Abstract
Highly reactive, low-molecular-weight diisocyanates (DIC) are the most commonly identified cause of occupational asthma (OA). Animal/clinical studies of DIC asthma have been more limited compared with atopic asthma, and an understanding of DIC pathogenesis is less clear. The aim of this study was to investigate in a mouse model, toluene diisocyanate (TDI, as 2,4-TDI isomer)-induced inflammatory reactions/cytokine profile changes in the lungs and accompanying changes in lymph node lymphocyte sub-populations. The study used female BALB/cJ/Han/IMP mice that were exposed first intra-nasally and then in an inhalation chamber to TDI or air. After the final exposure, bronchoalveolar lavage fluid (BALF) was collected and changes induced in inflammatory cell composition, levels of key cytokines (i.e. IL-4, TNFα, IFNγ), and lymphocyte sub-population profiles within auricular lymph nodes, were evaluated. Total number of cells in the BALF of treated mice was significantly higher than in control mice BALF. There was also a significant increase in BALF neutrophil and eosinophil levels with TDI mice compared to in controls; lymphocyte and macrophage numbers did not significantly differ. A significant increase in BALF levels of TNFα and IFNγ was also noted in mice exposed to TDI relative to levels in controls. BALF IL-4 levels were also increased, but the change from control was not significant. Lastly, the levels/percentages of CD3+CD4+ (T-helper [TH]) lymphocytes significantly increased in the lymph nodes of TDI-exposed groups while those of the CD3+CD8+ cells decreased as compared to in control mice. These studies, the first to assess TDI-induced changes in levels of three key cytokines in BALF in conjunction with changes in local lymph nodes following first an intra-nasal and then a general inhalation exposure to a low-level of TDI, confirm that TDI inhalation induces a pathology manifested by airway inflammation, TH cell-derived cytokine production, and shifts in lymph node lymphocytes sub-populations toward increases in TH cells.
Introduction
Toluene diisocyanate (TDI), used for the manufacture of polyurethane foams, paints, coatings, elastomers, adhesives, and many other products (NIOSH, Citation1996; Wisniewski et al., Citation2006), is a recognized human irritant and one of the leading causes of occupational asthma (OA) in industrialized countries (Chan-Yeung & Lam, Citation1986). It is estimated that as many as 5–20% of exposed workers develop asthma, characterized by symptoms like cough, chest tightness, short of breath, and wheezing, and which may persist indefinitely even in the absence of continued exposure (Johnson et al., Citation2004; Redlich & Karol, Citation2002).
Sensitization to a low-molecular-weight agent such as TDI is considered to result from a response of the immune system to haptens conjugated with endogenous proteins. However, the exact pathways and mechanisms of sensitization to such chemicals and the pathogenesis of the subsequent respiratory reactions are much less well understood, as they seem to differ from those of classic IgE-mediated asthma (Fisseler-Eckhoff et al., Citation2011; Raulf-Heimsoth & Baur, Citation1998; Sastre et al., Citation2003).
Classical and novel animal models have been used to investigate exposure determinants, epitope identity, and the role played by the immune system in OA in order to recapitulate disease phenotype and further the understanding of its pathogenesis. Data suggest that a murine model is the best experimental model for diisocyanate OA. Mice have a better-defined genome compared to guinea pigs and rats, and there is a greater availability of immunological reagents and different varieties of transgenic animals (Gelfand, Citation2002; Isenberg-Feig et al., Citation2003; Karol, Citation1991).
To date, the results published remain controversial regarding TDI-induced effects on T-helper lymphocyte type 1 (Th1) or 2 (Th2) responses. It is possible that the divergence in these results might be due partly to differing animal experimentation conditions. As such, there is a need for development of appropriate animal model TDI-induced asthma. Thus, the present study was undertaken to investigate an experimental murine model of TDI-induced asthma and to clarify the role of (Th1) or 2 (Th2) lymphocytes in any observed outcomes. In these studies, the cellular composition (reflecting inflammation) and concentrations of certain cytokines (interleukin [IL]-4, tumor necrosis factor [TNF]-α, and interferon [IFN]-γ) in bronchoalveolar lavage fluid (BALF), and changes among sub-populations of lymphocytes in host lymph nodes, were assessed to perform this clarification.
