Abstract
Aflatoxin M1 (AFM1) has been detected in many parts of the world both in raw milk and many dairy products, causing great economic losses and human disease. Unfortunately, there are few studies dealing with AFM1 immunotoxicity/interactions with lactic acid bacteria for potential application as a natural preventive agent. The aim of this study was to isolate (from dairy products) food-grade probiotic bacteria able to degrade/bind AFM1 in vitro and evaluate whether the same organism(s) could impart a protective role against AFM1-induced immunotoxicity in exposed Balb/c mice. Bacteria (Lactobacillus plantarum MON03 and L. rhamnosus GAF01) were isolated from Tunisian artisanal butter and then tested for abilities to eliminate AFM1 from phosphate-buffered saline (PBS) and reconstituted milk (containing 0.05, 0.10, and 0.20 µg AFM1/ml) after 0, 6, and 24 h at 37°C. Results showed that the selected bacteria could ‘remove’ AFM1 both in PBS and skimmed milk. The binding abilities of AFM1 by L. plantarum MON03 and L. rhamnosus GAF01 strains (at 108 CFU/ml) in PBS and reconstituted milk ranged, respectively, from 16.1–78.6% and 15.3–95.1%; overall, L. rhamnosus showed a better potential for removal than L. plantarum. ‘Removal’ appeared to be by simple binding; the bacteria/AFM1 complex was stable and only a very small proportion of mycotoxin was released back into the solution. L. rhamnosus GAF01 had the highest binding capacity and was selected for use in the in vivo study. Those results indicated that use of the organism prevented AFM1-induced effects on total white and red blood cells, and lymphocyte subtypes, after 15 days of host treatment. These studies clearly indicated that L. rhamnosus GAF01 was able to bind AFM1 in vitro and—by mechanisms that might also be related to a binding effect—counteract AFM1-induced immunotoxicity. Moreover, by itself, this bacterium was not toxic and could potentially be used as an additive in dairy products and in biotechnology for mycotoxin detoxification.
Introduction
Aflatoxin M1 (AFM1) is a hepato-carcinogenic metabolite (IARC, 1993) found in milk of animals that have consumed feeds contaminated with aflatoxin B1 (AFB1) produced by Aspergillus fungi (Park and Pohland, Citation1986; Moss, Citation1998). AFB1 quickly appears in cows (as AFM1 metabolite in blood after just 15 min (Moschini et al., Citation2006) and in milk at the first milking (Diaz et al., Citation2004; Masoero et al., Citation2007) after ingestion of aflatoxin-contaminated feed. We recently demonstrated that, in Beja province (Tunisia), milk and milk by-products were contaminated with high levels of AFM1 (3–4-fold above the permitted dose) (Abbès et al., Citation2012). AFM1 is such a major concern to humans because of its frequent occurrence in dairy products at concentrations high enough to cause adverse effects to health (IARC, Citation2002). As a result, guidance values for AFM1 in milk have been established, i.e., levels are limited to 0.05 and 0.50 µg/L in Europe and the US, respectively (Berg, Citation2003; Commission of the European Communities, Citation2006).
Effective detoxification methods would be useful in helping to remediate/mitigate the problem of AFM1 contamination in milk/by-products. Accordingly, adequate proof of efficacy of any novel measure would also be of pivotal importance. The ability/efficacy of food additives (i.e., using mineral and bioactive compounds) purported to detoxify in vivo or in vitro have been reviewed extensively elsewhere (Abbès et al., Citation2010; Ben Salah-Abbès et al., Citation2010). The detoxification of food products contaminated with mycotoxins is restricted due to problems regarding safety issues, possible losses in nutritional quality, limited efficacy, and cost (Bata and Lasztity, Citation1999). This has led to the search for alternative strategies such as the use of biological agents. There has been increasing interest in one concept stating that gastrointestinal tract micro-organisms can reduce the absorption of AFM1 in consumed foods. Consequently, numerous investigators have shown that some dairy strains of lactic acid bacteria and bifidobacteria were able to bind effectively to AFM1 in buffered solutions.
Thus, the aim of the present study was 2-fold: (a) to isolate lactobacillus bacteria from AFM1-contaminated artisanal butter and to test their ability to bind AFM1 (in a saline solution and reconstituted milk); and (b) to evaluate the ability of the isolated bacteria to mitigate/reduce any AFM1-induced immunotoxicities in exposed Balb/c mice.
Materials and methods
Chemicals
AFM1 solution (0.5 ng/ml, in methanol) was obtained from Sigma (St Louis, MO). Phosphate-buffered saline (PBS; 8 g NaCl, 1.2 g K2HPO4, 0.3 g KH2PO4) was prepared in 900 ml distilled water, adjusting the pH to 6.5, and then diluting to a volume of 1 L. All solvents used for AFM1 analyses were purchased from Merck (Darmstadt, Germany) and were of high performance liquid chromatography (HPLC) grade. In all analytical steps, water generated through a Millipore Synergy 18S Ultra-Pure Water System from Millipore (Molsheim, France) was used. AFM1 immunoaffinity columns containing specific monoclonal antibodies bound to a solid support material for AFM1 clean-up were obtained from Vicam (product code: G1007, Watertown, MA).
