Abstract
Cannabidiol (CBD) is a plant-derived cannabinoid that has been predominantly characterized as anti-inflammatory. However, it is clear that immune effects of cannabinoids can vary with cannabinoid concentration, or type or magnitude of immune stimulus. The present studies demonstrate that oral administration of CBD enhanced lipopolysaccharide (LPS)-induced pulmonary inflammation in C57BL/6 mice. The enhanced inflammatory cell infiltrate as observed in bronchoalveolar lavage fluid (BALF) was comprised mainly of neutrophils, with some monocytes. Concomitantly, CBD enhanced pro-inflammatory cytokine mRNA production, including tumor necrosis factor-α (Tnfa), interleukins (IL)-5 and -23 (Il6, Il23), and granulocyte colony stimulating factor (Gcsf). These results demonstrate that the CBD-mediated enhancement of LPS-induced pulmonary inflammation is mediated at the level of transcription of a variety of pro-inflammatory genes. The significance of these studies is that CBD is part of a therapeutic currently in use for spasticity and pain in multiple sclerosis patients, and therefore it is important to further understand mechanisms by which CBD alters immune function.
Introduction
Marijuana and cannabinoid compounds have historically been characterized as immunosuppressive. CBD, which is an abundant non-psychoactive congener in Cannabis sativa, exhibits anti-inflammatory or immunosuppressive actions in several models (Srivastava et al., Citation1998; Malfait et al., Citation2000; Costa et al., Citation2004, 2007; Carrier et al., Citation2006; Jan et al., Citation2007; El-Remessy et al., Citation2008; Kaplan et al., Citation2008; Mukhopadhyay et al., Citation2011; Ribeiro et al., Citation2012). In addition, CBD has also been demonstrated to be efficacious in the treatment of autoimmune disease; in part, due to its anti-inflammatory properties (Iuvone et al., Citation2009).
Despite the generalized notion that cannabinoids are immune suppressive, there are several reports that cannabinoids increase immune function under some conditions. For instance, low concentrations of the psychoactive cannabinoid △9-tetrahydrocannabinol (△9-THC) enhanced anti-CD3-mediated proliferation in splenocytes derived from adult mice, but not young mice (Nakano et al., Citation1993). Low concentrations of △9-THC, as well as the synthetic cannabinoids WIN55212-2 and CP55940, enhanced B-cell proliferation (Derocq et al., Citation1995). Immune enhancement with CBD has also been reported in a few studies. △9-THC and CBD enhanced interleukin (IL)-2 production in response to soluble anti-CD3 plus anti-CD28 stimulation which, as opposed to immobilized anti-CD3 plus anti-CD28 stimulation, results in a sub-optimal activation of T cells (Jan et al., Citation2002). More recently, we expanded these observations and demonstrated that CBD also enhanced cytokine (IL-2 and interferon [IFN]-γ) production by mouse splenocytes in response to low concentrations of phorbol ester plus calcium (Ca) ionophore, a stimulus also shown to induce sub-optimal cellular activation (Chen et al., Citation2012). We further determined that the mechanism by which CBD enhanced cytokine production in response to the sub-optimal activation signal correlated with nuclear factor of activated T cells (NFAT) transcription factor activation and increased intracellular Ca concentration.
The present study demonstrates that CBD enhanced immune function in vivo; in fact, this is the first demonstration that CBD enhances pro-inflammatory events induced by lipopolysaccharide (LPS) treatment. While initially unexpected, the results of the current study are consistent with many reports that cannabinoids differentially affect immune function depending on age, cell type, or type and/or magnitude of cellular activation (Pross et al., Citation1992; Nakano et al., Citation1993; Jbilo et al., Citation1999; Jan and Kaminski, Citation2001; Jan et al., Citation2002; Chen et al., Citation2012). With the use of cannabinoid-containing pharmaceuticals expanding beyond that of △9-THC-only preparations, including those containing CBD (Iuvone et al., Citation2009; Sastre-Garriga et al., Citation2011), it is critical to understand alterations in immune parameters mediated by CBD.
Materials and methods
Mice
Female wild-type C57BL/6 (5–8-weeks old at arrival) were obtained from Charles River (Portage, MI). Mice were housed at no more than five per cage in polycarbonate boxes and were given food (Teklad 8640 rodent chow; Harlan Teklad, Madison, WI) and water ad libitum. Rooms were maintained at 21–24°C and 40–60% humidity, with a 12-h light/dark cycle. All procedures were performed in accordance with guidelines set forth by the Institutional Animal Care and Use Committee at Michigan State University.
LPS and drug administration
For intranasal instillation, mice were ansesthetized with 4% Isoflurane plus 96% oxygen and received either saline (Sal) or LPS (Escherichia coli 055:B5, Cat. #L2880, lot 066K4096; Sigma, St. Louis, MO). LPS (10, 25, or 50 µg/mouse) was delivered at 15 µl/nostril. For the CBD co-treatment studies, mice received 75 mg/kg CBD in corn oil (CO) by oral gavage (delivered 0.1 ml/10 g body weight) or CO (vehicle) for 3 days. On Day 3, ~ 1 h before the last dose of CBD, mice also received either Sal or LPS (10 µg/mouse) as described above. Mice were then euthanized 2–48 h post-LPS administration.
