Abstract
The use of recombinant human proteins for the treatment of several diseases has increased considerably during the last decades. A major safety and efficacy issue of biopharmaceuticals is their potential immunogenicity. To prevent immunogenicity, biotechnology-derived proteins are engineered to be as human-like as possible. Immunogenicity is mainly determined in non-human primates (NHP), as they are considered to be the best predictive animal species for human safety, based on their close relatedness to man. As minipigs are increasingly used in the safety evaluation of (bio)pharmaceuticals, the predictive value of the minipig in immunogenicity testing was evaluated in this study, using anakinra as a model compound. Animals were treated subcutaneously with either placebo, low- (0.5 mg/kg), or high-dose (5 mg/kg) anakinra daily on 29 consecutive days. After the first and last dose, the pharmacokinetic (PK) profile of anakinra was evaluated. Antibodies directed to anakinra were measured on several time points during the treatment period. Furthermore, hematology, clinical chemistry, body weight, clinical signs, and histopathology of several organs were evaluated. No signs of toxicity were observed upon treatment with anakinra. PK parameters were comparable with those found in human and NHP studies performed with anakinra. All animals developed anti-anakinra antibodies. The results obtained in minipigs were comparable to those observed in monkeys. For anakinra, the predictive value of the minipig for immunogenicity testing was found to be comparable to that seen in NHP. However, more studies evaluating additional biopharmaceutical products are needed to support the use of the minipig as an alternative model for (immuno)toxicity testing, including immunogenicity.
Introduction
Since the approval of the first monoclonal antibody Muromonab-CD3 by the United States Food and Drug Administration (US FDA) in 1986, biopharmaceuticals for treatment of human diseases have experienced a tremendous growth. Although biotechnology-derived proteins have become increasingly more human-like, small differences in e.g., primary sequence, protein folding, and post-translational modification can still render them to be recognized as foreign (Schellekens, Citation2010) and are therefore potentially immunogenic. Immunogenicity of a protein drug refers to the ability of the protein to elicit an anti-drug immune response (Getts et al., Citation2010). It has been stated that all proteins are potentially immunogenic (Amy Rosenberg, FDA), and that only the vigor and the impact of the immunogenicity response may vary (Chirino et al., Citation2004; Singh, Citation2011). Immunogenicity of newly-developed biopharmaceuticals is an issue of concern as it can influence safety and efficacy of the respective biopharmaceutical in man. Immunogenicity induced by biologics in man can comprise humoral and cellular immune responses and can have an impact on efficacy (e.g., altered pharmacokinetics or pharmacodynamics) and on safety (e.g., recognition and neutralization of endogenous counterparts, immune complex formation or general immune system effects) (Brinks et al., Citation2011; Buttel et al., Citation2011).
The mechanisms by which immunogenicity is induced are still not elucidated. However, it is evident that the incidence and disposition of an immunogenic response is dependent on both intrinsic (protein-specific) factors such as protein structure, presence of T-cell epitopes, aggregation, impurities; and extrinsic (host-specific) factors such as patient’s genetics, immune competence and co-medications, administration route, dose and frequency (Kromminga et al., Citation2005; Buttel et al., Citation2011). The standard clinical endpoint indicative for ongoing immune responses against therapeutic proteins is the presence of anti-drug antibodies (ADAs).
Prediction of immunogenicity in humans based on animal studies has proven to be difficult (Wierda et al., Citation2001; Bugelski et al., Citation2004). As biotechnology-derived proteins are engineered to be as human-like as possible, they are likely to be immunogenic in non-clinical test species (Ponce et al., Citation2009). Moreover, inherent differences between the functional immune systems of different test species always exist. Altogether this indicates, and it also has been proven, that animal models are virtually never predictive for immunogenicity in patient populations (Bugelski and Treacy, Citation2004; Ponce et al., Citation2009; Brinks et al., Citation2011). Despite these shortcomings, assessment of immunogenicity in animals still provides important information for interpretation of toxicology, pharmacology and pharmacokinetic (PK) data. Furthermore, assessment of immunogenicity in animal studies can be used to study the potentially unwanted effects of an induced antibody response.
Because non-clinical safety of biologically-derived pharmaceuticals should be studied in animal species in which the drug has biological activity, immunogenicity is almost by default determined in non-human primates (NHP), and less frequently in dogs, rabbits, or rodents. Besides the anticipated legislative limits of the use of primates (especially in Europe) and the ethical and sociopolitical drawbacks and high costs of NHP, they are not necessarily the most predictive species for human safety. However, it is evident that, for the best predictive safety evaluation for humans, the most relevant test species should be selected. To increase the options of potentially relevant test species for testing (immuno)toxicity, including immunogenicity of biotherapeutics, the predictive value of immunogenicity testing was evaluated in minipigs for human safety.
The minipig has been used in non-clinical safety testing for almost three decades, but only recently its use in safety assessment of biopharmaceuticals has been suggested based on the expanded knowledge (Bode et al., Citation2010; Forster et al., Citation2010a, b, c), showing that various physiological aspects of minipigs resemble the human situation more closely than other animal species (e.g., PK, skin structure, GI-tract physiology, urogenital system, and the cardiovascular system) (Bode et al., Citation2010). Moreover, from information in the public domain as well as publications from the European Medicines Agency (EMA) and the FDA, it is clear that the minipig is fully recognized and accepted by regulatory authorities worldwide (van der Laan et al., Citation2010). The immune system of the pig has anatomical and organizational specificities, but is functionally similar to other mammalian species (Bode et al., Citation2010).
