Abstract
A gold nanoparticle (GNP) probe-based assay (GNPA) modified from the bio-barcode assay (BCA) was developed for ultrasensitive detection of ricin, a potential biothreat agent. In the GNPA, a chain of ricin was captured by a GNP probe coated with polyclonal antibodies and single-stranded signal DNA. A magnetic microparticle (MMP) probe coated with ricin A chain monoclonal antibody was then added to form an immuno-complex. After being magnetically separated, the immuno-complex containing the single-stranded signal DNA was characterized by PCR and real-time PCR. A detection limit of 10−2 fg/ml was determined for the ricin A chain; this is eight orders of magnitude more sensitive than that achieved with an ELISA and two orders more sensitive than that obtained with the BCA. The coefficients of variation (CV) of the intra- and inter-assay values ranged from 3.82–6.46%. The results here show that this novel assay is an ultrasensitive method for detection of ricin proteins and may be suitable for the ultrasensitive detection of other proteins.
Introduction
Ricin is a toxic glycoprotein readily isolated from Ricinus communis castor bean seeds (Roberts and Smith, Citation2004). Ricin consists of two chains (A and B) roughly of equal size that are connected by a disulfide bond, with a total molecular weight of ≈62 kDa. The A chain acts to inhibit protein synthesis, which ultimately leads to cell death by catalytically dupurinating the 28 S ribosomal RNA (Dertzbaugh et al., Citation2005). The B chain is a lectin responsible for binding to specific eukaryotic cell surface receptors, such as glycoproteins and glycolipids. Ricin is considered a likely agent for bioterrorism and is the only protein toxin listed in the Schedule 1 category of the Chemical Weapons Convention (CWC) (Rowe-Taitt et al., Citation2000; Atlas, Citation2002). Ricin can potentially be misused by anyone due to the wild growth of Ricinus communis plant. As such, a portable detection system for detection of minute amounts of ricin is desirable.
Current ricin detection methods include radioimmunoassay (Godal et al., Citation1981), rapid immuno-chromatography (Shyu et al., Citation2001), enzyme-linked immunosorbent assays (ELISA) (Poli et al., Citation1994), electrochemiluminescent-based technology (Garber and O’Brien, Citation2008), biosensors (Guglielmo-Viret and Thullier, Citation2007; Tran et al., Citation2008; Suresh et al., Citation2010), a silica coating magnetic nanoparticle-based silver enhancement immunoassay (Zhuang et al., Citation2010), liquid-crystal based sensors, and a quantitative polymerase chain reaction (PCR) assay (Melchior and Tolleson, Citation2010). The bio-barcode amplification (BCA) assay has become a powerful analytical tool for ultrasensitive measures of protein and nucleic acid targets (Nam et al., Citation2004; Agasti et al., Citation2010; Fournier-Wirth and Coste, Citation2010).
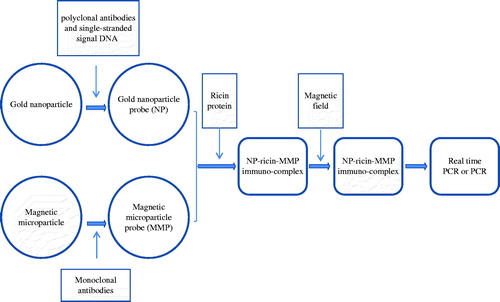
In a previous study, we developed a BCA for the detection of the ricin A chain (Yin et al., Citation2012). However, the conventional BCA protocol is complicated and time-consuming. To move beyond those problems, we report here development of a gold nanoparticle (GNP) probe-based assay (GNPA) () -- in effect a modified BCA -- that can be used for ultrasensitive and convenient detection of the ricin A chain.
Materials and methods
Reagents and materials
The proteins ricin, camphorin, and ebulitin were purchased from NOVASYGEN (Beijing, China). Ricin A chain antigen-specific antibody (Ab) was purchased from Abcam (Cambridge, UK). Ricin A chain antigen-specific monoclonal antibody (mAb) was purchased from Affinity Bioreagents (Rockford, IL). Other materials for use in the ELISA assay were purchased from Beijing CellChip Biotechnology Co. (Beijing, China). Tosyl-activated-functionalized magnetic microparticle (MMP) probe was purchased from Invitrogen (Carlsbad, CA). GNP (15 nm diameter) was purchased from Ted Pella (Altadena, CA). TaqTM was obtained from TaKaRa (Dalian, China). The iCycler Detection System was purchased from Bio-Rad (Hercules, CA).