Necropsy, tissue collection, and preparation
Mice were anesthetized with 0.1 ml 12% pentobarbital (Fatal Plus, Vortech Pharmaceuticals, Dearborn, MI), then euthanized via exsanguination of the abdominal aorta. Blood was collected into serum separator tubes (BD Medical, Franklin Lakes, NJ), stored on ice, and centrifuged at 3500 rpm for 15 min. The trachea was cannulated, and the heart/thymus/lung removed en bloc. The lungs were lavaged with two volumes of 0.9 ml Sal and pooled to collect bronchoalveolar lavage fluid (BALF). Cells in BALF were enumerated with a hemacytometer and a cytospin sample was prepared using a Shandon 3 Cytospin (Thermo Fisher Scientific, Waltham, MA) at 600 rpm for 10 min. Following BALF collection, the four right lung lobes were ligated, removed, and placed in RNA-Later (Qiagen, Valencia, CA) for subsequent RNA extraction and analysis. The left lung lobe was inflated intratracheally with 10% neutral buffered formalin (NBF) at a constant pressure of 30 cm. After at least 1 h, the left lung was ligated and stored in 10% NBF for subsequent histopathologic examination.
Determination of BALF cellularity
Slides were stained using the Diff-Quick Stain Set (Dade Behring, Newark, DE). A minimum of 150 total cells was enumerated across several fields of view, and the percentages of monocytes (macrophages and monocytes), lymphocytes, eosinophils, or neutrophils were determined. The concentration (cells/ml) of each cell type was calculated using the total cell hemacytometer count.
Preparation of lung sections for histopathological examination
Lungs were micro-dissected along the main axial airway and sectioned at airway generation 5 (G5, proximal) and 11 (G11, distal) as previously described (Farraj et al., Citation2003). All tissue sections were embedded in paraffin and stained with hematoxylin and eosin (H&E) for routine histopathogical examination, and a monoclonal rat anti-neutrophil antibody (NIMP-R14; Santa Cruz Biotechnology, Santa Cruz, CA) with immunoreactivity to mouse neutrophils.
RNA extraction and analysis
Right lung lobes (that had been stored in RNA Later) were weighed and placed in RLT buffer (Qiagen) containing 1% 2-mercaptoethanol (600 µl buffer for every 30 mg tissue). The lungs were then homogenized using a Pro 250 tissue homogenizer (Pro Scientific, Oxford, CT), centrifuged at 12,000 x g for 4 min, and stored at −80°C. RNA extraction was performed using the RNeasy RNA Isolation Kit from Qiagen according to the manufacturer’s instructions. All samples were treated with DNase using the RNase-free DNase Set (Qiagen) during the total RNA isolation. RNA was converted to cDNA using the High Capacity cDNA Archive Kit (Applied Biosystems, Foster City, CA). Quantitative real time polymerase chain reaction (QRT-PCR) was performed using a 7900 HT Thermocycler (Applied Biosystems). All Taqman primers and probe pairs were purchased from Applied Biosystems.
LC/MS/MS
Blood was collected from the abdominal aorta and serum was isolated using serum separator tubes (BD Medical). Serum was extracted with methanol (30 µl serum sample + 270 methanol) and centrifuged at 10,000 x g for 10 min at RT. Supernatants were analyzed by ultra-performance liquid chromatography/mass spectrometry/mass spectrometry (UPLC/MS/MS) using an Acquity UPLC/Quattro Premiere tandem quadrapole MS (Waters Corp., Millford, MA) at the Michigan State University Mass Spectrometry Facility. A standard curve with CBD prepared in methanol (at 6.3–630 ng/ml) was used to quantify the samples. When serum volumes allowed, serum samples were spiked prior to methanol extraction with either 31.5 or 315 ng/ml, and the percent (%) recoveries were calculated.
Statistical analysis
The mean ± SE was determined for each treatment group. Differences between means were determined with a 2-way analysis of variance. When significant differences were detected, treatment groups within time points were compared to the LPS plus CO control using Bonferroni’s test. For QRT-PCR data, Grubb’s outlier test was performed for each treatment group using Delta Ct (Ct target gene – Ct 18S). In addition, fold change values were transformed using natural log (fold change +1) prior to statistical analysis. Statistical analyses were performed using GraphPad Prism version 4.0a for Macintosh OSX (GraphPad software, San Diego, CA).
Results
Effect of intranasal LPS on inflammatory cells in the BALF
In order to verify that intranasally-instilled LPS induced pulmonary inflammation in C57BL/6 mice, time course and dose response experiments with LPS were conducted, and the magnitude of the inflammatory response in the lung was quantified using inflammatory cell infiltration in the BALF. LPS induced an increase in BALF inflammatory cells, which were predominantly neutrophils ( and ). Monocyte counts were not significantly altered with LPS treatment ( and ). Lymphocyte counts were negligible (≤ 104 cells/ml) and eosinophils were undetected in all studies (data not shown). LPS (at 50 µg/mouse) induced robust neutrophilia as early as 6 h post-LPS, which persisted through 48 h. Histological examination demonstrated that, despite persistent neutrophils in the BALF, inflammation in the parenchyma had begun to resolve by 48 h (data not shown), indicating that peak pulmonary inflammation occurred within 24 h. Since a relatively high dose of LPS (50 µg/mouse) was used to induce an inflammatory response in the time course study, a dose response with LPS was then conducted in order to determine the minimal dose requirement for LPS to induce an inflammatory response at 6 and 24 h post-LPS. At 6 h, there was relatively modest induction of neutrophilia with 10 and 25 µg LPS/mouse, with higher neutrophilia at 50 µg LPS/mouse (). By 24 h, all doses of LPS induced more robust neutrophilia than at 6 h. Based on the LPS time course and dose response, 10 µg LPS/mouse for 6 and 24 h were the conditions used to assess the effects of CBD on LPS-induced pulmonary inflammation.
