Abstract
The potential immunotoxicity of tabalumab was assessed as a component of standard pre-clinical toxicology studies in cynomolgus monkeys. To evaluate potential tabalumab-associated immunosuppression after antigen challenge, cynomolgus monkeys were administered placebo control or tabalumab in three immunotoxicological safety studies. Study 1, a 4-week pilot study, evaluated biweekly intravenous (IV) control, and 0.3, 1.0, 5.0, and 15.0 mg/kg tabalumab doses. Study 2 evaluated IV control, and 0.1, 1.0, and 30.0 mg/kg tabalumab doses biweekly for 6 weeks. Study 3 evaluated IV control and 0.1, 1.0, 30.0 mg/kg, and subcutaneous (SC) 30.0 mg/kg tabalumab biweekly for 6 months, with recovery (16 weeks) to monitor standard immunotoxicity endpoints. T-cell dependent primary and secondary antibody responses to tetanus toxoid antigen challenge (4-week and 6-week studies) or keyhole limpet hemocyanin (KLH; 6-week and 6-month studies) were evaluated as a measure of immunocompetence, together with quantitation of T- and B-cell subsets. In addition, anti-tabalumab antibody formation (6-week and 6-month studies) was assessed. The results indicated that, despite expected decreases in circulating B-cell populations, no changes in follicle histopathology or organ weights, except decreases in spleen weight (after 6-months of 30 mg/kg IV/SC treatment only), were attributed to tabalumab. Non-adverse microscopic decreases in size or number of germinal centers in spleen, mesenteric, and mandibular lymph nodes occurred, but without an effect on antibody responses to KLH or tetanus. At 16-weeks recovery, microscopic compound-related changes observed after 6 months of treatment were completely reversed (0.1 mg/kg group) and partially reversed (1.0 and 30.0 mg/kg groups), while peripheral blood B cells remained 66–72% reduced from baseline. Despite reduced germinal centres in lymphoid organs, and reductions in circulating B cells, T-cell-dependent humoral immunity was maintained following tabalumab administration in three safety studies in cynomolgus monkeys.
Introduction
Tabalumab is a fully human immunoglobulin G subclass 4 (IgG4) monoclonal antibody (MAb) that binds and neutralizes both soluble- and membrane-bound B-cell activating factor (BAFF), a B-cell survival factor (Manetta et al., Citation2014). Tabalumab was being investigated for the treatment of autoimmune diseases and cancer; however, those programs have been recently terminated. Dysregulated BAFF expression may contribute to autoimmune diseases via effects on abnormal B-cell activation, proliferation, survival, immunoglobulin secretion, and on certain B-cell malignancies by affecting B-cell proliferation and survival (Mackay et al., Citation2005; Mackay & Schneider, Citation2009). Thus, tabalumab may be of potential therapeutic benefit in these diseases.
BAFF (or ‘BLyS’) is a member of the tumor necrosis factor (TNF) superfamily and is expressed by and cleaved from monocytes, macrophages, dendritic cells, neutrophils, T-cells, stromal cells, astrocytes, fibroblast-like synovial cells, nurse-like cells, osteoclasts, and ductal epithelial cells (Kalled, Citation2006; Mackay et al., Citation2003). It is a transmembrane protein that is also secreted in soluble form and is a principal growth factor for survival during B-cell maturation and development. BAFF receptors (BR3, transmembrane activator and calcium-modulator and cyclophilin ligand interactor [TACI], and B-cell maturation antigen [BCMA]) are present on different immune cells including B-cell and T-cell subsets, and plasma cells (Mackay et al., Citation2005). Receptor engagement by BAFF leads to multiple immune cell functional activities including effects on peripheral B-cell survival and maturation, promotion of isotype switching to IgA, and co-stimulatory activity in T-cell activation; all are required in T-independent antibody responses (Mackay et al., Citation2005).
