Abstract
Functional innate immune assessments, including phagocytosis and respiratory burst, are at the forefront of immunotoxicology evaluation in pre-clinical animal species. Although in the clinic and in academic science, phagocytosis, and respiratory burst assessments have been reported for over two decades, the implementation of phagocytosis and respiratory burst analyses in toxicology safety programs is just recently gaining publicity. Discussed herein are general methods, both microtiter plate-based and flow cytometric-based, for assessing phagocytosis and respiratory burst in pre-clinical species including mouse, rat, dog, and monkey. This methods-centric discussion includes a review of technologies and descriptions of method applications, with examples of results from analyses testing reported inhibitors (rottlerin, wortmannin, and SB203580) of phagocytosis and respiratory burst. Justification of implementation, strategic experimental design planning, and feasibility aspects of evaluating test article effects on phagocytosis and respiratory burst function are described within the context of a case study. The case study involves investigation of the effects of a small molecule p38 kinase inhibitor, BMS-582949, on phagocytosis and respiratory burst functions in rat and monkey neutrophils and monocytes in vitro, as well as ex vivo in these innate immune cells from monkeys administered BMS-582949 during a 1-week repeat dose investigative study. The results of the in vitro and ex vivo assessments demonstrated that BMS-582949 inhibited phagocytosis and respiratory burst. These findings correlated with incidences of opportunistic infections observed in rat and monkey toxicity studies.
Introduction
Phagocytosis and respiratory burst functions are key contributors to innate immunity. These instrumental roles of engulfing foreign matter and rapidly releasing reactive oxygen species, an effective intracellular pathogen-killing mechanism, are carried out by innate immune cells including neutrophils, macrophages, and monocytes. Phagocytosis is an energy-requiring process in which foreign matter is detected in a non-specific manner. This matter attaches to the cell surface initiating the creation of a phagosome. The phagosome containing foreign particle(s) is then internalized and fuses with a lysosome to form a phagolysosome. Respiratory burst is the result of a complex network of signal transduction events often initializing with G-protein coupled receptor (GPCR) activation on the cell-surface membrane of the innate immune cells and culminating in the reduction of oxygen species to oxygen-free radicals by NAPDH oxidase (Lehmann et al., Citation2007; van der Vliet, Citation2008; Yona et al., Citation2008).
Assessment of phagocytosis and respiratory burst is not novel, as in the clinic these functions have been tested for decades to determine causes of increased risk of nosocomial infections and innate immunity-related genetic disorders (Schopfer and Douglas, 1976; Weinstein and Young, Citation1976; Schmaldienst and Horl, Citation1996). However, assessment of these functions in drug and chemical safety evaluations in pre-clinical species is recently gaining popularity. Reasons for investigating potential test article effects on phagocytosis/respiratory burst in safety testing include: (1) class of chemical/environmental toxicant predicts potential targeting of macrophage and/or neutrophil function; (2) pharmacology of a drug predicts potential targeting of innate immune function, e.g., target of drug is cytokine, chemokine, or kinase involved in macrophage/neutrophil activation; (3) observation of opportunistic infections upon drug treatment/environmental toxicant exposure; and (4) observation of exaggerated innate immune activation. Described herein are generalized methods used to assess phagocytosis and respiratory burst in innate immune cells with examples of corresponding results to provide ideas for assay training and verification of use. Furthermore, implementation of these methods in safety testing is described through presentation of a case study involving both in vitro and ex vivo phagocytosis and respiratory burst assessment.
General methods
Plate-based phagocytosis assay
Plate-based methods can be used to assess phagocytosis of, for example, adherent cells isolated from tissues including blood, peritoneal cavity fluid, or bronchial alveolar lavage fluid. While there are several plate-based phagocytosis assay methods (Wan et al., Citation1993; Foukas et al., Citation1998), the basic concept of measuring the amount of labeled foreign material engulfed by the cells is standard. Below is a general description of a plate-based phagocytosis assay method adapted from the manufacturer instructions of the Vybrant® Phagocytosis Kit (Molecular Probes, Invitrogen, Life Technologies, Grand Island, NY).
Cells (105 cells/well) are incubated for a minimum of 1 h in complete media (e.g., DMEM, with 10% fetal bovine serum [FBS]; Life Technologies) at 37°C in a 5% CO2 atmosphere to permit phagoctyic cell adherence. The plates are then washed with buffered saline (i.e. Hank’s Balanced Salt Solution [HBSS]) to dislodge any non-adherent cells. Labeled (e.g., fluorescein) opsonized Escherichia coli or polystyrene beads are then added to each well in the absence/presence of additional stimuli and/or immunomodulators/test article; the cells are then incubated at 37°C (in 5% CO2) for 60–100 min. The addition of mitogens like phorbol myristate acetate (PMA) or the chemotactic peptide N-formyl-methionine-leucine-phenylalanine (fMLP) increases the magnitude of phagocytic index, thereby potentially increasing the capacity to measure test article effect (Rosales and Brown, Citation1991; Chan et al., Citation2001). After the incubation, the culture supernatant is removed and the cells are washed with buffered saline; 0.4% trypan blue (Sigma, St. Louis, MO) is then added to each well to quench any external fluorescence. After 1 min, the dye is aspirated and the cells are then analyzed using a fluorimeter. Fluorescent microscopy can also be used for analysis if the engulfed material is fluorophore-labeled. An assessment of cell viability is recommended to ensure that data interpretation is not skewed due to test article-related cytotoxicity. This may be accomplished in the same wells using a viability indicator that fluoresces at a different wavelength than the fluorophore conjugated to the E. coli or beads, if applicable. Alternatively, dedicated test wells can be used to assess cell viability.
Overall, plate-based methods require low-end technological equipment and are relatively inexpensive. This method has been implemented to assess phagocytosis in numerous pre-clinical species including mouse, rat, dog, and monkey. A drawback of the plate-based method is that, without specific isolation of the cells being tested, populations of cells may not be pure, and therefore test article effects on phagocytosis function cannot be assessed within the context of one particular cell type unless a phagocytic cell line is used. Furthermore, this assay is seemingly not conducive to testing whole blood potentially due to fluorescence interference of unintended hemoglobin bound to the plate surface. This is potentially important when performing ex vivo analysis of test animal samples since the test article is voided during sample preparation.
