Abstract
In Brazil, scorpion envenomation is an important public health problem. The yellow scorpion, Tityus serrulatus (Ts), is considered the most dangerous species in the country, being responsible for the most severe clinical cases of envenomation. Currently, the administration of serum produced in horses is recognized and used as a treatment for accidents with scorpions. However, horse herds’ maintenance is costly and the antibodies are heterologous, which can cause anaphylaxis and Serum Sickness. In the present work, a human monoclonal fragment antibody, Serrumab, has been analysed. Toxin neutralizing effects of Serrumab were evaluated using a two-electrode voltage-clamp technique. The results show that Serrumab presented a high neutralizing effect against Ts β-toxins (Ts1, 43.2% and Ts2, 68.8%) and none or low neutralizing effect against α-toxins (Ts3, 0% and Ts5, 10%). Additional experiments demonstrated that Serrumab was also able to neutralize the action of toxins from other scorpion genus (Css II, 45.96% and Lqh III, 100%/β- and α-toxins, respectively). This work indicated that Serrumab is able to neutralize many toxins in Ts venom, and could being considered as a neutralizing antibody for formulating a human anti-scorpion serum in Brazil. Additionally, this work demonstrated that Serrumab could neutralize different toxins from distinct scorpion genus. All these results reinforce the idea that Serrumab is a scFv antibody with multiple neutralizing capacities and a promising candidate for inclusion in scorpion anti-venoms against different genera.
Introduction
Bites and stings by venomous animals cause severe medical emergencies around the world. In Brazil, scorpion envenomation is considered a public health problem. In 2010 alone, there were 50,126 cases of such envenomation, resulting in 88 deaths (0.2% lethality) (Saúde, 2012). The scorpion species that is considered the most dangerous in the country is Tityus serrulatus, which is responsible for most of the cases of scorpion envenomation (Petricevich & Lebrun, Citation2005).
Scorpion envenomation is attributable to the effects of the various components of the venom, most of which are peptides that present diverse types of biological activity (Possani et al., Citation1977; Sampaio et al., Citation1983). The most important and most widely studied components of scorpion venom are neurotoxins that act on Na+, K+, Ca++, and Cl− channels. Neurotoxins that are primarily responsible for the toxicological effects of the venom, and have been the most widely studied, are those that bind to voltage-gated sodium (Nav) channels (Possani et al., Citation1999). Neurotoxins that act on Nav channels are characterized by a long-chain, highly conserved structure, with four disulfide bridges, and can be classified into two groups: α-toxins that bind to Site 3 and β-toxins that bind to Site 4 (more information regarding Navs, as well as the binding and classification of toxins, can be found in Stevens et al. (Citation2011)).
In Brazil, scorpion anti-venom is currently produced at three locations (Barrio and Vital Brazil, Citation1949): the Butantan Institute, in the city of São Paulo; the Ezequiel Dias Fundation, in the city of Belo Horizonte; and the Vital Brazil Institute, in the city of Rio de Janeiro. Although this commercial anti-venom, which is an heterologous serum prepared in horses, has proven effective in reducing mortality in cases of scorpion envenomation, there are some obstacles to its use as a treatment for scorpion stings: the difficulty in obtaining large amounts of scorpion venom, which is the raw material for the production of scorpion anti-venom; the high cost of production, especially for maintaining the horses used in preparing the serum; the reduction in the lifespan of the horses, due to the high toxicity of the venom and the use of adjuvants; and the fact that only a few of the antibodies present in horse serum are specific to each venom inoculated, most of them being non-neutralizing and even harmful to humans (Abdelkader et al., Citation1991; Guglick et al., Citation1995; Wilde et al., Citation1996; Angulo et al., Citation1997; Theakston et al., Citation2003). In addition, in the final application of the serum (in humans), the horse antibodies can cause hypersensitivity reactions such as anaphylaxis and serum sickness (Shawler et al., Citation1985). Furthermore, there are concerns that infectious agents, especially viruses or prions, could be transmitted to humans via animal blood, although there have been no documented cases of such transmission during anti-venom treatment (Theakston et al., Citation2003).
Researchers from various countries have discussed and studied the possibility of producing a better scorpion anti-venom (Amaro et al., Citation2011; Chippaux & Goyffon, Citation1998; Mendes et al., Citation2008; Riano-Umbarila et al., Citation2011). Recently, our group published an article presenting a new single-chain variable-fragment (scFv) monoclonal human antibody, designated Serrumab prefix: serru- (from T. serrulatus); stem/suffix: -umab (fully human antibody) which was found to counter the adverse biochemical and immunological effects of T. serrulatus venom (Pucca et al., Citation2012). However, we were unable to explain how a monoclonal antibody could inhibit such effects. Therefore, the objective of the present study was to analyse the specificity of Serrumab against different sodium channel-specific toxins present in T. serrulatus venom, in order to explain its neutralizing properties. We also investigated the ability of Serrumab to inhibit the effects of such toxins in venom obtained from scorpions of other genera.