Preparation of magnetic microparticle (MMP) probe and gold nanoparticle (GNP) probe
To prepare MMP probe, the mAb specific to ricin A chain was bound to tosyl-activated-functionalized MMP (100 µl, 30 mg/ml aqueous solution, 2 × 109 beads/ml, 2.8 µm diameter) according to manufacturer protocols. To prepare the GNP, a single-stranded signal DNA was designed and synthesized: 5′-GTGACTGCAAGTCCATTGAGGTGAAATAGCCAGTTCTCG-TGGCATTTGCGTAATCCATGTATCGCTCGGTGGTTGCCAAC-TAGAGAAC-A10-(CH2)6-SH-3′. A 100 -μl suspension of the GNP was incubated with 10.5 µg ricin A chain antigen-specific Ab in an aqueous solution (pH 9.5) for 30 min. The particles were subsequently modified with thiolated-single-stranded signal DNA (2 µM final concentration) by slow salt aging (16 h) to a phosphate-buffered saline (PBS) solution (0.1 M NaCl in 0.01 M phosphate buffer, pH 7.0). Unbound thiolated-signal DNA was then removed by several rounds of centrifugation (13,000 × g for 20 min) at 4 °C and rinsing in PBS, followed by re-suspension in 0.1 M PBS.
GNP probe-based assay (GNPA) format
In the GNPA, 10 µl of ricin at different concentrations was added to 50 µl of MMP (5 mg/ml solution) and incubated with vigorous stirring for 1 h at 37 °C. After magnetically immobilizing the MMP, unbound antigens were removed by repeated washing with PBS. Washed MMP was then incubated with 50 µl GNP (0.1 nM) for 30 min at 37 °C with vigorous stirring. The GNP-bound MMP complex was then magnetically separated and washed 4-times with 500 µl of PBS. The sandwich complex containing the single-stranded signal DNA was then detected by PCR and TaqMan probe based real-time PCR methods.
PCR was performed in a final volume of 25 µl containing 5U Premix Ex TaqTM, 2 µM of forward primer (5′-GTTCTCTAGTTGGCAACCACC-3′), 2 µM of reverse primer (5′-GTGAC- TGCAAGTCCATTGAGG-3′), and 2 µl of the sandwich complexes solution under the following conditions: 95 °C for 10 s, followed by 35 cycles of 95 °C for 5 s, 52 °C for 20 s, and 72 °C for 20 s, and a final incubation at 72 °C for 5 min. Real-time fluorescence PCR detection was conducted in a reaction mixture (25 µl) containing 5 U TaqTM, 2 µM forward primer (5′-GTT-CTCTAGTTGGCAACCACC-3′), 2 µM reverse primer (5′-GTGACTGCAAGTCCATTGAGG-3′), 1 µM TaqMan probe (5′-FAM-AGTTCTCGTGGCATTTGCGTAATCC-TAMRA-3′), and 2 µl of sandwich complexes solution under the following conditions: 95 °C for 10 s, followed by 45 cycles of 95 °C for 5 s, 52 °C for 20 s. Real-time reactions were then analyzed using an iCycler Detection System. In the real-time PCR, standard template pGEM-DNA was prepared. In brief, the signal DNA was ligated into pGEM-T Easy Vector (Promega, Madison, WI) and used to transform Kan ultra-competent Escherichia coli DH5α cells. Positive clones were then selected by blue-white screening and sequenced by colony touch PCR (HuaDa, Beijing, China).
Assay specificity
To analyze specificity of the GNPA, camphorin and ebulitin were detected with the GNPA using the above protocol. Following amplification in the PCR detection format, 5 µl of amplified product was separated over 3% agarose gels. The gel was then stained with ethidium bromide, and the PCR products visualized under UV light.
Assay sensitivity
The sensitivity of the GNPA was evaluated using an antigen-capture ELISA method to detect ricin A chain at different concentrations. Flat bottom 96-well plates were incubated with 100 µl ricin A chain mAb (diluted 1:2500) and incubated overnight at 4 °C. After three washes with PBS, 100 µl 1% bovine serum albumin (BSA) blocking solution was added to the wells and the plates incubated at 37 °C for 60 min before being washed three times with PBS; 100 µl of ricin A chain samples at different concentrations were added to dedicated wells and incubated at 37 °C for 60 min. After three washes, 100 µl of horseradish peroxidase-conjugated mAb (diluted 1:2000) was added to each well and the plate incubated at 37 °C for 30 min. The wells were then developed by adding 3,3′,5,5′-tetramethylbenzidine (TMB) at room temperature. Reactions were stopped after 15 min by addition of 100 µl 2 M H2SO4. Absorbance in each well was then read at 450 nm in a Wellscan MK3 plate reader (Labsystems, Dragan, Finland).
