Abstract
The physiological functions of transforming growth factor (TGF)-β in cell signaling include regulation of developmental processes and cell growth. Tumor cells very often display altered regulation of the TGFβ signaling pathway, either by defects in TGFβ itself or in downstream components of the pathway. TGFβ can play a dual role in tumorigenesis, i.e. it can be either tumor-suppressive or tumor-promoting. TGFβ suppresses the growth of tumor cells; however, in advanced tumors, it is associated with induction of progression, resulting in poor prognosis for patients. The TGFβ negative regulation of cytotoxic cell function, together with the promotion of T-regulatory cell maturation, impairs anti-tumor responses. Recent studies have elucidated new roles for TGFβ signaling in the tumor microenvironment. Abrogation of proper signaling induces epithelial-to-mesenchymal transition with pro-metastatic functions, resulting in cancer progression. Thus, TGFβ signaling in the tumor microenvironment plays an important role in tumor initiation, progression, and metastasis by its capacity to regulate cross-talk between tumor cells and other components of the local environment.
Introduction
The malignant changes in healthy cells are sustained and accompanied by alteration of stromal cells and related fibrous structures, forming together the tumor stroma. The tumor cells together with the surrounding immune cells, cancer-associated fibroblasts (CAF), extracellular matrix (ECM) components, blood and lymphatic vessels, and nerves constitute the tumor microenvironment (TM). Many studies proved that the stromal component of TM plays not only a supportive but also a crucial role in cancer development: its components have the capacity to influence and even to deregulate the signaling pathways and interactions between normal and transformed cells in a continuous cross-talk. During embryogenesis, interactions between epithelial and mesenchymal cells in their local environment are essential for the development of tissues and the whole organism. However, in cancer, signaling pathways regulating these interactions are very often deregulated.
Transforming growth factor (TGF)-β, interacting in the TM, is considered as a critical regulator of tumor initiation and progression. TGFβ regulates processes supporting cancer invasiveness, regulation of immune cells of various types, activation, and chemotaxis of fibroblasts. An important mechanism favoring tumorigenesis is the induction of mesenchymal phenotype in the epithelial tumor cells, commonly known as an epithelial-to-mesenchymal transition (EMT). It was proved that this process is induced by prolonged exposition to TGFβ (Miettinen et al., Citation1994). By this observation, it was suggested that TGFβ plays a dual role in the carcinogenesis. In early phases, TGFβ attenuates proliferation of the tumor cells by activation of growth arrest and apoptosis, but, in advanced tumors, TGFβ activates EMT promoting tumor cells to be more aggressive and prone to achieve metastatic phenotype (Thiery et al., Citation2009). TGFβ also suppresses immune response of non-malignant cells and immune cells against cancer through its impact on their differentiation, proliferation, and survival (Li et al., Citation2006). It promotes angiogenesis and recruits immune cells producing cytokines that stimulate tumor progression (Turner et al., Citation1990; Wiseman et al., Citation1988). There are various experimental studies describing the role of TGFβ in initiation of cancer, but more precise investigations of its functions in the TM are still needed. The aim of this review is to address the role of TGFβ signaling in the regulation of TM and, particularly, how it contributes to the progression of cancer. Understanding the critical roles of TGFβ within the TM may provide new targets for design of therapeutics against cancer.
Basic principles of TGFβ signaling
We distinguish three TGFβ molecules: TGFβ1, TGFβ2, and TGFβ3. They are secreted as inactive homodimers and belong to the TGFβ protein superfamily. This includes 33 members in humans, such as activins, inhibins, bone morphogenic protein (BMP), growth and differentiation factors (GDF), glial cell line-derived neurotrophic factor (GDNF), and the above-mentioned TGFβ protein members (Massague, Citation2012; Piek et al., Citation1999). The most abundant isoform is TGFβ1, a 44-kDa protein, coded by TGFB1 gene located at chromosome 19 (chromosome 7 in mice). It is ubiquitously expressed in all tissues (Derynck et al., Citation1985). TGFβ2 is a 48-kDa protein coded by TGFB2 gene located at chromosome 1. TGFB2 is expressed in neurons and astroglial cells of embryonic tissues (Flanders et al., Citation1991), and it effects the heart, as well as other mesenchymal structures and development. In the adult mouse, it is expressed in almost all tissues, especially in the placenta, the male submaxillary gland and the lung, but not in the liver (Boyer et al., 1999; Miller et al., 1989). The TGFβ2 transcript attenuates T-cell maturation and immune responses in the TM, thus it is supporting tumor growth (Schwyzer & Fontana, Citation1985). TGFβ3 is a 47-kDa protein coded by TGFB3 gene located at chromosome 14 (chromosome 12 in mice). It functions as a regulator of palate development, in which it regulates cellular adhesion and formation of ECM (Proetzel et al., Citation1995), but it is also important for lung development and for wound healing in the skin (Bandyopadhyay et al., Citation2006; Kaartinen et al., Citation1995). TGFB3 is expressed mainly in the umbilical cord (Stewart et al., Citation1996).
TGFβ molecules, deposited in the extracellular matrix (ECM), interact with three TGFβ receptor types (TβRI, TβRII, and TβRIII). All TGFβ ligands differ in their binding affinity to TβRII. Both TGFβ1 and TGFβ3 bind to TβRII. TGFβ2 needs TβRI as a co-interacting partner for high-affinity interaction with TβRII, which binds alone to TβRIII (Derynck & Zhang, Citation2003). TβRI and TβRII are predominantly localized at the cell membrane in a homodimeric conformation. After binding TGFβ, TβRII is activated by autophosphorylation and forms a heterotetrameric complex with TβRI. Thereafter, TβRII transphosphorylates and activates TβRI. This mechanism allows TβRI to phosphorylate its downstream mediators SMAD2 and SMAD3 (Shi & Massague, Citation2003). Two types of TGFβ signaling cascades have been identified (). The canonical one is SMAD-dependent and the non-canonical one is SMAD-independent. In general, the canonical cascade involves phosphorylation of the carboxy-terminal serine residues of the SMAD2 and SMAD3 proteins that are receptor-regulated SMADs (also called receptor-SMADs; R-SMADs). Phosphorylation allows their oligomerization with SMAD4, also known as ‘co-SMAD’. This interaction is necessary for translocation of the complex to the nucleus (Schmierer & Hill, Citation2005) in order to modulate gene transcription. SMAD 7 competitively inhibits SMAD2/3 binding to TβRI (Inoue & Imamura, Citation2008).
Figure 1. Representation of main TGFβ1 downstream pathways related to tumor microenvironment. See also text.
