Abstract
Nanoparticle titanium dioxide (nano-TiO2) is a white pigment widely used in foods, sunscreens, and other cosmetic products. However, it remains unclear whether exposure to nano-TiO2 results in immunosuppressive effects or induces a contact hypersensitivity response. To address these data gaps, studies were conducted with the hypothesis that nano-TiO2 exposure could alter immune responses. After 28 days of oral gavage, nano-TiO2 (1.25–250 mg/kg in 0.5% methylcellulose) produced no significant effects on innate, humoral, or cell-mediated immune functions in female B6C3F1 mice. Furthermore, there were no effects on the weights of selected organs (spleen, thymus, liver, lung, and kidneys with adrenals). Following dermal exposure on the ears for 3 days, nano-TiO2 (2.5–10% w/v in 4:1 acetone:olive oil) did not affect auricular lymph node cell proliferation, although an irritancy response was observed following treatment with 5% and 10% nano-TiO2. Dermal sensitization (2.5–10%) on the back and subsequent challenge (10%) on the right ear with nano-TiO2 produced no significant effects on percentage ear swelling in the Mouse Ear Swelling Test (MEST). However, when nano-TiO2 was injected subcutaneously along the mid-line on top of the head at 125–250 mg/kg (in 0.5% methylcellulose), significant increases in auricular lymph node cell proliferation resulted. These results demonstrate that immune effects of nano-TiO2 exposure are route-of-exposure dependent, and they suggest that irritancy and/or potential hypersensitivity responses may occur following parenteral exposure or dermal administration of nano-TiO2 to compromised skin.
Introduction
Nanoparticle titanium dioxide (nano-TiO2) is a white pigment widely used in products such as paint, sunscreen, toothpaste, cosmetics, lipsticks, etc. (Duan et al., Citation2010; Furukawa et al., Citation2010; Newman et al., Citation2009). The US Food and Drug Administration (FDA) regulates its use as a food additive at levels not to exceed 1% by weight (FDA, Citation2010). Because of its whitening and photocatalytic effects, nano-TiO2 accounts for 70% of the total product volume of pigment worldwide, with an annual production of 5000–6400 tons (Baan et al., Citation2006; Mueller & Nowack, Citation2008). The Australian government has estimated that 70% of TiO2 sunscreens were formulated with nano-ingredients (TGA, Citation2006). TiO2 can be found in four major forms, e.g. ilmenite, rutile, anatase, and brookite; of these, anatase is frequently used in a variety of applications, including sunscreens (NMI, Citation2012), as a pigment in foodstuffs (CEC, 1995), and commercial manufacturing (Duan et al., Citation2010).
Due to its use in a large number of industrial applications, most research has focused on the toxicity and carcinogenicity of nano-TiO2 after inhalation exposure in the workplace (Liao et al., Citation2008; Newman et al., Citation2009). Titanium is often used in medical devices such as hip replacements and, over time, wear debris (small particles of titanium in the nano-to-micro-size range) can be released into the surrounding tissues and/or the bloodstream, resulting in parenteral exposure (Arys et al., Citation1998; Brien et al., Citation1992; Buly et al., Citation1992; Cunningham et al., Citation2002). Several groups have investigated nano-TiO2 for use in bone tissue engineering applications, representing another possible route of parenteral exposure (Gerhardt et al., Citation2007; Miyauchi et al. Citation2010; Smith et al. Citation2010a). In 2006, the International Agency for Research on Cancer (IARC) classified all forms of titanium dioxide as possibly carcinogenic to humans (Class 2B) on the basis of animal carcinogenicity data from inhalation studies, even though in vitro genotoxicity and carcinogenicity after oral exposure in B6C3F1 mice and F344 rats were negative (Ashby & Tennant, Citation1991; IARC, Citation2006; NCI, Citation1979; Shelby, Citation1988; Shelby & Zeiger, Citation1990; Witt et al., Citation2000). At present, significant gaps remain in the nano-TiO2 knowledge base, making quantitative risk assessments difficult for oral, dermal, and parenteral exposures. Therefore, evaluations of potential immune effects of nano-TiO2—for each of its relevant routes of exposure—are essential.
Jani et al. (Citation1994) demonstrated that TiO2 particles (500 nm diameter) translocated to systemic organs such as the liver, spleen, lungs, and peritoneal tissue (but not heart or kidney) after daily oral administration of 0.1 ml of a 2.5% (w/v) suspension (≈12.5 mg/kg) to female Sprague Dawley rats for 10 days. Others reported that female (but not male) CD-1 mice exposed to nano-TiO2 (25 and 80 nm diameter) in a single oral gavage at 5 g/kg had increased serum alanine aminotransferase (ALT):aspartate aminotransferase (AST) ratios and lactate dehydrogenase (LDH) levels, as well as increased relative liver weights (Wang et al., 2007). In addition, Duan et al. (Citation2010) reported that 30 days of oral exposure to 125–250 mg/kg nano-TiO2 anatase (5 nm diameter) produced decreases in several immunological and hematological parameters, including red blood cell counts, total white blood cell counts, various peripheral blood cell populations (T-lymphocytes [CD3+, CD4+ and CD8+], B-cells, and NK cells), and interleukin (IL)-2 levels in female CD-1 mice. Moreover, female CD-1 mice administered anatase nano-TiO2 at 5–150 mg/kg daily by intraperitoneal (IP) injection for 14 days showed an increase in both absolute and relative liver weights, liver enzymes (ALT, AST, LDH), and significant increases in both mRNA and protein levels of several cytokines (e.g. IL-1, IL-10, IL-4, and tumor necrosis factor [TNF]-α) (Wang et al., Citation2007). Li et al. (Citation2010) also reported that daily IP injection of 50–150 mg/kg nano-TiO2 into female CD-1 mice for 45 days induced splenic pathological changes, apoptosis, and elevated levels of reactive oxygen species and pro-inflammatory cytokines (IL-1, TNF-α). There was also evidence to suggest that mice exposed to nano-TiO2 by IP injection had enhanced inflammatory responses in a lipopolysaccharide (LPS)-induced septic brain model in as little as 30 min following the TiO2 injection (Shin et al., Citation2010).
While many reports have indicated that nano-TiO2 (20–100 nm diameter) does not penetrate through the stratum corneum of healthy skin, some reports have shown that nano-TiO2 can penetrate the upper layers of the stratum corneum with minimal penetration into the stratum granulosum (Borm et al., Citation2006; Furukawa et al., Citation2010; Gamer et al., Citation2006; Lademann et al., Citation1999; Mavon et al., Citation2007; Pflucker et al., Citation2001; Sadrieh et al., Citation2010; SCCP, Citation2007; Schulz et al., Citation2002). Moreover, 30 nm diameter nano-TiO2 has demonstrated prominent photocatalytic activity in promoting formation of protein tyrosine nitration after UV exposure in mouse skin homogenates in vitro (Lu et al., Citation2008). Protein tyrosine nitration has been linked to several cutaneous pathological events, including cutaneous inflammation, systemic sclerosis, and contact hypersensitivity (Dooley et al., Citation2006; Greenacre et al., Citation2002; Olmos et al., Citation2007; Szabo et al., Citation2001). Indeed, systemic hypersensitivity to titanium has been documented in humans following chronic exposure to dental and prosthetic implants (Müller & Valentine-Thon, Citation2006).