Materials and methods
Animals
Specific pathogen-free female BALB/cJ/Han/IMP mice (≈15–20 g, 6-weeks-old) were purchased from a breeding farm at the Nofer Institute of Occupational Medicine (Lodz, Poland). This particular strain of mice was selected for use in these studies based on data from the literature (Lee et al., Citation2001; Sun et al., Citation2006; Vanoirbeek et al., 2009a). All mice were housed in a conventional animal house with stable temperature (19–20 °C), relative humidity (55–60%), and a 12-h light/dark cycle. All animals had ad libitum access to standard rodent chow and filtered water. All mice were acclimated for 1 week prior to use in any experiment. During the experiment, body weight, overall health, and general behavior of the mice were assessed daily. The local Ethical Committee for Animal Experiments at the Nofer Institute of Occupational Medicine approved all procedures used herein.
Treatment protocol
At the time of the experiment, mice were randomly assigned into two groups: controls (n = 16) that were sensitized and challenged only with the solvent (ethyl acetate/olive oil [EAOO] 1:4); or exposed (n = 32) that were exposed to 2,4-TDI. All mice were sensitized by means of intra-nasal administration of 20 µl of a 3% (w/v) solution of TDI (Sigma, St. Louis, MO) dissolved in EAOO (1:4). Treatments were once daily for 5 consecutive days; after a 3-week interval, a second round of sensitizations was undertaken (Days 27–31) (Scheerens et al., Citation1996). Beginning 7 days after the end of the second treatment set (i.e. Day 38), mice were challenged whole-body to atmospheres containing 3% TDI dissolved in EAOO (1:4) for 4 h/day, 5 days/week, for 5 weeks. During each exposure, chamber TDI levels, humidity, and temperature was monitored. TDI levels were measured using a TSE System (TSE System Gmbh, Bad Homburg, Germany). Based on pilot studies, it was expected that the mice were thus exposed each time to ≈20 ppb TDI (≈0.14 mg TDI/m3 [resulting in an absorbed dose of ≈19 596 µg/mouse]), a level that approximates the NOAEL (no observed adverse effect level) for humans (Sećko, Citation2010).
Control mice were sensitized and challenged using the same protocols, but received only an atmosphere containing EAOO. One day after the final exposure (Day 74), the mice were euthanized via overdose of pentobarbital and their lungs then processed (see below) and their retro-auricular lymph nodes were collected for study.
Bronchoalveolar lavage (BAL)
To perform the lavage, a cannula was placed in the trachea and 3.2 ml of 0.9% NaCl solution (four portions, 0.8 ml each wash) was then used to lavage the lungs. Each wash was pooled, the total BAL fluid (BALF) volume was measured, and the BALF was then centrifuged at 1200 rpm for 10 min at 4 °C. The resultant supernatant was collected and stored at −70 °C for later study. The cell pellets were re-suspended in 1 ml phosphate-buffered saline (PBS, pH 7.4), and then aliquots removed and stained with toluidine blue for counting in a Bürker’s chamber. The number of cells/ml BALF was calculated; from this, the total number of cells recovered per mouse lung was derived. Other aliquots of the cells were placed on slides and then stained using May-Grunwald-Giemsa (MGG) and hematoxylin and eosin (H&E). Evaluation of the cytologic preparations was done by counting 300 non-epithelial cells and classifying them as macrophages, eosinophils, neutrophils, basophils, lymphocytes, or monocytes.
ELISA for culture supernatant cytokines
Levels of interleukin (IL)-4, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ were measured in undiluted supernatants of BAL fluid by a sandwich enzyme-linked immunosorbent assay (ELISA; R&D Systems, Abingdon, UK) according to manufacturer instructions. The sensitivity of the IL-4, TNFα, and IFNγ kits were < 2.0, 0.36, and < 2.0 pg/ml, respectively.
