Abstract
Hemiscorpius lepturus (H. lepturus), one of the most venomous scorpions in tropical and sub-tropical areas, belongs to the Hemiscorpiidae family. Studies of antibodies in sera against the protein component of the venom from this organism can be of great use for the development of engineered variants of proteins for eventual use in the diagnosis/treatment of, and prevention of reactions to, stings. In the present in vitro study, the proteins of H. lepturus venom, which could specifically activate the production of immunoglobulin G (IgG) in victims accidently exposed to the venom from this scorpion, were evaluated and their cross-reactivity with venoms from two other important scorpion species including Androctonus crassicauda and Mesobuthus eupeus assessed. H. lepturus venom was analyzed with respect to its protein composition and its antigenic properties against antibodies found in sera collected from victims exposed to the venom of this scorpion within a previous 2-month period. The cross-reactivity of the H. lepturus venom with those from A. crassicauda and M. eupeus was assessed using ELISA and immunoblotting. Electrophoretic analysis of the venom of H. lepturus revealed several protein bands with weights of 8–116 KDa. The most frequent IgG-reactive bands in the test sera had weights of 34, 50, and 116 kDa. A weak cross-reactivity H. lepturus of venom with venoms from A. crassicauda and M. eupeus was detected. The results of immunoblotting and ELISA experiments revealed that H. lepturus venom activated the host immune response, leading to the production of a high titer of antibodies. Clearly, a determination of the major immunogenic components of H. lepturus venom could be valuable for future studies and ultimately of great importance for the potential production of recombinant or hypo-venom variants of these proteins.
Introduction
Scorpion stings are a serious medical, social, and economic problem in many parts of the world, particularly in tropical and sub-tropical countries (Jalali et al., Citation2010; Khodadadi et al., Citation2012; Pipelzadeh et al., Citation2007). Scorpions are venomous arthropods in the class Arachnida and order Scorpiones. In Iran, 25 species of scorpions have been identified (Seyedian et al., Citation2010). One species that ranks high in terms of medical importance in Iran is Hemiscorpiidae lepturus; scorpions in the Buthidae family that are of great concern are in the genera Androctonus, Buthus, Mesobuthus, Buthotus, Parabuthus, and Leirus (Petricevich, Citation2010), and include in particular Androctonus crassicauda and Mesobuthus eupeus (Jalali et al., Citation2010; Pipelzadeh et al., Citation2007).
The usual clinical manifestations of envenomation by H. lepturus include dermo-necrotic reactions, anemia, hemolysis, renal failure, and cardiovascular and central nervous system disorders (Pipelzadeh et al., Citation2007). In the past, owing to technical difficulties and low venom production following milking of captured H. lepturus, the polyvalent end-product prepared by medical institutions did not contain antibodies against the venom from this specific scorpion, and there was widespread skepticism among clinicians about its effectiveness in treating stung patients. Recent attempts appear to have overcome this limitation, i.e. the current Fab2 anti-venom prepared in horse sera appears to contain anti-venom against this scorpion (according to the manufacturer’s pamphlet).
While there has been limited success in the development of sera anti-venoms against scorpion stings, improvements in the purity/safety of anti-venom and identification of clinically important toxic fractions of specific venoms that can be utilized as immunogens for production of efficient therapeutic antibodies are continuously being made with respect to envenomation treatment (Theakston et al., Citation2003). With regard to the latter aspect, previous studies have been performed in animal models including mice, rats, rabbits, or horses (Borchani et al., Citation2013; Heidarpour et al., Citation2012; Jalali et al., Citation2012; Mendes et al., Citation2008; Shahbazzadeh et al., Citation2007). However, to date, no studies have been conducted to characterize scorpion toxin immunogenicity using human serum. Further, identification of cross-reactivity among anti-venoms can save both time and cost for the preparation/administration of anti-venoms. Some previous studies have shown cross-reactivity occurs between venoms from different species of scorpions (and even between the venoms of various species of same family of scorpions) (D’Suze et al., Citation2007; Nishikawa et al., Citation1994; Riano-Umbarila et al., Citation2011).
