Abstract
Hepatitis C virus (HCV) infects primarily hepatocytes, leads to development of fibrosis and/or cirrhosis of the liver and is a significant factor for developing hepatocellular carcinoma (HCC). Evidence indicates that liver fibrosis contains uncontrolled inflammation as a part of its etiology. Normal cell-mediated immunity plays a central role in the mechanisms involved in viral clearance/persistence in the liver. In this context, cytokines modulate the immune system and exert direct anti-viral activity. To this end, this study investigated potential associations of serum IL-17 and IL-6 with exacerbation of hepatic damage in chronic HCV patients to determine their utility as prognostic markers for potential development of HCC. Chronic HCV-patients were recruited, divided into groups according to degree of liver damage, i.e. patients with peri-hepatic fibrosis, hepatic cirrhosis, or HCC, and had their blood collected for analysis of liver function and serum IL-6 and IL-17 levels. Interestingly, increases in serum IL-17 levels in the study groups were associated with aggravation of the clinical state from HCV to cirrhosis and then to HCC. Serum IL-6 levels followed a similar pattern. The association of both cytokines with progressive exacerbation of the initial HCV-induced liver damage was further confirmed by correlation analysis that revealed positive correlations between HCV RNA titer and IL-17 (+0.951, p < 0.05) and IL-6 (+0.85, p < 0.05). A receiver operating characteristics (ROC) analysis revealed their beneficial addition as promising biomarkers for a better prognostic profile of HCC. Interestingly, a significant progressive decline in the active vitamin D status was noted in all three clinical states, and these too were associated with progressive liver disease. This study confirms the necessity of adding screening for IL-6 and IL-17 and vitamin D to that of the classic marker AFP for patients with HCV and cirrhosis to hopefully permit clinicians to initiate measures that ultimately might mitigate/delay development of HCC in these infected patients.
Introduction
Hepatocellular carcinoma (HCC) has become the third most common malignancy worldwide, with a very poor prognosis, rendering it the fourth highest cause of cancer-related deaths. Hepatitis C virus (HCV) infects primarily hepatocytes, leads to the development of fibrosis or cirrhosis of the liver, and is a significant risk factor for the development of HCC, in a process termed as ‘inflammation–fibrosis–cancer axis’ deaths. In Africa, HCC is the dominant type of liver cancer (Soliman et al., Citation2010). HCC is the second most frequent cause of cancer incidence and mortality among men in Egypt (Lehman and Wilson, Citation2009). In addition, Egypt has the highest prevalence of HCV worldwide, with >90% of infections due to HCV genotype 4 (HCV-4), the most common variant of HCV in the Middle East and Africa (Elsharkawy and Mann, Citation2007; Kamal and Nasser, Citation2008).
In only 20% of the cases, the virus is spontaneously eliminated, while, in the remaining cases, a state of chronic infection is established. Following HCV exposure, immune system cells are recruited to the liver to aid in the control of viral replication. As a result, inflammatory liver damage is induced, which can then progress towards fibrosis and subsequent cirrhosis. These promotional effects apparently are exerted via hepatocyte regeneration (Bialecki and Di Bisceglie, Citation2005). As the cirrhosis precedes most cases of HCC, it is considered a pre-cancer condition and so necessitates an early detection and continuous screening of patients during follow-up visits.
Due to convenience, cost effectiveness and accuracy, serum tumor markers have been used as an effective and reliable method of detection of malignant tumors supplementary to ultrasonography and computer tomography. Using the appropriate single or combination of tumor markers may improve the effectiveness of screening HCC patients (Zhou et al., Citation2006). Currently, standard surveillance includes a combination of 6-monthly ultrasound scans (USS) and serum α-fetoprotein (AFP) measurement; the latter is considered the ‘gold standard’ for HCC detection. However, this strategy does not reliably detect the disease at its early stages (Hsia et al., Citation2007). Knowing that AFP may also be elevated in benign chronic liver diseases, e.g. chronic viral hepatitis and liver cirrhosis without HCC, it was suggested that the ‘gold standard’ approach lacked sensitivity and specificity (Tai et al., Citation2009). Although recent evidence indicates that the fucosilated fraction of AFP may be a more useful marker, AFP remains the onco-marker universally utilized and the standard for monitoring high-risk patients for HCC in clinical practice, especially patients with chronic liver disease (Johnson, Citation2001). Based on these uncertainties and conflicting opinions, it is clear that there is a critical need for newer markers, with great accuracy for the diagnosis of early HCC.
Evidence indicates that liver fibrosis incorporates uncontrolled inflammation as a part of its etiology. Kupffer cells that act as resident macrophages in the liver represent initial inflammatory effectors that stimulate a cascade (by releasing pro-inflammatory cytokines) that ultimately leads to tissue remodeling and fibrosis (Steib et al., Citation2007). There is an important role for cytokines and tissue microenvironments in the regulation and propagation of inflammatory responses and the homeostasis of organ functions (Denning et al., Citation2007).
In the liver, cytokines co-ordinate physiologic and pathologic processes such as liver growth and regeneration, as well as inflammatory processes during viral liver disease, liver fibrosis, and cirrhosis. The cell-mediated immune response plays a central role in hepatocellular necrosis and in the immunopathogenic mechanisms involved in viral clearance and persistence in liver disease of viral etiology as HCV chronic liver disease. In this context, cytokines modulate the immune system and exert direct antiviral activity. Specifically, interleukins (IL)-1, -2 and -6, as well as tumor necrosis factor (TNF)-α are all released in increased amounts during acute fulminant viral hepatitis; in this instance, they exert both pro-inflammatory and cytotoxic effects. Thus, T-cell immunoregulatory cytokines may play a key role in influencing the persistence of HCV infection (Falasca et al., Citation2006) and likely as well the progressivity of liver damage up to and including HCC that evolves.
