Abstract
This study sought to assess if chlorpyrifos (CPF) induced immunotoxic effects in orally-treated day-old broiler chicks. Groups of chicks received per os CPF diluted in xylene at 5, 10, and 20 mg/kg body weight (CPF-5, CPF-10, and CPF-20) orally daily for 15 days. Xylene and control groups received xylene alone (1 ml/kg BW) and physiological saline, respectively. At various times during/after the exposure regimens, different immune end-points were analyzed in the birds. Humoral immunity was examined by assessing antibody responses to sheep red blood cells. Cell-mediated immunity was measured via lymphoproliferative responses to avian tuberculin. Leukocyte phagocytic ability was measured using a carbon clearance assay. Results showed that CPF administered to broiler chicks caused a dose-dependent decrease in humoral immunity, cell-mediated immunity, and phagocytic activity. Dose- and time-related pathological changes were observed in bursa of Fabricius, spleen, and thymus in treated birds. These changes were mild, moderate, and severe, respectively, in the 5, 10, and 20 mg/kg CPF groups. The Bursa of Fabricius in treated birds showed increased inter-follicular connective tissue proliferation, severe moderate cytoplasmic vacuolation, edema, and degenerative changes such as pyknosis and fragmentation of nuclei that depleted the follicles of lymphoid cells. In the spleen, disorganization of follicular patterns, severe congestion, cytoplasmic vacuolation, degenerative changes, and hyperplasia of reticular cells were noted. The thymus in treated birds exhibited congestion, hyper-cellularity, and a presence of immature monocytes in the medullary region, as well as myoid cell necrosis. Taken together, these studies clearly demonstrated that chlorpyrifos could induce immunotoxicities in broiler birds.
Introduction
The use of synthetic chemicals is the quickest and easiest method of pest management; however, it imposes serious ecological and environmental effects (Ahmed et al., Citation2010; Khan et al., Citation2012; Muhammad et al., Citation2012). Chlorpyrifos (CPF) [O,O-diethyl-O-(3,5,6-trichloro-2-pyridyl)] phosphorothionate is a broad-spectrum organophosphate (OP) insecticide used extensively in agriculture and for residential pest control throughout the world (Ahmad et al., Citation2012; Saulsbury et al., Citation2009). It is primarily used to control pests invading grains, cotton, fruit, nut, and vegetable crops, as well as lawns and ornamental plants (Ambali et al., Citation2009). CPF is also used in treatment of livestock and poultry farms (Nakadai et al., Citation2006).
Extensive over-use of OP agents/CPF results in un-intentional accidental ingestion via direct exposure in the environment, as well as through interactions with contaminated ground water; this also results in increased risk of providing intoxicated feed to wild and farmed/domesticated animals (Martin & Forsyth, Citation1998; Reece & Handson, Citation1982). It is estimated that ≈672 million birds in the US get direct exposure each year to pesticides and 10% die due to acute toxicities (Mitra et al., Citation2011; Williams, Citation1997). Deaths induced by pesticides are difficult to estimate accurately as birds may die far away from the site of exposure/are eaten by scavengers (Hussain et al., Citation2011; Mitra et al., Citation2011), making total deaths under-reported.
If CPF is accidentally ingested, it is readily absorbed into the blood from the intestinal tract and ultimately (if not detoxified) becomes stored in fat tissues. Over time, CPF can eventually be moved out of fat stores and eliminated from the body (Hayes & Laws, Citation1990); the liver is the site where detoxification (and, oddly, as well as activation) of CPF takes place (Hartely & Kidd, Citation1983). In general, the major mechanism of action of CPF, like many OP, is inhibition of acetylcholinesterase activity, resulting in stimulation of cholinergic synapses throughout the peripheral and central nervous system (Moye & Pritsos, Citation2010; Wolf et al., Citation2010). Many of the pathological effects of OP and other anti-cholinesterases are derived from these effects on cholinergic pathways.
In addition, there is a wide-range of immunotoxic outcomes from OP; these include pathological changes and, oddly, symptoms of both hypersensitivity and immunosuppression (Duramad et al., Citation2006; Galloway & Handy, Citation2003; Voccia et al., Citation1999). Decreased humoral (Jeong et al., Citation1995), cell-mediated (Blakley et al., Citation1999), and non-specific immunity, along with increases in hyper-sensitivity and autoimmunity, are some other documented immunotoxicities arising from exposure to OP agents (Thrasher et al., Citation1993). With regard to CPF in particular, exposure of humans to CPF has been shown to cause impaired lymphocyte mitogenesis, increased percentages of CD26+ cells, increased circulating levels of autoantibodies, and altered levels of CD5+ thymocytes (Thrasher et al., Citation2002). Similar outcomes were noted in rats exposed to CPF, i.e. elevated CD5 and CD8 cell levels (Blakley et al., Citation1999), as well as impaired T-cell blastogenesis (Navarro et al., Citation2001). Taken together, these findings suggest that CPF exposure is associated with alterations to the immune system of mammals. In non-mammalian models, i.e. chicks, there is limited information. Thus, the present study was designed to explore the potential for immunotoxic effects of CPF in broiler chicks.