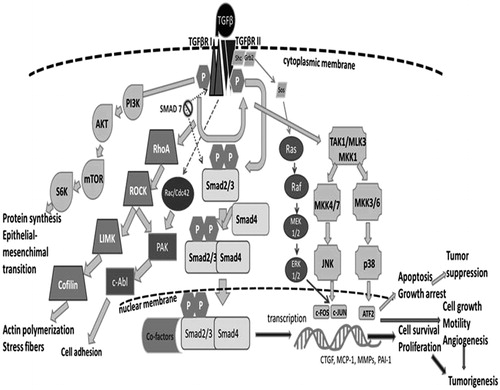
Non-canonical signaling involves activation of PI3K-AKT, RhoA, Rac1, Ras, Cdc42, Daxx, Par6, TAB1/TAK1, and MAPK pathways (Bierie & Moses, Citation2006). These pathways are more complex than the canonical one and involve more intensive cross-talk between them. Among them, the Rho-Rock1 and AKT pathways activated by TGFβ significantly contribute to migratory and invasive cellular phenotypes observed in various types of cancer (Dumont et al., Citation2003). Pleiotropic TGFβ ligands are involved in many other processes; for example, they suppress cell proliferation by repressing CDK4 expression and by activating the expression of CDK inhibitors (Ewen et al., Citation1995; Polyak et al., Citation1994). SMAD-dependent activation of Bcl-2 proteins is important for regulation of programmed cell death (Pardali & Moustakas, Citation2007). In addition, the regulation of cellular adhesions by TGFβ signaling is very important for tumorigenesis, mainly via decreases in E-cadherin and zonula adherens 1 production and through cyto-skeletal re-arrangements (Huber et al., Citation2005). Taken together, the above-mentioned facts highlight the double-edged character of TGFβ signaling.
TGFβ production and activation
TGFβ molecules are primarily synthesized as homodimers, stabilized by disulfide bridges and non-covalent interactions, and undergo intracellular processing before they act in signaling cascades (Dubois et al., Citation1995). First, these pro-proteins are cleaved in trans-Golgi apparatus by furin proteases to release truncated TGFβ dimer and a resting dimeric component called ‘latency-associated protein’ (LAP). Subsequently, LAP interacts with TGFβ to form the ‘small latent complex’ (SLC). Finally, SLC associates with latent TGFβ binding glycoprotein (LTBP) to form a 240 kDa large latent complex (LLC) (Miyazono et al., Citation1988). The LLC is secreted to the ECM network, where it is deposited in an inactive form (Rifkin, Citation2005). The LTBP protein is necessary for storing TGFβ in the ECM and, thus, plays a key role in TGFβ accumulation and release. The LTBP family includes four LTBP isomers (LTBP1–4) structurally similar to fibrillin. Each LTBP contains two types of cysteine-rich domains, i.e. an eight-cysteine domain and epidermal growth factor (EGF)-like repeats (Rifkin, Citation2005). All isomers of LTBP contribute to tumorigenesis in various types of cancer.
Many physiological processes and different factors activating extracellularly-deposited TGFβ from latent complex have been described in vivo so far, e.g. retinoid acid, integrins, matrix metalloproteases (MMP)-2, MMP-9, reactive oxygen species (ROS), irradiation, and thrombospondin-1 (TSP-1) (Barcellos-Hoff & Dix, Citation1996; Munger et al., Citation1999; Schultz-Cherry & Murphy-Ullrich, Citation1993; Yu & Stamenkovic, Citation2000). In addition, TGFβ can be activated by a decrease in the pH in a local environment. For example, an acidic environment is formed in vivo by osteoclasts attached to bone tissue during resorption. It was shown in in vitro experiments that the pH in this site was low enough to activate proteases that, in turn, allowed for the release of latent TGFβ complexes (Oursler, Citation1994). The protease plasmin also has numerous functions in the TGFβ activation cascade in vivo (Lyons et al., Citation1988). Specifically, LAP is proteolytically cleaved by plasmin, with a change of LTBP complex conformation and release of mature TGFβ from the complex. Retinoic acid can also activate latent TGFβ by similar processes (Kojima & Rifkin, Citation1993).
Interestingly, TSP-1 not only has an anti-angiogenic role, but it also appears to play a role in cancer initiation and progression through other mechanisms (Lawler & Detmar, Citation2004). Even mechanical tensions in the ECM can allow release of TGFβ from stored LTBP complex, a mechanism possible under tissue stiffening during chronic inflammation or tumor progression (Wipff et al., Citation2007; Wipff & Hinz, Citation2008). Each of the abovementioned factors interfere with the non-covalent interactions between LAP and mature TGFβ and, via this mechanism, they allow TGFβ to be released from its latent state.
Role of TGFβ in cancer
It is well known that components of the TGFβ signaling cascade are very often deregulated in various types of cancer. As noted above, TGFβ has a dual role in tumorigenesis; it can be a tumor-suppressing or tumor-promoting factor, depending on the stage of tumor development. Tumor suppression is promoted by repressing expression of c-Myc and cyklin-dependent kinase genes (CDKs) and by activating expression of CDK inhibitor genes p15, p21 and p27 (Datto et al., Citation1995; Hannon & Beach, Citation1994; Polyak et al., Citation1994). TGFβ is also able to down-regulate or inhibit expression of CDK4 and CDC25A genes (Iavarone & Massague, Citation1999). The second role of TGFβ, as a cancer promoter, is exerted through an inhibition of apoptosis and/or by a stimulation of proliferation.
Normally, TGFβ acts as a tumor suppressor in mature tissues and is generally produced in the TM. How then is it possible that tumor cells can proliferate in such suppressive environment? Cancer cells have evolved many strategies on how to use TGFβ for their survival. Typically, transformed cells can have mutated or disrupted TGFβ receptors or altered SMAD signaling pathways. Especially in breast, prostate, and colorectal carcinoma (CRC), alterations in the TGFβ signaling cascade can have prognostic significance (Bierie & Moses, Citation2006).
TGFBR2 is probably the most commonly affected gene from all genes coding components of the cascade. It codes one of the most important proteins of the cascade-TβRII, which recognizes and binds all isoforms of TGFβ. Repressed or down-regulated expression of TGFBR2 is found in many types of cancer and it is leading to increased tumor spreading. In addition, it is associated with the microsatellite instability in CRC. Hereditary and sporadic CRC tend to have high microsatellite instability in 10-bp poly-A sequence of TGFBR2, causing malfunction of TβRII (Kim et al., Citation2000). Apart from APC, K-RAS and TP53 genes, also microsatellite stable CRCs display mutations in TGFBR2. Moreover, TGFBR1, SMAD2, and SMAD4 genes are very commonly lost, mutated, or functionally attenuated. For example, TGF-β1T869C polymorphism is associated with 2.7-fold greater relative risk of developing squamous cell carcinoma, suggesting that also gene polymorphisms can affect the proper functions of TGFβ protein (Carneiro et al., Citation2013). Still, the real contribution of the gene polymorphisms on the development of various types of cancer still needs to be clarified.