Materials and methods
Toxins
The T. serrulatus toxins Ts1, Ts2, Ts3, and Ts5 were purified as previously described (Arantes et al., Citation1989, Citation1994; Sampaio et al., Citation1991). Centruroides suffusus suffusus (scorpion) toxin II (Css II) was kindly provided by Gerardo Corzo. Leiurus quinquestriatus hebraeus (scorpion) toxin III (Lqh III) was purchased from Latoxan (Valence, France).
Serrumab antibody
Serrumab (Patent Application BR102013005043-1) was obtained from the Griffin.1 phage antibody library, which comprises phagemids derived from synthetic single-chain variable fragments (scFvs). The library was generously provided by Dr Greg Winter (Medical Research Council, Laboratory of Protein Engineering, Cambridge, UK). Serrumab was produced and purified as previously described in Pucca et al. (Citation2012).
Sodium channel expression
The Nav channels 1.2, 1.3, and 1.6 were evaluated. The TipE BgNav channel from the cockroach Blattella germanica (the expression of proteins encoded by tipE results in robust sodium currents; Warmke et al., Citation1997) was also evaluated. For all channels, cRNA was synthesized from linearized plasmids using a large-scale transcription kit (T7 or SP6 mMESSAGE mMACHINE; Ambion, Austin, TX). Oocytes were harvested from anesthetized female Xenopus laevis frogs, as previously described (Tytgat et al., Citation1997), microinjected with 30–50 nl of each channel (Nanoject microinjector; Drummond Scientific, Broomall, PA) and incubated with ND-96 solution (96 mM NaCl, 2 mM KCl, 2 mM MgCl2, 1.8 mM Ca.Cl2, 5 mM HEPES [pH 7.4], supplemented with 50 mg gentamicin sulfate/L and 180 mg theophylline/L). Use of the frogs was in accordance with project number 122/2009.
Electrophysiological measurements
Sodium currents were recorded using the two-electrode voltage-clamp technique at room temperature (18–22 °C). Recordings were processed with a GeneClamp 500 amplifier, controlled by a pClamp data acquisition system (Axon Instruments, Foster City, CA). Whole-cell currents from oocytes were recorded at 2–5 days after injection. Currents and voltage electrodes had resistances of 0.5–1.0 MΩ, and the electrodes were filled with 3 M KCl. Currents were sampled at 20 kHz and filtered at 2 kHz using a 4-pole low-pass Bessel filter. Leak subtraction was performed with a P/4 protocol.
For the assays, 1 µM of each toxin (Ts1, Ts2, Ts3, Ts5, Css II, and Lqh III) was previously incubated with an equimolar amount of Serrumab for 1 h at 37 °C. The quantity of Serrumab was standardized previously; when the equimolar amount was doubled, no increase in neutralizing the effect of Ts1 was noted—a finding likely attributable to antigen-antibody reactions (Cromwell, Citation1925; Weir, Citation1963). The toxin-antibody was then added directly to the recording chamber, from a stock solution of ND-96, to the desired final concentration. Immediately after the toxin-antibody had been added to the chamber containing the oocyte, the bath solution was briefly stirred to obtain an homogenous final concentration.
For the activation protocols, 100-ms test depolarizations, ranging from −90 to +70, were applied from a holding potential of −100 mV, in 5-mV increments at 5-s intervals. For the inactivation protocols, employed double pulses, with a conditioning pulse, applied from a holding potential of −100 mV to a range of potentials from −90 mV to 0 mV, in 5-mV increments for 50 ms, immediately followed by a test pulse to 0 mV (or −5 mV). Data were normalized to the maximal Na+ current amplitude (Imax), plotted against the pre-pulse potential and fitted using the Boltzmann equation: INa/Imax = 1/[1 + exp((Vc − Vh)/kh)], where Vh was the voltage corresponding to half-maximal inactivation, Vc was the conditioning pre-pulse voltage, and kh was the slope factor. Each experiment was performed at least 4-times. Data were analyzed using Clampfit 8.1 (Molecular Devices, Sunnyvale, CA), Excel 2007 (Microsoft Corp., Redmond, WA), and OriginPro 8.0 (OriginLab Corp., Northampton, MA).
Toxin sequence alignment
For all toxins (Ts1, Ts2, Ts3, Ts5, Css II, and Lqh III), amino acid sequence alignment was performed using a ClustalW2 multiple sequence alignment tool (http://www.ebi.ac.uk/Tools/clustalw2/index.html). The Ts1 sequence was used as the standard upon which the identity and similarity percentages were based.