Materials and methods
Chemicals and other reagents
Technical grade chlorpyrifos (C9H11Cl3NO3PS; 92% purity) was a gift from M/S Ali Akbar Group of Industries (Lahore, Pakistan). Xylene was purchased from Merck (Darmstadt, Germany). Black India ink was obtained from Pelikan (Hanover, Germany). Avian tuberculin was bought from the Veterinary Research Institute (Lahore, Pakistan). Sheep red blood cells (SRBC) were prepared from blood obtained from a local abattoir. After centrifuging (150 × g, 15 min, room temperature), the RBC were suspended in normal saline to yield a 3% SRBC suspension. For the hemagglutination inhibition assay, Newcastle disease virus (NDV) antigen was purchased from M/S Intervet S.A. (Pty) Ltd. (Kempton Park, South Africa).
Experimental design
This experiment was planned in accordance with all national legislation concerning the protection of animal welfare and followed guidelines set by the Advanced Studies and Research Board (ASRB) of the University. The ASRB approved all experimental protocols. A total of 250 1-day-old broiler chicks were procured from a hatchery and kept in metal wire cages at ambient temperature (26 ± 2 °C) with 75% relative humidity. The birds were fed a corn soy basal feed containing 21% total protein (Hameed et al., Citation2013). After 5 days of acclimatization, birds were randomly allocated into five equal groups (n = 29/group) for the purpose of assessing potential dose-associated lethality, pathologic changes, and materials for use in a hemagglutination inhibition assay. For all remaining studies, i.e. antibody titres against sheep red blood cells, lymphoproliferative responses to avian tuberculin, and carbon clearance assay, a total of 21 birds/group were utilized.
Starting at Day 7-of-age, birds in the first three groups, i.e. CPF-5, CPF-10, and CPF-20, were to receive CPF diluted in xylene to deliver 5, 10, or 20 mg/kg BW by crop tubing. The oral LD50 of CPF in chicken was reported to be ≈18 mg/kg BW (Al-Badrany & Mohammad, Citation2007). As such, the highest dose (CPF-20) selected for use here approximated the oral LD50 value; the other two doses (CPF-10 and CPF-5) were ½ and ¼ of LD50, respectively. All birds were weighed every 7 days to adjust gavage volume; no bird ever received >1 ml/kg BW per instillation. Xylene and control groups received xylene (1 ml/kg BW) and physiological saline solution, respectively. All birds were monitored daily for any clinical signs of general toxicities or neuro-toxicities (i.e. changes in appearance, posture, behavior, respiration, consciousness, neurologic status, temperature, excretion, etc.) during the treatment period. Any birds that died during the course of the experiment were noted and the percent mortality/group calculated.
Treatments were daily for a period of 15 days (age of birds at treatment end = 22 days) and birds were monitored from the start of the treatments up to 45 days (up to 30 days past the final dosing; age of birds = 52 days). Immediately after the final treatment (Day 15) and again on post-treatment Days 15 and 30, sub-sets (n = 5) of birds in each group were first bled from the wing vein to obtain serum for use in the hemagglutination inhibition (HI) assays (see below) and then each bird was euthanized by cervical dislocation, weighed, and specific organs (i.e. spleen, bursa of Fabricius, thymus) then removed and weighed. Relative weights (i.e. as a percentage of body weight) of each organ were subsequently calculated.
Each bursa of Fabricius, spleen, and thymus was preserved in 10% buffered formalin and then processed by dehydration and paraffin embedding. For each organ, 4–5-µm thick sections were cut and stained with hematoxylin and eosin (Islam et al., Citation2012). Under light microscopy, a semi-quantitative evaluation on the basis of arbitrary scores (−−−− to ++++) was carried to characterize any histopathological lesions in the lymphoid organs. For each slide, at least 10 fields were randomly selected for such scoring and a cumulative value obtained for each bird and accordingly each treatment group. A certified pathologist read all the slides in a blinded manner.
Hemagglutination inhibition for Newcastle disease vaccine virus
To assess the humoral responses of the treated birds, chicks were vaccinated (B1 type, LaSota strain, live virus; Ceva Laboratories/Biomune Co., Libourne, France) against Newcastle disease (ND) when they were 2, 23, and 28 days old. In this study, blood samples collected from the wing vein on treatment Days 0 and 15 and on and post-treatment Days 15 and 30 (see above) were processed to yield serum that was then subjected to a hemagglutination inhibition (HI) assay (Chukwudi et al., Citation2012) to assess the extent of antibody formation against the ND in the birds. In brief, saline (50 µl) was added to each well of a round-bottom microtiter plate after which test serum (50 µl) was added to the first well of each dedicated row and serial 1:1 dilutions made until a final dilution of 1:1024 was achieved. Diluted (4HA) NDV antigen (50 µl) was then added to each well and the plate incubated for 30 min at room temperature. Washed naïve chicken RBC suspension (1%, 50 µl) was then added to each well and the plate was incubated at 37 °C for 30 min. Antibody titers were then interpreted based on the standard protocol of identifying the final well in a row that showed formation of agglutinated materials.