In spite of the above literature, there are only limited reports on how nano-TiO2 exposure affects overall immune function. This is particularly concerning due to the seemingly ubiquitous use of nano-TiO2 in a multitude of industrial applications, foods, and personal care products such as cosmetics and sunscreens. Furthermore, the cells of the immune system represent a primary potential target of nanoparticle-mediated toxicity, due to the high probability of nanoparticle uptake by immune cells such as phagocytes and antigen-presenting cells. Therefore, because of the tremendous potential for exposure of the general population to nano-TiO2 (primarily via the oral and dermal routes), the present studies evaluated the immunotoxic effects of nano-TiO2 following 28 days of oral gavage in B6C3F1 mice, in addition to irritancy, contact hypersensitivity, and systemic hypersensitivity effects following dermal or subcutaneous (SC) exposure for 3 days in BALB/c mice. Effects on humoral, cell-mediated, and innate immunity were assessed using the T-dependent antibody response (TDAR) to sheep erythrocytes (SRBC), the delayed-type hypersensitivity (DTH) response to C. albicans, and the functional activity of the fixed tissue macrophages of the mononuclear phagocytic system (MPS). Hypersensitivity responses were evaluated by using the local lymph node assay (LLNA), the irritancy (IRR) assay, the lymph node proliferation assay (LNPA), and the mouse ear-swelling test (MEST). Based upon previous studies reporting immunological effects following oral, dermal, and parenteral exposure to nano-TiO2, we hypothesized that, via each route of exposure, modulation of one or more functions of the immune system would become apparent.
Materials and methods
Test substances
Nano-TiO2, (anatase; <25 nm diameter; 99.7% metal basis, CAS no. 1317-70-0) was purchased from Sigma (St. Louis, MO). Nano-TiO2 was prepared in the appropriate vehicle for each assay, as indicated below. For the oral gavage studies, dosing solutions were prepared weekly, stored refrigerated, and stirred on a stir plate (using a magnetic stir bar) for at least 10 min prior to daily dosing. For hypersensitivity evaluations, dosing solutions were prepared daily. All nano-TiO2 solutions were sonicated for 2 min and then stirred for ≈24 h to achieve homogeneity.
Species selection, doses administered, and routes of exposure
For the oral gavage studies, female B6C3F1 mice (strain used by National Toxicology Program for immunotoxicity testing) were obtained from Taconic Farms (Germantown, NY). For the hypersensitivity studies, female BALB/c mice (strain used by the NTP for hypersensitivity testing) were obtained from Charles River Laboratories (Raleigh, NC). All mice were tested and found to be hepatitis- and Sendai virus-free. Mice arrived at 8–10 weeks-of-age and were quarantined for ≈1 week prior to being placed on study. All mice were housed and maintained at 21–24 °C and 40–70% relative humidity, with a 12-h light/dark cycle. Food pellets and tap water were provided ad libitum in all experiments. These studies were conducted in accordance with all applicable animal care and use laws, regulations, and guidelines, under a protocol approved by the Virginia Commonwealth University (VCU) Institutional Animal Care and Use Committee (IACUC) in an AAALAC-accredited facility.
The mice were randomized prior to the study and eight mice were assigned to each dose group. Female B6C3F1 mice were used in the TDAR, DTH, and MPS studies following oral administration of nano-TiO2 once a day for 28 days in a volume of 0.1 ml/10 g body weight. Nano-TiO2 was administered at doses ranging from 12.5–250 mg/kg in these studies. In one AFC study, two lower doses of nano-TiO2 (1.25 and 3.75 mg/kg) were also used. Female BALB/c mice were used in the LLNA, IRR, MEST, and LNPA studies. In the LLNA and IRR, nano-TiO2 exposure levels were 1, 2.5, 5, and 10% (w/v) administered dermally in a volume of 50 µl/mouse (25 µl to dorsal side of each ear). In the MEST, 2.5, 5, and 10% (w/v) nano-TiO2 doses were administered dermally (50 µl/mouse on shaved dorsal lumbar surface) during the sensitization phase, and 10% (w/v) was administered for challenge (25 µl to dorsal side of right ear). For the LNPA, 0.1 ml was administered subcutaneously (SC) along the mid-line on top of the head at doses of 12.5, 37.5, 125, and 250 mg/kg. The dosing regimen for each of these studies (LLNA, IRR, MEST, LNPA) is detailed in the methods sections below. Doses were selected based upon reports in the literature, at sufficiently high levels for purposes of hazard identification, without the likelihood of overt toxicity.
Vehicle controls
Methylcellulose (MTC; 0.5% w/v; Fisher Scientific, Fair Lawn, NJ; CAS No.9004-67-5) was used as the vehicle for oral gavage (TDAR, DTH, MPS) and SC injection (LNPA). MTC was prepared at the start of the study and stored refrigerated. Acetone:olive oil (4:1; AOO) was used as the vehicle for the LLNA, IRR, and MEST assays. Acetone (CAS No. 67-64-1) was bought from Sigma and extra virgin olive oil was purchased locally. AOO was prepared on the first day of dosing and stored in amber bottles at room temperature. All vehicle control animals were treated at the same time as the nano-TiO2 exposed animals, using the same dosing volumes and routes of exposure as the nano-TiO2 exposed animals for each study.
Positive controls
Cyclophosphamide (CPS, Sigma; CAS No. 6055-19-2) was prepared at 5 mg/ml in phosphate-buffered saline (PBS, pH 7.4) and administered at 50 mg/kg daily for the last 4 days of the exposure period by intraperitoneal (IP) injection (0.1 ml/10 g body weight) as a positive control for the TDAR and DTH assays. Maleic vinyl ether (MVE, Hercules, Wilmington, DE) was prepared at 5 mg/ml in sterile PBS and administered in a volume of 0.1 ml/10 g body weight (equivalent to dose of 50 mg/kg) by intravenous (IV) injection 24 h prior to study termination as a positive control for the MPS assay. For the LLNA, IRR, and MEST studies, solutions of 0.15% (v/v) 2,4-dinitrofluorobenzene (DNFB; Sigma, CAS No. 70-34-8) were prepared daily by dilution in AOO and administered at 50 µl/mouse (LLNA, IRR, MEST sensitization phase) or 25 µl/mouse (MEST challenge phase) as positive control. For the LNPA assay, DNFB was prepared daily at 0.1% (v/v) in 0.5% methylcellulose and administered by SC injection along the mid-line on top of the head at 100 µl/mouse as a positive control. For the LLNA, IRR, and MEST assays, hexyl cinnamic aldehyde (HCA; Sigma, CAS no. 101-86-0) solutions of 25% HCA (v/v) were prepared daily by dilution in AOO and administered in a volume of 50 µl/mouse (LLNA, IRR, MEST sensitization phase) or 25 µl/mouse (MEST challenge phase) as a positive control.
Toxicological parameters
Animal body weights were obtained weekly. On the day of assay, mice were weighed and were euthanized by CO2 inhalation. Female B6C3F1 mice were examined for gross pathology and the thymus, lungs, liver, spleen, and kidneys with adrenals were removed and weighed.
Spleen collection and single-cell suspension preparation
For the AFC assay, spleens were collected and placed in tubes containing 3 ml Earle’s Balanced Salt Solution (EBSS; Sigma) with 15 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES; Gibco, Grand Island, NY). Splenocyte suspensions were prepared by pressing each spleen between frosted ends of two microscope slides. The single-cell suspensions were centrifuged at 300 x g for 10 min and re-suspended in EBSS with 15 mM HEPES. Cell counts were performed using a ZBII Coulter counter in the presence of ZAP-OGLOBIN II lytic reagent (Coulter Corporation, Miami, FL).
Spleen IgM antibody response to the sheep erythrocytes (AFC)
Using a modification of the hemolytic plaque assay of Jerne & Nordin (Citation1963), the primary IgM response to SRBC was measured as described by White et al. (2010). Mice were IV sensitized with 7.5 × 107 SRBC (Lampire, Pipersville, PA) 4 days prior to study termination. On the day of euthanasia, spleen cell suspensions were prepared as described, and an aliquot of cells (100 μl) added to a test tube containing warm agar, guinea pig complement (1:4 dilution; Accurate Chemical, Westbury, NY), and 25 μl SRBC (1:2 dilution of cell pellet volume). The mixture was vortexed, plated in a petri dish, covered with a microscope coverslip, and incubated for 3 h at 37 °C. Plaques that developed were counted with a plaque viewer (Bellco, Vineland, NJ). Experimental results were ultimately expressed as specific activity (AFC/106 spleen cells) and total spleen activity (AFC/Spleen [×103]).
