Abstract
Neutrophils (PMN) play diverse regulatory and effector functions in the immune system through the release of reactive nitrogen species, including nitric oxide (NO). The enzyme responsible for NO synthesis in PMN is inducible nitric oxide synthase (iNOS) that is regulated by various signaling pathways, e.g. PI3K-Akt/PKB, and transcription factors. N-Nitrosodimethylamine (NDMA), a xenobiotic widespread in the human environment, affects immune cells. The study objective here was to examine the role of the PI3K-Akt/PKB pathway in induction of NO synthesis (with involvement of iNOS) in human PMN, as well as in autologous mononuclear cells (PBMC), exposed to NDMA. Isolated cells were incubated for 2 h with a sub-lethal dose of NDMA and then the expression of several select proteins in the cell cytoplasmic and nuclear fractions were determined by Western blot analyses. The results indicated that NDMA enhanced expression of iNOS, phospho-PI3K, and phospho-IκBα in the cytoplasmic fraction of the PMN and PBMC. The nuclear fraction of these cells also had a higher NF-κB expression. Moreover, in PMN, NDMA caused an increased expression of phospho-Akt (T308), phospho-Akt (S473), and phospho-IKKαβ in the cytoplasm, and c-Jun and FosB in the nuclear fraction. Blocking of PI3K caused a decrease in expression of all these proteins in NDMA-exposed PMN. However, inhibition of PI3K led to a drop in expression of iNOS, phospho-PI3K, and phospho-IκBα in the cytoplasm, and in NF-κB in the nuclear fraction, of PBMC. The results of these studies indicated to us that NDMA activates the PI3K-Akt/PKB pathway in human PMN and that this, in turn, contributes to the activation of transcription factors NF-κB, c-Jun, and FosB involved in NO production (through modulation of iNOS expression).
Introduction
N-Nitrosodimethylamine (NDMA) is used in some processes in the plastics industry, as well as in metallurgy and rubber production. NDMA also is found in tobacco products and foods, especially processed products (Lijinsky, Citation1992; Tricker & Preussmann, Citation1991). NDMA, a very toxic nitrosamine, possesses carcinogenic, mutagenic, and teratogenic potentials. NDMA toxicity is primarily associated with industrial exposure, mainly via inhalation; acute intoxications (most of criminal nature) have been reported (Pedal et al., Citation1982). In general, the long-term effects from exposure to NDMA, even at low doses, include organ damage and neoplastic transformation of cells (Belinsky et al., Citation1990; Souliotis et al., Citation1998). Fussgaenger and Ditschuneit (Citation1980) reported that a single occupational exposure to 50–100 mg NDMA led to liver damage and cirrhosis. Data about acute effects of NDMA have indicated that cytotoxicity occurred in hepatocytes in culture at 0.1 mM, and at 3–50 µg/ml in kidney/lung cells (Brendler et al., Citation1992; Jiao et al., Citation1993).
It is been suggested that NDMA also might affect immune cells. Nitric oxide (NO) is a versatile regulator of numerous bodily processes and a major signaling molecule. Inducible nitric oxide synthase (iNOS) is found in various cells, including neutrophils (PMN) (Beck et al., Citation1999; Mantovani et al., Citation2011; Nworu et al., Citation2011). iNOS activity, both at the level of synthesis and protein expression, can be regulated by endogenous and exogenous factors (Lirk et al., Citation2002; Moodley, Citation2002). Thus, it is plausible that NDMA could affect immune cells, in part, by affecting NO formation and that this in turn leads to modulated immune function in the exposed host.
It has been our contention that NDMA likely affects immune cells in a manner dependent on activation of various signal pathways. Our earlier studies in human PMN exposed to NDMA noted changes in these cells (i.e. in NO formation via iNOS) that were suggestive of a role for NF-κB and transcription factor AP-1 family members (activated by MAP p38 and JNK kinase) (Ratajczak-Wrona et al., Citation2013a, Citationb). Activation of AP-1 via de novo synthesis of its components, and through phosphorylation of already existing ones, can be stimulated by the action of various signaling pathways. According to data, heterodimer AP-1 (composed of c-Jun and c-Fos protein) shows the highest transcriptional efficacy (Foletta et al., Citation1998; Shaulian & Karin, Citation2002).
