosinophils, as well as in CD8+, B-, and NK cell levels. There were no apparent differences with respect to monocyte, neutrophil, basophil levels, or all T (CD3+)-lymphocyte levels. Again, these hosts displayed a significant relative decrease in CD4+-lymphocyte levels. Due to the significant shifts in levels CD8+- and CD4+-lymphocytes in the smoker/painters, it was not surprising that the CD4+:CD8+ ratio values associated with these subjects were decreased with respect to those of either the smoker or non-smoker /non-paintercontrols.
Serum IgE level analyses revealed no significant differences between the smoker/non-painter group and their non-smoking counterparts. Levels for these subjects were 169.07 (± 15.72) and 172.73 (± 15.89) IU/ml, respectively (). In contrast, IgE levels in the smoker/painters were significantly increased (to 304.53 [± 21.87] IU/ml) as compared with those of smoker/non-painters or non-smoker/non-painters. IgE levels in non-smoker/painters (186.13 [± 43.41] IU/ml) did not differ from either of these control populations; however, these values were significantly lower than those of their smoker/painter counterparts.
Figure 4. Immunoglobulin E (IgE) levels. Data shown are in terms of IU/ml and are mean [± SD] values from 15 workers per group. Data significantly different from (a) non-smoker/non-painter and (b) smoker/non-painter control values at p < 0.05 are indicated.
![Figure 4. Immunoglobulin E (IgE) levels. Data shown are in terms of IU/ml and are mean [± SD] values from 15 workers per group. Data significantly different from (a) non-smoker/non-painter and (b) smoker/non-painter control values at p < 0.05 are indicated.](/cms/asset/57fe51a4-e60e-4a35-b937-08388db7a01b/iimt_a_714005_f0004_b.gif)
The analyses of select interleukins in the serum collected from the test subjects demonstrated that smoking itself induced significant increases in serum IL-4 and -6 in the non-painters (). Serum IL-4 and -6 levels in the painters (regardless of smoking status) were both significantly increased relative to their respective non-smoker /non-painterand smoker/non-painter counterparts. With regard to IL-10, only smoker/painters had significantly decreased levels relative to either non-painter subject set; no similar effect was noted in the samples from the non-smoker/painters. It is interesting to note that among all the painters, the effect of smoking was only apparent (i.e., significant) when comparing levels of IL-4 and -6; it is not clear why this was not so for IL-10.
Figure 5. Interleukin-4, -6, and -10 levels. Data shown are in terms of pg/ml and are the mean [±SD] from 15 workers per group. Data that are significantly different from (a) non-smoker/non-painter and (b) smoker/non-painter control values at p < 0.05 are indicated.
![Figure 5. Interleukin-4, -6, and -10 levels. Data shown are in terms of pg/ml and are the mean [±SD] from 15 workers per group. Data that are significantly different from (a) non-smoker/non-painter and (b) smoker/non-painter control values at p < 0.05 are indicated.](/cms/asset/f796b04d-a5e6-49b9-a31a-6a893d4bae6f/iimt_a_714005_f0005_b.gif)
The analysis of the data also revealed that there was significant correlation between the measured immunologic parameters (i.e., changes in levels of WBC, lymphocytes, eosinophils, CD4, CD8, NK, B-cells, IL-4, and IL-6) and the period of exposure to paint in both sets of painters (i.e., smokers and non-smokers; ). This was also the case for the measured pulmonary function test values.
Table 3. Pearson correlation coefficient for various immune and respiratory parameters, and the length of exposure affected by paint in non-smoking and smoking house painters.
Discussion
The chemistry of paints has changed over time, and so have the potential health hazards for house painters. Paint is a generic name for a number of different products, and its potential toxicity depends on their types and solvents used in its manufacture. One of the two major groups of paints is latex paints, for which the resin is acrylic-, vinyl-, or styrene-based (water-based) and the solvent is water; the customary addition of glycol ethers and coalescent help the resins flow together, aiding in film formation. The other major group is the oil-based paints, in which the solvent is usually petroleum-based (such as toluene or xylene). The petroleum solvents encompass a wide variety of materials derived from crude oil, including many that are widely used in the home, such as paint thinner, spot remover, gasoline, kerosene, and lubricating oil (Scélo et al., Citation2009).