Antigen-specific IgM enzyme-linked immunosorbent assay (ELISA)
An IgM enzyme-linked immunosorbent assay (ELISA) was used to determine anti-SRBC IgM serum titres in the same mice used in the AFC assay. Blood was collected by cardiac puncture and allowed to clot in borosilicate glass tubes. The serum fraction was collected and frozen at −70 °C until thawed for analysis. The study was conducted as described by Temple et al. (Citation1993) with a slight modification as previously described (Smith et al., Citation2010b). Briefly, the day before the assay was conducted, Immulon II microtiter plates were coated with high-salt release SRBC membrane antigens and incubated at 4 °C overnight. Plates were then washed three times with Tween/PBS buffer (0.05% Tween 20 in PBS), and then incubated at room temperature with Tween/PBS solution for 1 h. Serum from each animal was diluted 1:4 in Tween/PBS buffer (1:2 for CPS-treated animals), and 100 µl of each sample was added to a dedicated well. Each serum sample was assayed as 10 serial 2-fold dilutions. Plates were incubated for 1.5 h at room temperature and then washed three times with Tween/PBS. Horseradish peroxidase-conjugated anti-mouse IgM antibody (100 µl; 1:500 dilution, SouthernBioTech, Birmingham, AL) was added to each well and the plates then incubated an additional 1.5 h at room temperature. Plates were then again washed three times before peroxidase substrate (2,2’-azino-di-[3-ethyl-benzthia-zoline-6-sulfonic acid], 0.2 mg/ml in 0.05 M phosphate-citrate buffer, 100 µl/well) was added, and, after 45 min, the absorbance in each well was read at 405 nm on an automated plate reader (Molecular Devices, Sunnyvale, CA). Titres were defined as the reciprocal of the serum dilution that produced an optical density (OD) of 0.5 and were determined for each mouse by interpolation using the linear portion of a log-log regression of OD vs reciprocal dilution (Kawabata et al., Citation1995).
Delayed-type hypersensitivity response (DTH) to Candida albicans
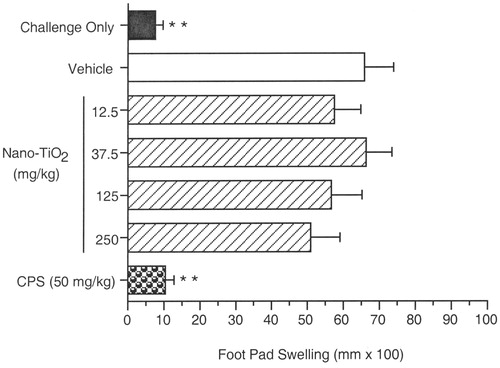
Delayed-type hypersensitivity responses to C. albicans were evaluated as described by Smith & White (Citation2010). Briefly, formalin-fixed C. albicans (AlerChek, Inc., Portland, ME) were diluted to 108 organisms/ml 0.9% NaCl and administered SC at 0.1 ml/mouse into the right flank on Day 21 of the study. On Day 29, pre-measurements of the right footpad were made with a digital micrometer (Mitutoyo Corp., Tokyo, Japan) and mice were then challenged SC with C. albicans antigen chitosan (CHT; [AlerChek, Inc.]) in that footpad (0.04 ml; 1 mg/ml). Twenty-four hours later, right footpad thickness was measured and footpad swelling calculated (average post-challenge thickness − average pre-challenge thickness). Data were reported in terms of footpad swelling (as mm × 100). Also included in each study was a group of mice challenged with CHT in the footpad on Day 29 but neither exposed to the test article nor sensitized with C. albicans. This ‘challenge only’ group (CO) was used to determine background footpad swelling resulting from non-specific trauma due to the CHT injection.
Mononuclear phagocyte system (MPS)
A modified MPS assay was conducted by measuring the phagocytosis of [51Cr]-labeled sheep erythrocytes ([51Cr]-SRBC) by fixed tissue macrophages in the liver, spleen, lungs, and thymus. This assay was performed as described by Auttachoat et al. (Citation2004), with modification. The [51Cr] used for labeling was purchased from PerkinElmer (Boston, MA). On Day 29, mice were injected IV with [51Cr]-SRBC (5 μl/g body weight; 5 × 109 SRBC/ml). After 60 min, the mice were euthanized by decapitation and exsanguinated, and then their liver, spleen, lungs, and thymus were removed to measure organ radioactivity as an indicator of [51Cr]-SRBC uptake. Results were expressed as percentage uptake (expressed as percentage of total counts injected) and as specific activity (CPM/mg tissue weight).
Combined local lymph node assay (LLNA) and irritancy assay (IRR)
A combined LLNA and IRR was conducted following a modification (Anderson et al., 2003) of a previously published procedure (Woolhiser et al., Citation1998). Prior to exposure, mice were weighed, randomized, and tattooed for identification. Before chemical application on the first day of the study, measures of ear thickness (pre-measurement; as mm × 10−2) were made using a modified micrometer (Mitutoyo). On Day 1, mice were treated with test substances (1–10% [w/v] nano-TiO2), vehicle (AOO), or positive controls (0.15% DNFB; 25% HCA) by applying a volume of 25 µl to the dorsum of each ear. Identical treatments were repeated for the next 2 days. On Days 4 and 5, the mice were rested. On Day 4, 24 h after the final treatment, measures of ear thickness (post-measurement) were made in the manner as the pre-treatment measures. The IRR results were expressed as percentage ear swelling using the formula {[(mean post-treatment ear thickness/mean pre-treatment ear thickness) × 100] − 100}. On Day 6, all mice were IV injected with 20 µCi [3H]-thymidine (PerkinElmer) via the lateral tail vein. Five hours later, the mice were euthanized, and the right and left auricular lymph nodes from each excised and placed in 4 ml cold PBS. Single-cell suspensions were made, and, following overnight incubation in 5% trichloroacetic acid at 4 °C, samples were analyzed in a β-counter (1214 Rackbeta LKB Wallac, Perkin Elmer Instruments, Boston, MA). Disintegrations per minute (DPM) for each mouse were calculated as (CPM − background count)/(counter efficiency). Results were expressed as lymph node DPM per mouse. Based on ICCVAM-recommended criteria (NIH, 1999), a chemical was classified as positive if at least one concentration reached or exceeded a stimulation index (SI) ≥3, with the results being statistically significant and dose-responsive.
Mouse ear swelling test (MEST)
The MEST evaluates the elicitation phase of the hypersensitivity response, with the end-point being ear swelling caused by an inflammatory reaction to a recall antigen. The assay here was conducted using a modification of the method of Gad et al. (Citation1986). Based on the results of the LLNA and IRR, mice were sensitized with 2.5–10% (w/v) nano-TiO2 and were challenged with 10% (w/v) nano-TiO2 in the MEST. On Day 1, the dorsal lumbar surface of each animal was shaved. Fifty microliters of vehicle (AOO), nano-TiO2, or 0.15% DNFB was applied to the prepared site via pipette (sensitization phase). Sensitization procedures were repeated on Days 2 and 3. Mice were rested on Days 4–7. On Day 8, the thickness of the right ear of each mouse was measured (pre-measurement), and the mice were then challenged (25 µl) on the dorsal side of the right ear pinna with vehicle (AOO), nano-TiO2, or 0.15% DNFB. Post-measurements of ear thickness were made 24 and 48 h after challenge in the same manner as the pre-measurements. The results were expressed in percentage ear swelling at both 24 and 48 h.
Lymph node proliferation assay (LNPA)
The LNPA measures a substance's potential to induce a systemic hypersensitivity response, with the end-point being a measure of [3H]-thymidine incorporation into proliferating lymphocytes in auricular draining lymph nodes. The assay was performed as described by Weaver et al. (Citation2005) with modification. Prior to exposure, mice were weighed, randomized, and tattooed for identification. On Day 1, mice were SC injected on the top of the head along the mid-line. The mice were then exposed to vehicle (0.5% methylcellulose), 12.5–250 mg/kg nano-TiO2, or the positive control (0.1% [≈30 mg/kg] DNFB). Identical treatments were repeated for the next 2 days. The mice were rested on Days 4 and 5. On Day 6, mice were injected IV with 20 µCi [3H]-thymidine and evaluated for lymph node cell proliferation as described above for the LLNA.