Data indicate that other signaling pathways, such as PI3K-Akt/PKB, play essential roles in iNOS activation and NO production (Kroncke et al., Citation1995; Kristof et al., Citation2006; Wright et al., Citation1997). In particular, the PI3K-Akt/PKB pathway plays a key role in regulation of cell proliferation, differentiation, metabolic homeostasis, and survival (Hawkins et al., Citation2010; Koyasu, Citation2003). In the pathway, activation of phosphatidylinositol 3-kinase (PI3K) converts phosphatidyl-inositol 4,5-bisphosphate (PIP2) into phosphatidylinositol.3.4.5.triphosphate (PIP3) in response to extra-cellular stimulus. PIP3 accumulation causes translocation of serine-threonine kinase Akt (protein kinase B [PKB]) and phosphoinositide-dependent kinase 1 (PDK1) from the cytosol to the cell membrane. Activated PDK1 phosphorylates Thr308 in the PKB.Akt kinase catalytic domain (Foster et al., Citation2003; Hanada et al., Citation2004). Full activation of Akt/PKB kinase also requires Ser473 phosphorylation in the C-terminal domain. With these phosphorylations, activated Akt/PKB kinase can then inhibit or activate various proteins (e.g. protein kinase IKK) and transcription factors like AP-1 (Hanada et al., Citation2004; Manning & Cantley, Citation2007; Romashkova & Makarov, Citation1999). Activated protein kinase IKK, via phosphorylation of IκB inhibitor, leads to its ubiquitination and degradation, and consequent release of NF-κB that translocates from the cytosol to nucleus, and induces gene transcription (Hayden & Ghosh, Citation2004). Additional data indicate that PI3K could induce iNOS dimerization and promote its activity in cells (Sakai et al., Citation2006). Further, a recent study showed that PI3K could also modulate iNOS expression through effects on the expression of a wide range of cytokines (Xia et al., Citation2012).
The objective of the current study was to examine the role of the PI3K-Akt/PKB pathway in induction of NO synthesis (with iNOS involvement) in human neutrophils exposed to NDMA. Contribution of this pathway to iNOS regulation was also assessed in mononuclear cells (PBMC) for comparison. Since the PI3K-Akt/PKB pathway activation manifests by a presence of phospho-PI3K, -AKT (Thr308) and -Akt (Ser473) proteins, their expression in the cytoplasm of PMN and PBMC was also used to confirm or exclude involvement of the pathway in NDMA-induced activation of iNOS. Likewise, investigation of the expression of transcription factors like NF-κB, c-Jun, and FosB induced by the pathway in PMN and PBMC were also done to ensure a more comprehensive knowledge of molecular mechanisms underlying NDMA-triggered induction of NO synthesis/release in leukocytes. Ultimately, the experiments here would allow for a clarification of potential causes of impaired innate responses in subjects who might be exposed to the xenobiotic NDMA on the job or in foods they consume.
Materials and methods
Reagents
NDMA, Griess reagent and BCIP/NBT Liquid substrate system were purchased from Sigma (Steinheim, Germany). Wortmannin was obtained from Calbiochem (San Diego, CA). Laemmli buffer, Tris-Buffered Saline (TBS)/Caseine buffer and TBS-T (containing: Tris-Buffered Saline and Tween 20) were purchased from BioRad Laboratories (Hercules, CA).
Antibodies
Monoclonal mouse antibodies against iNOS, PI3K, phospho-Akt (T308), NF-κB, c-Jun, FosB and β-actin, polyclonal goat antibodies against phospho-IKKαβ, phospho-IκBα, and phospho-PI3K protein, as well as polyclonal rabbit antibodies against Akt and phospho-Akt (S473) were purchased from Santa Cruz Biotechnology (Heidelberg, Germany). Goat anti-mouse IgG antibody conjugated with alkaline phosphatase (AP), donkey anti-goat IgG antibody conjugated with alkaline phosphatase and goat anti-rabbit IgG antibody conjugated with alkaline phosphatase were obtained from Vector Laboratories (Burlingame, CA). Monoclonal mouse antibody against the Poly(ADP-ribose)polymerase (PARP-1) was purchased from Calbiochem.
Isolation and incubation of PMN and PMBC
The study involved a group of 20 healthy (age 20–50 years) volunteer blood donors from the Regional Centre for Transfusion Medicine (Bialystok, Poland). Males were chosen to avoid possible influences due to endogenous hormones on the experimental findings. The Ethics Committee of the Medical University of Bialystok (R-I-002/335/2012) approved this study. All persons gave written informed consent prior to blood donations.