Lymph node cells
Retro-auricular lymph nodes recovered from each mouse were kept in Petri dishes in cold PBS until their cellular contents were released by straining the tissues over a 100-µm pore strainer (Becton Dickinson, San Jose, CA). After washing with PBS, cell viability was assessed via trypan blue exclusion and the number of cells was estimated in a Bürker chamber. Expression of CD4 or CD8 antigens on CD3+ cells was assessed with the use of a FACS Canto II Flow Cytometer and FACS Diva software (Becton Dickinson). Briefly, a sample of isolated lymph nodes cells (2 × 105 cells) in PBS was simultaneously incubated with 2.5 µg each of a monoclonal anti-mouse antibody against specific surface antigens, i.e. hamster anti-CD3ε (clone: 145-2C11), rat anti-CD4 (clone: RM4-5), and rat anti-CD8a (clone: 53-6.7) (all BD Pharmingen, San Jose, CA) for 30 min at room temperature in the dark. The cells were then washed three times in PBS and analyzed. Prior to each analysis, the system was calibrated using BD Cytometer Setup and Tracking Beads. A minimum of 20 000 events per sample was acquired.
Statistics
All data are shown as mean ± SD. Statistical analyses were done using a non-parametric Mann-Whitney test (Sigma Stat 3.5, San Jose, CA). A p value < 0.05 was considered significant.
Results
Pulmonary inflammation (BALF)
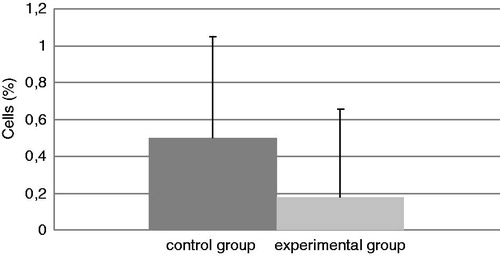
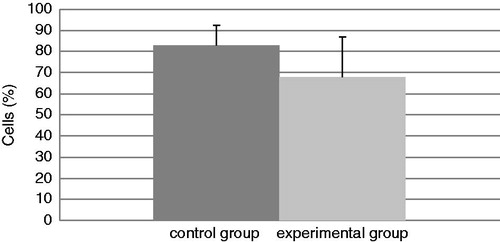
The total number of cells in the BALF of mice in each group at the end of the experiment is presented in . The experimental group had a significant increase in total cell counts of 188% relative to the values in the control mice. In addition, the levels of neutrophils (; an increase of 92%) and eosinophils (; in the control group there were no eosinophils) were significantly increased in BALF from TDI-challenged mice compared with that in BALF of their control counterparts. No differences were found in the amount of BALF lymphocytes and macrophages in the experimental mice compared with the control mice ( and ), even though the TDI mice had generally lower percentages of both cell types.
Figure 1. Total cell counts in BAL. Bars represent mean ± SD. n = 16/control group and n = 32/TDI group. *Value significantly different from control at p < 0.05.
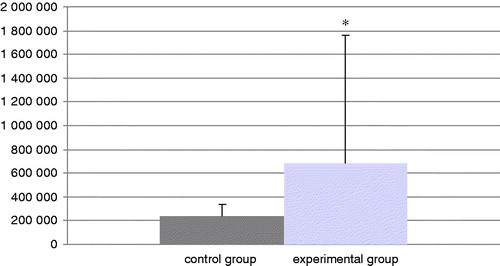
Figure 2. Relative percentage of neutrophils in all cells present in isolated BAL. Bars represent mean ± SD. n = 16/control group and n = 32/TDI group. *Value significantly different from control at p < 0.05.
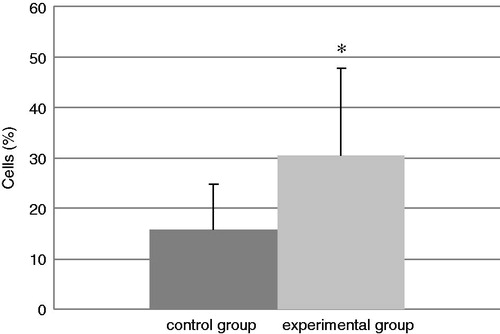
Figure 3. Relative percentage of eosinophils present in isolated BAL. Bars represent mean ± SD. n = 16/control group and n = 32/TDI group. *Value significantly different from control at p < 0.05.
