Abstract
In the past two decades, hematologic and immunologic disorders in humans have been increasingly reported as a result of pesticide exposures. Therefore, safety assessment is required to assess the effects on hematopoiesis and thus on the immune system in addition to routine toxicity evaluation. Currently, the data available on effects of pesticides on hematopoiesis in humans is limited. In the study here, cypermethrin and mancozeb were evaluated for their possible effects on hematopoiesis in vitro. Hematopoietic stem or progenitor cells from human cord blood were isolated and then exposed for 14 days to cypermethrin or mancozeb at non-cytotoxic doses (0.9–16 µM), and the effect on hematopoiesis screened via a methylcellulose-based clonogenic assay. Results indicated there were significant concentration-related decreases in clonogenic potentials of erythroid and granulocyte-macrophage colony formation. The inhibitory concentration (IC50) value with erythroid progenitors for cypermethrin was 8.7 [±0.2 µM; mean [± SE]) and for mancozeb 6.2 [±0.2] µM. Similarly, IC50 values with granulocyte-macrophage progenitors for cypermethrin and mancozeb were 19.2 [±1.0] and 8.1 [±0.2] µM, respectively. These data suggest that erythroid progenitors are perhaps more sensitive to these pesticides. Still, further studies are needed to understand the functional significance of these in vitro findings. For now, these data, albeit preliminary, emphasize the need to include an expanded battery of tests to understand effects on immune parameters in pre-clinical safety studies with pesticides. This study also emphasizes the utility of human cord blood in assessing potential effects on hematopoiesis in vitro.
Introduction
Pesticide effects on hematopoiesis and the immune system have been gaining attention in the last two decades due to their wide usage in agriculture, industries, and domestic purposes. Chronic exposure to pesticides indicates the risk of hematopoietic malignancies (Dennis, Citation1993; Hoshizaki et al., Citation1969; Loge, Citation1965; Merhi et al., Citation2007; Parent-Massin & Thouvenot, 1993; Vial et al., 1996) and might lead to the onset of different pathologies. Possible risk of hematological disorders such as leukemia, multiple myeloma, Hodgkin lymphoma, and non-Hodgkin lymphoma were explored (Merhi et al., Citation2007; Rudant et al., Citation2007) in agriculture workers, farmers, or employees in chemical industries. A cross-sectional study in cut-flower farmers indicated the hematotoxic effects due to pesticide exposure (Del Prado-Lu, Citation2007). Recently a study on dietary exposure to pesticide mixtures at below reference doses in a mouse model reported significant effects on red blood cells and hemoglobin levels (Demur et al., Citation2013). Further, studies in laboratory animals have shown the myelotoxicity even at low exposure levels, without having any overt signs of toxicity (Hong et al., Citation1991). These studies warrant the evaluation of pesticide exposure on hematopoiesis and thus on the immune system.
Cypermethrin and mancozeb are the two widely used pesticides worldwide in agriculture, households, and industries due to their low mammalian toxicity and short environmental persistence (Hasan, Citation2010; Hayes & Laws, Citation1990; Lorgue & Lechenet, Citation1996). Cypermethrin is a synthetic pyrethroid insecticide which showed significant effects on hematological (Kamal et al., Citation2007; Nair et al., 2010; Sayim et al., Citation2005; Yousef et al., Citation2003) and immunological parameters (Desi et al., Citation1986; Liu et al., 2006; WHO, Citation1989) in sub-chronic repeated dose studies in animals. Mancozeb, ethylenebisdithio carbamate, is another pesticide used extensively and reported to have adverse effects on various hematologic (Myoung-yun & Cheong, Citation2004; Wael, Citation2012) and immunologic parameters (Corsini et al., Citation2005). Although studies indicate the adverse effects of cypermethrin or mancozeb on hematological or immunological parameters, currently there were no data available on the direct effects of these two pesticides on hematopoiesis.
Clonogenic assays using hematopoietic stem or progenitor cells after exposure to various chemicals have proven to be sensitive indicators of toxicity (Noble & Sina, 1993; Parent-Massin, 2001; Parent-Massin et al., Citation2010). These assays have a higher degree of predictability than conventional evaluations like peripheral blood cell counts (Parchment et al., Citation1993) and bone marrow cytology for the assessment of hematotoxic potential. The use of validated in vitro hematotoxicity assays can refine and improve the in vivo testing required to identify the bone marrow as a most likely target tissue of a xenobiotic. Further, these assays using human cells aid considerably in risk assessment estimates of various chemical types (Gribaldo et al., Citation1996), including pesticides.