Thinners are mixtures of volatile organic solvents (e.g., toluene, acetone, mineral spirits, methyl ethyl ketone [MEK], and methyl isobutyl ketone [MIBK]) commonly used to dilute paints, ink, and adhesives, and as cleaning agents for a variety of purposes. Most of the solvents are volatile at room temperature and have considerable potential for toxicity (intentional by abuse or unintentional due to occupational exposure) by inhalation (Zaidi et al., Citation2007). As a result, concerns about health effects from exposure(s) to these agents led to a shift in their potential use by (professional) painters.
Construction or house painting was one of the first trades to make a large-scale shift from solvent-based (SB) to water-based (WB) products from the 1970s onwards. In Sweden, the use of solvent-based paint for professional indoor painting gradually decreased from 40% in 1970 to 10% in 1985. The Swedish painters union in 1987 urged their members to further minimize the use of solvent-based paints and to instead use water-based paints when possible. One advantage of water-based paints is that they reduce the exposure to organic solvents (Wieslander et al., Citation1994). The water-based paints have four basic components: pigments, binders, liquids, and additives. The latter commonly include thickeners, surfactants, defoamers, and biocides (such as methylisothiazolinone [MI], but not methylchloroisothiazolinone [MCI]) (Thyssen et al., Citation2006). As a result of this shift in utilization, by 1992, use of solvent-based paints among house painters in Sweden was only 4% of all paint consumption.
In addition to the organic solvents, many of the commonly used paints contain poly-urethane. The chemicals that comprise the polyurethanes are useful polymers in a large variety of technical/consumer products that are generally made from di-isocyanates and polyols or similar compounds. Toluene, 4,4′-methylenediphenyl, and 1,6′-hexamethylene di-isocyanate (TDI, MDI, and HDI, respectively) are useful for polyurethane products such as foam plastic products and polyurethane resin (Nakashima et al., Citation2002). The isocyanates are highly reactive compounds used in a variety of chemical manufacturing processes, including production of polyurethane paints and foams (Lesage et al., Citation1992). Exposure assessment is complicated by the variety of isocyanate compounds present in different occupational settings (Streicher et al., Citation2000).
The lungs represent the first line of defense against challenges by a variety of environmental dusts, gases, fumes, or vapors. In house painting/paint removal, the impact of task-related chemicals on respiratory health is difficult to estimate due to a typical multi-chemical use by painters and a consequent variety of irritative substances. During/after painting, some adverse short-term health effects such as headache, dizziness, eye, throat, and lung irritation have been observed. Apart from those general clinical manifestations, it is likely that the exposures to these paints/related products can impact on the lungs and their local immune system. Results from the present study, in fact, demonstrated that house painters may be at risk of immune- and pulmonary toxicities from job-related exposures. Further, these toxicities appear to be related to the duration of exposure to paint/paint removal products and exaggerated by cigarette smoking.
In the current study, 80% of all painters (non-smoker, smoker) examined displayed reticular patterns in both lung fields. These subjects also had increased bilateral bronchovascular marking, bilateral hilar congestion, and cardiothorathic ratios. These outcomes are comparable to those reported by Baur (Citation1995) in studies of painters from Germany; the X-ray films of those painters revealed reticular lung patterns, nodular opacities, and patchy infiltrates. The results of lung function tests here also demonstrated that paint exposure (by non-smoker, smoker subjects) was associated with significant reductions in PEF, FVC, FEV1, FEV1/FVC ratios and FEF25–75% values as compared with those for non-smoker/non-painter subjects. Compared to the smoker/non-painter group, only the PEF values of non-smoker/painters were still significantly lower. These results are in accordance with those from Pronk et al. (Citation2007), who found significant decreases in FEV1, FEV1/FVC, and flow-volume parameters among painters in the Netherlands. Among Canadian painters, Morgan and Reger (Citation2000) found that paint exposure and smoking induced airway obstruction and decreased FEV1. There is compelling evidence to indicate that the FEV1 is not affected until at least 25% of the lungs are involved. On the other hand, Kaya et al. (Citation2003) found that, while Iranian house painters had normal chest X-ray findings and FEV1/FVC values, actual FEV1 values in most of the subjects were abnormal.