PMN and PBMC were isolated from heparinized (10 U/ml – Heparin, Polfa, Lodz, Poland) whole blood by density centrifugation using Gradisol G gradient 1.115 g/ml (Polfa) (Zeman et al., Citation1988). This method enables simultaneous separation of two highly purified leukocyte fractions: PMN (containing 91% PMN) and PBMC (containing 94% lymphocytes). The purity of isolated PMN and PBMC was determined by MayGrunewald-Giemsa. Sera were obtained from blood samples collected without anti-coagulation agents.
The two highly purified PMN and PBMC fractions were each suspended at a concentration of 5 × 106 cells/ml in Hanks’ Balanced Salt Solution (Invitrogen, Carlsbad, CA) containing the subject’s own serum (7.4%, 20/270 µl), 100 U penicillin/ml, and 50 ng streptomycin/ml (Polfa Trachomin SA, Warsaw, Poland). The cells (200 µl aliquots) were then placed into wells of microplates (Microtest III-Falcon, BD Biosciences, Bedford, MA) and incubated for 2 h at 37 °C in a 5% CO2 incubator (NuaireTM US Autoflow, Plymouth, MN). PMN and PBMC in the wells were then treated with 20 µl NDMA to attain a final concentration of 0.74 µg NDMA/µl in the well; control wells received vehicle only. This dose was selected based on preliminary studies that determined the cytotoxic effects of NDMA on these cell types (Ratajczak-Wrona et al., Citation2013c); metabolic activity was decreased in the leukocytes only among cells incubated with ≥ 0.74 µg NDMA/µl. The PMN and PBMC were then cultured a further 2 h before culture supernatants were collected. Assessments of viability (via trypan blue exclusion) showed that PMN and PBMC were still >93% viable after the treatment.
To determine the role of PI3K pathway in the regulation of iNOS expression in PMN exposed to NDMA, selective inhibitors of this pathway were used. In these studies, cells were pre-incubated with 0.1 μM wortmannin – a selective PI3K inhibitor – for 1 h before addition of NDMA. Preliminary studies showed that the presence of the inhibitor did not affect cell viability.
Protein isolation and Western blot analyses
Cytoplasmic and nuclear extracts from PMN and PBMC (3 × 106 cells total/sample) were prepared using NucBusterTM Protein Extraction Kit (Calbiochem). Step-wise extraction delivered two distinct cellular protein fractions: cytoplasmic and nuclear. The concentration of protein in each was determined with a QubitTM Protein Assay Kit (Invitrogen). An antibody against PARP-1 (1:5000) and against β-actin (1:100) was used as an internal control within the nuclear and cytoplasmic fractions, respectively.
The extracts were suspended in Laemmli buffer, loaded at 20 µg/well, and then electro-phoresed over a 4% stacking and a 10% separating SDS-PAGE gel. The resolved proteins were electrotransferred onto 0.45 -µm pore-size nitrocellulose membranes (BioRad) then blocked with Tris-Buffered Saline (TBS)/Caseine buffer, washed with TBS-T (TBS with 0.05% Tween-20), and incubated with QentixTM Western Blot Signal Enhancer (Thermo Fisher Scientific, Rockford, IL). The membranes were then incubated for 10 min at room temperature in SNAP (Protein Detection System; Millipore, Billerica, MA) with 1:100 dilutions of primary monoclonal antibody against iNOS, PI3K, phospho-Akt (T308), NF-κB, c-Jun, FosB, or primary polyclonal antibodies against phospho-IKKαβ, phospho-IκBα, phospho-PI3K, Akt or phospho-Akt (S473). After washing with 0.1% TBS-T, the membrane was incubated at room temperature with alkaline phosphatase anti-mouse IgG Ab, anti-rabbit IgG Ab or anti-goat IgG Ab (1:200). Immuno-reactive bands were then visualized using a BCIP/NBT Liquid substrate system; intensities were determined using ImageJ software (Bethesda, MD) and reported as Arbitrary Units (AU).
Assay for nitrite production
Synthesis of NO was determined by an assay of the culture supernatant for nitrite, a stable reaction product of NO with molecular oxygen. Total NO concentration is commonly determined as a sum of the nitrite and nitrate concentrations present. NO production by PMN and PBMC was determined using an indirect method based on measurement of nitrite concentration in culture supernatants according to a Griess reaction (Schulz et al., Citation1999). In the samples analyzed, nitrate was reduced to nitrate in the presence of cadmium, and then converted to nitric acid that yielded a color reaction with Griess reagent. Nitrite concentrations were determined by spectrophotometric analysis at 540 nm with extrapolation from a standard curve prepared in parallel. Nitric oxide products were expressed as µM (106 cells in 270 µl supernatant).
