Abstract
The Yusho poisoning incident, caused by rice oil contaminated with polychlorinated biphenyls (PCBs), polychlorinated quarterphenyls (PCQs), and polychlorinated dibenzofurans (PCDFs) generated by heat-denatured PCBs, occurred in 1968 in western Japan. Although severe symptoms are rarely observed today, the levels of PCBs and PCDFs in the sera of Yusho patients remain high. The aryl hydrocarbon receptor (AhR), which also acts as a dioxin receptor, is a transcriptional regulator that mediates dioxin toxicity. Recent studies show that dioxin mediates its immune toxic effects via AhR and that AhR activation induces dysregulation of interleukin (IL)-17- producing T (TH17) cells. This study therefore hypothesized that Yusho patients would show dysregulated TH17 cell-mediated immune responses. To validate the hypothesis, levels of IL-17 and IL-22, each secreted by TH17 cells, along with IL-1β and IL-23 were measured in serum samples from 40 Yusho patients and 40 age-matched controls. Levels of tumor necrosis factor (TNF)-α potentially secreted by TH17 cell-stimulated neutrophils and macrophages were also measured. The results indicated that serum IL-17 levels, as well as those of IL-1β, IL-23, and TNFα, were significantly higher in Yusho patients than in controls. In contrast, serum IL-22 levels were significantly lower in the Yusho patients. These results suggest that Yusho patients have dysregulated TH17 cell-mediated immune responses that may be linked to inflammation.
Introduction
The Yusho incident that occurred in western Japan in 1968 involved a mass poisoning of 2000 individuals through contaminated food (Furue, Citation2005). This poisoning resulted from the accidental ingestion of rice bran oil contaminated with dioxin-like components, including polychlorinated biphenyls (PCBs). It was later found that the oil was also contaminated with polychlorinated dibenzofurans (PCDFs), quarterphenyls (PCQs), and other related compounds. Skin symptomae experienced by “Yusho patients” were acneiform eruptions and pigmentation of the skin, nails, and conjunctiva. Other symptoms included eye discharge, hyperemia, and eyelid swelling. In addition, general malaise, headache, nausea, vomiting, numbness of limbs, arthralgia, irregular menstruation, and growth retardation in infants and children were noticed. Most PCBs and PCDFs agents have a high affinity for fats. As such, they are not easily eliminated from the body and can be stored in adipose tissue for a long time (Furue, Citation2005). Therefore, even though more than 40 years have passed since the accidental poisoning, high PCBs, PCDFs, and PCQs levels are still detected in Yusho patients’ sera. Furthermore, a number of these patients still suffer from many of the Yusho-specific symptoms mentioned above.
The aryl hydrocarbon receptor (AhR) that acts as a dioxin receptor also acts as a transcriptional regulator to mediate dioxin toxicity (Esser et al., Citation2009). AhR activation also results in immunotoxicity. Most studies show that dioxin-mediated activation of AhR suppresses humoral, cellular, and innate immune responses (Esser et al., Citation2009; Stockinger, Citation2009). However, some reports showed the immune system could also be activated by dioxins such as 2,3,7,8-tetra-chlorodibenzo-p-dioxin (TCDD). For example, stimulated human synoviocytes of rheumatoid arthritis by TCDD induced inflammatory cytokines via its association with AhR (Kobayashi et al., Citation2008). Thus, it is thought that the immunotoxic effects mediated by dioxins are dependent on the cell type, species, or the activating ligand.
AhR are expressed by differentiated CD4+ effector T-cell sub-sets. In particular, interleukin (IL)-17-producing T-cells (TH17) show high expressions of AhR. Although regulatory T (Treg) cells also express AhR, the levels are extremely low compared to those on TH17 cells (Esser et al., Citation2009). Some AhR ligands, like 6-formylindolo-[3,2-β]-carbaxole (FICZ), interfere with Treg cell differentiation, but induce TH17 cell differentiation (Quintana et al., Citation2008). Thus, we hypothesized that Yusho patients may show dysregulated TH17-mediated immune responses. To investigate this, we measured levels of IL-17 and IL-22 secreted by TH17 cells in the serum of Yusho patients via ELISA. We also measured levels of several cytokines that regulate TH17 cell proliferation, and other inflammatory cytokines produced by TH17-stimulated neutrophils and macrophages.