Often in vitro hematotoxicity assays study the acute effect of toxicants on hematopoietic progenitors such as erythroid, granulocyte-macrophage, and megakaryocyte cells (Parent-Massin, 2001; Parent-Massin et al., Citation2010). These in vitro studies quantify the number of surviving progenitors as a function of exposure level, under optimal stimulatory conditions (Bradley and Metcalf, Citation1966; Metcalf, Citation1984). By mimicking in vivo physiological conditions, use of specific cytokine compositions in the medium allow for progenitors to differentiate into phenotypically-distinct colonies (Clarke et al. Citation2007; Parent-Massin et al., Citation2010).
In vitro models of hematopoiesis are promising for determining hematotoxic effects (Deldar & Parchment, 1997; Du et al., Citation1990; Parent-Massin, Citation2001; Pessina et al., Citation2001; Citation2003, Citation2010), even in the absence of information about the type of metabolism triggered in response to, and the target organ toxicity of, a test agent (Negro et al., Citation2001). Furthermore, these predictive in vitro assays have the potential to substantially reduce and refine animal usage, and could make possible human toxicology studies in preclinical settings (Gribaldo et al., Citation1998a,b; Pessina et al., Citation2001, Citation2002, Citation2010). The present study was designed to evaluate the potential effects of cypermethrin and mancozeb on hematopoiesis through lineage-specific colony formation in a clonogenic assay.
Materials and methods
Chemicals
Cypermethrin (>99%), mancozeb (>95%), benzo(a)pyrene (>96%), and urethane (>99%) were purchased from Sigma (St. Louis, MO). Stock solutions of each were prepared in dimethyl sulfoxide (DMSO; Sigma) and stored at −80 °C. Stock solutions were diluted in DMSO to the desired concentrations before further dilution in culture medium. Final culture levels of DMSO never exceeded 0.5%.
Hematopoietic progenitor cells
Upon obtaining informed consent, placental cord blood was collected after delivery into a heparinized tube. All experiments were approved by the 3rd Institutional Ethics Committee (held April 2, 2011). Isolation of mononuclear cells from the blood was then performed using Ficoll (ρ = 1.077 g/L) density gradient centrifugation. Briefly, heparinized cord blood was mixed with phosphate-buffered saline (PBS, pH 7.4) in a 1:1 ratio and slowly layered atop Ficoll-hypaque medium (1 mL of Ficoll for each 3 ml blood-PBS mix) held in a 50 ml centrifuge tube. Tubes were then centrifuged at 400 x g for 30 min and the buffy coat containing mononuclear cells then collected into a fresh centrifuge tube and washed twice with PBS (≈3 vol of the collected buffy coat). Cells were then re-suspended in Iscove’s modified Dulbecco’s medium (IMDM) and then cryopreserved in liquid nitrogen until use in the colony-forming cell assay or for cytotoxicity assessments.
Cytotoxicity assessment
Cord blood mononuclear cells were exposed overnight to at least eight serial doses of cypermethrin or mancozeb. Based on trypan blue dye exclusion, doses that caused > 10% cytotoxicity were excluded from further analysis. Preliminary cytotoxicity studies were performed using stem or progenitor cells of two donors per test chemical to assess biological variance.
Colony-forming unit (CFU) assay
Cord blood mononuclear cells were thawed and seeded into MethoCult™ H4034 Optimum (Stemcell Technologies, Vancouver, Canada) medium containing stem cell factor (SCF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interleukin (IL)-3, granulocyte colony-stimulating factor (G-CSF), and erythropoietin (EPO) to support proliferation of four major colony types; colony forming unit erythroids (CFU-E), burst-forming unit erythroids (BFU-E), colony-forming unit granulocyte and macrophages (CFU-GM), and multi-potential colony-forming unit (granulocyte, erythroid, macrophage, megakaryocytes; CFU-GEMM).
