Abstract
Liver injuries, liver tumor resection, and liver transplantation are known to be responsible for ischemia/reperfusion (I/R) injury that, in turn, gives rise to liver damage. This study was undertaken to investigate the possible protective effect of eugenol against the damage induced by I/R in rat livers as well as to explore possible mechanisms of action. Male rats were divided into four groups: sham-operated, I/R only, and two groups that received 10 or 100 mg eugenol/kg/day (Eug10 and Eug100, respectively) for 15 days by gavage and were then subjected to I/R, i.e. an ischemia induced for 45 min followed by re-perfusion for 6 h. The rats were euthanized and liver tissues and blood collected for examination. The results showed that I/R induced massive hepatic structural and functional damage. Eug10-treated rats had improvement in both liver function and structure, and inhibition of I/R-induced increases in serum myeloperoxidase (MPO), tumor necrosis factor (TNF)-α, as well as hepatic nuclear factor-κB (NF−κB) p65 and caspase-3 expression. Eug10 treatment also inhibited the degree of loss in reduced glutathione (GSH) and of rise in malondialdehyde (MDA) levels in liver tissues induced by I/R. In contrast, augmentation of liver damage induced by I/R was noted in Eug100-treated rats, with these hosts displaying significant increases in oxidant, inflammatory, and apoptotic markers relative to levels seen in I/R-only rats. The results of the present study provide the first evidence that a low dose of eugenol may protect the liver against I/R injury in part by decreasing levels of lipid peroxidation, down-regulating inflammatory mediators, and inhibiting apoptosis, and that a larger dose amplifies the liver injury via oxidant and inflammatory effects.
Introduction
Liver injuries, liver tumor resection, and liver transplantation are known to be responsible for ischemia/reperfusion (I/R) injury that, in turn, gives rise to liver damage (Sözen et al., Citation2011). Various mechanisms have been proposed to explain how I/R injury occurs. During re-perfusion, an inflammatory process occurs that results from neutrophil recruitment and accumulation in the liver. Activated neutrophils then cause endothelial and hepatocellular damages via release of reactive oxygen species (ROS) (Jaeschke, Citation2006; Okaya & Lentsch, Citation2003). This pathology triggers a series of deleterious effects that include oxidative modification of lipid and proteins, induction of hepatocyte apoptosis, and release of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α (Selzner et al., Citation2003). The ROS also activate tyrosine kinases and result in the phosphorylation of IκB and subsequent translocation of nuclear factor-κB (NF-κB) to the nucleus (Llacuna et al., Citation2010) that further enhances formation of proteins/products that may ultimately contribute to massive tissue destruction.
Eugenol (4-allyl-2-methoxyphenol) is a natural phenolic extract that has drawn much attention for its various desirable pharmacological functions. As such, it is broadly used in daily life and some medical practices (Zhang et al., Citation2013). Eugenol is the major component in the essential oil of many aromatic plants, including clove (Zyzygium aromaticum). Its mixture with zinc oxide is used in dentistry as a filling material, a pulp-capping agent, or as a sedative (Markowitz et al., Citation1992). In traditional medicine, eugenol has been used for treatment of colic, chronic diarrhea, and other gastrointestinal disorders (Pruthi, Citation1976). It is considered non-mutagenic, non-carcinogenic, and generally recognized as a safe product by the Food and Drug Administration. Eugenol is known to possess anti-oxidant, analgesic, and neuroprotective properties (Park et al., Citation2011; Yogalakshmi et al., Citation2010). In addition, eugenol and related compounds exhibited anti-inflammatory activities, e.g. inhibition of lipopolysaccharide (LPS)-stimulated NF-κB activation, cytokine release, and cyclooxygenase-2 expression by macrophages (Murakami et al., Citation2005) and inhibited activity of 5-lipoxygenase in polymorphonuclear cells (Raghavenra et al., Citation2006). In light of these previous protective effects of eugenol, the current study was undertaken to investigate its possible effects against hepatic I/R injury in a rat model.
Materials and methods
Drugs and reagents
Eugenol was purchased from Sigma Aldrich (St. Louis, MO). The required doses used in the studies were dissolved in olive oil immediately prior to use based on data from Yogalakshmi et al. (Citation2010) and Said (Citation2011). All antibodies and related products used for the immunohistochemical staining protocols were obtained from Vector (Burlingame, CA). All other reagents used in these studies were from Sigma unless otherwise noted.
Animals and experimental design
Male Wistar rats (8–10-weeks-old, 200–220 g) were obtained from the Faculty of Veterinary Medicine at Zagazig University. On arrival, rats were housed at constant environmental conditions (room temperature 25 [±2]°C with a 12-h light/dark cycle and 50% humidity). Rats were provided ad libitum access to standard rodent chow diet and filtered water. The rats were allowed to acclimate for 1 week prior to any use in experiments. All experimental procedures were approved by the local authorities, i.e. the Ethical Committee for Animal Handling at Zagazig University, and were according to the guidelines set forth by the National Institutes of Health (USA).