Antibody titer against sheep red blood cells (SRBC)
On treatment Day 3, 1 ml of the 3% SRBC suspension was injected intravenously into a separate set of five birds in each group (i.e. primary dosing). A booster dose was then injected 14 days later (i.e. post-treatment Day 2). Blood samples were collected (without anti-coagulant) from the wing vein both 7 and 14 days after the primary dose and also 7 and 14 days after the booster dose. Each time, serum was separated using standard protocols and stored at −20 °C until analysis of antibody responses. At that time, each serum sample was inactivated at 56 °C for 30 min and then analyzed for total anti-SRBC antibodies using the method of Delhanty & Solomon (Citation1966).
Lymphoproliferative response to avian tuberculin
On treatment Day 12, another set of five birds/group received 0.2 ml avian tuberculin (0.5 mg/ml saline) by injection into the intra-digital space between the 3rd and 4th digit of their right foot. Normal saline (0.2 ml) was injected in the same space in the left foot as the ‘self’-control. An identical treatment was carried out on another set of five birds/group at post-treatment Day 12. In all cases, skin thickness between the two digits (of both right and left foot) was measured via a micrometer screw gauge (to assess host cell-mediated immune response) 24, 48, and 72 h post-injection. Thickness was also measured immediately prior to either injection to provide a baseline value. The cutaneous hypersensitivity response at each timepoint was calculated as [toe web thickness after injection – before injection] OR [avian tuberculin response right foot – saline response left foot] (Akhtar et al., Citation2008).
Carbon clearance assay
A supernatant fraction of Black India Ink (Pelikan® 4001) was obtained by centrifugation (3000 × g) for 30 min. On treatment Day 12, another set of three birds/group received ink (1 ml/kg BW) via injection into the right wing vein. An identical treatment was carried out on another set of three birds/group at post-treatment Day 12. On each of those days, blood samples (200 µl/bird) were then collected from the left wing vein prior to (0 min) and 3 and 15 min post-ink injection. Each blood sample was immediately transferred into 4 ml of 1% sodium citrate and centrifuged (50 × g, 25 °C) for 4 min. The relative amount of carbon particles remaining in the supernatant was then measured at a wavelength of 640 nm in a Spectro 20D Plus RS-232C spectrophotometer (Labmed Inc., Los Angeles, CA), using the 0 min sample of each bird as zero value for its respective sample (Sarker et al., Citation2000).
Statistical analysis
Data were subjected to a one-way analysis of variance (ANOVA) and the means of different groups were compared by LSD using a Statistix® statistical package (M/S Analytical Software, Tallahassee, FL). Statistical significance was accepted at a p-level < 0.05.
Results
General observations
Birds in the CPF-20 (20 mg/kg BW) group showed more prominent/severe signs (++++) of cholinergic toxicity than did any birds in either the CPF-5 or CPF-10 groups. These other birds displayed clinical signs (++)/behavioral alterations of lesser intensity within 2 h of the first dose. Signs observed included salivation, lacrimation, frequent defecation, convulsions, and tremor. Within 2 weeks of the final exposure, signs of toxicity diminished.
Mortality over the course of the exposures was highest (≈34%, ) in the CPF-20 group followed by lower levels in the CPF-10, CPF-5, and xylene-only groups (21, 10, and 7%, respectively). The controls had the lowest mortality (≈3%).
Table 1. Mortality of broiler chicks administered orally various doses of chlorpyrifos.
Relative organ weights
No significant difference from control values was seen in relative weights of the thymus of treated birds on treatment Day 15 and post-treatment Days 15 and 30 (). Relative weights of the spleens were significantly reduced relative to control values, but only on post-treatment Day 15. In contrast, relative weights of the bursa of Fabricius were significantly reduced relative to control values on treatment Day 15 and post-treatment Day 15, but the effect was no longer apparent by post-treatment Day 30.
Table 2. Relative weights (% of BW) of organs from chicks given daily oral doses of CPF.
Antibody response to SRBC
At day 7 after the primary injection (treatment Day 10), total antibody titres against SRBC were decreased significantly in CPF-10 and -20 birds compared to values in the controls (2.6 and 1.4 vs 5.8, respectively); birds in CPF-5 and xylene groups did not differ significantly from controls (). At day 14 after primary injection (treatment Day 17), all CPF-treated birds had titres significantly lower than the controls (1.4, 1.2, and 0.6 vs 3.4 for control). On days 7 and 14 after the booster injection (treatment Days 24 and 31), total antibody titres against SRBC were significantly decreased in all CPF-treated birds compared to control values (CPF-5, -10, -20 vs control: Day 7, 3.2, 2.4, 3.4 vs 5.4; Day 14, 2.8, 2.2, 2.2 vs 4.4). In none of the assays did xylene-exposed birds yield titres that significantly differed from control levels.
Table 3. Anti-SRBC titers in chicks given daily oral doses of CPF.