To understand the biological variance, clonogenic assays were performed (in triplicate) using cells from the blood from three donors with each chemical separately, and following manufacturer instructions. Briefly, 20 µl of test solutions and 380 µl of cells (4 × 105 cells/ml) were added directly to a 4 ml Methocult tube for the clonogenic assay. Final levels of cypermethrin were 1, 2, 4, 8, or 16 µM and of mancozeb were 0.98, 1.95, 3.91, 7.81, and 15.62 µM. Benzo(α)pyrene (at 0.8, 1.6, 3.2, 6.4, 12.8 µM) and urethane (at 125, 250, 500, 750, 1000 µM) were included in the assays as positive and negative controls, respectively. After mixing, 1.1 ml of each Methocult-cell suspension was seeded into 35-mm dishes (in triplicate) and incubated at 37 °C under 5% CO2 and with >95% humidity for 14 days.
Colony scoring
After 14 days of incubation, all dishes were coded and a blinded scoring was performed on the different colonies using an inverted microscope. Based on morphology, BFU-E and CFU-GM colonies were scored at lower magnification (20×) and CFU-E and CFU-GEMM colonies at higher magnification (40×); 100× magnification was used to differentiate CFU-GM and CFU-GEMM colony types.
Data analysis
Cell proliferation was expressed in terms of percentage growth, with 100% corresponding to the number of colonies in control dishes. Concentrations that inhibited 50% growth (IC50) were interpolated using the Reed & Muench (Citation1938) formula. Data were expressed as mean ± SE of three individual experiments (n = 3) conducted in three different volunteers, in triplicate for each compound.
Results
Colony morphology
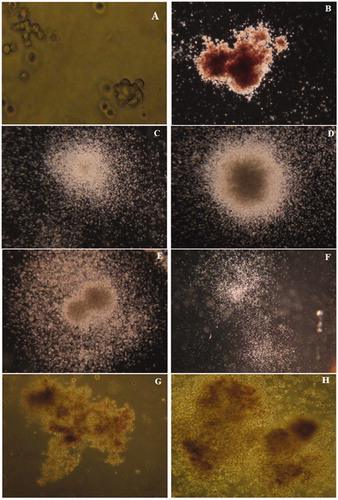
Four distinct colonies (), i.e. CFU-E, BFU-E, CFU-GM, and CFU-GEMM, containing morphologically recognizable progenies were observed after 14 days in this methyl-cellulose-based clonogenic assay. An isolated single colony containing ≈8–200 erythroblasts was recognized as a CFU-E (); those with >200 erythroblasts in single or multiple clusters were defined as BFU-E colonies (). CFU-GM was defined as an aggregate containing at least 50 or more granulocytes (CFU-G), macrophages (CFU-M), or cells of both lineages (CFU-GM) (). Colonies arising from a primitive CFU-GM progenitor contain thousands of cells in a single or multiple clusters. Four types of morphologically distinguishable CFU-GM colonies were observed in this assay; Colonies without an apparent nucleus (diffuse and spread colonies) (); compact colonies with a central dense nucleus and a peripheral halo (); multi-centric colonies with two or more dense nuclei with a common peripheral halo growing at the same depth (); and aggregates of several colonies with or without a peripheral halo (identified as multifocal colonies; ) were observed. Due to the primitive nature of CFU-GEMM progenitors, they produced large colonies of >500 cells containing erythroblasts and cells of at least two other recognizable lineages ().
Figure 1. Observed colonies in the methylcellulose-based clonogenic assay – after 14 days. (A) CFU-E (200×): An isolated single colony containing ≈8–200 erythroblasts. (B) BFU-E (20×): > 200 erythroblasts in a single or multiple clusters. (C–F) CFU-GM (20×): An aggregate containing at least ≥50 granulocytes (CFU-G), macrophages (CFU-M), or cells of both lineages. (G, H) CFU-GEMM (100×): > 500 cells containing erythroblasts and cells of at least two other recognizable lineages.