For the experiment, rats were randomly divided into four groups (12/group): Group 1 (sham-operated) rats served as control; Group 2 (I/R) rats received olive oil (vehicle of eugenol) in a volume of 2 ml/kg body weight by oral gavage daily for 15 days and were then subjected to I/R; Group 3 (Eug10) rats received 10 mg eugenol/kg/day by gavage for 15 days and were then subjected to I/R; and Group 4 (Eug100) rats received 100 eugenol/kg/day for 15 days and were then subjected to I/R. Rats were weighed daily so that appropriate dosages could be delivered; gavage volume in the studies never exceeded 2 ml/100 g body weight. The values of eugenol selected for use here were based on the findings of Yogalakshmi et al. (Citation2010), who showed that oral administration of 10 mg eugenol/kg/day for 15 days had a protective effect against thioacetamide-induced hepatotoxicity in rats, and Said (Citation2011), who demonstrated that oral administration of 100 mg eugenol/kg/day provided a protective effect against gentamycin-induced nephrotoxicity in rats.
Induction of hepatic I/R and post-I/R processing of rats
Rats were subjected to partial liver ischemia (70%) followed by reperfusion according to the method of Asakawa et al. (Citation1989). Rats were anesthetized by intraperitoneal injection of ethyl carbamate (1.3 g/kg) and ischemia induced by occluding the blood vessels and bile ducts to the left and median lobes with a traumatic vascular clamp. After 45 min of ischemia, the clamp was removed to start re-perfusion for 6 h. In the sham group, the rats were anesthetized and the blood vessels exposed but not occluded. At the end of the 6 h period, all rats were euthanized by CO2 asphyxiation and the left and median liver lobes as well as blood from the heart then collected. The liver tissues in each group were sub-divided into two equal sets (n = 6/set); one was to be homogenized while the other was designated for histopathological and immunohistochemical studies. Serum samples were prepared from the collected blood for use in measures of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) enzyme activities and tumor necrosis factor (TNF)-α levels. The left and median hepatic lobes were washed with ice-cold saline, blot-dried, and then used for determination of myeloperoxidase (MPO), reduced glutathione (GSH), and malondialdehyde (MDA) contents. For these assays, the liver samples were suspended in phosphate buffer (50 mmol/L, pH 6) at 5-times the tissue volume and processed in a Potter-Elvehjem homogenizer. The raw homogenate was then aliquoted and frozen at −85 °C until used in the various assays (Helewski et al., Citation2010).
Determination of liver enzyme activities and serum TNFαlevels
Serum ALT, AST, and LDH activities were assayed enzymatically using commercial kits purchased from Spinreact (Gerona, Spain). Serum TNFα levels were measured by radioimmunoassay kits (Albert Poole Biotechnology, Beijing, China). The level of sensitivity of the kit was 10 pg/ml.
Determination of hepatic MPO content and anti-oxidant and oxidative stress markers
Homogenate MPO levels were assessed using a commercial enzyme-linked immunosorbent assay (ELISA) kit (HBT, Uden, the Netherlands) according to manufacturer instructions. Homogenates-reduced GSH content was determined by the colorimetric method of Beutler et al. (Citation1963). Liver MDA levels were determined colorimetrically as described previously (Satoh, Citation1978) using a diagnostic kit from Bio Diagnostic Co., (Cairo, Egypt).
Histopathology
Dedicated specimens from six rats/group were taken from both left and median lobes of the liver, then fixed in 10% buffered formalin (pH 7.2), processed, and embedded in paraffin wax. Sections of 5-µm thickness were then generated and stained with H&E for subsequent light microscope examination (Bancroft & Gamble, Citation2002). Dr Selim (certified pathologist) read each slide in a blinded manner. A minimum of two slides per rat were read.
Immunohistochemical detection of the activated form of NF-κB (p65), caspase-3
Paraffin sections cut at 5-µm were stained using a modified Avidin-Biotin Peroxidase technique for NF-κB p65 and activated caspase-3 (Bantel et al., Citation2001; El-Khouly et al., Citation2012; Gown & Willingham, Citation2002). In brief, sections were de-paraffinized and rehydrated; to retrieve antigen, sections were incubated with 0.1% trypsin and 0.1% CaCl2 in Tris buffer (50 mmol/l, pH 7.4) at 37 °C for 120 min. The sections were then soaked for 30 min at room temperature in absolute methanol containing 0.3% hydrogen peroxide to eliminate endogenous peroxidase activity. The sections were then incubated with 1.5% non-immunized goat serum for 30 min at room temperature, then incubated with diluted primary antibody (1:500 dilution) for cleaved caspase-3 or NF-κB p65 (5–10 mg/ml) for 30 min at room temperature, The sections were then washed 3-times with phosphate-buffered saline (PBS, pH 7.4) for 30 min and then incubated with biotinylated goat anti-mouse immunoglobulin serum for 60 min. After being gently washed with PBS, the sections were incubated with avidin/biotin peroxidase complex. Ultimately, sites of peroxidase binding were detected using DAB (3,3′-diaminobenzidine) substrate. Tissue sections were then counterstained with hematoxylin and subjected to light microscopy analyses (as above) and morphometric measures (see below).