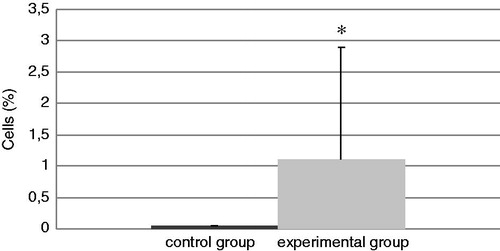
Cytokine levels in BALF
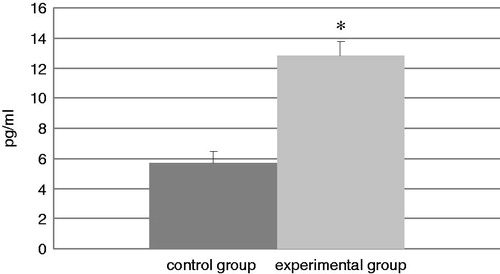
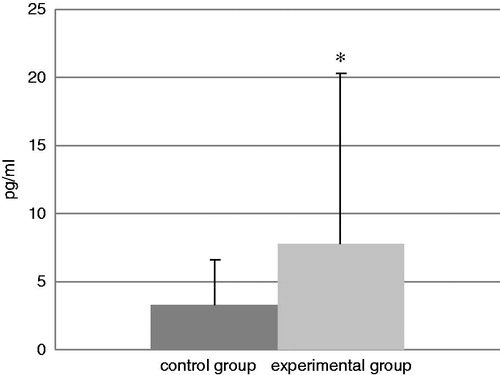
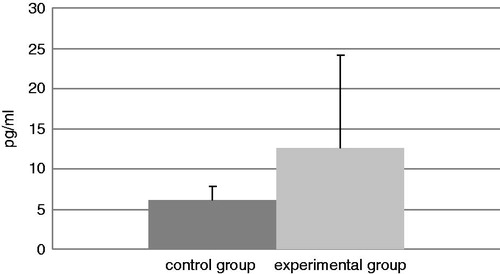
Data reflecting IL-4, TNFα, and IFNγ levels in BALF samples from mice in each group at the end of experiment are presented in, respectively, . The TDI-challenged mice showed a statistical increase in TNFα and IFNγ levels compared with those in the BALF of control mice. The levels of TNFα were increased in TDI mice by 125% relative to control levels and IFNγ levels were increased by 133%. In contrast, while the levels of IL-4 increased in the TDI group by 103%, the difference from the control levels was not statistically significant.
Figure 6. Levels of IL-4 in isolated BAL. Bars represent mean (pg/ml) ± SD. n = 16/control group and n = 32/TDI group.
Lymph node cells
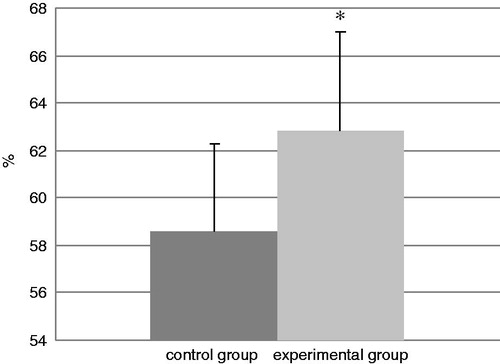
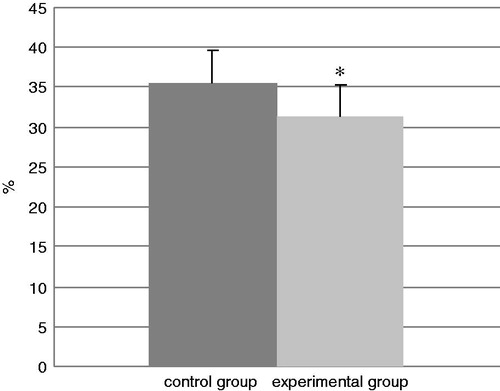
and show the percentages of various lymphocyte sub-populations in the retro-auricular lymph nodes removed on the last day of experiment (Day 74). It can be seen that the percentages of CD3+CD4+ lymphocytes were significantly increased in the lymph nodes of TDI-exposed mice from a control value of 58.6% to 62.8%. In contrast, the percentages of CD3+CD8+ lymphocytes were significantly decreased from a control value of 35.5% to 31.3%.