Another type of protein, E3 ligase Smurf2, is commonly up-regulated in squamous cell carcinomas with low levels of SMAD2 phosphorylation (Fukuchi et al., Citation2002). DNA methylation of TGFBR1 and TGFBR2 genes was observed in some cancers, suggesting the existence of epigenetic mechanisms regulating the pathway (Kang et al., Citation1999). An increased angiogenesis and invasion is induced by SMAD-independent up-regulation of MMP expression (Safina et al., Citation2007). Interestingly, TGFβ signaling in the malignant phenotype is able to regulate microRNA (miR) function. For example, hepatocellular carcinoma cells express CC-chemokine ligand 22 (CCL22) only when expression of miR-34a is inhibited by TGFβ (Yang et al., Citation2012). TGFβ increases the expression of miR-29a, which induces angiogenesis and represses the expression of phosphatase and tensin homolog (PTEN) (Wang et al., Citation2013). Important too is the TGFβ-induced expression of miR-494 in myeloid-derived suppressor cells (MDSC); this leads to increases in expression of CXC chemokine receptor and reduction in the expression of PTEN. These regulations also lead to increased expression of MMP3, MMP13, and MMP14 (Liu et al., Citation2012). Further, tumor-associated natural killer (NK) cells are silenced by TGFβ-inducible miR-183 (Donatelli et al., Citation2014).
The importance of TGFβ signaling in tumorigenesis has been studied in vitro by many investigators, mimicking conditions of tumors in patients by preparing TGFβ gene mutants or by directly treating the cancer cells with TGFβ. For example, Sartor et al. (Citation2010) proved that TGFβ had a capacity to increase expression of genes coding collagen type 1, collagen type 2, MMP2, MMP9, and lysyl oxidase homolog 4 in A549 lung adenocarcinoma cells. Those authors also observed increased expression of vascular endothelial growth factor A (VEGFA) and TSP-1. Advanced tumor stages are characterized by epithelial changes, but also by changes affecting the TM. Elevated TGFβ signaling is associated with increased metastases and poor prognosis for patients. Interestingly, loss of TGFβ signaling also correlates with increased metastases and progression and with poor prognosis (Forrester et al., Citation2005). Deregulation of TGFβ signaling is very often associated with stromal changes, such as activation of fibroblasts, deposition of ECM, increased angiogenesis and infiltration of immune cells (Bierie & Moses, Citation2006). Finally, in consideration of the heterogeneity of cancer cells in a tumor, it may be supposed that only part of these cells could be sensitive to TGFβ. However, this hypothesis still warrants further investigation. Interestingly, stromal changes induced by altered TGFB expression were found to increase metastatic activity of TGFβ unresponsive tumor cells (Finak et al., Citation2008). Thus, TGFβ can induce tumor progression directly or indirectly.
TGFβ regulation of immune cells
TGFβ is considered one of the most important regulators of proliferation and differentiation of immune cells deposited in a TM (). TGFβ is produced by and binds to many different types of immune cells, including macrophages, dendritic cells (DC), NK cells, B-cells, and T-cells. Cancer cells can also produce TGFβ; therefore, TGFβ has a capacity to modulate innate as well as adaptive immunity under both physiologic and cancer states (Yang et al., Citation2010).
Table 1. Regulation of immune cell function by TGFβ1.
In B-cells, TGFβ regulates expression of immunoglobulins, surface receptors, and major histocompatibility complex type II proteins (MHC II). These proteins are the direct markers of B-cell maturation and differentiation (Lebman & Edmiston, Citation1999). TGFβ also regulates T-cell maturation. It also inhibits proliferation of naïve CD4+ cells and T-cell expansion (Gilbert et al., Citation1997). Experiments on transgenic mice bearing dominant-negative TGFBR2 gene showed there was spontaneous T-cell differentiation leading to development of autoimmune diseases (Gorelik & Flavell, Citation2002). TGFβ favors tumor progression by suppressing T-cell production of perforins, granzymes, and other toxins. Thus, TGFβ negatively regulates both the expansion and cytotoxic activity of CD8+ T-cells, functions crucial to anti-tumor immunity (Thomas & Massague, Citation2005).
TGFβ also has a capacity to induce FoxP3 gene expression and subsequently to generate regulatory T (Treg)-cells. Together with interleukin (IL)-6, TGFβ induces TH17 cells that produce IL-17, important for activation of leukocytes (Shevach, Citation2009; Weaver et al., Citation2006). SMAD4−/− T-cells producing TH2-type cytokines that promote stromal expansion were found in gastrointestinal tumors (Kim et al., Citation2006). TGFβ inhibits the proper maturation of NK cells, which then lose their capacity to recognize non-self antigens, a process important for clearance of tumor cells (Marcoe et al., Citation2012). Moreover, TGFβ negatively regulates the ability of DC to present foreign antigens (Tanaka et al., Citation2010).
Proliferation of monocyte–macrophage lineage cells is suppressed mainly by TGFβ1 ligands (Chantry et al., Citation1989; Tsunawaki et al., Citation1988). In the TM, two types of macrophages can be found: M1 that deliver active anti-cancer functions and M2 type that promote tumor progression and metastasis. M2 are the most abundant inside tumors, and are also known as tumor-associated macrophages (TAM) (Mantovani et al., Citation2006). TGFβ is able to induce a shift of polarization from anti-tumor M1 to M2 TAM (Gong et al., Citation2012). Interestingly, in vitro inhibition of TGFβ signaling in TAM, blocking the TβRI and ligating the toll-like receptor 7 by an agonist reconverted M2 type macrophages into M1 type, provided new perspectives in cancer therapy (Peng et al., Citation2013). Recently, it was discovered that TGFβ has the capacity to transform anti-tumorigenic neutrophils (N1) into pro-tumorigenic neutrophils (N2) associated with production of MMP9 and chemokine CXCL1 (Fridlender et al., Citation2009). Interestingly, depletion of TGFβ results in reversible polarization of N2 neutrophils to N1 with an anti-tumor phenotype. Unexpected properties of TGFβ were recently described. TGFβ administered to mesenchymal stem cells (MSC), instead of leading to an increase, reversed their immunosuppressive activity upon T-cells. Moreover, TGFβ produced by MSC was found (in an autocrine manner) to inhibit inflammatory cytokine-induced inducible nitric oxide synthase (iNOS) expressed by MSCs themselves (Xu et al., 2014a).