Morphometric analyses
A Leica Qwin 500 (Cambridge, UK) image analyzer computer system was used to evaluate the area percentage of NF-κB p65 staining and the number of activated caspase-3 labeled hepatocytes using an interactive measure menu. The area percentage and standard measuring frame of a standard area equal to 118,476.6 mm2 were chosen from the parameters measuring 10 readings from five sections from each rat (from six rats/group). All measures were obtained at 400× magnification. The numbers of caspase-3 cells were then averaged and used to calculate the apoptosis index (AI) by the formula: AI = (apoptotic cells/total hepatic cells examined) × 100% (Qian et al., Citation2007).
Data analysis
One-way analysis of variance (ANOVA) was conducted across all groups, followed by a Tukey’s multiple comparison between all groups. All data are expressed as mean ± SEM. Significance was accepted at p values < 0.05. Graph Pad Prism-6 statistical software (La Jolla, CA) was used for all data analysis.
Results
In general, treatment with eugenol yielded bifurcated responses depending on the dose employed. Overall, the Eug10 regimen appeared to mitigate effects from subsequent I/R while Eug100 treatment led to a measure of amplification of I/R-induced effects in the rats. In no cases did eugenol treatment impact host body weights over the course of the study.
Effect on liver enzymes
illustrates that a 45 min ischemia followed by a 6 h re-perfusion resulted in a significant reduction in liver function, as illustrated by remarkable increases in serum levels of ALT (517.7%), AST (558.8%), and LDH (827.5%) compared to values in the sham-operated rat group. Eug10-treated rats had significant reductions in these parameters (respectively, 47, 62, and 82%) as compared to I/R rat values. However, the serum levels of both ALT and AST in Eug10-treated still rats were still significantly higher compared to those in sham-operated rats. In contrast, Eug100-treated rats displayed significant elevations in ALT, AST, and LDH (respectively, 15, 26, and 25%) compared to the I/R rats.
Table 1. Effect of eugenol treatment on serum levels of key marker enzymes of liver injury.
Effects on hepatic MPO
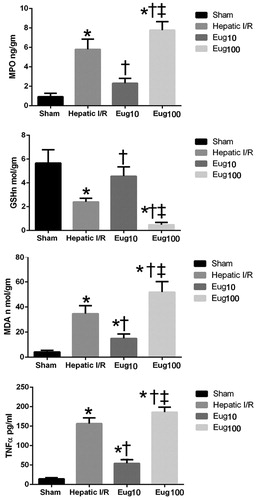
Rats subjected to I/R had significant increases in MPO content (544%) compared to values in sham-operated counterparts. Eug10-treated rats displayed significant reductions in MPO levels (60%) compared to levels in the I/R rats. Again, in contrast, Eug100-treated rats yielded significant increases in hepatic MPO (38%) relative to the I/R rat levels ().
Effect on anti-oxidant and oxidative stress parameters
Rats subjected to I/R showed significant reductions in liver-reduced GSH content (57%) and significant elevations in MDA levels (727%) compared to values in the sham rats. Eug10-treated rats had significant increases in liver-reduced GSH content (88%) and significant reductions in liver MDA (57%) when compared to values for the I/R rats. In Eug100-treated rats, significant reduction in GSH (83%) and increases in MDA (49%) were noted compared to values in the I/R rats ( and , respectively).
Effect on serum TNFα level
Serum levels of TNFα in I/R rats were significantly higher compared to in sham-operated rats. Compared to I/R host levels, Eug10-treated rats had significant reductions in serum TNFα (65%). In contrast, Eug100-treated rats had significant increases in TNFα levels (19%) compared to I/R counterparts ().
Histopathological changes in liver
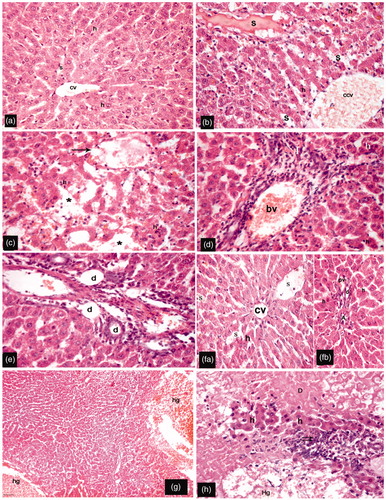
The livers of sham-operated rats showed hepatocytes arranged in a form of branching cords radiating from the central vein. Their hepatocytes were polygonal cells having acidophilic cytoplasm and rounded vesicular nuclei, and were separated by blood sinusoids (). Livers of rats exposed to I/R insult displayed dilated congested central veins and blood sinusoids as well as many hepatocytes with deeply stained acidophilic cytoplasm and dark stained nuclei (). Other sections showed distorted margins of central vein surrounded by hepatocytes with deeply stained cytoplasm and dark nuclei. Furthermore, multiple areas of parenchymal loss were observed (). Portal areas showed dilated congested blood vessels, intense perivascular cellular infiltration (), and bile ducts branching and proliferation (). Examination of liver sections from Eug10-treated rats revealed improvement of the histological changes seen in I/R hosts. Most of these rats had hepatocytes with an acidophilic cytoplasm and central rounded vesicular nuclei. In addition, their blood sinusoids were preserved, albeit still dilated (). Livers from Eug100-treated rats had a disorganized hepatic architecture with markedly dilated congested blood vessels (). In addition, multiple areas of degeneration, hemorrhage, and mononuclear cell infiltration were seen, and hepatocytes had deeply-stained nuclei and acidophilic cytoplasms ().
