Abstract
The genus Simulium, black fly (Diptera), comprises >1800 species worldwide, of which 67 species under six subgenera have been well studied in India. While at the extreme, black fly bites can cause onchocerciasis or river blindness, the majority of clinical observations indicate mainly severe pain and continuous itching at bite sites. This investigation experimentally observed that black fly salivary extract (BFSE) causes unique biologic effects including immunomodulation, anti-coagulation, and hypersensitivity reactions in Wistar rats. Salivary glands from black flies were isolated, extracted in saline, and then ≈800 ng extract (BFSE) subcutaneously injected into rats. To evaluate potential immunosuppressive activity of the BFSE, serum levels of interleukins [IL]-6 and -10 and tumor necrosis factor [TNF]-α were assayed. To assess the BFSE impact on coagulation, activated partial thromboplastin time (aPTT), prothrombin time (PT), and bleeding time, as well as generation of coagulation activated factors I, IX, and X were analyzed. Anaphylaxis induction was monitored via electrocardiogram (ECG) and measures of blood pressure and rectal temperature. The data showed that BFSE treatment resulted in a significantly prolonged aPTT, PT, and bleeding time and reversibly inhibited generation of coagulation activated factors I, IX, and X. The extract also led to a prolonged (up to 48 h) suppression of serum IL-6, IL-10, and TNFα production. While these results suggest that BFSE possesses anti-thrombotic, anti-coagulant, and immunomodulatory activities, importantly, they also indicate that the extract has a capacity to induce anaphylaxis and acute cardiotoxicity.
Introduction
Blood coagulation in mammals is a physiological response that is activated by a complex enzymatic cascade. Blood-feeding arthropods, at the time of biting, inject anti-hemostatic factors present in their saliva to facilitate feeding (Ribeiro & Francischetti, Citation2003). In India, the distribution of black flies is not properly documented. A study has reported 16 species of black fly in the Darjeeling area of India. Most black fly species belong to the immense genus Simulium; of these, S. Himalayas are most abundant, with S. rufibasis, grisescens, dentatum, ramosum, nigrifacies, and Biforaminiferum confined to certain pockets of the area (Datta, Citation1975). Another study noted that Arunachal Pradesh (India) has 61 species in the Tipulidae, Culicidae, Simuliidae, Bibionidae, and Sciaridae (sub-order Nematocera) families (Mitra et al., Citation2006).
Some Simulium species can transmit Onchocerca volvulus, causative parasites of river blindness (onchocerciasis) in Africa (WHO, Citation1995). Clinical manifestations of onchocerciasis include itching and disfiguring of skin, nodules on bony areas, serious eye lesions, and blindness; all of these are considered both public health problems and socioeconomic hazards (Opara et al., Citation2005; Youssefi et al., Citation2008). In countries like India, even despite evidence of O. volvulus not being reported so far, black flies still bite and crawl on the skin of individuals and livestock (Malhotra et al., Citation1986). The bites of the black fly induce a pronounced and persistent erythema due to the presence of saliva containing a wide range of physiologically active molecules. Not only does the saliva pose a threat as a carrier of pathogens, it contains potent toxins that can induce inflammation in the host. After being bitten by a black fly, continuous bleeding and a significant amount of blood loss are observed at the wound site. This is likely due to the presence in the saliva of several anti-coagulation factors that act against thrombin. To date, the precise identity of these anti-hemostatic factors remains unknown.
In the present investigation, Wistar rats were injected with isolated black fly saliva (BFSE) and subsequently analyzed for changes in activation of coagulation factors, any onset of anaphylaxis, as well as for fibrinolysis. The rats were also examined for circulating levels of some key immunoregulatory cytokines (i.e. interleukin [IL]-6 and -10, as well as tumor necrosis factor [TNF]-α) to examine the role that these products might play in any observed responses. While some might be concerned about the use of the rat as a model to examine the potential physiologic/immunotoxic effects of the BFSE, the European Medicine Agency (EMA) states that ‘although the predictive value of animal models is considered low, they should be considered for selected immunotoxicology studies. Assessment of immunogenicity in animals is useful to interpret non-clinical toxicology and pharmacology data (Brinks et al., Citation2011). Further, in a number of immunotoxicology studies, rats and other rodent species have been used as their immune responses have been shown to be quite comparable to the human response (Esquifino et al., Citation1996; Minor, 2011; Loveless et al., Citation2008). Through the use of this particular rat model, it was hoped that the present study might provide new insights into the mechanisms responsible for the coagulopathic effects of black fly saliva in vivo and data regarding its potential efficacy for use in specific future therapeutic interventions.
