Abstract
Rose Bengal (RB) has been used as a safe agent in clinical diagnosis. In addition, it is used as a photodynamic sensitizer for removing microorganisms and cancer cells. Recently, its preferential toxicity after direct exposure to cancer cells was proven. The present study focuses on anti-cancer and anti-inflammatory activities of RB. The toxicity of RB against AGS gastric cancer and NIH 3T3 fibroblast cell lines was studied using an MTT assay. Patterns of any cell death among the AGS cells were defined using Annexin-V and PI staining. In addition, the effect of RB on nitric oxide (NO) and prostaglandin E2 (PGE2) production induced by lipopolysaccha-ride in J774A.1 macrophages was determined. Modulation of inducible nitric oxide synthase (iNOS) and cyclooxygenase (COX)-2 expressions in the macrophages was also evaluated by Western blots. The results showed that AGS cells exhibited significant concentration-dependent decreases in growth in response to RB; these cells showed a greater growth inhibition than did non-malignant 3T3 cells, suggesting that anti-growth activity of RB could be cell-specific. Moreover, AGS cells exposed to RB exhibited a significant increase in apoptosis; only at high RB doses did the cells display significant levels of necrosis. While RB also caused a modest decrease in the growth of J774A.1 macrophages, the cells displayed remarkable decreases in NO production and iNOS expression without significant concurrent modulation in PGE2 production or COX-2 expression. The data from this study appears to suggest that RB differentially impacts on transformed cell lines, preferentially suppresses growth of a gastric cancer cell line through induction of apoptosis, and induces changes in cells that could reflect potential anti-inflammatory effects that might be induced in situ.
Introduction
Rose Bengal (RB: 4,5,6,7-tetrachloro-2′,4′,5′,7′-tetraiodofluoresceindisodium) is a water soluble xanthene dye capable for photocatalytic conversion of oxygen to singlet oxygen upon irradiation with green light (Ito & Kobayashi, Citation1997; Theodossiou & Hothersall, Citation2003). This characteristic enables RB to be utilized as a photodynamic sensitizer for the removal of a wide variety of micro-organisms and cancer cells (Nonaka et al., Citation2009; Panzarini et al., Citation2009; Dini et al., Citation2010; Kato et al., Citation2010). RB has a long history of safe usage with minimal side-effects (Wachter & Dees, Citation2003) in the systemic diagnosis of hepatic function (Delprat et al., Citation1924; Nordyke, Citation1965) as well as in the diagnosis of ophthalmic disorders (Marsh et al., Citation1976; Argueso & Tisdale, Citation2006).
Rose Bengal has been applied as a photodynamic agent for targeting different cancer cells (Umemura et al., Citation1999, Citation2004; Conlon & Berrios, Citation2007). One form of RB, i.e. Rose Bengal acetate (RBAc), causes phototoxicity in HeLa cervix adenocarcinoma cells after irradiation in part via alterations in mitochondrial membranes and induction of apoptosis (Panzarini et al., Citation2006, Citation2009; Soldani et al., Citation2007). Irradiation of RB with visible light generated intracellular singlet oxygen that, in turn, led to apoptosis in HL-60 human pro-myelocytic leukemia cells. The mechanism of apoptosis was seen as mediated by activation of caspases-3 and -8, release of cytochrome c, and activation of protein kinase C (Zhuang et al., Citation1998, Citation1999).
A preferential toxicity of RB against different cancer, but not normal, cells has been demonstrated (Mousavi et al., Citation2006, Citation2009; Agarwala et al., 2006; Agarwala, Citation2012; Koevary, Citation2012). Treatments resulted in significant concentration-dependent inhibition of the growth of ovarian and kidney cancer cells but few effects on normal human fibroblasts, suggesting specific anti-tumor activity. This growth inhibition was mediated through the induction of apoptosis and increased generation of reactive oxygen species (ROS) and H2O2 production (Koevary, Citation2012). Moreover, investigation of direct cytotoxic and pro-apoptotic effects of RB in MCF-7 cells showed significant reduction in cell growth through the induction of apoptosis and involvement of Bax protein expression (Mousavi et al., Citation2009). In addition, RB also was effective against a panel of melanoma cell lines, and an intra-lesion injection of RB was reported to induce a complete/partial response in clinical trials on subjects with different stage melanoma (Mousavi et al., Citation2006; Agarwala et al., 2006; Agarwala, Citation2012).
The epidemiologic observations that non-steroidal anti-inflammatory drugs (NSAID) – including cyclooxygenase (COX)-2 inhibitors and modulators of nitric oxide (NO) release – can prevent development of cancers has provided a clue to investigators seeking to identify new chemopreventive therapies (Wallace & Miller, Citation2000; Wallace & Del Soldato, Citation2003; Wallace et al., Citation2007; Wallace, Citation2008). Numerous reports have described anti-inflammatory and anti-cancer activities of various natural products (or their derivatives) in vitro (Lacour et al., Citation2009; Nworu et al., Citation2011, Citation2012; Vinod Prabhu and Guruvayoorappan, Citation2012; Karimian et al., Citation2013). Based on the epidemiologic and in vitro findings noted above, studies were undertaken here to ascertain if the means by which RB seemed to inhibit cancer cell growth in vitro was related to changes induced in NO formation and/or in COX-2 activity. Apart from examining effects on each of those parameters, measures of expression of inducible nitric oxide synthase (iNOS) and biosynthesis of prostaglandins (PG; production associated with COX-2 expression/activity) would also be needed to provide clarity about the specific mechanism of effect from RB. In this paper, we report the results of a study that assessed the anti-cancer (cell propagation) activity of RB against gastric cancer cells and whether this effect was related to any changes in inflammatory-related activities in the treated cells.