Figure 2. Representative H&E-stained sections of different groups. (a) Hepatocytes (h) arranged in plates radiating from the central vein (cv) and separated by blood sinusoids (s); hepatocytes are polygonal in shape, with central rounded vesicular nuclei and acidophilic cytoplasm (sham group). (b–e) I/R rats had: congested dilated central vein (ccv) and blood sinusoids (s); many hepatocytes (h) had deeply-stained acidophilic cytoplasm with darkly-stained nuclei (b); distorted margins of central vein (arrow) surrounded by hepatocytes with dark-stained nuclei; multiple areas of parenchymal loss (*) (c); portal areas with dilated congested blood vessels (bv) and intense perivascular cellular infiltration (I) with surrounding hepatocytes containing deeply acidophilic cytoplasm (d); and bile ductule (d) branching and proliferation (e). (f) Eug10-treated rats had: apparent normally hepatocytes (h); preserved central veins (cv) (fa), hepatic artery (A), portal vein (pv), and bile ducts (d); and blood sinusoids (s) that were pre-served but dilated (fb). (g, h) Eug100-treated rats had: large areas occupied by hemorrhage (hg) and a disorganized hepatic organization; hepatocytes with deeply stained acidophilic cytoplasm and dark nuclei; and massive areas of degeneration (D), hemorrhage (Hg), as well as intense cellular infiltration (double arrows). (a–h) Magnification 400×; (g) Magnification 100×.
Immunohistochemical detection of the activated form of NF-κB (p65) and caspase-3
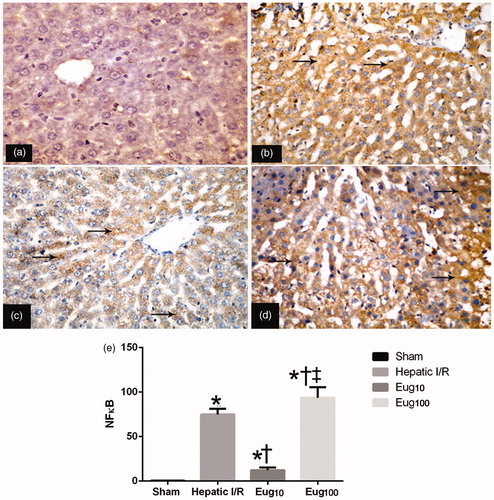
Levels of overall NF-κB in liver tissue samples of rats in all groups were assessed by examination of activated subunit p65 expression. The results indicated that I/R induced a significant increase in p65 levels in the livers, as evident from the intense brown staining relative to the staining seen in samples from sham-control rats (). Eug10-treated rats had significant reductions in NF-κB (p65) expression compared to I/R rats (), while Eug100-treated rats evidenced significantly increased expression (). The immunohistochemical staining in each case was quantified and the results summarized (). The data showed that I/R rats had a level of 30% NF-κB+ cells while the Eug10- and Eug100-treated rats had values of 13 and 51%, respectively.
Figure 3. Immunohistochemical detection of NF-κB (p65). Figures shown are representative micrographs for each group. (a) Section showing non-immunoreactivity in sample from the sham group. (b) Strong positive reactivity (arrow) in sample from I/R rat. (c) Mild positive staining (arrow) in sample from Eug10-treated rat. (d) Strong positive staining (arrow) were in Eug100-treated rat sample. (e) The staining when quantified. Data shown in (e) is mean percent NF-κB+ cells among all total hepatic cells counted (±SEM) fromsix rats/gruop. * p < 0.05 vs sham, † p < 0.05 vs I/R. ‡ p < 0.05 versus Eug10. Magnification = 400×.
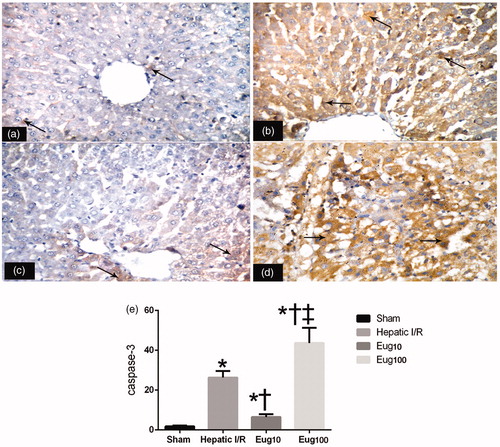
Regarding caspase-3, the I/R rats displayed significant increases in number of hepatocytes that showed activated caspase-3 in the cytoplasm () compared to what was seen among cells in samples from the sham-operated rats (). Eug10-treated rats showed significant reductions in this I/R-related expression (), while rats that received Eug100 had significant elevations (). Again, the staining was quantified and results summarized (). The data show that the apoptotic index in I/R rats was 30%; this was reduced to 9% in Eug10-treated rats but increased to 51% with the Eug100.