The present study was undertaken to investigate the antibody that was formed against. H. lepturus venom component(s) in envenomed patients. Investigation of the existence of antibodies in sera against the protein portion of the venom will be a great aid in the development of recombinant forms of proteins or hypo-venoms (venomoids, i.e. proteins with reduced toxicity) for the purposes of treatments (production of anti-venoms), prevention (vaccine), and diagnosis of this specific envenomation. In addition, in the present in vitro study, the proteins of H. lepturus venom that could specifically activate production of immunoglobulin G (IgG) in victims exposed to the venom were evaluated and assessed for IgG cross-reactivity with venom from two other important scorpion species, i.e. A. crassicauda and M. eupeus.
Materials and methods
Patients
Twenty patients (nine men, 11 women; mean age 22.7 ± 14.2 years) who each had been stung by H. Lepturus within a period 2 months prior to enrolling in this study were evaluated. Each subject had visited Razi Hospital (Ahvaz Jundishapur University of Medical Sciences) after the bite, been hospitalized for 2–4 days, and received an intra-venous anti-venom treatment (based on current Iranian protocol, i.e., two vials of 5 ml multivalent anti-venom for adult of ≈70 kg body weight). Five non-envenomed healthy subjects donated blood to provide negative control serum. Written consent was obtained from all participants before enrollment. The Human Ethics Committee of Ahvaz Jundishapur University approved the study protocol.
Preparation of venoms
Venoms from H. lepturus, A. crassicauda, and M. eupeus were obtained by electrical stimulation of captured scorpions as previously reported (Dent et al., Citation1980). Each extracted venom was lyophilized and stored in the dark at −70 °C for later use in the current studies.
SDS-PAGE and IgG-immunoblotting
The protein content in each scorpion venom sample was measured using the Bradford (Citation1976) method. Separation/characterization of proteins in each venom was done using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) over 12.5% acrylamide separation gels [under reducing and non-reducing conditions]. Molecular masses of bands in each gel were estimated using Image Lab Analysis Software (Bio-Rad, Hercules, CA) and comparisons against protein markers of known molecular weights (Amersham Low MW Calibration Kit; GE Healthcare, Little Chalfont, UK). Resolved proteins were then electrotransferred to polyvinylidene difluoride (PVDF) membranes (GE Healthcare). After washing and blocking, membranes were incubated with a serum pooled or individual sera from patients envenomed by H. lepturus or with control sera (1:5 dilutions in phosphate-buffered saline [PBS, pH 7.4]) for 3 h at room temperature. After gentle washing of the membrane with PBS to remove unbound antibodies/sera, the membranes were placed in a solution containing biotinylated anti-human IgG (Nordic-Mubio, Susteren, the Netherlands; 1:14,000 [v/v] in 1% bovine serum albumin [BSA] solution) and incubated for 2 h at room temperature. Unbound antibodies were then removed by gentle washing with PBS before the membrane was placed in a solution containing horseradish peroxidase (HRP)-streptavidin (Sigma, St. Louis, MO) for 1 h at room temperature. The bound horseradish peroxidase was, in turn, detected through the use of high-sensitivity liquid diaminobenzidine (Liquid DAB+) chromogen (DAKO, Produktionsvej, Denmark). The presence of the signal was then documented using ChemiDoc™ XRS+ (Bio-Rad, Hercules, CA).