Materials and methods
Chemical and culture reagents
Fetal bovine serum (FBS), Dulbecco’s modified Eagle’s medium (DMEM), penicillin and streptomycin were purchased from Gibco (Detroit, MI). Rose Bengal (RB), Escherichia coli lipopolysaccharide (LPS, serotype 0111:B4), sodium nitrite, N-(1-naphtyl) ethylene-diamine, sulfanilamide, 3-(4, 5 dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT), and dimethyl sulfoxide (DMSO) were purchased from Sigma (St. Louis, MO). An ELISA kit for measures of PGE2 was obtained from Assay Designs (Farmingdale, NY). An apoptosis detection kit was purchased from Abcam (Cambridge, MA). Protein bands of iNOS and COX-2 were detected on Western blots using monoclonal antibodies bought from Panomics, Inc. (Redwood City, CA); goat anti-rabbit horseradish peroxide-conjugated antibody was obtained from KOMA Biotech (Seoul, Korea). ECL-detection reagent was obtained from Amersham (Cardiff, UK).
Cell lines and cell culture
Cell lines were obtained from the National Cell Bank of Iran (Pasteur Institute, Tehran, Iran). J774A.1 monocyte-macrophage and AGS gastric adenocarcinoma cell lines were cultured in Dulbecco’s modified Eagle’s medium (DMEM); normal NIH 3T3 fibroblasts were cultured in RPMI 1640. Each medium was supplemented with 10% FBS, 2 mM L-glutamine, 100 U penicillin/ml, and 100 μg streptomycin/ml, and the cell lines were maintained in 95% air/5% CO2 humidified atmospheres at 37 °C. After harvest from their respective culture flasks, cells were counted and viability assessed via trypan blue exclusion.
Cell proliferation assay
Cell growth and proliferation was assessed via mitochondrial respiration-dependent reduction of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) to formazan (Mosmann, Citation1983; Mahmoudi et al., Citation2009; Zamanai Taghizadeh Rabe et al., 2011). Cells were seeded into a 96-well plate and treated with increasing concentrations of RB (1–300 μM). After 24 h of incubation at 37 °C in an incubator with 5% CO2, MTT solution (5 mg/ml) was added to each well; 3 h later, the medium from each well was removed and cells lysed with DMSO to dissolve the formazan crystals produced in viable cells. The optical density in each well was measured at 545 nm in an EL reader (Convergent Tachnologies, Marburg, Germany). The inhibitory rate of cell proliferation was calculated using: Growth inhibition (%) = 100 × [1 − (ODcontrol − ODtreated)/ODcontrol].
Apoptosis assay
Apoptosis among AGS cells was determined using control and treated cells stained with propidium iodide (PI) and fluorescein isothiocyanate-labeled Annexin V (Annexin V-FITC) (Emami et al., Citation2009; Ghazanfari et al., Citation2011). In brief, AGS cells were cultured overnight in 6-well culture plates (5 × 105 cells) and then treated with RB (1–300 μM) for 24 h. The cells were harvested, washed with phosphate-buffered saline (PBS, pH 7.4), re-suspended in Annexin V-FITC binding buffer (in Abcam kit) and incubated at room temperature (RT) for 5 min in the dark with Annexin-V FITC and PI solutions (in Abcam kit). The cells were then analysed within 1 h using a FACSCalibur flow cytometer (Becton Dickinson, Carlsbad, CA) and associated software. In each case, a minimum of 10,000 events was captured. Results were reported as percentages of Annexin V+ (early apoptosis), PI+ (necrosis), Annexin V+PI+ (double positive; late apoptosis), or Annexin V−PI− (double negative; non-staining) cells.
Cell viability assay
Viability of treated J774A.1 macrophages was also assessed via mitochondrial respiration-dependent reduction of MTT to formazan as described above. Cell viability was calculated as: Viability (%) = 100 × (ODcontrol − ODtreated)/ODcontrol.
Measurement of nitric oxide
J774A.1 macrophages were seeded in 24-well plates at 8 × 105 cells/well. After 3 h, the cells were treated with various concentrations of RB (1–100 μM) in the presence or absence of LPS (1 µg/ml) for 24 h. Nitrite accumulation (index of NO synthesis) in the culture medium was measured using Griess reagent. Briefly, an equal amount of cell culture supernatant was mixed with Griess reagent (equal volume 1% (w/v) sulphanilamide [in 5% (v/v) phosphoric acid] and 0.1% (w/v) naphtylenediamine) and incubated at RT for 20 min. The absorbance at 545 nm was then measured in the microplate reader. Nitrite concentrations (in µM) in each sample were calculated by extrapolation from a standard curve generated in parallel using sodium nitrite.
Measurement of PGE2
J774A.1 cells (125 × 103/well) were cultured in 24-well plates. After 2 h, cells were treated with RB (1–100 μM) in the presence or absence of LPS (1 µg/ml) for 24 h. The medium in each well was then collected and the cell-free supernatants measured for PGE2 in an ELISA kit, following manufacturer instructions. The sensitivity of the kit was 16 pg PGE2/ml.