Myelotoxicity
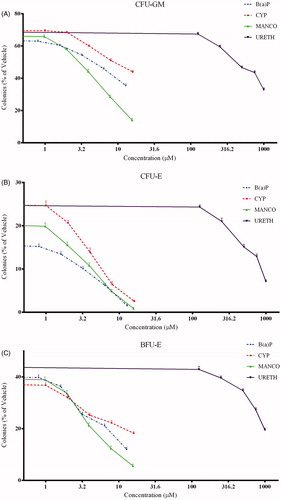
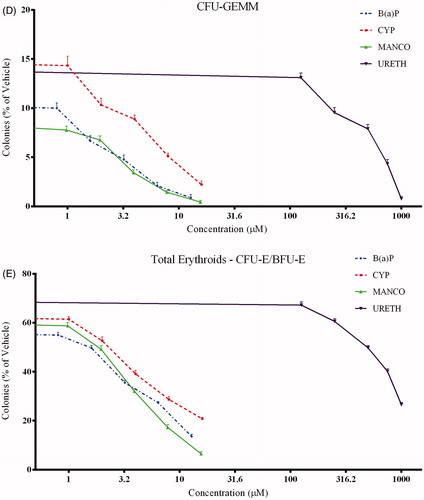
Both cypermethrin and mancozeb showed myelotoxic effects as evidenced by the inhibition of lineage-specific colonies CFU-E, BFU-E, CFU-GM, CFU-GEMM, and TOTAL-E in a concentration-related fashion (). A summary in of the degree of inhibition caused by these compounds is indicated in terms of relative IC50 values for both erythropoietic and granulopoietic lineage types (; calculated from three individual experiments) done using hematopoietic stem or progenitor cells from three different donors.
Figure 2. Effect on CFU resulting from in vitro exposure of human cord blood cells to cypermethrin, mancozeb, benzo(a)pyrene, or urethane. Values are expressed as a percentage of vehicle control and shown as mean (± SE) from three individual experiments (n = 3) conducted with samples from three volunteers (in triplicate for each compound). Effects on: (A) CFU-GM colonies; (B) CFU-E colonies; (C) BFU-E colonies; (D) CFU-GEMM colonies; and (E) Total erythroid progenitors (CFU-E and BFU-E).
Table 1. IC50 (µM) values with erythropoietic, granulopoietic, and primitive progenitor colonies.
Effect on granulopoiesis
The toxic effect of cypermethrin and mancozeb on cells with a granulopoietic lineage was assessed based on changes in formation of CFU-GM colonies. Although a decrease in the numbers of colonies was observed (), cypermethrin was not considered toxic to granulopoietic lineage-specific progenitors at the concentrations tested (i.e. the IC50 value was greater than the maximum concentration tested; ). However, mancozeb indicated a clear toxic effect with a lesser IC50 value () on this lineage-specific colony.
Effect on erythropoiesis
Erythroid (CFU-E/BFU-E) progenitors showed greater sensitivity to both test compounds (). IC50 values were ≈2–3 times lower than that seen with granulocyte-macrophage progenitors (CFU-GM) (). Total erythroid colonies (CFU-E and BFU-E) were also used to assess potential effects on erythropoiesis (); outcomes revealed a similar concentration-dependent decrease and lower IC50 values (). Of both chemicals, mancozeb had the greater toxic effect on cells of erythroid lineage than did cypermethrin (as was the case with cells critical to granulopoiesis).
Effect on primitive GEMM progenitors
Mixed-lineage colonies, like CFU-GEMM, develop from more primitive hematopoietic progenitors. The effect of both test compounds on these colonies was similar to that with cells involved in erythropoiesis (), as indicated by lower IC50 values than seen with granulopoietic progenitors (). Mancozeb was more toxic to the GEMM progenitors as well. Concentration-dependent responses with positive (benzo(a)pyrene) and negative (urethane) controls against the various colonies is presented in and their corresponding IC50 values are shown in .
Discussion
Pesticide-induced hematologic disorders have gained more attention in recent years (Fritschi et al., Citation2005; Landgren et al., Citation2009; Orsi et al., Citation2009), especially following chronic exposure at the workplace. Exposure to these agents might affect the hematopoiesis at one or more specific lineages leading to various hematological disorders. Cypermethrin and mancozeb are two pesticides widely used worldwide and classified as probable human carcinogens by the United States Environmental Protection Agency (USEPA, Citation1995). In addition, animal studies have shown that these two pesticides exert significant effects on various hematologic parameters (Cox, Citation1986; Myoung-yun & Cheong, Citation2004; Sayim et al., Citation2005; Wael, Citation2012; Yousef et al., Citation2003). For those latter reasons, the myelotoxic effects of these two compounds – with respect to erythropoiesis and granulopoiesis – were evaluated in the present study.