Statistics
Results were analyzed using Statistica version 9.1. (StatSoft, Inc., Tulsa, OK). Data distribution normality was determined using a Kolmogorov-Smirnov test. Since data were not normally distributed, for comparison of variations between assayed groups, Mann-Whitney U non-parametric tests were applied to un-related results. A p-value ≤0.05 was accepted as statistically significant. All data are presented as mean ± SE.
Results
Assessment of protein expression by Western blot
The exposure of neutrophils and mononuclear cells to N-Nitrosodimethylamine (NDMA) resulted in a simultaneous increase in expression of iNOS, phospho-PI3K, and phospho-IκBα in the cytoplasmic fraction as compared to in cells incubated without NDMA (). Moreover, the cytoplasmic fraction of PMN showed higher expressions of phospho-Akt (T308), phospho-Akt (S473) and phospho-IKKαβ. However, the expressions of these proteins were lower in the cytoplasmic fraction of the mononuclear cells ().
Figure 1. Expression of iNOS, phospho-PI3K, PI3K, phospho-Akt (T308), phospho-Akt (S473), phospho-IKKαβ, and phospho-IκBα in PMN and PBMC. PMN (left four columns) and PBMC (right four columns) were treated with or without wortmannin (0.1 µM) for 1 h before addition of NDMA (0.74 µg/µl). (a) Cytoplasmic fractions obtained from those cells were used for assessment of iNOS, phospho-PI3K, PI3K, phospho-Akt(T308), phospho-Akt(S473), Akt, phospho-IKKαβ, and phospho-IκBα protein levels via Western blot analyses. Results shown are representative of five independent experiments. (b) Band intensity was quantified using ImageJ software and expressed in Arbitrary Units. Value significantly different between * cells without and with NDMA (p < 0.05) or # NDMA-treated pre-incubated with or without inhibitor (p < 0.05).
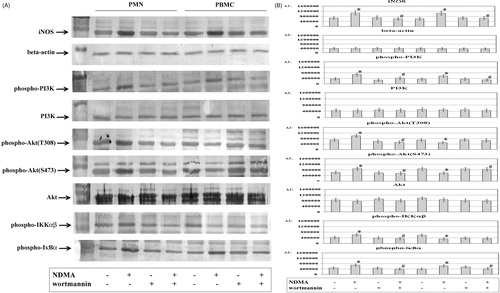
To confirm the involvement of PI3K in the induction of iNOS expression in PMN and PBMC exposed to NDMA, experiments were conducted with a selective inhibitor of this kinase. In the presence of the PI3K inhibitor, the expression of iNOS was found to be lower in the cytoplasmic fraction of PMN and PBMC exposed to NDMA as compared to the cells without the inhibitor. In the two groups, the expressions of phospho-PI3K and phospho-IκBα were decreased (). At the same time, the cytoplasm of neutrophils had a lower level of phospho-Akt (T308), phospho-Akt (S473), and phospho-IKKαβ. The use of wortmannin caused a higher expression of phospho-Akt (S473) in cytoplasm of PBMC exposed to NDMA, as compared to the cells without the inhibitor. Moreover, these cells exhibited no changes in the expression of phospho-Akt (T308) and phospho-IKKαβ as compared to cells without inhibitor.
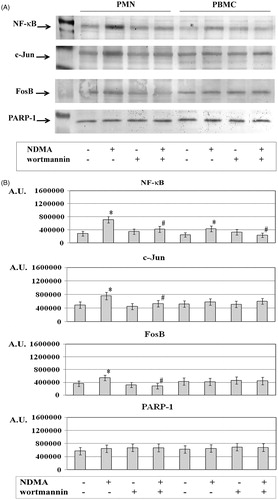
The treatment of neutrophils and mononuclear cells with NDMA also resulted in higher expression of NF-κB in the nuclear fraction as compared to in fractions from cells that did not receive the xenobiotic (). The nuclear fraction of neutrophils also exhibited a higher expression of c-Jun and FosB. However, expression of these transcription factors in the nuclear fraction of PBMC was similar to that in untreated cells. The incubation with the PI3K inhibitor resulted in a lowering of the expression of NF-κB in the nuclear fraction of both cell types exposed to NDMA. At the same time, the nuclear fractions of neutrophils had a lower level of c-Jun and FosB. In comparison, no changes were noted in levels of these items in nuclear fractions of PBMC.
