Abstract
B1a B-cells are concentrated in peritoneal and pleural cavities, are producers of ‘natural auto-antibodies’, and have been implicated in autoimmune responses. Their numbers are increased in humans and mice with systemic autoimmune diseases, but their role in the immune pathology is not known. Asbestos causes pulmonary, pleural, and peritoneal pathologies by accessing these tissues after inhalation. Amphibole asbestos has been shown to elicit immune dysfunction, including chronic inflammation, fibrosis, and autoantibody production. This study tested the hypothesis that asbestos affects immune dysfunction by activating B1a B-cells to traffic to secondary lymphatic tissue. C57Bl/6 mice were exposed to amphibole asbestos (Libby 6-Mix) either endotracheally or intraperitoneally, and the B1a B-cells in pleural or peritoneal compartments were tested by multi-parameter flow cytometry. Adoptive transfer of peritoneal lymphocytes from CD45.1 transgenic to wild-type mice was used to track the migration. The percentage and numbers of B1a B-cells in pleural and peritoneal cavities decreased 3–6 days following exposure. During that time, asbestos exposure led to a decrease in cells expressing alpha-4 (α4) integrin and MHC II antigen. Peritoneal cells treated in vitro showed decreased α4 integrin with no change in CD5, IgM, or MHC II antigen. Therefore, B1a cells (IgM+, CD5+, MHC II+) traffic from the peritoneal cavity following loss of α4 integrin expression. Following adoptive transfer into the peritoneum of asbestos-exposed mice, CD45.1+ B1a cells were detected in the spleen and mesenteric lymph nodes after 3 days, peaking at 6 days. Interestingly, the percentage of splenic suppressor B-cells (IgM+, CD5+, CD11b+, CD1d+) decreased following amphibole exposure, demonstrating that the B1a cells did not contribute to an increased pool of suppressive B-cells. These results show that B1a B-cells respond to asbestos exposure by trafficking to secondary lymphatic tissue where they may affect ultimate immune dysfunction.
Introduction
Studies of the amphibole asbestos-exposed population of Libby MT led to the discovery that high titers and frequencies of positive anti-nuclear antibody (ANA) tests were associated with exposure (Pfau et al., Citation2005), and that there was an increased risk for systemic autoimmune diseases (SAID) in that population (Noonan et al., Citation2006). A mouse model was established to further explore the initiating events and outcomes of this immune dysregulation, and that work demonstrated positive ANA and a mild lupus-like disease in amphibole exposed C57BL/6 mice, as well as similar patterns of specific autoantibodies in the mouse and human sera (Pfau et al., Citation2008, Citation2009). These mice have also been shown to be susceptible to Libby asbestos-induced inflammation and fibrosis, both of which also indicate chronic immune dysfunction (Smartt et al., Citation2010). To date, there is no clear understanding of the etiology of the unresolved immune dysfunction that leads to these pathologies, and therefore no truly effective therapies.
Immune cells in the peritoneal and pleural cavities are of particular interest for asbestos toxicology because the mode of exposure, inhalation, means that these cells potentially interact with the fibers since there is considerable evidence of translocation of fibers to these sites (reviewed in Broaddus et al., Citation2011; Bunderson-Schelvan et al., Citation2011). Libby amphibole in particular is associated with pleural disease (Peipins et al., Citation2003; Rohs et al., Citation2008; Larson et al., Citation2010; Antao et al., Citation2012). Specialized sub-sets of cells in the peritoneal and pleural cavities include B1 B-cells. This group is further broken down to B1a and B1b, based on surface marker expression and some functional differences. B1a B-cells are long-lived, self-renewing lymphocytes found primarily in the peritoneal and pleural cavities, and are major producers of IgM ‘natural autoantibodies’. Previous studies have indicated the possible involvement of B1a cells in autoimmune disease due to their reactivity to self-antigen and their expanded numbers in autoimmune mice and humans (Youinou and Lydyard, Citation2001; Xu et al., Citation2004; Morbach et al., Citation2006). Kendall et al. (Citation2004) found that the onset of autoimmune Type 1 diabetes in autoimmune prone mice can be delayed by the elimination of B1a cells from the peritoneal cavity. They also found that cells invading the pancreas in these mice are phenotypically similar to B1a peritoneal cells, implicating them in the pathology. Importantly, depletion of B1 cells also protected mice from disease in a lupus model (Murakami et al., Citation1995). Another study showed an expansion of B1a cell numbers in the spleens of lupus-prone mice compared to the wild-type (Mohan et al., Citation1998). However, there is also evidence to the contrary, in that B1a cells rarely secrete IgG, which is more likely to be pathogenic than ‘natural’ IgM, and studies have shown that the primary source of autoantibodies in lupus appear to be B2 cells (Youinou and Renaudineau, Citation2007). Further, a genetic study did not support a direct role of B1a B-cells in producing the autoantibodies in all mouse models of lupus (Atencio et al., Citation2004). Nevertheless, a recent study clearly demonstrated that dsDNA autoreactive B1a B-cells expand and traffic to secondary lymphoid tissues, class switching to IgG, during lupus development in NZB/W F1 mice (Enghard et al., Citation2010).
Once activated, B1a cells migrate out to spleen and lymph nodes, and sites of inflammation, where they expand and differentiate into autoantibody-producing cells (Fagarasan et al., Citation2000; Yang et al., Citation2007). In addition, through IgM cross-linking, toll-like receptor (TLR) pathways, and by activation with cytokines, they are capable of production of large amounts of autoantibodies (Tuscano et al., Citation2003; Lau et al., Citation2005; Ishida et al., Citation1992), and of undergoing isotype class switching in a T-cell-independent manner (Murakami and Honjo, Citation1995; Hillion et al., Citation2005; Ishida et al., Citation2006; Kaminski and Stavnezer, 2006). The other critical role of B1a cells is antigen presentation, where antigens are acquired by a variety of receptors, processed, and presented on MHC molecules to T-cells. In models of systemic autoimmune diseases, B1a cells appear to have excellent antigen-presenting cell (APC) capabilities, and express high levels of MHC antigen as well as co-stimulatory molecules such as B7-1 and B7-2 (Mohan et al., Citation1998; Bamba et al., Citation2005). Their broad reactivity via surface immunoglobulin and their array of TLR receptors for conserved elements such as DNA and other nuclear material raise the possibility that B1a cells could effectively activate self-reactive T-cells to common ANA. Therefore, it is essential to explore models in which the specific role of these cells in autoimmune responses can be elucidated.