Although much is known about the porcine immune system, in fact the basic knowledge of the porcine immune system is more extensive than that of the dog or Old World monkeys (Bode et al., Citation2010), the pig lags behind when it comes to knowledge about their use in immunotoxicity (including immunogenicity testing) and immunopharmacology testing of biopharmaceuticals for human safety. To our knowledge such data are not available in the public domain.
In earlier studies we investigated the possibilities of immunotoxicity testing in minipigs with well-known immunosuppressive agents using immune pathology and several immune function tests, as requested by the ICH note for guidance for immunotoxicity testing of human pharmaceuticals (2005). Both immune pathology and immune function tests were successfully implemented in the minipig (Penninks et al., Citation2012). The aim of the present study was to investigate the feasibility of the minipig as an alternative species for immunogenicity testing of biopharmaceuticals, as no data on immunogenicity testing in minipigs are available in the public domain. In a separate study we have studied the immunogenicity and PK of two anti-tumor necrosis factor (TNF)-α monoclonal antibodies in minipigs; viz adalimumab and infliximab, and the results for the investigated parameters were comparable with those found in NHP (van Mierlo et. al. submitted for publication).
In this study, Kineret® (generic name: anakinra) was used as a model biopharmaceutical after it was established that anakinra interacts with pig interleukin (IL)-1α using the D10 bioassay. Anakinra is a recombinant, non-glycosylated form of the human IL-1 receptor antagonist (IL-1Ra), and differs from native human IL-1Ra in that it has the addition of a single methionine residue at its amino terminus. Male and female minipigs were subcutaneously treated for 29 consecutive days with placebo or anakinra, and the PK of anakinra was evaluated after the first and last injection. Also, the immunogenicity of anakinra was determined by measuring the anti-drug antibody (ADA) response. The results obtained were compared with those obtained in non-human primates (NHP).
Materials and methods
Minipigs
Male and female Göttingen minipigs (aged 3.5–4.5 months, with body weight of 6.4–8.3 kg for males and 6.2–7.5 kg for females at study start) were provided by Ellegaard Göttingen Minipigs A/S (Dalmose, Denmark). The welfare of the animals was maintained in accordance with the general principles governing the use of animals in experiments of the European Communities (Directive 86/609/EEC) and Dutch legislation (Experiments on Animals Act, 1997).
Animals were housed in groups of three, sorted by treatment group (males and females separated) under conventional conditions. Available information on litter origin of the animals is depicted in Supplemental Table 1. The experimental room was ventilated with ≈ 10 air changes per hour and was maintained at a temperature of 20–24°C and a relative humidity of 45–65%. The animals were fed a commercial diet (SMP (E) SQC; SDS Special Diets Services, Whitham, UK) and received a measured amount of feed (ca. 120–150 g) twice daily. The animals were provided ad libitum with tap water suitable for human consumption (quality guidelines according to Dutch legislation based on EC Council Directive 98/83/EC). The study was started after acclimatization to the laboratory conditions for ca. 2 weeks.
D10 cell bio-assay
D10 cells were cultured in culture medium (RPMI-1640 enriched with 5% fetal calf serum, 100 U penicillin/streptavidin/ml, 1 ng hIL-1/ml, and 1 ng hIL-2/ml (all R&D Systems, Abingdon, UK). To determine the biological activity of anakinra in blocking pig IL-1α, culture medium containing 6 ng recombinant pig (rp)IL-1Rα/ml was pre-incubated for 2 h (37°C, 5% CO2) with anakinra (concentration range 0.074–54 ng/ml) to give anakinra an advantage to compete with rpIL-1α in binding to rpIL-1Rα. Cells were then washed and re-suspended in IL-1-free medium. Cells (seeded at 5000/well) were then cultured for 4 days (in triplicate) in the presence of increasing concentrations of rpIL-1α (concentration range in culture = 10–10,000 pg/ml) or the pre-incubated mixture of rpIL-1Rα with the different anakinra levels (concentrations in culture = 3 ng rpIL-1Rα/ml and 0.037–27.0 ng anakinra/ml). Total volume in each culture was 100 µl/well. After 4 days of incubation (37°C, 5% CO2), 25 µl of a 5 mg/ml solution of MTT [3-(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide] was added to each well. After 3 h of continued incubation, 100 µl of 10% (v/v) SDS in 50% DMF (dimethylformamide) was added to each well and the plates incubated overnight at 37°C. Thereafter, the absorption in each well was measured at 590 nm using a Victor2 Multilabel Counter (Perkin Elmer, Groningen, the Netherlands).
Study design
Treatments
Animals (three/gender/group) were treated with either water for injection (placebo control; Group 1), 0.5 mg anakinra/kg body weight (BW) (Group 2), or 5.0 mg anakinra/kg BW (Group 3). The animals were treated for 29 consecutive days by subcutaneous injection in the neck region of anakinra or placebo. Dose volumes were adjusted weekly to the latest recorded BW for each individual animal. For treatment of the high-dose animals, anakinra was injected as provided. For the low-dose treated animals, the anakinra solution was diluted 10-fold in water for injection prior to injection. On each treatment day, a fresh batch of test substances was prepared.