Materials and methods
Study protocol
The study protocol was approved by the Japanese Ministry of Health, Labor, and Welfare, and all subjects provided informed consent prior to enrolment in the study. In 2004, the Japanese Ministry of Health Labor and Welfare proposed a set of criteria for Yusho patients; these were based on clinical symptoms such as acneiform eruptions, pigmentation, and eyelid swelling, as well as serum high levels of PCBs, PCQs, or PCDFs. In our study, blood samples from 40 Yusho patients who met the criteria were obtained in 2005. In parallel, blood samples were obtained from 40 healthy Japanese residents from the same locality of similar age and gender (normal controls). The Yusho patient mean age was 73.6 ± 8.3 years and that of the controls was 69.9 ± 9.8 years. The blood samples of Yusho patients were analyzed for PCBs by saponification in 1 M NAOH ethanol solution, extraction with n-hexane column chromatography on silica gels, and then gas chromatography with electron capture detection (Masuda et al., Citation1985). The values of PCB obtained represent the concentrations of total coplanar PCBs, and were mainly comprised of 3,3′,4,4′-tetra-chlorinated biphenyl (CB), 3,3′,4,4′,5-penta-CB, and 3,3′,4,4′,5,5′-hexa-CB in the present study.
Sample collection
Fresh venous blood samples (8 ml/subject) were collected at 9 AM into serum (silicone-coated) Vacutainer tubes (BD, Fukuoka, Japan). Each sample was allowed to clot and then centrifuged to obtain the serum. All serum samples were stored at −70 °C until analyzed. All patients and controls were non-smokers. None of the Yusho patients had been treated with steroids, prostanoids, or other immunosuppressants, and none had a recent history of infection, collagen diseases, or abnormal liver function at the time of sampling.
Measures of serum cytokines
Serum IL-17, IL-22, IL-1β, IL-23, and TNFα levels were measured using specific ELISA kits, according to manufacturer instructions (R&D Systems, Minneapolis, MN). Each sample was tested in duplicate. The levels of sensitivity of the purchased IL-17, IL-22, IL-1β, IL-23, and TNFα kits were, respectively, 1.4, 15.0, 4.0, 39.0, and 0.5 pg/ml.
Statistical analysis
All statistics in the study were performed using Graph Pad Prism® software. A Mann-Whitney U-test was used to compare serum cytokines levels between Yusho patients and controls. In addition, a Spearman’s rank correlation was used to analyze correlation between levels of PCBs, PCQs, or PCDFs and serum cytokines levels. Outliers were not eliminated. A p value < 0.05 was considered statistically significant.
Results
Cytokines secreted by TH17 cells
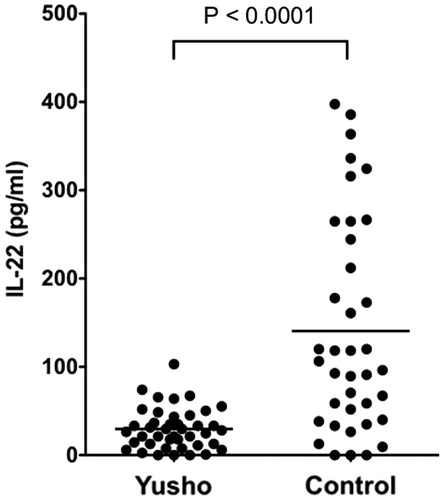
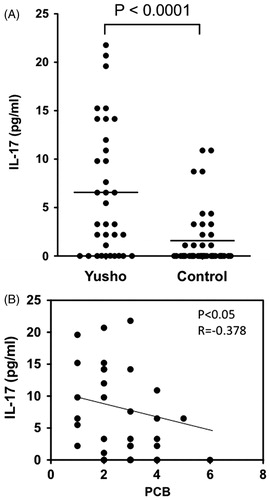
Serum IL-17 levels were significantly higher in Yusho patients (6.75 [±6.78] pg/ml) than in controls (1.59 [±2.99] pg/ml; p < 0.0001; ). There was a weak negative correlation between IL-17 levels and PCB levels in the serum obtained from Yusho patients (p < 0.05, r = −0.378; ). In contrast, serum IL-22 levels were significantly lower in Yusho patients (29.68 [±23.04] pg/ml) than in controls (140.59 [±120.49] pg/ml, p < 0.0001; ). There was no correlation between PCB, PCDF, or PCQ levels and IL-22 levels (data not shown).