This study tested the hypothesis that if B1a B-cells play a role in asbestos-induced immune dysfunction, asbestos exposure would result in activation and trafficking of B1a B-cells to secondary lymphoid organs. The results strongly supported this hypothesis by demonstrating surface marker changes indicative of activation, and migration of adopted B1a B-cells to spleen and lymph nodes.
Materials and methods
Mice
Mice used in this study were wild type C57BL/6 mice and CD45.1 transgenic C57BL/6 mice (B6.SJL-Ptprca Pepcb/BoyJ strain, The Jackson Laboratory, Bar Harbor, ME). Mice were housed at a constant temperature (22 °C) and humidity (45%), with a 12-h light–dark cycle, in specific pathogen-free conditions. Male and female mice between 6–10 weeks-of-age were used for experiments. The University of Montana and Idaho State University Institutional Care and Use Committees approved all protocols.
Asbestos and control fibers
Asbestos (Libby amphibole, LA) from the Libby, MT exposure sites was provided by the U.S. Geological Survey and is a mixture of amphibole fibers collected from six different contaminated sites (‘6-Mix’). NYCO minerals (Willsboro, NY) provided the non-cytotoxic non-fibrogenic control fiber Wollastonite. Dr Ann Wylie (University of Maryland) kindly provided Korean tremolite asbestos. Tremolite exposure has been shown to have immunologic effects on C57BL/6 mice, including autoantibody production (Pfau et al., Citation2008). In addition, Escherichia coli lipopolysaccharide (Type 0111:B4 LPS; Sigma, St. Louis, MO) at 10 μg/mouse was instilled intraperitoneally (IP) in 100 μl of sterile phosphate-buffered saline (PBS) for some experiments. All fiber suspensions were prepared fresh prior to administration by suspension in sterile PBS (pH 7.4). Suspensions were then sonicated (Branson Ultrasonics, Danbury, CT) for 1 min prior to use in order to reduce aggregation of the fibers as shown previously (Webber et al., Citation2008). Dosages of fibers were determined based on (a) sub-lethal dosages in in vitro toxicity studies (see below), and (b) work by others extrapolating in vivo exposures in animal models (Kane, Citation1992; Aust et al., Citation2011; Broaddus et al., Citation2011).
Particulate matter exposure
Exposures (IP) were performed using 100 μg of fibers suspended in 100 μl sterile PBS. This suspension was then injected into the peritoneal cavity of the mouse. Control mice received 100 μl sterile PBS. For endotracheal (ET) exposures, mice were anesthetized using Isoflurane, and then placed with their head tipped back and tongue gently pulled aside with cotton-padded forceps. This position permitted injection of a suspension of 60 μg fibers in 60 μl sterile PBS into the lungs through the mouth using a blunted curved 23-gauge (G) needle.
Cell harvesting
Mice were euthanized by CO2 asphyxiation immediately before cell harvesting. Peritoneal cells were harvested using the method described by Berry and Martinic (Citation2005). Briefly, the ventral skin was cut to expose the peritoneal lining; 5–10 ml of warmed culture media (DMEM + 10% FBS) was injected into the peritoneal cavity. After agitating the peritoneal cavity for 60–90 s, the media was withdrawn using a syringe and 18-G needle. Cells were then kept on ice until use. The lower volume of wash (5 ml) was used for immunoglobulin detection.
To harvest pleural cells the peritoneal cavity was cut open up to the ribcage and down the sides to expose the anterior region of the thorax. The liver was gently displaced in order to expose the diaphragm and intact pleural cavity. A 25-G needle was used to inject 1–2 ml of warmed culture media (DMEM + 10% FBS) into the pleural space by inserting the needle ventral and left of the mouse's midline. While holding up the sternum, the ribcage was gently tapped for ≈20 s to loosen the cells. A small ventral hole was cut in the diaphragm and the lavage fluid was withdrawn with a thin-tipped sterile Pasteur pipette. Cells were then placed on ice until use.
Cell counts and flow cytometry
The peritoneal and pleural fluids were centrifuged at 1500 rpm for 5 min to pellet the cells. The supernatant was decanted and the cells re-suspended in 1 ml PBS. A cell count was obtained using a Coulter Counter (Z-Series, Beckman-Coulter) and the cells were then aliquoted to vials (1 × 106 cells/vial) for staining. The cells were centrifuged and re-suspended in 100 μl of a solution of 3% Bovine Serum Albumin (BSA; Sigma) in PBS (staining buffer) containing Fc Block (BD Biosciences, San Jose, CA). Appropriate fluorochrome-conjugated antibodies (BD Biosciences) were then added (see ), the tube was vortexed, and the cells then incubated in the dark at 4 °C for 25 min. The stained samples were then analyzed on a FACS Aria or a FACS Calibur Flow Cytometer (BD Biosciences), with the cell population gates pre-determined from Fluorescence Minus One (FMO) staining or isotype controls. Data were collected on 30,000 events per sample, unless noted otherwise. The percentage of events in the positive regions and the median fluorescence intensity (MedFI) of the gated populations were obtained and compared between asbestos-treated and control mice.
Table 1. Antibodies used to distinguish cell sub-sets.
Ex vivo cell culture
Peritoneal cavity cells from wild type C57Bl/6 mice were collected and counted as previously described. The cells were then divided evenly among the 12 wells of two 6-well plates and asbestos was added at final concentrations in cell culture media as indicated in each figure, with triplicate wells for each treatment. The plates were incubated for indicated times at 37 °C, after which the non-adherent cells (lymphocytes) were collected from each well by vigorously pipetting and washing each individual well with 3 ml PBS. The cells were then washed and stained with appropriate antibodies (see ) as described previously.