Measures of specific IgG to H. lepturus venom proteins (ELISA)
To evaluate levels of specific IgG to H. lepturus venom proteins in envenomed patients, an indirect ELISA was utilized. In brief, wells of an ELISA microplate (Nunc A/S, Roskilde, Denmark) were coated with 120 µl H. lepturus venom (at 100 µg/well in coating buffer [15 mM Na2CO3, 35 mM NaHCO3, pH 9.6]) for 16 h at 4 °C. After blocking with 140 µl of PBS-2% BSA solution for 1 h at 37 °C, the plates were incubated with 100 µl patient sera for 3 h at room temperature [with gentle shacking]. Each well then received 100 µl goat biotinylated anti-human IgG (Nordic-Mubio; diluted 1:12,000 in PBS-1% BSA) solution and was incubated at room temperature for 2 h. The wells were then gently washed 5-times with T-PBS (PBS containing 0.05% Tween 20) to remove unbound anti-IgG and then each received 100 µl HRP-streptavidin (diluted 1:16,000 in PBS- 1% BSA) before the plate was incubated for 1 h at room temperature. After five washes with T-PBS, each well received 100 µl tetramethylbenzidine (TMB-H2O2; Sigma-Aldrich, St. Louis, MO) substrate solution and the plate was incubated at room temperature for 20 min before the reaction was stopped by addition of 100 µl of 3 M HCl. Thereafter, the absorbance in each well was assessed at 450 nm using a Sunrise™ microplate reader (TECAN, Mannedorf, Switzerland). All results were expressed as optical density (OD) units. Based on the mean value of five negative controls (i.e. < 0.1 OD units), any OD value greater than 4-times the median value from the negative controls was considered indicative of a positive sample.
ELISA and immunoblotting inhibition assays
To assess cross reactivity of H. lepturus venom with venoms from M. eupeus and A. crassicauda, an ELISA inhibition assay was carried. In brief, 50 µl of pooled serum from six envenomed patients (Patients 1, 3, 6, 7, 9, and 11) who had IgG antibodies to H. lepturus was pre-incubated with 50 µl of different concentrations of venom from H. lepturus, A. crassicauda, or M. eupeus (at 0.01, 0.1, 1, 10, 100, 1000 µg/ml) for 3 h at room temperature. The solution was then placed in a flat-bottomed microtiter plate that had been coated with H. lepturus venom (100 µg/well). The ELISA procedure used thereafter was the same as that described above for measures of H. lepturus specific IgG.
An immunoblotting inhibition assay was also performed (as described above). In brief, electrophoretically-resolved H. lepturus proteins were transferred to a PVDF membrane. After blocking membrane, the membranes strips were incubated for 2 h at room temperature with a solution containing 100 µl of pooled sera (from patients 1, 3, 6, 7, 9, and 11) that had been pre-incubated with an equal volume of H. lepturus, A. crassicauda, or M. eupeus for 3 h (each at 100 µg/ml as inhibitor). Pooled sera that had not undergone neutralization were used as negative control. Each membrane was then washed 3-times with PBS, and then incubated with biotin-labeled anti-human IgG (Nordic-Mubio; 1:14,000 [v/v] in PBS) for 2 h at room temperature, then incubated with HRP-streptavidin (1:16,000 [v/v] in PBS) for 1 h at room temperature. Bound HRP was detected using DAB and documented with GELDoc™ XRS+.
Statistics
All the data was analyzed by SPSS Version 18.0 software (Chicago, IL). A chi-square test was used to compare the relationship between variables. p values less than 0.05 was considered significant.
Results
Protein profiles of venoms from H. lepturus, A. crassicauda, and M. eupeus scorpions
The analysis of venom from H. lepturus revealed several protein bands with molecular weights (MW) in the range of ≈8–116 kDa (); the predominant proteins had apparent MWs of 8, 10, 15, 34, 39, 50, 68, and 100 kDa. The A. crassicauda venom yielded at least 10 bands with MWs in the range of 9–116 kDa; the majority of these had MWs >35 or < 10 kDa. M. eupeus venom yielded several protein bands with MWs in the range of ≈10–117 kDa. Compared to H. lepturus venom, there were more bands with greater intensity and exhibiting MWs >37 or <14 kDa.
Figure 1. Coomassie-stained gel containing SDS-PAGE resolved proteins of crude venoms from H. lepturus, A. crassicauda, and M. eupeus under reducing and non-reducing conditions. Lane MW = molecular weight markers; Lane HL = H. lepturus; Lane AC = A. crassicauda; Lane ME = M. eupeus.