Flow cytometric-based phagocytosis assay
As with the plate-based method, the general concept of a flow cytometry-based phagocytosis assay is measurement of fluorophore-labeled foreign material engulfed by phagocytic cells. This method can be used for isolated adherent cells as well as whole blood collected into tubes coated with sodium or lithium heparin. If using whole blood, it is recommended that the assay be performed within 4 h post-collection for optimal phagocytic function. If applicable, cells or whole blood are incubated with the test article at 37°C. Fluorescein (or alternative fluorophore)-labeled opsonized bacteria or polystyrene beads (≈107 bacteria or beads/ml) are then added and the mixtures incubated at 37°C. After incubation, a non-permeable quenching solution such as trypan blue is added to inactivate any fluorescein label (of bacteria or beads) that was not internalized. After washing, erythrocytes present are lysed with a lyse/fix solution (e.g., FACS lyse, BD BioSciences, San Jose, CA). After washing, the DNA in the remaining cells is stained with a cell permeable dye that intercalates double-stranded DNA (e.g., propidium iodide) to permit leukocyte identification and the samples are analyzed using a flow cytometer. Regions are drawn and gates set on neutrophils and monocytes, based on forward and side light scatter. Mean (or median) fluorescence intensity (MFI) data is then compiled for each population in the appropriate channel for the fluorophore used. In each case, a minimum of 1000 events/sample is collected to assure measured result accuracy.
This method, in general, follows a method adapted from the commercially available PhagoTest® kit (Orpegen Pharma, Heidelburg, Germany). This kit recommends 100 µl whole blood/test sample. Decreasing assay volumes by 50% enables use of a polypropylene 96-well deep-well block (2.2 ml capacity) facilitating liquid handling and minimizing blood collection from animals. Although initially marketed for human testing, this method is easily adapted for pre-clinical species including mouse, rat, dog, and monkey. Since flow cytometric-based methods can be performed in whole blood, they are amenable to in vitro and ex vivo analyses. If assessing whole blood, multiple time points can easily be added to a standard toxicity study per animal for non-rodent species. Tail vein bleeding of rats is not recommended, as results are not optimal, most likely due to the pre-bleed heating of the tail. Blood collected via cardiac puncture seemingly results in optimal data for rodents. A significant positive aspect of a flow cytometric-based method compared to a plate-based phagocytosis assay method is that cells can be analyzed within cell context as viable neutrophils and monocytes are easily identified based on light scatter. A down-side for this method is that it is more expensive than plate-based methods since it requires a flow cytometer and operator resources.
Plate-based respiratory burst assay
This method can assess respiratory burst of, for example, adherent cells isolated from tissues including blood, peritoneal cavity fluid, or bronchioalveolar lavage fluid. There are several plate-based respiratory burst assay methods: some of these can be specific to detect/measure free oxygen radicals, nitric oxides, or [hydrogen] peroxide species (Cathcart et al., Citation1983; Rosenkranz et al., Citation1992). The overall concept of most of these methods is the measurement of fluorescence elicited by oxidation of a fluorogenic substrate. Below is a general description of a plate-based respiratory burst assay method.
Cells (1 × 105 cells/well) are incubated for a minimum of 1 h in complete media (with or without test article) at 37°C (in 5% CO2). The plates are gently washed with media or buffered saline to remove non-adherent cells. Probe (substrate) solution (e.g., 6-carboxy-2,7-dichloro-hydrofluorescien diacetate in buffered saline) is added and the plates are incubated at 37°C (in 5% CO2) for 30 min to allow for internalization. Respiratory burst stimulants (e.g., phorbal esters, E. coli, zymosan [yeast cell wall glucan]) in the presence/absence of immunomodulators (unless pre-treated with immunomodulator/test article) are then added. Of note, the presence of media in the preceding steps may blunt the magnitude of super-oxide production and/or detection. Thereafter, fluorescence in each well is measured in a microplate fluorimeter.
Overall, plate-based methods are relatively inexpensive. This method has been implemented to assess respiratory burst function in numerous pre-clinical species including mouse, rat, dog, and monkey. A drawback of the plate-based method is that without specific isolation of the cells being tested or use of a cell line, respiratory burst cannot be precisely assessed within the context of one particular cell type. As described above for the phagocytosis plate-based method, this method is also not conducive to testing whole blood.
Flow cytometric-based respiratory burst assay
As with the plate-based method, the general concept of a flow cytometric-based respiratory burst assay is measurement of fluorescence elicited by oxidation of a fluorogenic substrate. This method can be used for isolated adherent cells as well as whole blood collected in sodium or lithium heparin. If using whole blood, it is recommended that the assay be performed within 4 h post-collection for optimal burst function. If applicable, cells or whole blood should be incubated with test article at 37°C (in 5% CO2). Following treatment, respiratory burst can be stimulated by addition of mitogens such as PMA or opsonized E. coli. After a short (5–10 min) incubation, a fluorogenic substrate (e.g., dihydrorhodamine [DHR]-123 or 6-carboxy-2,7-dichlorohydrofluorescien diacetate) is added. The erythrocytes present are then lysed with a lyse/fix solution (e.g., FACS lyse, BD BioSciences). Following further washing steps, DNA in all remaining cells is stained with propidium iodide to permit leukocyte identification, and the samples are then analyzed via flow cytometry. Regions are drawn and gates are set on neutrophils and monocytes, based on forward and side light scatter. MFI data for each population are compiled in the appropriate channel for fluorphore used. In each case, a minimum of 1000 events/sample is collected to assure measured result accuracy.
This method, in general, follows a method adapted from a commercially-available kit, PhagoBurst® (Orpegen Pharma). This kit recommends 100 µl of whole blood per test sample. Decreasing assay volumes by 50% enables use of a 96-well deep block plate facilitating liquid handling and minimizing blood collection from animals. Although initially marketed for human testing, this method is easily adapted for pre-clinical species, including mouse, rat, dog, and monkey; it is also amenable to in vitro and ex vivo analyses. As with the phagocytosis whole blood assay, tail vein bleeding of rats is not recommended as results are not optimal, most likely due to the pre-bleed heating of the tail.
Methods verification
Before implementing these methods for safety testing, several verification experiments were performed. Outlined below are representative examples that, in general, follow the methods described above. Thus, only details necessary to provide additional relevant information and an understanding of how the experiments demonstrated suitable application of the methods are reported. In addition, results for the experiments are shown in varying manners to convey different suggestions for data analysis and presentation. Statistical analyses were not performed on these test results.