Figure 1. Serum IL-17 levels. (A) IL-17 levels in serum samples obtained from Yusho patients and controls. Serum IL-17 levels were significantly higher in the Yusho patients (p < 0.0001). (B) A weak negative correlation was observed between IL-17 levels and PCB levels in the sera of Yusho patients (p < 0.05, r = −0.378). The values of PCBs obtained represent the concentrations of total coplanar PCBs that are mainly of 3,3′,4,4′-tetra-chlorinated biphenyl (CB), 3,3′,4,4′,5-penta-CB, and 3,3′,4,4′,5,5′-hexa-CB in the present study.
Inflammatory cytokines possibly secreted by neutrophils and macrophages activated by TH17 cells
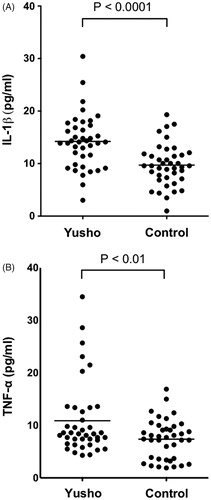
Serum IL-1β levels were significantly higher in Yusho patients (14.22 [±5.16] pg/ml) than in control subjects (9.71 [±3.95] pg/ml, p < 0.0001; ). Furthermore, the Yusho patients exhibited significantly elevated serum TNFα levels (10.89 [±6.98] pg/ml) compared to controls (7.39 [±3.77] pg/ml, p < 0.01; ). There was no correlation between PCB, PCDF, or PCQ levels and either levels of IL-1β or TNFα (data not shown).
Cytokines that regulate TH17 cell proliferation and differentiation
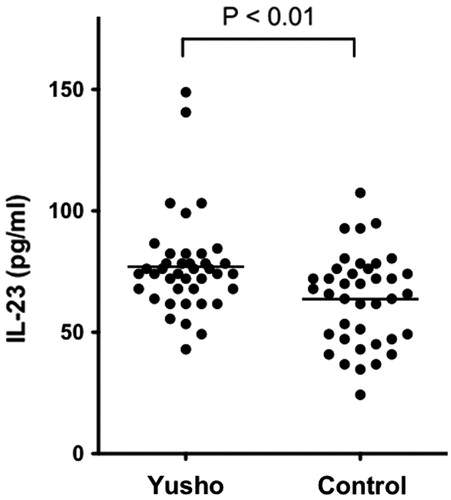
Serum IL-23 levels were significantly higher in Yusho patients than in controls (76.99 [±20.14] pg/ml vs 63.71 [±18.75] pg/ml, respectively, p < 0.01; ).
Discussion
illustrates how cytokines might regulate TH17 cell differentiation and function. The present study revealed that Yusho patients had higher serum levels of IL-17 than controls. Since IL-17 is the major cytokine secreted by TH17 cells, these results suggest that TH17 cells are activated in Yusho patients. Furthermore, as IL-17 activates both macrophages and neutrophils (Barin et al., Citation2012; Katz et al., Citation2001), we also measured the cytokines secreted by these cell types. Serum IL-1β and TNFα levels were higher in Yusho patients than in controls, indicating a presence of activated macrophages and neutrophils. It is surprising that active inflammatory cytokines production was observed in Yusho patients almost 40 years after the initial poisoning by PCBs or PCDFs. Dioxin-like components, once released into the circulation little-by-little from reservoirs such as fat stores, may perturb the host immune system. Moreover, it may be related to the high levels of IL-1β and IL-23, both of which regulate TH17 cell proliferation and differentiation (Kurebayashi et al., Citation2013). Overall, these results suggested that TH17 cells are activated in Yusho patients.
Figure 5. A proposed mechanism of TH17 cell activation and subsequent inflammatory responses in Yusho patients. Yusho patients ingested several dioxins such as PCBs or PCDFs in contaminated rice oil that then acted as ligands for AhR expressed by TH17 cells. Binding to AhR may have activated TH17 cells, which then secreted large amounts of IL-17 that stimulated neutrophils and macrophages that, in turn, secreted IL-1β and TNFα to induce inflammatory responses. High levels of IL-1β and IL-23 induce TH17 cell differentiation from TH0 cells. However, the TH17 cells in Yusho patients might secrete IL-17 but not IL-22. Cytokines measured in this study are in bold. Neut, neutrophil; MΦ, macrophage.