IL-6 is a pleiotropic pro-inflammatory cytokine with both differentiation and growth -promoting effects for target cells. IL-6 regulates the synthesis of a broad spectrum of acute-phase proteins in the liver and plays a role in immune responses that may lead to viral clearance. Moreover, IL-6 serves to block apoptosis, by modulating the transcription of several liver-specific genes during inflammatory processes. Up-regulated expression of the IL-6 gene appears to be involved in pathological conditions, and it has been hypothesized that activation of the IL-6 gene might trigger initial events leading to oncogenic transformation (Lee et al., Citation1998). Recently, IL-17 and IL-17 producing cells (TH17) have been identified as a key inflammatory cytokine involved in a number of autoimmune diseases including rheumatoid arthritis, experimental auto-immune encephalomyelitis (EAE), and colitis (Yen et al., Citation2006; Weaver et al., Citation2007).
Korn et al. (Citation2009) identified IL-6 and transforming growth factor (TGF)-β as differentiation factors for TH17 cells, and that both cytokines together induce the release of large amounts of IL-17 from naïve T-cells. It is now known that IL-6 is critical for the induction of TH17 lineage commitment, and so the production of IL-17. Indeed, other cytokines including IL-2, -15, -18, and -21 can also stimulate IL-17 production from activated human T-cells and peripheral blood mononuclear cells (PBMC). IL-17-secreting TH17 cells play a protective role in certain bacterial infections, but they are primarily mediators of inflammation. Few studies have looked into the role of TH17 cells in liver diseases; of these, there are reports of an elevated presence in patients with acute hepatic injury (Yasumi et al., Citation2007), in infection-induced hepatic granulomas (Heninger et al., Citation2006; Rutitzky et al., Citation2008), and in animal models of hepatic ischemia-reperfusion (Caldwell et al., Citation2005). A plethora of studies has investigated the association of circulating levels of IL-17 with individual liver diseases, including hepatitis B (Wang et al., Citation2011; Zhang et al., Citation2010), alcoholic hepatitis (Lemmers et al., Citation2009), autoimmune hepatitis (Zhao et al., Citation2011), and HCC (Wang et al., Citation2010; Zhang et al., Citation2009). Nevertheless, the role of TH17 cells in the progression of HCV to cirrhosis and then to HCC remains unclear. To this end, this study sought to investigate the potential association between serum IL-17 levels and the exacerbation of hepatic damage in chronic HCV patients. It was hoped that the results might bolster the case for assessing IL-17 levels as a prognostic marker for the onset of HCC.
Materials and methods
Subjects
Prior to initiation, this study received approval by the Ethical Committee of the Faculty of Medicine at Cairo University. The study enrolled 200 Egyptian patients from the Hepatology Outpatient Clinic at the Endemic Disease Hospital at Cairo University. The average age was 30–65 years, including both genders. Informed consent was obtained from all patients and controls. Inclusion criteria were based on a thorough history-taking and a clinical and pathological examination. Patients were considered eligible if they suffered from HCV-related chronic liver disease for a minimum of 7 years (as new patients or under follow-up). Patients with HBV or co-infection with HBV and HIV were excluded. All included subjects underwent a complete medical and laboratory evaluation including tests for liver function, the presence of HCV antibodies and AFP level complementary to abdominal ultrasonography. Abdominal ultrasonography was performed on all patients.
According to the virologic and biochemical results, patients were classified into three sub-groups, each containing 50 patients. The groups were: HCV-patients with peri-hepatic fibrosis; patients with hepatic cirrhosis; and patients with HCC. A control group was composed of 50 healthy volunteers who presented with no active disease or medical disorders.
Blood sampling and biochemical analyses
Venous blood samples were obtained (after overnight fasting) from all patients/controls. Samples were allowed to clot and sera were then separated by centrifugation (3500 rpm, 20 min, 25 °C) and divided into two aliquots: one part was used for biochemical laboratory tests and the remaining was stored at −20 °C for later determinations of vitamin D metabolites (25-hydroxy-vitamin D and 1,25-dihydroxy-vitamin D) and cytokine (IL-6 and -17) levels.
25-Hydroxy-vitamin D determinations were done using a commercial kit purchased from Medgenix Diagnostics (Fleurus, Belgium) (Mawer, Citation1980). 1,25-dihydroxy-vitamin D measures were carried out using a kit purchased from Incstar Corporation (Stillwater, MN) (Hollis, Citation1986). Liver function tests (e.g., serum bilirubin, albumin. ALP, GGT, ALT, AST, cholesterol, and PT) were determined using a Synchron CX5 autoanalyzer (Beckman Coulter Instruments, Brea, CA). AFP was analyzed using an Elecsys 2010 auto-analyzer system (Roche Diagnostics, Basel, Switzerland). IL-6 and IL-17 levels were determined by enzyme immunoassay (Biosource International, Grand Island, NY). Sensitivities of the IL-6 and IL-17 kits were 1.5 and 5 pg/ml, respectively.
The diagnosis of HCV infection was confirmed by measures of serum HCV-RNA titer using a quantitative RT-Polymerase chain reaction (RT-PCR) and TaqMan technology (Scott and Gretch, Citation2007). Only HCV genotype IV-infected subjects were included in the study. Typically, an RT-PCR assay has a limit of quantification (LOQ) of 25 IU/ml and a limit of detection (LOD) of 10–15 IU/ml; in the assays used here for HCV-RNA testing, the LOQ was 24 IU/ml and the LOD 12 IU/ml.