The most important regulator of inflammation, NF-κB, is also negatively regulated by TGFβ1 via activation of inhibitor of kappa B (IκB) protein, with down-regulation of the pro-inflammatory and pro-metastatic functions of NF-κB. However, in some studies on cell lines, a possible double-faceted effect of TGFβ was noted, with TGFβ either promoting or inhibiting NF-κB functions (Arsura et al., Citation1996; Han et al., Citation1998). A recent study on gastric cancer development showed how TGFβ effects are microenvironmental. In a TGFβ mutant, with impeded binding of TGFβ to the latent TGFβ binding protein (Tgfb1−/C33S), generalized inflammation and increased tumorigenesis developed. By introducing a second mutation into, and thereby subsequent suppression of, the recombination activator gene 2 (Tgfb1−/C33S; Rag2−/−), the inflammatory and pro-carcinogenic effects did not appear. Those authors indicated this experiment showed how changes in tumor onset were more directly associated with inflammatory processes, rather than with the loss of TGFβ protein. This experiment also highlights the role of TGFβ in controlling inflammation (Ota et al., 2014).
TGFβ as a regulator of tumor microenvironment
The tumor microenvironment is very dynamic and the active crosstalk within the various types of involved cells (both cancer and non-cancer cells) permits a tumor to establish and progress, escaping host immunosurveillance and anti-cancer responses. The TM is commonly a hypoxic area with a low pH, conditions supporting DNA damage and suppressed repair (Bristow et al., 2008). Moreover, it is fueled by persistent inflammation that importantly contributes in supporting and promoting tumor development and spread (Balkwill & Mantovani, Citation2001). Normal TGFβ1 master regulation of inflammation in physiological conditions turns to inhibitory and re-modeling functions in the TM, frustrating the anti-cancer response efficacy. Further, the organ microenvironment can affect progression of tumors, as recently described for experimental hepatocellular carcinoma (HCC). For example, when human HCC cells were inoculated in the subcutaneous or in the liver of nu/nu mice, based on the different sites of development, TGFβ1 mRNA levels were found to be significantly lower in liver tumors than in subcutaneous tumors, and these levels correlated with higher tumor weight and less pulmonary metastasis for the orthotopic cancers (Li et al., Citation2013).
TGFβ was for the first time observed as a regulator of TM when Bhowmick et al. (Citation2004) found that deletion of the TGFBR2 gene in mouse fibroblasts was inducing transformation of adjacent prostate and stomach epithelia. In vivo, various epithelial cells also displayed deletion of TGFBR2, and this deletion resulted in increased tumor progression and metastatic growth (Yang & Moses, 2008). Moreover, hepatocyte growth factor (HGF) and HGF receptor MET are very often up-regulated in tissues displaying TGFβ down-regulation, suggesting an important role of paracrine signaling in these tissues. Recently, an experiment was designed in which induction of TGFβ in CAF stimulated production of IL-11, thereby triggering STAT3. Mice treated with TGFBR1 inhibitor were not able to form metastases (Calon et al., Citation2012).
TGFβ is also involved in regulation of chemokines, chemokine receptors, and angiogenesis. For example, breast cancer cells increasingly produce TGFβ, which induce production of angiopoetin-like 4 proteins, thereby enhancing formation of metastases in lungs (Padua et al., Citation2008). However, loss of TβRII in these cancer cells correlates with recruitment of F4/80+ cells that produce pro-inflammatory proteins CXCL1 and CXCL5 (Yang & Moses, Citation2008). This complete loss of TGFβ signaling in epithelial cells correlates with reduced survival in patients with breast cancer, especially estrogen-receptor-positive patients (Bierie et al., Citation2009). Even the loss of TβRIII contributes to tumor progression. Hanks et al. (Citation2013) elucidated a new mechanism in melanoma and breast cancer cells in which loss of tumor-produced TβRIII induced the production of indoleamine 2,3-dioxygenase in plasmacytoid DC and of CCL22 chemokines in myeloid DC, thereby mediating Treg infiltration and suppression of anti-tumor immunity. Further, hypoxic conditions (a characteristic marker of TM) were seen to promote breast cancer as a result of mesenchymal stem cell secretion of TGFβ (Hung et al., Citation2013).
Other patterns have been observed in gastric carcinoma and in colon cancer models. In gastric cancer SNU16mAd cells, an SMAD-dependent pathway activates production of integrins through protein kinase Cδ (PKCδ), thereby enhancing invasiveness of the tumor cells (Lee et al., Citation2005). In cis-Apc+/Δ716 Smad+/– mice, an increased recruitment of CCR1+ myeloid cells with promotion of colon cancer cell invasiveness was found (Kitamura et al., Citation2007). The blockage of TGFβ was found to increase expression of pro-inflammatory cytokine genes such as IL-5, IL-6, and IL-13. While this can lead to the negative effects about promotion of tumor progression described by Mantovani et al. (2006), it also increases the response against tumor elicited by specific immunotherapy (Kim et al., Citation2006). Once again, the context and timing of cytokine network activity is critical. Increased inflammation in the TM was also observed in various non-GI cancers (e.g. head and neck carcinomas), and this was put in relation to deregulation of TGFβ signaling. Crosstalk between TGFβ and IL-1 signaling pathways appears to be very common in some cancer cell lines (Lu et al., Citation2007).
Lastly, TGFβ is important for EMT regulation, a key process leading to tumor invasion and metastases formation (Thiery, Citation2002). EMT occurs during wound healing, normal cell development, and abnormally in cancer progression in which epithelial cells differentiate into mesenchymal cells (Thiery et al., Citation2009). EMT is associated with transition of primordial epithelial cells during gastrulation, generating neural crest cells and formation of endocardial tissue. Epithelial cells can transform into fibroblasts during wound healing, regeneration, and fibrosis. EMT is also a characteristic process accompanying cancerogenesis (Zeisberg & Neilson, Citation2009). The EMT implies disruption of tight junctions and delocalization of tight junction proteins, disruption of adherent junctions, and re-organization of actin fibers. Epithelial cells display mesenchymal markers and show spindle-like morphology (Thiery, Citation2002). TGFβ is responsible for EMT maintenance through production of protein surviving that stabilizes tubulin and Aurora B, resulting in inhibition of cell cycle arrest and apoptosis (Lee et al., Citation2013). Moreover, colon cancer cells are able to transform normal fibroblasts into CAF by secretion of TGFβ (Hawinkels et al., Citation2014). CLIC4 (chloride intracellular channel 4) is a downstream effector of the TGFβ signaling pathway, regulating transition of normal fibroblasts to activated pro-metastatic myofibroblasts through p38 signaling. Renal, ovarian, and breast cancers showed increased production of CLIC4, which should be considered as a new target of anti-tumor therapy (Shukla et al., Citation2013; Suh et al., Citation2007). TGFβ is also responsible for tumor recurrence through IL-8-dependent activation of cancer stem-like cells, as was shown in patients with breast cancer (Bhola et al., Citation2013). EMT can also be initiated in epidermal keratinocytes by ROS-stimulated TGFβ secretion and MAPK activation (Fukawa et al., Citation2012).