Test compound
Anakinra is a recombinant, non-glycosylated form of the human IL-1 receptor antagonist (IL-1Ra), and differs from native human IL-1Ra in that it has the addition of a single methionine residue at its amino terminus. The molecule consists of 153 amino acids and has a molecular weight of 17.3 kD. It is produced by recombinant DNA technology using an E. coli bacterial expression system (FDA, Citation2001; Regulatory Affairs Canada, Citation2008). Anakinra (Kineret®; Amgen Europe B.V., Breda, the Netherlands) was provided in pre-filled syringes (100 mg in 0.670 ml total volume).
Clinical observations
Pen-side observations to detect signs of ill health and reaction to treatment as well as moribund or dead animals were conducted at least once daily throughout the study, until necropsy. Special attention was given to injection site reactions. The body weight of each animal was recorded on Day −5, Day 0, and once weekly thereafter until the end of the study (last body weight measurement on day of sacrifice).
Blood sampling
Blood samples from all animals were collected via the neck vein. On sampling days, blood was collected prior to administration of the study substances (except of course for blood samples used for PK analysis). The blood was sampled into tubes coated with K2-EDTA as an anti-coagulant for hematology determinations, into heparinized tubes for clinical chemistry determinations, into tubes containing citrate for determination of prothrombin time and fibrinogen and into coagulation tubes for antibody analysis.
For the PK analyses, a blood sample was collected into tubes coated with K2-EDTA as an anti-coagulant from animals treated with anakinra on Days 0 and 28 (at pre-dose and 2, 3, 4, 6, 8, 12, and 24 h following dose administration). Blood for PK analysis was also collected on Days 7, 14, and 21 prior to anakinra injection.
Necropsy and pathology
On Day 30, the animals were sedated by intramuscular (IM) dosing of Domitor (medetomidine HCl [1 mg/ml] given at 0.5 ml/10 kg BW) + Dormicum (midazolam [5 mg/ml] given at 0.5 ml/10 kg BW). The animals were then anesthetized via intracardial injection with sodium pentobarbital (60 mg/ml; dosing ≈ 1 ml/kg BW) until they fell into deep anesthesia. Thereafter, the animals were sacrificed by exsanguination via the axillary artery. At necropsy, the pigs were examined for external changes and grossly for pathological changes. The following organs were then collected for microscopic examination: thymus, spleen, lymph nodes distant from injection sites, lymph nodes draining the injection sites, adrenals, liver, kidneys, and injection sites. The thymus, spleen, adrenals, liver, and kidneys were also weighed. The relative organ weights (g/kg BW) were calculated on the basis of the terminal body weight of the animals. The collected organs were preserved in a neutral aqueous phosphate-buffered 4% solution of formaldehyde. The tissues were then embedded in paraffin wax, sectioned at 5-µm, and stained with hematoxylin and eosin. Histopathological examinations were then performed by light microscopy on the collected organs and tissues of all animals.
Hematology
The parameters determined for hematology were analyzed on an Advia 120 Hematology Analyzer (Den Haag, the Netherlands) and included hemoglobin, packed cell volume, red blood cell count, reticulocytes, thrombocyte count, and white blood cell count (total and differential). Pro-thrombin time was analyzed by Normotest (Technoclone GmbH, Vienna, Austria). The following parameters were calculated: mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC).
Clinical chemistry parameters
Clinical chemistry parameters were determined by using an AU-400 Analyzer System (Olympus, Hamburg, Germany). The parameters determined were: alkaline phosphatase activity (ALP), aspartate aminotransferase activity (ASAT), alanine aminotransferase activity (ALAT), γ-glutamyltransferase activity (GGT), lactate dehydrogenase (LDH), total protein, albumin, ratio of albumin-to-globulin (albumin/[total protein − albumin]), urea, creatinine, glucose, bilirubin (total), cholesterol (total), triglycerides, phospholipids, Ca, Na, K, Cl, and inorganic phosphate. All parameters, except for creatinine (Roche, Almere, the Netherlands), bilirubin (Randox, Antrim, UK), and phospholipids (Spinreact reagent, Girona, Spain) were determined using Olympus agents.