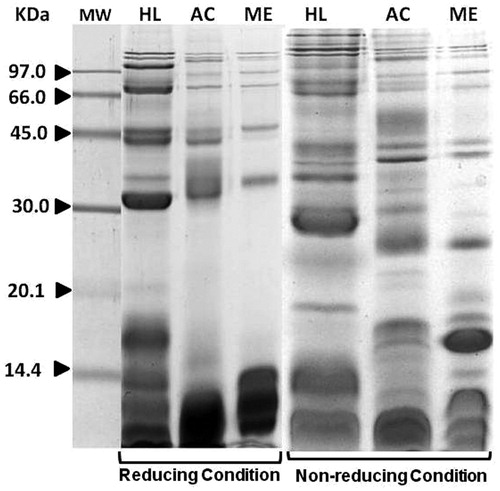
The results obtained also showed that overall migration patterns of H. lepturus venom proteins did not change considerably under non-reducing vs reducing conditions (). However, on the non-reduced gels, intensities of three bands with apparent MWs of 14.5, 20, and 34 kDa were greater than counterparts in the reduced gel. Similar results were also observed in the analyses of A. crassicauda and M. eupeus venoms under non-reducing conditions; however, in contrast to H. lepturus venom, several bands with MWs of 14–30 KDa now appeared that were not apparent under reducing conditions.
IgG-binding profile of H. lepturus venom using sera from envenomed patients
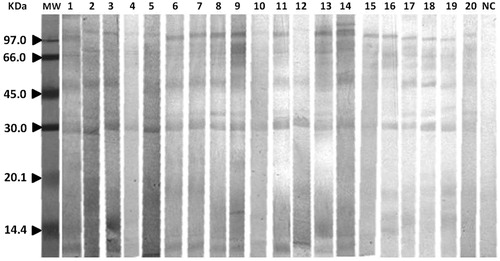
The levels of specific IgG against H. lepturus venom were determined in sera from 20 patients (). Each patient had significantly elevated levels of specific IgG against H. lepturus venom as indicated by OD450 values that were >0.3 (i.e. ≥4-fold higher than median value for negative controls). Thus, these samples were considered capable of binding.
Table 1. Demographic characteristics, extent of IgG antibody production, and degree of presence of hematuria among recruited H. lepturus stung patients.
The IgG reactivity with the H. lepturus venom protein bands separated by electrophoresis was determined by immunoblot assay. The specific IgG-binding fractions probed using the sera from all 20 envenomed patients are shown in . The results indicated there were several IgG-reactive bands with MWs of ≈30–116 kDa. also presents the apparent MW of each protein fraction and the prevalence of each among all the envenomed patients. The most frequent IgG-reactive bands in the patients’ sera were ≈34, 50, and 116 kDa. However, other IgG-reactive bands with MWs of 39 and 10 kDa were also noted in these sera. Control serum gave rise to no bands.
ELISA and immunoblotting inhibition assay
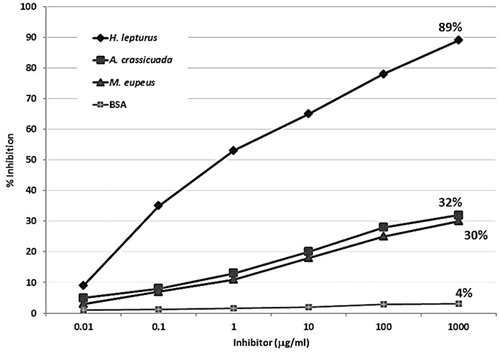
To investigate cross-reactivity of H. lepturus venom with A. crassicauda and M. eupeus venoms, an ELISA inhibition assay was conducted (). Almost complete inhibition was achieved with 100 µg H. lepturus venom/ml (positive control). Pre-incubation of pooled serum with a high dose (1000 µg/ml) of the venom revealed significant inhibition of IgG binding to all the protein components of H. lepturus venom. However, when A. crassicauda or M. eupeus venoms were used as inhibitors, poor inhibition of IgG reactivity to H. lepturus venom was noted as inhibitors (32 and 30%, respectively). In general, the ELISA results were similar to those of immunoblot inhibition assay.