Statistical analysis
All data were expressed as mean ± SD. All analyses utilized SPSS 15.0 statistical package for Windows (SPSS Inc., Chicago, IL). A one-way analysis of variance (ANOVA) was employed for comparisons of means of the different groups. A p-value <0.05 was accepted as statistically significant. Correlation analyses were done using Pearson’s correlation. A receiver operating characteristics (ROC) analysis was used to evaluate overall predictive value and cut-off level of the test.
Results
illustrates the deterioration of liver enzymatic function tests with progressively worse pathologic states of the livers of the patients. These trends are expressed through the significant increases in AST, ALT, and ALP activities in all patients, but to the greatest extent in those with HCV. Interestingly, values for these parameters were decreased significantly in the cirrhotic and the HCC patients relative to those with the HCV only (but did not significantly differ from one another), reflecting a loss of hepatocyte functions. Only GGT levels were significantly and progressively increased across the disease states.
Table 1. Laboratory data of patients with HCV, liver cirrhosis, and HCC.
Reflecting the deteriorated state of the hepatic enzymes’ functions, the synthetic functions that were assayed also displayed a similar pattern. The levels of both serum albumin and cholesterol were significantly decreased, and both bilirubin and PT increased in a manner that trended to parallel the disease states (i.e. changes from control levels were maximal in the HCC and cirrhotic patients). Lastly, each of these disease states also was reflected by significant decrements in serum vitamin D levels (measured as 25-hydroxy-vitamin D and 1,25-dihydroxy-vitamin D). Of all the subjects, the HCC patients had the maximal decrease in levels of these vitamin forms.
Interestingly, serum IL-17 levels increased in the study groups; the increases appeared to be associated with the progression of the pathologic state. Specifically, the values went from 6.4 (± 1.7; mean ng/ml [± SD]) in the HCV subjects, to 92.4 (±28.8) in those with cirrhosis, and then to 162.9 (±26) in subjects with HCC; control subjects had a mean IL-17 value of 1.2 ± 0.4 ng/ml (). Like with IL-17, serum IL-6 levels followed a similar pattern. In this case, the values went from 99.6 (±9.4; mean ng/ml [±SD]) in the HCV subjects, to 155.7 (±27.8) in those with cirrhosis, and then to 279.6 (±32.2) in subjects with HCC; control subjects had a mean IL-6 value of 68.3 ± 7.8 ng/ml (). For both cytokines, the levels associated with each pathologic state were significantly greater compared to the control and then to one another.
Table 2. Serum HCV-RNA titre and levels of IL-6, IL-17, and AFP in patients with HCV, liver cirrhosis, or HCC.
The association of both cytokines to the progressive exacerbation of HCV-induced liver damage was further confirmed by correlational analysis () that revealed a positive correlation of HCV RNA titer with IL-17 (+0.951, p < 0.05; ) () and IL-6 (+0.850, p < 0.05). In contrast, there was a negative correlation of vitamin D and active vitamin D with IL-17 (−0.67 and −0.76, respectively, p < 0.05) and IL-16 (−0.68 and −0.81, respectively, p < 0.05) (). These correlations aligned with progressive changes in other parameters, such as AFP levels and HCV RNA titer, that, when taken together, serve as prognostic markers of cirrhosis and HCC ().
Figure 1. Correlation between serum IL-17 levels and HCV titer. The data shows there is a positive correlation between IL-17 and HCV titers (r = +0.95).
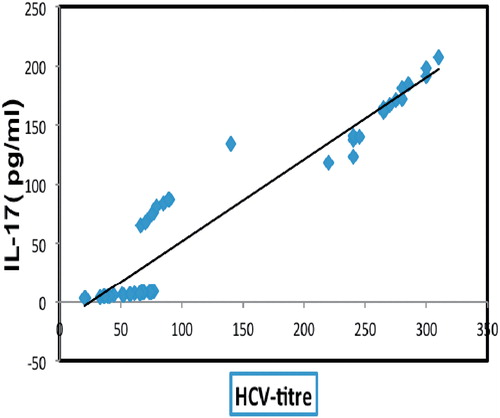
Table 3. Correlational analyses between IL-17 levels and classic hepatocellular markers among the studied groups.
The ROC analyses revealed 100% sensitivity and 92% specificity for predictive validity of IL-6 levels for cirrhotic vs HCC patients, with a best cut-off value of 216 ng/ml ( and ). The ROC analyses also revealed 100% sensitivity and 86% specificity for the predictive validity of IL-17 for cirrhotic vs HCC patients, with a best cut-off value of 108 ng/ml. While AFP displayed similar results to IL-17, IL-6 showed the better specificity/sensitivity combination. illustrates the prognostic validity of IL-6 and IL-17 compared to AFP in cirrhotic vs HCC patients. The prognostic validity of IL-17 and IL-6 compared to AFP in cirrhotic and HCV- infected patients is reported in ; in this case, the best cut-off values were 37.1 ng IL-17/ml, 122 ng IL-6/ml and 2719 for AFP.
Figure 2. ROC curve illustrating the sensitivity and specificity of the IL-6, IL-17, AFP values, and HCV titers in HCC-bearing and cirrhotic patients.