Statistical analysis
Results represent the mean ± standard error (SE). Statistical analysis of all data was conducted using JMP™ 8.0 (SAS Institute, Inc., Cary, NC) by first using Bartlett’s test for homogeneity of variances, followed by an analysis of variance (ANOVA) (parametric or non-parametric as necessary). Ad hoc pair-wise comparisons were made using Dunnett’s Test for parametric data and the Wilcoxon Rank Test for non-parametric data. Values significantly different from the appropriate control group at p ≤ 0.05 or p ≤ 0.01 were then identified.
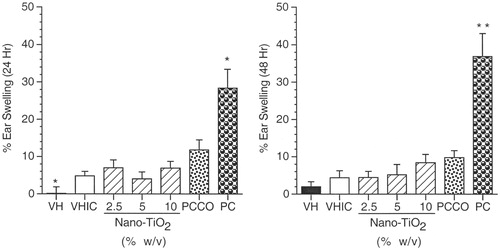
For the MEST, the vehicle control group was compared to the vehicle irritancy control (i.e. sensitized with vehicle; challenged with 10% nano-TiO2; VHIC) using the Student’s t-test, while nano-TiO2 treatment groups were compared to VHIC using Dunnett’s test; the positive control group was compared to the positive control challenge only (i.e. sensitized with vehicle; challenged with 0.15% DNFB; PCCO) group using the Student’s t test.
Results
General toxicology studies
There were no unexpected nano-TiO2-related deaths in these studies. In one study (MPS), three mice exposed to 12.5 mg/kg and one exposed to 125 mg/kg were humanely euthanized prior to study end due to gavage error. These mice did not display overt toxicity following gross histopathology observation, with the exception of observations associated with gavage error.
Exposure to nano-TiO2 had no effects on body weights or body weight changes in either B6C3F1 or BALB/c mice, and no signs of overt toxicity were observed throughout the studies (data not shown). Organ weight measurements of the mice from the AFC study were evaluated as both absolute organ weights and as organ-to-body weight ratio (relative weights) for the liver, spleen, lung, thymus, and kidneys with adrenals. No significant differences were noted between the vehicle controls and nano-TiO2-treated animals for any organs evaluated. Body and organ weight data for two separate studies are provided in Supplementary Tables S1 and S2. There was a slight decrease (13%) in liver weights of mice exposed to 125 mg/kg in Study 2 (Table S2). However, this decrease was not dose-related nor observed in the other study. Therefore, it was not considered biologically relevant. As expected, the positive control CPS induced significant decreases in spleen and thymus weights (Tables S1 and S2).
Spleen IgM AFC response to SRBC
As shown in (which shows combined results of two AFC studies), daily gavage with nano-TiO2 for 28 days did not significantly affect the AFC response to SRBC when evaluated as either specific activity or total spleen activity. CPS significantly suppressed the AFC response, as expected.
Figure 1. Spleen IgM antibody-forming cell (AFC) response to sheep erythrocytes. Female B6C3F1 mice were treated with nano-TiO2 for 28 days by oral gavage and were immunized with SRBC on Day 25 of the experimental period. Results are presented as mean (±SE) for the AFC/106 spleen cells (specific activity) and AFC/spleen (total spleen activity) outcomes. Results represented the mean obtained from combining two studies, each of which utilized seven or eight animals per group. Comparisons of the results of the vehicle control groups from the two studies were conducted using a Student’s t test, which indicated there were no significant differences (1689 [±186] versus 2058 [±261] for specific activity and 300 [±34] versus 399 [±66] for total spleen activity). Positive control (PC) is 50 mg/kg CPS. Level of statistical significance: **p ≤ 0.01 as compared to vehicle control group.
![Figure 1. Spleen IgM antibody-forming cell (AFC) response to sheep erythrocytes. Female B6C3F1 mice were treated with nano-TiO2 for 28 days by oral gavage and were immunized with SRBC on Day 25 of the experimental period. Results are presented as mean (±SE) for the AFC/106 spleen cells (specific activity) and AFC/spleen (total spleen activity) outcomes. Results represented the mean obtained from combining two studies, each of which utilized seven or eight animals per group. Comparisons of the results of the vehicle control groups from the two studies were conducted using a Student’s t test, which indicated there were no significant differences (1689 [±186] versus 2058 [±261] for specific activity and 300 [±34] versus 399 [±66] for total spleen activity). Positive control (PC) is 50 mg/kg CPS. Level of statistical significance: **p ≤ 0.01 as compared to vehicle control group.](/cms/asset/8a59eef3-5642-4a7c-a8fa-806869867150/iimt_a_844750_f0001_b.jpg)
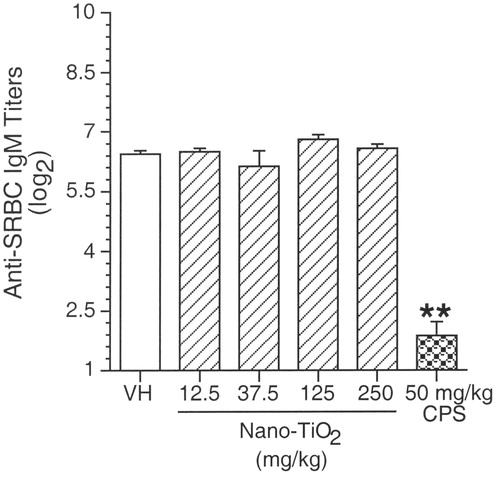
Anti-SRBC IgM enzyme-linked immunosorbent assay (ELISA)
The results of the antigen-specific serum IgM ELISA are shown in . Consistent with the results from the AFC studies, exposure to 12.5–250 mg/kg nano-TiO2 did not produce any significant effects when compared to the vehicle control. Mice exposed to the positive control (CPS) had a significant decrease in the serum IgM response to SRBC when compared to the vehicle control.
Figure 2. Serum IgM enzyme-linked immunosorbent assay (ELISA). Female B6C3F1 mice were treated with nano-TiO2 for 28 days by oral gavage and were immunized with SRBC on Day 25 of the experimental period. Results are presented as mean (±SE) for serum (log2) titres. Positive control was 50 mg CPS/kg. Level of statistical significance: **p ≤ 0.01 as compared to vehicle control group.
Delayed-type hypersensitivity (DTH) assay with C. albicans
Results are presented as mean footpad swelling (). Daily exposure to 12.5–250 mg/kg nano-TiO2 for 28 days did not affect the DTH response to C. albicans. As expected, footpad swelling of the challenge only group was significantly lower than the vehicle control group, which had received both sensitization and challenge. CPS, the positive control, significantly suppressed the percentage footpad swelling when compared to the vehicle control.
Mononuclear phagocyte system (MPS)
The MPS assay evaluated the phagocytic ability of fixed tissue macrophages of the liver, spleen, lungs, and thymus. Levels of [51Cr] in the kidneys were also assessed as an indicator of whether [51Cr]-labeled SRBC had lysed upon injection, which would result in the release of free [51Cr] into the blood stream, making all data uninterpretable. The results are shown in . There were no significant differences in phagocytic activity between nano-TiO2 treatment groups (12.5–250 mg/kg) and the vehicle control group. There was a slight increase in the liver weights of mice treated with 12.5 mg/kg (16.4%) and 250 mg/kg nano-TiO2 (17.2%), however these increases were not dose-responsive. Similar responses were also observed in the spleen weights, where there were significant increases in the spleen weights in mice treated with 12.5, 37.5, and 250 mg/kg, but not at 125 mg/kg nano-TiO2. The percentage increases were 21, 19.3, and 30.6% in mice treated with, respectively, 12.5, 37.5, and 250 mg/kg nano-TiO2. These increases were also not dose-dependent. These organ weight effects were not noted in two other studies evaluating organ weights, and are therefore not believed biologically relevant. As expected, MVE (positive control) significantly inhibited phagocytic activity of fixed macrophages of the liver when compared to activity of cells in the vehicle control.