Assay reproducibility
To determine reproducibility of the assay, the GNPA based on real-time PCR was carried out to detect ricin A chain protein at 1 fg/ml. The ricin A chain protein was tested on three separate occasions, with three identical samples of ricin A chain protein being tested each time.
Assay sample detection
Mimic samples were prepared by mixing 100 µl ricin A chain (102 fg/ml) in 900 µl of milk or water, respectively. The mimic samples were then processed using the GNPA and antigen-capture ELISA methods.
Results
Assay specificity
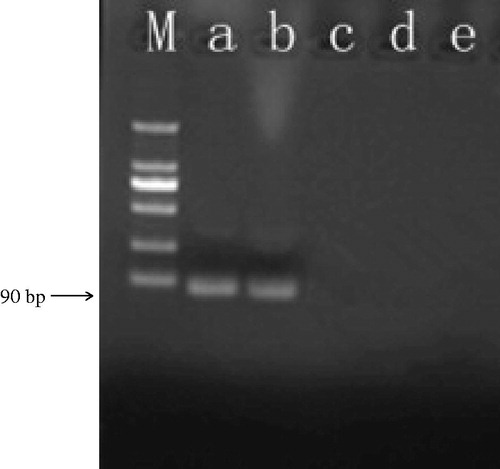
Ricin, camphorin, and ebulitin were detected by the GNPA. A 90 bp amplicon was observed in the tested ricin A chain samples but not noted with the camphorin and ebulitin, demonstrating the highly specific nature of the GNPA (). In the real-time PCR detection format, signal DNA was detected with the ricin A chain samples via real-time fluorescence PCR, but not with the camphorin and ebulitin samples ().
Figure 1. The GNPA protocol. The assay used MMP probes functionalized with monoclonal antibodies that recognize and bind target protein (i.e. ricin toxin). The ricin toxin was then functionalized with the nanoparticle (NP) probe that has been modified with ricin A chain antigen-specific antibodies and single-stranded signal DNAs. After being magnetically separated, the immune-complex containing the single-stranded signal DNA was characterized by PCR and by real-time PCR.
Assay sensitivity
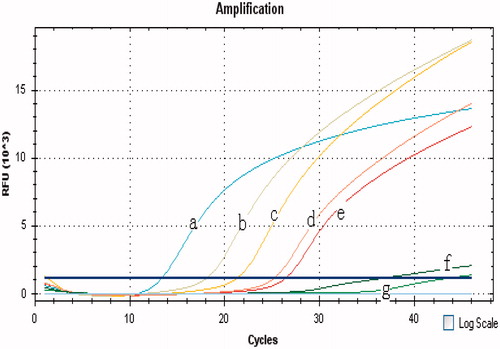
Ten-fold dilutions of ricin A chain were subjected to the GNPA and the antigen-capture ELISA methods to analyze assay sensitivity. The results demonstrated that >10−2 fg/ml of the ricin A chain was detected by the GNPA based on either PCR or real-time PCR; 106 fg/ml of ricin A chain was detected by the antigen-capture ELISA method. The sensitivity of the GNPA based on real-time PCR is shown in . Thus, the GNPA method is about eight orders of magnitude more sensitive than that of the antigen-capture ELISA method.
Figure 3. Specificity of the GNPA using real-time PCR detection. Signal DNA bound on GNP was analyzed using fluorescence real-time PCR. Real-time PCR results from: (Curves A, B) ricin A chain of two duplicates; (Curves C, D) camphorin and ebulitin protein; and (Curve E) blank control.
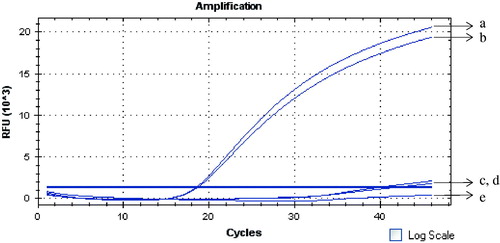
Figure 4. Sensitivity of the GNPA based on real-time PCR for the ricin A chain detection. Ten-fold dilutions of ricin A chain were subjected to the GNPA. Amplification plot of the signal DNA bound on GNP probe detected with real-time PCR showed that over 10−2 fg ricin A chain/ml were detected by the GNPA. (Curves A–F) amplification plots of 102, 101, 100, 10−1, 10−2, and 10−3 fg/ml ricin A chain detected by the GNPA. (Curve G) blank control.