Materials and methods
Collection of black flies and isolation of salivary extracts
Black flies (Simulium himalayense, ) were collected from three different locations in the West Kameng district of Arunachal Pradesh, India. Flies were caught in inverted test tubes when they settled on exposed parts of a human body. Salivary glands were subsequently isolated under a dissection microscope and preserved in phosphate-buffered saline (PBS, pH 7.4).
The salivary extracts from the glands were prepared by centrifugation at 8000 × g for 5 min, as described by Tsujimoto et al. (Citation2010). Total soluble protein content in the extract was then assessed using protein estimation kits (Bangalore Genei, Bangalore, India); on average, ≈600 ng protein was present per pair of salivary glands. The supernatant – termed blackfly salivary extract (BFSE) – was then aliquoted and preserved at −20 °C for later use in the experiments below. Previous pilot studies in the laboratories have shown that the aliquoted protein mixture was stable when maintained at −20 °C. For the actual experiments, several extracts were pooled to provide a parent stock (whose ‘new’ protein concentration was re-assessed to provide an accurate post-mixing value) from which dilutions could then be prepared and rats injected with a fixed amount of BFSE in a fixed volume (approximating a dose of 1 mg/kg body weight, i.e. 200 µl).
Animals
Wistar rats (male, 200–250 g, 7–8-weeks-old) were obtained from the Laboratory of Animal Resources at the Defence Research Laboratory (Tezpur, India). All rats were housed in a specific pathogen-free facility maintained at 25 [±2] °C and 70% relative humidity and with a 12-h light/dark cycle. All rats had ad libitum access to a standard rodent chow (Pranav Agrotech Ltd, Delhi, India) and filtered water. All rats were acclimatized for 1 week before use in experiments. All animals received care in compliance with the guide for the Care and Use of Laboratory Animals (National Institute of Health, USA). All experimental protocols were reviewed and approved by the Institutional Animal Ethics Committee (IAEC).
For the studies, rats were divided into vehicle- and BFSE-injected groups (n = 6/group). For the latter, ≈800 ng BFSE was injected subcutaneously (SC) in a 200 µl volume; controls received saline vehicle in the same volume (SC). This particular dose of BFSE was selected based upon our previous study (Borah et al., Citation2012).
At various timepoints after the single injection, the rats were subjected to analyses. Ultimately, at 48 h post-injection, all rats were euthanized by over-dose with ether (Himedia Chemicals, Mumbai, India) and then blood, vital organs, and skin (injection site) samples were recovered for analyses.
Blood collection and processing
Blood samples were obtained by venipuncture (tail vein) prior to the saliva injection and 1, 6, 12, 24, 36, and 48 h thereafter. Samples for use in the determination of serum interleukin (IL)-6, IL-10, and tumor necrosis factor (TNF)-α were placed into clot activator tubes (Peerless Biotech Pvt. Ltd, Chennai, India). Blood for routine hematological measures was placed into EDTA-coated Vacutainer tubes (Peerless). All blood samples (except those for cell counts) were immediately placed in ice and then centrifuged at 4 °C for 20 min at 1600 × g to generate serum or plasma. All serum and plasma samples were stored at −70 °C until assayed.