Statistics
Data are presented as mean ± standard error (SEM) of at least four independent experiments (n = 4). All data were tested for normality using a D’Agustino Pearson omnibus normality test. All data were tested for variance using Bonferroni test or Dunn’s test. Data following a Gaussian distribution were analyzed for significance using one-way ANOVA. Non-parametric data were analyzed for significance using the Kruskal-Wallis test. Differences were considered significant if the probability that their difference stemmed from chance was <5% (p < 0.05).
Results
Effects of Serrumab on T. serrulatus β-toxins
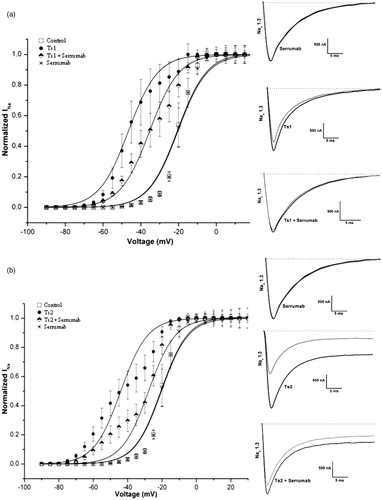
demonstrates an activation curve recorded from oocytes expressing the Nav 1.3 channel: under control conditions; with the Serrumab antibody alone; in the presence of 1 µM of Ts1 or Ts2 toxin; and in the presence of 1 µM of Ts1 or Ts2 toxin + an equimolar amount of Serrumab. The Nav 1.3 channel was chosen because it is known that Ts1 (unpublished data) and Ts2 (Cologna et al., Citation2012), both of which are β-toxins, have an effect on this channel. In equimolar amounts, Serrumab had a strong neutralizing effect—43.2% (ANOVA, Bonferroni test, p < 0.05) for Ts1 and 68.8% (Kruskal-Wallis, Dunn’s test, p < 0.05) for Ts2 (in which the V1/2 of control conditions is considered as 0% and the V1/2 of toxin conditions as 100%) (, respectively). However, when we increased the amount of Serrumab to double the equimolar amount, we observed no increase in the inhibitory effect (data not shown).
Figure 1. Neutralizing capacity of Serrumab against Tityus serrulatus beta toxins on the cloned mammalian voltage-gated sodium channel 1.3 expressed in Xenopus laevis oocytes. Steady-state activation curves (left panels) and representative whole-cell current traces (right panels) for (a) Ts1 and (b) Ts2: under control conditions (square); with Serrumab alone (star); with 1 µM toxin alone (black ball); and with 1 µM toxin + an equimolar amount of Serrumab (semi-filled diamond). Grey lines indicate traces under control conditions. Voltage dependence data were fitted with a Boltzmann function. The data were tested for their statistical significance using ANOVA, Bonferroni test, p < 0.05 (a) or Kruskal-Wallis, Dunn’s test, p < 0.05 (b).
Effects of Serrumab on T. serrulatus α-toxins
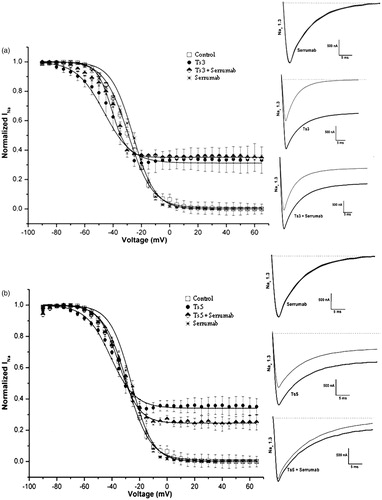
shows an inactivation curve recorded from oocytes expressing Nav 1.3 channel: under control conditions; with the Serrumab antibody alone; in the presence of 1 µM of Ts3 or Ts5 toxin; and in the presence of 1 µM of Ts3 or Ts5 toxin + an equimolar amount of Serrumab. The Nav 1.3 channel was chosen because it is known that Ts3 and Ts5, both α-toxins, have an effect on this channel (unpublished data). In equimolar amounts with Ts3, Serrumab had no neutralizing effect (). Serrumab only presented a weak neutralizing effect (10%) when it was pre-incubated with Ts5 (Kruskal-Wallis, Dunn’s test, p > 0.05) ().
Figure 2. Neutralizing capacity of Serrumab against Tityus serrulatus alpha toxins on the cloned mammalian voltage-gated sodium channel 1.3 expressed in Xenopus laevis oocytes. Steady-state inactivation curves (left panels) and representative whole-cell current traces (right panels) for (a) Ts3 and (b) Ts5: under control conditions (square); with Serrumab alone (star); with 1 µM toxin alone (black ball); and with 1 µM toxin + an equimolar amount of Serrumab (semi-filled diamond). Grey lines indicate traces under control conditions. Voltage dependence data were fitted with a Boltzmann function. The data were tested for their statistical significance using Kruskal-Wallis and Dunn’s test, p > 0.05.