Western blot analysis
Treated and untreated cells were lysed in freshly prepared lysis buffer (20 mM HEPES [pH 7.9], 400 mM NaCl, 0.1% NP-40, 10% glycerol, 1 mM sodium vanadate, 1 mM sodium fluoride, 1 mM dithiothreitol (DTT), 1 mM phenylmethylsulphonyl fluoride (PMSF), and protease inhibitor cocktail) for 45 min on ice. The protein concentrations of each cytosolic extract were determined using a Bradford assay. Equal volumes of lysate (containing 30–50 μg protein/ml) were mixed with Laemmli buffer and then loaded into wells in 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and electrophoresed. Western blotting was performed by transferring proteins from the gel to a polyvinylidene difluoride membrane at 240 mA for 40 min at RT. The filter was then blocked overnight at 4 °C with 5% skim milk in PBS. After extensive washing with PBS, the membrane was then incubated for 2 h at RT with primary antibody (rabbit anti-mouse iNOS polyclonal antibody [1:200 dilution] and rabbit anti-mouse COX-2 polyclonal antibody [1:500 dilution]). Thereafter, after gently rinsing away unbound primary antibodies, each membrane was incubated with secondary antibody (goat anti-rabbit-HRP-conjugated antibody [1:10,000 dilution]) for 1 h at room temperature. β-actin protein (and appropriate antibody for detection) was used as the gel loading control. Subsequently, each blot was extensively washed with PBS and ultimately developed using enhanced chemiluminescence (ECL) detection reagents (Pierce, Rockford, IL). Each blot was then analyzed using a Kodak Imaging Densitometer (Wayne, NJ).
Statistical analyses
Data are reported as mean ± SEM of three independent determinations; all experiments were performed at least three times. Statistical analysis was performed using analysis of variance (ANOVA) tests and multiple comparisons were made using a Bonferroni’s test. p Values < 0.05 were considered statistically significant.
Results
Evidence of growth inhibition and death pattern in AGS cells after Rose Bengal treatment
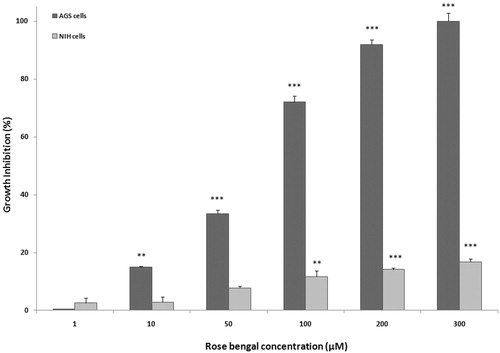
An MTT assay was performed in AGS and NIH 3T3 cells to determine the potential cytotoxicity of Rose Bengal. Interestingly, the effects on each cell line were significantly different. As shown in , Rose Bengal (RB; 1–300 µM) dose-dependently inhibited AGS cell growth. The AGS cells appeared very sensitive to the effects of the RB while, in contrast, the 3T3 cells appeared unaffected at RB doses > 100 µM. These differences in outcomes were suggestive of potential cell-specific effects for the RB.
Figure 1. Growth inhibitory effect of RB. Effects of a 24 h RB (1–300 µM) treatment on AGS and NIH 3T3 cell growth were determined using MTT. Results are shown as mean ± SEM (n = 3 per dose/cell line). p Values for extent of inhibition versus untreated control: **p < 0.01, and ***p < 0.001.
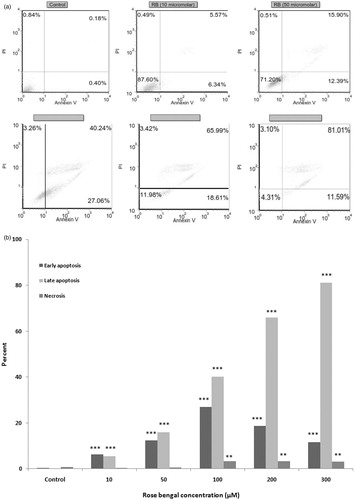
To examine a potential mechanism responsible for RB-mediated cell growth inhibition, cellular death patterns were evaluated after treatment of AGS cells with RB (using flow cytometry). As shown in , RB induced apoptosis in AGS cells. The results showed that early apoptosis (right lower quadrant of dot plot) in untreated control (0.4%) was significantly increased after treatment with 10, 50, 100, or 200 µg RB/ml (6.3, 12.4, 27.1, 18.6, and 11.6%, respectively). Late apoptosis (right upper quadrant) in untreated controls (0.18%) was significantly increased after treatment with these doses of RB (5.6, 15.9, 40.2, 66.0, and 81.1%, respectively). Necrosis (left upper quadrant) in untreated control (0.84%) was increased slightly after treatment with these doses of RB (0.5, 0.5, 3.3, 3.4, and 3.1%, respectively).
Figure 2. Apoptosis induced by RB in AGS cells. (a) AGS cells were treated with different concentrations of RB (1–300 µM) for 24 h, collected and stained with Annexin V-FITC and PI, and then assessed by flow cytometry. In representative dot-plots from each treatment, the x-axis indicates Annexin-V+ cells and the y-axis PI+ cells. (b) Percentages of early apoptotic, late apoptotic, and necrotic cells among all AGS cells at the different RB concentrations. **p < 0.01 and ***p < 0.001.