In vitro cell death and apoptosis assays
Experiments were set up as above in culture plates, and prepared for staining with selected antibodies to determine B1a B-cells (see ). AnnexinV-FITC solution (5 μl, BD Pharmingen) was added to each experimental and control vial following the last washing step. After a 15 min incubation time, propidium iodide was added, and the samples were analyzed on the FACS Aria Flow Cytometer. Normal peritoneal cells were used for FMO samples and positive controls. For a positive control for cell death, 5 μl hydrogen peroxide (H2O2) was added to 1 × 106 cells/ml media suspension and the cells incubated at 37 °C for 2 h prior to analysis.
Detection of peritoneal immunoglobulins
IgM, IgA, and IgG levels in peritoneal fluids were assayed using a mouse isotyping ELISA kit (Pierce/ThermoScientific, Rockford, IL) according to manufacturer instructions. Kit sensitivity was 3 ng antibody/ml and was qualitative, based on kit positive and negative controls.
B1a B-cell trafficking
Female transgenic CD45.1 mice were used for the harvest of CD45.1+ lymphocytes from the peritoneal cavity. Peritoneal cells were collected as described above, and then plated in cell culture flasks for 3 h at 37 °C to allow macrophages to adhere. Media and non-adherent cells were then collected and cells pelleted by centrifugation and then counted using a Coulter Z-series cell counter.
Wild-type C57B1/6 mice were injected IP with 1 × 106 CD45.1+ cells. Mice were then exposed to asbestos and saline for the times indicated for each experiment. After asphyxiation using CO2, peritoneal cells, mesenteric lymph nodes, and spleens were collected. Cells were obtained from the lymph nodes by mashing the dissected tissue with the flat end of a 1 ml syringe plunger through a strainer cap, centrifuging the collected cells and retaining the pellet. The spleen was processed according to an established protocol that released the cells into Hank's Balanced Salt Solution (HBSS, Sigma) by mashing the tissue through a strainer, subsequently centrifuging the HBSS solution, and retaining the cell-containing pellet. Prior to cell staining, red blood cell lysis was performed on the spleen cells using 10× HBSS. Cell fractions were then stained for flow cytometry using the previously explained method and anti-CD45.1 antibody (BD Biosciences). Anti-CD43 (BD Biosciences) was also used in the staining to distinguish B-1 and B-2 cells in the spleen (Martin and Kearney, Citation2001).
Statistical analysis
Statistical significance between a single treatment group and control group was determined using an unpaired, two-tailed t-test. Multiple treatment groups were analyzed using an analysis of variance (ANOVA). Either Excel or Prism software was used for analysis and graphing. Statistical significance was defined as p < 0.05.
Results
Peritoneal and pleural B1a B-cells are reduced following asbestos treatment in vivo
Intraperitoneal treatment with amphibole asbestos led to reduced percentages () and numbers () of B1a B-cells in the peritoneal cavity. B1b cells did not significantly change except by increasing slightly in terms of percentage, consistent with the loss of B1a cells (). The numbers of T-cells or B2 cells did not change significantly (data not shown). Wollastonite caused no change in these values from control levels. Interestingly, at 24 h, no reduction in B1a cells was apparent due to the asbestos exposure, but there was already a decrease as a result of LPS exposure (), consistent with results from others that indicate that LPS-induced B1a cell trafficking is very rapid, occurring in less than 24 h (Ha et al., Citation2006).
Figure 1. Reduction in peritoneal B1a B-cells after 6-Mix exposure. B1a cells = IgM+, CD5+, CD23−; B1a cells = IgM+, CD5−, CD23− (all gated on lymphocytes). (a) Mice were treated with fibers by IP instillation (100 μg/mouse for fibers or 1 μg/mouse for LPS). At Day 7 post-treatment cells were isolated from hosts and analyzed; values presented are the percent of peritoneal B-cells (CD3- lymphocytes). (b) 24 h or 3 day in vivo [as in a]. Values shown were calculated by multiplying percent positive by the total number of cells. (c) Peritoneal B-cell populations after in vitro treatment at 25 μg/cm2 for 24 h or 3 days. n = 3–5 mice (or wells)/group; values shown in each figure are mean ± SEM. *p < 0.05 compared to Saline (a, b) or No Treatment (c) value at the same time point.
![Figure 1. Reduction in peritoneal B1a B-cells after 6-Mix exposure. B1a cells = IgM+, CD5+, CD23−; B1a cells = IgM+, CD5−, CD23− (all gated on lymphocytes). (a) Mice were treated with fibers by IP instillation (100 μg/mouse for fibers or 1 μg/mouse for LPS). At Day 7 post-treatment cells were isolated from hosts and analyzed; values presented are the percent of peritoneal B-cells (CD3- lymphocytes). (b) 24 h or 3 day in vivo [as in a]. Values shown were calculated by multiplying percent positive by the total number of cells. (c) Peritoneal B-cell populations after in vitro treatment at 25 μg/cm2 for 24 h or 3 days. n = 3–5 mice (or wells)/group; values shown in each figure are mean ± SEM. *p < 0.05 compared to Saline (a, b) or No Treatment (c) value at the same time point.](/cms/asset/fa83439c-105e-4f70-8923-91b733a0e069/iimt_a_796024_f0001_b.jpg)
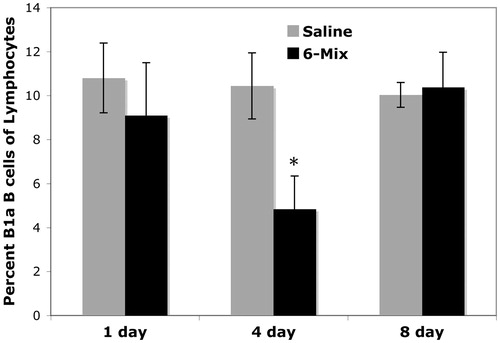
In vitro experiments using harvested peritoneal lymphocytes (macrophages removed by adherence) and asbestos dosages from 25–200 μg/cm2 showed no significant increase in apoptosis (Annexin V+) nor loss of cell numbers of B1a B-cells in the wells, indicating that the reduction in cell numbers in vivo was not due to cell death (data not shown). In addition, in vitro experiments showed no changes in the percentages of B1a B-cells (), suggesting that the loss of B1a B-cells in vivo was more likely due to trafficking rather than a phenotypic change. Endotracheal instillation similarly reduced the percentage of B1a B-cells in the pleural cavity within 4 days, with complete recovery by 8 days ().