Therapeutic perspectives
Since TGFβ plays dual roles in tumorigenesis, it would seem to be an intriguing prospective therapeutic target. It was demonstrated in several studies that loss of TGFβ signaling is not tumorigenic but can affect already pro-tumorigenic (inflammatory) microenvironments. Conversely, over-expression of TGFB genes is commonly associated with progression of aggressive tumors with pro-metastatic potential and poor patient prognosis. Many neutralizing antibodies and molecular inhibitors that suppress tumor-promoting functions of TGFβ have been discovered so far. However, it is very important to design drugs that do not affect the normal tumor suppressive properties of TGFβ (Kim et al., Citation2008).
Several clinical studies proved that TGFβ therapy can be safe and effective (Bogdahn et al., Citation2011; Schlingensiepen et al., Citation2011). The main advantages for reducing TGFβ are reported as a better host immune surveillance and better prognosis for patients after radio- or chemotherapy (Biswas et al., Citation2007). TGFβ modulation also has effects on TM, i.e. it induces T-cell-mediated anti-tumor responses by causing an increased infiltration of NK cells and T-cells into the TM. TGFβ also helps to reduce the suppressive capacity of Treg cells and to decrease production of IL-17 that inhibits apoptosis in tumor cells (Nakamura et al., Citation2001; Nam et al., Citation2008). Another study showed that even SMAD4-deficient tumors could be treated by TGFβ therapy, suggesting pro-tumorigenic functions of TGFβ depend on complex TGFβ signaling in the TM (Zhong et al., Citation2010). Immunotherapy with TGFβ and tumor necrosis factor (TNF)-α antagonists was found to be able to restore production of interferon (IFN)-α by tumor-associated DC, resulting in anti-tumor responses (Sisirak et al., Citation2013).
MED12, a key component of transcription MEDIATOR system, has become a new target for therapy. MEDIATOR is a protein system that integrates and transduces positive and negative regulatory information, from enhancers and operators to promoters, functioning through RNA polymerase II with modulation of its activity in promoter-dependent transcription (Myers & Kornberg, Citation2000). In vitro experiments showed that loss or suppression of MED12 is associated with EMT and drug resistance due to activation of TβRII. MED12−/− cells treated by TGFβ signaling inhibitors displayed restoration of drug responsiveness (Huang et al., Citation2012). Xu et al. (2014b) designed an experiment based on nanotechnology principles. Anti-TGFβ small interfering RNA was nanoparticle-delivered into the late-stage TM. This administration down-regulated TGFβ production, leading to enhanced therapeutic effects of a vaccine against melanoma tumors in C57BL/6 mice (Xu et al., 2014b).
Despite all the positive effects of TGFβ modulation, Achyut et al. (Citation2013) discovered that abrogation of TGFβ signaling in stromal cells of Tgfbr2fspKO mice increased expression of various inflammatory mediators (e.g. iNOS and cyclooxygenase 2), inducing genetic damage, and proliferation in the neighboring epithelial compartment. Expression of the downstream mediator of p53, Cdkn1a/p21 (p21), was reduced. This, when taken together with the increases in inflammation and inflammatory cell infiltration, could also enhance tumor progression. The different effects of TGFβ therapy highlight the fact that the TM is a very complex system (Burkholder et al., Citation2014). This brings up new challenges to more precisely recognize further roles for TGFβ in the TM as well as its different expressions and activities in relation to the various stages of tumors and TM evolution (Zarzynska, Citation2014).
Conclusions
TGFβ signaling is a fundamental pathway for normal development and functions of mature cells. TGFβ ligands are widely expressed in all tissues of the body. However, TGFβ is also an important factor in the tumorigenesis network. Interestingly, TGFβ plays both tumor- suppressive and -promoting roles. It is evident that cross-talk between different cells in a TM is essential for cancer progression and that TGFβ is a potent regulator of this cross-talk. It regulates tumor progression by mutual interactions between various components of TM, including fibroblasts, epithelial cells, stromal cells, immune cells, etc. An increase in the pro-metastatic capacity of tumor cells is induced through the EMT, yet also regulated by TGFβ signaling. These facts have led to a strong effort to target TGFβ in anti-tumor therapy; various very promising drugs that have been discovered so far. Despite this success, the exact roles of TGFβ in the TM still need further elucidation not only to permit the better design of new therapeutic approaches, but to also more precisely define strategies for intervention.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
The authors thank grant GAAV IAA500200917, CZ.1.05/2.1.00/03.0124 (Project ExAM), RVO 61388971, RVO 67985904 (CZ); Antonucci Fund and Castoldi Fund, Varese (IT), and the Cecchi Gustavo e C. s.r.l. and Studio Puccinelli Fund, Viareggio (IT).