Sampling of artisanal butter
Three samples of 1-month-old artisanal butter made from cow’s milk were collected from local producers from southern, central, and northern regions of Tunisia. The butter samples—designated as GAF01, MON03, and BEJ01—were intentionally collected in regions where it has been traditionally manufactured in households. Collected samples were kept in sterile bags and held at 4°C; all analyses were performed within the following 24 h after collection.
In vitro studies
Bacteria screening
Artisanal butter samples (10 g) were homogenized in 90 ml of sterile 2% (w/v) tri-sodium citrate dihydrate solution (pH 7.5) pre-heated to 45°C and then 10-fold serial dilutions were prepared in 0.85% (w/v) sterile saline. Using standard protocols, aerobic mesophilic bacteria were enumerated on nutrition agar (NA; Merck, GmbH, Darmstadt, Germany) and presumptive lactobacilli were incubated on Man-Rogosa-Sharpe broth (MRS) agar plates (Biorad, Paris, France) for 72 h at 30°C to permit isolation of lactic acid bacteria. All isolated colonies were cultured in MRS broth. Thereafter, to test for those organisms that might be able to bind AFM1, the toxin was added (at 1 mg/L) to the culture broth and incubated with shaking for 24 h. After incubation, levels of recoverable mycotoxin (i.e., non-pelleting with bacteria) were assessed via HPLC analysis of the broth supernatant. Those organisms shown to have bound/removed AFM1 were then conclusively identified using API 50CHL kit and 16S rDNA sequencing.
AFM1 removal in PBS and reconstituted milk by isolated bacteria and evaluation of AFM1/bacteria complex stability
Volumes of the culture broth (each containing ≈ 108 CFU/ml of given organism of interest) were centrifuged (3000 × g, 15 min) and the bacterial pellets washed with water. The pellets were then re-suspended in 5 ml PBS contaminated with AFM1 at 0.05, 0.10, or 0.20 µg/ml. The tubes were then vortexed and the suspensions incubated at 37°C for 0, 4, and 24 h. An AFM1 positive control (0.05, 0.10, and 0.20 µg/ml in PBS) in the absence of bacteria and negative controls (bacteria suspended in pure PBS) were also incubated in parallel to monitor the efficacy of bacterial AFM1 binding.
The binding ability of AFM1 by viable lactobacilli bacteria (108 CFU/ml) in reconstituted milk was also assessed in order to determine if there were any matrix effects on the efficacy of AFM1 removal. AFM1-free skimmed milk powder was suspended in sterile water (at 0.2 g/ml) and portions of the reconstituted milk were then contaminated with standard working solutions of AFM1 at three different concentrations, i.e., 0.05, 0.10, and 0.20 µg/ml. The Lactobacilli bacteria were then treated as described earlier, but, instead of PBS, the bacterial pellets were suspended in the contaminated skimmed milk. Each removal assay was carried out in triplicate. All designated tubes were centrifuged (15 min, 3000 × g) at the end of each incubation period; supernatants were collected, transferred to clean tubes, and then stored at 4°C until AFM1 analysis. Unbound AFM1 content in the supernatants were determined by HPLC (see below). Each removal assay was performed in triplicate.
The stabilities of any bacteria-AFM1 complexes in PBS and reconstituted milk were evaluated by determining the amount of AFM1 remaining bound after washing. After 6 h incubation, a supernatant sample was collected to determine binding. Bacterial pellets with bound AFM1 were washed and re-suspended in 5 ml fresh PBS and incubated for 30 min at 37°C. The bacteria were then re-pelleted and the supernatant collected for quantification of released AFM1. Ultimately, the bacteria with the highest percentages of binding and AFM1/bacteria complex stability were selected for use in the in vivo studies.
Determination of AFM1
AFM1 analyses were performed using HPLC that incorporated an immunoaffinity column. Briefly, with each butter sample, 10 g was placed in 125 ml 75% (v/v) aceto-nitrile/water for 5 min. After blending, the samples were filtered using Whatman filter paper, and the filtrate diluted with 80 ml PBS. A sample of the filtrate (10 µl) was then passed through the Vicam AFM1 immunoaffinity column (at 5 ml/min, followed by washing with 20 ml distilled water at 5 ml/min). Bound AFM1 was eluted with 1.5 ml acetonitrile followed by 1.5 ml distilled water, and collected in a clean vial. These two eluted samples were mixed and analyzed using an Agilent 1100 HPLC system (Agilent Technologies, Englewood, CO) containing an ACE C18 silica (5 µm i.d., 25 × 46 mm) column purchased from Advanced Chromatography Technologies (Aberdeen, Scotland); product measurements were made in-line spectrophotometrically at an OD of 435 nm. Quantification of AFM1 was done from measures of peak areas and extrapolating against a calibration curve prepared/analyzed in parallel using AFM1 standards (Sigma). Three replicate analyses were performed for each sample to determine AFM1 content. To verify the soundness of the assay in this protocol, recovery from bacteria-free PBS was determined for the test range of AFM1 used; mean recovery was in the range of 89–93%. The percentage of AFM1 bound to the bacteria was calculated using the formula: 100% × (1.00 − [peak area of AFM1 in the supernatant/peak area of AFM1 in positive control sample]).