Table 1. Functional activity of mononuclear phagocytic system in female B6C3F1 mice exposed to nano-TiO2 for 28 days.
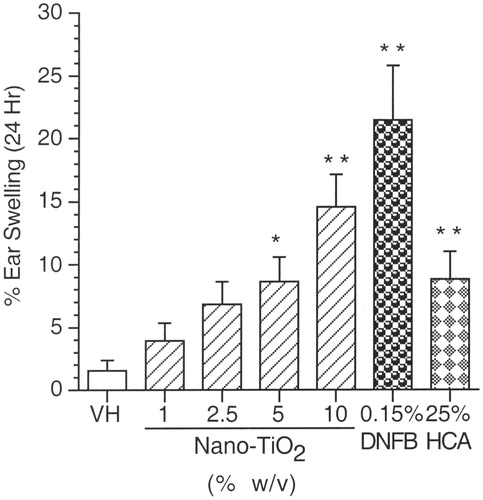
Irritancy assay (IRR)
Results of the irritancy studies are shown in . There was a dose-responsive increase in the percentage ear swelling of mice exposed to 1–10% nano-TiO2, reaching statistical significance at the two highest dose levels (i.e. 5% and 10% nano-TiO2) when compared to the vehicle control. Both positive controls, DNFB and HCA, produced significant increases in percentage ear swelling when compared to the vehicle control.
Figure 4. Irritancy response (IRR). Mean percentage ear swelling (±SE) for female BALB/c mice exposed to nano-TiO2 by dermal administration. VH = AOO; Positive controls (PC) are 0.15% DNFB and 25% Hexyl Cinnamic Aldehyde HCA. Levels of statistical significance: *p ≤ 0.05 and **p ≤ 0.01 compared to vehicle control group.
Local lymph node assay (LLNA)
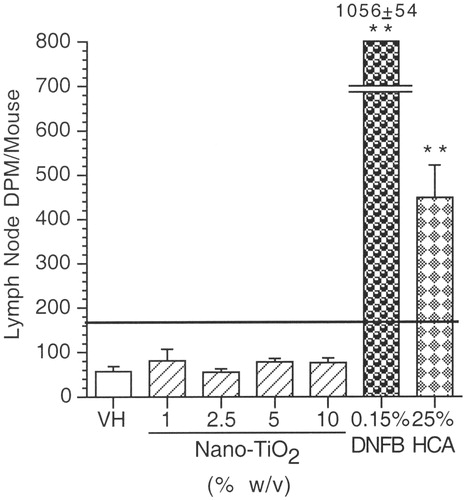
Dermal exposure to nano-TiO2 had no statistically significant effects on the proliferation of auricular lymph node cells when compared to the vehicle control (). As expected, significant increases in lymph node proliferation were observed in mice treated with the positive controls (DNFB and HCA) when compared to the vehicle control.
Figure 5. Local lymph node proliferation response (LLNA). Mean DPM (±SE) for female BALB/c mice exposed to nano-TiO2 by dermal administration. VH = AOO; Positive controls are 0.15% DNFB and 25% HCA. Level of statistical significance: **p ≤ 0.01 compared to vehicle control group. Solid horizontal line across graph indicates value where Stimulation Index (SI) = 3.
Mouse ear swelling test (MEST)
The MEST was conducted to evaluate further the sensitization potential of nano-TiO2. Nano-TiO2 treatment levels were 2.5, 5, and 10% for the sensitization phase, and 10% for the challenge phase. Results indicated that there were no exposure-related changes in mouse ear swelling when compared to the VHIC group (). A significant increase in percentage ear swelling at 24 h was observed for the VHIC group when compared to the VH control group, which was consistent with the irritant effects of 10% nano-TiO2 seen in the irritancy study (). As expected, animals sensitized and challenged with DNFB exhibited a statistically significant increase in percentage ear swelling when compared to animals in the PCCO group at both 24 and 48 h post-challenge ().
Figure 6. Mouse ear swelling test (MEST). Mean percentage ear swelling (±SE) for female BALB/c mice exposed to nano-TiO2 by dermal administration. Animals were sensitized with 2.5, 5, and 10% nano-TiO2, and challenged with 10% nano-TiO2. VH animals received 4:1 acetone:olive oil (AOO) for both sensitization and challenge, while VHIC animals received AOO at sensitization and 10% nano-TiO2 at challenge. PCCO (positive control challenge only) animals received AOO at sensitization and 0.15% DNFB at challenge, while PC (positive control) animals received 0.15% DNFB for both sensitization and challenge. Levels of statistical significance: *p ≤ 0.05 and **p ≤ 0.01 compared to appropriate control group.
Lymph node proliferation assay (LNPA)
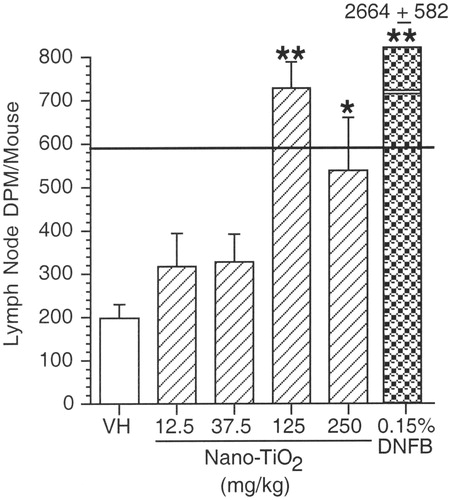
In contrast to the results seen in the LLNA, exposure by SC injection of nano-TiO2 at 125–250 mg/kg to female BALB/c mice induced a significant increase in the LNPA when compared to the vehicle control (). Lymph node proliferation in mice exposed to 125 mg/kg exceeded the 3-fold threshold of the vehicle control response. At the 250 mg/kg dose, a statistically significant increase was also observed; however, the 3-fold threshold was not exceeded. Exposure to DNFB induced a significant increase, as expected, when compared to the vehicle control.
Figure 7. Lymph node proliferation assay (LNPA). Mean DPM (±SE) for female BALB/c mice exposed to nano-TiO2 by SC administration. VH = 0.5% methylcellulose. Levels of statistical significance: *p ≤ 0.05 and **p ≤ 0.01 compared to vehicle control group. Solid horizontal line across graph indicates Stimulation Index (SI) = 3.
Discussion
Exposure of the general population to nano-TiO2 can occur by several routes, including oral and dermal, because nano-TiO2 is used as a food additive in toothpaste, capsules, and cosmetics (Duan et al., Citation2010; Furukawa et al., Citation2010; Newman et al., Citation2009). Another potential route of human exposure is parenteral following medical device implantation or in the future from tissue engineered products (Arys et al., Citation1998; Brien et al., Citation1992; Buly et al., Citation1992; Cunningham et al., Citation2002; Gerhardt et al., Citation2007; Miyauchi et al, Citation2010; Smith et al., Citation2010a). The daily consumption of nano-TiO2 for adults is estimated to be ≈0.5–1.3 mg/kg/day, while children are believed to receive even greater doses (EFSA, Citation2004; Weir et al., Citation2012). Human exposure levels to nano-TiO2 from personal care products, including sunscreens, toothpastes, and cosmetics, are unknown. However, nano-TiO2 levels as high as 90 µg per mg of lotion have been found in certain sunblocks (Weir et al., Citation2012) that, if applied at the recommended density of 2 mg lotion per cm2 exposed skin (Stokes & Diffey, Citation1997) could result in exposures of 24 mg/kg per application for a 60 kg individual applying the lotion to half of their body surface area.
According to the manufacturer’s Certificate of Analysis (Sigma-Aldrich, Citation2009), the dry titanium dioxide nanoparticles used in these studies were spherical, with a batch-specific average particle size of 20 nm. We acknowledge that nanoparticle characterizations were not conducted for the nano-TiO2 preparations used in these studies. However, the results of these studies should not be summarily dismissed, as humans are exposed to both well-dispersed and aggregated nanoparticles having varied physicochemical properties.