References
- Achyut, B. R., Bader, D. A., Robles, A. I., et al. 2013. Inflammation-mediated genetic and epigenetic alterations drive cancer development in the neighboring epithelium upon stromal abrogation of TGFβ signaling. PLoS Genet. 9:e1003251
- Arsura, M., Wu, M., and Sonenshein, G. E. 1996. TGFβ1 inhibits NF-κB/Rel activity inducing apoptosis of B-cells: Transcriptional activation of IκBα. Immunity 5:31–40
- Balkwill, F., and Mantovani, A. 2001. Inflammation and cancer: Back to Virchow? Lancet 357:539–545
- Bandyopadhyay, B., Fan, J., Guan, R., et al. 2006. “Traffic control” role for TGFβ3: Orchestrating dermal and epidermal cell motility during wound healing. J. Cell. Biol. 172:1093–1105
- Barcellos-Hoff, M. H., and Dix, T. A. 1996. Redox-mediated activation of latent TGFβ1. Mol. Endocrinol. 10:1077–1083
- Bhola, N. E., Balko, J. M., Dugger, T. C., et al. 2013. TGFβ inhibition enhances chemotherapy action against triple-negative breast cancer. J. Clin. Invest. 123:1348–1358
- Bhowmick, N. A., Chytil, A., Plieth, D., et al. 2004. TGFβ signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science 303:848–851
- Bierie, B., and Moses, H. L. 2006. Tumor microenvironment: TGFβ - The molecular Jekyll and Hyde of cancer. Nat. Rev. Cancer 6:506–520
- Bierie, B., Chung, C. H., Parker, J. S., et al. 2009. Abrogation of TGFβ signaling enhances chemokine production and correlates with prognosis in human breast cancer. J. Clin. Invest. 119:1571–1582
- Biswas, S., Guix, M., Rinehart, C., et al. 2007. Inhibition of TGFβ with neutralizing antibodies prevents radiation-induced acceleration of metastatic cancer progression. J. Clin. Invest. 117:1305–1313
- Bogdahn, U., Hau, P., Stockhammer, G., et al. 2011. Targeted therapy for high-grade glioma with the TGFβ2 inhibitor trabedersen: Results of a randomized and controlled Phase IIb study. Neuro Oncol. 13:132–142
- Boyer, A. S., Ayerinskas, I. I., Vincent, E. B., et al. 1999. TGFβ2 and TGFβ3 have separate and sequential activities during epithelial-mesenchymal cell transformation in the embryonic heart. Dev. Biol. 208:530–545
- Bristow, R. G., and Hill, R. P. 2008. Hypoxia and metabolism: Hypoxia, DNA repair and genetic instability. Nat. Rev. Cancer 8:180–192
- Burkholder, B., Huang, R. Y., Burgess, R., et al. 2014. Tumor-induced perturbations of cytokines and immune cell networks. Biochim. Biophys. Acta 1845:182–201
- Calon, A., Espinet, E., Palomo-Ponce, S., et al. 2012. Dependency of colorectal cancer on a TGFβ-driven program in stromal cells for metastasis initiation. Cancer Cell 22:571–584
- Carneiro, N. K., Oda, J. M., Losi Guembarovski, R., et al. 2013. Possible association between TGFβ1 polymorphism and oral cancer. Int. J. Immunogenet. 40:292–298
- Chantry, D., Turner, M., Abney, E., and Feldmann, M. 1989. Modulation of cytokine production by TGFβ. J. Immunol. 142:4295–4300
- Datto, M. B., Li, Y., Panus, J. F., et al. 1995. TGFβ induces the cyclin-dependent kinase inhibitor p21 through a p53- independent mechanism. Proc. Natl. Acad. Sci. USA. 92:5545–5549
- Derynck, R., Jarrett, J. A., Chen, E. Y., et al. 1985. Human TGFβ complementary DNA sequence and expression in normal and transformed cells. Nature 316:701–705
- Derynck, R., and Zhang, Y. E. 2003. Smad-dependent and Smad-independent pathways in TGFβ family signaling. Nature 425:577–584
- Donatelli, S. S., Zhou, J. M., Gilvary, D. L., et al. (2014). TGFβ-inducible microRNA- 183 silences tumor-associated natural killer cells. Proc. Natl. Acad. Sci. USA 111:4203–4208
- Dubois, C. M., Laprise, M. H., Blanchette, F., et al. 1995. Processing of TGFβ1 precursor by human furin convertase. J. Biol. Chem. 270:10618–10624
- Dumont, N., Bakin, A. V., and Arteaga, C. L. 2003. Autocrine TGFβ signaling mediates Smad- independent motility in human cancer cells. J. Biol. Chem. 278:3275–3285
- Ewen, M. E., Oliver, C. J., Sluss, H. K., et al. 1995. p53-dependent repression of CDK4 translation in TGFβ-induced G1 cell-cycle arrest. Genes Dev. 9:204–217
- Finak, G., Bertos, N., Pepin, F., et al. 2008. Stromal gene expression predicts clinical outcome in breast cancer. Nature Med. 14:518–527
- Flanders, K. C., Lüdecke, G., Engels, S., et al. 1991. Localization and actions of TGFβ in the embryonic nervous system. Development 113:183–191
- Forrester, E., Chytil, A., Bierie, B., et al. 2005. Effect of conditional knockout of the Type II TGFβ receptor gene in mammary epithelia on mammary gland development and polyomavirus middle T antigen-induced tumor formation and metastasis. Cancer Res. 65:2296–2302
- Fridlender, Z. G., Sun, J., Kim, S., et al. 2009. Polarization of tumor-associated neutrophil phenotype by TGFβ: “N1” versus “N2” TAN. Cancer Cell 16:183–194
- Fukawa, T., Kajiya, H., Ozeki, S., et al. 2012. Reactive oxygen species stimulates epithelial mesenchymal transition in normal human epidermal keratinocytes via TGFβ secretion. Exp. Cell Res. 318:1926–1932
- Fukuchi, M., Fukai, Y., Masuda, N., et al. 2002. High-level expression of the Smad ubiquitin ligase Smurf2 correlates with poor prognosis in patients with esophageal squamous cell carcinoma. Cancer Res. 62:7162–7165
- Gilbert, K. M., Thoman, M., Bauche, K., et al. 1997. TGFβ1 induces antigen-specific unresponsiveness in naive T-cells. Immunol. Invest. 26:459–472
- Gong, D., Shi, W., Yi, S. J., et al. 2012. TGFβ signaling plays a critical role in promoting alternative macrophage activation. BMC Immunol. 13:31
- Gorelik, L., and Flavell, R. A. 2002. TGFβ in T-cell biology. Nat. Rev. Immunol. 2:46–53
- Han, S. H., Yea, S. S., Jeon, Y. J., et al. 1998. TGFβ1 promotes IL-2 mRNA expression through the up-regulation of NF-κB, AP-1 and NF-AT in EL4 cells. J. Pharmacol. Exp. Ther. 287:1105–1112
- Hanks, B. A., Holtzhausen, A., Evans, K. S., et al. 2013. Type III TGFβ receptor down-regulation generates an immunotolerant tumor microenvironment. J. Clin. Invest. 123:3925–3940
- Hannon, G. J., and Beach, D. 1994. pl5INK4B is a potential effector of TGFβ-induced cell cycle arrest. Nature 371:257–261