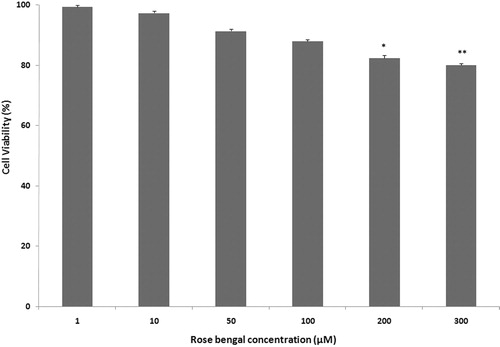
Effects of Rose Bengal on J774A.1 macrophage viability
Viability of J774.A1 macrophages after 24 h RB (1–300 µM) treatment was assessed (). Higher concentrations of RB caused significant decreases in the viability of the J774.A1 cells. Therefore, non-toxic concentrations of RB (i.e. < 100 µM) were used for studying the potential anti-inflammatory effects of RB in this cell line.
Effects of Rose Bengal on J774A.1 LPS-induced  production
production
To evaluate any effect of RB on nitric oxide (NO) production in LPS-stimulated J774A.1 macrophages, nitrite (index of NO) in culture medium was measured (). Basal levels of NO in unstimulated J774.A1 macrophages were 0.55 [±0.04] µM; LPS stimulation increased production to 21.40 [±0.20] µM. Treatment with RB significantly inhibited the stimulated production in a concentration-dependent manner. Treatment with Rose Bengal (1, 10, 50, or 100 µM) led to LPS-induced levels of NO formation of only 17.10 [±0.55], 14.30 [±0.17], 13.16 [±0.08], and 8.90 [±0.90], respectively.
Figure 4. Evaluation of NO and PGE2 production by J774A.1 macrophages. (a) NO and (b) PGE2 production was evaluated after the cells were stimulated for 24 h with LPS alone or in combination with RB (1–100 µM). Results are shown as mean ± SEM (n = 3 per dose). **p < 0.01 and ***p < 0.001 compared to LPS-stimulated macrophages without RB.
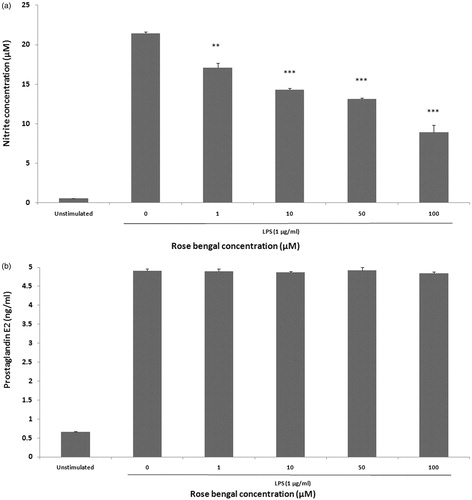
Effects of Rose Bengal on J774A.1 LPS-induced PGE2 production
To determine any modulatory effect of RB on LPS-induced production of PGE2, J774.A1 macrophages were treated with LPS in the presence or absence of RB and PGE2 levels in the medium then assessed by ELISA. Stimulation with LPS alone caused an appreciable increase in PGE2 level (4.91 [±0.04] ng/ml) from unstimulated levels (0.65 [±0.01] ng/ml). However, treatment with increasing levels of Rose Bengal (1, 10, 50, or 100 µM) imparted no discernible effect on PGE2 production ().
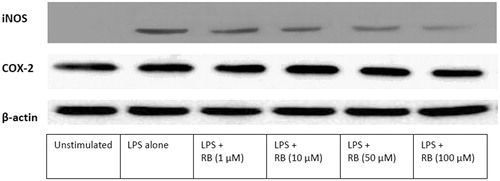
Effect of Rose Bengal on J774A.1 LPS-induced iNOS and COX-2 expression
To determine whether RB (1, 10, 50, or 100 µM) could modulate LPS induction of the presence of iNOS and COX-2, expression of each enzyme was evaluated via Western blot analysis (). Densitometric analysis results revealed that, although RB caused an inhibition of LPS-induced iNOS expression, the RB had no significant effect on induced COX-2 expression. These profiles were consistent with the above-noted effects of RB on NO and PGE2 production.
Discussion
Use of Rose Bengal (RB) has been explored for > 15 years as a photodynamic sensitizer for potential use in cancer chemotherapy (Zhuang et al., Citation1998, Citation1999; Umemura et al., Citation1999, Citation2004; Panzarini et al., Citation2006, Citation2009; Conlon & Berrios, Citation2007; Soldani et al., Citation2007), as well as in the killing of viruses, bacteria, or protozoa (Dahl et al., Citation1988; Lenard et al., Citation1993). More recently, it was reported that RB treatment of various cancer cell lines resulted in significant growth inhibition and induction of apoptosis (Mousavi et al., Citation2006, Citation2009; Agarwala et al., 2006; Agarwala, Citation2012; Koevary, Citation2012). In light of these observations, we thought it interesting to examine the toxicity of RB against gastric cancer cells and to ascertain if any effects observed in these (and other transformed cells) might be related to changes in cell formation/release of pro-inflammatory factors.
