Abstract
In vitro gene profiling studies have associated the molecular pathways of Nrf2-Keap1 and Toll-like receptor (TLR) signaling with skin sensitization. In this study, the role of these pathways in the regulation of protein biomarkers for skin sensitization was further elucidated using transient gene knock-down of key components of the signaling cascades in HaCaT cells after exposure to dinitrochlorobenzene (DNCB). The effect of targeting these pathways was established through evaluation of heme oxygenase1 (HMOX1) and interleukin (IL)-8 production. These experiments showed that Nrf2 is not involved in regulating HMOX1 after exposure to DNCB, but that activation of TLR signaling moderates the expression of HMOX1. The regulation of IL-8 depended on Nrf2, but also on the Toll/interleukin-1 receptor (TIR)-domain-containing adapter-inducing interferon-β (TRIF) adaptor protein in TLR signaling. This study provides new insights into the regulation of HMOX1 and IL-8, but the exact regulating mechanisms remain to be further elucidated.
Introduction
The murine local lymph node assay (LLNA) and guinea pig tests, such as the guinea pig maximization test and Buehler test, are used to identify chemicals with skin sensitizing potential. Recent changes in legislation have prompted the development of alternative methods to determine skin sensitizing potential. These methods are mainly based on measuring biomarkers, either genes or proteins, or chemical reactions (Vandebriel and van Loveren, Citation2010). Many molecular processes involving skin sensitization have been identified (Martin et al., Citation2011; van der Veen et al., Citation2011), the role of these mechanisms in driving biomarker expression is often not known. In recent gene profiling studies it was shown both in dendritic cells as well as in keratinocytes that the cytoprotective Nrf2-Keap1 and the innate immunity-related Toll-like receptor (TLR) signaling pathways are triggered upon exposure to chemical sensitizers (Ade et al., Citation2009; Johansson et al., Citation2011; van der Veen et al., Citation2013b). In this study, the role of these pathways in driving the biomarkers heme oxygenate1 (HMOX1) and interleukin (IL)-8 was further established.
Under normal conditions, Nrf2 transcription factor is sequestered to the cytoplasm by Keap1. After exposure to reactive oxygen species or nucleophilic chemicals, conformational changes in Keap1 results in release of Nrf2. In the nucleus, Nrf2 complexes with various small MAF proteins to form a DNA binding complex that recognizes anti-oxidant responsive elements (ARE) in the promoter region of cytoprotective genes, such as HMOX1 (Jaiswal, Citation2004; Kensler et al., Citation2007). In mice lacking Nrf2, an increased response to sensitizers was observed, indicating a protective effect of Nrf2 (El Ali et al., Citation2013; van der Veen et al., Citation2013a).
The TLR signaling pathway is activated after triggering TLR on a cellular membrane. As the intracellular domain of TLR is not sufficient for continued signaling, adaptor proteins, such as MyD88 or TRIF (Toll/interleukin-1 receptor [TIR]-domain-containing adapter-inducing interferon-β), are recruited. In turn, these proteins activate TRAF6, which triggers activation of NF-κB through the MAP kinases p38 and JNK. In addition, the adaptor protein TRIF can induce Interferon-Regulatory-Factors (IRF) without assistance from TRAF6 (O’Neill and Bowie, Citation2007). Previous studies showed that mice lacking both TLR2 and TLR4 were unable to mount an immune response towards a skin-sensitizing chemical (which also holds true for mice lacking MyD88), while TRIF knockout mice were not affected (Martin et al., Citation2008; Klekotka et al., Citation2010). These findings suggest that the TLR and the underlying signaling processes are instrumental to sensitization, for example by initiating an inflammatory response through production of pro-inflammatory cytokines.
The biomarkers IL-8 and HMOX1 have are initiated in vitro by both keratinocytes and DC-like cell lines after exposure to skin sensitizers (Ade et al., Citation2009; Arkusz et al., Citation2010; Mitjans et al., Citation2010; Frankart et al., Citation2012; van der Veen et al., 2013b). Production of IL-8 in dendritic cells has been shown to depend on MAP kinase activity (Nukada et al., Citation2008) that is mainly initiated by MyD88 (O’Neill and Bowie, Citation2007), but the mechanism underlying IL-8 production in keratinocytes has not been described. In addition, several transcription factors have been linked to the expression of HMOX1, primary among which is Nrf2 (Alam and Cook, Citation2007). Using transient knockdown of Nrf2, Keap1, MyD88, TRIF and TRAF6 in the HaCaT keratinocyte cell line, we studied the role of these pathways in the regulation of IL-8 and HMOX1.