Electrocardiogram blood pressure and rectal temperature recording
All rats received a dose of ketamine (60 mg/kg, IV; Neon Laboratory Ltd, Mumbai, India) prior to being subjected to a resting electrocardiogram (ECG) recording using an ECG-100 C system (NICO-100 C, Biopac Systems Inc., Goleta, CA) at a speed of 25 mm/s. For each analysis, monitoring electrodes were placed on the skin of the lower chest (area had been shaved prior to start of study) and connected to system transducers and amplifiers. Heart rate (bpm) for each fully awake rat was calculated by dividing the mean RR (R wave to R wave) interval by 60. Heart rate variability (HRV) was calculated based on each RR interval. Rectal temperature was monitored at each timepoint using a 6-channel tail thermometer (INCO, Ambala, India).
Assays of serum/plasma parameters
Levels of IL-6, IL-10, and TNFα in serum samples, as well as levels of coagulation factor I, IX, and X, were assessed using specific enzyme-linked immune assay (ELISA) kits (Cayman Chemicals, Ann Arbor, MI) and following manufacturer instructions. All ELISA outcomes were measured using a 96-well microplate reader (Transasia, Mumbai). Routine hematologic and coagulation tests were performed using standard kits from Tulip Diagnosis (Goa, India) and a fully automated cell counter (Milet Sesloesing Laboratory, MS-4, Paris, France) according to manufacturer guidance. Coagulation tests were performed using a channel coagulometer (Trivitron, Chennai) and standard kits (Diagnostica Stago, Franconville, France).
Histopathology
At 48 h after injection of the BFSE or vehicle, all rats were euthanized, and samples of abdominal skin and all major organs collected into 10% neutral-buffered formalin and processed for microscopic analyses. Sections of abdominal skin (including injection sites with and without any apparent lesions) were stained and cross-sectional areas measured using Coslab Image Software (Coslab, Ambala). Fifty transversely-sectioned cells with central nuclei from each of two slides/rat (i.e. for 100 total cells/rat) were subsequently evaluated for pigmentation. All histopathologic analyses were performed in a blinded manner by a certified pathologist.
Statistical analysis
Statistical analyses were conducted using SPSS for WINDOWS (Cary, NC). Analysis of variance (ANOVA) tests were used in the analyses. When a variable was not normally distributed, an appropriate transformation (e.g. log or square root) was used to better achieve approximate normality and analyses were performed on the transformed variables. When the data could not be normalized, a non-parametric analysis (Student-Newman-Keul’s test) was performed. Differences were significant at p values <0.05. All results were expressed as mean ± SD.
Results
Physiological effects
Injection of the rats with the BFSE resulted in reversible physiologic responses. Diastolic arterial blood pressures decreased maximally at 20 min post-injection (with mean decrease of 30 [±5] mmHg as compared to baseline values) and gradually returned to normal thereafter. At 1 h after extract injection, rectal temperature was increased and then gradually returned to normal values after 220 min. The maximal increase in body temperature was 1.8 [±0.6] °C. Control rats that received vehicle only showed no physiologic alterations. By 48 h post-injection of extract, mean body weight of the rats increased by 2% due to fluid retention.
Hematology
Hematological parameters such as levels of hemoglobin, red blood cells, white blood cells, and platelets, as well as mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, mean platelet volume, and red cell indices were found to be within the clinical range of rats in the experimental group within the first hour after BFSE injection. Thereafter, WBC counts fell (to ∼400/pl); these counts remained markedly depressed for 12 h and then rebounded to reach higher-than-normal levels by 24 h. Platelet counts also fell after the first hour, and continued to decline to the 12 h point, and remained depressed even at the 24 h mark (data not shown).
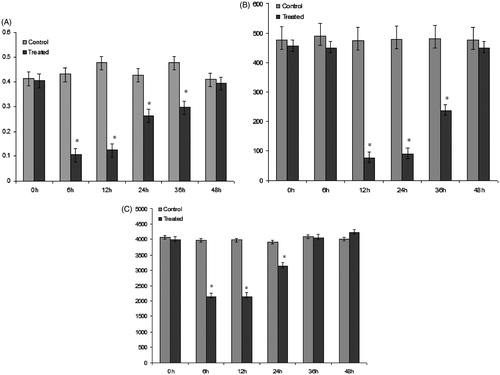
Activation of coagulation and fibrinolysis
At 6 h after injection with the extract, significant changes were observed in prothrombin time, activated partial thromboplastin time (data not shown), and in plasma levels of coagulation factors I, IX, X, prothrombin, and fibrinogen, as compared to baseline measurements of control rats (). Significant elevations of these markers were not evident until 6 h after the injection and maximal levels were attained at the 12 h mark.