Belimumab, a humanized IgG1λ anti-B-lymphocyte stimulator (BLyS) MAb was the first BLyS antagonist MAb to be tested in clinical trials of systemic lupus erythematosus (SLE). Treatment reduced the number of circulating CD20+ B cells along with reductions in anti-dsDNA IgG antibody levels and total IgG levels (Furie et al., Citation2011). Treatment also reduced populations of activated and naïve B cells and short-lived plasma cells, but not T-cells and memory B cells. In three clinical trials, the most common serious adverse reactions were serious infections (6.0 and 5.2%, respectively, in patients receiving belimumab and placebo), and due to its mechanism of action belimumab may interfere with the response to immunization (GlaxoSmithKline, Citation2014). In pre-clinical studies, changes in circulating and lymphoid organ cell populations were well characterized; however, consequences of the observed changes in B-cell compartments on in vivo immune responsiveness were not evaluated (Baker et al., Citation2003; Halpern et al., Citation2006; Vugmeyster et al., Citation2006).
Many recently developed therapies target B cells with the intent of reducing autoimmune antibody responses (Sabahi & Anolik, Citation2006), with the majority aimed at depleting B cells. An important safety concern for B-cell depleting agents is the level of immunosuppression that may occur and pre-dispose some individuals to opportunistic infections, as was noted in patients treated with ocrelizumab, an anti-CD20 MAb (Mysler et al., Citation2013). However, in contrast to anti-CD20 antibodies that kill B cells, BAFF antagonists act indirectly by selectively modifying naïve B-cell development, which results in a reduction, but not depletion, of B cells and spares memory B cells (Stohl et al., Citation2012). In theory, this class of B-cell modulating agents could be less immunosuppressive overall than the anti-CD20 class of immunotherapeutics, a concept that is supported by studies in mice that show BAFF antagonism eliminates naïve B cells, but spares most elements of acquired and natural immunity (Scholz et al., Citation2008). However, recent clinical trials with BAFF/TACI antagonists have also observed issues with increased rates of infection (Vincent et al., Citation2013).
The consequences of unintended immunosuppression can range from increased infection risk and reductions in therapeutic vaccine responses to increased incidence of malignancies. Therefore, understanding pre-clinical (animal) immunotoxicology findings and their relevance to humans is important. A recent publication (Martin & Bugelski, Citation2012) evaluated several immuno-modulatory targets in non-human primates (NHP) compared to humans and showed good concordance between NHP immune endpoint changes and human pharmacodynamic parameter effects. However, there was limited concordance between NHP adverse effects with human adverse effects. To address key immune function endpoints that were of relevance in our studies, we focused a literature search on immunomodulatory agents used for the treatment of autoimmune diseases (). While not exhaustive in nature, the pre-clinical results of these highlighted biologicals, obtained at exposure levels at or well above anticipated clinical levels, indicated some immunotoxicity potential for each therapeutic agent. Importantly, as shown by the immune assessment comparisons in , this prediction was verified during clinical studies at therapeutic dose levels and durations. Overall, this correlative information supports the general relevance of animal immunotoxicity screening in identifying potential human immunomodulatory drug effects.
Table 1. Examples of correlation of pre-clinical immunotoxicological findings with clinical immunomodulatory effects.
The objective of this paper was to present the results of a series of three pre-clinical NHP studies that investigated the immunotoxic potential of tabalumab. Study 1 was a pilot toxicity study with 4 weeks of tabalumab dosing (4-week study); Studies 2 (6 weeks of tabalumab dosing [6-week study]) and 3 (6 months of tabalumab dosing [6-month study]) were Good Laboratory Practice (GLP) toxicology studies with detailed immunotoxicology endpoints. As part of the immunotoxicology testing strategy for tabalumab, reproductive and developmental immunotoxicology studies were also performed, but will be reported separately. Collectively, these studies focused on immunophenotyping of peripheral blood lymphocyte subsets, evaluation of immune organ histology, and functional T-cell dependent humoral responses to immunogen (KLH and tetanus toxoid) challenge.