Measurement of anakinra in minipig plasma
Anakinra plasma levels were quantified using a Time-Resolved Fluorescence (TRF)-ELISA. DELFIA® Yellow plates (Perkin Elmer) were coated overnight at room temperature (RT) with 100 µl monoclonal anti-human IL-1Ra/IL-1F3 (10 µg/ml in phosphate-buffered saline [PBS, pH 7.4]; R&D Systems). After washing the plates three times (each wash with 250 µl 0.05% Tween-20 in Tris-Buffered Saline (TBS) [50 mM Tris, 50 mM NaCl, pH 7.5]), the plates were blocked for minimally 1 h at RT with 1% bovine serum albumin (BSA) in TBS. The plates were then washed again three times using 0.05% Tween-20/TBS. Calibration and serum samples (diluted 10- or 100-times in TBS/1% BSA) were then added to dedicated wells (100 µl/well; in duplicate) and the plates incubated for 2 h (at RT) protected from light. After washing the plates three times using 0.05% Tween-20/TBS, 100 µl biotinylated polyclonal anti-human IL-1ra/IL-1F3 (1:2000 in assay buffer; R&D Systems) was added to each well and the plates were incubated for 1 h (at RT) protected from light. Thereafter, the plates were washed again three times (0.05% Tween-20/TBS) before 100 µl Streptavidine-Eu3+ (150 ng/ml; Perkin Elmer, Brussels, Belgium) in assay buffer was added to each well and the plates incubated (at RT) for 30 min protected from light. After washing the plates six times (0.05% Tween-20/TBS), 200 µl DELFIA® Enhancement Solution (Perkin Elmer, Brussels, Belgium) was added and the plates incubated for a final 1 h (at RT) protected from light and while under continuous shaking. Fluorescence from each well was then measured at 615 nm (excitation 340 nm) using the Victor2 Multilabel Counter. Calibration samples were fitted to an un-weighed 4-parameter algorithm using WorkOut software (Perkin Elmer); the resulting calibration curve was used to calculate the anakinra concentrations in all samples.
Pharmacokinetic (PK) evaluation
Pharmacokinetic parameters were calculated by non-compartmental regression analysis using WinNonLin 6.2 (Phoenix; Pharsight, St. Louis, MO). The area under the plasma concentration vs time curve was extrapolated to infinity by mixed-log linear regression analysis of the final part of the curve. A concentration of 0 µg/ml was assigned to all plasma samples which were below the lower limit of quantification of the analysis method, as anakinra was assumed not to be endogenously present.
Measurement of anti-anakinra antibodies in minipig serum
Antibodies against anakinra in blood samples recovered from the minipigs were detected by a direct ELISA assay. In brief, Corning high binding plates were coated overnight at 2–10°C with 100 µl of a solution of 2 µg anakinra/ml in coating buffer (50 mM carbonate buffer [pH 9.6]). After washing the plates three times with wash buffer (0.05% [v/v] Tween-20 in PBS [pH 7.4]), the plates were blocked for minimally 1 h at RT with block buffer [1% (w/v) BSA/PBS]. The plates were then washed three times with wash buffer. Calibration curves (goat anti-human IL-1ra antibody used as reference), quality control samples, and study samples were then added to dedicated wells and the plates were incubated for 2 h at RT. The plates were then washed three times with wash buffer. Wells with calibration samples were subsequently incubated for 30 min at RT with donkey anti-goat IgG-HRP (horseradish peroxidase; 1:12,500; Santa Cruz, Heidelberg, Germany); wells with assay cut-point and study samples were incubated for 30 min at RT with rabbit anti-swine IgG-HRP (1:10,000; Rockland Immunochemicals, Gilbertsville, PA). Thereafter, the plates were washed three times (wash buffer) and 100 µl TMB (3,3′,5,5′-tetramethylbenzidine; Kem-en-Tec, Taastrup, Denmark) solution was added to each well and the plates incubated for at least 5 min before the reaction was stopped by addition of 100 µl of 1 M H2SO4 (Merck, Darmstadt, Germany) to each well. Absorbance in each well was then measured at 450 nm using a SpectraMax M5 plate reader (Molecular Devices, Workingham, UK). The optical density (OD) in each well was directly proportional to the amount of anti-anakinra antibody in the sample. The reference standard dilution series were fitted to an un-weighed 4-parameter algorithm using the SoftMax Pro GXP software (Molecular Devices). The resulting calibration curve was used to calculate anti-anakinra antibody concentrations in each pig sample. As the concentrations are calculated based on a standard curve using a goat anti-human IL-1ra antibody, depicted concentrations of pig-anti-anakinra antibodies should be considered estimated concentrations.
Statistical analysis
Statistical analyses were performed using Prism Software (Graphpad Software Inc., La Jolla, CA). For organ weights, body weight, ADA, hematology, and clinical chemistry data, 2-way analysis of variance (ANOVA) was performed including sex and treatment as a main factor and its interaction. If the 2-way ANOVA yielded a significant effect (p < 0.05) for treatment and sex or the interaction between treatment and sex, inter-group comparisons with the placebo control group were made by a Bonferroni multiple comparisons test. For ADA also a comparison was made between high- and low-dose anakinra treated groups. If the 2-way ANOVA yielded a significant effect (p < 0.05) for treatment only, we simplified the model because terms were involved that are not significant, and a 1-way ANOVA was performed for males and females combined. If the 1-way ANOVA yielded a significant effect (p < 0.05), inter-group comparisons with the placebo control group were made by a Bonferroni multiple comparisons test.
Incidences of histopathological changes were analyzed by Fisher’s exact probability test. Tests were performed as 2-sided tests, with results taken as significant where the probability of the results yielded a p-value < 0.05.
Results
Anakinra exerts biological activity to pig IL-1α in the D10 cell bio-assay
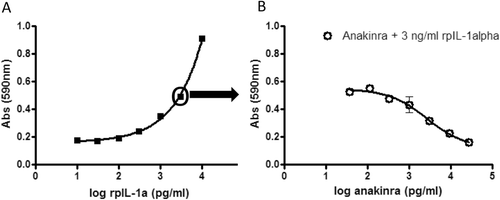
To demonstrate that anakinra is biologically active in pigs, a bioassay was used in which it was shown that anakinra blocks pig IL-1α-induced D10 cell proliferation. As D10 cells are dependent on IL-1α for their growth, using the cell bioassay it was shown that in the presence of 3 ng IL-1α/ml, anakinra could prevent D10 cell proliferation. As is shown in , less MTT was converted when anakinra was added to the culture than in its absence, indicating that anakinra actively blocked IL-1α of pig origin.