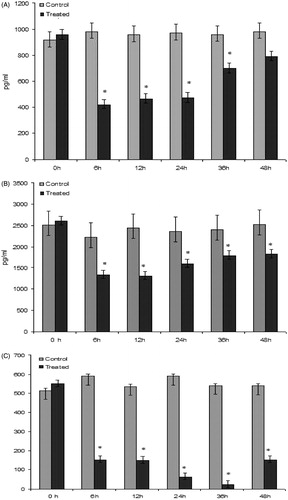
Serum levels of pro- and anti-inflammatory cytokines
The role of induced cellular expression of some key pro- and ant-inflammatory cyotkines in BFSE-elicited activation of coagulation was also investigated. In the treated rats, significant reductions in both inflammatory and pro-inflammatory cytokines, i.e. IL-6, IL-10, and TNFα, were noted at 6 h (and maximal levels were observed at 12 h) post-injection compared to levels seen in control rats. It can be seen that, with time, levels of IL-6 and TNFα progressively increased but did not reach control levels even after 48 h (). In contrast, levels of IL-10 appeared to get progressively lower, with a modicum of rebounding in the period from 36–48 h post-BFSE injection.
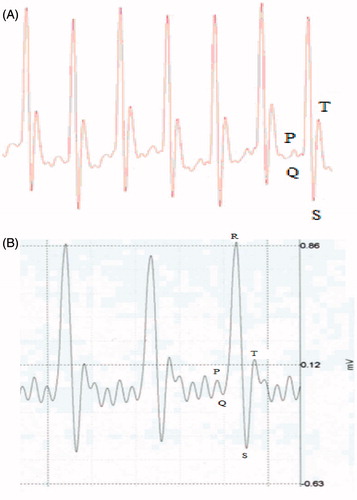
ECG profile
The R-wave amplitudes were similar for both groups at baseline on 0 h. However, at 1 h after injection of the extract, there was an increase in R-wave amplitude, suggesting a cardiac ischemic insult (). Furthermore, there was an ≈4% shortening of RR intervals, an ≈10% elevation in the ST wave, and heart rates were increased in the treated rats (at baseline) compared to outcomes noted in the vehicle control counterparts. The extract-treated rats also displayed a shortening of PR intervals without changes in heart rate. Changes of the QRS intervals in BFSE-treated hosts were also observed, an outcome that was suggestive of signs of abnormal intra-ventricular conduction. Another observable difference between the BFSE-treated and control rats existed at the QRS intervals. Lastly, QT intervals of BFSE-injected rats were wider as compared to in the control rats; this indicated that severe ventricular re-polarization had been caused by the BFSE. Interestingly, many of these alternations were not observed at 6 h post-injection or thereafter. Taken together, these results indicated that, at least with this single dose of the extract, the BFSE could cause an acute cardiotoxicity, but one that was ultimately resolved.
Histopathology
Histologically, shallow, crateriform, cutaneous ulcers were developed soon after the sub-cutaneous injection with the extract was performed (). A few lesions were associated with an exuded fibrin and necrosis of the upper dermis and of the superficial portions of hair follicles; sometimes, lesions were observed at the injection sites. Very few signs of intradermal hemorrhage were noted at the lesions. In addition, ill-defined large foci of dense granulomatous infiltrates – comprised of large histiocytes and histiocytic foamy dermal cells with lymphocytes and mast cell infiltration – were observed. A few plasma cells and histiocytic giant cells were also noted.
Figure 5. Histopathology of the injection site epidermis 48 h after SC injection of the BFSE (at ≈800 ng/rat). (a, b) Representative tissue samples from control rats showing normal architecture of epidermis with no sign of inflammation, necrosis, or congestion. (c, d) Representative tissue samples from treated rats showing acute, multifocal dermal necrosis, and deposition of fibrin (arrow) (H&E, 100×[a and c] and 1000× [b and d]).