Discussion
Although animal models are not always closely reflective of human responses, it could certainly improve our understandings of the cellular and molecular mechanism associated with TDI-induced allergic disorders (Tarlo, Citation2003). Early studies using murine models demonstrated the involvement of the immune system in diisocyanate OA, as topical exposure to TDI resulted in the production of TDI-specific IgE antibodies and contact hypersensitivity. It was lately shown that CD4+ and CD8+ T-lymphocytes were important effector cells in these responses. However, these early models lacked evidence of respiratory inflammation (Dearman et al., Citation1992, Citation1996; Scheerens et al., Citation1996; Tse et al., Citation1979).
Newer models of murine diisocyanate OA vary greatly with regard to mice strain, the diisocyanate used, the route, timing, and minimum diisocyanate dose required to produce sensitization, as well as the inflammatory and physiologic responses noted. In the present study we used a murine model of TDI-induced asthma previously developed in our laboratory (Świerczyńska-Machura et al., Citation2012). Based on reports in the literature, the genetic background of a given mouse strain has a significant impact on the phenotypic outcome associated with TDI-induced asthma. de Vooght et al. (Citation2010), who developed TDI-induced asthma models using seven different mouse strains, concluded that phenotypical characteristics of chemically induced occupational asthma in humans were best reflected by BALB/c mice. Thus, in our protocol, using BALB/cJ/Han/IMP mice, it was seen that repetitive TDI airway exposure induced marked increases in inflammatory leukocytes, especially neutrophils and some eosinophils, in BALF.
There are similar observations to those provided by Sun et al. (Citation2006) who demonstrated that a neutrophil-dominant inflammation (with a few eosinophils also infiltrating) occurred in the peri-bronchial and peri-vascular regions of the lungs of TDI-exposed BALB/c mice. Moreover, in a murine model of TDI asthma applied by Lee et al. (Citation2001, Citation2011), intra-nasal sensitization with TDI followed by respiratory challenge with ultrasonically-nebulized TDI resulted in airway hyper-responsiveness, inflammatory infiltrates around the bronchioles, and a predominantly neutrophilic response. While Herrick et al. (Citation2002) have published about a murine model of HDI asthma wherein BALB/c mice were epicutaneously sensitized with HDI followed by intranasal respiratory challenge with HDI-albumin, this approach resulted in BALF eosinophilia and a marked lung inflammation comprised mostly of lymphocytes and eosinophils.
Although the data concerning the studies provided on animal models are controversial, there are many papers where, in TDI-induced asthma in human subjects, a neutrophilia in BAL fluid was found associated with asthmatic reactions induced by isocyanate challenge (Fabbri et al., Citation1987). Neutrophils could be an important source of pro-inflammatory cytokines and proteolytic enzymes (Cassatella, Citation1995; Sibille & Marchandise, Citation1993).
In the experiment here, the role of inflammatory mediators in TDI-induced asthma was also investigated. TDI is capable of inducing different types of immune reactions, depending on T-lymphocyte polarization toward T-helper Type 1 (TH1) or 2 (TH2) cells. TH1 lymphocytes promote cell-mediated immunity and are defined by their secretion of cytokines, mainly IFNγ and TNFα. TH2 cells are recognized by their secretion of interleukins, such as IL-4, IL-5, and IL-13, that support humoral immune responses. Because of this, concentrations of some TH1 and TH2 cytokines in BALF from the mice here were assessed. The study here showed that there were non-significant increases in levels of IL-4 but significantly increases in levels of IFNγ and TNFα in the BALF of the TDI mice.
Even at this date, published results concerning the effects on T-lymphocyte responses still seem to conflict. Ban et al. (Citation2006) described four models of TDI-induced asthma, and showed that the immune-mediated mechanisms depended on the route of TDI administration. Topical application followed by intra-tracheal instillations of TDI resulted in local and systemic TH2-dominated immune responses associated with a rise in IL-4, -5, and -13 levels local lymph nodes. In contrast, when TDI was administered by inhalation only, it failed to provoke a vigorous TH2 response, with only a slight increase in IL-5 production and in serum total IgE levels being induced.