Figure 2. Expression of NF-κB, c-Jun, and FosB in PMN and PBMC. PMN (left four columns) and PBMC (right four columns) were treated with or without wortmannin (0.1 µM) for 1 h before addition of NDMA (0.74 µg/µl). (a) The nuclear fractions obtained from those cells were used to detect for NF-κB, c-Jun, and FosB protein levels by Western blot analyses. Results shown are representative of five independent experiments. (b) Band intensity was quantified using ImageJ software and expressed in Arbitrary Units. Value significantly different between * cells without and with NDMA (p < 0.05) or # NDMA-treated pre-incubated with or without inhibitor (p < 0.05).
Assessment of total NO concentration in PMN supernatants
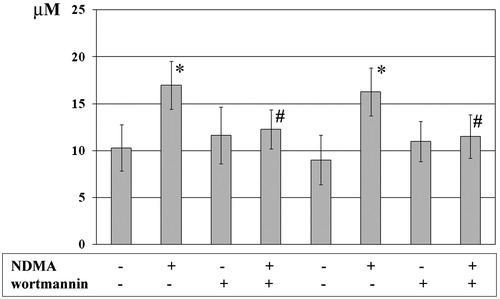
The exposure of neutrophils and mononuclear cells to NDMA confirmed earlier observations (Ratajczak-Wrona et al., Citation2011) and showed there was an increased release of nitric oxide (NO) as compared to among PMN that were not exposed (change from 10.31 [±2.45] to 16.99 [±2.55] µM (p < 0.05); ). Similarly, among the PBMC, there was a change from 9.01 [ ± 2.64] to 16.27 [±2.54] µM (p < 0.05). To determine any contribution of PI3K to the observed induction of NO production (via implied iNOS involvement), NO levels were assessed in the presence of a selective PI3K inhibitor (i.e. wortmannin, 0.1 µM). The cultures of PMN and mononuclear cells pre-incubated with the inhibitor and then NDMA displayed lower total NO levels as compared to those observed with NDMA-treated cells that did not receive the wortmannin. In the case of the PMN, the level was now reduced to near control values (i.e. 12.27 [±2.07] µM (p < 0.05)); for PBMC, the level was reduced to 11.51 [±2.33] µM (p < 0.05).
Figure 3. Concentrations of total NO from PMN and PBMC. PMN (left four columns) and PBMC (right four columns) were treated with or without wortmannin (0.1 µM) for 1 h before addition of NDMA (0.74 µg/µl). Two hours after addition of NDMA, culture medium was collected for use in measures of nitrite levels (as marker of NO production). Value significantly different between * cells without and with NDMA (p < 0.05) or # NDMA-treated pre-incubated with or without inhibitor (p < 0.05). NO products were expressed as µM and data are shown as mean (±SE) of 20 experiments.
Discussion
Studies conducted by various authors have indicated that NDMA affects immune cells, e.g. mononuclear cells and neutrophils (PMN) (Holsapple et al., Citation1985; Jablonski et al., Citation2011). It has been found that NDMA can impact on PMN phagocytic activity, oxygen metabolism, and functions associated with production and the release of immunologically-active molecules (Jablonska et al., Citation1996; Jablonski et al., Citation2007).
Research has revealed the involvement of PI3K in PMN migration, chemotaxis, phagocytosis, and oxygen burst (Hawkins et al., Citation2010). We noted increased expressions of phospho-PI3K, phospho-Akt (T308) and phospho-Akt (S473) in the cytoplasmic fraction of PMN exposed to NDMA, which confirms the involvement of NDMA in the activation of the PI3K-Akt/PKB pathway in these cells. However, the increase in expression of phospho-PI3K with a simultaneous decrease in phospho-Akt T308 and S473 expression in the cytoplasm of PBMC exposed to NDMA may suggest another mechanism is regulating these proteins in these cells.
Based on the noted changes in NO formation and iNOS expression in a presence of wortmannin in PMN and mononuclear cells exposed to NDMA, it may be assumed that the synthesis of NO with involvement of iNOS is associated with PI3K activation. However, in PMN the process also involves Akt kinase. The differences observed in PI3K-Akt/PKB pathway activity between the study cells may be a result of an effect of NDMA (and/or metabolites) on protein kinase C (PKC) in the mononuclear cells. Some data indicate a reduced level of PKC is accompanied by a decrease in Akt/PKB activity (Jakubowicz-Gil, Citation2009). It is believed PKC reduces levels of Hsp90 protein indispensable for Akt/PKB stabilization (Okhrimenko et al., Citation2005).