- Hawinkels, L. J., Paauwe, M., Verspaget, H. W., et al. 2014. Interaction with colon cancer cells hyperactivates TGFβ signaling in cancer-associated fibroblasts. Oncogene 33:97–107
- Huang, S., Hölzel, M., Knijnenburg, T., et al. 2012. MED12 controls the response to multiple cancer drugs through regulation of TGFβ receptor signaling. Cell 151:937–950
- Huber, M. A., Kraut, N., and Beug, H. 2005. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr. Opin. Cell Biol. 17:548–558
- Hung, S. P., Yang, M. H., Tseng, K. F., and Lee, O. K. 2013. Hypoxia-induced secretion of TGFβ1 in mesenchymal stem cell promotes breast cancer cell progression. Cell Transplant. 22:1869–1882
- Iavarone, A., and Massague, J. 1999. E2F and histone deacetylase mediate TGFβ repression of cdc25A during keratinocyte cell cycle arrest. Mol. Cell Biol. 19:916–922
- Inoue, Y., and Imamura, T. 2008. Regulation of TGFβ family signaling by E3 ubiquitin ligases. Cancer Sci. 99:2107–2112
- Kaartinen, V., Voncken, J. W., Shuler, C., et al. 1995. Abnormal lung development and cleft palate in mice lacking TGFβ3 indicates defects of epithelial-mesenchymal interaction. Nat. Genet. 11:415–421
- Kang, S. H., Bang, Y. J., Im, Y. H., et al. 1999. Transcriptional repression of the TGFβ type I receptor gene by DNA methylation results in the development of TGFβ resistance in human gastric cancer. Oncogene 18:7280–7286
- Kim, B. G., Li, C., Qiao, W., et al. 2006. Smad4 signaling in T-cells is required for suppression of gastrointestinal cancer. Nature 441:1015–1019
- Kim, S., Buchlis, G., Fridlender, Z. G., et al. 2008. Systemic blockade of TGFβ signaling augments the efficacy of immunogene therapy. Cancer Res. 68:10247–10256
- Kim, S. J., Im, Y. H., Markowitz, S. D., and Bang, Y. J. 2000. Molecular mechanisms of inactivation of TGFβ receptors during carcinogenesis. Cytokine Growth Factor Rev. 11:159–168
- Kitamura, T., Kometani, K., Hashida, H., et al. 2007. SMAD4-deficient intestinal tumors recruit CCR1+ myeloid cells that promote invasion. Nat. Genet. 39:467–475
- Kojima, S., and Rifkin, D. B. 1993. Mechanism of retinoid-induced activation of latent TGFβ in bovine endothelial cells. J. Cell Physiol. 155:323–332
- Lawler, J., and Detmar, M. 2004. Tumor progression: The effects of thrombospondin-1 and -2. Int. J Biochem. Cell Biol. 36:1038–1045
- Lebman, D. A., and Edmiston, J. S. 1999. The role of TGFβ in growth, differentiation, and maturation of B-lymphocytes. Microbes Infect. 1:1297–1304
- Lee, J., Choi, J. H., and Joo, C. K. 2013. TGFβ1 regulates cell fate during epithelial-mesenchymal transition by up-regulating survivin. Cell Death Dis. 4:e714
- Lee, M. S., Kim, T. Y., Kim, Y. B., et al. 2005. The signaling network of TGFβ1, protein kinase Cδ, and integrin underlies the spreading and invasiveness of gastric carcinoma cells. Mol. Cell Biol. 25:6921–6936
- Li, G., Qin, L., Ye, Q., Dong, Q., et al. 2013. Organ microenvironment affects growth and metastasis of hepatocellular carcinoma via the TGFβ/Smad pathway in mice. Exp. Ther. Med. 5:133–137
- Li, M. O., Wan, Y. Y., Sanjabi, S., et al. 2006. Transforming growth factor-β regulation of immune responses. Annu. Rev. Immunol. 24:99–146
- Liu, Y., Lai, L., Chen, Q., et al. 2012. MicroRNA-494 is required for the accumulation and functions of tumor- expanded myeloid-derived suppressor cells via targeting of PTEN. J. Immunol. 188:5500–5510
- Lu, T., Tian, L., Han, Y., et al. 2007. Dose-dependent crosstalk between the TGFβ and IL-1 signaling pathways. Proc. Natl. Acad. Sci. USA. 104:4365–4370
- Lyons, R. M., Keski-Oja, J., and Moses, H. L. 1988. Proteolytic activation of latent TGFβ from fibroblast-conditioned medium. J. Cell Biol. 106:1659–1665
- Mantovani, A., Schioppa, T., Porta, C., et al. 2006. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev. 25:315–322
- Marcoe, J. P., Lim, J. R., Schaubert, K. L, et al. 2012. TGFβ is responsible for NK cell immaturity during ontogeny and increased susceptibility to infection during mouse infancy. Nat. Immunol. 13:843–850
- Massague, J. 2012. TGFβ signaling in context. Nat. Rev. Mol. Cell. Biol. 13:616–630
- Miettinen, P. J., Ebner, R., Lopez, A. R., and Derynck, R. 1994. TGFβ-induced trans-differentiation of mammary epithelial cells to mesenchymal cells: Involvement of Type I receptors. J. Cell. Biol. 127:2021–2036
- Miller, D. A., Lee, A., Pelton R. W., et al. 1989. Murine transforming growth factor-beta 2 cDNA sequence and expression in adult tissues and embryos. Mol. Endocrinol. 3:1108–1114
- Miyazono, K., Hellman, U., Wernstedt, C., and Heldin, C. H. 1988. Latent high molecular weight complex of TGFβ1. Purification from human platelets and structural characterization. J. Biol. Chem. 263:6407–6415
- Munger, J. S., Huang, X., Kawakatsu, H., et al. 1999. The integrin alpha v beta 6 binds and activates latent TGFβ1: A mechanism for regulating pulmonary inflammation and fibrosis. Cell 96:319–328
- Myers, L. C., and Kornberg, R. D. 2000. Mediator of transcriptional regulation. Annu. Rev. Biochem. 69:729–749
- Nakamura, K., Kitani, A., and Strober, W. 2001. Cell contact-dependent immunosuppression by CD4+CD25+ regulatory T-cells is mediated by cell surface-bound TGFβ. J. Exp. Med. 194:629–644
- Nam, J. S., Terabe, M., Kang, M. J., et al. 2008. TGFβ subverts the immune system into directly promoting tumor growth through IL-17. Cancer Res. 68:3915–3923
- Ota, M., Horiguchi, M., Fang, V., et al. 2014. Genetic suppression of inflammation blocks the tumor-promoting effects of TGF-β in gastric tissue. Cancer Res. 74:2642–2651
- Oursler, M. J. 1994. Osteoclast synthesis and secretion and activation of latent TGFβ. J. Bone Miner. Res. 9:443–452
- Padua, D., Zhang, X. H., Wang, Q., et al. 2008. TGFβ primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell 133:66–77
- Pardali, K., and Moustakas, A. 2007. Actions of TGFβ as tumor suppressor and pro-metastatic factor in human cancer. Biochim. Biophys. Acta 1775:21–62