Our data was consistent with the idea that sub-chronic TDI sensitization involves a mixed TH1/TH2 response. Other authors have noted significant increases in expression of TH2 cytokines IL-4 and IL-5, as well as the TH1 cytokine IFNγ, in mice exposed to 20 ppb TDI for 6 weeks (4 h/day) in inhalation chambers (Matheson et al., Citation2005). In the current study, there were significant increases in TNFα in the BALF of TDI-exposed mice. Earlier reports showed that TNFα is likely a central mediator of airway inflammation and bronchial hyper-responsiveness in asthma (Oosterhout & Nijkamp, Citation1993). Using TNFα-deficient mice, Matheson et al. (Citation2002) identified TNFα as an integral pro-inflammatory cytokine in disease development. Increased levels of airway IL-4 and TNFα mRNA, a predominantly neutrophilic inflammation into the lungs, and a migration of airway dendritic cells to the draining lymph nodes were specifically documented. We also noted a significant increase of IFNγ in the BALF of TDI-exposed mice. Other investigators have shown that, in IFNγ-deficient mice, there was reduced extent of allergic inflammation, lower levels of specific antibodies in the serum, and less severe pathologic changes in the epithelium of the lung tissue (Lee et al., Citation2011; Matheson et al., Citation2005; Vanoirbeek et al., 2009b).
Many experiments have been conducted using lymphocytes from TDI-sensitized mice to determine the role of specific immunity in the response to TDI. Studies in humans have found both CD4+ and CD8+ T-lymphocytes presence in the airways of asthmatics (Krug et al., Citation1996; Magnan et al., Citation2000; van Rijt & Lambrecht, Citation2001). In several studies, elevated levels of induced immune cells, i.e. CD4+ but also CD8+ T-lymphocytes, and different cytokines were demonstrated in biopsies, bronchoalveolar lavage (BAL), and the sputum of patients with isocyanate-induced asthma (Boulet et al., Citation2007; Maestrelli et al., Citation1994, Citation1997; Piirilä et al., Citation2008; Wisnewski et al., Citation2008). The majority of T-lymphocyte clones derived from the bronchial mucosa of patients with OA present a CD8 phenotype, which produce IFNγ and IL-5 (Maestrelli et al., Citation1994; Wisnewski et al., Citation2003). Experimental data also showed that the TH and the Tcytotoxic lymphocytes appear to be important for ventilatory, inflammatory, and immunologic responses in mouse models of TDI-induced asthma, since these responses are lacking in CD4- and CD8-knockout mice (Matheson et al., Citation2005). However, in our experiments, an increased percentage of CD4+ and a decreased percentage of CD8+ T-lymphocytes were found in the lymph nodes of the TDI-exposed hosts. This seemed to lend support to the idea that CD4+ lymphocyte production was more crucial than CD8+ cells in the pathogenesis of diisocyanate-induced asthma.