The goal of this study was to clarify the role of Nrf2-Keap1 and TLR signaling pathways in regulating these biomarkers. We hypothesized that targeting Nrf2 would decrease HMOX1 expression, while targeting Keap1, the negative regulator of Nrf2, would increase HMOX1 expression due to the increased availability of Nrf2. As the TLR-signaling cascade is thought to affect IL-8 levels through the activation of NF-κB (Gales et al., Citation2013), knocking down the key proteins in this cascade would limit IL-8 production. In addition, the role of Nrf2 in IL-8 regulation was studied, as was the involvement of TLR signaling in HMOX1 regulation.
Materials and methods
Cell culturing and exposure
The human keratinocyte cell line HaCaT (Cell Line Services, Heidelberg, Germany) was cultured in T-flasks (Corning, Amsterdam, the Netherlands) in complete Dulbecco’s Modified Eagle Medium (DMEM) containing GlutaMAXTM-1, 4500 mg D-Glucose/L, and 25 mM HEPES (GlutaMAXTM, GIBCO, Carlsbad, CA), supplemented with 10% decomplemented fetal calf serum (FCS; Integro, Zaandam, the Netherlands), 100 U penicillin/ml, 100 µg streptomycin/ml, and 100 µM Non-Essential Amino Acids (each GIBCO) at 37 °C in a 5% CO2 incubator. For exposure, cells were seeded in 12 well-plates at a density of 4.5 × 105 cells/well. DNCB (Sigma-Aldrich, Zwijndrecht, the Netherlands) (Supplementary Table I) was administered in duplicate to the cells at their 80% Cell Viability (CV80) concentration. The effect of DNCB on cell viability was assessed colorimetrically by adding WST-1 (Roche, Woerden, the Netherlands) to the cells after 24 h of exposure.
Transient knockdown and exposure
To knock down MyD88, Keap-1, TRIF, and TRAF6, a transfection mix was prepared by adding 20 μl of DharmaFECT 4 and target specific siRNA (Thermo Fisher Scientific, Landsmeer, the Netherlands; to a final concentration of 120 nM) to 2 ml serum-free DMEM. The transfection mix was incubated on an orbital shaker at RT for ≈15 min. After cell harvesting, ≈2.5 × 106 cells were added to the transfection mix. The transfected cells were incubated for 48 h in T-flasks, after which the transfected HaCaT cells were seeded in 12-well plates (Costar®, Corning) with a cell density of 2.5 × 105 cells/well. The cells were allowed to adhere for 24 h, after which they were exposed to DNCB or vehicle (ethanol).
The method described above did not provide sufficient knock down of Nrf2. Therefore, Nrf2 knock down was performed in a different manner. Specifically, HaCaT cells were seeded into 12-well plates at a cell density of 7.5 × 104 cells/well and transfected with 50 nM Nrf2 Smartpool or 50 nM non-targeting siRNA using 6 µl DharmaFECT 4 per well (Thermo Scientific). The cells were then incubated for 96 h and then exposed to DNCB or vehicle (EtOH).
For analysis of changes in gene regulation and determining the knockdown efficiency, cells were exposed for 4 h, after which the exposure medium was removed and RNAprotect cell reagent (Qiagen®, Venlo, the Netherlands) was added. To analyze changes in protein level, cells were exposed for 24 h, after which the supernatant was collected for measures of IL-8 levels and the cells were lysed in a solution of phosphate-buffered saline (PBS [pH 7.5]) + 0.5% Triton X-100 to permit subsequent analysis of HMOX-1 levels. Only results of experiments with sufficient knockdown efficiency, i.e. >75% knock down of mRNA, were considered for further analysis.
Protein measurements
IL-8 levels in supernatants were determined using a Human IL-8 ELISA Ready-SET-Go! ELISA Kit (eBioscience, Vienna, Austria) according to manufacturer’s instructions. HMOX1 levels in cell lysates were assayed using a Human HMOX1 DuoSet IC ELISA kit (R&D Systems, Abingdon, UK) according to manufacturer’s instructions. To normalize the results, protein levels were compared to non-targeting cells exposed to ethanol. The levels of sensitivity of the IL-8 and HMOX1 kits were 2 pg IL-8/ml and 156 pg HMOX1/ml.