In vivo studies
Animals and treatment
Forty-eight male Balb/c mice (SEXUEL, St. Doulchard, France) were used (25 ± 0.3 g, 6-week-old) here. All mice were housed (12/cage) in a pathogen-free facility maintained at 23 ± 1°C with a 50 ± 5% relative humidity and a 12-h light/dark cycle. All mice were provided were ad libitum access to standard granulated chow (certified mycotoxin-free) and filtered drinking water. Mice were cared for under the Tunisian Code of Practice for the Care and Use of Animals for Scientific Purposes. Experimental protocols were approved in accordance with the guidelines of the Ethical Committee of NIGEB, Tehran.
At the start of the experiment, mice were randomly allocated into four treatment groups (12/group) as follows: (A) control group of distilled water, (B) AFM1 alone (100 µg/kg BW), (C) bacteria alone (1 mg/kg BW = 2 × 108 cfu/ml), and (D) AFM1+ bacteria. Thereafter, each host received the indicated treatment(s) orally (by gavage; without any need for anesthesia) daily for 2 weeks (sub-chronic study). Twenty-four hours after the final treatment, blood samples were collected from retro-orbital plexus for analyses of immune system-related endpoints (see below).
Measures of total white and red blood cells, and lymphocyte T-cell subtypes
Total white and red blood cells (WBC and RBC, respectively) were determined using a Coulter STKS blood counter (Coulter Electronics, Ltd., Luton, UK). In order to perform subtype analyses using 2-color flow cytometry, 1 ml of heparinized blood per mouse was incubated for 20 min at room temperature together with 0.05 ml of a suspension containing monoclonal antibodies conjugated with fluorescein isothiocyanate (FITC) or phycoerythrin (PE) against CD4, CD8, CD54, or CD56 surface markers (Becton/Dickinson, San Jose, CA). Any erythrocytes present were lysed with 0.02 ml FACSLysing solution (Becton Dickinson) for 10 min at 4°C. The samples were then analyzed on a FACScan flow cytometer (Becton Dickinson) equipped with Cellquest software. A minimum of 10,000 events/sample was analyzed. Lymphocytes (n = 2000) were measured and the percentage of positive cells in this population (gate) was assessed.
Statistical analysis
Data were expressed as mean ± SD of three independent experiments (in vitro study), and analyzed for statistical significance by Student’s t-test using the general linear model (n = 6 for in vivo tests). The criterion for significance was set at p < 0.05.
Results
In vitro study
AFM1 concentration in butter used for lactobacilli bacteria isolation
The presence of AFM1 in butter used for bacteria isolation was detected at concentrations ranging between 54.3–112.9 ng/L. The AFM1 levels in 12 (32%) of the positive samples were higher than the maximum tolerance limit (i.e., 50 ng/L) accepted by Tunisia and European Union nations. The rationale for this study was to select lactobacilli bacteria that could tolerate a high level of AFM1.
Isolation of micro-organisms capable of removal AFM1
Colonies (40) isolated from various samples for potential AFM1-binding activity were screened. Among them, two strains (from butter samples MON03 and GAF01) that were able to bind the most AFM1 were isolated and identified using an API 50CHL kit. The organisms (i.e., Lactobacillus) most apt at binding AFM1 in the MON03 and GAF01 were phenotyped and designated L. plantarum MON03 and L. rhamnosus GAF01, respectively. Moreover, 16S rDNA sequencing showed that these isolated organisms had 99% homology with L. plantarum and L. rhamnosus standards (data not shown).
Determination of AFM1 binding ability by the selected bacteria
The AFM1-binding abilities of viable test strains are summarized in . The binding abilities of AFM1 by L. plantarum MON03 and L. rhamnosus GAF01 strains (at 108 CFU/ml) in PBS and reconstituted milk ranged from 16.1 (± 1.4)–78.6 (± 7.1)%, and 15.3 (± 1.5)–95.1 (± 8.3)%, depending on contamination level and incubation period. L. rhamnosus GAF01 was the better binder, with ≈ 95% binding both in PBS and reconstituted milk. The differences in binding ability of L. plantarum MON03 and L. rhamnosus GAF01 at 108 CFU/ml were significant for all incubation times (0, 6, and 24 h) and with all toxin concentrations ().
Table 1. Percentage of AFM1 removal from PBS and reconstituted milk by Lactobacillus plantarum MON03 and Lactobacillus rhamnosus GAF01 strains.
Figure 1. High performance liquid chromatography (HPLC) chromatograms. (a) Untreated (positive control) aflatoxin M1 (at 0.15 µg AFM1/ml)-phosphate buffered saline (PBS) solution; (b) AFM1-free PBS solution; or (c) AFM1 (0.15 µg/ml)-PBS solution after 6 h treatment with Lactobacillus rhamnosus GAF01 at 108 CFU/ml.