Antibody response against Newcastle disease vaccine antigen
On treatment Day 15, antibody titres against Newcastle disease vaccine antigen in birds in all three CPF groups were significantly decreased compared to titres in control birds (). Values for CPF-5, -10, and -20 birds averaged 5.8, 5.0, and 2.8, while those of the controls were 7.2. By post-treatment Days 15 and 30, antibody titres had recovered somewhat and only values associated with the CPF-20 birds remained significantly lower than the controls. In none of the three assays did xylene-exposed birds yield titres significantly different from control bird levels.
Table 4. Anti-Newcastle disease vaccine titres in chicks given daily oral doses of CPF.
Lymphoproliferative response to avian tuberculin
On treatment Day 12, the response to avian tuberculin at 24 and 48 h post-injection was decreased significantly (to >50% relative to control levels) in all CPF-treated birds; responses in birds that received xylene did not differ from the control levels (). By 72 h, all responses against avian tuberculin were not significantly different among the various groups. By post-treatment Day 12, responses to the avian tuberculin were not impacted by the CPF treatments at any of the three post-tuberculin injection timepoints analyzed.
Table 5. Lymphoproliferative response (mm) in chicks given daily oral doses of CPF.
Carbon clearance assay
At either timepoint tested (i.e. treatment Day 12 or post-treatment Day 12), the extent of the phagocytic response against injected carbon particles at 3 min after injection did not differ significantly among any test group (). In contrast, at 15 min post-particle injection, phagocytosis was seen as having been increased significantly (i.e. >100%) in each CPF-treated host. Once again, treatment with xylene alone imparted no effect.
Table 6. In vivo phagocytic response (OD increase %; carbon clearance assay) in chicks given daily oral doses of CPF.
Histopathology
Each bursa of Fabricius, spleen, and thymus from the control birds exhibited normal morphology. In contrast, birds that received CPF exhibited dose- and time-related pathologic changes in these organs. Overall, the changes were mildest in birds in the CPF-5 group, but ranged from moderate-to-severe in birds in the CPF-10 and -20 groups (). Similarly, lesions in the lymphoid organs were mild-to-moderate in intensity at treatment Day 15, while severe changes were noted at post-treatment Day 15. However, at post-treatment Day 30, these degenerative changes had mostly resolved.
Table 7. Histologic changes in bursa of Fabricius, spleen, and thymus due to oral CPF dosing.
Of particular note, in the bursa of birds treated with the higher CPF doses, there was mild congestion, increased inter-follicular connective tissue proliferation, and moderate-to-severe cytoplasmic vacuolation (). Demarcation between cortex and medulla was lost. In some cases, edema fluid accumulation () and/or degenerative changes such as pyknosis and fragmentation of cell nuclei () resulted in depletion of follicle lymphoid cells ( and ). In the spleens, each CPF regimen resulted (increasingly worse-so at the highest dose) in increased disorganization of follicular patterns and congestion (), cytoplasmic vacuolation, degenerative changes (like pyknosis and fragmentation of cell nuclei) (), as well as increased hyperplasia among reticular cells. The thymus of treated birds, primarily among the birds that received the highest CPF dose, exhibited mild-to-moderate congestion (), hyper-cellularity, a presence of immature monocytes in the medullary region, and myoid cell necrosis (). Other findings noted were vacuolation of cytoplasm and degeneration and perforation of myoid cells ().
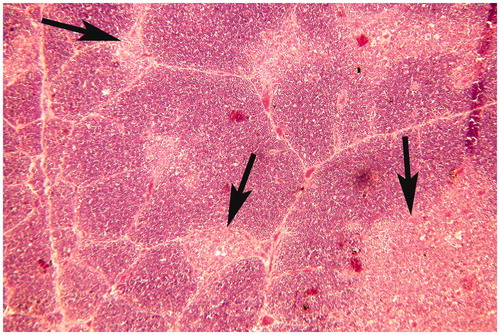
Figure 1. Bursa of Fabricius of broiler chick administered chlorpyrifos (20 mg/kg BW) at post-treatment Day 15. Representative photomicrograph shows vacuolar degeneration (arrow heads), proliferation of fibrous connective tissue between lymphoid follicles (arrows), and follicles depleted from lymphoid tissue (D). H&E stain. Magnification = 200×.
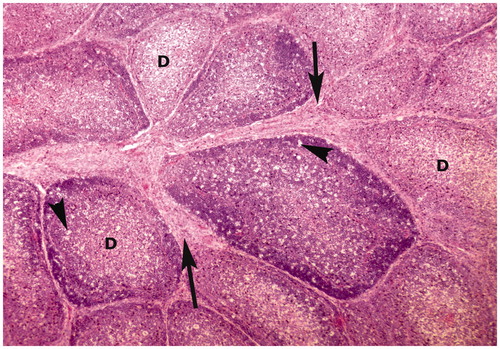
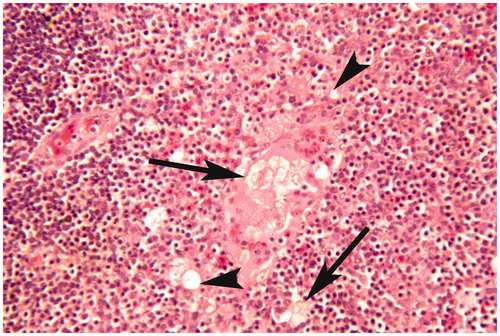
Figure 2. Bursa of Fabricius of broiler chick administered chlorpyrifos (20 mg/kg BW) at post-treatment Day 15. Representative photomicrograph shows necrosis of follicle in the center, edema in the follicle (arrow heads), and proliferation of fibrous connective tissue around the lymphoid follicles (arrows). H&E stain. Magnification = 400×.