Figure 1. Characterization of LPS-induced pulmonary inflammation. LPS or saline (Sal) was instilled intranasally and inflammatory cell infiltration in the BALF was assessed. Values shown are mean cells/ml ± SE (≥ 6 animals/group). (A) Neutrophil and (B) monocyte counts over time in response to 50 µg LPS/mouse. (C) Neutrophil and (D) monocyte counts in response to various doses of LPS at 6 and 24 h post-instillation. NA, naive (untreated); Sal, 2 h post-Sal. *p < 0.05 as compared to Sal.
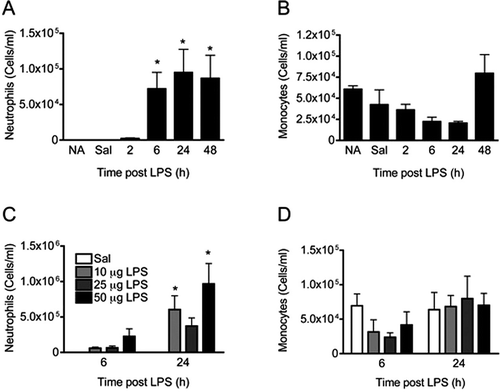
Effect of CBD on LPS-induced inflammatory cells in the BALF
Based on previous observations with CBD in vivo in which CBD possessed anti-inflammatory actions in response to LPS (Carrier et al., Citation2006; El-Remessy et al., Citation2008; Ribeiro et al., Citation2012), we hypothesized that CBD administered by oral gavage would also suppress LPS-induced pulmonary inflammation. However, CBD modestly enhanced the number of inflammatory cells at both 6 and 24 h post-LPS. The inflammatory cell infiltrate was predominantly due to neutrophils, particularly at the 6 h timepoint (). LPS treatment alone at 6 h post-LPS resulted in a modest decrease in monocyte counts, but CBD enhanced the presence of monocytes at 24 h post-LPS ().
Figure 2. Effect of CBD on LPS-induced pulmonary inflammatory cell infiltration. Mice were administered CBD (75 mg/kg/day) in corn oil by oral gavage for 3 days. On the third day, ~ 1 h before the last dose of CBD, mice received either Sal or LPS (10 µg/mouse) intranasally. Values shown are mean cells/ml ± SE (≥ 6 animals/group). Results are representative of two separate experiments. * p < 0.05 as compared to LPS/Oil.
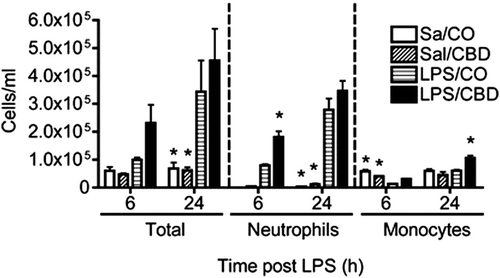
Descriptive pulmonary histopathology
Intranasal treatment with LPS induced a mild-to-moderate acute centriacinar alveolitis, consisting of a mixed inflammatory cell infiltrate of predominately neutrophils and lesser numbers of mononuclear cells (monocytes/macrophages and lymphocytes; ). Interstitial edema and mixed inflammatory cell accumulation was also present in the perivascular and peribronchiolar interstitium of centriacinar regions of the lung. Treatment with CBD alone had no histologic effect on the lungs (), but CBD significantly enhanced the distribution and severity of the LPS-induced pulmonary lesions (). Specifically the neutrophilic alveolitis was more prominent in centriacinar regions of these co-treated mice, with increased accumulation of neutrophils predominantly in the airspaces of the proximal alveolar ducts, along with thickening of associated alveolar septa due to Type II epithelial cell proliferation ().
Figure 3. Effect of CBD on LPS-induced acute pulmonary centriacinar inflammation. Mice were treated as outlined in the legend for . Left lung lobes (6 h post-LPS) were fixed and sections were stained with NIMP-R14 anti-neutrophil antibody and counter-stained with hematoxylin. (A) Sal/Oil; (B) LPS/Oil; (C) Sal/CBD; (D) LPS/CBD. Arrows depict positive staining for neutrophils in the centriacinar region of the lung. a, alveoli; ad, alveolar duct; tb, terminal bronchiole. Neutrophilic inflammation is present in (B) and (D), with enhanced neutrophilic influx in the proximal alveolar ducts and associated alveoli in D compared to B.