Results here revealed that RB caused profound inhibition of gastric cancer cell propagation, but had little impact on normal transformed fibroblasts. Determining the pattern of cell death in gastric cancer cells revealed that the RB induced primarily early apoptosis at doses of ≤ 100 µM and late apoptosis at concentrations of 100–300 µM. Necrosis was not markedly increased in RB-treated cells until very high doses were utilized in these experimental conditions. It seems that apoptotic cell death was the dominant mechanism underlying the anti-growth activity of RB against the gastric cancer cells. In previous reports, RB decreased the viability of SK-Mel-28, IgR3, and Me 4405 melanoma cells, but not fibroblast cells (Mousavi et al., Citation2006). Unlike in the findings here, apoptosis did not markedly contribute in RB-mediated toxicity of melanoma cells and non-apoptotic cell death was in fact the main form of cell death. In another study (Mousavi et al., Citation2009), RB was able to induce toxicity in MCF-7 cells, but not in non-malignant cell lines. In this case, RB-induced apoptosis was only partially involved in the outcome. More recently, it was shown that ovarian cancer cells exhibited remarkable inhibition in growth in response to RB treatment and that this effect was due mainly to induction of apoptosis (Koevary, Citation2012).
Production of inflammatory mediators by resident and infiltrating macrophages plays a pivotal role in the outcome of tumor progression (Grivennikov et al., Citation2010). Therefore, compounds possessing anti-inflammatory potential, in combination with any anti-cancer activity, are attractive as potential therapeutics. It is well established that there are strong correlations between inflammatory responses and development of cancers (Chang et al., Citation2009). In addition, non-steroidal anti-inflammatory drugs often seem to possess protective effects against different cancers (Ulrich et al., Citation2006). Thus, it is reasonable to suggest that the presence of inflammation can induce or facilitate carcinogenic processes.
Macrophages play a central role in inflammatory responses. Evaluations of LPS-induced inflammatory responses in/by macrophages are widely used in vitro to study anti-inflammatory effects of different agents (Emami et al., Citation2010; Ha et al., Citation2012; Kuan et al., Citation2012). The enzyme nitric oxide synthase (NOS) that produces nitric oxide (NO, via conversion of L-arginine to L-citrulline) is a key regulator of carcinogenesis and tumor progression because it modulates some signal transduction pathways in cancer cells via its NO product (Jenkins et al., Citation1995; Landar & Darley-Usmar, Citation2003). Over-expression of NO and increased iNOS activity and its expression in gastric tumor tissues is associated with angiogenesis within the solid tumor, acceleration of tumor growth, metastasis, and, ultimately, a poor survival rate among gastric cancer patients (Rajnakova et al., Citation1997; Thomsen & Miles, Citation1998; Doi et al., Citation1999; Feng et al., Citation2002; Zhang et al., Citation2011). Therefore, compounds with the dual capabilities of inhibiting the cancer cell proliferation and host inflammatory responses, particularly iNOS activity/NO formation, should be exceptional anti-cancer agents. To our knowledge, few pharmacological studies have examined the biologic properties of RB. Based on the expanded understanding that inflammation plays a crucial role in tumor progression, in particular gastric cancers, we sought to evaluate the anti-inflammatory/anti-cancer activities of RB. The studies reported here clearly indicated that RB inhibited on human gastric (AGS) cancer cell proliferation, in part, via induction of apoptosis in the cells.
Due to the pivotal role of immune cells and cytokines in cancer-related inflammation, we investigated the inhibitory potential of RB on inflammatory pathways. We demonstrated that RB (in a concentration-dependent manner) significantly reduced the production of pro-inflammatory NO in LPS-induced J774A.1 macrophages without causing cell toxicity. However, RB was not able to affect levels of PGE2 produced by these cells. Similarly, while the expression of iNOS was markedly decreased by RB treatment, there was no significant impact on COX-2 expression. These results illustrate that RB imparts anti-inflammatory effects that appear to be mediated through inhibition of iNOS, but not COX-2, pathways in stimulated macrophages.
Cytotoxicity as a result of a substantial NO formation is known to initiate apoptosis, and is often characterized by changes in expression of pro- and anti-apoptotic Bcl-2 family members (e.g., Bax, Bak, Bcl-2), cytochrome c relocation, activation of caspases, chromatin condensation, and DNA fragmentation. However, the presence of NO may impart cell protection as well. In part, this seems due to effects on transcription/translation of protective proteins, including COX-2. With regard to the cell type studied here, a protective/anti-apoptotic effect from COX-2 has been confirmed in colon epithelial cells (Williams et al., Citation1997). Thus, crosstalk between protective vs destructive signaling pathways that are under the influence of NO ultimately is key to resolving the potential for apoptotic death vs survival (reviewed in Brune et al., 1999) in the AGS cells.
Still, the data here regarding induction of apoptosis in the RB-treated gastric cancer cells appear incongruent. Since RB caused decreased NO formation, it would be expected these cells should display less apoptotic events – based on the above. Similarly, with less NO, it would have been expected these cells would express less COX-2. As this was not the case, the cells should have been protected by an enhanced presence of this enzyme and not displayed increased apoptotic frequencies. Therefore, the question remains: how does RB induce apoptosis if not through expected increases in NO/reductions in COX-2? The MTT data used to assess overall changes in cell proliferation may provide the needed clue.
As the MTT assay is based on the ability of mainly mitochondrial succinate dehydrogenase to reduce the tetrazolium salt to formazan (Wang et al., Citation2010), it could be that the RB-treated cells underwent damage to their mitochondria. If so, there was a great potential for release of numerous apoptogens from the organelles (reviewed in Vaux, Citation2011; Kim et al., Citation2012; Katsuyama et al., 1998). These include apoptosis inducing factor (AIF), endonuclease G (EndoG), adenylate kinase 2 (AK2), and factors (e.g. Smac (second mitochondrial activator of caspases), Diablo (direct IAP binding protein with low pI, HtrA2/Omi)) that are binders of inhibitor of apoptosis ([X]IAP) proteins. Planned studies here will examine expression of these apoptogens to ascertain if RB was in fact inducing outright mitochondrial damage and if this was a likely mechanism to explain induced increases in apoptosis in the RB-treated gastric cancer cells (Oshima et al., 1996, 2004, 2005, 2006, 2011).