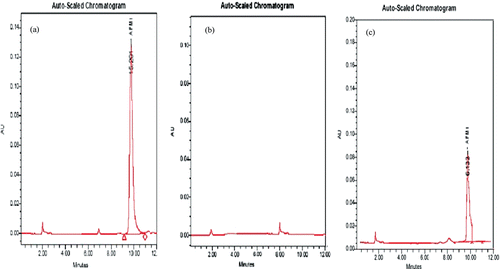
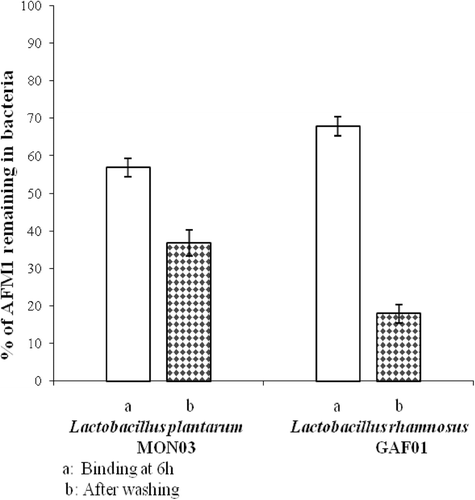
Binding was not irreversible and small amounts of AFM1 were released back into the buffered solution, especially for L. rhamnosus GAF01. When washing the bacteria/AFM1 complexes, 18.5% of bound AFM1 by L. plantarum MON03 was released back to the solution (). In comparison, washing of L. rhamnosus GAF01/AFM1 complexes resulted in 5.1% of bound AFM1 being released back.
Figure 2. Effect of washing on bacteria:AFM1 complex. Initial binding was determined after bacteria (108 CFU/ml) and AFM1 (0.05 µg/ml) were incubated together for 6 h at 37°C. Bacteria/AFM1 complexes formed were subjected to centrifugation and then washing with 5 ml PBS, before the amount of AFM1 released was determined as outlined in the Methods. Results shown are the mean (± SD) from triplicate samples.
In vivo study
General observations
The outcome of the in vitro study indicated that L. rhamnosus GAF01 was more suitable for use in the in vivo study due to its highest capability to bind AFM1. Moreover, L. rhamnosus GAF01 also showed good complex stability with AFM1.
After 2 weeks of AFM1 treatment, as expected, a general toxicity was observed in the mice. Specifically, body and lymphoid organ weights were significantly decreased in the AFM1-treated mice (data not shown). In addition, the deaths of two mice (one on Day 10 and one on Day 12; ≈16% overall mortality) were noted in the AFM1 treated group. In contrast, no overt general toxicities were observed from L. rhamnosus GAF01 alone or in combination with AFM1.
Immunological study
The current results concerning relative levels of T-cells (and subtypes) in mice in the various treatment groups indicated that daily treatment (for 14 days) with 100 µg AFM1/kg BW induced immune system damage. This was reflected as significant decreases in the relative levels of CD4+, CD8+, CD54+, and CD56+ cells in these hosts (). On the other hand, treatment with L. rhamnosus GAF01 did not alter blood T-cell levels. Treatment with AFM1 + L. rhamnosus GAF01 resulted in a significant improvement in the total counts of immune system cells toward control levels, although these values were still different from the values of control CD4+ and CD8+ cell counts.
Figure 3. Influence of AFM1 and Lactobacillus rhamnosus GAF01 (alone or in combination) on lymphocyte sub-types. Balb/c mice received daily (for 14 days) by gavage distilled water (open bar), AFM1 (100 µg/kg BW) (solid bar), L. rhamnosus GAF01 (1 mg/kg BW) (shaded bold bar), or AFM1 + L. rhamnosus GAF01 (shaded fine bar). Lymphocyte counts were then performed on blood samples collected on Day 15 of the experiment (i.e., 1 day after final exposure in each regimen). Results are expressed as mean [± SE] from n = 12 mice/group. A Student’s t-test was used to compare differences between groups: aValue significantly/bnot significantly different from control at p ≤ 0.05.
![Figure 3. Influence of AFM1 and Lactobacillus rhamnosus GAF01 (alone or in combination) on lymphocyte sub-types. Balb/c mice received daily (for 14 days) by gavage distilled water (open bar), AFM1 (100 µg/kg BW) (solid bar), L. rhamnosus GAF01 (1 mg/kg BW) (shaded bold bar), or AFM1 + L. rhamnosus GAF01 (shaded fine bar). Lymphocyte counts were then performed on blood samples collected on Day 15 of the experiment (i.e., 1 day after final exposure in each regimen). Results are expressed as mean [± SE] from n = 12 mice/group. A Student’s t-test was used to compare differences between groups: aValue significantly/bnot significantly different from control at p ≤ 0.05.](/cms/asset/0eea1276-3d0a-4afc-bdab-861daf208dec/iimt_a_718810_f0003_b.gif)
Total WBC and RBC levels () were also decreased in the AFM1 only-treated hosts. In contrast, total WBC and RBC counts in mice treated with L. rhamnosus GAF01 alone were comparable to the controls. The addition of L. rhamnosus GAF01 to the AFM1 treatment regimen resulted in a significant improvement in both total WBC and RBC counts, although again, these values still differ (albeit not significantly) from the control group levels.