![Figure 5. Histopathology of the injection site epidermis 48 h after SC injection of the BFSE (at ≈800 ng/rat). (a, b) Representative tissue samples from control rats showing normal architecture of epidermis with no sign of inflammation, necrosis, or congestion. (c, d) Representative tissue samples from treated rats showing acute, multifocal dermal necrosis, and deposition of fibrin (arrow) (H&E, 100×[a and c] and 1000× [b and d]).](/cms/asset/43a63682-d3de-472b-814b-535bfbd26bc0/iimt_a_809038_f0005_b.jpg)
Discussion
The present investigation showed that BFSE, a unique anti-coagulant mixture, caused reductions of partial thromboplastin time (PTT), prothrombin time (PT), and coagulation factors I, IX, and X within 6 h; however, by 36 h post-injection, these parameters gradually recovered and tended to normal values. Further, this complex affected the presence of both pro- (i.e. IL-6 and TNFα) and anti-inflammatory (IL-10) cytokines in the treated hosts. Serum levels of both the pro-inflammatory and anti-inflammatory cytokines were reduced within 6 h; however, again by 36 h post-injection, these levels of the pro-inflammatory cytokines gradually recovered and trended to normal levels. In contrast, levels of anti-inflammatory IL-10 became further reduced over this period and only began to rebound in the final 12 h of analyses. Thus, overall, the major important finding of this study was that BFSE – at the dose utilized – was an anti-coagulant and an immunomodulant.
A bite by the Simulium Himalayas black fly is normally followed by severe itching and bleeding. Black fly bite reactions can be life-threatening and have been reported to produce a severe hypersensitivity reaction in humans, causing intense itching, local swelling, and soreness (Malhotra et al., Citation1986). Here, BFSE resulted in reproducible dose-dependent elevations in serum TNFα and IL-6 levels, as well as marked acceleration of thrombin generation in vivo. Previous reports suggested that BFSE extracts caused hypersensitivity and allergic dermatitis in horses (Hellberg et al., Citation2009). Pathophysiologic studies indicated that the hypersensitivity induced was in fact IgE-mediated (Orange et al., Citation2004). While these studies showed that the BFSE induced changes in key cytokines associated with inflammatory responses, a potential impact on certain types of cells involved in immune responses overall, and in hypersensitivity reactions in particular, were also possible. Effects of exogenous agents on the functions of monocytes and macrophages are usually critical events in any induced immunomodulation, including immunosuppression. The fact that levels of IL-6, IL-10, and TNFα – cytokines produced mainly by activated monocytes/macrophages, were significantly impacted allows us to assume that the BFSE likely also imparted some effects on these cells in the injected rats. Furthermore, skin lesions at the extract injection sites displayed characteristics of an acute (or delayed) cutaneous hypersensitivity reaction. This included clear signs of mast cell infiltration.
In this study, a potential role for BFSE as an anti-coagulant (by inhibiting thrombin or coagulation factors X) was evidenced by a prolongation of clotting time in plasma-based PT and APTT assays. Further, the inhibitory effects of black fly extract on FXa generation and thrombin generation support its role as a potential anti-coagulant agent. It is well known that FXa has no effect on platelet activation; however, once assembled into the pro-thrombinase complex, it triggers enormous amounts of thrombin (Davie et al., Citation1991; Schaffartzik et al., Citation2010). Thrombin (final enzyme in clotting cascade) is responsible for clot formation platelet activation (Davie et al., Citation1991; Hoffman & Monroe, Citation2005). Based on the results showing that BFSE could inhibit Factor X and thrombin generation, it would seem the anti-coagulant activity of BFSE was initiated via inhibition of the penultimate and final enzymes in the clotting cascade.