Figure 2. Reduction in pleural B1a B-cells after intratracheal exposure to 6-Mix. Mice were exposed through endotracheal instillation (at 60 μg 6-Mix/mouse) in 60 μl sterile saline and then cells were harvested at the indicated times post-treatment. Harvested pleural cells were stained for B1a B-cells (IgM+, CD5+, CD23−). Values shown are mean (±SEM) percent positive of all gated lymphocytes. n = 5/group. *p < 0.05 compared to saline value at the same time point.
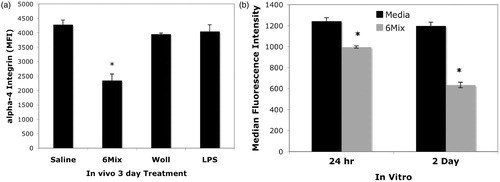
α4 Integrin expression is reduced following asbestos treatment in vivo and in vitro
In order to demonstrate that the reduction in B1a cell numbers was due to trafficking, we first measured expression of α4 integrin, which occurs when B1a B-cells prepare to leave the peritoneal cavity (Ha et al., Citation2006). shows that α4 integrin expression on peritoneal B1a B-cells declined significantly after 3 days with asbestos, but not with wollastonite. Reduced α4 integrin expression also occurred in vitro, by 24 h, suggesting an early change in expression, prior to trafficking (). Also, consistent with the early activation and recovery of peritoneal B1a cells after LPS treatment, after 3 days, α4 integrin expression on LPS-treated B1a cells was unchanged from control levels.
Figure 3. α4 Integrin expression on B1a B-cells from the peritoneal cavity after treatment. (a) In vivo exposure, single dose of 100 μg/mouse, cell harvest at Day 3 post-treatment, n = 3 mice/group. (b) In vitro exposure, 25 μg/cm2, 24 h and 2 days of culture, n = 4 in each treatment group. In both figures, values shown are mean ± SEM. *p < 0.05 compared to Saline (a) or Media (b) at the same time point.
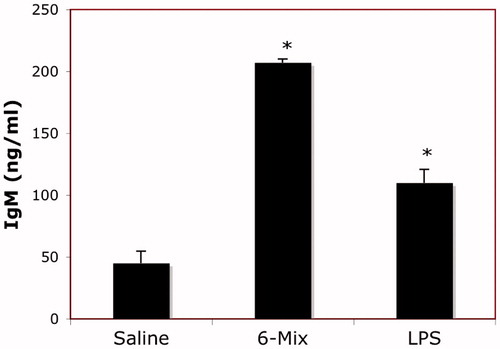
6-Mix increased peritoneal IgM production in vivo
Instillation (IP) of 6-Mix led to significant elevation in the amount of IgM in peritoneal fluid (). In contrast, there was no elevation of either IgA or IgG detected.
Figure 4. Asbestos exposure increased peritoneal IgM production. Mice were instilled IP once with 100 μg 6-Mix, or LPS (1 μg/mouse), or saline vehicle. After 3 days, peritoneal fluid was then collected and assayed for immunoglobulin using a mouse isotyping kit. n = 3/group; values shown are mean ± SEM. *p < 0.05 compared to saline value.
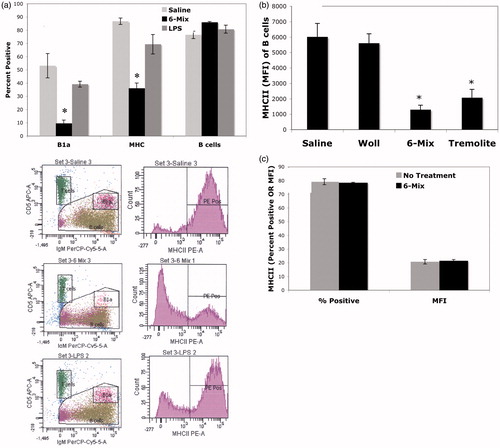
Peritoneal treatments with asbestos also led to reduced numbers of cells expressing MHC II antigen in vivo, but no change in expression in vitro
demonstrates a loss of MHC II antigen expression on peritoneal B-cells when exposure occurred in vivo. In this experiment, similar to that shown in , the percent of total B-cells did not change. However, there was a dramatic loss of B1a B-cells (). Because the expression of MHC II antigen on the remaining B1a B-cells remained very high (Mean [±SEM]): Saline 98.5% [±0.23], 6-Mix 94% [±4.7], LPS 98.2% [±2.3]), the data suggested to us that the reason for the loss was due to trafficking of B1a B-cells and not a change in expression after exposure. Further, when total peritoneal cells were harvested and treated in vitro for 3 days, there was no change in the percent of B-cells expressing MHC II antigen nor of the MFI of the B-cells in culture ().
Figure 5. Percentage of MHC II+ B-cells was decreased in the peritoneum after exposure, consistent with a loss of B1a B-cells. Peritoneal cells were harvested (a) 3 days or (b) 7 days after host exposure and then stained. B1a cells = IgM+, CD5+, CD23−; B-cells = All lymphocytes minus T-cells (T = CD5+, IgM−). Representative histograms are presented below (a) demonstrating the reduction in B1a B-cells concurrent with loss of MHCII+ B-cells. (b) Similar outcomes as in (a) and showing similar effect of pure Korean tremolite but not the control fiber wollastonite (Woll). n = 4 mice/group. (c) In vitro experiments showed no change in expression of MHC II antigen, suggesting that the changes in vivo were due to trafficking. Peritoneal cells were cultured with or without 6-Mix for 3 days and then stained as in (a). n = 3 wells/treatment group. In each figure, values shown are mean ± SEM. *p< 0.05.