Gene expression
cDNA was produced with the High Capacity cDNA Reverse Transcription kit (Life Technologies, Carlsbad, CA) according to manufacturer instructions. TaqMan Gene Expression analysis was performed using a 7500 Fast Real-Time PCR System (Life Technologies), according to manufacturer’s instructions and using 10 ng cDNA and TaqMan® Fast Universal PCR Master mix (Life Technologies) in a total volume of 10 µl. Relative quantification was done by the comparative CT method (ddCt) in Microsoft Excel, wherein the housekeeping genes HPRT1 and β2 M were used.
Results
Effects of DNCB exposure
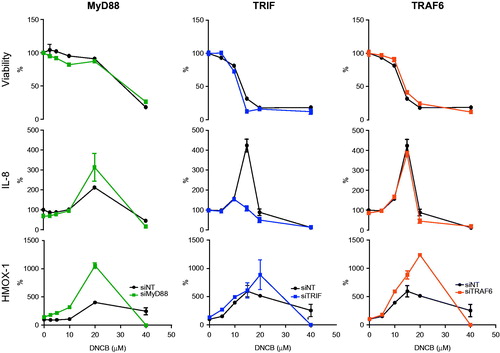
To evaluate the findings in knockdown studies of the normal response to DNCB, effects of DNCB on HaCaT cells treated with non-targeting siRNA were established ( and ). After 24 h of exposure to DNCB, the viability of HaCaT cells decreased to ∼20 µM DNCB, which was the CV80 concentration. This concentration also triggered a marked increase in formation of IL-8. Production of HMOX1 was already apparent after exposure to 5 µM DNCB; HMOX1 levels increased dose dependently, although they decreased at concentrations ≥20 µM due to cell death. Changes to this pattern of expression in knockdown experiments could be attributed to the knockdown target.
Figure 1. Knockdown efficiency of the targets. Knockdown efficiency was routinely checked at the mRNA level and compared to that in the non-targeting control.
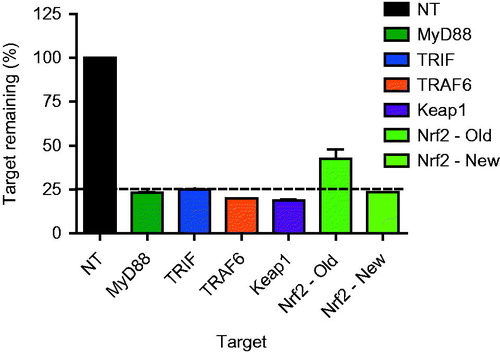
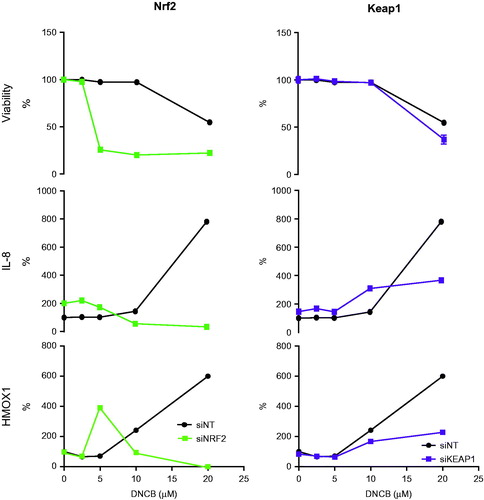
Figure 2. Effects of knockdown of Nrf2-Keap1 pathway related targets on viability and on IL-8 and HMOX1 protein levels. HaCaT cells were exposed to a range of DNCB concentrations. After 24 h, effects on cell viability and on IL-8 and HMOX-1 production were evaluated. Effects in targeted cells were compared to control cells (siNT) (n = 3).
Knockdown efficiency
Knockdown efficiency was routinely checked and an experiment was approved when an efficiency of >75% was achieved at the mRNA level. Efficiency was also confirmed at the protein level using Western blots (data not shown). In the case of MyD88, TRAF6, TRIF, and Keap1, the knockdown protocol proved sufficiently effective; however, this was not the case for Nrf2. To ensure sufficient knockdown of Nrf2, the number of trypsinizing steps between initial knockdown and exposure to DNCB was reduced in an adjusted targeting protocol ().