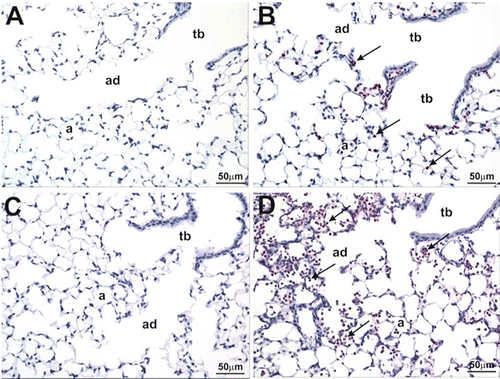
Figure 4. Effect of CBD on type II epithelial cell proliferation. Mice were treated as outlined in the legend for . Left lung lobes (24 h post-LPS) were fixed and sections were stained with hematoxylin and eosin. (a) Sal/CBD; (b) LPS/CBD. Dashed arrow depicts neutrophils; arrow depicts type II epithelial cell proliferation. a, alveoli; ad, alveolar duct.
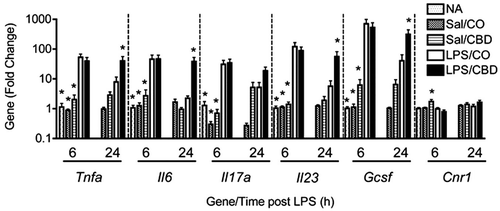
Effect of CBD on LPS-induced cytokine RNA in the lungs
With the demonstration that CBD enhanced LPS-induced pulmonary inflammation, the effect of CBD on LPS-induced inflammatory gene mRNA from the lungs was evaluated. LPS robustly induced mRNA expression of tumor necrosis factor-α (Tnfα), interleukins (IL)-6, -17A, and -23 (Il6, Il17a, and Il23) and granulocyte colony stimulating factor (Gcsf) at 6 h (), but there was no effect of CBD on LPS-induced pro-inflammatory gene expression at 6 h. At 24 h post-LPS, the induction of Tnfα, Il6, Il17a, Il23, and Gcsf was significantly lower as compared to 6 h, and CBD significantly enhanced the mRNA expression of all the above genes, except Il17a. Since the mRNA expression profile in response to LPS and/or CBD was remarkably similar for all inflammatory genes assessed, gene expression for cannabinoid receptor 1 (Cnr1) was determined (). Neither LPS nor CBD affected Cnr1 gene expression, providing evidence that the effects observed by LPS and/or CBD were specific for inflammatory genes, as opposed to global gene expression changes.
Figure 5. Effect of CBD on LPS-induced pro-inflammatory cytokines. Mice were treated as outlined in the legend for . Total RNA was isolated from right lung lobes and QRT-PCR was performed. Bars represent fold-change relative to naive (NA) group at 6 h (NA; not analyzed at 24-h timepoint). Results are representative from two separate experiments. * p < 0.05 as compared to LPS/Oil at each respective timepoint.
Determination of serum CBD levels
Since the effects of CBD enhancing LPS-induced pulmonary inflammation were unexpected, we assessed serum levels of CBD using LC/MS/MS to ensure absorption occurred following oral administration. As seen in , at 6 h post-saline or LPS (corresponding to 5 h post-CBD), serum CBD levels were ~ 40 ng/ml, regardless of LPS co-administration. These levels dropped below the limits of detection 24 h post-Sal or LPS (corresponding to 23 h post-CBD), indicating that the parent compound was completely metabolized and/or excreted by 23 h post-oral administration. For samples with adequate serum volume, CBD spikes were done prior to extraction in order to evaluate the rigor of the extraction procedure. There was higher variability with the low spike as compared to the high spike, which is likely due to both higher variability at the low end of the curve and the relatively low number of samples available for analysis. As a comparison, serum concentrations of the psychoactive cannabinoid △9-THC have been made in separate studies following oral gavage dosing for 3 days. In one study, the mean (± SE) △9-THC serum concentration was 56.9 (± 8.6) ng/ml (n = 7 mice) at 1.5 h following the last dose on Day 3; in another study, the mean (± SE) △9-THC serum concentration was 13.4 (± 3.1) ng/ml (n = 2 mice) at 6 h following the last dose on Day 3. The △9-THC dose in both studies was 75 mg/kg/day, similar to the present CBD study.
Table 1. Serum levels of CBD.
Discussion
In the present studies, CBD exhibited an unexpected enhancement of LPS-induced pulmonary inflammation as measured by inflammatory cell infiltrate into the BALF, histopathology, and expression of pro-inflammatory gene expression in the lung. This study is in contrast to several other studies in which CBD was anti-inflammatory (Berdyshev et al., Citation1998; Carrier et al., Citation2006; El-Remessy et al., Citation2008; Ribeiro et al., Citation2012); in particular, there have been a few studies in which CBD suppressed pulmonary inflammation in response to LPS (Berdyshev et al., Citation1998; Ribeiro et al., Citation2012). A major difference between the present study and others was that CBD was delivered by oral gavage in the present study as opposed to intranasal instillation (Berdyshev et al., Citation1998) or intraperitoneal injection (Ribeiro et al., Citation2012). Oral administration is the second most relevant administration route following inhalation, since marijuana is often consumed in foods or teas, and the CBD/△9-THC therapeutic Sativex® is delivered via oromucosal spray (Sastre-Garriga et al., Citation2011). It is likely that CBD bioavailability to the lung via intranasal or intraperitoneal administration would be higher due to extensive first-pass metabolism following oral gavage. Indeed, although CBD was detected in serum at ~ 5 h post-CBD administration, it was undetectable by 24 h (). Together, these data suggest that the differential effect of CBD on LPS-mediated inflammation might be a result of differences in tissue dose or due to metabolite(s).