Furthermore, the adsorption of proteins to materials following exposure to biological systems has been well established and occurs rapidly, in a few seconds to a few minutes (Cedarvall et al., Citation2007; Monopoli et al., Citation2012; Ratner, Citation2004). The formation of this protein corona fundamentally alters the surface characteristics of administered nanoparticles and irrevocably changes both particle–cell and particle–particle interactions, such that it is highly unlikely that the particle characteristics pre-exposure are identical to those post-exposure. Indeed, the physical characteristics (including size and aggregation/dispersion state) comprising the ‘synthetic identity’ of a nanomaterial preparation are distinct from its ‘biological identity’ (Walkey et al., Citation2012). Although pre-adsorption of proteins onto the NM surface can be accomplished prior to exposure, it is unknown whether this is sufficient to prevent further adsorption once the material is exposed to a living system. Although it has been suggested that nanoparticle size dictates the density of adsorbed serum proteins (Walkey et al., Citation2012), at present there is no definitive correlation between the ‘synthetic’ and ‘biological’ identities of nanoparticle formulations and observed in vivo effects (Ma-Hock et al., Citation2013). Lastly, human exposure to nanoparticles rarely, if ever, occurs to well-defined nanoparticle preparations. Thus, the results of the present studies are relevant, even in the absence of a particle characterization. However, future studies in which the physical and chemical characteristics are determined for nano-TiO2 solutions similar to those used in these studies will be important in imparting greater significance to the results presented here.
Many reports have indicated that the penetration of particles into the stratum corneum is limited by particle size, and nano-TiO2 does not appear to penetrate through the stratum corneum of healthy skin (Borm et al., Citation2006; Furukawa et al., Citation2010; Gamer et al., Citation2006; Lademann et al., Citation1999; Mavon et al., Citation2007; Pflucker et al., Citation2001; Sadrieh et al., Citation2010; SCCP, Citation2007; Schulz et al., Citation2002). However, developments in alternative drug delivery systems at the nanoscale level have also introduced the concept of a nanoemulsion containing oil, surfactant, and water to improve the bioavailability of hydrophobic materials (Newman et al., Citation2009). Due to the potential of local dermal exposure as well as systemic exposure by other routes, our studies also evaluated the potential of nano-TiO2 to induce systemic hypersensitivity after SC injection into mice.
In general, the WHO (Citation1969) has indicated the LD50 of TiO2 for rats is >12,000 mg/kg body weight after oral administration. This is not surprising in light of reports that the vast majority of orally ingested nano-TiO2 (even at doses >1000 mg/kg) is excreted in the feces and is not absorbed or distributed systemically (Cho et al., Citation2013). However, Jani et al. (Citation1994) showed that TiO2 (500 nm diameter) may translocate to organs, including the liver, spleen, lungs, and peritoneal tissue, but not the heart or kidney after oral administration. Overall, our results have shown that nano-TiO2 (at 1.25–250 mg/kg) did not induce overt toxicity in the major organs as demonstrated by no significant effect on the percentage and absolute organ weights of the spleen, thymus, liver, lung, and kidneys.
Duan et al. (Citation2010) suggested that nanoparticulate anatase TiO2 (5 nm diameter) at 125–250 mg/kg produced decreases in body weight and increases in liver, kidney, spleen, and thymus weights in CD-1 mice after oral administration for 30 days. Such changes were not observed in the study here. This may be a result of difference in particle size (20 nm vs 5 nm), strain differences (B6C3F1 vs CD-1), or vehicle differences (0.5% methylcellulose vs 0.5% hydroxypropylmethylcellulose). Here, 0.5% methylcellulose was used as the vehicle (instead of 0.5% hydroxypropylmethylcellulose as used by others) because this vehicle has historically been shown not to cause immune suppression in B6C3F1 mice. Wang et al. (Citation2007) indicated that CD-1 mice demonstrated increased liver weights (absolute and relative) exposed to TiO2 nanoparticles (25 and 80 nm) at 5 g/kg in a single oral gavage. However, this dose is 20-times greater than the highest dose used in the current study, and the aim of the Wang et al. study was primarily to characterize nanoparticulate TiO2 tissue distribution and retention in liver, spleen, kidneys, and lungs.
Duan et al. (Citation2010) also indicated that nanoparticulate TiO2-treated CD-1 mice displayed a reduction in IL-2 activity, white blood cells, red blood cells, hemoglobin, mean corpuscular hemoglobin concentration, thrombocytes, reticulocytes, T- and B-lymphocytes, and NK cells when treated with 125–250 mg TiO2/kg. However, the Duan et al. study did not determine whether the decrease in those factors could also affect overall immune function. Toward that end, we investigated immunotoxicological effects by using holistic functional assays to assess innate, humoral, and cell-mediated immunity. The current studies demonstrated that nano-TiO2 at 1.25–250 mg/kg produced no significant effects on humoral immune responses, macrophage function, or cell-mediated immune responses, as assessed through AFC, MPS, and DTH responses.
The present studies also evaluated the potential hypersensitivity effects of nano-TiO2 in mice after dermal and SC exposure. No effects were observed in the LLNA, suggesting that nano-TiO2 is not a contact sensitizer. The MEST confirmed this result. Although nano-TiO2 did not produce any contact sensitizing effects at concentrations ranging from 1–10% (w/v), there was a dose-responsive increase in percentage ear swelling in the irritancy study, with statistically significant increases observed at the 5 and 10% dose levels (equivalent to 125 and 250 mg/kg/day, respectively, for a 20 g mouse). These results may be due to the inability of nano-TiO2 to penetrate beyond the upper layer of the stratum corneum after dermal administration to healthy skin (Borm et al., Citation2006; Furukawa et al., Citation2010; Gamer et al., Citation2006; Lademann et al., Citation1999; Mavon et al., Citation2007; Pflucker et al., Citation2001; SCCP, Citation2007; Schulz et al., Citation2002).
In contrast to results following dermal exposure, systemic (parenteral) exposure by SC administration at 125–250 mg/kg produced significant increases in lymph node proliferation in the LNPA study. The LNPA assay is a modification of the LLNA that is designed to test the ability of many substances, especially drugs, to induce immune-mediated systemic hypersensitivity (Weaver et al., Citation2005). In addition, the LNPA is recommended over the LLNA when evaluating the hypersensitivity potential of nanoparticles (Dobrovolskaia et al., Citation2009). A previous study by Hussain et al. (Citation2012) evaluated the ability of nano-TiO2 pre-treatment to modulate the hypersensitivity response to a potent known sensitizer, 2,4-dinitrochlorobenzene. Results from their control animals (which received TiO2 but no subsequent chemical exposure) indicated that SC injection of TiO2 did not affect lymphocyte proliferation in the LNPA at doses up to 0.4 mg/ml (100 µl; equivalent to ≈2 mg/kg). The doses used in those studies were much lower than those evaluated herein, and the absence of effects in the LNPA in the Hussain et al. report are consistent with results of our studies.
Results from the present study demonstrate that, if exposure is parenteral at sufficiently high doses, nano-TiO2 can produce significant increases in lymphocyte proliferation. However, a limitation of this study is the inability to firmly establish whether the effects observed in the LNPA are primarily due to non-specific irritancy or to systemic hypersensitivity. It is well established that non-sensitizing irritants can induce proliferation in the LLNA, thereby confounding the ability to distinguish between irritants and sensitizers. However, responses that exceed a stimulation index (SI) of 3-fold over control are typically associated with sensitizers and not irritants (Anderson et al., Citation2011), although exceeding an SI of 3 does not confirm that a chemical is truly a sensitizer. Although hypersensitivity to implanted titanium has been documented in humans (Müller & Valentine-Thon, Citation2006) and is consistent with a possible systemic hypersensitivity response to nano-TiO2 in the present study, further study is warranted to more clearly delineate the irritant vs systemic hypersensitivity of nano-TiO2 following parenteral administration.