Figure 4. Influence of AFM1 and Lactobacillus rhamnosus GAF01—alone or in combination—on blood cell counts. Balb/c mice received daily (for 14 days) by gavage distilled water (open bar), AFM1 (100 µg/kg BW) (solid bar), L. rhamnosus GAF01 (1 mg/kg BW) (shaded bold bar), or AFM1 + L. rhamnosus GAF01 (shaded fine bar). Erythrocyte and leukocyte counts were then performed on blood samples collected on Day 15 of the experiment (i.e., 1 day after final exposure in each regimen). Results are expressed as mean [± SE] from n = 12 mice/group. A Student’s t-test was used to compare differences between groups: aValue significantly/bnot significantly different from control at p ≤ 0.05.
![Figure 4. Influence of AFM1 and Lactobacillus rhamnosus GAF01—alone or in combination—on blood cell counts. Balb/c mice received daily (for 14 days) by gavage distilled water (open bar), AFM1 (100 µg/kg BW) (solid bar), L. rhamnosus GAF01 (1 mg/kg BW) (shaded bold bar), or AFM1 + L. rhamnosus GAF01 (shaded fine bar). Erythrocyte and leukocyte counts were then performed on blood samples collected on Day 15 of the experiment (i.e., 1 day after final exposure in each regimen). Results are expressed as mean [± SE] from n = 12 mice/group. A Student’s t-test was used to compare differences between groups: aValue significantly/bnot significantly different from control at p ≤ 0.05.](/cms/asset/33a41ebe-564c-491a-a5d4-1f8409b3cd09/iimt_a_718810_f0004_b.gif)
Discussion
Aflatoxin M1 (AFM1) is an immunotoxic metabolite found in milk of animals that have consumed feeds contaminated with aflatoxin B1 (AFB1). Aflatoxins are produced by Aspergillus fungi that grow (in temperate, tropical, and subtropical climates) at a high incidence on foodstuffs/grains (Abbès et al., Citation2008). Since AFM1 is a potent toxin and may pose a threat to animal and human health, practical methods to detoxify/inactivate AFM1-contaminated milk and by-products are in great demand. In the present study, following studies to isolate bacteria from higher contaminated Tunisian traditional butter samples that could bind with AFM1, we evaluated the possible protective effect of Lactobacillus rhamnosus GAF01 against AFM1-induced immunotoxicity in Balb/c mice as an animal model. There is very limited literature on the occurrence of AFM1 (levels) in butter and milk products in Tunisia (Abbès et al., Citation2012). In this study, 12 out of 40 traditional butter samples tested were contaminated; all contaminated samples had a level of AFM1 > 50 ng/L.
The in vitro portion of our study showed the binding abilities by L. plantarum MON03 and L. rhamnosus GAF01 strains (at 108 CFU/ml) toward AFM1 in PBS. While L. rhamnosus GAF01 was the best binder with ≈95% binding, the binding abilities of L. plantarum MON03 and L. rhamnosus GAF01 (at 108 CFU/ml) were almost immediate and significant for all incubation times (i.e., 0, 6, and 24 h) and toxin concentrations tested. Our study clearly indicates that a bacteria population of 108 CFU/ml is very suitable for binding AFM1 in PBS and reconstituted milk. Moreover, our study demonstrated that the matrix did not influence the AFM1 binding capacity of the bacteria. The amount was similar to that reported for trichothecene binding by some strains of Lactobacillus and Propionibacterium (El-Nezami et al., Citation1998). Similarly, Line and Brackett (Citation1995) indicated that populations of ≥108 CFU/ml were necessary for significant removal of AFB1. El-Nezami et al. (Citation1998) reported that a higher minimum (≈2 × 109 CFU/ml) was required for the effect.
Information on the binding ability of mycotoxins by dairy strains of lactic acid bacteria and bifidobacteria is available for AFB1, but very limited for AFM1. Our study indicated a higher percentage binding ability against AFM1 by the bacteria used than those published by Pierides et al. (Citation2000), who found that the AFM1 binding abilities of viable Lactobacillus strains within 15–16 h ranged from 18–54%. Similarly, Peltonen et al. (Citation2001) investigated the AFB1 binding ability of 12 Lactobacilli, five Bifidobacteriae, and three Lactococci strains. In their study, Lactobacillus strains bound 17–60%, Bifidobacterium strains 18–49%, and Lactococcus strains 6–41% of the AFB1 provided.