Moreover, the present study done with PBMC exposed to NDMA revealed increased phosphorylation of Ser473 residues accompanied by no change in phosphorylation of Thr308 in the Akt/PKB kinase domain after blocking of PI3K. The increased expression of phospho-Akt (S473) protein in these cells could be a consequence of autocatalytic phosphorylation. It was suggested that phosphorylated Thr and Ser in the Akt/PKB kinase domains could be found independent of one another, not only due to involvement of kinases but also of autocatalytic events (Hanada et al., Citation2004).
Available data show that activity of the proteins that form transcription factor AP-1 can be regulated at the transcription and post-transcription levels, and through phosphoylation of its components by various pathways (Karin et al., Citation1997). The current study showed the involvement of the PI3K-Akt/PKB pathway in activation of c-Jun and FosB in human PMN exposed to NDMA. Both our present study and previous observations suggest a convergence of various signal transduction pathways, e.g. MAP kinases and PI3K-Akt/PKB pathway, within human PMN exposed to NDMA, resulting in AP-1 activation. Taking into account the effect of NDMA on human PMN, including inducing NO production and iNOS activation, we also evaluated the role of c-Jun and FosB in induction of iNOS in mononuclear cells. Lack of change in expression of these transcription factors in PBMC exposed to NDMA suggested they were not activated. The study results and our earlier findings also indicated that NDMA activated the NF-κB pathway in PMN (Ratajczak-Wrona et al., Citation2013b). The current data showing enhanced expression of phospho-IKKαβ and -IκBα in the cytoplasm of PMN exposed to NDMA suggested activation of the pathway via IKK kinase (Wu et al., 2004; Nishikori, Citation2005; Wu & Miyamoto, Citation2007). However, reduced expression of both phospho-IKKαβ and -IκBα in the cytoplasm, as well as NF-κB in the nuclear fraction, of PMN exposed to NDMA (noted after blocking of PI3K) indicated Akt kinase involvement in activation of the NF-κB pathway. The essential component of IKK kinase activation is a transitory binding to Akt kinase that, in turn, contributes to its activation (Romashkova & Makarov, Citation1999).
The current findings are the first to show that the effect of NDMA on human mononuclear cells leads to NF-κB pathway activation in an IKK kinase-independent manner (Bonizzi & Karin, Citation2004; Perkins, Citation2007). In the light of our earlier study that revealed that the action of NDMA on human PBMC activated MAP kinases in the cells, p38 kinase seems of special importance (Ratajczak-Wrona et al., Citation2011). According to some data, cells may display atypical activation of the NF-κB pathway via p38 kinase, which by activating casein kinase (CK2) leads to IκBα phosphorylation within the C-terminal PEST domain; in the classical mechanism, the N-terminal fragment is involved (Perkins, Citation2007; Wu & Miyamoto, Citation2007). Some data indicate a different role of PI3K in induction of iNOS expression. Diaz-Guerra et al. (Citation1999) conducted a study on the LPS-stimulated macrophage cell line RAW 264.7 and found that PI3K inhibition led to enhancement of iNOS expression and NO production via NF-κB activation. Moreover, those researchers excluded involvement of Akt kinase in regulation of NO synthesis in the cells.
In conclusion, the present studies indicated that in human PMN, NDMA activated the PI3K-Akt/PKB pathway that, in turn, participated in activation of transcription factors c-Jun, FosB and NF-κB, each of which is involved in enhancing NO production (via iNOS). On the other hand, production of NO (with involvement of iNOS) by mononuclear cells exposed to the xenobiotic was apparently regulated by PI3K activity that, in turn, affected NF-κB pathway activation. These changes in PI3K activation in cells due to NDMA may cause disorders in both non-specific and specific immune responses. In particular, increases in NO production by cells due to NDMA could contribute to oxidative stress, a factor in the pathophysiology of numerous immune disorders. Our observations indicate complex and multi-stage regulation of iNOS and NO production in human leukocytes exposed to NDMA. Further studies are necessary to identify interactions between many cellular regulatory pathways impacted by this nitrosamine and to also determine the significance of each respective pathway in the regulation of NO production.
Declaration of interest
The study was supported by Medical University of Bialystok, Poland (Project no: 124-06502 F). The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
The authors thank Mrs Malgorzata Walko-Lachowicz for expert technical assistance.