Predictive capacity of IL-8
Production of IL-8 in HaCaT was a possible biomarker for skin sensitization, as was shown in a pilot study using five chemicals; only the included sensitizers induced IL-8 production (Supplementary Figure I). In a subsequent experiment, the set of chemicals was expanded to include 41 chemicals. The results showed a prediction accuracy of 78%, a level comparable to the LLNA (Supplementary Table I).
Nrf2-Keap1 pathway
The Nrf2 and Keap1 proteins were targeted to elucidate their role in IL-8 and HMOX1 regulation (). In the case of Keap1 knockdown, viability was affected in a similar way as in non-targeted cells treated with DNCB. In the absence of Keap1, DNCB-induced HMOX1 production decreased compared to that in non-targeting control cells. In Keap1-targeted cells, the IL-8 levels are higher than in controls at DNCB concentrations <10 µM, but lower at 20 µM.
The Nrf2 knockdown increases the sensitivity of HaCaT cells to DNCB, as cell death already occurs at a lower concentration of DNCB. After Nrf2 knockdown, HMOX1 was not affected at the protein or RNA levels ( and ), but the peak shifted from 20 to 5 µM DNCB due to the increased sensitivity of the cells to DNCB. Production of IL-8 was reduced after knockdown of Nrf2, even though the IL-8 level in cells exposed to ethanol was higher after Nrf2 knockdown.
Toll-like receptor signaling pathway
To elucidate the role of TLR signaling in IL-8 and HMOX1 production, MyD88, TRIF and TRAF6, proteins involved in early TLR signal transduction, were targeted (). No effects on viability were observed for any of these targets. HMOX1 levels increased after targeting MyD88, TRIF and TRAF6, indicating that these proteins are negative regulators ofHMOX1. At the mRNA level, this increase was not observed (). TRIF was essential for IL-8 production at higher DNCB concentrations (i.e. 10 and 15 µM), as production was reduced after knockdown compared to that in non-targeting control exposures. Targeting MyD88 increases IL-8 production, particularly at 20 µM DNCB. Knockdown of TRAF6 did not affect IL-8 protein levels.
Figure 4. Effects of knockdown of Toll-like receptor signaling pathway related targets on viability and on IL-8 and HMOX1 protein levels. HaCaT cells were exposed to a range of DNCB concentrations. After 24 h, effects on cell viability and on IL-8 and HMOX1 production were evaluated. Effects in targeted cells were compared to the response range observed in control cells (shaded area) (n = 3).
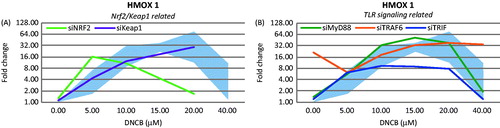
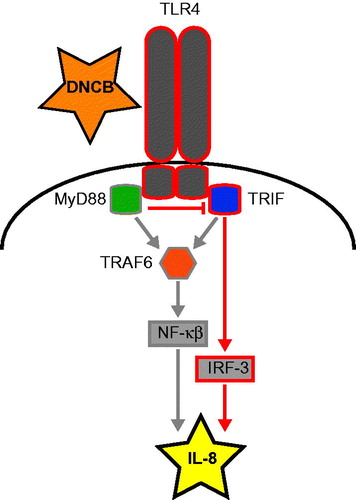
Figure 5. Hypothesis for regulation of IL-8 after DNCB exposure. After DNCB triggers TLR4—possibly through Danger Associated Molecular Patterns—both MyD88 and TRIF can be recruited for signal transduction. This triggers activation of transcription factors IRF-3 by TRIF and NF-κB through TRAF6. MyD88 and TRIF compete for binding to TLR4; TRAF6 is not involved in IL-8 regulation, indicating an important role for TRIF in up-regulation of IL-8 following DNCB exposure.