In conclusion, our studies on a murine model of TDI-induced asthma confirmed that low-level subchronic TDI inhalation induced a pathology that was consistent with allergic asthma, one manifested by airway inflammation, production of TH-lymphocyte-derived cytokines, and shifts in lymphocyte sub-population profiles in lymph nodes. The results reported here were in line with previous studies and confirmed our theory that the previously-documented divergences in outcomes reported in the literature were most likely a result of different animal experimental conditions, e.g. sensitization method employed and dose applied. However, these studies did not provide definitive answers to the question about the nature of the lymphocyte activation in TDI-induced asthma.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ban, M., Morel, G., Langonné, I., et al. 2006. TDI can induce respiratory allergy with TH2-dominated response in mice. Toxicology 218:29–47
- Boulet, L. P., Lemière, C., Gautrin, D., and Cartier, A. 2007. New insights into occupational asthma. Curr. Opin. Allergy Clin. Immunol. 7:96–101
- Cassatella, M. A. 1995. The production of cytokines by polymorphonuclear neutrophils. Immunol. Today 16:21–26
- Chan-Yeung, M., and Lam, S. 1986. Occupational asthma. Am. Rev. Respir. Dis. 133:686–703
- de Vooght, V., Vanoirbeek, J. A., Luyts, K., et al. 2010. Choice of mouse strain influences the outcome in a mouse model of chemical-induced asthma. PLoS ONE. 5:e12581
- Dearman, R. J., Basketter, D. A., and Kimber, I. 1992. Variable effects of chemical allergens on serum IgE concentration in mice. Preliminary evaluation of a novel approach to the identification of respiratory sensitizers. J. Appl. Toxicol. 12:317–323
- Dearman, R. J., Moussavi, A., Kemeny, D. M., and Kimber, I. 1996. Contribution of CD4+ and CD8+ T-lymphocyte subsets to cytokine secretion patterns induced in mice during sensitization to contact and respiratory chemical allergens. Immunology 89:502–510
- Fabbri, L. M., Boschetto, P., Zoca, E., et al. 1987. Bronchoalveolar neutrophilia during late asthmatic reactions induced by toluene diisocyanate. Am. Rev. Respir. Dis. 136:36–42
- Fisseler-Eckhoff, A., Bartsch, H., Zinsky, R., and Schirren, J. 2011. Environmental isocyanate-induced asthma: Morphologic and pathogenetic aspects of an increasing occupational disease. Int. J. Environ. Res. Public Health 8:3672–3687
- Gelfand, E. W. 2002. Pro: Mice are good model of human airway disease. Am. J. Respir. Crit. Care Med. 166:5–6
- Herrick, C. A., Xu, L., Wisnewski, A. V., et al. 2002. A novel mouse model of diisocyanate-induced asthma showing allergic-type inflammation in the lung after inhaled antigen challenge. J. Allergy Clin. Immunol. 109:873–878
- Isenberg-Feig, H., Justice, J. P., and Keane-Myers, A. 2003. Animal models of allergic asthma. Curr. Allergy Asthma Rep. 3:70–78
- Johnson, V. J., Matheson, J. M., and Luster, M. I. 2004. Animal models for diisocyanate asthma: Answer for lingering questions. Curr. Opin. Allergy Clin. Immunol. 4:105–110
- Karol, M. H. 1991. Comparison of clinical and experimental data from an animal model of pulmonary immunologic sensitivity. Ann. Allergy 66:485–489
- Krug, N., Madden, J., Redington, A. E., et al. 1996. T-Cell cytokine profile evaluated at the single cell level in BAL and blood in allergic asthma. Respir. Cell Mol. Biol. 14:319–326
- Lee, S. H., Jang, A. S., Kwon, J. H., et al. 2011. Mesenchymal stem cell transfer suppresses airway remodeling in a toluene diisocyanate-induced murine asthma model. Allergy Asthma Immunol. Res. 3:205–211
- Lee, Y. C., Song, C. H., Lee, H. B., et al. 2001. A murine model of toluene diisocyanate-induced asthma can be treated with matrix metallo-proteinase inhibitor. J. Allergy Clin. Immunol. 108:1021–1026
- Maestrelli, P., Del Prete, G. F., De Carli, M., et al. 1994. CD8 T-cell clones producing IL-5 and IFNγ in bronchial mucosa of patients with asthma induced by toluene diisocyanate. Scand. J. Work Environ. Health 20:376–381