Figure 4. Immunohistochemical detection of caspase-3. Figures shown are representative micrographs for each group. (a) Activated caspase-3 was only present in a few hepatocytes (arrow) around the central vein in the livers of the sham rats. (b) Many hepatocytes (arrow) were immunostained in the liver samples from the I/R group. (c) A few hepatocyes around the central vein (arrow) were stained in samples from the Eug10-treated rats (d). Many hepatocytes (arrow) were stained in liver sections from the Eug100-treated rats. (e) The staining when quantified. Data shown in (e) is mean apoptosis indices (±SEM) from six rats/group. * p < 0.05 vs sham, † p < 0.05 vs I/R, ‡ p < 0.05 versus Eug10. Magnification = 400×.
Discussion
Hepatic I/R injury is observed following major liver surgery, transplantation, and trauma. It may cause metabolic and structural damage to the liver (Shin et al., Citation2008). This remains a significant problem in surgical procedures, and is a limiting factor in liver transplantation (He et al., Citation2005). It was reported that apoptosis of hepatocytes is a critical mechanism contributing to hepatic I/R injury (Sun et al., Citation2004). The mechanisms of apoptosis in I/R injury usually include production of oxygen radical species (Oshimi & Miyazaki, Citation1995) and initiation of inflammatory cascades leading to liver damage (Sözen et al., Citation2011).
The results of the current study provide noticeable evidence that oral administration of 10 mg eugenol/kg/day for 15 days prior to ischemia protected the liver from I/R injury. This protective effect was evidenced as significant reductions in serum ALT, AST, and LDH levels and improvements in the microscopic histologic features of the livers of Eug10-treated rats (compared to I/R rats). These outcomes were also associated with significant reductions in oxidative stress, inflammatory responses, and markers of apoptosis. On the other hand, 100 mg eugenol/kg/day for 15 days prior to hepatic I/R had a deleterious effect, as manifested by significant elevation in liver enzymes and serum TNFα. Moreover, there were increases in hepatic content of MDA, MPO activity, a depletion of hepatic GSH, and marked histologic deterioration of the liver as compared to what was seen among the I/R rats.
The present study revealed significant elevation in serum concentrations of ALT, AST, and LDH following I/R insult (as compared to sham rats) together with significant reductions in liver GSH content, elevation in MDA and MPO content, increased NF-κB expression, and concomitant elevations in serum TNFα levels. Moreover, histologic examination of livers from the I/R rats revealed dilated congested central veins and blood sinusoids, and hepatocytes that showed signs of apoptosis (had deeply stained acidophilic cytoplasm and dark stained nuclei). This was confirmed by the increase in numbers of hepatocytes with positive immunoreactivity for activated caspase 3. These outcomes were in line with other reports showing increased TNFα and NF-κB in a hepatic I/R model (Mahmoud et al., Citation2012).
An elevated LDH activity indicates necrotic cell death of hepatocytes while high ALT levels suggest damage to hepatocytes membranes that can also cause cell death. Increased AST concentrations indicate damage of both mitochondrial and cell membranes (Helewski et al., Citation2010). Ischemia starts a series of biochemical reactions that cause a cell dysfunction that may progress to cell death. Restoration of blood flow to ischemic tissue is the requisite to remove toxic metabolites produced during the ischemia. However, severe metabolic disorders may appear due to the infusion of various inflammatory mediators formed during ischemia into the systemic circulation, and re-perfusion may cause further tissue damage (Carden & Granger, Citation2000). Our study also revealed there were increases in mononuclear cellular infiltrates into the affected sites. This finding was also reported by several authors who noted that inflammatory mediators produced in hepatocytes, like TNFα -- which increased in our experiment -- most likely acted in a paracrine manner to enhance liver injury and mononuclear cell infiltration (Al-Rawi, Citation2007; Bu et al., Citation2005). The improvement in liver function and pathology seen in Eug10-treated rats compared to I/R rats suggested a possible protective effect of eugenol against hepatic I/R insult.
To explore possible mechanisms responsible for this protective effect, our study revealed a significant reduction in MPO activity in Eug10-treated rats. MPO, an enzyme found primarily in neutrophils (indeed infiltration of neutrophils into tissues is commonly assessed by changes in activity of MPO) was increased in the liver of rats after I/R. This suggested stimulation of an inflammatory response and may indicate that neutrophil accumulation and lipid peroxidation contributed to I/R-induced liver injury (Sözen et al., Citation2011). Previously, it was reported that activated neutrophils located in inflammatory foci and secreting MPO into the extracellular space could also convert hydroperoxides to free radicals, triggering local lipid peroxidation (Cetinkaya et al., Citation2006). The ability of eugenol to reduce MPO activity was illustrated by Yogalakshmi et al. (Citation2010), who reported that eugenol reduced the elevated MPO activity in thioacetamide-induced hepatotoxicity.