The immune system plays a crucial role in maintaining health overall, and in the lungs in particular. Accumulating evidence indicates that this system can be the target for toxic effects caused by a variety of chemicals, including those found in paint/paint removal products. Any induced immunomodulation, in turn, may be expressed as immunosuppression or immuno-enhancement in situ. The former may manifest as decreased resistance to opportunistic viral, bacterial, or fungal agents or increased susceptibility to cancer. Conversely, immunoenhancement may be expressed by an increase in autoimmune or allergic reactions (Selgrade, Citation2007). Because of the critical role that immune cells play in many of the respiratory pathologies noted above (and in the other cited studies) in the painters, the current study also examined the relationship between occupational exposures to paints/removal agents and changes in the hosts’ immune systems.
The present study revealed that exposure to paint resulted in lymphocytosis, primarily among CD8+ T-lymphocytes. This lymphocytosis (and leukocytosis) seen in the peripheral blood could be due to paint-induced increases in histamine release/inflammatory mediator production from stimulated mast cells (secondary to other modulating events in painters’ immune responses; Seo et al., Citation2008). Finotto et al. (Citation1991) found that exposure to toluene di-isocyanate (a componant of paint) induced an increase in the number of CD8+ lymphocytes and eosinophils, but no significant change in CD3+ levels. In contrast, Herrick et al. (Citation2003) found that di-isocyanate exposure led to decreases in CD4+ levels and in host CD4+:CD8+ ratios.
The di-isocyanates present themselves as interesting potential causative agents for many of the symptoms noted in the painters studied here. Di-isocyanates are an important cause of occupational asthma (Bakke et al., Citation2001) and hypersensitivity pneumonitis (HP) (Baur, Citation1995). There are reports suggesting that, in some workplaces, HP may be a more frequent consequence of di-isocyanate exposure than originally thought (Vandenplas et al., Citation1993). In general, isocyanates cause acute inflammatory reactions. These agents react with proteins to produce isocyanate–protein conjugates; as a consequence, it was not surprising that high serum levels of IgE and IgG antibodies against di-isocyanates (actually, the de novo complexes) have been detected in exposed subjects (Kaya et al., Citation2003). Exposure to isocyanates also can induce hemorrhagic pneumonitis, a condition likely mediated by circulating antibodies (IgG, IgE) and immune complexes (Mapp et al., Citation1997). In the present study, serum IgE levels in painters were significantly increased (regardless of smoking status) in comparison to levels in either the smoker or non-smoker non-painter control subjects.
In addition, paint exposure appeared to result in significantly increased serum IL-4, and -6 levels, regardless of smoking status; this outcome was in accordance with that reported by Karagözler et al. (Citation2002), who found that pro-inflammatory cytokine levels (including IL-6) were significantly elevated in the sera of painters. In comparison, levels of anti-inflammatory IL-10 were seen to be significant decreased (relative to control subject values) in smoker/painters only. These particular patterns may help to explain why there are increases in respiratory symptoms among the painters (both smoker and non-smoker). For example, IL-4 is a cytokine with many roles, including enhancing chemotaxis, increasing IgE production, increasing B-lymphocyte formation, and spurring on T-lymphocyte proliferation while concomitantly causing decreases in macrophage killing activity and cytokine production (Olver et al., Citation2007). Over-production of IL-4 has been found to be associated with increases in the onset/progression of allergies (Hershey et al., Citation1997). Unlike IL-4, IL-6 acts as both a pro- and anti-inflammatory cytokine. IL-6 supports growth of B-lymphocytes, is antagonistic to regulatory T (Treg)-lymphocytes, and causes an IL-10-dependent inhibition of CD4+ T-lymphocyte expansion. Lastly, anti-inflammatory IL-10 has stimulatory effects towards certain T-lymphocytes and mast cells, but also stimulates B-lymphocyte maturation and antibody production (Pestka et al., Citation2004). Thus, in the painters here, the changes in the relative status of the levels of these three critical cytokines could provide a platform for over-stimulation of the immune system (system and locally, i.e., in lungs) that, in turn, could result in prolonged inflammation and with repeated/prolonged stimulation, scarring of tissues/compromising of lung function. Further studies of the lungs of painters (i.e., using lavage cell/fluid analyses) will be critical to more precisely resolving this issue.