In vitro data here with human hematopoietic stem/progenitor cells indicate that erythropoietic (CFU-E and BFU-E) progenitors were more sensitive to both cypermethrin and mancozeb (each reduced numbers of CFU-E and BFU-E) than were myeloid (CFU-GM) progenitors. It was also evident that, due to its significant effects on both erythroid and myeloid colonies, the myelotoxic effect of mancozeb was slightly higher than that of cypermethrin, as based on this in vitro clonogenic assay.
Numerous animal studies have reported dose-dependent decreases in hematologic end-points such as erythrocyte count, packed cell volume, as well as both hemoglobin and hematocrit levels, that were accompanied by significant increases in mean corpuscular volume and mean corpuscular hemoglobin due to cypermethrin (Kamal et al., Citation2007; Nair et al., Citation2010; Sayim et al., Citation2005) or mancozeb (Cox, Citation1986) exposure. These changes were attributed due to disruptive action of either pesticide on erythropoietic tissues. The observed results, i.e. decreased numbers of erythroid (CFU-E/BFU-E) colonies, in our current in vitro clonogenic assay correlated well with those in vivo findings and indicated that the cypermethrin or mancozeb directly affected erythroid progenitor cells. It is important to note that the range of in vitro doses tested here was in accordance with those earlier in vivo studies. In those studies, significant effects on hematologic parameters were seen using cypermethrin at doses ranging from 25–300 mg/kg BW when administered by oral or intraperitoneal routes (Kamal et al., Citation2007; Sayim et al., Citation2005). Similarly, mancozeb exposure of laboratory animals – at 250–1500 mg/kg BW casued significant effects (Myoung-yun & Cheong, Citation2004).
While our studies did demonstrate effects from direct exposure of the cord blood mononuclear cells to each pesticide, we feel that questions about relevance of the doses used needs should be addressed. Our in vitro results demonstrated inhibitory effects on hematopoietic progenitors after exposure to 0.9–16 µM levels of the parent agents. Occupational exposure to pyrethroids and carbamates often results in increases in body burdens of the toxicants that are, in turn, reflected at milligram levels of select metabolites of the parent agent (for cypermethrin, 3-phenoxybenzoate; for mancozeb, ethylene thiourea) in the blood/urine of the workers (Corsini et al., Citation2005; Leng & Gries, Citation1999; Lu et al., 2013). As such, at even just a single milligram of each metabolite, this would yield corresponding levels of 4.7 (3-phenoxybenzoate) and 9.8 (ethylene thiourea) µM in the human blood/urine of the exposed works. Thus, the doses of cypermethrin or mancozeb used in the current experiments were likely to have been on par with levels of the parent + metabolite found in the workers routinely exposed to either pesticide. Nevertheless, the true relationship between cumulative quantity of metabolites produced by continuous exposures to these pesticides in workers can and should be estimated by others using mathematical toxico-kinetic modeling to better estimate what types of doses of these pesticides should be used/would be unquestionably relevant in the types of in vitro studies performed here.
The IC50 values summarized in indicate clear lineage-specific toxicities from the two pesticides. While mancozeb inhibited erythroid colony formation in a dose-related manner (albeit with a greater effect on both CFU-E/BFU-E progenitor types), the effect of cypermethrin was much stronger against the CFU-E type. With regard to myeloid type progenitors, mancozeb again differed in the degree of toxicity imparted, i.e. effects on myeloid (CFU-GM) colonies were apparent at less than half the dose needed to achieve the same effect with the cypermethrin. The data also clearly showed the two pesticides caused variable effects on different stages of hematopoiesis. It is interesting to recall that CFU-GEMM colonies develop from more primitive hematopoietic progenitor cells, and thus any effect on these colonies could be related to effects on erythropoiesis, granulopoiesis, and/or megakaryopoiesis. The data in the current study illustrated a clear relationship between effects on CFU-GEMM colonies and then upon erythroid (CFU-E/BFU-E) or myeloid (CFU-GM) colonies by the two pesticides. However, we were not able to clearly establish any relationship between the outcomes and effects on megakaryopoiesis due to technical limitations (i.e. identifying megakaryocytes versus macrophages requires use of specific staining procedures and non-methylcellulose-based cultures). Still, based on the in vitro results, we can confidantly still assert that cypermethrin or mancozeb appear to have an ability to directly affect hematopoietic progenitor cells.