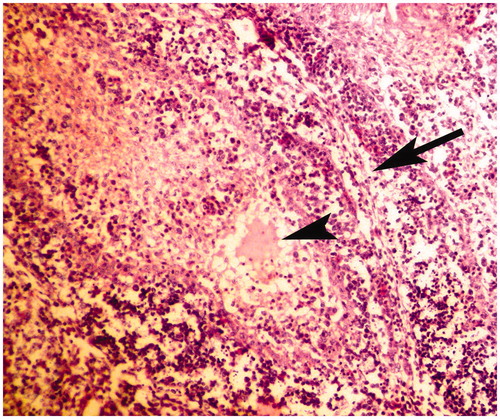
Figure 3. Bursa of Fabricius of broiler chick administered chlorpyrifos (20 mg/kg BW) at post-treatment Day 15. Representative photomicrograph shows fragmentation of nuclei (apoptotic changes) in lymphoid follicle (arrows). H&E stain. Magnification = 400×.
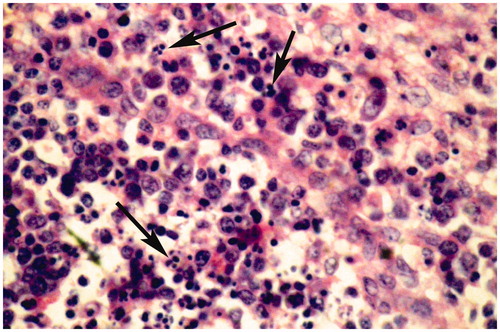
Figure 4. Spleen of broiler chick administered chlorpyrifos (10 mg/kg BW) at day 15 of treatment. Representative photomicrograph shows congestion and degenerative changes. H&E stain. Magnification = 40×.
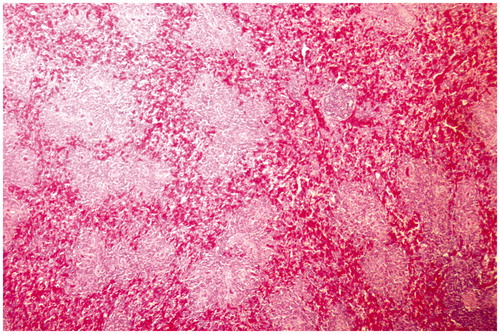
Figure 5. Spleen of broiler chick administered chlorpyrifos (20 mg/kg BW) at post-treatment Day 15. Representative photomicrograph shows congestion and fragmentation of nuclei (apoptotic changes) (arrows). H&E stain. Magnification = 400×.
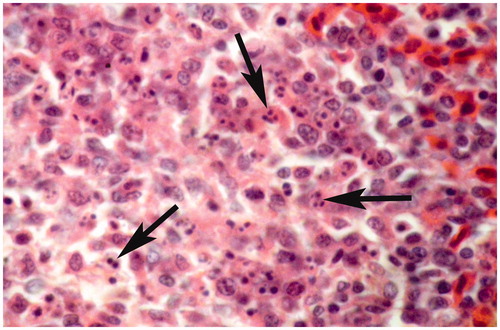
Figure 6. Thymus of broiler chick administered chlorpyrifos (10 mg/kg BW) at day 15 of treatment. Representative photomicrograph shows congestion and degenerative changes. H&E stain. Magnification = 40×.
Discussion
It is well documented that over-use of CPF results in accidental exposures of wild, domesticated, and farmed animals and birds (Martin & Forsyth, Citation1998) that can result in death (Hussain et al., Citation2011; Mitra et al., Citation2011). Data limitations (i.e. reliability of counts) are a major impediment toward risk assessment of different environmental pollutants, especially pesticides like CPF, in many species, including birds (Tariq et al., Citation2007). While there is a wide range of data illustrating the immunotoxic effects of OP agents (Duramad et al., Citation2006; Galloway & Handy, Citation2003; Jeong et al., Citation1995) and how CPF in particular can affect the immune systems of mammals (Blakley et al., Citation1999; Navarro et al., Citation2001; Thrasher et al., Citation2002), such information in broiler chicks is for the most part non-existent.