References
- Beck, K. F., Eberhardt, W., Frank, S., et al. 1999. Inducible NO synthase: Role in cellular signaling. J. Exp. Biol. 202:645–653
- Belinsky, S. A., Devereux, T. R., and Anderson, M. W. 1990. Role of DNA methylation in the activation of proto-oncogenes and the induction of pulmonary neoplasia by nitrosamines. Mutat. Res. 233:105–116
- Bonizzi, G., and Karin, M. 2004. The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 25:280–288
- Brendler, S. Y., Tompa, A., Hutter, K. F., et al. 1992. In vivo and in vitro genotoxicity of several N-nitrosamines in extra-hepatic tissues of the rat. Carcinogenesis. 13:2435–2441
- Díaz-Guerra, M. J., Castrillo, A., Martín-Sanz, P., and Boscá, L. 1999. Negative regulation by phosphatidylinositol 3-kinase of inducible nitric oxide synthase expression in macrophages. J. Immunol. 162:6184–6190
- Foletta, V. C., Segal, D. H., and Cohen, D. R. 1998. Transcriptional regulation in the immune system: All roads lead to AP-1. J. Leukocyte Biol. 63:139–152
- Foster, F. M., Traer, C. J., Abraham, S. M., and Fry, M. J. 2003. The phosphoinositide (PI) 3-kinase family. J. Cell Sci. 116:3037–3040
- Fussgaenger, R. D., and Ditschuneit, H. 1980. Lethal exitus of a patient with N-nitrosodimethylamine poisoning, 2.5 years following the first ingestion and signs of intoxication. Oncology. J. 37:273–277
- Hanada, M., Feng, J., and Hemmings, B. A. 2004. Structure, regulation and function of PKB/AKT -- a major therapeutic target. Biochim. Biophys. Acta 1697:3–16
- Hawkins, P. T., Stephens, L. R., Suire, S., and Wilson, M. 2010. PI3K signaling in neutrophils. Curr. Top. Microbiol. Immunol. 346:183–202
- Hayden, M. S., and Ghosh, S. 2004. Signaling to NF-κB. Gene Dev. 18:2195–2224
- Holsapple, M. P., Bick, P. H., and Duke, S. S. 1985. Effects of N-nitrosodimethylamine on cell mediated immunity. J. Leukocyte Biol. 37:367–381
- Jablonska, E., Jablonski, J., and Pietruska, Z. 1996. Function of neutrophils (PMN) in nitrosodimethylamine (NDMA)-exposed rats. Bromatol. Chem. Toksykol. 29:85–89
- Jablonski, J., Jablonska, E., and Leonik, A. 2011. The effect of N-nitrosodimethylamine (NDMA) on Bax and Mcl-1 expression in human neutrophils. Environ. Contam. Tox. 87:638–642
- Jablonski, J., Jablonska, E., and Moniuszko-Jakoniuk, J. 2007. The effect of N-nitrosodimethylamine on TRAIL and DR5 expression in human neutrophils: Preliminary study. Immunopharmacol. Immunotoxicol. 29:287–296
- Jakubowicz-Gil, J. 2009. Inhibitors of PI3K-Akt/PKB-mTOR pathway in glioma therapy. Post. Biol. Kom. 36:189–201
- Jiao, J., Glickman, B. W., Anderson, M. W., and Zielinska, M. 1993. Mutational specificity of N-nitrosodimethylamine: Comparison between in vivo and in vitro assays. Mutat. Res. 301:27–31
- Karin, M., Liu, Z., and Zandi, E. 1997. AP-1 function and regulation. Curr. Opin. Cell Biol. 9:240–246
- Koyasu, S. 2003. The role of PI3K in immune cells. Nat. Immunol. 4:313–319
- Kristof, A. S., Fielhaber, J., Triantafillopoulos, A., et al. 2006. Phosphatidyl-inositol 3-kinase-dependent suppression of the human inducible nitric-oxide synthase promoter is mediated by FKHRL1. J. Biol. Chem. 281:23958–23968
- Kroncke, K. D., Fehsel, K., and Kolb-Bachofen, V. 1995. Inducible nitric oxide synthase and its product nitric oxide, a small molecule with complex biological activities. Biol. Chem. H-S. 376:327–343
- Lijinsky, W. (Ed.). 1992. Chemistry and biology of N-nitroso compounds. Cambridge: University Press Cambridge
- Lirk, P., Hoffmann, G., and Rieder, J. 2002. Inducible nitric oxide synthase; Time for reappraisal. Curr. Drug Targets 1:89–108
- Manning, B. D., and Cantley, L. C. 2007. AKT/PKB signaling: Navigating downstream. Cell 129:1261–1274
- Mantovani, A., Cassatella, M. A., Costantini, C., and Jaillon, S. 2011. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat. Rev. Immunol. 11:519–531