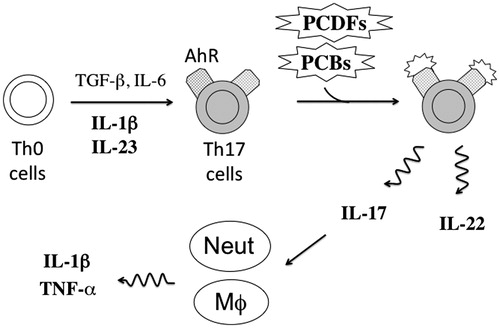
Interestingly, another study showed that rheumatoid factor levels were higher in the serum of Yusho patients than in those of controls (Nagayama et al., Citation2001). As activated TH17 cells play a key role in rheumatoid arthritis (Maddur et al., Citation2012), Yusho patients should be monitored for rheumatoid arthritis. Moreover, systemic lupus erythematosus, inflammatory bowel diseases, or psoriasis vulgaris, pathologies in which activated TH17 cells also play a key role, should also be mentioned in Yusho patients.
Precise mechanisms underlying TH17 cell activation in Yusho patients are unclear. In mice models, activated TH17 cells also express high levels of AhR (Quintana et al., Citation2008). FICZ, a tryptophan product and not a dioxin, has been shown to act as an endogenous AhR ligand that induced TH17 cell differentiation and activation when present with certain combinations of cytokines (Quintana et al., Citation2008). Accordingly, taken together with our results, PCBs, PCQs, and/or PCDFs may act as AhR ligands and play a role in driving TH17 cell proliferation and activation in Yusho patients. Quintana et al. (Citation2008) also showed that TCDD, a different type of dioxin, could induce differentiation and activation of Treg cells, but not TH17 cells; this outcome was likely opposite to the dioxins-like components’ effects on TH17 cells in Yusho patients. There is a report of a dioxin and dioxin-like species-specific activation of AhR in vitro. For example, in Panc1 and HEK293 cell lines, TCDD induced higher levels of AhR-related gene-expression, such as that of CYP1A1 and PCB126. In contrast, PCB126 showed more enhanced induction of AhR-related genes than did TCDD in Hepa1c1c7 cell lines (Zhang et al., Citation2008). Thus, there seems to be a difference in AhR activation among the various types of dioxins and dioxin-like components. Further study, such as in vitro analysis of TH17 cell differentiation induced by PCBs, PCQs, or PCDFs, would likely help to clarify the relationship between dioxin species and T-cell activation in Yusho patients.
Meanwhile, as with TCDD, it could be hypothesized that PCBs, PCDFs, and/or PCQs induced Treg cell differentiation in Yusho patients. Recent studies revealed IL-17-producing FOXP3+ regulatory T (Tr17)-cells. Tr17 cells express Foxp3 and display immunosuppressive function, and participate in innate immunity by producing IL-17 as well (Li et al., Citation2012; Voo et al., Citation2009). Tr17 cells have been identified in human peripheral blood and lymphoid tissues (Voo et al., Citation2009). Moreover, it was shown that Tr17 cells were differentiated from CD4+FOXP3+ Treg cells when cultured with antigen-presenting cells and cytokines such as IL-1β, -2, -21, and -23 (Koenen et al., Citation2008; Voo et al., Citation2009). Taken together with our results showing that IL-1β and IL-23 levels were elevated in Yusho patients, PCBs, PCDFs, and/or PCQs might have induced Tr17 cells in these individuals.
In this study, serum IL-22 levels were lower in Yusho patients than in controls. This result was not in accordance with results expected from simple TH17 cell activation, because activated TH17 cells produce both IL-22 as well as IL-17 (Zenewicz & Flavell, Citation2011). However, production of IL-17 and IL-22 seems not to be strictly coordinated and rather depends on species of stimuli, cell type, or pathways induced. For example, human TH17 cells that were stimulated by IL-23 and prostaglandin E2 displayed enhanced IL-17 production but decreased IL-22 production (Chizzolini et al., Citation2008). It was also seen that human CD4+ T-cells when activated by TCDD produced high levels of IL-22 but not IL-17 (Ramirez et al., Citation2010). As for the results here, unlike what was seen with TCDD in vitro, the PCBs, PCDFs, PCQs possibly could have induced an up-regulation of IL-17 and down-regulation of IL-22 formation in the Yusho patients. As such, the precise mechanism of regulation of IL-17 and IL-22 in Yusho patients remains uncertain; however, in vitro studies with PCBs, PCQs, and/or PCDFs could help reveal the mechanism underlying CD4+ T-cell activation and differentiation in Yusho patients.