Agriculture workers, farmers, and employees in chemical industries are usually at higher risk for chronic exposure to pesticides. A wide disparity in experimental designs, simultaneous exposures to multiple compounds, and a lack of information on occupational exposure levels have to date limited comparisons between in vitro and in vivo data for the toxicity of pesticides, including mancozeb and cypermethrin. Because of this, it is obvious that novel protocols that can accurately reflect toxicities (including immunologic or hematologic) through the use of various levels of pesticides (or any other toxic agent) or various exposure scenarios [single substance versus mixtures], and thus better reflect occupational exposures, are needed. The in vitro clonogenic assay employed here to assess hematotoxicity has the potential to be just such a protocol.
Once validated for a wide spectrum of test toxicants, not only will use of this novel assay mean that substantially fewer numbers of animals would need to be consumed for any similar type of evaluation (an increasingly important ethical consideration), its use of human cells could provide data of clearer scientific relevance for those most at risk of toxicity from exposures (i.e. workers). In addition, establishing this assay as a reliable method to assess toxicity for any given test agent would eventually permit a bridging of already-existing data obtained in animal models with that from clinical studies (Clarke & Atkinson, Citation2010; Ficheux et al., Citation2012; Parchment et al., Citation1993; Pessina et al., Citation2010; van den Heuvel et al., Citation1999).
Conclusions
The present study sought to demonstrate that pesticides that are indiscriminately used likely could pose a potential risk for myelotoxicity. Furthermore, the results here suggest the in vitro model tested to evaluate myelotoxic potentials might be translatable to pre-clinical settings.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
This work was supported by the International Institute of Biotechnology and Toxicology. The authors thank the management of the International Institute of Biotechnology and Toxicology for funding and supporting the research, and staff of Ranga Nursing Home for a continuous supply of cord blood samples. This work would have not been possible without the moral support from Dr Srivatsa Prakhya; special thanks to him.
References
- Bradley, T. R., and Metcalf, D. 1966. The growth of mouse bone marrow cells in vitro. Aus. J. Exp. Biol. Med. Sci. 44:287–300
- Clarke, E., and Atkinson, E. 2010. Predicting drug-induced myelotoxicity CFC assays using primary bone marrow cells show utility in toxicity screening. Genetic Engineer. Biotech. News 30:4
- Clarke, E., Pereira, C., Chaney, R., et al. 2007. Toxicity testing using hematopoietic stem cell assays. Regen. Med. 2:947–956
- Corsini, E., Birindelli, S., Fustinoni, S., et al. 2005. Immunomodulatory effects of the fungicide mancozeb in agricultural workers. Toxicol. Appl. Pharmacol. 208:178–185
- Cox, R. H. 1986. Three-month dietary toxicity study in dogs with mancozeb. Unpublished report No. HLA 417-416 from Hazleton Labs., Vienna, VA. Submitted to WHO by Rohm and Haas Company, Spring House, PA (as Rohm and Haas Report No. 86RC-7)
- Del Prado-Lu, J. L. 2007. Pesticide exposure, risk factors and health problems among cutflower farmers: A cross-sectional study. J. Occup. Med. Toxicol. 2:9
- Deldar, A., and Parchment, R. E. 1997. Pre-clinical risk assessment for hematotoxicity: Animal models and “in vitro” systems. In: Comprehensive Toxicology, (Sipes, I. G., McQueen, C. A., and Gandolfi, A. J., Eds.). New York: Pergamon Press, pp. 303–320
- Demur, C., Métais, B., Canlet, C., et al. 2013. Dietary exposure to low dose of pesticides alone or as a mixture: The biological metabolic fingerprint and impact on hematopoiesis. Toxicology 308:74–87