Figure 1. Anakinra blocks IL-1α from pig origin in a cell-based bio-assay. D10 cells were cultured with varying concentrations of rpigIL-1α (A) or with 3 ng/ml rpigIL-1α plus varying concentrations of anakinra (B). After a 4-day incubation, MTT was added to the cultures for 3 h followed by 10% SDS/50% DMF. Absorbance in each well was then measured at 590 nm. For each treatment group, mean (± SE) is depicted. O = concentration rpigIL-1α used in (B).
Clinical observations and body weights
At the injection sites a number of clinical signs (subcutaneous nodules or redness surrounding injection site) were observed in several animals of all groups. During the course of the study, all animals gained weight (data not shown). Body weight increase was not significantly altered by treatment.
Hematology and clinical chemistry
No treatment-related effects on red blood cell counts and coagulation parameters were observed (see Supplementary Table 2), except for a significant drop in hemoglobin (Hb) levels in both male (high-dose anakinra) and female (low- and high-dose anakinra) animals on Day 29 of the study. This drop in Hb levels correlates with a significant drop in packed cell volume (PCV) in the same groups. Also a slightly decreased red blood cell count is observed, but only in male animals treated with high-dose anakinra. These effects could be attributed to the multiple blood samples collected for PK determinations. White blood cell counts did not reveal any treatment-related effects other than a non-significant increased number of eosinophils in males (less pronounced also in females) treated with anakinra, albeit not dose-related (see Supplementary Table 3). No treatment-related effects on clinical chemistry parameters were observed (see Supplementary Table 4).
Organ weights
Group mean relative organ weights are depicted in . No significant group-differences in absolute and relative organ weights were observed, except for a significantly increased thymus weight (both absolute and relative; see also Supplemental Table 5) in the low-dose anakinra treated female animals compared to the placebo control group. This was caused by an increased thymus weight in two animals in this group. The thymus of the third animal in this group was not measured as a large number of hemorrhagic events were observed macroscopically. Increased thymus weight is observed more often in minipigs when numerous blood samples are taken via the neck vein (based on in-house experience).
Table 1. Mean relative organ weights and terminal body weights following anakinra treatment.
Anti-anakinra antibodies
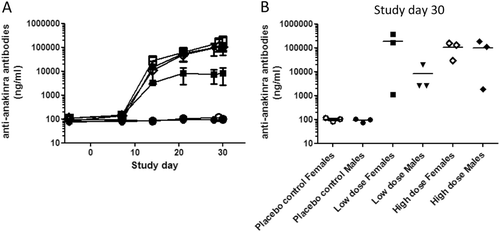
As expected the placebo-treated animals did not develop an anti-anakinra antibody response. All animals treated with anakinra, both low and high dose, developed an antibody response directed to anakinra from Day 14 onwards, albeit low in some animals. The serum levels of anti-anakinra antibodies are depicted in . Low dose anakinra-treated male animals produced on average less anti-drug antibodies (ADA) than the high-dose treated male animals, albeit not statistically significant. In female animals no difference in ADA was observed between low- and high-dose treated animals. High-dose male and low- and high-dose female animals produced ADA that seemed to have reached a plateau at the end of the study.
Figure 2. Serum levels of anti-anakinra antibodies. Blood for serum preparation was taken on several study days prior to treatment and analyzed for antibodies to anakinra. As the concentrations are calculated based on a standard curve using a goat anti-human IL-1ra antibody, depicted concentrations of pig-anti-anakinra antibodies should be considered estimated concentrations. (A) For each treatment group, mean ± SEM is depicted. -o-, Placebo control females; -•-, Placebo control males; -□-, Low dose anakinra females; -▪-, Low dose anakinra males; -◊-, High dose anakinra females; -▪-, High dose anakinra males. Day 7: 2-way ANOVA, placebo control vs low dose, p < 0.05. Furthermore, no significant differences are observed between groups. (B) Individual data anti-anakinra antibodies on study day 30.
PK of anakinra
Plasma levels of anakinra were determined at several time points after the first and last injection. As can be seen in , 24 h after the first (Day 0) and final (Day 28) injection, plasma anakinra levels in the animals had declined to nearly pre-dose levels. The calculated PK parameters are presented in . In general, repeated dosing resulted in increased half-life and mean-residence time, and decreased clearance, for anakinra in both genders and at both dose levels. At Day 0, dose-proportional kinetic behaviors were observed. No gender-dependent differences were observed at both dose levels. Remarkably, at Day 28, a much higher inter-individual variation in the kinetic behavior of anakinra was noted.
Table 2. Pharmacokinetic parameters at Day 0 and Day 28 for anakinra following daily dosing at 0.5 and 5.0 mg/kg body weight to male and female Göttingen minipigs.