Our studies also showed that the binding that occurred was not completely irreversible as very small amounts of AFM1 were released back into the buffered solution, especially for L. rhamnosus GAF01. Specifically, when L. rhamnosus GAF01/AFM1 complexes were washed, ≈5% of the bound AFM1 was released back to the solution. This is consistent with findings for the efficient AFB1-binding strain L. rhamnosus GG (Haskard et al., Citation2001), but contrasts with results from Peltonen et al. (Citation2001). Those authors noted that AFB1 was not bound strongly by Lactobacilli strains, and that 28–94% of bound AFB1 went back into the solution. Thus, L. rhamnosus GAF01 was more suitable for use in in vivo studies due to its demonstrably high(est) capability to bind AFM1 and to maintain a good complex stability.
The results of the blood T-cell determination studies revealed that treatment with a daily oral dose of AFM1 induced immune system damage as evidenced by significant decreases in CD4+, CD8+, CD54+, and CD56+ levels in these hosts. Moreover, our findings indicated that the 14-day AFM1 treatment also impacted on the numbers of red and white blood cells. This is not all that remarkable in that the parent compound of AFM1 (i.e., AFB1) is immunotoxic (Meissonnier et al., Citation2008; Abbès et al., Citation2010). Regrettably, very few studies have been performed examining the effects of AFM1 on immune functions. Recently, Russo et al. (Citation2010) demonstrated the immunotoxic effects of AFM1 on macrophage functions. Further, no work is available regarding immunotoxicity of AFM2 even thought both AFM1 and AFM2 are obtained from AFB1 and AFB2 (via hydroxylation) and excreted into a host’s milk.
The studies here showed that a co-treatment with the isolated L. rhamnosus GAF01 enhanced blood T-cell levels (except for CD54+ cells) to those somewhat comparable to values seen with the controls. Why (cells with) this one cell marker was not affected is not clear at this time. While CD54 represents the intercellular molecule of adhesion (ICAM-1) protein responsible for cell co-operation, an impact on the number of CD54+ cells appeared to also not be impacted by treatments with other Lactobacilli (i.e., L. paracasei; Jahreis et al., Citation2002). In contrast to the CD54+ cells, the effects noted here on the other T-cell subtypes are in keeping findings by other investigators. In rats with induced enterocolitis, the concentration of CD4+ and CD8+ cells in the intestinal lamina propria were increased to a more normal level by administration of L. plantarum (Mao et al., Citation1996). In another study, L. paracasei NCC2461 induced the development of a population of CD4+ T-cells with low proliferative capacity and that were induced to produce transforming growth factor (TGF)-b and interleukin (IL)-10 (Von der Weid et al., Citation2001). In the current study, treatment with AFM1 + L. rhamnosus GAF01 (at 1 g/kg BW) resulted in a significant improvement in immune system cell total counts (towards control levels), although in the end they were still different than control values. Nevertheless, these findings suggested that the apparent biosorption (i.e., tight binding) of AFM1 to the L. rhamnosus GAF01 resulted in a reduction of AFM1 bioavailability in the gastrointestinal tract and so a mitigation of potential effects upon T-cells in these hosts. Whether there are other non-binding-related attributes from having the L. rhamnosus GAF01 in the host that impart other effects on the immune system to combat the toxicities that could be induced by AFM1 remains to be determined.
Many micro-organisms, including bacteria, yeasts, molds, actinomycetes, and algae, are able to remove or degrade small amounts of aflatoxin in food/feed (Styriak et al., Citation2001). Results of several studies suggest that binding is the main mechanism of detoxification against aflatoxins (Niderkorn et al., Citation2006; Gratz, Citation2007). Strains L. rhamnosus GG and LC-705 seem to be the most effective in such detoxifications (Lahtinen et al., Citation2004). However, the binding mechanism itself still remains not thoroughly understood. It was suggested that carbohydrate-rich mannoproteins or glucans might be involved in the binding, as levels of complex formation appear to be strain- and toxin-specific (Shetty and Jespersen, Citation2006). Raju and Devegowda (Citation2000) attributed the binding of aflatoxins by yeast cell walls to mannan oligosaccharides, while zearalenone binding was attributed to glucan components (Yiannikouris et al., Citation2004). Haskard et al. (Citation2001) suggested that AFB1 binds predominantly to carbohydrate and protein components of viable cells. Clearly, as noted by Shetty and Jespersen (Citation2006), systematic studies are still needed to understand the precise binding mechanisms.
Metabolism of aflatoxins can be considered as a second mechanism for detoxification. Very few studies have addressed the potential role and importance of the gastrointestinal tract in AFB1 metabolism. Studies in rats identified free hydroxylated metabolites of AFB1, i.e., AFM1 and AFL (Gratz, Citation2007). In the presence of probiotics, an increased excretion of AFM1 was observed, suggesting a reduced AFB1 uptake and hepatic metabolism. Some of the metabolites that diffuse(d) back into the lumen were likely also bound by the probiotics and excreted in the feces. L. rhamnosus GG is known to bind AFM1 as equally efficiently as AFB1 (Kabak and Var, Citation2004). Because both AFM1 and AFM2 are excreted into the milk of cows fed aflatoxin-contaminated feed, this implies some measure of gastrointestinal metabolism of the mycotoxins (El-Nezami et al., Citation2000). The data here strongly suggest that the intestinal metabolism of AFM1 is an important process and that probiotic bacteria impact upon these processes.