Effects of Serrumab on other toxins
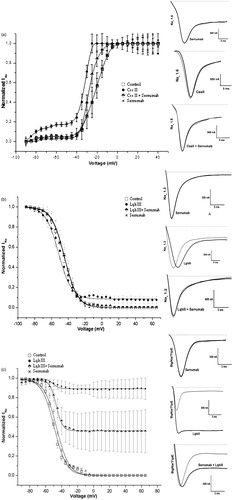
demonstrates an activation curve recorded from oocytes expressing the Nav 1.6 channel (for Css II), together with inactivation curves recorded from oocytes expressing the channels Nav 1.2 (3b) and TipE BgNav (3c), both for Lqh III: under control conditions; with the Serrumab antibody alone; in the presence of 1 µM of toxin; and in the presence of 1 µM of toxin + an equimolar amount of Serrumab. These channels were chosen because of their known susceptibility to the effects of Css II (a β-toxin) and Lqh III (an α-like toxin) (Estrada et al., Citation2011). In equimolar amounts, Serrumab neutralized 46.0% of the effect of Css II (Kruskal-Wallis, Dunn’s test, p < 0.05) (), although its design was not performed against C. suffusus suffusus venom, whereas it neutralized 100.0% of the effect of Lqh III (Kruskal-Wallis, Dunn’s test, p < 0.05) (). Although the neutralizing capacity of Serrumab against LqhIII was high, the effect that Lqh III alone had on the inactivation curve was low; this can explain why Serrumab completely neutralized the effect of the toxin on the Nav 1.2 channel. In the TipE BgNav channel, the effect of the Lqh III toxin was greater and, as expected, the neutralizing capacity of Serrumab was lower, with 50.0% of the Lqh III effect being inhibited ().
Figure 3. Neutralizing capacity of Serrumab against toxins from the venom of other scorpions on the cloned mammalian voltage-gated sodium (Nav) channels 1.2 and 1.6, as well as on a tipE-encoded insect Nav channel from the cockroach Blattella germanica (BgNav/tipE), expressed in Xenopus laevis oocytes. Steady-state activation and inactivation curves (left panels) and representative whole-cell current traces (right panels) for (a) Centruroides suffusus suffusus toxin II (Css II) on Nav channel 1.6, (b) Leiurus quinquestriatus hebraeus toxin III (Lqh III) on Nav channel 1.2, and (c) Lqh III on the BgNav/tipE channel: under control conditions (square); with Serrumab alone (star); with 1 µM toxin alone (black ball); and with 1 µM toxin + an equimolar amount of Serrumab (semi-filled diamond). Grey lines indicate traces under control conditions. Voltage dependence data were fitted with a Boltz-mann function. The data were tested for their statistical significance using Kruskal-Wallis and Dunn’s test, p < 0.05.
Neutralizing effects of Serrumab on β- and α-toxins
shows the neutralizing effects of Serrumab on the β-toxins tested (Ts1, Ts2, and Css II). The neutralizing effects of Serrumab on the α- and α-like toxins tested (Ts3, Ts5, and Lqh III) are shown in .
Figure 4. Proportional neutralizing capacity of Serrumab against beta toxins. Bars show percentage of Serrumab neutralization of toxins induced modulation of activation of the channels, quantified by the reduction of the shift in V1/2 of the activation curves (, and ). Inhibition was measured in the mammalian voltage-gated sodium channels 1.3 (for Ts1 and Ts2) and 1.6 (for Centruroides suffusus suffusus toxin II—Css II). The values obtained with the toxin alone were used as the standard (100%). The data are represented as the observed percentage of Serrumab-induced inhibition of toxin activty.
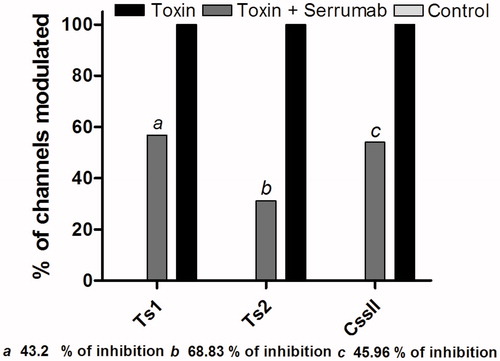
Figure 5. Proportional neutralizing capacity of Serrumab against alpha toxins. Bars show percentage of Serrumab neutralization of toxins induced modulation of the inactivation of the channels, quantified as a reduction in the area under the curve of the inactivation curves (, 2b, 3b, and 3c). Inhibition was measured in the mammalian voltage-gated sodium (Nav) channels 1.3 (for Ts3 and Ts5) and 1.2 (for Leiurus quinquestriatus hebraeus toxin III - Lqh III), as well as in the tipE-encoded insect Nav channel from the cockroach Blattella germanica (also for Lqh III). The control cells inactivation values were used as the control (100%). The data are represented as the observed percentage of Serrumab-induced inhibition of toxin activty.