Adoptively transferred B1a B-cells traffic to the spleen and mesenteric lymph nodes
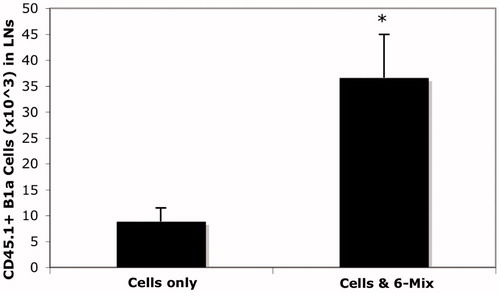
To clearly demonstrate that peritoneal B1a cells were trafficking out, CD45.1 transgenic mice were used as donors. Because there is a small resident population of B1a B-cells in the spleen, and because they are known to replicate while in the spleen (Yang et al., Citation2007), it was essential to use a model that would allow us to enumerate only B1a cells that had migrated there from the peritoneum. Peritoneal cells from CD45.1 mice were harvested and plated for adherence of the macrophages, and then the non-adherent cells (lymphocytes) were collected and used as donor cells into the peritoneum of wild type recipients. The numbers of trafficked B1a B-cells after 3 and 6 days in hosts that were treated with or without 6-Mix are shown in . contains representative histograms from a separate experiment. The top panels show the loss of CD45.1 B1a cells from the peritoneum after asbestos exposure, and the lower panel illustrates an increase in CD45.1+ B1a B-cells in the spleen. Spleens from 6-Mix exposed (IP) mice were significantly enlarged relative to the saline-exposed mice after 6 days (). It is highly unlikely that the limited number of cells that trafficked could account for that much change. However, this outcome was suggestive of additional trafficking and/or proliferation in response to the trafficked cells or other signals within that same time frame. Because peritoneal B1a B-cells have been shown to traffic to local lymph nodes as well as the spleen (Fagarasan et al., Citation2000), mesenteric lymph nodes were collected and assessed for the arrival of the adopted peritoneal CD45.1 cells. The results shown in clearly indicate trafficking of B1a B-cells to these lymph nodes.
Figure 6. 6-Mix asbestos induces trafficking of B1a B-cells to spleen. Peritoneal lymphocytes from CD45.1 transgenic mice were adopted into wild-type mice with or without 100 μg of 6-Mix. (a) Spleens were harvested after 3 or 6 days, and splenocytes then counted and analyzed for expression of CD45.1 (PE), IgM (PerCP-Cy5.5), and CD5 (APC) gating on lymphocytes; the percent positive for all three markers was multiplied by total cell number to yield number of trafficked cells. n = 6/group; values shown are mean ± SEM. *p < 0.05 compared to cells only at same timepoint. (b) Representative histograms showing loss of CD45.1+ cells from peritoneum at 4 days after host treatment with asbestos (top right, compared to no treatment in top left), and an increase of CD45.1+ cells in the spleen at 6 days post-exposure (bottom right, compared to no treatment in bottom left).
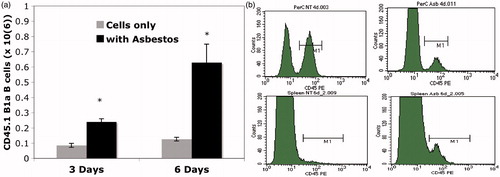
Figure 7. Spleens double in size within 6 days of 6-Mix asbestos exposure. Prior to preparing splenocytes for flow cytometry, spleens were gently dried and then weighed. All mice were the same age with no significant differences in size. n = 6–8 mice/group. *p < 0.05 compared to the saline group.
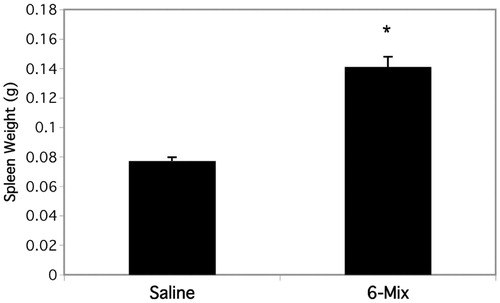
Figure 8. 6-Mix asbestos increased B1a B-cell trafficking to mesenteric lymph nodes. Peritoneal lymphocytes from CD45.1 transgenic mice were adopted into wild-type mice by IP injection with or without 100 μg 6-Mix asbestos. Mesenteric lymph nodes were then harvested and analyzed for expression of CD45.1 (PE), IgM (PerCP-Cy5.5) and CD5 (APC) gating on lymphocytes: the percent positive for all three markers was multiplied by total cell number to get number of trafficked cells. n = 8/group; values shown are mean ± SEM. *p < 0.05 compared to the saline group.
Asbestos exposure (IP) decreased suppressor B-cell population in the spleen
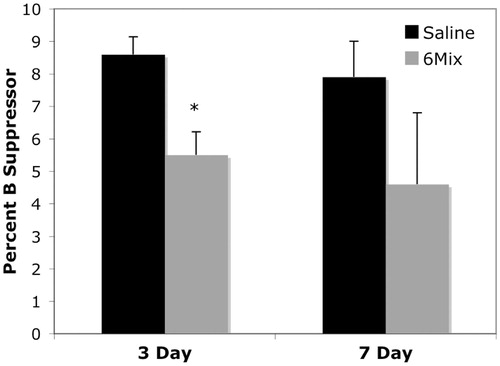
Suppressor B-cells in the spleen following 6-Mix exposure (IP) were identified by the expression of IgM, CD5, CD11b, and CD1d (Bouaziz et al., Citation2008; Yanaba et al., Citation2008; DiLillo et al., 2009). shows the percentage of lymphocytes that expressed all four markers. The data indicate that this population was reduced in spleens of the treated mice, at least after 3 days.
Figure 9. 6-Mix asbestos decreased the percentage of suppressor B-cells in splenic lymphocytes. Following IP exposure with or without 100 μg 6-Mix, peritoneal cells were collected at 3 and 7 days post-exposure and stained for IgM+ CD5+ CD11b+ CD1d+ suppressor B-cells. Data shows the percent B-suppressor cells (i.e. positive for all four markers) in the lymphocyte gate. n = 3/group; values shown are mean ± SEM. *p < 0.05 compared to the saline group at the same time point.
Discussion
Amphibole asbestos exposure has been shown to induce anti-nuclear autoantibodies (ANA) in mice and humans (Pfau et al., Citation2005, Citation2008). In order to address critical data gaps in our understanding of the mechanisms involved, the C57BL/6 mouse was used as a model for studying the immune dysfunction that may influence the devastating outcomes of asbestos exposure, including interstitial and pleural fibrosis, and possibly systemic autoimmune diseases (Pfau et al., Citation2008, Citation2011; Smartt et al., Citation2010).