Lastly, it is well known that envenomation by insects can produce anaphylaxis. Envenomation by honeybees causes multi-organ dysfunction as a result of direct toxic effects and also as secondary outcomes associated with systemic anaphylaxis (Mathew et al., Citation2011). A similar observation was noted in the case when BFSE was injected into the rats; these hosts evidenced electrocardiographic changes and an appearance of acute cardiac injury (but no infarction) as indicated by an absence of Q waves in serial electrocardiograms (along with near-normal levels of cardiac enzymes; data not shown here). Furthermore, an ≈10% elevation in the ST-segment in the experimental rats suggested that, in fact, these hosts had undergone a myocardial ischemic event reflective of what can occur sometimes in association with anaphylaxis. In addition, the ST wave was elevated in the extract-treated group, an outcome that was considered to be a coronary vasospasm and often referred to as reflective of acute coronary syndrome(s) (Yanagawa et al., Citation2012). Such syndromes have been described as potential, yet rare, complications of any type of anaphylactic reaction, including those arising as a consequence of an insect sting (Del Furia et al., Citation2008). The study here also provided evidence that a concurrence of acute coronary syndrome might be associated with likely activation of mast cells in the host rats. Although the studies here did not specifically measure select parameters that could verify such activation (i.e., increases in IgE in serum, increases in circulating levels of mast cell granule products), mast cell infiltration was clearly evident and, as such, the role of mast cells in the induced cardiotoxicities requires further study.
Conclusion
The studies reported here attempted to correlate known clinical and histological features associated with Simulium Himalayas black fly bites with variations in select physio-biological parameters as well as the timing of their appearance in hosts treated with the insect saliva. In particular, this study evaluated the immuno- and cardiotoxicity of a single injection of black fly salivary gland extract (BFSE) in rats. In the rats treated with BFSE, there was clear evidence of alterations in pro- and anti-inflammatory cytokine status, as well as in levels of clotting factors. The observed changes induced by BFSE were strongly indicative of an overall immunomodulatory activity for this material. Further, histopathologic analyses of skin around the injection site evidenced mast cell infiltration. Taken together, these results suggest that in vivo exposure to BFSE – at the dose used here – aused immunomodulation in the host. The studies here also yielded ECG profiles that indicated that the exposed rats underwent induced coronary vasospasms and anaphylactic reactions. Whether or not the above-noted changes in the immune end-points studied here contributed to any of the cardiac changes also seen in these hosts remains to be determined. Apart from that, future research will also seek to better define potential toxicities (and mechanisms of action) of this biologic material. This information will be critical to investigators who seek to design and formulate this material as a potential immunomodulant/anti-coagulant for use in future therapeutic interventions.
Declaration of interest
This study was supported by the Defense Research and Development Organization (DRDO), Ministry of Defense, Government of India. The authors report no conflicts of interest and that they alone are responsible for the content of this paper.
| Abbreviations | ||
| BFSE, | = | Black fly salivary extracts |
| aPTT, | = | activated partial thromboplastin time |
| PT, | = | prothrombin time |
| IL-6, | = | Interleukin-6 |
| IL-10, | = | Interleukin-10 |
| TNF-α, | = | Tumor Necrosis Factor-α |
| sc, | = | subcutaneous |
| ECG, | = | Electrocardiogram |
Notice of Correction
The version of this article published online ahead of print on 23 July 2013 contained an error in the first line of the abstract. The sentence “The genus Simulium, black fly (Diptera), comprises 41800 species and eight sub-species distributed in northeastern India.” should have read “The genus Simulium, black fly (Diptera), comprises >1800 species worldwide, of which 67 species under six subgenera have been well studied in India.” The error has been corrected for this version.