Conclusion
In summary, results from these studies demonstrated that immune effects after exposure to nano-TiO2 solutions are route-dependent. Oral gavage of nano-TiO2 at doses up to 250 mg/kg in 0.5% methylcellulose produced no significant effects on humoral immune responses, macrophage function, or cell-mediated immune responses when evaluated using, respectively, the AFC assay, MPS assay, and DTH response to C. albicans. In addition, there was no significant effect on lymph node proliferative responses or percentage ear swelling evaluated by LLNA and MEST assays after dermal exposure to 1–10% nano-TiO2. However, mice exposed dermally to 5–10% nano-TiO2 demonstrated an increase in percentage ear swelling, suggesting that extremely high concentrations of nano-TiO2 may cause dermal irritation. Finally, an SC injection of nano-TiO2 resulted in a significant increase in lymph node cell proliferation at 125 and 250 mg/kg nano-TiO2, suggesting irritant and/or potential hypersensitivity responses following parenteral exposure or dermal administration of nano-TiO2 to compromised skin.
Declaration of interest
This research project was supported in part by NIEHS Contract N01-ES-55530.
Acknowledgements
The authors would like to thank Ronnetta D. Brown, Deborah L. Musgrove, Michael Montague, Anthony Brown, Julia Nims, and Andre Savage for their technical assistance.
References
- Anderson, P. K., Woolhiser, M. R., Rase, J. M., and Holsapple, M. P. 2003. Integration of primary irritation with the LLNA. The Toxicologist 72:492
- Anderson, S. E., Siegel, P. D., and Meade, B. J. 2011. The LLNA: A brief review of recent advances and limitations. J. Allergy 2011:424203
- Arys, A., Philippart, C., Dourov, N., et al. 1998. Analysis of titanium dental implants after failure of osseointegration: Combined histological, electron micro-scopy, and X-ray photoelectron spectroscopy approach. J. Biomed. Mater. Res. 43:300–312
- Ashby, J., and Tennant, R. W. 1991. Definitive relationships among chemical structure, carcinogenicity and mutagenicity for 301 chemicals tested by the U.S. NTP. Mutat. Res. 257:229–306
- Auttachoat, W., Chitsomboon, B., Peachee, V. L., et al. 2004. Immunomodulation by Don Din Daeng (Aeginetia indica Roxb.) extracts in female B6C3F1 mice: II. Humoral immunity, innate immunity and hematology. Int. Immunopharmacol. 4:1381–1390
- Baan, R., Straif, K., Grosse, Y., et al. 2006. Carcinogenicity of carbon black, titanium dioxide, and talc. Lancet Oncol. 7:295–296
- Borm, P. J., Robbins, D., Haubold, S., et al. 2006. The potential risks of nanomaterials: A review carried out for ECETOC. Part. Fibre Toxicol. 3:11
- Brien, W.W., Salvati, E. A., Betts, F., et al. 1992. Metal levels in cemented total hip arthroplasty. A comparison of well-fixed and loose implants. Clin. Orthoped. Related Res. 264:66–74
- Buly, R. L., Huo, M. H., Salvati, E., et al. 1992. Titanium wear debris in failed cemented total hip arthroplasty. An analysis of 71 cases. J. Arthroplasty 7:315–323
- CEC (Commission of the European Communities). 1995. Commission directive 95/45/EC of 26 July 1995 laying down specific purity criteria concerning colors for use in foodstuffs. Available online at: http://ec.europa.eu/food/fs/sfp/addit_flavor/flav13_en.pd [last accessed 29 July 2013]
- Cedarvall, T., Lynch, I., Lindman, S., et al. 2007. Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc. Natl. Acad. Sci. USA 104:2050–2055
- Cho, W. S., Kang, B. C., Lee, J. K., et al. 2013. Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration. Part. Fibre Toxicol. 10:9
- Cunningham, B. W., Orbegoso, C. M., Dmitriev, A. E., et al. 2002. The effect of titanium particulate on development and maintenance of a postero-lateral spinal arthrodesis: An in vivo rabbit model. Spine 27:1971–1981
- Dobrovolskaia, M. A., Germolec, D. R., and Weaver, J. L. 2009. Evaluation of nanoparticle immunotoxicity. Nat. Nanotechnol. 4:411–414
- Dooley, A., Gao, B., Bradley, N., et al. 2006. Abnormal nitric oxide metabolism in systemic sclerosis: Increased levels of nitrated proteins and asymmetric dimethylarginine. Rheumatology (Oxford) 45:676–684
- Duan, Y., Liu, J., Ma, L., et al. 2010. Toxicological characteristics of nanoparticulate anatase titanium dioxide in mice. Biomaterials 31:894–899
- EFSA (European Food Safety Authority). 2004. Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food on a request from the Commission related to the safety in use of rutile titanium dioxide as an alternative to the presently permitted anatase form. Available online at: http://efsa.europa.eu/en/efsajournal/doc/163.pdf [last accessed 9 September 2013]
- Furukawa, F., Doi, Y., Suguro, M., et al. 2010. Lack of skin carcinogenicity of topically applied titanium dioxide nanoparticles in the mouse. Food Chem. Toxicol. 49:744–749
- Gad, S. C., Dunn, B. J., Dobbs, D. W., et al. 1986. Development and validation of an alternative dermal sensitization test: The mouse ear-swelling test (MEST). Toxicol. Appl. Pharmacol. 84:93–114
- Gamer, A. O., Leibold, E., and van Ravenzwaay, B. 2006. The in vitro absorption of microfine zinc oxide and titanium dioxide through porcine skin. Toxicol. In Vitro 20:301–307
- Gerhardt, L. C., Jell, G. M., and Boccaccini, A. R. 2007. Titanium dioxide (TiO2) nanoparticles filled poly(D,L-lactid acid) (PDLLA) matrix composites for bone tissue engineering. J. Mater. Sci. Mater. Med. 18:1287–1298
- Greenacre, S. A., Rocha, F. A., Rawlingson, A., et al. 2002. Protein nitration in cutaneous inflammation in the rat: Essential role of inducible nitric oxide synthase and polymorphonuclear leukocytes. Br. J. Pharmacol. 136:985–994
- Hussain, S., Smulders, S., de Vooght, V., et al. 2012. Nano-titanium dioxide modulates the dermal sensitization potency of DNCB. Part. Fibre Toxicol. 9:15
- IARC (International Agency for Research on Cancer). 2006. Monographs on the evaluation of carcinogenic risks to humans. Carbon Black, Titanium Dioxide and Non-Asbestiform Talc. Vol 93. Geneva: World Health Organization
- Jani, P. U., McCarthy, D. E., and Florence, A. T. 1994. Titanium Dioxide (rutile) particle uptake from the rat GI tract and translocation to systemic organs after oral administration. Int. J. Pharmaceut. 105:157–168
- Jerne, N. K., and Nordin, A. A. 1963. Plaque formation in agar by single antibody-producing cells. Science 140:405–406
- Kawabata, T. T., Babcock, L. S., Gauggel, D. L., et al. 1995. Optimization and validation of an ELISA to measure specific guinea pig IgG1 antibody as an alternative to the in vivo passive cutaneous anaphylaxis assay. Fundam. Appl. Toxicol. 24:238–246
- Lademann, J., Weigmann, H., Rickmeyer, C., et al. 1999. Penetration of titanium dioxide microparticles in a sunscreen formulation into the horny layer and the follicular orifice. Skin Pharmacol. Appl. Skin Physiol. 12:247–256
- Li, N., Duan, Y., Hong, M., et al. 2010. Spleen injury and apoptotic pathway in mice caused by titanium dioxide nanoparticules. Toxicol. Lett. 195:161–168
- Liao, C. M., Chiang, Y. H., and Chio, C. P. 2008. Model-based assessment for human inhalation exposure risk to airborne nano/fine titanium dioxide particles. Sci. Total Environ. 407:165–177
- Lu, N., Zhu, Z., Zhao, X., et al. 2008. Nano titanium dioxide photo-catalytic protein tyrosine nitration: A potential hazard of TiO2 on skin. Biochem. Biophys. Res. Commun. 370:675–680