The doses of CBD and LPS in this study differ from other reports. For instance, in the study by Ribeiro et al. (Citation2012), the LPS dose was 2 µg/mouse, assuming a 20-g mouse. This is 5-times lower than the LPS dose administered in the present study with CBD (10 µg LPS/mouse). However, ambient LPS exposures to humans in air or house dust can be significant, and even higher for those involved in animal handling (up to 1000 ng LPS/mg organic dust) (Simpson et al., Citation1999; Harkema and Wagner, Citation2005). The CBD dose of 75 mg/kg was based on previous studies in which similar doses of either △9-THC or CBD suppressed humoral immune function (Kaplan et al., Citation2008; Springs et al., Citation2008), and at least for the △9-THC studies, resulted in △9-THC serum concentrations that are consistent with humans following marijuana smoking (Azorlosa et al., Citation1992). It was reported that chronic CBD treatment of up to 1500 mg/day (~ 21 mg/kg dose based on 70-kg human) is well-tolerated in humans (Bergamaschi et al., Citation2011); therefore, a dose of 75 mg/kg for 3 days is reasonable. Blood CBD concentrations have not been reported as often as △9-THC, likely because CBD contributes little to the psychoactive effects of marijuana (Long et al., Citation2010). A few studies have reported CBD concentrations ranging from 5–70 ng/ml following CBD, marijuana, or marijuana extract administration (Agurell et al., Citation1981; Ohlsson et al., Citation1986; Nadulski et al., Citation2005; Karschner et al., Citation2011). However, it is best to compare the present study in which oral CBD was administered to those studies using oral CBD-only preparations, as opposed to marijuana or extracts. Thus, following administration of 40 mg CBD in cookies (resulting in ~ 0.6 mg/kg dose based on 70-kg human), CBD concentrations were up to 11 ng/ml at 1-h post-administration (Agurell et al., Citation1981). In another study, oral administration of 600 mg CBD (~ 9 mg/kg), CBD concentrations were 20 ng/ml at 2-h post-administration (Bhattacharyya et al., Citation2009). Overall, these results demonstrate that the LPS and CBD doses used in the present study are appropriate.
There are several mechanisms by which CBD could potentially enhance LPS-induced pulmonary inflammation. First, and as suggested above, a metabolite of CBD could exhibit pro-inflammatory effects. A distinct but related mechanism involves the observation that CBD inhibits various metabolizing enzymes, such as cytochrome P450 isozymes (Bornheim et al., Citation1993) or fatty acid amide hydrolase (FAAH) (de Petrocellis et al., Citation2011). Interestingly, both cytochrome P450 and FAAH metabolize anandamide (Snider et al., Citation2010), which has been shown to exacerbate inflammation under some conditions (Molina-Holgado et al., Citation1998; Altinsoy et al., Citation2011). Second, despite the fact that CBD is a plant-derived cannabinoid, it does not exhibit high affinity for the CB1 or CB2 receptors (Matsuda et al., Citation1990; Munro et al., Citation1993), suggesting the involvement of other receptors. Several studies have shown that the myriad effects of CBD can be mediated via 5-HT1A (Rock et al., Citation2012), adenosine A2A (Ribeiro et al., Citation2012), α3 glycine (Xiong et al., Citation2012), PPARγ (Esposito et al., Citation2011), or TRP cation channels (de Petrocellis et al., Citation2011; Hegde et al., Citation2011). Additional studies are required to investigate if these mechanisms are involved in the pro-inflammatory effects of CBD.
Another possible mechanism by which CBD might exhibit differential inflammatory effects is via modulation of the transcription factor, NFAT. We have recently identified that CBD differentially affects T-cell cytokine production depending, at least in part, on the magnitude of cellular activation (Chen et al., Citation2012). Specifically, in response to optimal stimulation in which IL-2 or IFNγ were robustly produced, CBD suppressed cytokine production, but in response to sub-optimal stimulation in which IL-2 or IFNγ were modestly produced (or not at all), CBD enhanced cytokine production. The differential effect of CBD on cytokine production was also observed at the mRNA level, and correlated with activation of NFAT (Chen et al., Citation2012), which is critical for both Il2 and Ifng production (Macian, Citation2005). Interestingly, several of the pro-inflammatory cytokines enhanced by CBD in the present study are also regulated in part by NFAT, including Il12p40, a gene that encodes a heterodimer partner comprising IL-23 (Zhu et al., Citation2003) and Tnfα (Klein et al., Citation2006; Lawrence et al., Citation2011). Thus, perhaps the magnitude of the immune response induced by LPS in this study was sub-optimal, resulting in enhanced pro-inflammatory cytokine production and inflammation by CBD co-administration. This is an intriguing possibility given that the lowest LPS dose tested was used in the CBD studies and that the C57BL/6 mouse is a relatively resistant strain to LPS-induced inflammation (Alm et al., Citation2010a, b).