Figure 3. Anakinra plasma concentration vs time curves. Data shown are from Göttingen minipigs following a subcutaneous low (-o-) and high (-•-) dose (females) and low (-Δ-) and high (-▴-) dose (males) at Day 0 and Day 28. Mean values for each group are depicted.
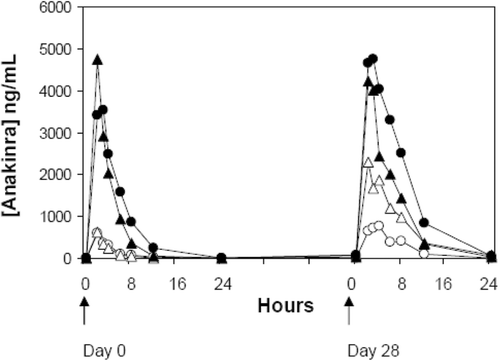
When looking at individual animals, a slight correlation (R2 = 0.6) was observed between antibody levels and clearance rate of anakinra. For one female low-dose animal on Day 28, significantly lower anakinra levels in plasma were observed compared to other animals in the same dose group. This animal had a very high ADA level, which might be correlated to the low anakinra level in the plasma.
Pathology
Macroscopy
Macroscopic examination at necropsy did not reveal distinct treatment-related gross changes. The injection site of one low-dose female appeared red. All other gross changes were unremarkable.
Microscopy
Microscopic examination revealed treatment-related inflammation at the injection site of 6/6 male and 4/6 female animals treated with anakinra without a distinct dose-relationship. The inflammation consisted of a mix of inflammatory cell types (mononuclear and granulocytic inflammatory cells), but consisted predominantly of plasma cells. Inflammation was restricted to the sub-dermal layers, mainly deep in the adipose tissue beneath the muscular layer, but to a lesser extent also in the muscle itself and in the subcutis (adipose tissue between the muscular layer and the dermis). This inflammation was not observed in placebo-treated animals. Necrosis and granulation tissue was associated with severe inflammatory cell infiltration in one low-dose female. Fiber regeneration was present in a few placebo control males and one low-dose anakinra male and was considered related to the inoculation procedure rather than caused by the test substance.
Increased germinal center development/enhanced activation was observed in the draining superficial cervical lymph nodes and spleens of animals injected with anakinra, but also in one placebo control; in addition, it was observed in the popliteal lymph nodes, which were distant from the inoculation site. Therefore, the biological significance of these histological findings in relation to the treatment is doubtful.
Discussion
As biotechnology-derived proteins are nowadays engineered to be as human-like as possible, they are likely to be recognized by the immune system of all pre-clinically used test species resulting in immunogenicity. This implicates that animal models are unlikely to be predictive for immunogenicity in man. Despite this caveat, immunogenicity testing in animals still has important value for interpretation of toxicology, pharmacology, and pharmacokinetic (PK) data. As non-clinical safety of biologically-derived pharmaceuticals should be studied in a relevant species, immunogenicity is mainly determined in non-human primates (NHP). However, NHP need not necessarily be the most predictive species for human safety. To increase the number of potentially relevant test species for testing (immuno)toxicity, including immunogenicity of biotherapeutics, and more specifically to potentially replace the NHP in non-clinical safety evaluation, we evaluated the predictive value of immunogenicity testing of biotherapeutics in minipigs for human safety. Despite the increasing role of minipigs in non-clinical safety testing, there is still a significant gap in knowledge concerning the value of the minipig for testing biotechnology-derived products (Bode et al., Citation2010). Therefore, more research is needed to provide sufficient comparative experimental data for a rigorous evaluation of the predictivity of the minipig for human drug safety, and to evaluate whether minipigs may better reflect certain human drug-induced toxicities than traditionally used non-rodent toxicology models.
In this study, the feasibility of the minipig as an alternative species for immunogenicity testing of biotechnology-derived drugs was investigated. Anakinra, a recombinant non-glycosylated form of the human IL-1 receptor antagonist (IL-1Ra), was used as a model biopharmaceutical. Minipigs were subcutaneously dosed with 0.5 or 5 mg anakinra/kg for 29 consecutive days. For comparison, in man anakinra/Kineret® is administered by daily subcutaneous injection of 100 mg (corresponding to 1.4 mg/kg for 70 kg subject). In man, the most often reported side effect (in ≈ 70% of patients) is mild injection site reactions, characterized by edema, erythema, ecchymosis, inflammation, and pain (FDA, Citation2000) (see also package insert and www.medicines. org.uk/EMC/medicine/23104/SPC/kineret). Comparable to the human situation, injection site reactions were temporary and occasionally observed in 11 of 18 (61%) minipigs during the course of the study. However, injection site reactions were observed in minipigs of all groups equally, including the placebo-treated controls. Microscopically, inflammatory cell infiltrate was observed in 10 out of 12 (83%) minipigs treated with anakinra, indicative of injection site reactions that were not always visible by clinical external observation. In a 4-week toxicity study of anakinra in Rhesus monkeys using dose groups of 10, 100, and 200 mg anakinra/kg/day, clinically visible injection site reactions were only observed in animals treated with 200 mg anakinra/kg/d (FDA, Citation2000). In this monkey study, at necropsy macroscopic evaluation showed minimal-to-moderate hemorrhage and thickening at the injection site(s) in all dose groups including the placebo control group. On histologic evaluation, a dose–effect relation was observed in severity of injection site reactions.