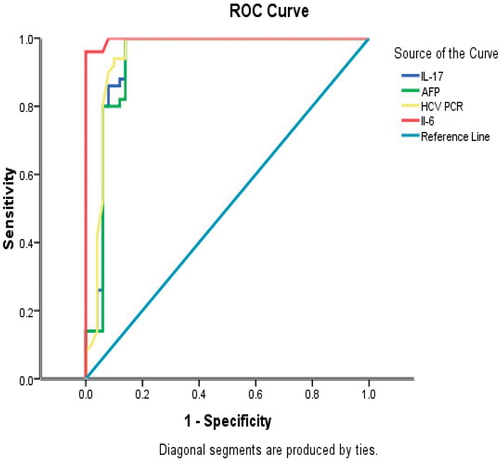
Table 4. Prognostic validity of IL-6, IL-17, AFP, and HCV titers (by PCR) in differentiating between cirrhotic and HCC states.
Table 5. Prognostic validity of IL-6, IL-17, AFP, and HCV titers (by PCR) in differentiating between cirrhotic vs HCV-infected states.
Discussion
Progressive hepatic fibrosis is part of a common pathway noted with most chronic liver injuries, caused primarily by HBV or HCV. This pathway leads to cirrhosis and an increased risk of liver failure and/or hepatocellular carcinoma (HCC) formation. More than 70% of acute HCV infections become chronic; 20% of chronic hepatitis C (CHC) patients may develop liver cirrhosis within 20 years, with or without hepatic de-compensation or onset of HCC. Evidence indicates that liver fibrosis incorporates an uncontrolled inflammation as a part of its etiology. The complex multi-cellular processes/functions that are involved in the progression from hepatic inflammation, to fibrosis, and ultimately to the development of HCC, has been termed the ‘inflammation-fibrosis-cancer axis’ (Elsharkawy and Mann, Citation2007).
In the present study, we measured serum cytokine levels (IL-6 and IL-17) and AFP in HCV-infected patients with progressive levels of liver damage, i.e., pre-hepatic fibrosis, cirrhosis, and HCC. All outcomes were compared to those measured in healthy controls in order to evaluate the potential prognostic value of these markers. In most solid malignancies, tumor stage at presentation determines prognosis and the therapeutic strategy. However, most patients with HCC have a concomitant disease (such as HCV-induced liver cirrhosis) and complex interactions exist between the two, creating implications for prognoses and treatment choices. In this study, we report significant progressive elevations in circulating IL-6 and IL-17 levels that were concomitant with the progression of hepatic exacerbation from HCV-induced liver fibrosis to cirrhosis and ultimately to HCC. Such findings are in alignment with escalating levels of viral load and AFP as well. These promising findings may open a new horizon for prognostic and diagnostic oncologic markers.
Levels of the classic tumor marker AFP were significantly and progressively increased among the studied groups of HCV-patients. This is in accordance with the findings of Abdel-Wahab et al. (Citation2008), who evaluated AFP during the three stages of liver diseases. Other studies comparing AFP levels in HCC patients vs those in cirrhotic patients revealed similar results (El-Houseini et al., Citation2005; Spadaro et al., 2006). However, there are some reports that have indicated that AFP has low sensitivity and specificity for its high false-positive and false-negative rates and is, therefore, only of limited utility in differentiating HCC from benign hepatic disorders (Zhou et al., Citation2006); these authors noted that patients with acute exacerbation of viral hepatitis but no HCC may also have markedly increased AFP levels. To improve sensitivity and specificity of any clinical detection, other biomarkers have been utilized (in addition to AFP) including measures of α-fucosidase lysosomal enzyme (Giardina et al., Citation1992) and of hepatocyte-produced pro-inflammatory cytokines (such as IL-2, -6, and -8) (Falasca et al., Citation2006). In the current study, AFP—as a differentiating marker between cirrhotic and HCC-bearing patients—had a 100% sensitivity and 86% specificity. This finding led us to test the utility of IL-6 and IL-17 for providing even higher specificity for use in differentiation between disease states.
Evaluation of circulating IL-6 levels in our study groups revealed a progressive increase among the addressed three liver stages (e.g. HCV-induced liver fibrosis, cirrhosis, and HCC) and a positive correlation with the viral load and serum levels of AFP and vitamin D. To the best of our knowledge, this is the first study to compare and evaluate IL-6 levels and prognostic significance across these three particular liver pathologic states. Furthermore, the ROC analysis applied in the current study revealed high sensitivity (100%) and specificity (92%) for the predictive validity of IL-6 in differentiating cirrhotic vs HCC vs HCV-infected health states.
It was previously reported that the activation of the IL-6 gene might trigger certain initial events that lead to oncogenic transformation (Lee et al., Citation1998). It has also been postulated that this activation could affect chronic disease progression and correlate with the stage of liver cirrhosis (Porta et al., Citation2008). Falasca et al. (Citation2006) documented higher plasma levels of IL-6 in patients with HCV than with HBV; both classes of patients had IL-6 levels greater than those in uninfected control subjects. These authors suggested that, in the course of chronic HCV hepatitis, an increase of pro-inflammatory cytokines is typically more profusely evident and associated with illness duration and HCV-RNA level. Our current findings are in accordance with those of Falasca et al., and indicate an important pathogenic role for humoral immunity during the liver injury that evolves in HCV patients. The changes in IL-6 status noted here, i.e. the increments that mirror the progression of pathology, are also in accordance with findings by Ataseven et al. (Citation2006), who reported significant increases in serum IL-6 levels in HCC patients (as compared to in control subjects), and by Porta et al. (Citation2008) and Soresi et al. (Citation2006), who noted increases in serum IL-6 levels in HCC patients as compared to in cirrhotic patients. Albeit that these findings are consistent across several studies, it remains to be elucidated how HCC cells (especially in advanced stages of the disease) may produce and secrete IL-6 to stimulate their growth.