Figure 3. Inhibition of IgG-binding to H. lepturus venom by ELISA using venoms from A. crassicauda and M. eupeus scorpions. Control experiments were performed using only BSA.
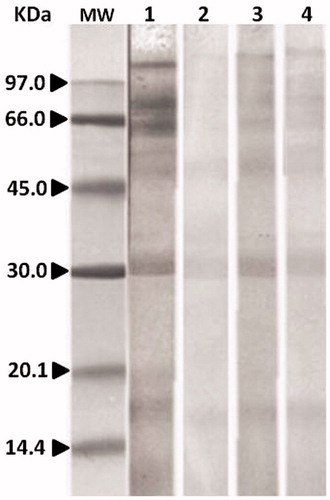
To evaluate IgG cross-reactivity between H. lepturus and A. crassicauda or M. eupeus venom, immunoblotting inhibition assays were carried out with H. lepturus venom as the solid phase. As shown in , IgG reactivity to most of the protein components of H. lepturus venom was inhibited when H. lepturus venom was used as an inhibitor (negative control, lane 2). Further, complete inhibition of IgG binding to the 30, 46, 66, and 116 kDa components of H. lepturus venom was observed when the sera samples were pre-incubated with 200 µg H. lepturus venom/ml. However, only partial inhibition of IgG reactivity to H. lepturus venom proteins was detected when A. crassicauda/M. eupeus venoms were used as inhibitors (32 and 30%, respectively).
Figure 4. Immunoblotting inhibition assays. Lane MW = molecular weight marker. Lane 1: H. lepturus venom incubated with pooled serum without inhibitor (negative control). Lane 2: H. lepturus venom incubated with pooled serum (from patients 1, 3, 6, 7, 9, and 11) containing 100 µg/ml of H. lepturus venom as inhibitor (positive control). Lane 3: H. lepturus venom incubated with pooled serum containing 100 µg/ml M. eupeus as inhibitor. Lane 4: H. lepturus venom incubated with pooled serum containing 100 µg/ml A. crassicauda as inhibitor.
Discussion
Despite the recent developments in the production of available polyvalent anti-venom, epidemiological findings have shown that the mortality rate among H. lepturus victims has not significantly changed (Pipelzadeh et al., Citation2007). Furthermore, there is still a lack of general consensus on the effectiveness of anti-venom in the treatment of scorpion sting envenomation, particularly those caused by H. lepturus scorpion. This may be attributed, among several factors, to late referral (because of painlessness), low immunogenic potency (Hmila et al., Citation2012; Jalali et al., Citation2012), or low distribution kinetics (Krifi et al., Citation2005; Seyedian et al., Citation2010). In the present in vitro study, the protein profiles of the venoms from H. lepturus, A. crassicauda, and M. eupeus scorpions were compared under both reducing and non-reducing conditions. The capacity of the envenomed patients to produce antibodies against H. lepturus scorpion venom components was assessed using ELISA and Western blotting. Lastly, IgG cross-reactivity against H. lepturus, A. crassicauda, and M. eupeus venoms was evaluated.
The results showed that the dominant protein bands had apparent molecular weights of 8, 10, 15, 34, 39, 50, 68, and 100 kDa. Among these bands, the protein bands of 34, 50, and 116 kDa were the major components that elicited immune responses in the human subjects. These findings were somewhat similar to those in a previous study of polyvalent anti-venom produced in horse sera (Seyedian et al., Citation2010), suggesting that the immune responses in both humans and horses yielded similar IgG antibodies towards these immunogenic bands. A recent study by Borchani et al. (Citation2011a, Citationb) demonstrated that a protein component with a weight of 34 kDa (named heminecrolysin) was a specific toxic component that provoked production of an antigen with strong immunogenic potential against hemolysis and dermo-necrosis. The findings in the current study suggest it is possible to produce more specific potent anti-venom against the toxin from the H. lepturus scorpion.