In minipigs, as in Rhesus monkeys after 4 weeks of daily administration of anakinra (FDA, Citation2000), no further treatment-related clinical or pathological findings were observed. Also, no clear effects of anakinra were observed on haematological/clinical chemistry parameters. Both in minipigs and in man, small increased numbers of eosinophils were observed (package insert). In Rhesus monkeys, a trend towards increased lymphocyte numbers and decreased neutrophil numbers was observed. These effects were, however, not statistically significant and were only observed in the monkeys treated with 200 mg anakinra/kg/day and not in the lower (10 and 100 mg anakinra/kg/day) dose groups. Furthermore, no anakinra-related effects on clinical chemistry data and hematology were found in the Rhesus monkey studies (FDA, Citation2000).
In minipigs, no clear effects of anakinra were observed on organ weights, except for a significantly increased thymus weight (both absolute and relative) in the low-dose anakinra-treated female animals compared to the placebo control group. However, as the difference in thymus weight was only observed in the low-dose anakinra female animals and was neither in the high-dose treated females nor in anakinra-treated males, an anakinra-related effect on thymus weight is considered unlikely.
Evaluation of the pharmacokinetic data revealed that in minipigs the Cmax was reached 2–4 h after injection, whereas in the human situation Cmax was reached slightly later, at 3–9 h after a subcutaneous bolus injection (FDA, Citation2001; EMEA, Citation2006). The half-life of anakinra in plasma was slightly shorter in the minipig compared to man (1.5–2.4 h at Day 0 and 2.7–4.2 h at Day 28 in minipigs vs 4–6 h in man) (FDA, Citation2001). It should, however, be considered that the differences in half-life could be explained by the disease state, as has been observed in rats (Zuurmond et al., Citation2011) and man (FDA, Citation2000).
All minipigs produced anti-drug antibodies (ADA) to anakinra from Day 14 onwards. No clear dose-related ADA response was observed, although in the low dose anakinra-treated males the ADA levels were slightly lower than in the high-dose males and low- and high-dose females, which were all comparable. In a study with rhesus monkeys, 3/3 males and 1/3 females treated subcutaneously with 5 mg anakinra/kg twice per day showed ADA from Day 14 until sacrifice on Day 29, with titers ranging from 1:50–1:400 (FDA, Citation2000). In clinical studies, 3.8–57.2% of patients were reported to be sero-positive for ADA to anakinra at one or more time points (FDA, Citation2001; FDA Arthritis Drugs Advisory Committee, 2001). As anakinra is a recombinant human protein, it is expected that the incidence of an immune response in animals is greater than that in man, due to species differences in protein structure and therewith the perceived foreignness of the protein. In female minipigs the ADA production was comparable in low- and high-dose treated animals, reaching a plateau level of ADA by the end of the study period. In male minipigs the low-dose treated animals produced less ADA than the high-dose treated animals.
The fact that antibody titers reached a plateau level after a certain period (ca. 3 weeks in this study) might be related to the short half-life of the drug. Due to a short half-life, the presence of the drug might be too short to induce more antibody production. Another possibility is that, when anakinra treatment continues, tolerance might be induced, leading to a decreased ADA titer. Antibody formation can cause increased or decreased clearance of the therapeutic proteins, although the former effect is the most common. In the minipig, the average anakinra clearance decreased following repeated dosing, but no apparent relationship between clearance at Day 28 and ADA formation was observed.
The goal of the study was to test the feasibility of the minipig to be used in pre-clinical immunotoxicity studies, with a focus on immunogenicity and to compare these results with those obtained in non-human primates (NHP). From the data obtained in minipigs, NHP, and man it is clear that both in minipigs and NHP almost all animals developed ADA against anakinra in contrast to much lower numbers in patients. As anakinra is a recombinant human protein, it is obvious that higher percentages of ADA-positive hosts are observed in the two animal species compared to the human patient population. The Tmax and half-life of anakinra were almost comparable in minpigs (Tmax ± 2 h and half-life between 1.5–2.4 h) and NHP (Tmax ± 2 h and half-life between 1.9–2.7 h), values that were somewhat lower than for human patients (Tmax ± 3–7 h and half-life between 4–6 h). Differences in PK between man and the animal test species might be partly influenced by the disease status of the human test population as it has been shown in a rat adjuvant arthritis model that disease status had a significant effect on pharmacokinetic behavior of anakinra (Zuurmond et al., Citation2011).
Conclusion
As the immunogenicity results obtained in the minipig using the human recombinant protein anakinra were overall comparable to those obtained in NHP, the results are supportive for the increasing role minipigs may play in safety assessment of human drugs, including drug-induced immunogenicity. However, more studies with additional biopharmaceutical products are needed to support the role of the minipig as an alternative predictive model for testing (immuno)toxicity, including immunogenicity.
Notice of Correction
This paper published online on 07 November 2012 contained an error in the title. The word “immunogenicity” was misspelled as “immunogecity”. This error has been corrected for this version.
Supplementary Material
Download PDF (73.6 KB)Acknowledgements
The authors would like to thank all colleagues who contributed to this study.
Declaration of interest
Niels-Christian Ganderup is employed at Ellegaard Göttingen Minipigs A/S, breeder and provider of minipigs. The authors alone are responsible for the content and writing of the paper.