Growing evidence shows that oxidative stress is involved in liver damage after I/R. In the present study, I/R injury induced a significant increase in levels of liver MDA, an end-product of free radical formation and lipid peroxidation as well as an index of ROS-mediated injury (Draper & Hadley, Citation1991) that results from an imbalance between radical generating and scavenging systems leading to cell membrane impairment or DNA damage (McCord, Citation2000). This imbalance also can result in enhanced rates of protein degradation, eventually resulting in cell lysis (García et al., Citation1997). Together with a depletion of liver GSH content (Mahmoud et al., Citation2012), these events would lead to a spiral of increasing oxidative damage, inflammation, and cell death. In the present study, administration of a low dose of eugenol led to significant reductions in hepatic MDA content and increments in GSH compared to levels in the I/R hosts, indicating a reduction in oxidative stress and an enhanced anti-oxidant state in these hosts’ livers. This is inconsistent with previous findings (Prasad & Muralidhara, Citation2013) who reported that eugenol ameliorated acrylamide-induced neuropathy via attenuation of MDA levels and restoration of reduced GSH levels in rats.
Our study showed a significant presence of NF-κB and significant increase in the numbers of hepatocytes with activated caspase-3 in I/R rats. Because ROS activate tyrosine kinases that phosphorylate IκB, resulting in translocation of NF-κB to the nucleus, this results in the transcription of several genes (Dutta et al., Citation2006; Hayden & Ghosh, Citation2004) that could further impact on the damage already induced by the I/R. Interestingly, among these genes is manganese superoxide dismutase (MnSOD), a protective enzyme (Llacuna et al., Citation2010) used to inactivate ROS as superoxide anion. However, even in this case of purported helpfulness, the SOD end-product is hydrogen peroxide that ultimately requires GSH to be inactivated. It is understandable then that the depletion of GSH by I/R observed in our study likely aggravated the overall damage. Further, with all the increases in presence of ROS, it is not surprising that the cells would also be triggered to undergo apoptotic cell death. Accordingly, increases in caspase-3 activity during re-perfusion would be expected (Cursio et al., Citation1999).
The Eug10-treated rats here had significant reductions in hepatic NF-κB and caspase-3 expression and reduced serum TNFα levels compared to values in the I/R rats. Other investigators have cited that eugenol effectively improved functional and structural pulmonary changes that could be induced by LPS (Magalhães et al., Citation2010) and reduced collagen-induced arthritis (Grespan et al., Citation2012) in rats via inhibition of TNFα release and NF-κB activation. Fouad and Yacoubi (Citation2011) showed that eugenol prevented doxorubicin-induced activation of caspase-3 in cardiomyocytes. It was, thus, somewhat surprising that eugenol at 100 mg/kg/day not only failed to protect the rats from I/R insult but also exerted a deleterious effect on the liver. This was apparent from significant elevation of liver enzymes, serum TNFα levels, hepatic MPO activity, MDA content, as well as NF-κB and caspase-3 expression, and a depletion of GSH compared with what was noted in I/R rats. Moreover, microscopy revealed marked deterioration in the liver manifested by disorganized hepatic architecture with congestion, multiple areas of hemorrhage, focal necrosis, and mononuclear cellular infiltration.
It has been reported that high concentrations of eugenol contained in 10 ml clove oil may induce hepatotoxicity via depletion of GSH in liver cells (Janes et al., Citation2005). In that case, Thompson et al. (Citation1989) reported that incubation of 10 µM eugenol with polymorphonuclear cells was likely to release MPO that, in turn, catalyzed oxidation of eugenol to a reactive intermediate -- possibly a quininemethide. Because GSH inhibited formation of this metabolite, any GSH depletion associated with induction of oxidative stress could be one mechanism leading to deterioration of the liver when a large dose of eugenol is used. This would be in keeping with the finding of Yoo et al. (Citation2005), who showed that an anti-cancer property of eugenol against leukemia cells was attributed to increased ROS generation in tandem with a depletion of GSH that led to a fall in mitochondrial membrane potentials and caspase-3 activation. However, those results were in apparent contradiction to a study by Said (Citation2011) that showed that eugenol at 100 mg/kg exerted a protective effect against gentamycin-induced nephrotoxicity in rats.
In general, treatment with eugenol yielded bifurcated responses depending on the dose employed. Overall, the Eug10 regimen appeared to mitigate effects from subsequent I/R while Eug100 treatment led to a measure of amplification of I/R-induced effects in the rats. From these findings, it can be concluded that eugenol -- at certain dosages -- has a potential therapeutic effect against hepatic I/R injury. This effect appears to be mediated, in part, by decreases in lipid peroxidation, down-regulation of inflammatory mediators, and an inhibition of apoptosis. Still, the data here clearly show that care should be taken during any potential use of eugenol as administration of large doses aggravated damage produced by hepatic I/R insult itself.