- Dennis, D. W. 1993. Human health effects of agrichemical use. Human Pathol. 24:571–576
- Desi, I., Dobronyi, L., and Varga, L. 1986. Immuno-, neuro-, and general toxicologic animal studies on a synthetic pyrethroid: Cypermethrin. Ecotoxicol. Environ. Safety 12:220–232
- Du, D., Donna, A., Volpe, C., et al. 1990. Effects of L-phenylalaninemustard and L-buthionine sulfoximine on murine and human hematopoietic cells in vitro. Cancer Res. 50:4038–4043
- Ficheux, A. S., Sibiril, Y., Le Garrec, R., and Parent-Massin, D. 2012. In vitro myelotoxicity assessment of the emerging mycotoxins Beauvericin, Enniatin b and Moniliformin on human hematopoietic progenitors. Toxicon 59:182–191
- Fritschi, L., Benke, G., Hughes, A. M., et al. 2005. Occupational exposure to pesticides and risk of non-Hodgkin's lymphoma. Am. J. Epidemiol. 162:849–857
- Gribaldo, L., Bueren, J., Deldar, A., et al. 1996. The use of “in vitro” systems for evaluating haematotoxicity. Report of recommendations of ECVAM Workshop 14. ATLA 24:211–231
- Gribaldo, L., Casati, S., Figliuzzi, L., and Marafante, E. 1998a. In vitro myelotoxicity of environmental contaminants. Environ. Toxicol. Pharmacol. 6:135–141
- Gribaldo, L., Piccirillo, M., Casati, S., et al. 1998b. Drug sensitivity of Granulocyte-Macrophage precursors (GM-CFU) from fresh murine bone marrow and from long-term bone marrow cultures. Toxicol. In Vitro 12:39–45
- Hasan, H. O. 2010. Fungicides and Their Effects on Animals, Fungicides, (Carisse, O., Ed.). ISBN: 978–953–307–266–1, US: InTech
- Hayes, W. J., and Laws, E. R. (Eds.). 1990. Handbook of Pesticide Toxicology, Vol. 3, Classes of Pesticides. New York: Academic Press, Inc
- Hong, H. L., Yang, R. S., and Boorman, G. A. 1991. Residual damage to hematopoietic system in mice exposed to a mixture of groundwater contaminants. Toxicol. Lett. 57:101–111
- Hoshizaki, H., Niki, Y., Tajima, H., et al. 1969. A case of leukemia following exposure to insecticides. Acta Hematol. Japan 32:672–677
- Kamal, S. M., Khan, A., Rizvi, F., et al. 2007. Effect of cypermethrin on clinico-hematological parameters in rabbits. Pakistan Vet. J. 27:171–175
- Landgren, O., Kyle, R. A., Hoppin, J. A., et al. 2009. Pesticide exposure and risk of monoclonal gammopathy of undetermined significance in the Agricultural Health Study. Blood 113:6386–6391
- Leng, G., and Gries, W. 1999. Methods in Biotechnology. In: Pesticide Protocols, Vol. 19 ( Martínez Vidal, J. L., and Garrido Frenich, A., Eds.). Totowa, NJ: Humana Press Inc
- Liu, P., Song, X., Yuan, W., et al. 2006. Effects of cypermethrin and methyl parathion mixtures on hormone levels and immune functions in Wistar rats. Arch. Toxicol. 80:449–457
- Loge, J. P. 1965. Aplastic anemia following exposure to benzene hexachloride (lindane). JAMA 193:110–114
- Lorgue, G., and Lechenet, J. (Eds.). 1996. Clinical Veterinary Toxicology. London: Blackwell Science, pp. 5–194
- Lu, D., Wang, D., Feng, C., et al. 2013. Urinary concentrations of metabolites of pyrethroid insecticides in textile workers, Eastern China. Environ. Int. 60:137–144
- Merhi, M., Raynal, H., Cahuzac, E., et al. 2007. Occupational exposure to pesticides and risk of hematopoietic cancers: Meta-analysis of case-control studies. Cancer Causes Control. 18:1209–1226
- Metcalf, D. 1984. The basic biology of hematopoiesis. In: Haematopoietic Colony Stimulating Factors, (Metcalf, D., Ed.). Amsterdam: Elsevier, pp. 1–26
- Myoung-yun, P., and Cheong, A. 2004. Effects of subacute oral administration of mancozeb on immunopathological parameters and splenocyte proliferation in mice. J. Environ. Toxicol. 19:367–373