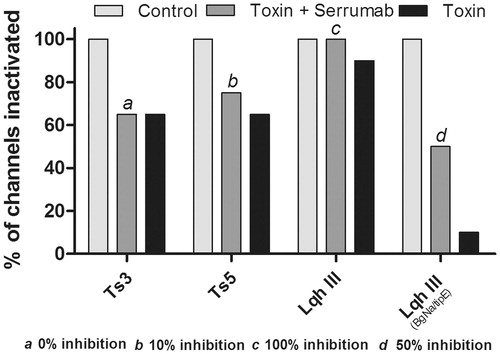
Figure 6. Multiple sequence alignment of long-chain scorpion toxins. The aligment was performed with six sequences. Swiss-Prot accession numbers (Acc#) are followed by the names. Cysteine residues are highlighted in grey. Percentages of identity (Id%) and similarity (Si%) were calculated on the basis of the Ts1 sequence, which was taken as 100%. The aligment was performed with the ClustalW2 multiple sequence alignment tool.

Amino acid sequence alignment of toxins
The amino acid sequence of the toxins Ts1, Ts2, Ts3, Ts5, CssII, and LqhIII shows that the scorpion toxins presented high homology, sequence identity more than 30% compared to Ts1, which is the acceptable range of sequence identity for the implementation of molecular modeling by sequence similarities. Ts2 presented the highest identity (72.6%) and similarity (80.6%) to Ts1.
Discussion
Most of the toxic components of scorpion venom are neurotoxins that bind to sodium channels (Rodriguez de la Vega and Possani, Citation2005). Therefore, most research aimed at producing efficient anti-venoms has focused on antibodies against the major sodium-channel toxins. The β-toxins are the main targets, because they shift the voltage dependence of activation to more negative membrane potentials via a voltage sensor-trapping mechanism and cause a reduction in peak sodium current (Catterall et al., Citation2007). The β-toxins are also responsible for the principal signs and symptoms of toxicity in mammals (Bertazzi et al., Citation2003; Correa et al., Citation1997; Pessini et al., Citation2003; Vasconcelos et al., Citation2005).
In T. serrulatus venom, the major β-toxin is Ts1, which has been well studied (Polikarpov et al., Citation1999). In the present study, Serrumab, a scFv antibody against T. serrulatus crude venom, inhibited 43.2% of the activation effect of Ts1 on sodium channels. This neutralizing effect was expected, because the phage display selection was performed with the crude venom, of which Ts1 accounts for 14.6% (quantified using UNICORN software v.5.2, GE Healthcare). Therefore, many Ts1 molecules were exposed to the Griffin.1 library during the selection procedure (Pucca et al., Citation2012). The Serrumab/Ts1 results obtained in the present study corroborate with those obtained by in a previous study using crude venom. Because Serrumab was able to inhibit ∼50% of the effect of the major toxin, we can presume that it would counter some effects of scorpion envenomation. Other authors have shown that Ts1 plays an essential role in envenomation (Bertazzi et al., Citation2003; Correa et al., Citation1997; Teixeira et al., Citation2010; Vasconcelos et al., Citation2005).
Serrumab was also able to neutralize 68.83% of the effect of the Ts2 toxin. In addition to being classified as a β-toxin in the Nav 1.3 channel (Cologna et al., Citation2012), Ts2 presents 72.6% identity and 80.6% similarity with Ts1 in the primary sequence (see Figure 6), which might explain how a monoclonal antibody can inhibit more than one toxin. In addition, Ts2 accounts for 6.02% (quantified using UNICORN) of the crude venom; this may explain the specificity of the antibody during the rounds of selection. Although the differences were minimal, it is intriguing that Serrumab showed a greater neutralizing capacity for Ts2 than for Ts1. That finding is probably related to a difference between the two toxins in terms of affinity for binding to the sodium channel. It is well known that Ts1 has a high sodium channel-binding affinity (Barhanin et al., Citation1984; Vijverberg & Lazdunski, Citation1984). However, further studies are needed in order to determine whether Ts2 shows such an affinity.