Declaration of interest
The authors report no conflict of interests. The authors alone are responsible for the content and writing of the paper.
| Abbreviations | ||
| I/R, | = | ischemia/reperfusion; |
| ALT, | = | alanine aminotransferase; |
| AST, | = | aspartate aminotransferase; |
| LDH, | = | lactic dehydrogenase; |
| MPO, | = | myeloperoxidase; |
| MDA, | = | malondialdehyde; |
| NF-κB, | = | nuclear factor-κB; |
| GSH, | = | reduced glutathione; |
| LPS, | = | lipopolysaccharide; |
| MnSOD, | = | manganese superoxide dismutase. |
References
- Al-Rawi, M. M. 2007. Effect of Trifolium sp. flowers extracts on the status of liver histology of STZ-induced diabetic rats. Saudi J. Biol. Sci. 14:21–28
- Asakawa, H., Jeppsson, B., Mack, P., et al. 1989. Acute ischemic liver failure in the rat: A reproducible model not requiring portal decompression. Eur. Surg. Res. 21:42–48
- Bancroft, J. D., and Gamble, M., (Eds.). 2002. Theory and Practice of Histological Techniques, 5th ed. London: Churchill Livingstone
- Bantel, H., Lugering, A., Poremba, C., et al. 2001. Caspase activation correlates with the degree of inflammatory liver injury in chronic hepatitis C virus infection. Hepatology 34:758–767
- Beutler, E., Duron, O., and Kelly, B. M. 1963. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 61:882–888
- Bu, D. X., Erl, W., De Martin, R., et al. 2005. IKKβ-dependent NF-κB pathway controls vascular inflammation and intimal hyperplasia. FASEB J. 19:1293–1295
- Carden, D. L., and Granger, D. N. 2000. Pathophysiology of ischemia-re-perfusion injury. J. Pathol. 190:255–266
- Cetinkaya, A., Bulbuloglu, E., Kurutas, E. B., and Kantarceken, B. 2006. N-acetylcysteine ameliorates methotrexate-induced oxidative liver damage in rats. Med. Sci. Monit. 12:BR274–278
- Cursio, R., Gugenheim, J., Ricci, J. E., et al. 1999. A caspase inhibitor fully protects rats against lethal normothermic liver ischemia by inhibition of liver apoptosis. FASEB J. 13:253–261
- Draper, H. H., and Hadley, M. 1991. Malonaldehyde determination as index of lipid peroxidation. Meth. Enzymol. 186:421–431
- Dutta, J., Fan, Y., Gupta, N., et al. 2006. Current insights into the regulation of programmed cell death by NF-κB. Oncogene 25:6800–6816
- El-Khouly, D., El-Bakly, W. M., Awad, A. S., et al. 2012. Thymoquinone blocks lung injury and fibrosis by attenuating bleomycin-induced oxidative stress and activation of nuclear factor-κB in rats. Toxicology 302:106–113
- Fouad, A. A., and Yacoubi, M. T. 2011. Mechanisms underlying protective effect of eugenol in rats with acute doxorubicin cardiotoxicity. Arch. Pharm. Res. 34:821–828
- García, J. J., Reiter, R. J., Guerrero, J. M., et al. 1997. Melatonin prevents changes in microsomal membrane fluidity during induced lipid peroxidation. FEBS Lett. 408:297–300
- Gown, A. M., and Willingham, M. C. 2002. Improved detection of apoptotic cells in archival paraffin sections: Immunohistochemistry using antibodies to cleaved caspase-3. J. Histochem. Cytochem. 50:449–454
- Grespan, R., Paludo, M., Lemoside, P., et al. 2012. Anti-arthritic effect of eugenol on collagen-induced arthritis experimental model. Biol. Pharm. Bull. 35:1818–1820
- Hayden, M. S., and Ghosh, S. 2004. Signaling to NF-κB. Genes Dev. 18:2195–2224
- He, X. S., Ma, Y., Wu, L. W., et al. 2005. Dynamical changing patterns of glycogen and enzyme histochemical activities in rat liver graft undergoing warm ischemia injury. World J. Gastroenterol. 11:2662–2665
- Helewski, K. J., Kowalczyk-Ziomek, G. I., Czecior, E., et al. 2010. Administration of low doses of TNFα protects rat liver from ischemic damage and re-perfusion injury. J. Physiol. Pharmacol. 61:273–278
- Jaeschke, H. 2006. Mechanisms of liver injury. II. Mechanisms of neutrophil-induced liver cell injury during hepatic ischemia reperfusion and other acute inflammatory conditions. Am. J. Physiol. 290:G1083–G1088
- Janes, S. E., Price, C. S., and Thomas, D. 2005. Essential oil poisoning: N-acetylcysteine for eugenol-induced hepatic failure and analysis of a national database. Eur. J. Pediatr. 164:520–522