In the present study, an indirect ELISA was developed. The results obtained were comparable to those of the immunoblotting experiments, which confirmed that the IgG antibodies produced in human subjects were specific against the venom from H. lepturus scorpion. To date, no studies have compared the protein components of the venoms from key scorpion species using human sera. In the present study, these protein components were evaluated under both reducing and non-reducing conditions. The SDS-PAGE results indicated that the pattern of migration of the protein components of the H. lepturus venom was partially changed by the reducing conditions. Unlike H. lepturus, however, the pattern of migration of the venoms from A. crassicauda and M. eupeus were significantly changed by these conditions. This was likely due in great part to the presence of inter-/intra-chain disulfide bonds. The SDS-PAGE results in the current study also showed that treatment of venoms from the selected scorpions with 2-mercaptoethanol could have led to a change in epitope availability for the recognition by antibodies.
Not only did the intensity (i.e. quantity) of protein components in the three venoms vary, but their capacity to bind to and inhibit the IgG antibodies in the sera of our human subjects (i.e. quality) did as well. Moreover, the results also showed that the H. lepturus venom had an immunogenic potential and provoked IgG production against components mainly with weights of 30–116 kDa (primarily the 34, 50, and 116 kDa forms). There was weak cross-reactivity between the venoms from A. crassicauda and M. eupeus and the IgG antibodies in the sera of H. lepturus-envenomed patients. This was an expected observation because, phylogenetically, while H. lepturus belongs to the family Hemiscorpiidae and both A. crassicauda and M. eupeus belong to the family Buthidae, they each belong to the common order scorpions. However, a recent in vitro study demonstrated that toxic effects of these scorpions differed (Khodadadi et al., Citation2012).
Unlike the venoms from A. crassicauda and M. eupeus, that from H. lepturus has been reported to produce dose-dependent lysis of human red blood cells (RBC) and evince phospholipase activity (Petricevich, Citation2010; Pipelzadeh et al., Citation2006). Thus, not only do the protein components in the venoms from these three scorpion species have different immunogenic profiles and different protein compositions, they also induce differing toxicities.
Conclusions
The results in this study indicated the possibility of human production of antibodies against certain protein components of H. lepturus venom, especially those with molecular weights of 34, 50, and 116 KDa. Still, further research is needed to evaluate the effectiveness of these antibodies (mostly IgG type) in neutralizing the toxicity of the venom. In addition, the nature of these immunogenic proteins has to be better clarified and the probability of cross-reactivity against other venoms assessed. Ultimately, this information will be of great use in the ultimate production of engineered recombinant or hypo-venom variants of these venomoid proteins.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. Financial support was provided by the Toxicology Research Center at the Ahvaz Jundishapur University of Medical Sciences (Grant # TRC-9205).
Notice of Correction
The version of this article published online ahead of print on 20 June 2014 contained an error in the affiliation details. Dr Babak Vazirianzadeh should have been affiliated with the “Department of Medical Entomology and Vector Control, School of Public Health, Ahwaz Jundishapur University of Medical Sciences, Ahvaz, Iran,” and not the “Department of Mycoparasitology, Ahwaz Jundishapur University of Medical Sciences, Ahvaz, Iran”. The error has been corrected for this version
Acknowledgements
This article is derived from the thesis of Ms Khanbashi.