In the present study, no significant changes in relative weights of the thymus were seen in the chlorpyrifos (CPF)-treated birds. However, relative weights of the spleen and the bursa of Fabricius were decreased as compared to values in control hosts. This is in contrast with findings in CPF-treated rats where splenic weights were increased compared to control host values (Jeong et al., Citation2006); however, splenic weights in F1 offspring were decreased significantly. A combination of 50% chlorpyrifos and 5% cypermethrin has also been reported to cause reductions in weights of the thymus and spleen in RIR chicks (Gowri et al., Citation2010). Such reductions could be due to peroxidative damage, leading to necrosis in the lymphoid tissues of the treated hosts (Fatima et al., Citation2000; Jahan et al., Citation2012). We must note that, in our study there were appreciable changes in diet consumption by chicks due to the CPF; this alone might have led to a decrease in body weights (and accordingly organ:body indices) of the birds. Moreover, organ weight reduction with CPF treatment in the present study could be particular to this species, as previous studies were carried out with rats (Ehrich et al., Citation2004; Jeong et al., Citation2006), fish (Fatima et al., Citation2000; Siwicki et al., Citation1990), and RIR chicks (Gowri et al., Citation2010) noted non-significant reductions in weights of the spleen and thymus of treated hosts.
The present study indicated there was a significantly decreased antibody response against SRBC and Newcastle disease vaccine virus in groups administered CPF as compared to that seen with birds in the control group. Such outcomes would be in keeping with finding in rodent models; decreased humoral immunity (Blakley et al., Citation1999) and marked suppression of humoral immune functions were noted in rats/mice exposed to OP (e.g. fenitrothion, fenthion, diazinon, and EPN) agents (Moon et al., Citation1986). With the OP parathion, a reduction in the number of IgM plaque-forming cells was documented in exposed mice that had been immunized with SRBC (Casale et al., Citation1984). A suppressed primary humoral IgM response to SRBC ex vivo and in vivo was also seen in mice treated with parathion (Pruett et al., Citation1992). Contrary to these reports, Hinton et al. (Citation1987) reported no significant effect on antibody production responses in rats treated with the OP triphenyl phosphate. Since OP are neurotoxins that hamper neuronal development and also induce concomitant immunologic deficits (Naz et al., Citation2011), it is not without bases for us to conclude that the immunomodulation noted in the treatment groups here might be due to disturbances in the nervous systems of these birds.
Intradermal administration of avian tuberculin into animals stimulates T-lymphocyte division (with minimal effects on B-lymphocytes) and induces increases in thickness at the site of administration. The current study showed that administration of the tuberculin gave rise to significantly lower response in CPF-treated birds. Decreased T-lymphocyte proliferation in response to phytohemagglutinin (PHA) and Concanavalin A (Thrasher et al., Citation2002) and mitogen (Rodgers & Ellofson, Citation1992) in humans exposed to CPF or malathion has been documented. While the precise mechanism for this effect is still not clear, it might be that serine hydroxylases associated with lymphocyte (and monocyte) cell membranes may be affected in a manner that ultimately leads to structural/functional alterations in immune cells, including an ability to proliferate in response to antigens.
In chickens, carbon clearance assays have been used to assess phagocytic activity in situ (Awais & Akhtar, 2012; Haunshi et al., Citation2002). The present study showed there were significantly delayed phagocytic responses against carbon particles in birds that had been exposed to CPF. The current data is in line with the findings of Queiroz et al. (1999), who reported a considerable reduction in ability to kill Candida albicans by phagocytes (i.e. neutrophils) from workers exposed to carbamate/OP pesticides. The authors concluded that exposure to those insecticides likely led to changes in phagocyte function, even though the workers presented with no impairment in cholinesterase (ChE) activity. Reduced phagocytic activity by splenic macrophages in RIR chicks exposed to CPF has also been reported (Gowri et al., Citation2010).
In the present study, spleen and thymus showed congestion, derangements, cytoplasmic vacuolation, necrosis, and decreased population of mononuclear cells, which concurred with reported findings (Malik et al., Citation2004). Bursa of Fabricius showed congestion, lymphoid cells depletion, increased inter-follicular connective tissue proliferation, vacuolar degeneration, necrotic changes, and fragmentation of nuclei. The results were in accordance with Krishnamoorthy et al. (Citation2007), who also observed lymphoid depletion in follicles, mild inter-follicular fibrosis, lack of cortico-medullary differentiation, and lymphocytolysis in bursa of Fabricius of birds. The CPF has been reported to induce fragmentation of DNA in immune cells of humans in vitro (Nakadai et al., Citation2006). In treatment groups, bursa of Fabricius and spleen showed fragmentation of the nucleus of lymphocytes that were not reported earlier in vivo. This might be due to damage of nucleic acids produced in result of oxidative stress attributed to free radicals by activating the transmembrane glycoprotein death receptor pathway. The cascade of caspases released leads to activation of endonucleases which results in DNA digestion into fragments and ultimately packed into vesicles. Histopathological damage to lymphoid organs might be due to the inflection of nervous system results into altered production of lymphocytes, phosphorylation and oxidative damage (Galloway & Handy, Citation2003).