Assay reproducibility
The reproducibility of the GNPA was evaluated by detection of the ricin A chain. The results indicated that the coefficients of variation (CV) of the threshold cycle (Ct) values were 6.17, 3.82, and 6.46 (intra-test) and 5.48 (inter-test) ().
Table 1. Reproducibility of the GNPA for detection of ricin A chain.
Assay sample detection
Both mimic samples were subjected to the GNPA and antigen-capture ELISA. The results showed that each mimic sample tested positive in the GNPA based on PCR and real-time formats, but tested negative in the antigen-capture ELISA method.
Discussion
Nanomaterials have some unique physical, chemical, and biological properties that are fundamentally different from those of the corresponding bulk material and could be widely used in medical testing (Rosi and Mirkin, Citation2005). In the past decade, nanoparticle-based assays have been adapted to improve the sensitivity and specificity of medical testing and could provide new tools for clinical diagnosis due to their potential for high degrees of sensitivity, specificity, multiplexing capabilities, and ability to operate without enzymes (Rosi and Mirkin, Citation2005). Gold nanoparticles are especially attractive for biodetection due to their high surface area (Du et al., Citation2009; Khalavka et al., Citation2009). The BCA utilizes double-stranded oligonucleotide-modified gold nanoparticles for signal amplification and magnetic nanoparticles for easy separation from the samples (Nam et al., Citation2003, Citation2004; Yin et al., Citation2012).
In this study, the GNPA modified from the traditional bio-barcode amplification (BCA) assay was developed for detecting ricin A chain and PCR and real-time PCR were used as the assay readouts. In our previous study, 30-nm diameter GNP were synthesized and used to prepare a GNP probe. Further, double-stranded DNA were bound to the GNP, and after the immunocomplex was formed, signal DNA annealed to DNA strands covalently bound to the GNP were released by heating and characterized by PCR and real-time fluorescence PCR (Yin et al., Citation2012). In the assay, 15-nm diameter GNP were used to prepare the probe because they are more stable than larger GNP (data not shown). Then, single-stranded signal DNA was modified on the GNP probe and the sandwich complexes containing signal DNA bound on GNP were detected directly. In addition, the length of the signal DNA was designed to be ≈90 bp and was adapted to the TaqMan probe-based real-time PCR; this improved the specificity of the assay further and decreased the risk of DNA contamination due to the closed nature of the system and the absence of post-PCR handling of the amplified material.
These results indicated that only ricin A chain samples were detected in the GNPA and no cross-reactivity was observed with the closely-related camphorin and ebulitin samples. A lower detection limit of 10−2 fg/ml was measured for the ricin A chain. The sensitivity of this assay is significantly higher than that of conventional ELISA, with a measured detection limit of 106 fg ricin A chain/ml (). Moreover, recent studies showed the sensitivities of surface plasmon resonance analysis, a quantitative PCR assay, and a liquid-crystal based sensor for ricin detection to be 10 pg/ml, 30 pg/ml, and 10 μg/ml of ricin, respectively (Driskell et al., Citation2008; Melchior et al., Citation2010). In addition, our previous study showed that the sensitivity of the traditional BCA was 1 fg/ml of ricin A chain protein (Yin et al., Citation2012). Therefore, the GNPA reported here is more sensitive than the traditional BCA and other methods for detecting ricin.
There is one potentially important issue that was not addressed in the current study. Specifically, it is unclear if the amount of signal DNA and antibodies coated to the gold nanoparticle and microparticle probes might/could vary from lot-to-lot. To address this possible event and potential effects on the efficacy of our novel assay, inter-lot comparisons will be made and effects on the measured outcomes here will be evaluated in an upcoming study.
In this GNPA, directly detecting the single-stranded signal DNA bound to the GNP probe simplified the procedure and shortened the exam time from that of the traditional BCA method. Both assay readouts of the GNPA, i.e. PCR and real-time fluorescence PCR, proved equally sensitive and demonstrated similar detection limits. Furthermore, using the real-time PCR method based on the TaqMan probe decreased the risk of DNA contamination due to the closed nature of the system and the absence of post-PCR handling of amplified material.