The significance of this study is that CBD-containing therapeutics are being developed for various indications, including spasticity and neuropathic pain associated with multiple sclerosis or cancer (Iuvone et al., Citation2009). A major benefit to developing CBD as a therapeutic is that it lacks many of the psychoactive side effects commonly associated with marijuana or other cannabinoid pharmaceuticals. Although CBD is generally well tolerated (Serpell et al., Citation2012), the results suggest that CBD could exacerbate inflammatory conditions under some conditions. Thus, additional studies examining CBD in various disease states are warranted.
Conclusions
CBD is generally anti-inflammatory and immune suppressive, but, under certain conditions, enhances cytokine production (Chen et al., Citation2012), and, as shown here, exacerbates inflammation. While all of the mechanisms for the differential effects of CBD are not yet understood, the cumulative effect of CBD in vivo likely involves the parent compound, metabolites, inhibition of certain metabolizing enzymes, and inhibition of NFAT activity. These studies provide further evidence that cannabinoids should be considered immune modulatory, rather than strictly immune suppressive.
Acknowledgments
The authors acknowledge funding from the Michigan State University Respiratory Research Initiative (to B.L.F.K., J.G.W. and J.R.H.) and NIH DA07908 (to N.E.K.). The authors would also like to thank Mrs Lori Bramble and Mr Robert Crawford for excellent technical assistance, and Dr A. Daniel Jones and Ms Lijun Chen at the Michigan State University Mass Spectrometry Facility. Finally, the authors thank Ms Kimberly Hambleton with assistance in manuscript submission.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Agurell, S., Carlsson, S., Lindgren, J. E., Ohlsson, A., Gillespie, H., Hollister, L. 1981. Interactions of △1-tetrahydrocannabinol with cannabinol and cannabidiol following oral administration in man. Assay of cannabinol and cannabidiol by mass fragmentography. Experientia 37:1090–1092.
- Alm, A. S., Li, K., Chen, H., Wang, D., Andersson, R., Wang, X. 2010a. Variation of lipo-polysaccharide-induced acute lung injury in eight strains of mice. Respir. Physiol. Neurobiol. 171:157–164.
- Alm, A. S., Li, K., Yang, D., Andersson, R., Lu, Y., Wang, X. 2010b. Varying susceptibility of pulmonary cytokine production to lipopolysaccharide in mice. Cytokine 49:256–263.
- Altinsoy, A., Dilekoz, E., Kul, O., Ilhan, S. O., Tunccan, O. G., Seven, I., Bagriacik, E. U., Sarioglu, Y., Or, M., Ercan, Z. S. 2011. A cannabinoid ligand, anandamide, exacerbates endotoxin-induced uveitis in rabbits. J. Ocular Pharmacol. Ther. 27:545–552.
- Azorlosa, J. L., Heishman, S. J., Stitzer, M. L., Mahaffey, J. M. 1992. Marijuana smoking: Effect of varying △9-tetrahydrocannabinol content and number of puffs. J. Pharmacol. Exp. Ther. 261:114–122.
- Berdyshev, E., Boichot, E., Corbel, M., Germain, N., Lagente, V. 1998. Effects of cannabinoid receptor ligands on LPS-induced pulmonary inflammation in mice. Life Sci. 63:125–129.
- Bergamaschi, M. M., Queiroz, R. H., Zuardi, A. W., Crippa, J. A. 2011. Safety and side effects of cannabidiol, a Cannabis sativa constituent. Curr. Drug Safety 6:237–249.
- Bhattacharyya, S., Fusar-Poli, P., Borgwardt, S., Martin-Santos, R., Nosarti, C., O’carroll, C., Allen, P., Seal, M. L., Fletcher, P. C., Crippa, J. A., Giampietro, V., Mechelli, A., Atakan, Z., Mcguire, P. 2009. Modulation of mediotemporal and ventrostriatal function in humans by △9-tetrahydrocannabinol: A neural basis for the effects of Cannabis sativa on learning and psychosis. Arch. Gen. Psychiatry 66:442–451.
- Bornheim, L. M., Kim, K. Y., Chen, B., Correia, M. A. 1993. The effect of cannabidiol on mouse hepatic microsomal cytochrome P450-dependent anandamide metabolism. Biochem. Biophys. Res. Commun. 197:740–746.
- Carrier, E. J., Auchampach, J. A., Hillard, C. J. 2006. Inhibition of an equilibrative nucleoside transporter by cannabidiol: A mechanism of cannabinoid immunosuppression. Proc. Natl. Acad. Sci. USA 103:7895–7900.
- Chen, W., Kaplan, B. L., Pike, S. T., Topper, L. A., Lichorobiec, N. R., Simmons, S. O., Ramabhadran, R., Kaminski, N. E. 2012. Magnitude of stimulation dictates the cannabinoid-mediated differential T-cell response to HIV gp120. J. Leukocyte Biol. (Aug 16. [Epub ahead of print].
- Costa, B., Colleoni, M., Conti, S., Parolaro, D., Franke, C., Trovato, A. E., Giagnoni, G. 2004. Oral anti-inflammatory activity of cannabidiol, a non-psychaactive constituent of cannabis, in acute carrageenan-induced inflammation in the rat paw. Naunyn Schmied. Arch. Pharmacol. 369:294–299.
- Costa, B., Trovato, A. E., Comelli, F., Giagnoni, G., Colleoni, M. 2007. The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur. J. Pharmacol. 556:75–83.