Plate-based phagocytosis assay
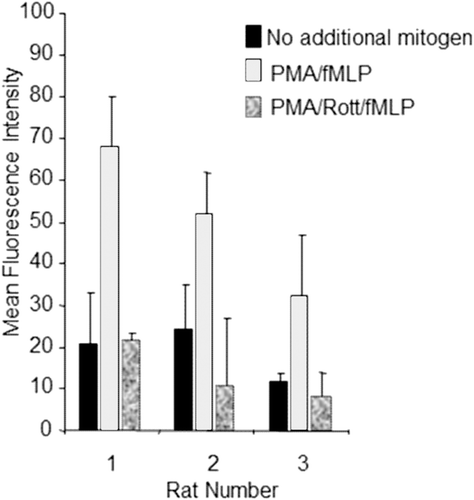
Alveolar lavage fluid was collected from Sprague Dawley rats following a standard procedure (Burleson et al., Citation1995). The cells were washed with HBSS by centrifugation of lavage fluid at 500 x g followed by re-suspension in HBSS and further centrifugation. Cells were counted and diluted to an appropriate concentration. In general, the method outlined above was then followed. Upon addition of fluorescein-labeled E. coli, adherent cells were treated with HBSS alone; 12.5 ng PMA/ml and 5 nM fMLP; or PMA, fMLP, and 10 µM rottlerin (a protein kinase C [PKC] inhibitor), and incubated at 37°C (in 5% CO2) for 1 h. Replicate wells were tested to ensure viability using a Vybrant® Cell Metabolic Assay (Molecular Probes). As shown in , addition of PMA (a potent PKC activator) and fMLP (a chemotactic peptide and activator of mitogen activated protein kinases [MAPKs]) enhanced phagocytosis, as indicated by a ≈2–3-fold increase in MFI (Torres et al., Citation1993). Rottlerin inhibited the mitogen-stimulated phagocytosis (), but did not affect phagocytosis in the absence of mitogen co-treatment (data not shown). Within the 1-h incubation, 10 µM rottlerin did not affect cell viability (data not shown), supporting the conclusion that the decreases in MFI were due to inhibition of phagocytosis and not due to cytotoxicity. These results demonstrated that this plate-based assay method was suitable to determine potential test article effects on phagocytosis in rat alveolar adherent cells.
Figure 1. Plate-based phagocytosis method test results. Adherent cells from rat alveolar lavage fluid (n = 3, x-axis) were treated with HBSS alone; 12.5 ng/mL PMA and 5 nM fMLP; or PMA, fMLP, and 10 µM rottlerin upon addition of fluoroscein labeled E. coli. Results are expressed as mean fluorescence intensity (y-axis). Error bars represent the standard deviation of triplicate wells. Rottlerin is abbreviated to Rott in the graph legend.
Flow cytometric-based phagocytosis assay
Peripheral blood from cynomolgus monkeys was collected into sodium heparin tubes. The whole blood was then aliquoted and incubated at 37°C (in 5% CO2) for 1 h in the absence or presence of SB203580 (a commercially available p38 kinase inhibitor) at 0.2, 1, 5, and 20 µM, prior to addition of fluorescein-labeled E. coli. Data was then acquired using a BD FACScalibur flow cytometer (BD BioSciences). Viable neutrophils and monocytes were analyzed independently after gating these cell populations on a forward vs side scatter analysis plot, using Cell Quest Pro software (BD Biosciences). demonstrates that SB203580 inhibits phagocytosis in both neutrophils and monocytes in a dose-dependent manner as previously reported, thus supporting the use of this method to determine potential test article effects on phagocytosis in monkey peripheral blood neutrophils and monocytes (Coxon et al., Citation2003).
Figure 2. Flow cytometric-based phagocytosis method test results. Whole blood was treated with 0.2, 1, 5, and 20 µM SB203580 prior to addition of fluoroscein-labeled E. coli. Graph depicts percent inhibition (y-axis) of MFI in SB203580-treated monkey whole blood relative to untreated control samples for neutrophils and monocytes. Data represent three independent assay days with blood from eight monkeys tested per day at each of the four SB203580 concentrations (escalating from left to right).
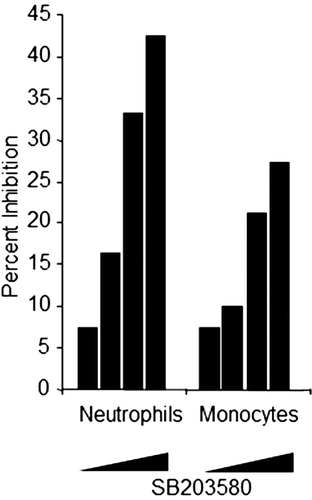
Plate-based respiratory burst assay
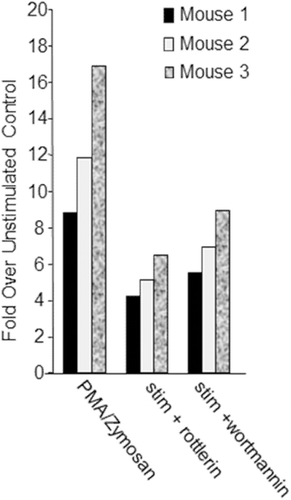
Adherent cells from CD1 mouse alveolar lavage fluid were treated with vehicle (DMSO ≤ 0.1%) or stimulated with 12.5 ng PMA/ml and 2 mg zymosan/ml in the presence or absence of known inhibitors of respiratory burst, expressly 10 µM rottlerin or 10 µM wortmannin, a phosphatidylinositol-3 kinase pathway inhibitor (Jackson et al., Citation1997; Dzik et al., Citation2010). Cell viability was not assessed in this experiment; however, the incubation with rottlerin or wortmannin was brief (≤ 30 min) as these agents were added at the same time as the stimulants. As shown in , rottlerin and wortmannin inhibit stimulated respiratory burst, supporting the use of this method in assaying respiratory burst function in adherent cells.
Figure 3. Plate-based respiratory burst test results. Adherent cells from CD1 mouse alveolar lavage fluid (n = 3, x-axis) were treated with vehicle (DMSO ≤ 0.1%) or stimulated with 12.5 ng/mL PMA and 2 mg/mL zymosan in the presence or absence of 10 µM rottlerin or 10 µM wortmannin. Respiratory burst stimulants were added at the same time as rottlerin or wortmannin. Data are represented as fold over unstimulated (vehicle control; y-axis); mean fluorescence values for cells stimulated with PMA and Zymosan in the presence or absence of either rottlerin or wortmannin were divided by mean fluorescence values resulting from the unstimulated cells per each mouse.