- Llacuna, L., Barcena, C., Bellido-Martin, L., et al. 2010. Growth arrest-specific protein 6 is hepatoprotective against murine ischemia/reperfusion injury. Hepatology 52:1371–1379
- Magalhães, C. B., Riva, D. R., DePaula, L. J., et al. 2010. In vivo anti-inflammatory action of eugenol on lipopolysaccharide-induced lung injury. J. Appl. Physiol. 108:845–851
- Mahmoud, M. F., El Shazly, S. M., and Barakat, W. 2012. Inhibition of TNFα protects against hepatic ischemia-reperfusion injury in rats via NF-κB-dependent pathway. Naunyn Schmied. Arch. Pharmacol. 385:465–471
- Markowitz, K., Moynihan, M., Liu, M., and Kim, S. 1992. Biologic properties of eugenol and zinc oxide- eugenol. A clinically oriented review. Oral Surg. Oral Med. Oral Pathol. 73:729–737
- McCord, J. M. 2000. The evolution of free radicals and oxidative stress. Am. J. Med. 108:652–659
- Murakami, Y., Shoji, M., Hirata, A., et al. 2005. Dehydrodiisoeugenol, an isoeugenol dimer, inhibits lipopolysaccharide-stimulated nuclear factor-κB activation and cyclooxygenase-2 expression in macrophages. Arch. Biochem. Biophys. 434:326–332
- Okaya, T., and Lentsch, A. B. 2003. Cytokine cascade and the hepatic inflammatory response to ischemia and reperfusion. J. Invest. Surg. 16:141–147
- Oshimi, Y., and Miyazaki, S. 1995. Fas antigen-mediated DNA fragmentation and apoptotic morphologic changes are regulated by elevated cytosolic Ca2+ level. J. Immunol. 154:599–609
- Park, S. H., Sim, Y. B., Lee, J. K., et al. 2011. The analgesic effects and mechanisms of orally administered eugenol. Arch. Pharm. Res. 34:501–507
- Prasad, S. N., and Muralidhara, M. 2013. Neuroprotective efficacy of eugenol and isoeugenol in acrylamide-Induced neuropathy in rats: Behavioral and biochemical evidence. Neurochem. Res. 38:330–345
- Pruthi, J. S., (Ed.). 1976. Species and Condiments, 1st ed. Delhi, India: National Book Trust, pp. 91–98
- Qian, J. M., Zhang, H., Wu, X. F., et al. 2007. Improvement of recipient survival after small size graft liver transplantation in rats with pre-ischemic manipulation or administering antisense against NF-κB. Transpl. Int. 20:784–789
- Raghavenra, H., Diwakr, B. T., Lokesh, B. R., and Naidu, K. A. 2006. Eugenol, the active principle from cloves, inhibits 5-lipoxygenase activity and leukotriene-C4 in human PMNL cells. Prostagland. Leukot. Essent. Fatty Acids 74:23–27
- Said, M. M. 2011. Protective effect of eugenol against gentamicin-induced nephrotoxicity and oxidative damage in rat kidney. Fundam. Clin. Pharmacol. 25:708–716
- Satoh, K. 1978. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta 90:37–43
- Selzner, N., Rudiger, H., Graf, R., and Clavien, P. A. 2003. Protective strategies against ischemic injury of the liver. Gastroenterology 125:917–936
- Shin, T., Kuboki, S., Huber, N., et al. 2008. Activation of peroxisome proliferator-activated receptor-γ during hepatic ischemia is age-dependent. J. Surg. Res. 147:200–205
- Sözen, S., Kisakürek, M., Yildiz, F., et al. 2011. The effects of glutamine on hepatic ischemia reperfusion injury in rats. Hippokratia 15:161–166
- Sun, K., Liu, Z. S., and Sun, Q. 2004. Role of mitochondria in cell apoptosis during hepatic ischemia-reperfusion injury and protective effect of ischemic post conditioning. World J. Gastroenterol. 10:1934–1938
- Thompson, D., Constantin-Teodosiu, D., Norbeck, K., et al. 1989. Metabolic activation of eugenol by myeloperoxidase and polymorphonuclear leukocytes. Chem. Res. Toxicol. 2:186–192
- Yogalakshmi, B., Viswanathan, P., and Anuradha, C. V. 2010. Investigation of anti-oxidant, anti-inflammatory and DNA-protective properties of eugenol in thioacetamide-induced liver injury in rats. Toxicology 268:204–212
- Yoo, C. B., Han, K. T., Cho, K. S., et al. 2005. Eugenol isolated from the essential oil of Eugenia caryophyllata induces a reactive oxygen species-mediated apoptosis in HL-60 human promyelocytic leukemia cells. Cancer Lett. 225:41–52
- Zhang, E., Xiao, M., Chen, C., and Xu, W. 2013. Study of anti-inflammatory activities of α-D-glucosylated eugenol. Arch. Pharm. Res. 36:109--115