Initial studies of a human amphibole asbestos-exposed population (Libby MT) led to the discovery of high titers and frequencies positive ANA tests (Pfau et al., Citation2005), and an increased risk for autoimmunity in that population (Noonan et al., Citation2006). There are also several other studies that suggest that amphibole asbestos can influence autoimmune responses, including ANA and rheumatoid factor (reviewed in Bunderson-Schelvan et al. (Citation2011)). However, most of the studies are small and, because of the rarity and latency of systemic autoimmune diseases, they did not allow for the analysis of an association between exposure and autoimmune disease. We then established a mouse model of Libby amphibole exposure to further explore the initiating events and outcomes of this immune dysregulation, and showed positive ANA and a mild lupus-like disease, as well as similar patterns of specific autoantibodies in the mouse and human sera (Pfau et al., Citation2008, Citation2009). In addition, we have shown that the presence of ANA was associated with more severe lung disease in Libby (Pfau et al., Citation2005), and that other autoantibodies to fibroblasts and mesothelial cells may contribute to fibrosis (Pfau et al., Citation2011; Marchand et al., Citation2012). Clearly, there is some immune dysregulation that raises the risk of auto-antibody production, but a much better understanding of the mechanisms leading to the auto-antibodies would be helpful in linking them with any disease process and actually translating that risk to humans.
In both mice and humans, B1a B-cells are a sub-set of B-cells that have been implicated in autoimmunity due to their reactivity to self-antigen and their expanded numbers in autoimmune mice and humans (Youinou and Lydyard, Citation2001; Xu et al. Citation2004; Morbach et al. Citation2006). B1a B-cells are long-lived, self-renewing lymphocytes found primarily in the peritoneal and pleural cavities, and are major producers of IgM ‘natural autoantibodies’. Evidence of a role in autoimmunity includes studies validating that their elimination results in amelioration of disease in mouse models of lupus and diabetes (Murakami et al., Citation1995; Kendall et al., Citation2004). Because asbestos is known to enter both the pleural and peritoneal cavities following exposure (reviewed in Broaddus et al., Citation2011; Bunderson-Schelvan et al., Citation2011), where B1a B-cells are concentrated, we hypothesized that their interaction could lead to activation and trafficking of B1a B-cells.
Experimental data showed a decrease of α4 integrin on B1a cells after IP treatment with amphibole asbestos. This integrin controls B-cell retention in the peritoneum (Ha et al., Citation2006; Berberich et al., Citation2008) and a decrease would indicate that B1a cells are detaching from the peritoneal cavity and have become more motile. This decrease occurred both in vivo and in vitro, suggesting that this is a phenotypic change that leads to the ability of these cells to traffic. This conclusion is supported by the decreases in B1a B-cells in the peritoneal cavity after treatment with either Libby 6-Mix or tremolite, an amphibole asbestos component of 6-Mix. Animals treated with saline or wollastonite did not have this decrease in B1a cell numbers. Wollastonite is a non-fibrogenic fiber that also has been shown not to induce ANA production in mice (Pfau et al., Citation2008), so it was used as a negative control. Broad ranges of all the fibers were tested in vitro for their ability to induce death (apoptosis/Annexin V or necrosis/LDH) in CH12.LX B1a-like cells (Rasmussen and Pfau, 2012) and peritoneal B-cells (data not shown), with no apparent effect up to 400 μg/ml (≈200 μg/cm2 in our in vitro model of 12-well plates). As we instilled only 100 μg/mouse, the instilled amount is far below the highest amount tested for cytotoxicity on both a mass/volume and mass/cm2 basis.
Intratracheal instillation also led to a reduction in B1a B-cells in the pleural cavity by 4 days, with recovery of these cells by 8 days, suggesting that a similar induction of trafficking occurs with pulmonary exposure. Data suggest that small fibers can travel to the pleural cavity within 3 days (Broaddus et al., Citation2011), so it is reasonable to see effects by 4 days. The reason for the rapid recovery of B1a B-cells is unknown, except that there is considerable lymphatic drainage through the pleura, allowing continual trafficking to and from this compartment (Broaddus et al., Citation2011). Experiments are underway to determine whether the B1a cells exiting the pleural cavity traveled to the lung, spleen, or to local lymph nodes. We hypothesize that they travel to lymph nodes, where their role is yet to be determined.
Adoptive transfer experiments using peritoneal cells from CD45.1 mice indicated that cells leaving the peritoneal cavity after asbestos exposure traffic to both the mesenteric lymph nodes and the spleen starting at 3–4 days and peaking at 6–7 days. This time-course is interesting because it is it so much slower than what appears to occur with lipopolysaccharide (LPS). Several studies on B1a trafficking have used LPS as the inducer, since B1 cells play a vital role in responses to infection (Ha et al., Citation2006; Yang et al., Citation2007). These cells express high levels of TLR4, a LPS receptor, and they have an important role in responding to gut-acquired bacteria. The response is very rapid, leading to activation and trafficking within 6 h via TLR4 signaling, including rapid reduction in α4 integrin expression followed by exodus to the spleen or inflammatory sites (Murakami et al., Citation1994; Ha et al., Citation2006; Yang et al., Citation2007). It was also shown that α4 integrin expression began to recover within 14 h, suggesting that the peritoneal B1a B-cell activation is transient and would shortly recover (Ha et al., Citation2006). Our data is consistent with all of this, in that LPS induced a rapid reduction in peritoneal cell B1a numbers with recovery by 3 days. This is contrasted by the response to asbestos, which was much slower. Therefore, asbestos is not signaling via TLR4 in this model, and is likely not a direct effect on the B-cells, but rather is due to signals produced by macrophages or other cells within the peritoneal cavity that respond directly to the presence of the fibers.