- de Petrocellis, L., Ligresti, A., Moriello, A. S., Allara, M., Bisogno, T., Petrosino, S., Stott, C. G., Di Marzo, V. 2011. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 163:1479–1494.
- Derocq, J. M., Segui, M., Marchand, J., Le Fur, G., Casellas, P. 1995. Cannabinoids enhance human B-cell growth at low nanomolar concentrations. FEBS Lett. 369:177–182.
- El-Remessy, A. B., Tang, Y., Zhu, G., Matragoon, S., Khalifa, Y., Liu, E. K., Liu, J. Y., Hanson, E., Mian, S., Fatteh, N., Liou, G. I. 2008. Neuroprotective effects of cannabidiol in endotoxin-induced uveitis: Critical role of p38 MAPK activation. Mol. Vis. 14:2190–2203.
- Esposito, G., Scuderi, C., Valenza, M., Togna, G. I., Latina, V., De Filippis, D., Cipriano, M., Carratu, M. R., Iuvone, T., Steardo, L. 2011. Cannabidiol reduces Aβ-induced neuro-inflammation and promotes hippocampal neurogenesis through PPARγ involvement. PloS one 6:e28668.
- Farraj, A. K., Harkema, J. R., Jan, T. R., Kaminski, N. E. 2003. Immune responses in the lung and local lymph node of A/J mice to intranasal sensitization and challenge with adjuvant-free ovalbumin. Toxicol. Pathol. 31:432–447.
- Harkema, J. R., Wagner, J. G. 2005. Epithelial and inflammatory responses in the airways of laboratory rats co-exposed to ozone and biogenic substances: Enhancement of toxicant-induced airway injury. Exp. Toxicol. Pathol. 57 (Suppl 1):129–141.
- Hegde, V. L., Nagarkatti, P. S., Nagarkatti, M. 2011. Role of myeloid-derived suppressor cells in amelioration of experimental autoimmune hepatitis following activation of TRPV1 receptors by cannabidiol. PloS one 6:e18281.
- Iuvone, T., Esposito, G., de Filippis, D., Scuderi, C., Steardo, L. 2009. Cannabidiol: A promising drug for neurodegenerative disorders? CNS Neurosci. Ther. 15:65–75.
- Jan, T. R., Kaminski, N. E. 2001. Role of mitogen-activated protein kinases in the differential regulation of IL-2 by cannabinol. J. Leukocyte Biol. 69:841–849.
- Jan, T. R., Rao, G. K., Kaminski, N. E. 2002. Cannabinol enhancement of interleukin-2 (IL-2) expression by T-cells is associated with an increase in IL-2 distal nuclear factor of activated T-cell activity. Mol. Pharmacol. 61:446–454.
- Jan, T. R., Su, S. T., Wu, H. Y., Liao, M. H. 2007. Suppressive effects of cannabidiol on antigen-specific antibody production and functional activity of splenocytes in ovalbumin-sensitized BALB/c mice. Int. Immunopharmacol. 7:773–780.
- Jbilo, O., Derocq, J. M., Segui, M., Le Fur, G., Casellas, P. 1999. Stimulation of peripheral cannabinoid receptor CB2 induces MCP-1 and IL-8 gene expression in human promyelocytic cell line HL60. FEBS Lett. 448:273–277.
- Kaplan, B. L., Springs, A. E., Kaminski, N. E. 2008. The profile of immune modulation by cannabidiol (CBD) involves deregulation of nuclear factor of activated T-cells (NFAT). Biochem. Pharmacol. 76:726–737.
- Karschner, E. L., Darwin, W. D., Goodwin, R. S., Wright, S., Huestis, M. A. 2011. Plasma cannabinoid pharmacokinetics following controlled oral △9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clin. Chem. 57:66–75.
- Klein, M., Klein-Hessling, S., Palmetshofer, A., Serfling, E., Tertilt, C., Bopp, T., Heib, V., Becker, M., Taube, C., Schild, H., Schmitt, E., Stassen, M. 2006. Specific and redundant roles for NFAT transcription factors in the expression of mast cell-derived cytokines. J. Immunol. 177:6667–6674.
- Lawrence, M. C., Naziruddin, B., Levy, M. F., Jackson, A., McGlynn, K. 2011. Calcineurin/nuclear factor of activated T-cells and MAPK signaling induce TNFα gene expression in pancreatic islet endocrine cells. J. Biol. Chem. 286:1025–1036.
- Long, L. E., Chesworth, R., Huang, X. F., Mcgregor, I. S., Arnold, J. C., Karl, T. 2010. A behavioral comparison of acute and chronic △9-tetrahydrocannabinol and cannabidiol in C57BL/6JArc mice. Int. J. Neuropsychopharmacol. 13:861–876.
- Macian, F. 2005. NFAT proteins: Key regulators of T-cell development and function. Nat. Rev. Immunol. 5:472–484.
- Malfait, A. M., Gallily, R., Sumariwalla, P. F., Malik, A. S., Andreakos, E., Mchoulam, R., Feldmann, M. 2000. The non-psychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc. Natl. Acad. Sci. USA 97:9561–9566.
- Matsuda, L. A., Lolait, S. J., Brownstein, M. J., Young, A. C., Bonner, T. I. 1990. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature (London) 346:561–564.