Conclusion
The results here showed that AFM1 causes physiological disorders (i.e., at the level of WBC overall, and T-cells specifically) in Balb/c mice. These studies also illustrated how Lactobacillus rhamnosus isolated from Tunisian artisanal butter would bind AFM1 in vitro and decrease its immunotoxic potential in vivo. Thus, the isolated L. rhamnosus GAF01 can be considered as a good candidate for use as an in vivo detoxification agent during food consumption (i.e., when taken as a probiotic).
Acknowledgement
The authors would like to acknowledge the Academy of Sciences for the Developing World (TWAS) and the United Nations Educational, Scientific, and Cultural Organization (UNESCO) for the financial support granted to the first author to carry out this study as a part of TWAS-UNESCO Associateship Scheme 2010–2012 at the National Institute of Genetic Engineering and Biotechnology (NIGEB), Iran. The work was supported also by the Tunisian Ministry of Higher Education and Scientific Research (Unit of Immunology, Environmental Microbiology, and Cancerology) and the Higher Institute of Biotechnology of Beja (Animal Biotechnology Department).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abbès, S., Ben Salah-Abbès, J., Bouraoui, Y., Oueslati, S., Oueslati, R. 2012. Natural occurrence of aflatoxins (B1 and M1) in feed, plasma and raw milk of lactating dairy cows in Beja, Tunisia, using ELISA. Food Addit. Contam. 5:11–15.
- Abbès, S., Ben Salah-Abbès, J., Hetta, M. M., Ibrahim, M., Abdel-Wahhab, M. A., Bacha, H., Oueslati, R. 2008. Efficacy of Tunisian montmorillonite for in vitro aflatoxin binding and in vivo amelioration of physiological alterations. Appl. Clay Sci. 42:151–157.
- Abbès, S., Salah-Abbès, J. B., Abdel-Wahhab, M. A., Oueslati, R. 2010. Immunotoxicological and biochemical effects of aflatoxins in rats prevented by Tunisian montmorillonite with reference to HSCAS. Immunopharmacol. Immunotoxicol. 32:514–522.
- Bata, A., Lasztity, R. 1999. Detoxification of mycotoxin-contaminated food and feed by microorganisms. Trends Food Sci. Techol. 10:223–228.
- Ben Salah-Abbès, J., Abbès, S., Abdel-Wahhab, M. A., Oueslati R. 2010. Immunotoxicity of zearalenone in Balb/c mice in a high subchronic dosing study counteracted by Raphanus sativus extract. Immunopharmacol. Immunotoxicol. 32:628–636.
- Berg, T. 2003. How to establish international limits for mycotoxins in food and feed? Food Control 14:219–224.
- Diaz, D. E., Hagler, W. M. Jr., Blackwelder, J. T., Eve, J. A., Hopkins, B. A., Anderson, K. L., Jones, F. T., Whitlow, L. W. 2004. Aflatoxin binders II: Reduction of aflatoxin M1 in milk by sequestering agents of cows consuming aflatoxin in feed. Mycopathologia 157:233–241.
- El-Nezami, H., Kankaanpaa, P., Salminen, S., Ahokas., J. 1998. Ability of dairy strains of lactic acid bacteria to bind a common food carcinogen aflatoxin B1. Food Chem. Toxicol. 36:321–326.
- El-Nezami, H., Mykkanen, H., Kankaanpa, P., Salminen, S., Ahokas, J. 2000. Ability of Lactobacillus and Propionibacterium strains to remove aflatoxin B1 from the chicken duodenum. J. Food Protect. 63:549–552.
- El-Nezami, H. S. Chrevatidis, A., Auriola, S., Salminen, S., Mykkanen, H. 2002. Removal of common Fusarium toxins in vitro by strains of Lactobacillus and Propionibacterium. Food Addit. Contam. 19:680–686.
- European Communities (EC). 2006. Commission Regulation. 1881/2006 of December 12th Setting Maximum Levels of Certain Contaminants in Foods. Official Journal of European Communities. 1:364–365.
- Gratz, S. 2007. Aflatoxin binding by probiotics. Experimental Studies on Intestinal Aflatoxin Transport, Metabolism and Toxicity. Doctoral dissertation, School of Public Health and Clinical Nutrition, Clinical Nutrition and Food Health Research Centre, University of Kuopio, Finland.
- Haskard, C., El-Nezami, H. S., Kankaanpaa, P. E., Salminen, S. E., Ahokas, J. T. 2001. Surface binding of aflatoxin B1 by lactic acid bacteria. Appl. Environ. Microbiol. 67:2086–3091.
- IARC (International Agency for Research on Cancer). 1993. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 56. Some Naturally Occurring Substances, Food Items and Constituents, Heterocyclic Aromatic Aamines, and Mycotoxins. Lyon, France: World Health Organization, pp. 489.