We have demonstrated this indirect effect in a B1a cell-like model, the CH12.LX cell line (Rasmussen and Pfau, 2012). In that model, signals from peritoneal macrophages induced class switching and increased antibody production following asbestos exposure. We, therefore, hypothesized that peritoneal B1a B-cells might also respond to IP exposure by production of anti-bodies; this viewpoint was supported by the finding of a significantly increased expression of IgM within the peritoneal cavity after 3 days. We did not consistently see class switching to IgA or any IgG isotypes within the peritoneal cavity prior to trafficking, but this may occur following their departure, since adopted B1a B-cells trafficking in a lupus mouse model primarily showed class switching in the spleen and not in the peritoneal cavity (Enghard et al., Citation2010). IgM auto-antibodies have been shown to down-regulate pathogenic autoimmune responses (Gronwall et al., Citation2012), putting B1a B-cells in a possible position to serve a protective role. We therefore also tested whether the trafficking B1a B-cells contributed to an increase in the splenic suppressor B-cell population, and found that in fact this population was decreased following asbestos exposure. Certainly, while an interesting acute finding in exposed mice, this does not rule out the possibility of a long-term protective role for these B1a B-cells. Further studies are underway to explore suppressive B-cells in long-term exposure models.
Another critical role for B1a cells is antigen presentation, as these cells appear to have excellent APC capabilities, and express high levels of MHC antigen as well as co-stimulatory molecules like B7-1 and B7-2 (Mohan et al., 1998; Sato et al., Citation2004; Bamba et al., Citation2005). Our results also showed there was a very high level of expression of MHC II antigen on the peritoneal B1a cells, and a departure of B1a B-cells in response to asbestos that led to an overall reduction in MHC II antigen expression on the remaining peritoneal B-cells. Using our current model, it was not possible to determine the functional activities of the trafficked cells, so this will be a critical next step.
Taken together, these results of these studies indicated that exposure to asbestos does cause physiological changes in B1a B-cell populations in both the pleural and peritoneal compartments. This, in turn, leads to detachment and migration of these cells to the spleen and lymph nodes wherein they may play roles in autoantibody production, antigen presentation, or modulation of immune responses.
Declaration of interest
The authors report no conflicts of interest. This work was funded by a pilot grant from the ITHS/WWAMI, as well as funding from the National Institutes of Health under Award Numbers P20 RR017670 COBRE (Holian, University of Montana) and P20 RR016454 (Bohach, University of Idaho, Idaho State University). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Some of the work was also supported by Undergraduate Research Fellowships from Idaho State University (C. Fowers, C. Peterson).
Acknowledgements
We gratefully acknowledge Dr Leonore Herzenberg (Stanford University) for her advice and discussions regarding this work.
References
- Antao, V. C., Larson, T. C., and Horton, D. K. 2012. Libby vermiculite exposure and risk of developing asbestos-related lung and pleural diseases. Curr. Opin. Pulm. Med. 18:161–167
- Atencio, S., Amano, H., Izui, S., and Kotzin, B. L. 2004. Separation of the New Zealand Black genetic contribution to lupus from New Zealand Black determined expansions of marginal zone B and B1a cells. J. Immunol. 172:4159–4166
- Aust, A. E., Cook, P. M., and Dodson, R. F. 2011. Morphological and chemical mechanisms of elongated mineral particle toxicities. J. Toxicol. Environ. Health 14:40–75
- Bamba, H., Ishigaki, H., Ishida, H., et al 2005. B1-B-cells are the main antigen-presenting cells in CpG-ODN-stimulated peritoneal exudate cells. Microbiol. Immunol. 49:89–95
- Berberich, S., Dähne, S., Schippers, A., et al 2008. Differential molecular and anatomical basis for B-cell migration into the peritoneal cavity and omental milky spots. J. Immunol. 180:2196–2203
- Berry, K., and Martinic, G. 2005. Harvesting murine peritoneal cells: A methodology for the untrained research worker. Contemp. Top. Lab. Anim. Sci. 44:50–51
- Bouaziz, J. D., Yanaba, K., and Tedder, T. F. 2008. Regulatory B-cells as inhibitors of immune responses and inflammation. Immunol. Rev. 224:201–214
- Broaddus, V. C., Everitt, J. I., Black, B., and Kane, A. B. 2011. Non-neoplastic and neoplastic pleural endpoints following fiber exposure. J. Toxicol. Environ. Health 14:153–178
- Bunderson-Schelvan, M., Pfau, J. C., Crouch, R., and Holian, A. 2011. Non-pulmonary outcomes of asbestos exposure. J. Toxicol. Env. Health 14:122–152
- DeLillo, D. J., Matsushita, T., and Tedder, T. F. 2009. B10 cells and regulatory B-cells balance immune responses during inflammation, autoimmunity, and cancer. Ann. NY Acad. Sci. 1183:38–57
- Enghard, P., Humrich, J. Y., Chu, V. T., et al 2010. Class switching and consecutive loss of dsDNA-reactive B1a B-cells from the peritoneal cavity during murine lupus development. Eur. J. Immunol. 40:1809–1818
- Fagarasan, S., Watanabe, N., Honjo, T. 2000. Generation, expansion, migration and activation of mouse B1 cells. Immunol. Rev. 176:205–215
- Gronwall, C., Vas, J., and Silverman, G. J. 2012. Protective roles of natural IgM antibodies. Front. Immunol. 3:66
- Ha, S. A., Tsuji, M., Suzuki, K., et al 2006. Regulation of B1 cell migration by signals through Toll-like receptors. J. Exp. Med. 203:2541–2550