Although Nav α-toxins (site 3 toxins) are not the toxins primarily responsible for the envenomation profile, they are known to contribute and to intensify the β-toxins toxic effects. Their binding to Nav channels impairs fast inactivation, thereby enhancing recovery from inactivation (Stevens et al., Citation2011). Here it was found that Serrumab was unable to neutralize the inactivation effect of the site 3 toxin Ts3. In comparison with the other T. serrulatus toxins, Ts3 showed the lowest identity and similarity with Ts1. The differences between these amino acid residues could explain the variations in the epitope binding with Serrumab and in its neutralizing capacity. In addition, Ts3 accounts for only 1.05% (quantified using UNICORN) of the crude venom, which could also explain the fact that Serrumab showed a low capacity to neutralize the effects of this toxin.
Serrumab presented little capacity to neutralize the effect of the α-toxin Ts5, the activity of which was inhibited by only 10% after the addition of Serrumab. In the primary sequence alignment, Ts5 showed higher identity and similarity with Ts1 (Figure 6) than did Ts3 (39.1% and 56.2% vs 36.4 and 51.5, respectively). Although those differences were minor, it is well known that isolated amino acids are crucial for antibody binding (Cohen et al., Citation2004, Citation2005; Karbat et al., Citation2007). In addition, Ts5 accounts for a higher proportion of the crude venom than does Ts3 (5.80% versus 1.05%, quantified using UNICORN).
In summary, Serrumab presented a high neutralizing capacity against Ts1 and Ts2. Despite being a monoclonal scFv antibody, Serrumab also presented a low neutralizing capacity against Ts5. Therefore, it can be classified as an antibody with multiple neutralizing capacities. These are not novel findings. In 2011, Amaro et al. (Citation2011) produced a human scFv antibody specific to Ts1. Those authors demonstrated that the monoclonal antibody scFv 15e, produced against Ts1, was also capable of recognizing toxins from Tityus species other than T. serrulatus, providing evidence of the strong homology among these toxins.
The studies here found that Serrumab neutralized the effect of Css II, a β-toxin from the venom of the scorpion C. suffusus suffusus, to a degree similar to that observed for Ts1 and Ts2, both of which are also β-toxins. The greater inhibition can be justified by the great homology with Ts toxins. Therefore, Serrumab presents high neutralizing capacity against β-toxins from a distinct scorpion genus. Against Lqh III, an α-like toxin from the venom of the scorpion L. quin-questriatus hebraeus, Serrumab presented the highest neutralizing capacity, inhibiting 100% of the toxin effect. However, the effect of the toxin in the inactivation was low, casting doubt on the neutralizing capacity of Serrumab. Therefore, an inactivation assay was performed using TipE BgNav, an insect channel, in which the action of α-toxins is known to be higher (Billen et al., Citation2010). The study showed that the Lqh III toxin had a stronger effect in the insect channel and that Serrumab presented a correspondingly lower neutralizing capacity, inhibiting 50% of the effect.
The main purpose of this work was to establish ex vivo proof of the neutralizing effect of Serrumab and an in-depth characterization of the interaction at the level of the biological target, i.e., the voltage-gated Na channel. The results indicate that Serrumab is capable of neutralizing many of the toxins found in T. serrulatus venom and can, therefore, be considered a neutralizing antibody for use in production of human scorpion anti-venom (in Brazil). In addition, these studies have demonstrated that Serrumab can recognize toxins from the venom of other scorpion genera. Taken together, these findings support our previous in vivo assertion that Serrumab has multiple scorpion neutralizing capacities. Unfortunately, in vivo assays using isolated toxins were not performed based on the difficulty of obtaining sufficient quantities of toxins using a conventional purification method; however, we are working on production of recombinant toxins to solve this problem.
Conclusions
In summary, the data highlight that Serrumab can be characterized as a promising candidate for inclusion in scorpion anti-venoms targeting various genera and might come to be considered a ‘universal antibody’ against scorpion venoms. This could represent an innovative alternative to the scorpion anti-venom currently available.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
JT was supported by the following grants: G.0433.12 (F.W.O. Vlaanderen), IUAP 7/10 (Inter-University Attraction Poles Program, Belgian State, Belgian Science Policy) and OT/12/081 (KULeuven).
Acknowledgments
The authors are grateful to Dr Ruth Verplaetse for help in data analysis. This study received financial support from the Fundação de Amparo à Pesquisa do Estado de São Paulo and Instituto Nacional de Ciência e Tecnologia em Toxinas (FAPESP and INCTTOX, São Paulo Research Foundation and National Institute of Toxin Science and Technology; Grant no. 573790/2008-6); the Fundação de Apoio ao Ensino, Pesquisa e Assistência (FAEPA, Foundation for the Support of Instruction, Research and Treatment); the Fundação Waldemar Barnsley Pessoa (Waldemar Barhsley Pessoa Foundation); and the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Office for the Advancement of Higher Education), which provided a Programa de Doutorado no país com Estágio no Exterior (PDEE, Doctoral Study at Home/Fellowship Abroad Program) scholarship to MBP (Grant no. BEX 1095/11-0).