Discussion
Targeting Nrf2 for knockdown increased sensitivity to DNCB-induced cytotoxicity. This confirmed the cytoprotective effect of Nrf2 activation against DNCB, which is in line with observations in mice (El Ali et al., Citation2013; van der Veen et al., Citation2013a). HMOX1 is thought to be regulated by Nrf2 after exposure to sensitizers (Ade et al., Citation2009). However, HMOX1 was not affected by Nrf2 knockdown, indicating that the regulation of HMOX1 was also dependent on other transcription factors, an outcome recently also observed in the KeratinoSens assay (Emter et al., Citation2013). Targeting the AP-1, NF-κB, and HSF1 transcription factors can help to further understand the molecular mechanisms that regulate HMOX1 after exposure to skin sensitizers (Alam and Cook, Citation2007)
The expression of IL-8 is a promising biomarker for skin sensitizing potential. To illustrate the predictive capacity of IL-8, a set of 41 chemicals was tested. While those results indicated that measuring IL-8 production as a predictive marker was comparable to employing the LLNA, this novel prediction model still will need to be further defined to increase its accuracy and reliability. Production of IL-8 was reduced after knockdown of Nrf2, confirming the important role for Nrf2 in IL-8 regulation (Zhang et al., Citation2005). HMOX1 levels were decreased after targeting Keap1 compared to what was noted after non-targeting control exposures. This indicated that Keap1 was involved in up-regulation of this protein. In addition, the expression levels of the HMOX1 gene were similar between the control and Keap1 knock-down cells, indicating that Keap1 may influence mRNA stability or mRNA translation. It is probable that the increased availability of Nrf2 after Keap1 knockdown could explain the increased levels of IL-8 at low concentrations of DNCB. Nevertheless, the mechanisms that caused reductions in IL-8 production at high DNCB concentrations remain unclear.
Targeting the TLR signaling pathway did not affect the sensitivity to DNCB, but after targeting any of the TLR signaling pathway constituents an increase in HMOX1 production was observed, indicating this pathway moderated HMOX1 expression. Induction of IL-8 was affected differently between the TLR signaling targets. These results indicate that the TLR adaptor protein TRIF, but not MyD88 or TRAF6, is instrumental in regulating IL-8 in HaCaT cells. It therefore seems that IL-8 regulation after DNCB exposure is likely to be NF-κB independent, as TRAF6 is not involved in signaling and that IRFs activated by TRIF might be essential (). This hypothesis was supported by studies using hyaluronic acid (HA)-exposed TRIF and MyD88 knockout mice (Black et al., Citation2013). HA is a Danger Associated Molecular Pattern recognized by TLR2 and TLR4 and is released after exposure to sensitizers (Esser et al., Citation2012). In addition, HA has been found to promote DC migration from the skin (Muto et al., Citation2014). Similar to the regulation of IL-8 in the current study, production of interferon (IFN)-β was abolished in TRIF knockout mice, while it was increased in MyD88 knockout mice (Black et al., Citation2013). This suggested to us that HA was also a cause of IL-8 production after sensitizer exposure.
The increase in IL-8 after MyD88 knockdown indicated a role for TLR4, which was able to initiate a signaling cascade through both MyD88 and TRIF. As MyD88 and TRIF compete for binding to TLR4, targeting of MyD88 would increase the signaling through TRIF, leading to increased IL-8 production (). On the other hand, sensitization experiments with TRIF and MyD88 knockout mice showed that MyD88 (but not TRIF) was essential for sensitization of mice (Klekotka et al., Citation2010). It is possible that MyD88 is essential in regulation of other important inflammatory mediators and key events of skin sensitization, i.e. activation of dendritic cells, as the majority of the other TLR use MyD88 as their adaptor protein. In addition, our results were obtained in a human keratinocyte cell line, limiting the crosstalk between cell types. The molecular mechanisms in HaCaT may, therefore, deviate from those in mice and humans. Further, the influence of unspecific cytotoxicity in HaCaT cells on the release of IL-8 and HMOX1—which might contribute to the observed effects—is not known. Nevertheless, these findings substantiate the importance of TLR signaling in skin sensitization.
Supplementary material available online
Supplementary Tables I and Figure I.
Supplemental Material.pdf
Download PDF (107.1 KB)Acknowledgements
The authors would like to acknowledge E. Gremmer of the RIVM for his excellent technical support.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. This study was funded by a grant of the Netherlands Genomics Initiative/Netherlands Organization for Scientific Research (NWO) No.: 050-060-510.