References
- Bode, G., Clausing, P., Gervais, F., Loegsted, J., Luft, J., Nogues, V., Sims, J. 2010. The utility of the minipig as an animal model in regulatory toxicology. J. Pharmacol. Toxicol. Meth. 62:196–220.
- Brinks, V., Jiskoot, W., Schellekens, H. 2011. Immunogenicity of therapeutic proteins: The use of animal models. Pharm. Res. 28:2379–2385.
- Bugelski, P. J., Treacy, G. 2004. Predictive power of preclinical studies in animals for the immunogenicity of recombinant therapeutic proteins in humans.Curr. Opin. Mol. Ther. 6:10–16.
- Buttel, I. C., Chamberlain, P., Chowers, Y., Ehmann, F., Greinacher, A., Jefferis, R., Kramer, D., Kropshofer, H., Lloyd, P., Lubiniecki, A., Krause, R., Mire-Sluis, A., Platts-Mills, T., Ragheb, J. A., Reipert, B. M., Schellekens, H., Seitz, R., Stas, P., Subramanyam, M., Thorpe, R., Trouvin, J. H., Weise, M., Windisch, J., Schneider, C. K. 2011. Taking immunogenicity assessment of therapeutic proteins to the next level. Biologicals 39:100–109.
- Chirino, A. J., Ary, M. L., Marshall, S. A. 2004. Minimizing the immunogenicity of protein therapeutics. Drug Discov. Today 9:82–90.
- EMEA. 2006. Kineret: EPAR - Scientific Discussion.
- FDA. 2000. Center for drug evaluation and research and center for biologics evaluation and research. Application number 103950/0.
- FDA. 2001. Center for drug evaluation and research and center for biologics evaluation and research. Application number 103950/0; Approved labeling.
- FDA (Food and Drug Administration) Arthritis Drugs Advisory Committee. 2001. Kineret TM (anakinra) FDA Arthritis Drugs Advisory Committee Briefing Package.
- Forster, R., Ancian, P., Fredholm, M., Simianer, H., Whitelaw, B. 2010a. The minipig as a platform for new technologies in toxicology. J. Pharmacol. Toxicol. Meth. 62:227–235.
- Forster, R., Bode, G., Ellegaard, L., van der Laan, J. W. 2010b. The RETHINK project on minipigs in the toxicity testing of new medicines and chemicals: Conclusions and recommendations. J. Pharmacol. Toxicol. Meth. 62:236–242.
- Forster, R., Bode, G., Ellegaard, L., van der Laan, J. W. 2010c. The RETHINK project -minipigs as models for the toxicity testing of new medicines and chemicals: An impact assessment. J. Pharmacol. Toxicol. Meth. 62:158–159.
- Getts, D. R., Getts, M. T., McCarthy, D. P., Chastain, E. M., Miller, S. D. 2010. Have we over-estimated the benefit of human(ized) antibodies? MAbs 2:682–694.
- ICH. 2005. ICH S8. Immunotoxicity Studies for Human Pharmaceuticals.
- Kromminga, A., Schellekens, H. 2005. Antibodies against erythropoietin and other protein-based therapeutics: An overview. Ann. NY Acad. Sci. 1050:257–265.
- Penninks, A. H., van Mierlo, G. J. 2012. Immunotoxicity studies in minipigs. In: The Minipig in Biomedical Research (McAnulty, P. A., Dayan, A. D., Ganderup, N. C.Hastings, K. L., Eds.). New York: CRC Press/Taylor and Francis Group, pp. 397–414.
- Ponce, R., Abad, L., Amaravadi, L., Gelzleichter, T., Gore, E., Green, J., Gupta, S., Herzyk, D., Hurst, C., Ivens, I. A., Kawabata, T., Maier, C., Mounho, B., Rup, B., Shankar, G., Smith, H., Thomas, P., Wierda, D. 2009. Immunogenicity of biologically-derived therapeutics: Assessment and interpretation of nonclinical safety studies. Regul. Toxicol. Pharmacol. 54:164–182.
- Regulatory Affairs Canada. 2008. Product Monograph PrKineret.
- Schellekens, H. 2010. The immunogenicity of therapeutic proteins. Discov. Med. 9:560–564.
- Singh, S. K. 2011. Impact of product-related factors on immunogenicity of biotherapeutics.J. Pharm. Sci. 100:354–387.
- van der Laan, J. W., Brightwell, J., McAnulty, P., Ratky, J., Stark, C. 2010. Regulatory acceptability of the minipig in the development of pharmaceuticals, chemicals, and other products. J. Pharmacol. Toxicol. Meth. 62:184–195.
- Wierda, D., Smith, H. W., Zwickl, C. M. 2001. Immunogenicity of biopharmaceuticals in laboratory animals. Toxicology 158:71–74.
- Zuurmond, A. M., Koudijs, A., van, E. B., Doornbos, R. P., van Manen-Vernooij, B. C., Bastiaans, J. H., Penninks, A. H., van Bilsen, J. H., Cnubben, N. H., Degroot, J. 2011. Integration of efficacy, pharmacokinetic, and safety assessment of IL-1 receptor antagonist in a pre-clinical model of arthritis. Regul. Toxicol. Pharmacol. 59:461–470.