Conclusion
The results of the present study suggested to us that TH17 cells mediate inflammatory responses in Yusho patients. However, this study was a small-sized study. Further large-scale studies of the immune responses in the Yusho patients are required.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This study has been supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Health Labor and Welfare, Japan, and the Yusho Research Commission of Nagasaki Prefecture.
References
- Barin, J. G., Baldeviano, G. C., Talor, M. V., et al. 2012. Macrophages participate in IL-17-mediated inflammation. Eur. J. Immunol. 42:726–736
- Chizzolini, C., Chicheportiche, R., Alvarez, M., et al. 2008. Prostaglandin E2 synergistically with IL-23 favors human TH17 expansion. Blood 112:3696–3703
- Esser, C., Rannug, A., and Stockinger, B. 2009. The aryl hydrocarbon receptor in immunity. Trends Immunol. 30:447–454
- Furue, M. 2005. Overview of Yusho. J. Dermatol. Sci. Suppl. 1:S03–S10
- Katz, Y., Nadiv, O., and Beer, Y. 2001. IL-17 enhances TNFα-induced synthesis of IL-1, -6, and -8 in skin and synovial fibroblasts: Possible role as a “fine-tuning cytokine” in inflammation processes. Arthritis Rheum. 44:2176–2184
- Kobayashi, S., Okamoto, H., Iwamoto, T., et al. 2008. A role for the aryl hydrocarbon receptor and the dioxin TCDD in rheumatoid arthritis. Rheumatology (Oxford) 47:1317–1322
- Koenen, H. J., Smeets, R. L., Vink, P. M., et al. 2008. Human CD25highFoxp3pos regulatory T-cells differentiate into IL-17-producing cells. Blood 112:2340–2352
- Kurebayashi, Y., Nagai, S., Ikejiri, A., and Koyasu, S. 2013. Recent advances in understanding molecular mechanisms of development and function of TH17 cells. Genes Cells 18:247–265
- Li, L., Patsoukis, N., Petkova, V., and Boussiotis, V. A. 2012. Runx1 and Runx3 are involved in the generation and function of highly suppressive IL-17-producing T-regulatory cells. PLoS One. 7:e45115
- Maddur, M. S., Miossec, P., Kaveri, S. V., and Bayry, J. 2012. TH17 cells: Biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am. J. Pathol. 181:8–18
- Masuda, Y., Kuroki, H., Haraguchi, K., and Nagayama, J. 1985. PCB and PCDF congeners in the blood and tissues of Yusho and Yu-cheng patients. Environ. Health Perspect. 59:53–58
- Nagayama, J., Tsuji, H., Iida, T., et al. 2001. Effects of contamination level of dioxins and related chemicals on thyroid hormone and immune response systems in patients with “Yusho”. Chemosphere 43:1005–1010
- Quintana, F. J., Basso, A. S., Iglesias, A. H., et al. 2008. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature 453:65–71
- Ramirez, J. M., Brembilla, N. C., Sorg, O., et al. 2010. Activation of aryl hydrocarbon receptor reveals distinct requirements for IL-22 and IL-17 production by human T-helper cells. Eur. J. Immunol. 40:2450–2459
- Stockinger, B. 2009. Beyond toxicity: Aryl hydrocarbon receptor-mediated functions in the immune system. J. Biol. 8:61
- Voo, K. S., Wang, Y. H., Santori, F. R., et al. 2009. Identification of IL-17-producing FOXP3+ regulatory T-cells in humans. Proc. Natl. Acad. Sci. USA 106:4793–4798
- Zenewicz, L. A., and Flavell, R. A. 2011. Recent advances in IL-22 biology. Int. Immunol. 23:159–163
- Zhang, S., Rowlands, C., and Safe, S. 2008. Ligand-dependent interactions of the Ah receptor with co-activators in a mammalian two-hybrid assay. Toxicol. Appl. Pharmacol. 227:198–206