Conclusion
The present study provides evidence that paint exposure is associated with pulmonary toxicity in the form of abnormalities in pulmonary function and chest radiograms. Paint exposure is also associated with immunotoxicity in the form of modulation in peripheral lymphocyte sub-populations, and of serum (select) cytokine and IgE levels. Based on these findings, for painters, special attention should be paid to work methods and personal (respiratory) protection. A shift from epoxy- / urethane-, and other solvent-based paints to those that are water-based should also be further encouraged.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Bakke, J. E., Olof Nore´n, J., Thorud, S., Aasen, T. B. 2001. International Consensus Report on Isocyanates-Risk Assessment and Management. November 20–22, 2001, Furnes, Norway. Available online at: www.arbeidstilsynet.no/binfil/download.php?tid527871, accessed 22 June 2011.
- Baur, X. 1995. Hypersensitivity pneumonitis (extrinsic allergic alveolitis) induced by isocyanates. J. Allergy Clin. Immunol. 95:1004–1010.
- Cohn, L., Elias, J. A., Chupp, G. L. 2004. Asthma: Mechanisms of disease persistence and progression. Annu. Rev. Immunol 22:789–815.
- Cotes, J. E., Peslin, R., Yernault, J. C. 1983. Dynamic lung volumes and forced ventilatory flow rates. Bull. Eur. Physiopathol. Resp. 19:22–27.
- Dykewicz, M. S. 2009. Occupational asthma: Current concepts in pathogenesis, diagnosis, and management. J. Allergy Clin. Immunol. 123:519–528.
- Finotto, S., Fabbri, L. M., Rado, V., Mapp, C. E., Maestrelli, P. 1991. Increase in numbers of CD8 positive lymphocytes and eosinophils in peripheral blood of subjects with late asthmatic reactions induced by toluene di-isocyanate. Br. Ind. Med. 48:116–121.
- Herrick, C. A., Das, J., Xu, L., Wisnewski, A. V., Redlich, C. A., Bottomly, K. 2003. Differential roles for CD4 and CD8 T-cells after di-isocyanate sensitization: Genetic control of TH2-induced lung inflammation. J. Allergy Clin. Immunol. 111:1087–1094.
- Hershey, G. K., Friedrich, M. F., Esswein, L. A., Thomas, M. L., Chatila, T. A. 1997. The association of atopy with a gain-of-function mutation in the alpha subunit of the IL-4 receptor. New Engl. J. Med. 337:1720–1725.
- Kanaji, N., Bandoh, S., Nagamura, N., Chang, S. S., Ishikawa, H., Yokomise, H., Katsuki, N., Haba, R., Kushida, Y., Ishida, T. 2007. Lipoid pneumonia showing multiple pulmonary nodules and reversed halo sign. Resp. Med. Extra 3:98–101.
- Karagözler, A. A., Mehmet, N., Batçioglu, K. 2002. Effects of long-term solvent exposure on blood cytokine levels and antioxidant enzyme activities in house painters. J. Toxicol. Environ. Health 65:1237–1246.
- Kaya, M., Salan, A., Tabakog, E., Aydog, N., Berkarda, S. 2003. The bronchoalveolar epithelial permeability in house painters as determined by 99mTc DTPA aerosol scintigraphy. Ann. Nuclear Med. 17:305–308.
- Kilpeläinen, M., Terho, E. O., Helenius, H., Koskenvuo, M. 2001. Validation of a new questionnaire on asthma, allergic rhinitis, and conjunctivitis in young adults. Allergy 56:377–384.
- Kogevinas, M., Anto, J. M., Sunyer, J., Tobias, A., Kromhout, H., Burney, P. 1999. Occupational asthma in Europe and other industrialized areas: A population-based study. Lancet 353:1750–1754.
- Lesage, J., Goyer, N., Desjardins, F., Vincent, J. Y., Perrault, G. 1992. Workers’ exposure to isocyanates. Am. Ind. Hyg. Assoc. J. 53:146–153.
- Mapp, C. E., Balboni, A., Baricordi, R., Fabbri, L. M. 1997. Human leukocyte antigen associations in occupational asthma induced by isocyanates. Am. J. Respir. Crit. Care Med. 156:S139–S143.
- Mendoca, E. M., Algranti, E., Pires de Freitas, J. B., Rosa, E. A., Freire, J. A., Santos, U. P. 2003. Occupational asthma in the City of Sao Paulo, 1995–2000, with special reference to gender analysis. Am. J. Ind. Med. 43:611–617.
- Morgan, W. K., Reger, R. B. 2000. Rise and fall of the FEV1. Chest 118:1639–1644.