- Moodley, Y. P. 2002. The role of inducible nitric oxide in health and disease. Curr. Diag. Pathol. 8:297–304
- Nishikori, M. 2005. Classical and alternative NF-κB activation pathways and their roles in lymphoid malignancies. J. Clin. Exp. Hematopathol. 45:15–24
- Nworu, C. S., Akah, P. A., Okoye, F. B., et al. 2011. The leaf extract of Spondias mombin L. displays an anti-inflammatory effect and suppresses inducible formation of tumor necrosis factor-α and nitric oxide (NO). J. Immunotoxicol. 8:10–16
- Okhrimenko, H., Lu, W., Xiang, C., et al. 2005. Protein kinase Cϵ regulates the apoptosis and survival of glioma cells. Cancer Res. 65:7301–7319
- Pedal, I., Besserer, K., Goerttler, K., et al. 1982. Fatal nitrosamine poisoning. Arch. Toxicol. 50:101–112
- Perkins, N. D. 2007. Integrating cell-signaling pathways with NF-κB and IKK function Mol. Cell Biol. 8:49–62
- Ratajczak-Wrona, W., Jablonska, E., Garley, M., et al. 2011. Effect of N-nitrosodimethylamine on inducible nitric oxide synthase expression and production of nitric oxide by neutrophils and mononuclear cells: The role of JNK signaling pathway. APMIS. 119:431–441
- Ratajczak-Wrona, W., Jablonska, E., Garley, M., et al. 2013a. Role of AP-1 family proteins in regulation of inducible nitric oxide synthase (iNOS) in human neutrophils. J. Immunotoxicol. 10:32–39
- Ratajczak-Wrona, W., Jablonska, E., Garley, M., et al. 2013b. The role of MAP kinases in the induction of iNOS expression in neutrophils exposed to NDMA: The involvement transcription factors. Adv. Med. Sci. 58:(In Press - the print version will be published in December 2013 in Volume 58)
- Ratajczak-Wrona, W., Jablonska, E., Garley, M., et al. 2013c. Activation of the JAK/STAT pathway in human neutrophils by NDMA. Turk. J. Biol. (In Press; Available online at: http://mistug.tubitak.gov.tr/bdyim/kabul.php?dergi=biy)
- Romashkova, J. A., and Makarov, S. S. 1999. NF-κB is a target of AKT in anti-apoptotic PDGF signalling. Nature 401:86–90
- Sakai, K., Suzuki, H., Oda, H., et al. 2006. Phosphoinositide 3-kinase in nitric oxide synthesis in macrophages: Critical dimerization of inducible nitric-oxide synthase. J. Biol. Chem. 281:17736–17742
- Schulz, K., Kerber, S., and Kelm, M. 1999. Reevaluation of the Griess method for determining NO/NO2- in aqueous and protein containing samples. NO-Biol. Chem. 3:225–234
- Shaulian, E., and Karin, M. 2002. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 4:E131–136
- Souliotis, V. L., van Delft, J. H., Steenwinkel, M. J., et al. 1998. DNA adducts, mutant frequencies and mutation spectra in λ lacZ transgenic mice treated with N-nitrosodimethylamine. Carcinogenesis 19:731–739
- Tricker, A. R., and Preussmann, R. 1991. Carcinogenic N-nitrosamines in the diet: Occurrence, formation, mechanisms, and carcinogenic potential. Mutat. Res. 259:277–289
- Wright, K., Ward, S. G., Kolios, G., and Westwick J. 1997. Activation of phosphatidylinositol 3-kinase by Interleukin-13. An inhibitory signal for inducible nitric-oxide synthase expression in epithelial cell line HT-29. J. Biol. Chem. 272:12626–12633
- Wu, S., Tan, M., Hu, Y., et al. 2004. Ultraviolet light activates NF-κB through translational inhibition of IκB synthesis. J. Biol. Chem. 279:24898–24902
- Wu, Z. H., and Miyamoto, S. 2007. Many faces of NF-κB signaling induced by genotoxic stress. J. Mol. Med. (Berlin). 85:1187–1202
- Xia, X., Hu, X., Xu, H., et al. 2012. Phosphatidylinositol 3-kinase inhibitor suppresses inducible nitric oxide synthase expression in bronchiole epithelial cells in asthmatic rats. Mol. Cell. Biochem. 359:293–299
- Zeman, K., Tchorzewski, H., and Majewska, E. 1988. Simple and fast method of simultaneous isolation of lymphocytes and the polymorphonuclear cells from peripheral blood. Immunol. Pol. 13:217–220