- Hillion, S., Rochas, C., Youinou, P., and Jamin, C. 2005. Expression and reexpression of recombination activating genes: Relevance to development of autoimmune states. Ann. NY Acad. Sci. 1050:10–18
- Ishida, D., Su, L., Tamura, A., et al 2006. Rap1 signal controls B-cell receptor repertoire and generation of self-reactive B1a cells. Immunity 24:417–427
- Ishida, H., Hastings, R., Kearney, J., and Howard, M. 1992. Continuous anti-IL-10 antibody administration depletes mice of Ly-1 B-cells but not conventional B-cells. J. Exp. Med. 175:1213–1220
- Kaminski, D. A., and Stavnezer, J. 2006. Enhanced IgA class switching in marginal zone and B1 B-cells relative to follicular/B2 B-cells. J. Immunol. 177:6025–6029
- Kane, A. B. 1992. Animal models of mesothelioma induced by mineral fibers: Implications for human risk assessment. Prog. Clin. Biol. Res. 374:37–50
- Kendall, P. L., Woodward, E. J., Hulbert, C., and Thomas, J. W. 2004. Peritoneal B-cells govern the outcome of diabetes in non-obese diabetic mice. Eur. J. Immunol. 34:2387–2395
- Larson, T. C., Meyer, C. A., Kapil, V., et al 2010. Workers with Libby amphibole exposure: retrospective identification and progresssion of radiographic changes. Radiology 255:924–933
- Lau, C. M., Broughton, C., Tabor, A. S., et al 2005. RNA-associated autoantigens activate B-cells by combined B-cell antigen receptor/Toll-like receptor 7 engagement. J. Exp. Med. 202:1171–1177
- Marchand, D. L., St-Hilaire, S., Putnam, E. A., et al 2012. Mesothelial cell and anti-nuclear autoantibodies associated with pleural abnormalities in an asbestos exposed population of Libby MT. Toxicol. Lett. 208:168–173
- Martin, F., and Kearney, J. F. 2001. B1 cells: Similarities and differences with other B-cell subsets. Curr. Opin. Immunol. 13:195–201
- Mohan, C., Morel, L., Yang, P., and Wakeland, E. K. 1998. Accumulation of splenic B1a cells with potent antigen-presenting capability in NZM2410 lupus-prone mice. Arthritis Rheum. 41:1652–1662
- Morbach, H., Singh, S. K., Faber, C., et al 2006. Analysis of RAG expression by peripheral blood CD5+ and CD5- B-cells of patients with childhood systemic lupus erythematosus. Ann. Rheum. Dis. 65:482–487
- Murakami, M., Tsubata, T., Shinkura, R., et al 1994. Oral administration of lipopolysaccharide activates B-1 cells in the peritoneal cavity and lamina propria of gut and induces autoimmune symptoms in an autoantibody transgenic mouse. J. Exp. Med. 180:111–121
- Murakami, M., and Honjo T. 1995. Involvement of B-1 cells in mucosal immunity and auto-immunity. Immunol. Today 16:534–539
- Murakami, M., Yoshioka, H., Shirai, T., et al 1995. Prevention of auto-immune symptoms in autoimmune-prone mice by elimination of B-1 cells. Int. Immunol. 7:877–882
- Noonan, C. W., Pfau, J. C., Larson, T. C., and Spence, M. R. 2006. Nested case-control study of autoimmune disease in an asbestos-exposed population. Environ. Health. Perspect. 114:1243–1247
- Peipins, L. A., Lewin, M., Campolucci, S., et al 2003. Radiographic abnormalities and exposure to asbestos-contaminiated vermiculite in the community of Libby, Montana, USA. Environ. Health Perspect. 111:1753–1759
- Pfau, J. C., Blake, D. J., and Fritzler, M. 2009. Autoantibody profiles of an asbestos-exposed population. In: Autoimmunity: Role, Regulation and Disorders (Vogel, F. L., and Zimmerman, L. F., Eds.). New York: Nova Biomedical Books, pp. 245–268
- Pfau, J. C., Li, S., Holland, S., and Sentissi, J. J. 2011. Alternation of fibroblast phenotype by asbestos-induced autoantibodies. J. Immunotoxicol. 8:159–169
- Pfau, J. C., Sentissi, J. J., Li, S., et al 2008. Asbestos-induced autoimmunity in C57BL/6 mice. J. Immunotoxicol. 5:129–137
- Pfau, J. C., Sentissi, J. J., Weller, G., and Putnam, E. A. 2005. Assessment of autoimmune responses associated with asbestos exposure in Libby, Montana, USA. Environ. Health. Perspect. 113:25–30
- Rasmussen, D. L., and Pfau, J. C. 2012. Asbestos activates CH12.LX B-lymphocytes via macro-phage signaling. J. Immunotoxicol. 9:129–140
- Rohs, A. M., Lockey, J. E., Dunning, K. K., et al 2008. Low-level fiber-induced radiographic changes caused by Libby vermiculite: A 25-year follow-up study. Am. J. Respir. Crit. Care Med. 177:630–637
- Sato, T., Ishikawa, S., Akadegawa, K., et al 2004. Aberrant B1 cell migration into the thymus results in activation of CD4 T-cells through its potent antigen-presenting activity in the development of murine lupus. Eur. J. Immunol. 34:3346–3358
- Smartt, A. M., Brezinski, M., Trapkus, M., et al 2010. Collagen accumulation over time in the murine lung after exposure to crocidolite asbestos or Libby amphibole. Environ. Toxicol. 25:68–76
- Tuscano, J. M., Harris, G. S., and Tedder, T. F. 2003. B-Lymphocytes contribute to autoimmune disease pathogenesis: Current trends and clinical implications. Autoimmun. Rev. 2:101–108
- Webber, J. S., Blake, D. J., Ward, T. J., and Pfau, J. C. 2008. Separation and characterization of respirable amphibole fibers from Libby, Montana. Inhal. Toxicol. 20:733–740
- Xu, Z., Butfiloski, E. J., Sobel, E. S., and Morel, L. 2004. Mechanisms of peritoneal B-1a cells accumulation induced by murine lupus susceptibility locus Sle2. J. Immunol. 173:6050–6058
- Yanaba, K., Bouaziz, J. D., Haas, K. M., et al 2008. A regulatory B-cell subset with a unique CD1dhiCD5+ phenotype controls T-cell-dependent inflammatory responses. Immunity 28:639–650
- Yang, Y., Tung, J. W., Ghosn, E. E., and Herzenberg, L. A. 2007. Division and differentiation of natural antibody-producing cells in mouse spleen. Proc. Natl. Acad. Sci. USA 104:4542–4546
- Youinou, P., and Lydyard, P. M. 2001. CD5+ B-cells in non-organ-specific autoimmune diseases: a fresh look. Lupus 10:523–525
- Youinou, P., and Renaudineau, Y. 2007. The paradox of CD5-expressing B-cells in systemic lupus erythematosus. Autoimmun. Rev. 7:149–154