- Ma-Hock, L., Strauss, V., Treumann, S., et al. 2013. Comparative inhalation toxicity of multi-wall carbon nanotubes, graphene, graphite nanoparticles, and low surface carbon black. Part. Fibre Toxicol. 10:23
- Mavon, A., Miquel, C., Lejeune, O., et al. 2007. In vitro percutaneous absorption and in vivo stratum corneum distribution of an organic and a mineral sunscreen. Skin Pharmacol. Physiol. 20:10–20
- Miyauchi, T., Yamada, M., Yamamoto, A., et al. 2010. The enhanced characteristics of osteoblast adhesion to photofunctionalized nanoscale TiO2 layers on biomaterials surfaces. Biomaterials 31:3827–3839
- Monopoli, M. P., Åberg, C., Salvati, A., and Dawson, K. A. 2012. Biomolecular coronas provide the biological identity of nanosized materials. Nat. Nanotechnol. 7:779–786
- Mueller, N. C., and Nowack, B. 2008. Exposure modeling of engineered nanoparticles in the environment. Environ. Sci. Technol. 42:4447–4453
- Müller, K., and Valentine-Thon, E. 2006. Hypersensitivity to titanium: Clinical and laboratory evidence. NeuroEndocrinol Lett. 27:31–35
- National Cancer Institute, Carcinogenesis, Technical Report Series No.97. 1979. Bioassay of titanium dioxide for possible carcinogenicity (CAS No. 3463-67-7) NCI-CG-TR-97. Available online at: http://ntp.niehs.gov/ntp/htdocs/LT_rpts/tr097.pdf [last accessed 10 Sep 2013]
- Newman, M. D., Stotland, M., and Ellis, J. I. 2009. The safety of nanosized particles in titanium dioxide- and zinc oxide-based sunscreens. J. Am. Acad. Dermatol. 61:685–692
- NIH. 1999. The murine local lymph node assay: A test method for assessing the allergic contact dermatitis potential of chemicals/compounds. NIH Publication No. 99-4494
- NMI (National Measurement Institute). 2012. XRD Phase Analysis of TiO2 Sunscreens. Australian Government, NMI. Available from: http://libcloud.s3.amazonaws.com/93/5a/1/2746/NMI-report-XRD-anatase.pdf [last accessed 29 July 2013]
- Olmos, A., Giner, R. M., Recio, M. C., et al. 2007. Effects of plant alkylphenols on cytokine production, tyrosine nitration and inflammatory damage in the efferent phase of contact hypersensitivity. Br. J. Pharmacol. 152:366–373
- Pflucker, F., Wendel, V., Hohenberg, H., et al. 2001. The human stratum corneum layer: An effective barrier against dermal uptake of different forms of topically applied micronised titanium dioxide. Skin Pharmacol. Appl. Skin Physiol. 14:92–97
- Ratner, B. D. 2004. Biomaterials Science: An Introduction to Biomaterials in Medicine, Chapter 3, 2nd ed (Ratner, B. D., Hoffman, A. S., Schoen, F. J., and Lemons, J. E., Eds.). London: Elsevier Academic Press
- Sadrieh, N., Wokovich, A. M., Gopee, N.V., et al. 2010. Lack of significant dermal penetration of titanium dioxide from sunscreen formulations containing nano- and submicron-size TiO2 particles. Toxicol. Sci. 115:156–166
- SCCP (Scientific Committee on Consumer Products). 2007. SCCP/1147/07: Opinion on safety of nanomaterial in cosmetic products. Adopted by the SCCP after the public consultation on the 14th Plenary of 18 December 2007. Available online at: http://ec.europa.eu/health/ph_risk/committees/04_sccp/docs/sccp_o_123.pdf [last accessed 10 Sep 2013]
- Schulz, J., Hohenberg, H., Pflucker, F., et al. 2002. Distribution of sunscreens on skin. Adv. Drug Deliv. Rev. 54:S157–S163
- Shelby, M. D. 1988. The genetic toxicity of human carcinogens and its implications. Mutat. Res. 204:3–15
- Shelby, M. D., and Zeiger, E. 1990. Activity of human carcinogens in the Salmonella and rodent bone-marrow cytogenetics tests. Mutat. Res. 234:257–261
- Shin, J. A., Lee, E. J., Seo, S. M., et al. 2010. Nanosized titanium dioxide enhanced inflammatory responses in the septic brain of mouse. Neuroscience 165:445–454
- Sigma-Aldrich. 2009. Certificate of Analysis: Titanium(IV) oxide, anatase, nanopowder, <25 nm particle size. St. Louis, MO: Sigma-Aldrich
- Smith, C. M., Roy, T. D., Bhalkikar, A., et al. 2010a. Engineering a titanium and polycaprolactone construct for a biocompatible interface between the body and artificial limb. Tissue Eng. Part A 16:717–724
- Smith, D. C., Smith, M. J., and White, K. L. Jr. 2010b. Systemic immunosuppression following a single pharyngeal aspiration of 1,2:5,6-dibenzanthracene in female B6C3F1 mice. J. Immunotoxicol. 7:219–231
- Smith, M. J., and White, K. L. Jr. 2010. Establishment and comparison of delayed-type hypersensivity models in the B6C3F1 mouse. J. Immunotoxicol. 7:308–317
- Stokes, R., and Diffey, B. 1997. How well are sunscreen users protected? Photodermatol. Photoimmunol. Photomed. 13:186–188
- Szabo, E., Virag, L., Bakondi, E., et al. 2001. Peroxynitrite production, DNA breakage, and poly(ADP-ribose) polymerase activation in a mouse model of oxazolone-induced contact hypersensitivity. J. Invest. Dermatol. 117:74–80
- Temple, L., Kawabata, T. T., Munson, A. E., and White, K. L. Jr. 1993. Comparison of ELISA and plaque-forming cell assays for measuring the humoral immune response to SRBC in rats and mice treated with benzo[a]pyrene or cyclophosphamide. Fundam. Appl. Toxicol. 21:412–419
- TGA. 2006. A review of the scientific literature on the safety of nanoparticulate titanium dioxide or zinc dioxide in sunscreens. Australian Government, OTC Medicines Section. Available online at: http://www.tga.gov.au/npmeds/sunscreen-zotd.pdf [last accessed 10 Sep 2013]
- U.S. Food and Drug Administration (FDA). 2010. CFR-Code of Federal Regulations Title 21, Volume 1-Food and Drugs, Chapter 1-Food and Drug Administration. Department of Health and Human Services. Subchapter A-general, part 73- listing of color additives exempt from certification. Revised on April 1, 2010. 21 CFR73.575. Available online at: http://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=73.575 [last accessed 10 Sep 2013]
- Walkey, C. D., Olsen, J. B., Guo, H., et al. 2012. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J. Am. Chem. Soc. 134:2139–2147
- Wang, J., Zhou, G., Chen, C., et al. 2007. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol. Lett. 168:176–185
- Weaver, J. L., Chapdelaine, J. M., Descotes, J., et al. 2005. Evaluation of a lymph node proliferation assay for its ability to detect pharmaceuticals with potential to cause immune-mediated drug reactions. J. Immunotoxicol. 2:11–20
- Weir, A., Westerhoff, P., Fabricius, L., et al. 2012. Titanium dioxide nanoparticles in food and personal care products. Environ. Sci. Technol. 46:2242–2250
- White, K. L., Musgrove, D. L., and Brown, R. D. 2010. The sheep erythrocyte T-dependent antibody response (TDAR). Methods Mol. Biol. 598:173--184
- Witt, K. L., Knapton, A., Wehr, C. M., et al. 2000. Micronucleated erythrocyte frequency in peripheral blood of B6C3F1 mice from short-term, prechronic, and chronic studies of the NTP carcinogenesis bioassay program. Environ. Mol. Mutagen. 36:163–194
- Woolhiser, M. R., Hayes, B. B., and Meade, B. J. 1998. A combined murine local lymph node and irritancy assay to predict sensitization and irritancy potential of chemicals. Toxicol. Mech. Meth. 8:245–256
- World Health Organization. 1969. FAO Nutrition Meeting Report Series No. 46 A: Toxicological evaluation of some food colors, emulsifers, stabilizers, anti-caking agents, and certain other substances. Geneva: WHO/FOOD ADD/70.36