References
- Borchani, L., Sassi, A., Ben Gharsa, H., et al. 2013. Pathological effects of heminecrolysin, a dermo-necrotic toxin from Hemiscorpius lepturus scorpion venom are mediated through its lysophospholipase D activity. Toxicon. 68:30–39
- Borchani, L., Sassi, A., Ben Yekhlef, R., et al. 2011a. Heminecrolysin, a potential immunogen for monospecific anti-venom production against Hemiscorpius lepturus scorpion. Toxicon. 58:681–688
- Borchani, L., Sassi, A., Shahbazzadeh, D., et al. 2011b. Heminecrolysin, the first hemolytic dermo-necrotic toxin purified from scorpion venom. Toxicon. 58:130–139
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254
- D’Ssuze, G., Moncada, S., Gonzalez, C., et al. 2007. Antigenic cross-reactivity between sixteen venoms from scorpions belonging to six genera. Clin. Toxicol. (Philadelphia). 45:158–163
- Dent, M. A., Possani, L. D., Ramirez, G. A., and Fletcher, P. L Jr. 1980. Purification and characterization of two mammalian toxins from the venom of the Mexican scorpion Centruroides noxius Hoffmann. Toxicon. 18:343–350
- Heidarpour, M., Ennaifer, E., Ahari, H., et al. 2012. Histopathological changes induced by Hemiscorpius lepturus scorpion venom in mice. Toxicon. 59:373–378
- Hmila, I., Cosyns, B., Tounsi, H., et al. 2012. Pre-clinical studies of toxin-specific nanobodies: Evidence of in vivo efficacy to prevent fatal disturbances provoked by scorpion envenoming. Toxicol. Appl. Pharmacol. 264:222–231
- Jalali, A., Bavarsad-Omidian, N., Babaei, M., et al. 2012. The pharmaco-kinetics of Hemiscorpius lepturus scorpion venom and Razi anti-venom following intra-muscular administration in rat. J. Venom Res. 3:1–6
- Jalali, A., Pipelzadeh, M. H., Sayedian, R., and Rowan, E. G. 2010. A review of epidemiological, clinical and in vitro physiological studies of envenomation by the scorpion Hemiscorpius lepturus (Hemiscorpiidae) in Iran. Toxicon. 55:173–179
- Khodadadi, A., Pipelzadeh, M. H., Vazirianzadeh, B., et al. 2012. An in vitro comparative study upon the toxic properties of the venoms from Hemiscorpius lepturus, Androctonus crassicauda, and Mesobuthus eupeus scorpions. Toxicon. 60:385–390
- Krifi, M. N., Savin, S., Debray, M., et al. 2005. Pharmacokinetic studies of scorpion venom before and after anti-venom immunotherapy. Toxicon. 45:187–198
- Mendes, T. M., Dias, F., Horta, C. C., et al. 2008. Effective Tityus serrulatus anti-venom produced using Ts1 component. Toxicon. 52:787–793
- Nishikawa, A. K., Caricati, C. P., Lima, M. L., et al. 1994. Antigenic cross-reactivity among venoms from several species of Brazilian scorpions. Toxicon. 32:989–998
- Petricevich, V. L. 2010. Scorpion venom and the inflammatory response. Med. Inflamm. 2010:903295
- Pipelzadeh, M. H., Jalali, A., Taraz, M., et al. 2007. An epidemiological and a clinical study on scorpionism by the Iranian scorpion Hemiscorpius lepturus. Toxicon. 50:984–992
- Pipelzadeh, M. H., Dezfulian, A. R., Jalali, M. T., and Mansouri, A. K. 2006. In vitro and in vivo studies on some toxic effects of the venom from Hemiscorpious lepturus scorpion. Toxicon. 48:93–103
- Riano-Umbarila, L., Contreras-Ferrat, G., Olamendi-Portugal, T., et al. 2011. Exploiting cross-reactivity to neutralize two different scorpion venoms with one single chain antibody fragment. J. Biol. Chem. 286:6143–6151
- Seyedian, R., Pipelzadeh, M. H., Jalali, A., et al. 2010. Enzymatic analysis of Hemiscorpius lepturus scorpion venom using zymography and venom-specific anti-venin. Toxicon 56:521–525
- Shahbazzadeh, D., Srairi-Abid, N., Feng, W., et al. 2007. Hemicalcin, a new toxin from the Iranian scorpion Hemiscorpius lepturus which is active on ryanodine-sensitive Ca2+ channels. Biochem. J. 404:89–96
- Theakston, R. D., Warrell, D. A., and Griffiths, E. 2003. Report of a WHO workshop on the standardization and control of anti-venoms. Toxicon. 41:541–557