Predicting a pro-inflammatory paradigm similar to that for IL-6, this study attempted to investigate and then prove an association between increased circulating IL-17 levels and the progressive exacerbation of liver damage in the chronic HCV-induced ‘inflammation–fibrosis–cancer axis’. The studies here revealed that a progressive increase in IL-17 was correlated with elevations in serum viral load, AFP, IL-6, as well as significant decreases in vitamin D and its active metabolite. An association between elevated IL-17 levels and separate types of hepatic insults has been questioned in the following studies, but neither addressed the issue from the vantage of hepatocellular progression from HCV-induced fibrosis, to cirrhosis, to HCC. Yasumi et al. (Citation2007) demonstrated the usefulness of serum IL- 6 and IL-17 levels in evaluating the severity of acute hepatic injury, and emphasized the need to investigate any pathological role for IL-17 in acute hepatitis.
Jimenez-Sousa et al. (Citation2010) showed that HCV infection induced the activation of a broad range of immune mediators participating in both innate and adaptive TH1 and TH17 cytokine and chemokine responses to a virus, along with mediators involved in fibrogenesis. These authors conjectured that increases in IL-17 could reflect T-lymphocyte mobilization and proliferation in response to an HCV infection, and thereby constituted a pivotal, anti-viral response. Zhao et al. (Citation2011) confirmed these findings and further explained that the increased hepatic expressions of IL-6, IL-17, and other cytokines were associated with increased inflammation and fibrosis. IL-17 induces IL-6 expression via AMPK signaling pathways in hepatocytes; the release of IL-6, in turn, may further stimulate TH17 cells, leading to a positive feedback loop. Several previous studies investigated the significance of IL-17 increases in each pathologic liver state separately. Serum IL-17 levels were significantly increased in HCV-patients with or without cirrhosis (Fathy et al., Citation2011). The values were significantly higher in cirrhotic states and correlated well with the severity of the cirrhosis (Jimenez-Sousa et al., Citation2010). In other studies, the comparison was made between HCC and cirrhotic patients; here, the levels in the HCC patients were significantly higher than those in cirrhotic counterparts (El Husseiny et al., Citation2012; Wang et al., Citation2010, Citation2011; Zhang et al., Citation2009). The ROC analyses performed in the current study showed that there was a high sensitivity and specificity for the predictive validity of IL-17 in differentiating between cirrhotic vs HCC states (100% sensitivity and 86% specificity). Knowing that IL-6 displayed a higher specificity (i.e. 92%) than IL-17 for use in these determinations/differentiations should encourage clinicians to include both cytokine (along with the conventional AFP) in their screening profiles for HCC.
Several studies using ROC analysis claimed a prognostic value for a number of interleukins, such as sIL-2R, IL-2, -6, -8, and -18, complementary to AFP in HCC patients. Elnemr et al. (Citation2012) revealed a significant increase in AFP levels in patients with liver disease compared to in controls as well as in HCC vs cirrhotic patients. At a 20 ng/ml cut-off value, the sensitivity and sensitivity of AFP was, respectively, 75 and 90% for differentiating HCC from cirrhosis. Similar findings were noted in Shimizu et al. (Citation2002), Durazo et al. (Citation2008), and Bertino et al. (2011).
Similar to our results, Porta et al. (Citation2008) reported that IL-6 titers were 4-fold higher in HCC vs cirrhotic patients and 25-fold higher than in healthy controls. As for AFP titers, the highest levels were seen in cancer patients. ROC curves analysis demonstrated that IL-6 was significantly more discriminant than AFP, with ‘optimal’ cut-off values of 7.9 pg/ml (sensitivity = 0.83, specificity = 0.83, efficiency = 0.83). The authors concluded that IL-6 could be considered a promising tumor marker for HCC, with a diagnostic value that is significantly increased when combined with measures of AFP.
With regard to the other interleukins noted above, Mohran et al. (Citation2011) concluded that serum IL-18 (complementary to AFP) was a suitable marker for diagnosis of HCV-related HCC, especially in cases with AFP levels below diagnostic value. Mohran et al. reported that the best cut-off value for IL-18 in diagnosis of HCC was 500 pg/ml (84% sensitivity, 86.7% specificity, and an area-under-ROC curve value of 0.675). In parallel, Welling et al. (2012) indicated that there was a significant increase in serum IL-8 levels in HCC vs cirrhotic patients, whereas there were no notable differences in levels of IL-1β, -2, -4, -6, -7, -10, -12, -13, -15, -17, or -21, or of VEGF. IL-8 predicted an HCC presence with an area-under-ROC curve value of 0.68. These authors concluded that HCC patients with the highest IL-8 levels had worse survival rates and recommended assessments of IL-8 during HCC. Chan et al. (Citation2012) reported that high IL-10 levels portended worse overall survival in patients with HCC. Serum IL-10 level was identified as an independent prognostic factor for unresectable HCC.