- Peng, J., Tsang, J. Y., Li, D., et al. 2013. Inhibition of TGFβ signaling in combination with TLR7 ligation re- programs a tumoricidal phenotype in tumor-associated macrophages. Cancer Lett. 331:239–249
- Piek, E., Heldin, C. H., and Ten Dijke, P. 1999. Specificity, diversity, and regulation in TGFβ superfamily signalling. FASEB J. 13:2105–2124
- Polyak, K., Kato, J. Y., Solomon, M. J., et al. 1994. p27Kip1, a cyclin-Cdk inhibitor, links TGFβ and contact inhibition to cell cycle arrest. Genes Dev. 8:9–22
- Proetzel, G., Pawlowski, S. A., Wiles, M. V., et al. 1995. TGFβ3 is required for secondary palate fusion. Nat. Genet. 11:409–414
- Rifkin, D. B. 2005. Latent transforming growth factor (TGF)-β binding proteins: Orchestrators of TGFβ availability. J. Biol. Chem. 280:7409–7412
- Safina, A., Vandette, E., and Bakin, A. V. 2007. ALK5 promotes tumor angiogenesis by up-regulating matrix metalloproteinase-9 in tumor cells. Oncogene 26:2407–2422
- Sartor, M. A., Mahavisno, V., Keshamouni, V. G., et al. 2010. ConceptGen: A gene set enrichment and gene set relation mapping tool. Bioinformatics 26:456–463
- Schlingensiepen, K. H., Jaschinski, F., Lang, S. A., et al. 2011. TGFβ2 gene silencing with trabedersen (AP 12009) in pancreatic cancer. Cancer Sci. 102:1193–1200
- Schmierer, B., and Hill, C. S. 2005. Kinetic analysis of Smad nucleo-cytoplasmic shuttling reveals a mechanism for TGFβ-dependent nuclear accumulation of Smads. Mol. Cell Biol. 25:9845–9858
- Schultz-Cherry, S., and Murphy-Ullrich, J. E. 1993. Thrombospondin causes activation of latent TGFβ secreted by endothelial cells by a novel mechanism. J. Cell Biol. 122:923–932
- Schwyzer, M., and Fontana, A. 1985. Partial purification and biochemical characterization of a T-cell suppressor factor produced by human glioblastoma cells. J. Immunol. 134:1003–1009
- Shevach, E. M. 2009. Mechanisms of FoxP3+ T-regulatory cell-mediated suppression. Immunity 30:636–645
- Shi, Y., and Massague, J. 2003. Mechanisms of TGFβ signaling from cell membrane to the nucleus. Cell 113:685–700
- Shukla, A., Edwards, R., Yang, Y., et al. 2013. CLIC4 regulates TGFβ-dependent myofibroblast differentiation to produce a cancer stroma. Oncogene 33:842–850
- Sisirak, V., Vey, N., Goutagny, N., et al. 2013. Breast cancer-derived TGFβ and TNFα compromise IFNγ production by tumor-associated plasmacytoid dendritic cells. Int. J. Cancer 1:771–778
- Stewart, A. A., Haley, J. D., Qu, G. Y., et al. 1996. Umbilical cord TGFβ3: Isolation, comparison with recombinant TGFβ3 and cellular localization. Growth Factors 13:87–98
- Suh, K. S., Crutchley, J. M., Koochek, A., et al. 2007. Reciprocal modifications of CLIC4 in tumor epithelium and stroma mark malignant progression of multiple human cancers. Clin. Cancer Res. 13:121–131
- Tanaka, H., Hinto, O., Yashiro, M., et al. 2010. TGFβ signaling inhibitor, SB-431542, induces maturation of dendritic cells and enhances anti-tumor activity. Oncol. Rep. 24:1637–1643
- Thiery, J. P. 2002. Epithelial-mesenchymal transitions in tumor progression. Nat. Rev. Cancer 2:442–454
- Thiery, J. P., Acloque, H., Huang, R. Y., and Nieto, M. A. 2009. Epithelial-mesenchymal transitions in development and disease. Cell 139:871–890
- Thomas, D. A., and Massague, J. 2005. TGFβ directly targets cytotoxic T-cell functions during tumor evasion of immune surveillance. Cancer Cell 8:369–380
- Tsunawaki, S., Sporn, M., Ding, A., and Nathan, C. 1988. Deactivation of macrophages by TGFβ. Nature 334:260–262
- Turner, M., Chantry, D., and Feldmann, M. 1990. TGFβ induces production of IL-6 by human peripheral blood mononuclear cells. Cytokine 2:211–216
- Wang, J., Wang, Y., Ma, Y., et al. 2013. TGFβ-regulated microRNA-29a promotes angiogenesis through targeting the phosphatase and tensin homolog in endothelium. J. Biol. Chem. 288:10418–10426
- Weaver, C. T., Harrington, L. E., Mangan, P. R., et al. 2006. TH17: An effector CD4 T-cell lineage with regulatory T-cell ties. Immunity 24:677–688
- Wipff, P. J., and Hinz, B. 2008. Integrins and the activation of latent TGFβ1 - an intimate relationship. Eur. J. Cell Biol. 87:601–615
- Wipff, P. J., Rifkin, D. B., Meister, J. J., and Hinz, B. 2007. Myofibroblast contraction activates latent TGFβ1 from the extracellular matrix. J. Cell Biol. 179:1311–1323
- Wiseman, D. M., Polverini, P. J., Kamp, D. W., and Leibovich, S. J. 1988. Transforming growth factor (TGF)-β is chemotactic for human monocytes and induces their expression of angiogenic activity. Biochem. Biophys. Res. Commun. 157:793–800
- Xu, C., Yu, P., Han, X., et al. 2014a. TGFβ promotes immune responses in the presence of mesenchymal stem cells. J. Immunol. 192:103–109
- Xu, Z., Wang, Y., Zhang, L., and Huang, L. 2014b. Nanoparticle-delivered TGFβ siRNA enhances vaccination against advanced melanoma by modifying tumor microenvironment. ACS Nano 8:3636–3645
- Yang, L., and Moses, H. L. 2008. TGFβ: Tumor suppressor or promoter? Are host immune cells the answer? Cancer Res. 68:9107–9111
- Yang, L., Pang, Y., and Moses, H. L. 2010. TGFβ and immune cells: An important regulatory axis in the tumor microenvironment and progression. Trends Immunol. 31:220–227
- Yang, P., Li, Q. J., Feng, Y., et al. 2012. TGFβ-miR-34a-CCL22 signaling-induced Treg cell recruitment promotes venous metastases of HBV+ hepatocellular carcinoma. Cancer Cell 22:291–303
- Yu, Q., and Stamenkovic, I. 2000. Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGFβ and promotes tumor invasion and angiogenesis. Genes Dev. 14:163–176
- Zarzynska, J. M. 2014. Two faces of TGF-β1 in breast cancer. Mediators Inflamm. 2014:141747
- Zeisberg, M., and Neilson, E. G. 2009. Biomarkers for epithelial-mesenchymal transitions. J. Clin. Invest. 119:1429–1437
- Zhong, Z., Carroll, K. D., Policarpio, D., et al. 2010. Anti-TGFβ receptor II antibody has therapeutic efficacy against primary tumor growth and metastasis through multi-effects on cancer, stroma, and immune cells. Clin. Cancer Res. 16:1191–1205