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ahmad, L., Khan, A., and Khan, M. Z. 2012. Pyrethroid-induced reproductive toxicopathology in non-target species. Pak. Vet. J. 32:1–9
- Ahmed, N. S., Mohamed, A. S., and Abdel-Wahhab, M. A. 2010. Chlorpyrifos-induced oxidative stress and histological changes in retinas and kidney in rats: Protective role of ascorbic acid and α-tocopherol. Pest. Biochem. Physiol. 98:33–38
- Akhtar, M., Hafez, M. A., Haq, A., et al. 2008. Immunomodulatory and protective effects of sugar cane juice in chickens against Eimeria infection. Turk. J. Vet. Anim. Sci. 32:463–467
- Al-Badrany, Y. M., and Mohammad, F. K. 2007. Effects of acute and repeated oral exposure to the organophosphate insecticide chlorpyrifos on open-field activity in chicks. Toxicol. Lett. 174:110–116
- Ambali, S. F., Abbas, S. O., Shittu, M., et al. 2009. Effects of gestational exposure to chlorpyrifos on implantation and neonatal mice. J. Cell. Anim. Biol. 3:50–57
- Awais, M. M., and Akhtar, M. 2012. Evaluation of some sugarcane (Saccharum officinarum L.) extracts for immunostimulatory and growth promoting effects in industrial broiler chickens. Pak. Vet. J. 32:398–402
- Blakley, B. R., Yole, M. J., Brousseau, P., et al. 1999. Effects of chlorpyrifos on immune function in rats. Vet. Human Toxicol. 41:140–144
- Casale, G. P., Cohen, S. D., and Dicapua, R. A. 1984. Parathion-induced suppression of humoral immunity in inbred mice. Toxicol. Lett. 23:239–247
- Chukwudi, O. E., Chukwuemeka, E. D., and Mary, U. 2012. Newcastle disease virus shedding among healthy commercial chickens and its epidemiological importance. Pak. Vet. J. 32:354–356
- Delhanty, J. J., and Solomon, J. B. 1966. The nature of antibodies to goat erythrocytes in the developing chicken. Immunology 11:103–113
- Duramad, P., Tager, I. B., Leikauf, J., et al. 2006. Expression of TH1/TH2 cytokines in human blood after in vitro treatment with chlorpyrifos, and its metabolites, in combination with endotoxin LPS and the allergen Der p1. J. Appl. Toxicol. 26:458–465
- Ehrich, M., Hancock, S., Ward, D., et al. 2004. Neurologic and immunologic effects of exposure to corticosterone, chlorpyrifos, and multiple doses of tri-o-tolyl phosphate over a 28-d period in rats. J. Toxicol. Environ. Health 67:431–457
- Fatima, M., Ahmad, I., Sayeed, I., et al. 2000. Pollutant-induced over-activation of phagocytes is concomitantly associated with peroxidative damage in fish tissues. Aquat. Toxicol. 49:243–250
- Galloway, T., and Handy, R. 2003. Immunotoxicity of organophosphorous pesticides. Ecotoxicology 12:345–363
- Gowri, U. K., Patel, P. V., and Suresh, B. 2010. Evaluation of pesticides induced developmental immunotoxicity in RIR chicks. J. Cell. Tissue Res. 10:2229–2234
- Hameed, M. R., Khan, M. Z., Khan, A., and Javed, I. 2013. Ochratoxin induced pathological alterations in broiler chicks: Effect of dose and duration. Pak. Vet. J. 33:145–149
- Hartely, D., and Kidd, H., (Eds.). 1983. The Agrochemicals Handbook. Nottingham, England: Royal Society of Chemistry
- Haunshi, S., Sharma, D., Nayal, L. M. S., et al. 2002. Effect of naked neck gene (NA) and frizzle gene (F) on immunocompetence in chickens. Br. Poult. Sci. 43:28–32
- Hayes, W. J., and Laws, E. R., (Eds.). 1990. Handbook of Pesticide Toxicology. New York: Academic Press
- Hinton, D. M., Jessop, J. J., Arnold, A., et al. 1987. Evaluation of immunotoxicity in a subchronic feeding study of triphenyl phosphate. Toxicol. Ind. Health 3:71–89
- Hussain, R., Mahmood, F., Khan, M. Z., et al. 2011. Pathologic and genotoxic effects of atrazine in male Japanese quail (Coturnix japonica). Ecotoxicology 20:1–8
- Islam, M. N., Khan, M. Z., Jahan, M. R., et al. 2012. Histomorphological study on prenatal development of the lymphoid organs of native chickens of Bangladesh. Pak. Vet. J. 32:175–178
- Jahan, N., Rahman, K. U., Ali, S., et al. 2012. Cardioprotective potential of gemmomodified extract of Terminalia arjuna against chemically induced myocardial injury in rabbits. Pak. Vet. J. 32:255–259
- Jeong, S. H., Kim, B. Y., Kang, H. G., et al. 2006. Effect of chlorpyrifos-methyl on steroid and thyroid hormones in rat F0- and F1-generations. Toxicology 220:189–202
- Jeong, T. C., Jordan, S. D., Matulka, R. A., et al. 1995. Role of metabolism and by esterase and cytochrome P450 in cocaine-induced suppression of antibody response. J. Pharmacol. Exp. Ther. 272:407–416