Lastly, the results showed that AGS cells exhibited significant decreases in growth in response to RB, while the non-malignant 3T3 cells and J774A.1 macrophages did not. This clearly suggested the anti-growth activity of RB was cell-specific. Why this is so is uncertain. Differences in uptake of RB could be examined to determine if there was selective ‘exclusion’ of the agent by the 3T3 cells and J774A.1 cell types. However, it is unlikely differences in uptake were the basis for outcomes here; as noted earlier, RB was previously shown to readily enter a variety of cell types (HeLa cells, HL-60 leukemia cells, etc.). It is more likely that differences in metabolic activities among the three cell types could have had a major role in the differential effects of RB. This is plausible in that there are clearly differences in effects of RB in transformed vs normal cells (see Introduction) and because different cancer/tumor cell types display variations in metabolic parameters (Herman et al., Citation2011). The role of cell metabolism as a factor in RB-mediated toxicity remains to be determined in our studies.
Conclusions
In summary, the studies here demonstrated that Rose Bengal could impart an anti-inflammatory effect on cells via inhibition of nitric oxide production and iNOS expression. Moreover, RB showed preferential cytotoxicity activity here against gastric cancer cells and that this effect was mediated, in part, by induction of apoptosis. Since inflammation and associated signaling pathways are related to carcinogenic processes, this anti-cancer cell growth activity in combination with an anti-inflammatory effect makes RB a potential candidate for treatment of cancers/inflammation-related diseases. Clearly, further studies are needed to confirm the pharmacology of RB in more detail in animal models of inflammation and cancer.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
The authors would like to thank the authorities in the research council of Mashhad University of Medical Sciences (MUMS) for their financial support (Grant #85497).
References
- Agarwala, S. S. 2012. PV-10, Rose Bengal: International therapy for metastatic melanoma. Melanoma. Lett. 30:6–9
- Agarwala, S. S., Thompson, J. F., Smithers, B. M., et al. 2006. Chemoablation of metastatic melanoma with intra-lesional PV-10. Pigment. Cell. Melanoma. Res. 23:883
- Argueso, P., and Tisdale, A. 2006. Mucin characteristics of human corneal-limbal epithelial cells that exclude the Rose Bengal anionic dye. Invest. Ophthalmol. Vis. Sci. 47:113–119
- Brune, B., von Knethen, A., and Sandau, K. B. 1999. Nitric oxide (NO): An effector of apoptosis. Cell. Death. Differ. 6:969–975
- Chang, H. Y., Sneddon, J. B., Alizadeh, A. A., et al. 2009. Gene expression signature of fibroblast serum response predicts human cancer progression: Similarities between tumors and wounds. PLoS Biol. 42:7
- Conlon, K. A., and Berrios, M. 2007. Site-directed photoproteolysis of 8-oxoguanine DNA glycosylase 1 (OGG1) by specific porphyrin-protein probe conjugates: A strategy to improve effectiveness of photodynamic therapy for cancer. J. Photochem. Photobiol. 87:9–17
- Dahl, T. A., Midden, W. R., and Neckers, D. C. 1988. Comparison of photodynamic action by Rose Bengal in Gram-positive and Gram-negative bacteria. Photochem. Photobiol. 48:607–612
- Delprat, G. D., Epstein, N. N., and Kerr, W. J. 1924. A new liver function test: Elimination of Rose Bengal when injected into circulation of human subjects. Arch. Intern. Med. 34:533–541
- Dini, L., Inguscio, V., Tenuzzo, B., and Panzarini, E. 2010. Rose Bengal acetate photodynamic therapy-induced autophagy. Cancer. Biol. Ther. 10:1048–1055
- Doi, C., Noguchi, Y., Marat, D., Saito, A., et al. 1999. Expression of nitric oxide synthase in gastric cancer. Cancer. Lett. 144:161–167
- Emami, S. A., Zamanai Taghizadeh Rabe, S., Ahi, A., et al. 2009. Study of the cytotoxic and pro-apoptotic activity of Artemisia annua extracts. Pharmacol. Online. 3:1062–1069
- Emami, S. A., Zamanai Taghizadeh Rabe, S., Iranshahi, M., et al. 2010. Sesquiterpene lactone fraction from Artemisia khorassanica inhibits inducible nitric oxide synthase and cyclooxygenase-2 expression via inactivation of NF-κB. Immunopharmacol. Immunotoxicol. 32:688–695
- Feng, C. W., Wang, L. D., Jiao, L. H., et al. 2002. Expression of p53, inducible nitric oxide synthase and vascular endothelial growth factor in gastric precancerous and cancerous lesions: Correlation with clinical features. BMC. Cancer. 2:8–13