Conclusion
In conclusion, a modified BCA assay, GNPA, was developed for the highly sensitive and convenient detection of the ricin A chain protein, and the assay is suitable for the detection of the ricin A chain protein in mimic samples. Moreover, this assay format may be suitable for the ultrasensitive detection of other proteins.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This work was supported by the China Postdoctoral Science Foundation (20090451505).
References
- Agasti, S. S., Rana, S., Park, M. H., et al. 2010. Nanoparticles for detection and diagnosis. Adv. Drug. Deliv. Rev. 62:316–328
- Atlas, R. M. 2002. Bioterriorism: From threat to reality. Annu. Rev. Microbiol. 56:167–185
- Dertzbaugh, M. T., Rossi, C. A., Paddle, B. M., et al. 2005. Monoclonal antibodies to ricin: In vitro inhibition of toxicity and utility as diagnostic reagents. Hybridoma (Larchmont) 24:236–243
- Driskell, J. D., Seto, A. G., Jones, L. P., et al. 2008. Rapid microRNA (miRNA) detection and classification via surface-enhanced Raman spectroscopy (SERS). Biosens. Bioelectron. 24:923–928
- Du, P., Li, H., Mei, Z., and Liu, S. 2009. Electrochemical DNA biosensor for the detection of DNA hybridization with the amplification of Au nanoparticles and CdS nanoparticles. Bioelectrochemistry 75:37–43
- Fournier-Wirth, C., and Coste, J. 2010. Nanotechnologies for pathogen detection: Future alternatives? Biologicals 38:9–13
- Garber, E. A., and O'Brien, T. W. 2008. Detection of ricin in food using electrochemiluminescence-based technology. Journal AOAC Intl. 91:376–382
- Godal, A., Olsnes, S., and Pihl, A. 1981. Radioimmunoassays of abrin and ricin in blood. J. Toxicol. Environ. Health 8:409–417
- Guglielmo-Viret, V., and Thullier, P. 2007. Comparison of an electrochemiluminescence assay in plate format over a colorimetric ELISA, for the detection of ricin B chain (RCA-B). J. Immunol. Meth. 328:70–78
- Khalavka, Y., Becker, J., and Sönnichsen, C. 2009. Synthesis of rod-shaped gold nano-rattles with improved plasmon sensitivity and catalytic activity. J. Am. Chem. Soc. 131:1871–1875
- Melchior, W. B. Jr., and Tolleson, W. H. 2010. A functional quantitative polymerase chain reaction assay for ricin, Shiga toxin, and related ribosome-inactivating proteins. Biochemistry 396:204–211
- Nam, J. M., Stoeva, S. I., and Mirkin, C. A. 2004. Bio-barcode-based DNA detection with PCR-like sensitivity. J. Am. Chem. Soc. 126:5932–5933
- Nam, J. M., Thaxton, C. S., and Mirkin, C. A. 2003. Nanoparticle-based bio-barcodes for the ultra- sensitive detection of proteins. Science. 301:1884–1886
- Poli, M. A., Rivera, V. R., Hewetson, J. F., and Merrill, G. A. 1994. Detection of ricin by colorimetric and chemiluminescence ELISA. Toxicon 32:1371–1377
- Roberts, L. M., and Smith, D. C. 2004. Ricin: The endoplasmic reticulum connection. Toxicon 44:469–472
- Rosi, N. L., and Mirkin, C. A. 2005. Nanostructures in biodiagnostics. Chem. Rev. 105:1547–1562
- Rowe-Taitt, C. A., Cras, J. J., Patterson, C. H., et al. 2000. A gangli- oside-based assay for cholera toxin using an array biosensor. Anal. Biochem. 281:123–133
- Shyu, R. H., Shyu, H. F., Liu, H. W., and Tang, S. S. 2001. Colloidal gold-based immuno-chromatographic assay for detection of ricin. Toxicon 40:255–258
- Suresh, S., Gupta, A. K., Rao, V. K., et al. 2010. Amperometric immunosensor for ricin by using on graphite and carbon nanotube paste electrodes. Talanta 81:703–708
- Tran, H., Leong, C., Loke, W. K., et al. 2008. Surface plasmon resonance detection of ricin and horticultural ricin variants in environmental samples. Toxicon 52:582–588
- Yin, H. Q., Jia, M. X., Yang, S., et al. 2012. A nanoparticle-based bio- barcode assay for ultrasensitive detection of ricin toxin. Toxicon 59:12–16
- Zhuang, J., Cheng, T., Gao, L., et al. 2010. Silica coating magnetic nanoparticle-based silver enhancement immunoassay for rapid electrical detection of ricin toxin. Toxicon 55:145–152