- Nakashima, K., Takeshita, T., Morimoto, K. 2002. Review of the occupational exposure to isocyanates: Mechanisms of action. Environ. Health Prev. Med. 7:1–6.
- Olver, S., Apte, S., Baz, A., Kienzle, N. 2007. The duplicitous effects of IL-4 on tumor immunity: How can the same cytokine improve or impair control of tumor growth? Tissue Antigens 69:293–298.
- Pestka, S., Krause, C. D., Sarkar, D., Walter, M. R., Shi, Y., Fisher, P. B. 2004. Interleukin-10 and related cytokines and receptors. Annu. Rev. Immunol. 22:929–979.
- Pronk, A., Preller, L., Raulf-Heimsoth, M., Jonkers, I. C., Lammers, J. W., Wouters, I. M., Doekes, G., Wisnewski, A. V., Heederik, D. 2007. Respiratory symptoms, sensitization, and exposure response relationships in spray painters exposed to isocyanates. Am. J. Respir. Crit. Care Med. 176:1090–1097.
- Scélo, G., Metayer, C., Zhang, L., Wiemels, J. L., Aldrich, M. C., Selvin, S., Month, S., Smith, M. T., Buffler, P. A. 2009. Household exposure to paint and petroleum solvents, chromosomal translocations, and the risk of childhood leukemia. Environ. Health Perspect. 117:133–139.
- Selgrade, M. K. 2007. Immunotoxicity: The risk is real. Toxicol. Sci.100:328–332.
- Seo, M., Koji, I., Tetsunori, O., Kumiko, K., Masahiko, S., Naoki, I., Hiroichi, N., Hisamitsu, N. 2008. Enhancing effect of chlorinated organic solvents on histamine release and inflammatory mediator production. Toxicology 243:75–83.
- Simpson, C., Garabrant, D., Torrey, S., Robins, T., Franzblau, A. 1996. Hypersensitivity pneumonitis-like reaction and occupational asthma associated with 1,3-bis(isocyanatomethyl) cyclohexane pre-polymer. Am. J. Ind. Med. 30:48–55.
- Streicher, R. P., Reh, C. M., Key-Schwartz, R. J., Schlecht, P., Cassinelli, M., O’Connor, P. F. 2000. Determination of airborne isocyanate exposure: Considerations in method selection. Am. Ind. Hyg. Assoc. J. 61:544–556.
- Thyssen, J. P., Sederberg-Olsen, N., Thomsenand, J. F., Menne, T. 2006. Contact dermatitis from methylisothiazolinone in a paint factory. Contact Dermatitis 54:322–324.
- Tornling, G., Alexandersson, R., Hedenstierna, G., Plato, N. 1990. Decreased lung function and exposure to di-isocyanates (HDI and HDIBT) in car repair painters: Observations on re-examination 6 years after initial study. Am. J. Ind. Med. 17:299–310.
- Vandenplas, O., Cartier, A., Lesage, J., Cloutier, Y., Perreault, G., Grammer, L. C., Shaughnessy, M. A., Malo, J. L. 1993a. Prepolymers of hexamethylene di-isocyanate as a cause of occupational asthma. J. Allergy Clin. Immunol. 91:850–861.
- Vandenplas, O., Malo, J. L., Dugas, M., Cartier, A., Desjardins, A., Lévesque, J., Shaughnessy, M. A., Grammer, L. C. 1993b. Hypersensitivity pneumonitis-like pneumonitis reaction among workers exposed to diphenylmethane [correction to pipheylmethane] di-isocyanate (MDI). Am. Rev. Respir. Dis. 147:338–346.
- White, M. C. Baker, E. L. 1988. Measurements of respiratory illness among construction painters. Br. J. Ind. Med. 45:523–531.
- Wieslander, G., Norback, D., Edling C. 1994. Occupational exposure to water-based paint and symptoms from the skin and eyes. Occup. Environ. Med. 51:181–186.
- Wisnewski, A. V., Redlich, C. A. 2001. Recent developments in di-isocyanate asthma. Curr. Opin. Allergy Clin. Immunol. 1:169–175.
- Zaidi, S. A., Shaw, A. N., Patel, M. N., Shah, V. V., Rajendran, D., Shah, B. P., Sinha, S. N., Gandhi, S. J., Saiyed, H. N. 2007. Multi-organ toxicity and death following acute unintentional inhalation of paint thinner fumes. Clin. Toxicol. 45:287–289.