- Khan, A., Ahmad, A., and Khan, M. Z. 2012. Hemato-biochemical changes induced by pyrethroid insecticides in avian, fish and mammalian species. Int. J. Agric. Biol. 14:834–842
- Krishnamoorthy, P., Vairamuthu, S., Balachandran, C., and Muralimanohar, B. 2007. Pathology of lymphoid organs in chlorpyriphos and T-2 toxin fed broiler chicken. Int. J. Poult. Sci. 6:71–76
- Malik, G., Dahiya, J. P., and Gera, S. 2004. Biochemical studies on chlorpyrifos toxicity in broiler chickens. Indian J. Anim. Sci. 74:473–476
- Martin, P. A., and Forsyth, D. J. 1998. Effects of exposure to vegetation sprayed with dimethoate or chlorpyrifos on mallard ducklings (Anas platyrhynchos). Ecotoxicology 7:81–87
- Mitra, A., Chatterjee, C., and Mandal, F. B. 2011. Synthetic chemical pesticides and their effects on birds. Res. J. Environ. Toxicol. 5:81--96
- Moon, C. K., Yun, Y. P., Lee, S. H., and Lee, Y. S. 1986. Effects of some organophosphates pesticides on the murine immune system following subchronic exposure. Arch. Pharm. Res. 9:175–181
- Moye, J. K., and Pritsos, C. A. 2010. Effects of chlorpyrifos and aldicarb on flight activity and related cholinesterase inhibition in homing pigeons Columba livia: Potential for migration effects. Bull. Environ. Contam. Toxicol. 84:677–681
- Muhammad, F., Javed, I., Akhtar, M., et al. 2012. Quantitative structure activity relationship and risk analysis of some pesticides in the cattle milk. Pak. Vet. J. 32:589–592
- Nakadai, A., Li, Q., and Kawada, T. 2006. Chlorpyrifos induces apoptosis in human monocyte cell line U937. Toxicology 224:202–209
- Navarro, H. A., Basta, P. V., Seidler, F. J., and Slotkin, T. A. 2001. Neonatal chlorpyrifos administration elicits deficits in immune function in adulthood: A neural effect. Brain Res. Dev. 130:249–252
- Naz, S., Rana, S. A., Javed, M., and Rehman, K. U. 2011. Toxicological effects of brodifacoum and food energy inhibitor on some physiological parameters in house rats (Rattus rattus). Pak. Vet. J. 31:219–222
- Pruett, S. B., Han, Y., Munson, A. E., and Fuchs, B. A. 1992. Assessment of cholinergic influences on a primary humoral immune response. Immunology 77:428–435
- Queiroz, M. L., Fernandes, M. D., and Valadares, M. C. 1999. Neutrophil function in workers exposed to organophosphate and carbamate insecticides. Int. J. Immunopharmacol. 21:263–270
- Reece, R. L., and Handson, P. 1982. Observations on the accidental poisoning of birds by organophosphate insecticides and other toxic substances. Vet. Record 111:453–455
- Rodgers, K. E., and Ellofson, D. D. 1992. Mechanism of modulation of murine peritoneal cell function and mast cell degranulation by low doses of malathion. Agents Actions 35:57–63
- Sarker, N., Tsudzuki, M., Nishibori, M., et al. 2000. Cell mediated and humoral immunity and phagocytic ability in chicken lines divergently selected for serum immunoglobulin M and G levels. Poult. Sci. 79:1705–1709
- Saulsbury, M. D., Heyliger, S. O., Wang, K., and Johnson, D. J. 2009. Chlorpyrifos induces oxidative stress in oligodendrocyte progenitor cells. Toxicology 259:1–9
- Siwicki, A. K., Cossarini-Dunier, M., Studnicka, M., and Demael, A. 1990. In vivo effect of the organophosphorus insecticide trichlorphon on immune response of carp (Cyprinus carpio). II. Effect of high doses of trichlorphon on non-specific immune response. Ecotoxicol. Environ. Safety 19:99–105
- Tariq, M. I., Afzal, S., Hussain, I., and Sultana, N. 2007. Pesticides exposure in Pakistan: A review. Environ. Int. 33:1107–1122
- Thrasher, J. D., Heuser, B., and Broughton, A. 2002. Immunological abnormalities in humans chronically exposed to chlorpyrifos. Arch. Environ. Health 57:181–187
- Thrasher, J. D., Madison, R., and Broughton, A. 1993. Immunologic abnormalities in humans exposed to chlorpyrifos: Preliminary observations. Arch. Environ. Health 48:89–93
- Voccia, I., Blakley, B., Brousseau, P., and Fournier, M. 1999. Immunotoxicity of pesticides: A review. Toxicol. Ind. Health 15:119–132
- Williams, T. 1997. Silent scourge: Legal pesticides continue to kill millions of our birds. Audubon 99:28–35
- Wolf, C., Riffel, M., Weyman, G., et al. 2010. Telemetry-based field studies for assessment of acute and short-term risk to birds from spray applications of chlorpyrifos. Environ. Toxicol. Chem. 29:1795–1803