- Molina-Holgado, F., Molina-Holgado, E., Guaza, C. 1998. The endogenous cannabinoid anandamide potentiates IL-6 production by astrocytes infected with Theiler’s murine enceph-alomyelitis virus by a receptor-mediated pathway. FEBS Lett. 433:139–142.
- Mukhopadhyay, P., Rajesh, M., Horvath, B., Batkai, S., Park, O., Tanchian, G., Gao, R. Y., Patel, V., Wink, D. A., Liaudet, L., Hasko, G., Mechoulam, R., Pacher, P. 2011. Cannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell death. Free Rad. Biol. Med. 50:1368–1381.
- Munro, S., Thomas, K. L., Abu-Shaar, M. 1993. Molecular characterization of a peripheral receptor for cannabinoids. Nature (London) 365:61–65.
- Nadulski, T., Sporkert, F., Schnelle, M., Stadelmann, A. M., Roser, P., Schefter, T., Pragst, F. 2005. Simultaneous and sensitive analysis of THC, 11-OH-THC, THC-COOH, CBD, and CBN by GC-MS in plasma after oral application of small doses of THC and cannabis extract. J. Anal. Toxicol. 29:782–789.
- Nakano, Y., Pross, S., Friedman, H. 1993. Contrasting effect of △9-tetrahydrocannabinol on IL-2 activity in spleen and lymph node cells of mice of different ages. Life Sci. 52:41–51.
- Ohlsson, A., Lindgren, J. E., Andersson, S., Agurell, S., Gillespie, H., Hollister, L. E. 1986. Single-dose kinetics of deuterium-labelled cannabidiol in man after smoking and intravenous administration. Biomed. Environ. Mass Spectrom. 13:77–83.
- Pross, S. H., Nakano, Y., Widen, R., Mchugh, S., Newton, C. A., Klein, T. W., Friedman, H. 1992. Differing effects of △9-tetrahydrocannabinol (THC) on murine spleen cell populations dependent upon stimulators. Int. J. Immunopharmacol. 14:1019–1027.
- Ribeiro, A., Ferraz-De-Paula, V., Pinheiro, M. L., Vitoretti, L. B., Mariano-Souza, D. P., Quinteiro-Filho, W. M., Akamine, A. T., Almeida, V. I., Quevedo, J., Dal-Pizzol, F., Hallak, J. E., Zuardi, A. W., Crippa, J. A., Palermo-Neto, J. 2012. Cannabidiol, a non-psycho-tropic plant-derived cannabinoid, decreases inflammation in a murine model of acute lung injury: Role for the adenosine A2A receptor. Eur. J. Pharmacol. 678:78–85.
- Rock, E. M., Bolognini, D., Limebeer, C. L., Cascio, M. G., Anavi-Goffer, S., Fletcher, P. J., Mechoulam, R., Pertwee, R. G., Parker, L. A. 2012. Cannabidiol, a non-psychotropic component of cannabis, attenuates vomiting and nausea-like behaviour via indirect agonism of 5-HT1A somatodendritic autoreceptors in the dorsal raphe nucleus. Br. J. Pharmacol. 165:2620–2634.
- Sastre-Garriga, J., Vila, C., Clissold, S., Montalban, X. 2011. THC and CBD oromucosal spray (Sativex®) in the management of spasticity associated with multiple sclerosis. Exp. Rev. Neurother. 11:627–637.
- Serpell, M. G., Notcutt, W., Collin, C. 2012. Sativex long-term use: An open-label trial in patients with spasticity due to multiple sclerosis. J. Neurol. (Aug 10. [Epub ahead of print]).
- Simpson, J. C., Niven, R. M., Pickering, C. A., Oldham, L. A., Fletcher, A. M., Francis, H. C. 1999. Comparative personal exposures to organic dusts and endotoxin. Ann. Occup. Hyg. 43:107–115.
- Snider, N. T., Walker, V. J., Hollenberg, P. F. 2010. Oxidation of the endogenous cannabinoid arachidonoyl ethanolamide by the cytochrome P450 monooxygenases: Physiological and pharmacological implications. Pharmacol. Rev. 62:136–154.
- Springs, A. E., Karmaus, P. W., Crawford, R. B., Kaplan, B. L., Kaminski, N. E. 2008. Effects of targeted deletion of cannabinoid receptors CB1 and CB2 on immune competence and sensitivity to immune modulation by △9-tetrahydrocannabinol. J. Leukocyte Biol. 84:1574–1584.
- Srivastava, M. D., Srivastava, B. I., Brouhard, B. 1998. △9-Tetrahydrocannabinol and cannabidiol alter cytokine production by human immune cells. Immunopharmacology 40:179–185.
- Xiong, W., Cui, T., Cheng, K., Yang, F., Chen, S. R., Willenbring, D., Guan, Y., Pan, H. L., Ren, K., Xu, Y., Zhang, L. 2012. Cannabinoids suppress inflammatory and neuropathic pain by targeting alpha3 glycine receptors. J. Exp. Med. 209:1121–1134.
- Zhu, C., Rao, K., Xiong, H., Gagnidze, K., Li, F., Horvath, C., Plevy, S. 2003. Activation of the murine IL-12 p40 promoter by functional interactions between NFAT and ICSBP. J. Biol. Chem. 278:39372–39382.