Interestingly, a significant progressive decline in active vitamin D levels was witnessed in all three clinical states analyzed (compared to in their control counterparts). Further, this outcome was associated with progressive exacerbation of the hepatic disease states. These results indicated that a decrease in vitamin D levels could reflect the severity of hepatocellular injury and so serve as a new hepatic biomarker in progressive liver diseases (El Husseiny et al., Citation2012; Schaalan et al., Citation2012). Even so, these findings are consistent with previous results, indicating that vitamin D inadequacy is common in non-cholestatic chronic liver diseases and correlates with disease severity (Babbs et al., Citation1988; Fisher and Fisher, Citation2007). In other earlier studies, a deficient vitamin D status was linked to severe fibrosis and low sustained virologic responses (SVR) during interferon (IFN)-γ-based therapies (Abu Mouch et al., Citation2010; Petta et al., Citation2010).
Vitamin D is considered a critical regulator of immunity, playing a role in both innate and cell-mediated immune responses (Deluca and Cantorna, Citation2001). Vitamin D suppresses production of T-helper (TH)-1 lymphocyte type cytokines, such as IFNγ and IL-2, and consequently leads to enhanced production of TH2 cytokines like IL-4 and IL-5 that promote humoral immune responses. Vitamin D also enhances innate immunity by directly inducing gene expression of anti-microbial peptides, such as cathelicidin and β-defensin-2, in various cell types (Holick and Chen, Citation2008). Further, vitamin D deficiency was associated with several immune-mediated diseases and increased susceptibility to infection and cancer (Holick and Chen, Citation2008; Lange et al., Citation2009). Recently, vitamin D insufficiency (defined as 25-hydroxy-vitamin D serum level of 20–29 ng/ml) has been proposed as a predictor of failure of treatment of chronic hepatitis C with PEG-IFNα and ribavirin (Abu Mouch et al., Citation2011; Petta et al., Citation2010). Moreover, severe vitamin D deficiency (defined by a 25-hydroxy-vitamin D serum level <20 ng/ml) is a common feature of chronic hepatitis C, even in the absence of advanced liver fibrosis (Lange et al., Citation2011).
Unraveling the association of vitamin D decreases to the upsurge in IL-17 levels in the hepatic diseases studied here revealed that vitamin D exhibits an immunomodulatory effect via activation of T-regulatory (Treg) lymphocytes. Interesting preliminary data indicate that 1,25-dihydroxy-vitamin D suppresses TH17-driven cytokine responses (Colin et al., Citation2010), induces formation/activation of Treg lymphocytes (Krstic, Citation2010), stimulates IL-4 production (TH2), and enhances natural killer-T (NKT)-cell functions; the differentiation and maturation of B-lymphocytes is also inhibited (Deluca and Cantorna, Citation2001; Mahon et al., Citation2003; Muller et al., Citation1988; Shirakawa et al., Citation2008). This helps to explain the negative correlation observed between serum IL-17 and active vitamin D levels noted here. To this end, recommendations about the importance of providing vitamin D supplements to hepatic disease patients are warranted. Apart from that, this study also concludes that there is clearly a need to integrate the screening of cytokine profiles (specifically of IL-6 and IL-17) and vitamin D levels into the classic AFP profiling during clinical assessments of patients with HCV and cirrhosis in order to increase the prognostic and diagnostic sensitivity and specificity and hopefully aid in the prevention of development of HCC.
Acknowledgment
The authors want to thank Professor Dr Inas El Attar, Professor at the National Cancer Institute, Cairo, for her guidance about as well as the performance and interpretation of the ROC analyses.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abdel-Wahab, M., Mostafa, M., Sabry, M., et al. 2008. Aflatoxins as a risk factor for hepatocellular carcinoma in Egypt: Mansoura Gastroenterology Center study. Hepatogastroenterology 55:1754–1759
- Abu Mouch, S., Fireman, Z., Jarchovsky, J., and Assy, N. 2010. Vitamin D supplement improve SVR in chronic hepatitis C (genotype I) naive patients treated with PEG, interferon, and ribavirin. 45th Annual Meeting of European Association for Study of the Liver (EASL 2010). Vienna, Austria
- Abu Mouch, S., Fireman, Z., Jarchovsky, J., et al. 2011. Vitamin D supplement improves sustained virologic response in chronic hepatitis C (genotype 1)-naive patients. World J. Gastroenterol. 17:5184–5190
- Ataseven, H., Bahcecioglu, I. H., Kuzu, N., et al. 2006. The levels of ghrelin, leptin. TNFα, and IL-6 in liver cirrhosis and hepatocellular carcinoma due to HBV and HDV infection. Mediat. Inflamm. 4:78380
- Babbs, C., Smith, A., Hunt, L. P., et al. 1988. Type III pro-collagen peptide: A marker activity and prognosis in primary biliary cirrhosis. Lancet 1:1021–1024