References
- Abdelkader, S. V., Gudding, R., and Nordstoga, K. 1991. Clinical chemical constituents in relation to liver amyloidosis in serum-producing horses. J. Comp. Pathol. 105:203–211
- Amaro, I., Riano-Umbarila, L., Becerril, B., and Possani, L. D. 2011. Isolation and characterization of a human antibody fragment specific for Ts1 toxin from Tityus serrulatus scorpion. Immunol. Lett. 139:73–79
- Angulo, Y., Estrada, R., and Gutierrez, J. M. 1997. Clinical and laboratory alterations in horses during immunization with snake venoms for the production of polyvalent (Crotalinae) anti-venom. Toxicon 35:81–90
- Arantes, E. C., Prado, W. A., Sampaio, S. V., and Giglio, J. R. 1989. A simplified procedure for the fractionation of Tityus serrulatus venom: isolation and partial characterization of TsTX-IV, a new neurotoxin. Toxicon 27:907–916
- Arantes, E. C., Riccioppo Neto, F., Sampaio, S. V., et al. 1994. Isolation and characterization of TsTX-V, a new neurotoxin from Tityus serrulatus scorpion venom which delays the inactivation of Na+ channels. Biochim. Biophys. Acta 1199:69–75
- Barhanin, J., Ildefonse, M., Roughier, O., et al. 1984. Tityus gamma toxin, a high affinity effector of the Na+ channel in muscle, with a selectivity for channels in the surface membrane. Pflugers Arch. 400:22–27
- Barrio, A., and Vital Brazil, G. 1949. Ein neues Verfahren der Giften Nahme ber Spinnen (A new method of poisons name on spiders). Experientia 6:112--113
- Bertazzi, D. T., De Assis-Pandochi, A. I., Azzolini, A. E., et al. 2003. Effect of Tityus serrulatus scorpion venom and its major toxin, TsTX-I, on the complement system in vivo. Toxicon 41:501–508
- Billen, B., Debaveye, S., Beress, L., et al. 2010. Phyla- and subtype-selectivity of CgNa, a Na channel toxin from the venom of the giant Caribbean Sea anemone Condylactis gigantea. Front. Pharmacol. 1:133
- Catterall, W. A., Cestele, S., Yarov-Yarovoy, V., et al. 2007. Voltage-gated ion channels and gating modifier toxins. Toxicon 49:124–141
- Chippaux, J. P., and Goyffon, M. 1998. Venoms, anti-venoms, and immunotherapy. Toxicon 36:823–846
- Cohen, L., Karbat, I., Gilles, N., et al. 2004. Dissection of the functional surface of an anti-insect excitatory toxin illuminates a putative “hot spot” common to all scorpion β-toxins affecting Na+ channels. J. Biol. Chem. 279:8206–8211
- Cohen, L., Karbat, I., Gilles, N., et al. 2005. Common features in the functional surface of scorpion β-toxins and elements that confer specificity for insect and mammalian voltage-gated sodium channels. J. Biol. Chem. 280:5045–5053
- Cologna, C. T., Peigneur, S., Rustiguel, J. K., et al. 2012. Investigation of the relationship between structure and function of Ts2, a neurotoxin from Tityus serrulatus venom. FEBS J. 279:1495–1504
- Correa, M. M., Sampaio, S. V., Lopes, R. A., et al. 1997. Biochemical and histopathological alterations induced in rats by Tityus serrulatus scorpion venom and its major neurotoxin tityustoxin-I. Toxicon 35:1053–1067
- Cromwell, H. W. 1925. Quantitative relations between antigen and antibody in the precipitin reaction. J. Infect. Dis. 37:321--328
- Estrada, G., Restano-Cassulini, R., Oriz, E., et al. 2011. Addition of positive charges at the C-terminal peptide region of CssII, a mammalian scorpion peptide toxin, improves its affinity for sodium channels Nav1.6. Peptides 32:75–79
- Guglick, M. A., Macallister, C. G., Ely, R. W., and Edwards, W. C. 1995. Hepatic disease associated with administration of tetanus antitoxin in eight horses. J. Am. Vet. Med. Assoc. 206:1737–1740
- Karbat, I., Turkov, M., Cohen, L., et al. 2007. X-Ray structure and mutagenesis of the scorpion depressant toxin LqhIT2 reveals key determinants crucial for activity and anti-insect selectivity. J. Mol. Biol. 366:586–601
- Mendes, T. M., Dias, F., Horta, C. C., et al. 2008. Effective Tityus serrulatus anti-venom produced using the Ts1 component. Toxicon 52:787–793