- Ghazanfari, T., Zamanai Taghizadeh Rabe, S., Tabasi, N., and Mahmoudi, M. 2011. Study of cytotoxicity and pro-apoptotic effect of medical mushroom Pleurotus florida in cancer cell lines. Pharmacol. Online. 3:774–783
- Grivennikov, S. I., Greten, F. R., and Karin, M. 2010. Immunity, inflammation, and cancer. Cell. 140:883–899
- Gupta, R. A., and Bios, R. N. 2001. Colorectal cancer prevention and treatment by inhibition of cyclo-oxygenase-2. Nat. Rev. Cancer. 1:11–21
- Ha, S. K., Park, H. Y., Eom, H., et al. 2012. Narirutin fraction from citrus peels attenuates LPS-stimulated inflammatory response through inhibition of NF-κB and MAPKs activation. Food. Chem. Toxicol. 50:3498–3504
- Herman, A. B., Savage, V. M., and West, G. B. 2011. A quantitative theory of solid tumor growth, metabolic rate, and vascularization. PLoS One. 6:e22973
- Ito, T., and Kobayashi, K. 1997. A survey of in vivo photodynamic activity of xanthenes, thiazines, and acridines in yeast cells. Photochem. Photobiol. 26:581–587
- Jenkins, D. C., Charles, I. G., and Thomsen, L. L. 1995. Roles of nitric oxide in tumor growth. Proc. Natl. Acad. Sci. USA. 92:4392–4396
- Karimian, P., Kavoosi, G., and Amirghofran, Z. 2013. Anti-inflammatory effect of Mentha longifolia in lipopolysaccharide-stimulated macrophages: Reduction through inhibition of inducible synthase. J. Immunotoxicol. 10:393–400
- Kato, H., Komagoe, K., Inoue, T., and Katsu, T. 2010. In situ monitoring of photodynamic inactivation of the membrane functions of bacteria using electrochemical sensors. Anal. Sci. 26:1019–1021
- Kim, G. S., Jung, J. E., Narasimhan, P., et al. 2012. Release of mitochondrial apoptogenic factors and cell death are mediated by CK2 and NADPH oxidase. J. Cereb. Bloodflow Metab. 32:720–730
- Koevary, S. B. 2012. Selective toxicity of Rose Bengal to ovarian cancer cells in vitro. Int. J. Physiol. Pathophysiol. Pharmacol. 4:99–107
- Kuan, Y. H., Huang, F. M., Li, Y. C., and Chang, Y. C. 2012. Pro-inflammatory activation of macrophages by bisphenol A-glycidyl-methacrylate involved NF-κB activation via PI3K/Akt pathway. Food Chem. Toxicol. 50:4003–4009
- Lacour, S., Antonios, D., Gautier, J. C., and Pallardy, M. 2009. Acetaminophen and lipopolysaccharide act in synergy for the production of pro-inflammatory cytokines in murine RAW264.7 macrophages. J. Immunotoxicol. 6:84–93
- Landar, A., and Darley-Usmar, V. M. 2003. Nitric oxide and cell signaling: Modulation of redox tone and protein modification. Amino Acids. 25:313–321
- Lenard, J., Rabson, A., and Vanderoef, R. 1993. Photodynamic inactivation of infectivity of human immunodeficiency virus and other enveloped viruses using hypericin and Rose Bengal: Inhibition of fusion and syncytia formation. Proc. Natl. Acad. Sci. USA. 90:158–162
- Mahmoudi, M., Zamanai Taghizadeh Rabe, S., Ahi, A., and Emami, S. A. 2009. Evaluation of cytotoxic activity of different Artemisia khorasanica samples on cancer cell lines. Pharmacol. Online. 2:778–786
- Marsh, R. J., Fraunfelder, F. T, McGill, J. I., and Phil, D. 1976. Herpetic corneal epithelial disease. Arch. Ophthalmol. 94:1899–1902
- Mosmann, T. 1983. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Meth. 983:55–63
- Mousavi, S. H., Tavakkol-Afshari, J., Brook, A., and Jafari-Anarkooli, I. 2009. Direct toxicity of Rose Bengal in MCF-7 cell line: Role of apoptosis. Food Chem. Toxicol. 47:855–859
- Mousavi, S. H., Zhang, X. D., Sharifi, A. M., and Hersey, P. 2006. Study of Rose Bengal-induced cell death in melanoma cells in the absence of light. IJBMS. 9:216–222
- Nonaka, M., Yamamoto, M., Yoshino, S., et al. 2009. Sono-dynamic therapy consisting of focused ultrasound and a photosensitizer causes a selective anti-tumor effect in a rat intracranial glioma model. Anticancer. Res. 29:943–950
- Nordyke, R. A. 1965. Surgical vs. nonsurgical jaundice: Differentiation by a combination of Rose Bengal I131 and standard liver-function tests. JAMA. 194:949–953
- Nworu, C. S., Akah, P. A., Okoye, F. B., et al. 2011. The leaf extract of Spondias mombin L. displays an anti-inflammatory effect and suppresses inducible formation of TNFα and nitric oxide (NO). J. Immunotoxicol. 8:10–16
- Nworu, C. S., Nwuke, H. C., Akah, P. A., et al. 2012. Extracts of Ficus exasperate leaf inhibit topical and systemic inflammation in rodent and suppress LPS-induced expression of mediators of inflammation in macrophages. J. Immunotoxicol. Ahead of print
- Oshima, M., Dinchuck, J. E., and Kargman, S. L. 1996. Suppression of intestinal polyposis in Apc knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell. 87:803–809