- Maestrelli, P., Occari, P., Turato, G., et al. 1997. Expression of IL-4 and IL-5 proteins in asthma induced by toluene diisocyanate (TDI). Clin. Exp. Allergy 27:1292–1298
- Magnan, A. O., Mely, L. G., Camilla, C. A., et al. 2000. Assessment of the TH1/TH2 paradigm in whole blood in atopy and asthma. Increased IFNγ-producing CD8+ T-cells in asthma. Am. J. Respir. Crit. Care Med. 161:1790–1796
- Matheson, J. M., Johnson, V. J., and Luster, M. I. 2005. Immune mediators in a murine model for occupational asthma: Studies with toluene diisocyanate. Toxicol Sci. 84:99–109
- Matheson, J. M., Lemus, R., Lange, R. W., et al. 2002. Role of TNF in toluene diisocyanate asthma. Am. J. Respir. Cell Mol. Biol. 27:396–405
- NIOSH. 1996. Preventing asthma and death from diisocyanate exposure. Public Health Service, CDC/NIOSH Alert: 96–111. Atlanta, GA: NIOSH
- Oosterhout, A. J., and Nijkamp, F. P. 1993. Role of cytokines in bronchial hyper-responsiveness. Pulm. Pharmacol. 6:225–236
- Piirilä, P. L., Meuronen, A., Majuri, M. L., et al. 2008. Inflammation and functional outcome in diisocyanate-induced asthma after cessation of exposure. Allergy 63:583–591
- Raulf-Heimsoth, M., and Baur, X. 1998. Patho-mechanisms and pathophysiology of isocyanate-induced diseases. Summary of present knowledge. Am. J. Ind. Med. 34:137–148
- Redlich, C. A., and Karol, M. H. 2002. Diisocyanate asthma: Clinical aspects and immunopathogenesis. Int. Immunopharmacol. 2:213–224
- Sastre, J., Vandenplas, O., and Park, H. S. 2003. Pathogenesis of occupational asthma. Eur. Resp. J. 22:364–373
- Scheerens, H., Buckley, T. L., Davidse, E. M., et al. 1996. Toluene diisocyanate-induced in vitro tracheal hyper-reactivity in the mouse. Am. J. Respir. Crit. Care Med. 154:858–865
- Sećko, R. 2010. Documentation of maximum admissible values of occupational exposure. PiMOŚP 2(64). Warsaw: Central Institute for Labor Protection
- Sibille, Y., and Marchandise, F. X. 1993. Pulmonary immune cells in health and disease: Polymorphonuclear neutrophils. Eur. Resp. J. 6:1529–1543
- Sun, L. Z., Elsayed, S., Bronstad, A. M., et al. 2006. Airway inflammation and bronchial remodeling in toluene diisocyanate-exposed BALB/c mouse model. Scan. J. Immunol. 65:118–125
- Świerczyńska-Machura, D., Walusiak-Skorupa, J., Nowakowska-Świrta, E., et al. 2012. Immunological determinants in a murine model of toluene diisocyanate-induced asthma. Int. J. Occup. Environ. Health 25:492–498
- Tarlo, S. M. 2003. Occupational asthma: A valid model for adult asthma? Curr. Opin. Allergy Clin. Immunol. 3:91–94
- Tse, C. S., Chen, S. E., and Bernstein, I. L. 1979. Induction of murine reaginic antibodies by toluene diisocyanate. An animal model of immediate hypersensitivity reactions to isocyanates. Am. Rev. Respir. Dis. 120:829–835
- Vanoirbeek, J. A., de Vooght, V., Nemery, B., and Hoet, P. H. 2009a. Multiple challenges in a mouse model of chemical-induced asthma lead to tolerance: Ventilatory and inflammatory responses are blunted, immunologic humoral responses are not. Toxicology 257:144–152
- Vanoirbeek, J. A., Tarkowski, M., de Vooght, V., et al. 2009b. Immunological determinants in a mouse model of chemical-induced asthma after multiple exposures. Scand. J. Immunol. 70:25–33
- van Rijt, L. S., and Lambrecht, B. N. 2001. Role of dendritic cells and TH2 lymphocytes in asthma: Lessons from eosinophilic airway inflammation in mouse. Miscrosc. Res. Tech. 53:256–272
- Wisnewski, A. V., Herrick, C. A., Liu, Q., et al. 2003. Human γδ T-cell proliferation and IFNγ production induced by hexamethylene diisocyanate. J. Allergy Clin. Immunol. 112:538–546
- Wisnewski, A. V., Liu, Q., Liu, J., and Redlich, C. A. 2008. Human innate immune responsem to hexa-methylene diisocyanate (HDI) and HDI-albumin cojugates. Clin. Exp. Allergy 38:957–967
- Wisniewski, A. V., Redlich, C. A., Mapp, C. E., and Bernstein, D. I., (Eds.). 2006. Poly-isocyanates and their pre-polymers. In: Asthma in the Workplace, 3rd Edition. New York: Taylor and Francis Group, pp. 481–504