Flow cytometric-based respiratory burst assay
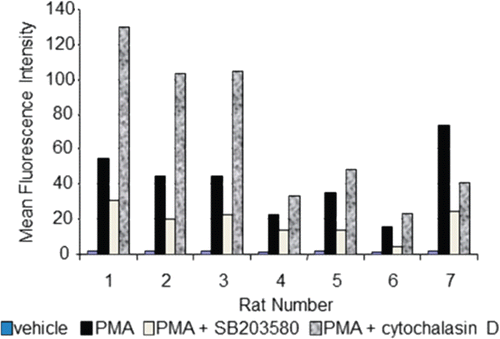
Peripheral blood from Sprague Dawley rats was collected into tubes containing sodium heparin. Whole blood was aliquoted and incubated at 37°C (5% CO2) for 1 h in the absence or presence of 20 µM SB203580 or 5 µg cytochalasin D/ml (an inhibitor of actin polymerization reported to inhibit phagocytosis yet enhance respiratory burst) prior to addition of PMA (Klaus, Citation1973; Takeshige and Minakami, Citation1981; Elliot and Winn, Citation1986; Voloshina et al., Citation2009). Data was then acquired using a BD FACScalibur. Viable neutrophils and monocytes (data not shown) were analyzed independently after gating these cell populations on a forward vs side scatter analysis dot plot using Cell Quest Pro software (BD Biosciences). demonstrates that 20 µM SB203580 inhibited respiratory burst by ≈50% in the neutrophils in six of seven rats and, as reported in the literature, cytochalasin D enhanced PMA-stimulated respiratory burst. Thus, this assay is robust enough to demonstrate both inhibition and enhancement of a stimulated respiratory burst.
Figure 4. Flow cytometric-based respiratory burst results. Whole blood from Sprague Dawley rats (n = 7, x-axis) was incubated for 1 h in the absence or presence of 20 µM SB203580 or 5 µg/mL cytochalasin D, prior to addition of respiratory burst stimulant, PMA. Data are presented as MFI (y-axis). Even though data is presented in an uncalculated format (i.e. percent inhibition or fold change), standard deviation is not represented since samples were only analyzed in duplicate.
Case study
Observations
Drug-related bacterial infections occurred with two small molecule p38 kinase inhibitors, BMS-582949 and its phosphocarbamate pro-drug BMS-751324; these presented in Sprague Dawley rats as bacterial skin infections and abscesses of the preputial/clitoral gland, and in Cynomolgus monkeys as bacterial rhinitis, bacterial pericarditis, bacteremia, and/or infections of the skin or gastrointestinal tract (Price, Citation2010). These infections were not seen in every animal of a particular dose-group or in every study at similar dose levels, and were sporadic regardless of study duration (Price, Citation2010). Study durations for the p38 kinase inhibitor, BMS-582949, toxicology program included up to a 6-month repeat dose toxicity study in rats and to a 1-year repeat dose toxicity study in monkeys. Bacterial rhinitis was observed in monkeys within a 2-week oral repeat toxicity study and the incidence and severity of infections were higher in monkeys compared to in rats (Price, Citation2010).
Hypothesis
The primary hypothesis for the development and the sporadic nature of these findings in non-clinical species was that, in the context of immunomodulation by a p38 inhibitor, opportunistic pathogens may manifest clinically relevant infections. Inhibition of p38 has been implicated in decreased neutrophil and monocyte functions, including phagocytosis and respiratory burst, and these innate immune functions are imperative for first-line defense against infection (Schnyder et al., Citation1998; Ward et al., Citation2000; Coxon et al., Citation2003; Wang et al., Citation2006a, b).
In vitro assessments
An investigative study was conducted to evaluate the effects of BMS-582949 on phagocytosis and respiratory burst functions in rat and monkey neutrophils and monocytes in vitro. Approximately 2700 samples were analyzed by flow cytometric methods to rigorously meet the scientific study objectives. The overall study design was two-part, range finding assessments and definitive assessments, for both rat and monkey species and phagocytosis and respiratory burst evaluations. Range-finding assessment parameters included evaluating multiple drug pre-treatment times, BMS-582949 concentrations, stimulants, and stimulation times. It is recommended that for every test article and/or test species evaluated these parameters be considered for investigation. An aim of the range-finding assessments was to determine what stimulants and their concentrations and stimulation times provided a similar magnitude in respiratory burst for rat and monkey so that inhibition of phagocytosis and respiratory burst could be more accurately compared between the species. Although experimental designs for range-finding assessments are described in this manuscript, data are not reported here since the purpose of describing the range-finding assessments is to share with the readership ideas of parameters to test when designing in vitro phagocytosis and respiratory burst experiments, and the actual data is irrelevant concerning the demonstration of addressing the case-study hypothesis. Once optimal drug-concentration range and assay parameters were identified, definitive assessments were conducted. Definitive assessment parameters included animal-to-animal and day-to-day variability, and calculation of IC30 values using four animals/species/sex. In general, test samples were performed in triplicate. The methods used for both phagocytosis and respiratory burst evaluation were the flow cytometry-based methods described above using PhagoTest® and PhagoBurst® Test kits (Orpegen Pharma), respectively.
In general, manufacturer instructions were followed, with the exception of volumes, some incubation times, and stimulant concentrations. Assay volumes were reduced by 50%; this allowed for decreased volumes of blood to be collected from the monkeys and also facilitated use of 96-deep well blocks (2.2 ml volume/well). In addition, stimulation time for respiratory burst was decreased from 10 min to 8 min based on prior methods development work and not re-assessed herein. In pre-clinical species’ samples stimulated for > 8 min, assay variability increased and light-scatter gating of neutrophils and monocytes was sub-optimal, as the populations greatly shifted in side scatter and there were decreased cell numbers within these populations. Other modifications made to the instructions are described below. Analyses were performed using a BD FACScalibur flow cytometer. Neutrophil and monocyte populations were gated based on forward and side scatter using Cell Quest Pro software. Data was compiled individually for both cell types, based on fluorescence intensities in the FL1 (488 or green) channel. In general, triplicates were performed for each test sample.