- Bertino, G., Neri, S., Bruno, C. M., et al. 2011. Diagnostic and prognostic value of α-fetoprotein, des-γ-carboxy-prothrombin, and squamous cell carcinoma antigen immunoglobulin M complexes in hepatocellular carcinoma. Minerva Med. 102:363–371
- Bialecki, E. S., and Di Bisceglie, A. M. 2005. Clinical presentation and natural course of hepatocellular carcinoma. Eur. J. Gastroenterol. Hepatol. 17:485–489
- Caldwell, C. C., Okaya, T., Martignoni, A., et al. 2005. Divergent functions of CD4 T-lymphocytes in acute liver inflammation and injury after ischemia-reperfusion. Am. J. Physiol. 289:G969–976
- Chan, S. L., Mo, F. K., Wong, C. S., et al. 2012. A study of circulating IL-10 in prognostication of un-resectable hepatocellular carcinoma. Cancer 118:3984–3992
- Colin, E. M., Asmawidjaja, P. S., van Hamburg, J. P., et al. 2010. 1,25-dihydroxyvitamin D3 modulates TH17 polarization and IL-22 expression by memory T-cells from patients with early rheumatoid arthritis. Arthritis Rheum. 62:132–142
- Deluca, H. F., and Cantorna, M. T. 2001. Vitamin D: Its role and uses in immunology. FASEB J. 15:2579–2585
- Denning, T. L., Wang, Y. C., Patel, S. R., et al. 2007. Lamina propria macrophages and dendritic cells differentially induce regulatory and IL-17-producing T-cell responses. Nat. Immunol. 8:1086–1094
- Durazo, F. A., Blatt, L. M., Corey, W. G., et al. 2008. Des-γ-carboxyprothrombin, α-fetoprotein and AFP-L3 in patients with chronic hepatitis, cirrhosis, and hepatocellular carcinoma. J. Gastroenterol. Hepatol. 23:1541–1548
- El-Houseini, M. E., Mohammed, M. S., Elshemey, W. M., et al. 2005. Enhanced detection of hepatocellular carcinoma. Cancer Control 12:248–253
- El Husseiny, N. M., Fahmy, H. M., Mohamed, W. A., and Amin, H. H. 2012. Relationship between vitamin D and IL-23, IL-17, and MCP-1 as markers of fibrosis in hepatitis C+ Egyptians. World J. Hepatol. 4:242–247
- Elnemr, D. M., Abdel-Azeez, H. A., Labib, H. A., and Abo-Taleb, F. M. 2012. Clinical relevance of serum endoglin level in Egyptian hepatocellular carcinoma patients. Clin. Lab. 58:1023–1028
- Elsharkawy, A. M., and Mann, D. A. 2007. Nuclear factor-κB and the hepatic inflammation- fibrosis-cancer axis. Hepatology 46:590–597
- Falasca, K., Ucciferri, C., Dalessandro, M., et al. 2006. Cytokine patterns correlate with liver damage in patients with chronic hepatitis B and C. Ann. Clin. Lab. Sci. 36:144–150
- Fathy, A., Ahmed, A. S., Metwally, L., and Hassan, A. 2011. T-Helper Type 1/T-helper Type 17-related cytokines in chronic Hepatitis C patients before and after interferon and ribavirin therapy. Med. Princ. Pract. 20:345–349
- Fisher, L., and Fisher, A. 2007. Vitamin D and parathyroid hormone in outpatients with non-cholestatic chronic liver disease. Clin. Gastroenterol. Hepatol. 5:513–520
- Giardina, M. G., Matarazzo, M., Varriale, A., et al. 1992. Serum α-L-fucosidase. A useful marker in the diagnosis of hepatocellular carcinoma. Cancer 70:1044–1048
- Heninger, E., Hogan, L. H., Karman, J., et al. 2006. Characterization of the Histoplasma capsulatum-induced granuloma. J. Immunol. 177:3303–3313
- Holick, M. F., and Chen, T. C. 2008. Vitamin D deficiency: A worldwide problem with health consequences. Am. J. Clin. Nutr. 87:1080S–1086S
- Hollis, B. W. 1986. Assay of circulating 1,25-dihydroxy Vitamin D involving a novel single-cartridge extraction and purification procedure. Clin. Chem. 32:2060–2063
- Hsia, C. Y., Huo, T. I., Chiang, S. Y., et al. 2007. Evaluation of IL-6, IL-10, and human hepatocyte growth factor as tumor markers for hepatocellular carcinoma. Eur. J. Surg. Oncol. 33:208–212
- Jimenez-Sousa, M. A., Almansa, R., de la Fuente, C., et al. 2010. Increased TH1, TH17, and pro-fibrotic responses in hepatitis C-infected patients are down-regulated after 12 weeks of treatment with pegylated interferon plus ribavirin. Eur. Cytokine Network 21:84–91
- Johnson, P. J. 2001. The role of serum α-fetoprotein estimation in the diagnosis and management of hepatocellular carcinoma. Clin. Liver Dis. 5:145–159
- Kamal, S. M., and Nasser, I. A. 2008. Hepatitis C genotype 4: What we know and what we don't yet know. Hepatology 47:1371–1383
- Korn, T., Bettelli, E., Oukka, M., and Kuchroo, V. K. 2009. IL-17 and TH17 cells. Annu. Rev. Immunol. 27:485–517
- Krstić, G. 2010. TH17 mediators and vitamin D status. Crit. Care 14:410
- Lange, C. M., Bojunga, J., Ramos-Lopez, E., et al. 2011. Vitamin D deficiency and a CYP27B1-1260 promoter polymorphism are associated with chronic hepatitis C and poor response to IFNα-based therapy. J. Hepatol. 54:887–893
- Lange, N. E., Litonjua, A., Hawrylowicz, C. M., and Weiss, S. 2009. Vitamin D, the immune system, and asthma. Expert Rev. Clin. Immunol. 5:693–702
- Lee, Y., Park, U. S., Choi, I., et al. 1998. Human IL-6 gene is activated by hepatitis B virus-X protein in human hepatoma cells. Clin. Cancer Res. 4:1711–1717