References
- Borah, S., Goswami, S., Agarwal, M., et al. 2012. Clinical and histopathological study of Simulium (blackfly) dermatitis from North-Eastern India – a report. Int. J. Dermatol. 51:63–66
- Brinks, V., Jiskoot, W., and Schellekens H. 2011. Immunogenicity of therapeutic proteins: The use of animal models. Pharm. Res. 28:2379–2385
- Datta, M. 1975. Ecology of black flies (Diptera: simuliidae) in Darjeeling area India. Proc. Plant Sci. 81:7–19
- Davie, E. W., Fujikawa, K., and Kisiel, W. 1991. The coagulation cascade: Initiation maintenance and regulation. Biochemistry 30:10363–10370
- Del Furia, F., Matucci, A., and Santoro, G. M. 2008. Anaphylaxis-induced acute ST-segment elevation myocardial ischemia treated with primary percutaneous coronary intervention: Report of two cases. J. Invasive Cardiol. 20:E3–E7
- Esquifino, A. I., Selgas, L., Arce, A., et al. 1996. Twenty-four-hour rhythms in immune responses in rat submaxillary lymph nodes and spleen: Effect of cyclo-sporine. Brain Behav. Immun. 10:92–103
- Hellberg, W., Mellor, P. S., Torsteinsdóttir, S., and Marti, E. 2009. Insect bite hypersensitivity in the horse: Comparison of IgE-binding proteins in salivary gland extracts from Simulium vittatum and Culicoides nubeculosus. Vet. Immunol. Immunopathol. 15:62–67
- Hoffman, M. M., and Monroe, D. M. 2005. Re-thinking the coagulation cascade. Curr. Hematol. Rep. 4:391–396
- Loveless, S. E., Hoban, D., Sykes, G., et al. 2008. Evaluation of the immune system in rats and mice administered linear ammonium perfluorooctanoate. Toxicol. Sci. 105:86–96
- Malhotra, P. R., Sarkar, P. K., and Bhuyan, M. 1986. Relative effectiveness of four commercial repellents against Simuliids. Curr. Sci. 55:142–143
- Mathew, A., Chirspal, A., and David, T. 2011. Acute myocardial injury and rhabdomyolysis caused by multiple bee stings. J. Assoc. Physicians India 59:518–520
- Minor, P. 2011. Summary report of a meeting on the estimation of the potency of inactivated polio vaccine. Biologicals 18:243–244
- Mitra, B., Lahiri, A. R., and Mujherjee, M. 2006. Insecta: Diptera: nematocera. In: Fauna of Arunachal Pradesh. (Alfred, J. R., Ed.). New Delhi, India: Zoological Survey of India, pp. 225–255
- Opara, K. N., Fagbemi, B. O., Ekwe, A., and Okenu, D. M. 2005. Status of forest onchocerciasis in the Lower Croos River basin Nigeria: Entomologic profile after five years of vermectin intervention. Am. J. Trop. Med. Hyg. 73:371– 376
- Orange, J. S., Song, L. A., Twarog, F. J., and Schneider, L. C. 2004. A patient with severe black fly (Simuliidae) hypersensitivity referred for evaluation of suspected immunodeficiency. Ann. Allergy Asthma Immunol. 92:276–280
- Ribeiro, J. M., and Francischetti, I. M. 2003. Role of arthropod saliva in blood feeding: Sialome and post-sialome perspectives. Annu. Rev. Entomol. 48:73–88
- Schaffartzik, A., Marti, E., Crameri, R., and Rhyner, C. 2010. Cloning, production and characterization of antigen 5-like proteins from Simulium vittatum and Culicoides nubecu-losus, the first cross-reactive allergen associated with equine insect bite hypersensitivity. Vet. Immunol. Immunopathol. 137:76–83
- Tsujimoto, H., Gray, E. W., and Champagne, D. E. 2010. Black fly salivary gland extract inhibits proliferation and induces apoptosis in murine splenocytes. Parasite Immunol. 32:275–284
- WHO (World Health Organization). 1995. Onchocerciasis and Its Control. Report of WHO Expert Committee on onchocerciasis control. Technical Report Series. Geneva: World Health Organization, pp. 1–104
- Yanagawa, Y., Tajima, M., Ohara, K., et al. 2012. A case of cardiac arrest with ST elevation induced by contrast medium. Am. J. Emerg. Med. 30:2083. e3–e4
- Youssefi, M. R., Aminpour, A., and Arabkhazaeli, F. 2008. Dermatitis caused by Simulium (Black fly) bite. Iran. J. Parasitol. 3:48–53