For definitive assessment data, repeated measures analysis of variance (ANOVA) procedures were utilized to assess differences between vehicle control and in vitro treatment of BMS-582949 at 0.05, 0.5, 5, and 50 (phagocytosis) µM. The Dunnett multiple-comparison t-test procedure was utilized for the comparisons. Two-sided test statistics were calculated at the 5% significance level. Percent inhibition was calculated as follows: 100 − [(mean fluorescence intensity of test sample/mean fluorescence intensity of vehicle control) × 100]. An estimated inhibitory concentration at 30% (IC30) value was calculated, when appropriate, using linear regression methods. Incidence of ≥ 30% inhibition and IC30 values were considered a cut-off for biologically relevant inhibition in this assay as correlations of disease- and/or chemical-induced inhibition of innate immune-cell function by ≥ 30% with increased incidences of bacterial infections have been reported in the literature (Schopfer and Douglas, 1976; Weinstein and Young, Citation1976; Schmaldienst and Horl, Citation1996; Hübell et al., Citation1999; Butcher et al., Citation2001, Citation2003).
In vitro phagocytosis evaluation experimental design and results
The parameters tested for the range finding assessment portion of the phagocytosis evaluation are shown in , and the assay parameters chosen for the definitive assessments based on the range finding results are shown in .
Table 1. Phagocytosis range-finding assessment experiments.
Table 2. Phagocytosis definitive assessment parameters.
Definitive assessment results demonstrated that BMS-582949 inhibited phagocytosis in monkey and rat neutrophils in a dose-dependent manner (; briefly reviewed in Price, Citation2010). Phagocytosis function was significantly (p ≤ 0.05) decreased in rat and monkey neutrophils at 0.5 µM (0.2 µg/ml), 5 µM (2.1 µg/ml), and 50 µM (21 µg/ml). At 5 and 50 µM the median percent inhibitions were higher for monkeys (37 and 44%, respectively) than rats (16 and 27%, respectively). The incidence of ≥ 30% inhibition was also higher in monkeys (). The species differences in median percent inhibition and incidence of ≥ 30% inhibition are reflected in the higher IC30 values for rat (62 µM, 25 µg/ml) than monkey (23.2 µM, 9.4 µg/ml). Regardless of the group median differences between monkey and rat, there are several individual incidences of ≥ 30% inhibition of neutrophil phagocytosis observed in both monkey and rat () at 5 µM (2.1 µg/ml) BMS-582949, which is 0.1–10× the Cmax values achieved in animals with infections (Price, Citation2010). There were no BMS-582949-related effects on monocyte phagocytosis function demonstrated in monkeys or rats (data not shown).
Table 3. Incidence of inhibition of neutrophil phagocytosis function.
Figure 5. Effects of BMS-582949 on phagocytosis function in monkey and rat neutrophils in vitro (x-axis). Data are presented as percent inhibition of phagocytosis in vehicle control samples independently calculated for each animal (y-axis). For monkeys, n = 8 (four/sex) and for rat, n = 16 (eight/sex), except n = 15 (eight male and seven female) for rat at 0.05 µM treatment. Triplicate (except no replicates for two of eight monkeys and one of 15 rats at 0.05 µM) MFI values were averaged for each parameter per assay prior to percent inhibition calculation. Each individual monkey data point is a median of three independent assays. Each individual rat data point represents a single assessment per rat. Color-coded bars represent the median of the individual data per BMS-582949 treatment concentration.
In vitro respiratory burst evaluation experimental design and results
The parameters tested for the range finding assessment portion of the respiratory burst evaluation are shown in and the assay parameters chosen for the definitive assessments based on the range finding results are shown in .
Table 4. Respiratory burst range-finding assessment experiments.
Table 5. Respiratory burst definitive assessment parameters.
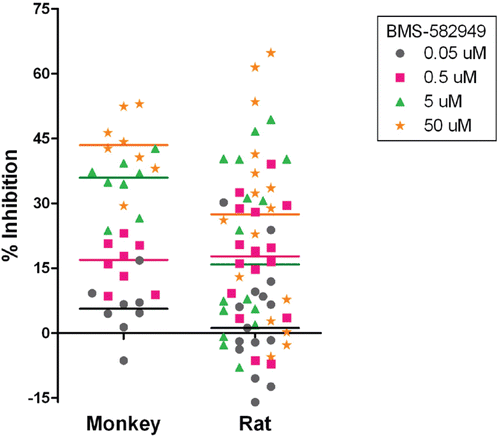
As demonstrated in , BMS-582949 inhibited the respiratory burst function of monkey and rat neutrophils in a dose-dependent manner (briefly reviewed in Price, Citation2010). Respiratory burst function was significantly (p ≤ 0.05) decreased compared to vehicle control at 0.5 µM (0.2 µg BMS-582949/ml), and 5 µM (2.1 µg BMS-582949/ml) in monkey and rat cells. At 0.5 µM, the median percent inhibition of PMA and E. coli stimulated respiratory burst was greater in monkeys (40 and 30%, respectively) than in rats (39 and 25%, respectively). However, at 5 µM, the median percent inhibition of PMA- and E. coli-stimulated respiratory burst was greater for rat neutrophils (67 and 57%, respectively) than for those of cells from monkeys (58 and 51%, respectively). The comparably heightened effect observed in the rats as compared to monkeys at 5 µM may be due to the potential peak of inhibition being between 0.5 and 5 µM for some monkeys. In the range-finding assessments, for some samples a slight decline was observed after the peak of inhibition within the upper-end of the expanded concentration ranges. IC30 values were calculated from the median percent inhibition values and are reported in . There was minimal difference between monkey and rat IC30 values for PMA stimulated respiratory burst, but the monkey IC30 value was notably lower than that of the rat for E. coli stimulated respiratory burst. However, as shown in , there was a high incidence of ≥ 30% respiratory burst inhibition at 0.5 and 5 µM in both species.
Table 6. IC30 values for BMS-582949 effects on neutrophil respiratory burst function.
Table 7. Incidence of neutrophil respiratory burst function inhibition.
Figure 6. Effects of BMS-582949 on respiratory burst function in monkey and rat neutrophils in vitro. n = 8 (four/sex) per stimulation condition and per species (x-axis). Data are presented as percent inhibition of stimulated respiratory burst in vehicle-control samples independently calculated for each animal (y-axis). Triplicate MFI values were averaged for each parameter per assay prior to percent inhibition calculation. Each individual monkey data point is a median of three independent assays. Each individual rat data point represents a single assessment per rat. Color-coded bars represent the median of the individual data per BMS-582949 treatment concentration.