- Nair, R. R., Abraham, M. J., Nair, N. D., et al. 2010. Hematological and biochemical profile in sublethal toxicity of cypermethrin in rats. Int. J. Biol. Med. Res. 1:211–214
- Negro, G. D., Bonato, M., and Gribaldo, L. 2001. In vitro bone marrow granulocyte-macrophage progenitor cultures in the assessment of hematotoxic potential of the new drugs. Cell. Biol. Toxicol. 17:95–105
- Noble, C., and Sina, J. F. 1993. Usefulness of the in vitro bone marrow colony-forming assay in cellular toxicology. Toxicol. In Vitro 6:187–195
- Orsi, L., Delabre, D., Monnereau, A., et al. 2009. Occupational exposure to pesticides and lymphoid neoplasms among men: Results of a French case-control study. Occup. Environ. Med. 66:291–298
- Parchment, R. E., Huang, M., and Erickson-Miller, C. L. 1993. Roles for in vitro myelotoxicity tests in pre-clinical drug development and clinical trial planning. Toxicol. Pathol. 21:241–250
- Parent-Massin, D. 2001. Relevance of clonogenic assays in hematotoxicology. Cell. Biol. Toxicol. 17:87–94
- Parent-Massin, D., and Thouvenot, D. 1993. In vitro study of pesticide hematotoxicity in human and rat progenitors. J. Pharmacol. Toxicol. Meth. 30:203–207
- Parent-Massin, D., Hymery, N., and Sibiril, Y. 2010. Stem cells in myelotoxicity. Toxicology 267:112–117
- Pessina, A., Albella, B., Bayo, M., et al. 2002. In vitro tests for hematotoxicity: Prediction of drug-induced myelosuppression by the CFU-GM assay. ATLA 30:75–79
- Pessina, A., Albella, B., Bayo, M., et al. 2003. Application of the CFU-GM assay to predict acute drug-induced neutropenia: An international blind trial to validate a prediction model for the Maximum Tolerated Dose (MTD) of myelosuppressive xenobiotics. Toxicol. Sci. 75:355–367
- Pessina, A., Albella, B., Bueren, J., et al. 2001. Pre-validation of a model for predicting acute neutropenia by colony forming unit granulocyte/macrophage (CFU–GM) assay. Toxicol. In Vitro 15:729–740
- Pessina, A., Bonomi, A., Cavicchini, L., et al. 2010. Pre-validation of the rat CFU-GM assay for in vitro toxicology applications. Altern. Lab. Anim. 38:105–117
- Reed, L. J., and Muench, H. A. 1938. A simple method of estimating fifty percent endpoints. Am. J. Hyg. 27:493–497
- Rudant, J., Menegaux, F., Leverger, G., et al. 2007. Household exposure to pesticides and risk of childhood hematopoietic malignancies: The ESCALE study (SFCE). Environ. Health Perspect. 115:1787–1793
- Sayim, F., Yavasoglu, N. U., Uyanikgil, Y., et al. 2005. Neurotoxic effects of cypermethrin in Wistar rats: A Hematological, biochemical and histo-pathological study. J. Health Sci. 51:300–307
- USEPA (U.S. Environmental Protection Agency). 1995. List of Chemicals Evaluated for Carcinogenic Potential. Memo from Stephanie Irene to Health Effects Division Branch Chiefs Washington, D.C.: Office of Pesticide Programs. Health Effects Division
- van den Heuvel, R. L., Leppens, H., and Schoeters, G. E. 1999. Lead and catechol hematotoxicity in vitro using human and murine hematopoietic progenitor cells. Cell Biol. Toxicol. 15:101–110
- Vial, T., Nicolas, B., and Descotes, J. 1996. Clinical immunotoxicity of pesticides. J. Toxicol. Environ. Health 48:215–229
- Wael, M. A. 2012. Hematological and biochemical effects of metalaxyl fungicide on albino mice. Am. J. Biochem. 2:62–66
- WHO (World Health Organization). 1989. International Program on Chemical Safety: WHO Task Group Meeting on Environmental Health Criteria for Cypermethrin. Cypermethrin. Published under joint sponsorship of United Nations Environment Program, the International Labor Organization, and World Health Organization. Environ. Health Criteria 82. Geneva: WHO
- Yousef, M. I., El-Demerdasha, F. M., Kamelb, K. I., and Al-Salhena, K. S. 2003. Changes in some hematological and biochemical indices of rabbits induced by isoflavones and cyper-methrin. Toxicology 189:223–234