- Oshima, H., Hiokik, K., Popivanova, B. K., et al. 2011. PGE2 signaling and bacterial infection recruits tumor-promoting macrophages to mouse gastric tumors. Gastroenterology. 140:596–607
- Oshima, H., Mastunaga, A., and Fujimura, T. 2006. Carcinogenesis in mouse stomach by simultaneous activation of Wnt signaling and PGE2 pathway. Gastroenterology. 131:1086–1095
- Oshima, M., Oshima, H., and Inaba, T. 2004. Hyperplastic gastric tumors induced by activated macrophages in COX-2/mPGES-1 transgenic mice. EMBO. J. 23:1669–1678
- Oshima, M., Oshima, H., and Mastunaga, A. 2005. Hyperplastic gastric tumors with spasmolytic polypeptide-expressing metaplasia caused by TNFα-dependent inflammation in cyclooxy-genase-2/microsomal PGE2 synthase-1 transgenic mice. Cancer. Res. 65:9147–9151
- Panzarini, E., Tenuzzo, B., and Dini, L. 2009. Photodynamic therapy-induced apoptosis of HeLa cells. Ann. N.Y. Acad. Sci. 1171:617–626
- Panzarini, E., Tenuzzo, B., Palazzo, F., et al. 2006. Apoptosis induction and mitochondria alteration in human HeLa tumor cells by photoproducts of Rose Bengal acetate. J. Photochem. Photobiol. B. 83:39–47
- Rajnakova, A., Goh, P. M., Ngoi, S. S., et al. 1997. Expression of differential nitric oxide synthase isoforms in human normal gastric mucosa and gastric cancer tissue. Carcinogenesis. 18:1841–1845
- Soldani, C., Croce, A. C., Bottone, M. G., et al. 2007. Apoptosis in tumor cells photosensitized with Rose Bengal acetate is induced by multiple organelle photodamage. Histochem. Cell Biol. 128:485–495
- Theodossiou, T., and Hothersall, J. S. 2003. Firefly luciferin-activated Rose Bengal: In vitro photodynamic therapy by intracellular chemiluminescence in transgenic NIH 3T3 cells. Cancer. Res. 15:1818–1821
- Thomsen, L. L., and Miles, D. W. 1998. Role of nitric oxide in tumor progression: Lessons from human tumors. Cancer Metast. Rev. 17:107–118
- Ulrich, C. M., Bigler, J., and Potter, J. D. 2006. Non-steroidal anti-inflammatory drugs for cancer prevention: Promise, perils, and pharmacogenetics. Nat. Rev. Cancer. 6:130–140
- Umemura, S., Kawabata, K., Sugita, N., et al. 2004. Sono-dynamic approaches to tumor treatment. Int. Cong. Series. 1274:164–168
- Umemura, S., Yumita, N., Umemura, K., and Nishigaki, R. 1999. Sono-dynamically-induced effect of Rose Bengal on isolated sarcoma 180 cells. Cancer Chemother. Pharmacol. 43:389–393
- Vaux, D. L. 2011. Apoptogenic factors released from mitochondria. Biochim. Biophys. Acta. 1813:546–550
- Vinod Prabhu, V., and Guruvayoorappan, C. 2012. Anti-inflammatory and anti-tumor activity of marine mangrove Rhizophora apiculata. J. Immunotoxicol. 9:341–352
- Wachter, E., and Dees, C. 2003. Topical Rose Bengal: Pre-clinical evaluation of pharmaco-kinetics and safety. Lasers Surg. Med. 32:101–110
- Wallace, J. L. 2008. Prostaglandins, NSAIDs, and gastric mucosal protection: Why doesn’t the stomach digest itself. Physiol. Rev. 88:1547–1565
- Wallace, J. L., and Del Soldato, P. 2003. The therapeutic potential of NO-NSAIDs. Fund. Clin. Pharmacol. 17:11–20
- Wallace, J. L., and Miller, M. J. 2000. Nitric oxide in mucosal defense: A little goes a long way. Gastroenterology. 119:512–520
- Wallace, J. L., Caliendo, G., Santagada, V., et al. 2007. Gastrointestinal safety and anti-inflammatory effects of a hydrogen sulfide-releasing diclofenac derivative in the rat. Gastroenterology. 132:261–271
- Wang, P., Henning, S. M., and Heber, D. 2010. Limitations of MTT and MTS-based assays for measurement of anti-proliferative activity of green tea polyphenols. PLoS One. 5:e10202
- Williams, C. S., Smalley, W., and DuBois, R. N. 1997. Aspirin use and potential mechanisms for colorectal cancer prevention. J. Clin. Invest. 100:1325–1329
- Zamanai Taghizadeh Rabe, S., Mahmoudi, M., Ahi, A., and Emami, S. A. 2011. Anti-proliferative effects of extracts from Iranian Artemisia species on cancer cell lines. Pharmaceutical. Biol. 49:962–969
- Zhang, W., He, X. J., Ma, Y. Y., et al. 2011. Inducible nitric oxide synthase expression correlates with angiogenesis, lyphangiogenesis, and poor prognosis in gastric cancer patients. Human Pathol. 42:1275–1282
- Zhuang, S., Lynch, M., and Kochevar, I. 1998. Activation of protein kinase C is required for protection of cells against apoptosis induced by singlet oxygen. FEBS. Lett. 437:158–162
- Zhuang, S., Lynch, M., and Kochevar, I. 1999. Caspase-8 mediates caspase-3 activation and cytochrome c release during singlet oxygen-induced apoptosis of HL-60 Cells. Exp. Cell Res. 250:203–212