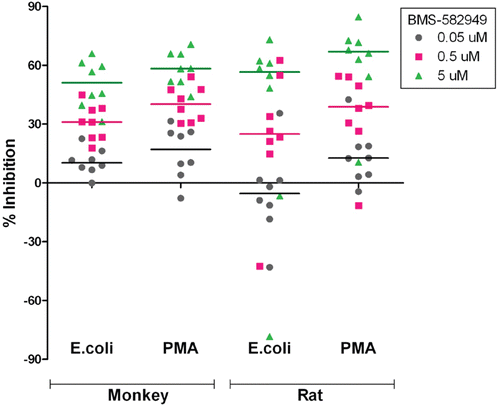
BMS-582949-induced inhibition of respiratory burst function in monocytes was also observed in a dose-dependent manner, but to a lesser extent as compared to neutrophil respiratory burst (; briefly reviewed in Price, Citation2010). Statistically significant (p ≤ 0.05) differences from controls in respiratory burst function were achieved in monkey blood stimulated with PMA, not E. coli, following pre-treatment with 0.5 and 5 µM BMS-582949. At these doses, the median percent inhibition values of PMA stimulated respiratory burst were 22 and 29%, respectively, for monkeys. The statistical inference and median percent inhibition values suggest a minimal potential biological relevance of BMS-582949 effect on monocyte respiratory burst on overall immune status in monkeys and rats; however, the observed susceptibility to bacterial infections in pre-clinical species was sporadic in nature and this observation correlates with the incidence of inhibition ≥ 30% in monkey and rat samples ().
Table 8. Incidence of inhibition of monocyte respiratory burst function.
Figure 7. Effects of BMS-582949 on respiratory burst function in monkey and rat monocytes in vitro. n = 8 (four/sex) per stimulation condition and per species (x-axis). Data are presented as percent inhibition of stimulated respiratory burst in vehicle-control samples independently calculated for each animal (y-axis). Triplicate MFI values were averaged for each parameter per assay prior to percent inhibition calculation. Each individual monkey data point is a median of three independent assays. Each individual rat data point represents a single assessment per rat. Color-coded bars represent the median of the individual data per BMS-582949 treatment concentration.
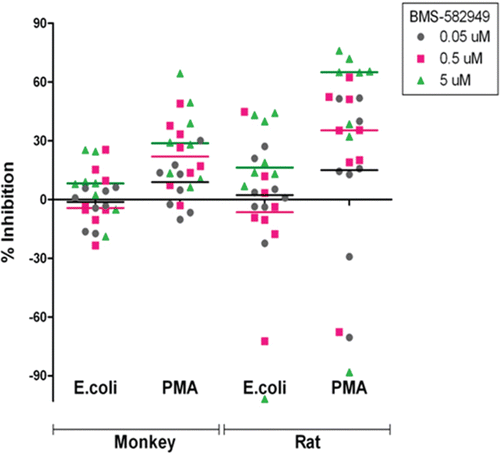
The rat monocyte data was highly variable resulting in unmet statistical significance of differences in fluorescence intensity values between vehicle and drug-treated samples. However, the median percent inhibition for PMA-stimulated respiratory burst (≈35 and 65% at 0.5 and 5 µM, respectively) was higher in cells from rats than that of cells from monkeys, which was statistically different from control. This numerical difference in percent inhibition of PMA-stimulated respiratory burst data is further demonstrated by the calculated IC30 values, 2.7 µM for rat and 4.8 µM for monkey, and the high incidences of 30% or greater inhibition (). IC30 values for BMS-582949 effect on rat and monkey E. coli-stimulated respiratory burst in monocytes were not calculated as the slope estimate derived from the linear regression function used to describe the relationship between percent inhibition and BMS-582949 concentration was not significantly different from ‘0’ (p ≤ 0.10, rat) or the range of percent inhibition data observed did not include 30% (monkey). Nonetheless, there were individual rats (one of eight at 0.5 µM and three of eight at 5 µM, three of eight rats collectively) that demonstrated a greater than 30% inhibitory effect of BMS-582949 on E. coli-stimulated respiratory burst ().
Ex vivo assessments
To further evaluate potential BMS-582949 effects on phagocytosis and respiratory burst, an in vivo cynomolgus monkey study was performed that included ex vivo functional assessments. In brief, four dose groups with six males per group were orally administered vehicle (5% [w/v] propylene glycol, 5% [w/v] polyethylene glycol 300, 5% [w/v] glycerin, 1.5% Methocel E5, 9% [w/v] 1N hydrochloric acid, and 1.5% [w/v] anhydrous alcohol in reverse osmosis water) or BMS-582949 at 1, 10, or 75 mg/kg daily for 7 days (Price, Citation2010). In a 1-year monkey repeat dose toxicity study, infections were observed at 10 and 75 mg/kg/day (Price, Citation2010). Blood (≈ 2 ml) was collected in sodium heparin tubes twice pre-tested on study days 4, 5, and 8–12 (test reversibility) for phagocytosis and respiratory burst analyses. During the dosing phase, blood was collected at the drug’s approximate Cmax (2–4 h post-dose). The methods used for both phagocytosis and respiratory burst evaluation were the flow-cytometric based methods described above using the PhagoTest® and PhagoBurst® Test kits (Orpegen Pharma), respectively. Assay conditions used were the same as those discussed previously for in vitro definitive assessments ( and 5; monkey) without the BMS-582949 pre-treatment. Stimulation of blood was initiated within 90 min of blood collection. Analyses were then performed using a BD FACScalibur flow cytometer. Neutrophils and monocytes were gated based on forward and side scatter using Cell Quest Pro software. Data was compiled individually for both cell types based on fluorescent intensities in the FL1 (488 or green) channel and repeated measures analysis of variance (ANOVA) procedures were utilized to assess differences between vehicle control and BMS-582949 treatment groups. A Dunnett multiple-comparison t-test procedure was utilized for comparisons and two-sided test statistics were calculated at the 5% significance level.