- Lehman, E. M., and Wilson, M. L. 2009. Epidemic hepatitis C virus infection in Egypt: Estimates of past incidence and future morbidity and mortality. J. Viral Hepat. 16:650–658
- Lemmers, A., Moreno, C., Gustot, T., et al. 2009. The IL-17 pathway is involved in human alcoholic liver disease. Hepatology 49:646–657
- Mahon, B. D., Wittke, A., Weaver, V., and Cantorna, M. T. 2003. Targets of vitamin D depend on differentiation and activation status of CD4+ T-cells. J. Cell. Biochem. 89:922–932
- Mawer, E. B. 1980. Clinical implications of measurements of circulating vitamin D metabolites. Clin. Endocrinol. Metab. 9:63–79
- Mohran, Z. Y., Ali-Eldin, F. A., and Abdel Aal, H. A. 2011. Serum IL-18: Does it have a role in diagnosis of hepatitis C virus-related hepatocellular carcinoma? Arab J. Gastroenterol. 12:29–33
- Muller, K., Svenson, M., and Bendtzen, K. 1988. 1α,25-Dihydroxyvitamin D3 and a novel vitamin D analogue MC903 are potent inhibitors of human IL-1 in vitro. Immunol. Lett. 17:361–365
- Petta, S., Cammà, C., Scazzone, C., et al. 2010. Low Vitamin D serum level is related to severe fibrosis and low responsiveness to IFN-based therapy in genotype I chronic hepatitis C. Hepatology 51:1158–1167
- Porta, C., De Amici, M., Quaglini, S., et al. 2008. Circulating IL-6 as a tumor marker for hepatocellular carcinoma. Ann. Oncol. 19:353–358
- Rutitzky, L. I., Bazzone, L., Shainheit, M. G., et al. 2008. IL-23 is required for the development of severe egg-induced immunopathology in schistosomiasis and for lesional expression of IL-17. J. Immunol. 180:2486–2495
- Schaalan, M. F., Mohamed, W. A., and Amin, H. H. 2012. Vitamin D deficiency: Correlation to IL-17, IL-23, and PIIINP in hepatitis C virus genotype 420. World J. Gastroenterol. 18:3738–3744
- Scott, J. D., and Gretch, D. R. 2007. Molecular diagnostics of hepatitis C virus infection: A systematic review. JAMA 297:724–732
- Shimizu, A., Shiraki, K., Ito, T., et al. 2002. Sequential fluctuation pattern of serum des-γ-carboxy prothrombin levels detected by high-sensitive electrochemiluminescence system as an early predictive marker for hepatocellular carcinoma in patients with cirrhosis. Int. J. Mol. Med. 9:245–250
- Shirakawa, A. K., Nagakubo, D., Hieshima, K., et al. 2008. 1,25-dihydroxyvitamin D3 induces CCR10 expression in terminally differentiating human B-cells. J. Immunol. 180:2786–2795
- Soliman, A. S., Hung, C. W., Tsodikov, A., et al. 2010. Epidemiologic risk factors of hepatocellular carcinoma in a rural region of Egypt. Hepatol. Int. 4:681–690
- Soresi, M., Giannitrapani, L., D'Antona, F., et al. 2006. IL-6 and its soluble receptor in patients with liver cirrhosis and hepatocellular carcinoma. World J. Gastroenterol. 12:2563–2568
- Spadaro, A., Ajello, A., Luigiano, C., et al. 2006. Low utility of plasma nociceptin/orphanin FQ in the diagnosis of hepatocellular carcinoma. World J. Gastroenterol. 12:4716–4720
- Steib, C. J., Gerbes, A. L., Bystron, M., et al. 2007. Kupffer cell activation in normal and fibrotic livers increases portal pressure via thromboxane A2. J. Hepatol. 47:228–238
- Tai, W. C., Hu, T. H., Wang, J. H., et al. 2009. Clinical implications of α-fetoprotein in chronic hepatitis C. J. Formosa Med. Assoc. 108:210–218
- Wang, L., Chen, S., and Xu, K. 2011. IL-17 expression is correlated with hepatitis B-related liver diseases and fibrosis. Int. J. Mol. Med. 27:385–392
- Wang, W. W., Wang, Z. M., Liu, Y. Y., et al. 2010. Increased level of TH17 cells in peripheral blood correlates with the development of hepatocellular carcinoma. Zhonghua Zhong Liu Za Zhi. 32:757–761
- Weaver, C. T., Hatton, R. D., Mangan, P. R., and Harrington, L. E. 2007. IL-17 family cytokines and the expanding diversity of effector T-cell lineages. Annu. Rev. Immunol. 25:821–852
- Welling, T. H., Fu, S., Wan, S., et al. 2012. Elevated serum IL-8 is associated with presence of hepatocellular carcinoma and independently predicts survival. Cancer Invest. 30:689–697
- Yasumi, Y., Takikawa, Y., Endo, R., and Suzuki, K. 2007. Interleukin-17 as a new marker of severity of acute hepatic injury. Hepatol. Res. 37:248–254
- Yen, D., Cheung, J., Scheerens, H., et al. 2006. IL-23 is essential for T-cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Invest. 116:1310–1316
- Zhang, J. P., Yan, J., Xu, J., et al. 2009. Increased intra-tumoral IL-17-producing cells correlate with poor survival in hepatocellular carcinoma patients. J. Hepatol. 50:980–989
- Zhang, J. Y., Zhang, Z., Lin, F., et al. 2010. IL-17-producing CD4+ T-cells increase with severity of liver damage in patients with chronic hepatitis B. Hepatology 51:81–91
- Zhao, L., Tang, Y., You, Z., et al. 2011. IL-17 contributes to the pathogenesis of autoimmune hepatitis through inducing hepatic IL-6 expression. PLoS One 6:e18909
- Zhou, L., Liu, J., and Luo, F. 2006. Serum tumor markers for detection of hepatocellular carcinoma. World J. Gastroenterol. 12:1175–1181