References
- Ade, N., Leon, F., Pallardy, M., et al. 2009. HMOX1 and NQO1 genes are up-regulated in response to contact sensitizers in dendritic cells and THP-1 cell line: Role of the Keap1/Nrf2 pathway. Toxicol. Sci. 107:451–460
- Alam, J., and Cook, J. L. 2007. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am. J. Respir. Cell Mol. Biol. 36:166–174
- Arkusz, J., Stepnik, M., Sobala, W., and Dastych, J. 2010. Prediction of the contact sensitizing potential of chemicals using analysis of gene expression changes in human THP-1 monocytes. Toxicol. Lett. 199:51–59
- Black, K. E., Collins, S. L., Hagan, R. S., et al. 2013. Hyaluronan fragments induce IFNβ via a novel TLR4-TRIF-TBK1-IRF3-dependent pathway. J. Inflamm. (London) 10:23
- El Ali, Z., Gerbeix, C., Hemon, P., et al. 2013. Allergic skin inflammation induced by chemical sensitizers is controlled by the transcription factor Nrf2. Toxicol. Sci. 134:39–48
- Emter, R., van der Veen, J. W., Adamson, G., et al. 2013. Gene expression changes induced by skin sensitizers in the KeratinoSens cell line: Discriminating Nrf2-dependent and Nrf2-independent events. Toxicol. In Vitro 27:2225–2232
- Esser, P. R., Wolfle, U., Durr, C., et al. 2012. Contact sensitizers induce skin inflammation via ROS production and hyaluronic acid degradation. PLoS One 7:e41340
- Frankart, A., Coquette, A., Schroeder, K. R., and Poumay, Y. 2012. Studies of cell signaling in a reconstructed human epidermis exposed to sensitizers: IL-8 synthesis and release depend on EGFR activation. Arch. Dermatol. Res. 304:289–303
- Gales, D., Clark, C., Manne, U., and Samuel, T. 2013. The Chemokine CXCL8 in carcinogenesis and drug response. ISRN Oncol. 2013:859154
- Jaiswal, A. K. 2004. Nrf2 signaling in coordinated activation of anti-oxidant gene expression. Free Radic. Biol. Med. 36:1199–1207
- Johansson, H., Lindstedt, M., Albrekt, A. S., and Borrebaeck, C. A. 2011. A genomic biomarker signature can predict skin sensitizers using a cell-based in vitro alternative to animal tests. BMC Genomics 12:399
- Kensler, T. W., Wakabayashi, N., and Biswal, S. 2007. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 47:89–116
- Klekotka, P. A., Yang, L., and Yokoyama, W. M. 2010. Contrasting roles of the IL-1 and IL-18 receptors in MyD88-dependent contact hypersensitivity. J. Invest. Dermatol. 130:184–191
- Martin, S. F., Dudda, J. C., Bachtanian, E., et al. 2008. Toll-like receptor and IL-12 signaling control susceptibility to contact hypersensitivity. J. Exp. Med. 205:2151–2162
- Martin, S. F., Esser, P. R., Weber, F. C., et al. 2011. Mechanisms of chemical-induced innate immunity in allergic contact dermatitis. Allergy 21:1398–9995
- Mitjans, M., Galbiati, V., Lucchi, L., et al. 2010. Use of IL-8 release and p38 MAPK activation in THP-1 cells to identify allergens and to assess their potency in vitro. Toxicol. In Vitro 24:1803–1809
- Muto, J., Morioka, Y., Yamasaki, K., et al. 2014. Hyaluronan digestion controls DC migration from the skin. J. Clin. Invest. 124:1309–1319
- Nukada, Y., Miyazawa, M., Kosaka, N., et al. 2008. Production of IL-8 in THP-1 cells following contact allergen stimulation via mitogen-activated protein kinase activation or TNFα production. J. Toxicol. Sci. 33:175–185
- O’Neill, L. A., and Bowie, A. G. 2007. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 7:353–364
- van der Veen, J., Vandebriel, R. J., van Loveren, H., and Ezendam, J. 2011. Keratinocytes, Innate Immunity and Allergic Contact Dermatitis – Opportunities for the Development of In Vitro Assays to Predict the Sensitizing Potential of Chemicals. In: Contact Dermatitis. (Ro, Y. S., Ed.). Croatia: Rijeka, pp. 39–58
- van der Veen, J. W., Gremmer, E. R., Vermeulen, J. P., et al. 2013a. Induction of skin sensitization is augmented in Nrf2-deficient mice. Arch. Toxicol. 87:763–766
- van der Veen, J. W., Pronk, T. E., van Loveren, H., and Ezendam, J. 2013b. Applicability of a keratinocyte gene signature to predict skin sensitizing potential. Toxicol. In Vitro 27:314–322
- Vandebriel, R. J., and van Loveren, H. 2010. Non-animal sensitization testing: State-of-the-art. Crit. Rev. Toxicol. 40:389–404
- Zhang, X., Chen, X., Song, H., et al. 2005. Activation of the Nrf2/anti-oxidant response pathway increases IL-8 expression. Eur. J. Immunol. 35:3258–3267