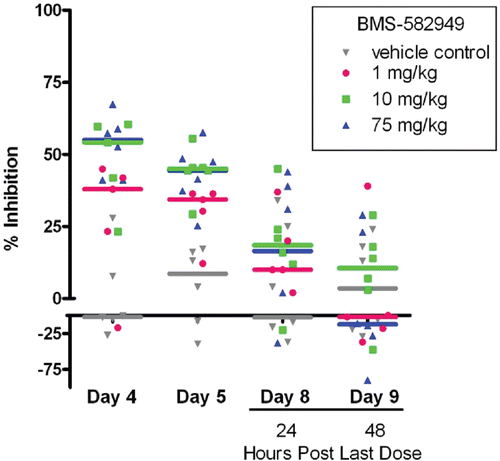
After four daily doses of BMS-582949 (at 10 and 75 mg/kg), significant (p ≤ 0.05) decreases (54 and 56% inhibition relative to control, respectively) in phagocytosis by neutrophils were noted (). After five daily doses, there were significant decreases (36% inhibition relative to control) in phagocytosis at 1 mg BMS-582949/kg as well. Twenty-four hours after the final dose (study day 8), there were no significant decreases in phagocytosis. However, there were numerical differences at both 10 and 75 mg BMS-582949/kg at 24 h post-final dose that were fully recovered by 48 h post-final dose. There were no significant decreases noted at any point in monocyte phagocytic activity.
Figure 8. Effects of BMS-582949 on phagocytosis in monkey neutrophils. Monkeys were administered vehicle or BMS-582949 at 1, 10, and 75 mg/kg for 7 days and peripheral blood neutrophils were analyzed for phagocytosis on study days 4, 5, 8, and 9 (x-axis). Data are presented as percent inhibition of the per day averaged vehicle-control sample MFI (y-axis). Triplicate MFI values were averaged for each test parameter. Data were normalized to pre-test MFI values prior to calculation of percent inhibition. Color-coded bars represent the group median percent inhibition.
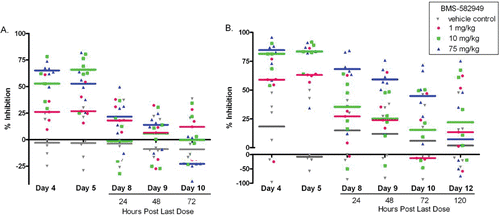
In neutrophils (at doses of 10 and 75 mg/kg BMS-582949), E. coli- and PMA-stimulated respiratory burst were significantly inhibited (53 and 66% and 82 and 85% inhibition relative to control, respectfully) after four daily doses (study day 4) and at 1 mg/kg on study day 5 (64% inhibition for PMA-stimulated respiratory burst (; Price, Citation2010). Recovery was dose-dependent: PMA- and E. coli-stimulated respiratory burst functions were fully recovered at all doses 120 and 72 h post-final dose, respectively. Although there were statistically significant decreases in monocyte E.coli- and PMA-stimulated respiratory burst at 10 and 75 mg/kg on Day 5 relative to vehicle control, after normalizing the data to pre-test values and calculating percent inhibitions, drug-related differences were not demonstrated (data not shown).
Figure 9. Effects of BMS-582949 on (a) E.coli or (b) PMA stimulated respiratory burst in monkey neutrophils. Monkeys were administered vehicle or BMS-582949 at 1, 10, and 75 mg/kg for 7 days and peripheral blood neutrophils were analyzed for respiratory burst function on study days 4, 5, 8, 9, 10, and 12 (PMA stimulation only day 12; x-axis). Data are presented as percent inhibition of the per day averaged vehicle-control sample MFI (y-axis). Triplicate MFI values were averaged for each test parameter. Data were normalized to pre-test MFI values prior to calculation of percent inhibition. Color-coded bars represent the group median percent inhibition. Figure was modified from Price (Citation2010).
Of note, a bacterial infection was identified in this study. A high-dose (75 mg BMS-582949/kg) monkey had severe cellulitis of a finger. A blood culture collected from this monkey tested positive for enterococcus and staphylococcus. In addition, liquid feces, indicative of intestinal infection, were observed in all high-dose monkeys.
Case study summary
In vitro phagocytosis and respiratory burst assessments demonstrated that BMS-582949 inhibits these functions at concentrations which were similar to drug exposures in animals that had infections in toxicity studies. For example, 5 µM BMS-582949 is 0.1–10× the Cmax values achieved in animals with infections (Price, Citation2010). In general, inhibition of these functions was greater in monkeys than rats, which correlates with the observation that severity and incidence of observed infections were higher in monkeys compared to rats. Moreover, ex vivo analyses demonstrated that phagocytosis and respiratory burst were inhibited at doses that resulted in infections in a 1-year monkey study. In both the in vitro and ex vivo assessments, respiratory burst was inhibited more than phagocytosis and inhibition was greater in neutrophils than monocytes.
In summary, the in vitro and ex vivo phagocytosis and respiratory burst assessment results supported the hypothesis that in the context of immunomodulation (decreased phagocytosis and respiratory burst) by a p38 inhibitor, opportunistic pathogens may manifest clinically-relevant infections.
Methodology conclusions
The methods described herein for assessing phagocytosis and respiratory burst are suitable for evaluating test article effects on these important innate immune functions. Common, commercially available immunomodulators can be used to verify the technical expertise and suitability of these methods. These assessments can be performed in vitro or ex vivo. For each test article and species tested, multiple assay parameters should be evaluated to ensure optimal assay conditions. If feasible, in vitro assessments provide a facile platform for testing numerous parameters and these conditions can translate into ex vivo evaluations as demonstrated by the case study described herein. Flow cytometric-based methods are more amenable to ex vivo assessments compared to plate-based methods as whole blood can be analyzed by flow cytometric-based methods. Although investigation of test article effects can be performed in investigative studies, the 96-well format of the plate- and flow cytometric-based methods facilitate addition of these functional end-points in standard toxicology studies with minimal logistical obstacles and can be utilized across pre-clinical species.
Acknowledgments
The authors would like to acknowledge Kuburat Bello for her technical help with the phagocytosis and respiratory burst assessments described herein and the biotechnologists in Drug Safety Evaluation at Bristol-Myers Squibb for their unwavering dedication to animal care and study functions. The first author would also like to thank the participants of the 20th Summerschool in Immunotoxicology, Beaune, France for the robust discussion on this subject and their comments which may have been included within this manuscript.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Burleson, G. R., Dean, J. H., Munson, A. E. (Eds.) 1995. Methods in Immunotoxicology. Chapter 2. In: Isolation of Alveolar Macrophages, Peritoneal Macrophages, and Kupffer Cells. Vol. 2. (Lewis, J. G., Ed.). New York, NY: Wiley-Liss, Inc., pp. 15–26.
- Butcher, S. K., Chahal, H., Nayak, L., Sinclair, A., Henriquez, N. V., Sapey, E., O’Mahony, D., Lord, J. M. 2001. Senescence in innate immune responses: Reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. J. Leukocyte Biol. 70:881–886.