- Pessini, A. C., De Souza, A. M., Faccioli, L. H., et al. 2003. Time-course of acute-phase response induced by Tityus serrulatus venom and TsTX-I in mice. Int. Immunopharmacol. 3:765–774
- Petricevich, V. L., and Lebrun, I. 2005. Immunomodulatory effects of the Tityus serrulatus venom on murine macrophage functions in vitro. Med. Inflamm. 2005:39–49
- Polikarpov, I., Junior, M. S., Marangoni, S., et al. 1999. Crystal structure of neurotoxin Ts1 from Tityus serrulatus provides insights into the specificity and toxicity of scorpion toxins. J. Mol. Biol. 290:175–184
- Possani, L. D., Alagon, A. C., Fletcher, P. L. Jr., and Erickson, B. W. 1977. Purification and properties of mammalian toxins from the venom of Brazilian scorpion Tityus serrulatus Lutz and Mello. Arch. Biochem. Biophys. 180:394–403
- Possani, L. D., Becerrilo, B., Delepierre, M., and Tytgat, J. 1999. Scorpion toxins specific for Na+-channels. Eur. J. Biochem. 264:287–300
- Pucca, M. B., Zoccal, K. F., Roncoloato, E. C., et al. (2012). Serrumab: A human monoclonal antibody that counters the biochemical and immunological effects of Tityus serrulatus venom. J. Immunotoxicol. 9:173--183
- Riano-Umbarila, L., Contreras-Ferrat, G., Olamendi-Portugal, T., et al. 2011. Exploiting cross-reactivity to neutralize two different scorpion venoms with one single chain antibody fragment. J. Biol. Chem. 286:6143–6151
- Rodriguez de la Vega, R. C., and Possani, L. D. 2005. Overview of scorpion toxins specific for Na+ channels and related peptides: Biodiversity, structure-function relationships, and evolution. Toxicon 46:831–844
- Saúde, Portal da. (Health Portal) 2012. Acidentes por animais peçonhentos: Escorpiões, Aspectos Epidemiológicos. (Accidents by venemous animals: Scorpions, epidemiological aspects). Available from: http://portalsaude.saude.gov.br/portalsaude/index.cfm?portal=pagina.visualizarTexto&codConteudo=5818&codModuloArea=783&chamada=acidentes-por-escorpioes [date accessed 30 July 2013]
- Sampaio, S. V., Arantes, E. C., Prado, W. A., et al. 1991. Further characterization of toxins T1iv (Tstx-Iii) and T2iv from Tityus serrulatus scorpion venom. Toxicon 29:663–672
- Sampaio, S. V., Laure, C. J., and Giglio, J. R. 1983. Isolation and characterization of toxic proteins from the venom of the Brazilian scorpion Tityus serrulatus. Toxicon 21:265–277
- Shawler, D. L., Bartholemew, R. M., Smith, L. M., and Dillman, R. O. 1985. Human immune response to multiple injections of murine monoclonal IgG. J. Immunol. 135:1530–1535
- Stevens, M., Peigneur, S., and Tytgat, J. 2011. Neurotoxins and their binding areas on voltage-gated sodium channels. Front. Pharmacol. 2:71
- Teixeira, V. F., Conceicao, I. M., Lebrun, I., et al. 2010. Intra-hippocampal injection of TsTX-I, a β−scorpion toxin, causes alterations in electroence-phalographic recording and behavior in rats. Life Sci. 87:501–506
- Theakston, R. D., Warrell, D. A., and Griffiths, E. 2003. Report of a WHO Workshop on the standardization and control of anti-venoms. Toxicon 41:541–557
- Tytgat, J., Maertens, C., and Daenens, P. 1997. Effect of fluoxetine on a neuronal, voltage-dependent potassium channel (Kv1.1). Br. J. Pharmacol. 122:1417–1424
- Vasconcelos, F., Lanchote, V. L., Bendhack, L. M., et al. 2005. Effects of voltage-gated Na+ channel toxins from Tityus serrulatus venom on rat arterial blood pressure and plasma catecholamines. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 141:85–92
- Vijverberg, H. P., and Lazdunski, M. 1984. A new scorpion toxin with a very high affinity for sodium channels. An electrophysiological study. J. Physiol. (Paris) 79:275–279
- Warmke, J. W., Reenan, R. A., Wang, P., et al. 1997. Functional expression of Drosophila para sodium channels. Modulation by the membrane protein TipE and toxin pharmacology. J. Gen. Physiol. 110:119–133
- Weir, D. M. 1963. Antigen-antibody reactions. Mod. Trends Immunol. 55:53–85
- Wilde, H., Thipkong, P., Sitprija, V., and Chaiyabutr, N. 1996. Heterologous anti-sera and anti-venins are essential biologicals: Perspectives on a worldwide crisis. Ann. Intern. Med. 125:233–236