- IARC. 2002. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 82. Aflatoxins: B1, B2, G1, G2, M1. In: Some Traditional Herbal Medicines, Some Mycotoxins, Naphthalene, and Styrene. Lyon: World Health Organization 82, pp. 171–175.
- Jahreis, G., Vogelsang, H., Kiessling, G., Schuberta, R., Bunte, C., Hammes, W. P. 2002. Influence of probiotic sausage (Lactobacillus paracasei) on blood lipids and immunological parameters of healthy volunteers. Food Res. Intl. 35:133–138.
- Kabak, B., Var, I. I. 2004. Binding of aflatoxin M1 by Lactobacillus and Bifidobacterium strains. Milchwissenschaft – Milk Sci. Intl. 59:301–303.
- Lahtinen, S. J., Haskard, C. A., Ouwehand, A. C., Salminen, S. J., Ahokas, J. T. 2004. Binding of aflatoxin B1 to cell wall components of Lactobacillus rhamnosus strain GG. Food Addit. Contam. 21:158–164.
- Line, J. E., Brackett, R. E. 1995. Factors affecting aflatoxin B1 removal by Flavobacterium aurantiacum. J. Food Protect. 58:91–94.
- Mao, Y., Nobeak, S., Kasravi, B., Adawir, D., Stenram, U., Molin, G., Jeppsson, B. 1996. The effect of lactobacilli strains and oat fiber on methotrexate-induced enterocolitis in rats. Gastroenterology 111:334–344.
- Masoero, F., Gallo, A., Moschini, M., Piva, G., Diaz, D. 2007. Carryover of aflatoxin from feed to milk in dairy cows with low or high somatic cell counts. Animal 1:1344–1350.
- Meissonnier, G. M., Pinton, P., Laffitte, J., Cossalter, A. M, Gong, Y. Y., Wild, C. P., Bertin, G., Galtier, P., Oswald, I. P. 2008. Immunotoxicity of aflatoxin B1: Impairment of the cell-mediated response to vaccine antigen and modulation of cytokine expression. Toxicol. Appl. Pharmacol. 231:142–149.
- Moschini, M., Masoero, F., Diaz, D. E., Gallo, A. 2006. Plasma aflatoxin concentrations over time in bolus-fed lactating dairy cows. J. Anim. Sci. 84 (Suppl 1):74.
- Moss, M. O. 1998. Recent studies of mycotoxins. J. Appl. Micro. Symp. Suppl. 84:62S–76S.
- Niderkorn, V., Boudra, H., Morgavi, D. 2006. Binding of Fusarium mycotoxins by fermentative bacteria in vitro. J. Appl. Micro. 101:849–856.
- Park, D. L., Pohland, A. E. 1986. A rationale for the control of aflatoxin in animal feeds. In: Mycotoxins and Phycotoxins (Steyn, P. S.Vleggaar, R., Eds.). Amsterdam: Elsevier Applied Science, pp. 473–482.
- Peltonen, K., El-Nezami, H., Haskard, C., Ahokas, J., Salminen, S. 2001. Aflatoxin B1 binding by dairy strains of lactic acid bacteria and bifidobacteria. J. Dairy Sci. 84:2152–2156.
- Pierides, M., El-Nezami, H., Peltonen, K., Salminen, S., Ahokas, J. 2000. Ability of dairy strains of lactic acid bacteria to bind aflatoxin M1 in a food model. J. Food Protect. 63:645–650.
- Raju, M., Devegowda, G., 2000. Influence of esterified glucomannan on performance and organ morphology, serum biochemistry and hematology in broiler exposed to individual and combined mycotoxicosis (aflatoxin, ochratoxin, and T-2 toxin). Br. Poult. Sci. 41:640–650.
- Russo, R., Bianco, G., Marzocco, S., De Simone, A., Autore, G., Severino, L. 2010. Effects of aflatoxin M1 and aflatoxin M2 on J774A.1 murine macrophage cell line. Toxicol. Lett. 196S:S37–S51.
- Shetty, P., Jespersen, L., 2006. Saccharomyces cerevisiae and lactic acid bacteria as potential mycotoxin decontaminating agents. Trends Food Sci. Technol. 17:48–55.
- Styriak, I., Conková, E., Kmet, V., Böhm, J., Razzazi, E. 2001. The use of yeast for microbial degradation of some selected mycotoxins. Mycotoxin Res. 17:24–27.
- Von der Weid, T., Bulliard, C., Schiffrin, E. J. 2001. Induction by a lactic acid bacterium of a population of CD4+ T-cells with low proliferative capacity that produce transforming growth factor-β and IL-10. Clin. Diagn. Lab. Immunol. 8:695–701.
- Yiannikouris, A., Francois, J., Poughon, L., DussapC., Bertin, G., Jeminet, G., Jouany, J. 2004. Adsorption of zearalenone by b-D-glucans in the Saccharomyces cerevisiae cell wall. J. Food Protect. 67:1195–1200.