- Butcher, S. K., Killampalli, V., Chahal, H., Kaya Alpar, E., Lord, J. M. 2003. Effect of age on susceptibility to post-traumatic infection in the elderly. Biochem. Soc. Trans. 31:449–451.
- Cathcart, R., Schwiers, E., Ames, B. N. 1983. Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Anal. Biochem. 134:111–116.
- Chan, H., Kedzierska, K., O’Mullane, J., Crowe, S. J., Jaworowski, A. 2001. Quantifying complement-mediated phagocytosis by human monocyte-derived macrophages. Immunol. Cell Biol. 79:429–435.
- Coxon, P. Y, Rane, M. J., Uriarte, S., Powell, D. W., Singh, S., Butt, W., Chen, Q., McLeish, K. R. 2003. MAPK-activated protein kinase-2 participates in p38 MAPK-dependent and ERK-dependent functions in human neutrophils. Cell Signal. 15:993–1001.
- Dzik, J. M., Zielinski, Z., Cielsla, J., and Walajtys-Rode, E. 2010. Trichinella spiralis infection enhances protein kinase C phosphorylation in guinea pig alveolar macrophages. Parasite Immunol. 32:209–220.
- Elliot, J. A., Winn, W. C. 1986. Treatment of alveolar macrophages with cytochalasin D inhibits uptake and subsequent growth of Legionella pneumophila. Infect. Immun. 51:31–36.
- Foukas, L. C., Katsoulas, H. L., Paraskevopoulou, N., Metheniti, A., Lambropoulou, M., Marmaras, V. 1998. Phagocytosis of Escherichia coli by insect hemocytes requires both activation of the Ras/mitogen-activated protein kinase signal transduction pathway for attachment and β3 integrin for internalization. J. Biol. Chem. 273:14813–14818.
- Hübell, K., Hegener, K., Schnell, R., Mansmann, G., Oberhäuser, F., Staib, P., Diehl, V., Engert, A. 1999. Suppressed neutrophil function as a risk factor for severe infection after cytotoxic chemotherapy in patients with acute non-lymphocytic leukemia. J. Ann. Hematol. 78:73–77.
- Jackson, J. K., Lauener, R., Duronio, V., Burt, H. M. 1997. The involvement of phosphatidylinositol 3-kinase in crystal induced human neutrophil activation. J. Rheumatol. 24:341–348.
- Klaus, G. G. 1973. Cytochalasin B. Dissociation of pinocytosis and phagocytosis by peritoneal macrophages. Exp. Cell Res. 79:73–78.
- Lehmann, D. M., Seneviratne, A. M. P. B., Smrcka, A. V. 2007. Small molecule disruption of G-protein subunit signaling inhibits neutrophil chemotaxis and inflammation. Mol. Pharmacol. 73:410–418.
- Price, K. D. 2010. Bacterial infections in cynomolgus moneys given small molecule immunomodulatory antagonists. J. Immunotoxicol. 7:128–137.
- Rosales, C., Brown, E. J. 1991. Two mechanisms for IgG Fc-receptor-mediated phagocytosis by human neutrophils. J. Immunol. 146:3937–3944.
- Rosenkranz, A. R., Schmaldienst, S., Stuhlmeier, K. M., Chen, W., Knapp, W., Zlabinger, G. J. 1992. A microplate assay for the detection of oxidative products using 2′,7′-dichlorofluorescin diacetate. J. Immunol. Meth. 156:39–45.
- Schmaldienst, S., Horl, W. H. 1996. Bacterial infections during immunosuppression-immunosuppressive agents interfere not only with immune response, but also with polymorphonuclear cell function. Nephrol. Dial. Transplant. 11:1243.
- Schnyder, B., Meunier, P. C, Car, B. D. 1998. Inhibition of kinases impairs neutrophil activation and killing of Staphylococcus aureus. Biochem. J. 331:489–495.
- Schopfer, K., Douglas, S. D. 1976. Neutrophil function in children with kwashiorkor. J. Lab. Clin. Med. 88:450–461.
- Takeshige, K., Minakami, S. 1981. Involvement of calmodulin in phagocytotic respiratory burst of leukocytes. Biochem. Biophys. Res. Com. 99:484–490.
- Torres, M., Hall, F. L., O’Neill, K. 1993. Stimulation of human neutrophils with formyl-methionyl-leucyl-phenylalanine induces tyrosine phosphorylation and activation of two distinct mitogen-activated protein-kinases. J. Immunol. 150:1563–1577.
- van der Vliet, A. 2008. NADPH oxidases in lung biology and pathology, host defense enzymes and more. Free Radic. Biol. Med. 44:938–955.
- Voloshina, E. V., Prasol, E. A., Grachev, S. V., Prokhorenko, I. R. 2009. Effect of cytochalasin D on the respiratory burst of primed neutrophils activated with a secondary stimulus. Dokl Biochem. Biophys. 424:13–15.
- Wan, C. P., Park, C. S., Lau, B. H. 1993. A rapid and simple microfluorometric phagocytosis assay. J. Immunol. Meth. 162:1–7.
- Wang, R., Town, T., Gokarn, V., Flavell, R. A., Chandawarkar, R. Y. 2006a. HSP70 enhances macrophage phagocytosis by interaction with lipid raft-associated TLR-7 and up-regulating p38 MAPK and PI3K pathways. J. Surg. Res. 136:58–69.
- Wang, X. M., Kim, H. P., Song, R., Choi, A. M. K. 2006b. Caveolin-1 confers anti-inflammatory effects in murine macrophages via the MKK3/p38 MAPK pathway. Am. J. Resp. Cell Mol. Biol. 34:434–442.
- Ward, R. A., Nakamura, M., McLeish, K. R. 2000. Priming of the neutrophil respiratory burst involves p38 mitogen-activated protein kinase-dependent exocytosis of flavocytochrome b558-containing granules. J. Biol. Chem. 275:36713–36719.
- Weinstein, R. J., Young, L. S. 1976. Neutrophil function in gram-negative rod bacteremia: The interaction between phagocytic cells, infecting organisms, and humoral factors. J. Clin. Invest. 58:8190–8199.
- Yona, S., Lin, H., Dri, P., Davies, J. Q., Hayhoe, R. P. G., Lewis, S. M., Heinsbroek, S. E., Brown, K. A., Perretti, M., Hamann, J., Treacher, D. F., Gordon, S., Stacey, M. 2008. Ligation of the adhesion-GPCR EMR2 regulates human neutrophil function. FASEB J. 22:741–751.