Materials and methods
The 4- and 6-week studies were executed at Shin Nippon Biological Laboratories (SNBL) USA Ltd. (Everett, WA) August 2002–April 2004; the 6-month study was executed at Covance Laboratories, Inc. (Madison, WI) November 2006–March 2008. Assessment of immunogenicity (anti-tabalumab antibodies) was performed at Eli Lilly and Company (Greenfield, IN) for the 6-week study and by Midwest BioResearch (Skokie, IL) for the 6-month study. The 6-week and 6-month studies were conducted in accordance with the Food and Drug Administration (FDA), 21 Code of Federal Regulations (CFR) Part 58, and Organization for Economic Cooperation and Development (OECD) GLP standards in place at the time of study initiation. The 4-week pilot study was not subject to requirements of any GLP standards; however, the study was conducted according to standard operating procedures of SNBL.
Animal care
Male and female cynomolgus monkeys (Macaca fasicularis) from China (4- and 6-week studies) and Vietnam (6-month study) were assigned to each study using a procedure designed to achieve body weight balance across treatment groups. Animals were housed individually in a monitored and controlled environment at 18–29 °C, with humidity maintained from 30–70%. All animals were acclimated for at least 7 days prior to study initiation. Animal procedures and housing were conducted in compliance with USDA Regulations and institutional guidelines and were approved by the Institutional Animal Care and Use Committee. Animals ranged in age from 2–6 years, with starting body weights of 2.2–4.9 kg for males and 2.2–3.9 kg for females. Animals were offered food daily (Purina Mills Laboratory Fiber Plus® Monkey Diet, Animal Specialties, Hubbard, OR for 4- and 6-week studies; Certified Primate Diet #2055C, Harlan Teklad for 6-month study) and water ad libitum.
Study procedures
The vehicle used in the 4-week study was phosphate buffered saline (PBS), 10 mM, pH 7.5. In the 6-week study, the vehicle used was 8 mM Citrate/0.016% Tween 80 + 0.6% (w/v) sodium chloride. The vehicle used in the 6-month study was 10 mM citrate buffer (pH 6.0 [±0.2]), 0.02% (w/v) polysorbate 80 with 150 mM sodium chloride prepared in sterile water for injection (USP; United States Pharmacopoeia). Tabalumab was administered per each study protocol as summarized in with doses (mg/kg) ranging from 0.3–15.0 in the 4-week study to 0.1–30.0 in the longer duration studies. All doses of tabalumab were administered intravenously with the exception of one group in the 6-month study that received tabalumab 30.0 mg/kg subcutaneously. The treatment phase in each study was followed by a recovery period (). In the 4- and 6-week studies, animals were anesthetized and then euthanized by exsanguination at the end of the recovery period (Days 193 and 156, respectively). In the 6-month study, four animals/sex/group were euthanized at the end of the 26-week dosing phase (Day 185); the last two animals/sex/group were euthanized at the end of the recovery phase (Day 297). Live-phase observations and evaluations included clinical signs, body weight, food consumption, electrocardiography (ECG), hematology, and chemistry endpoints. Necropsy evaluations included gross and microscopic pathology endpoints.
Table 2. Study characteristics and immunotoxicologic endpoints of Studies 1 (4-weeks), 2 (6-weeks), and 3 (6-months).
Hematology and immunophenotyping
For all three studies, EDTA-anti-coagulated whole blood samples were collected per the timing schedule in . Hematology parameters assessed included red and white blood cell counts, hematocrit, hemoglobin concentration, platelet count, mean corpuscular volume, mean corpuscular hemoglobin and hemoglobin concentration, reticulocyte count, and white blood cell differential. Hematology parameters were examined using an Advia 120 hematology analyzer (Bayer Corporation, Tarrytown, NY). Blood samples for immunophenotyping were maintained at ambient room temperature until stained with antibodies. The antibodies used were against the following surface antigens: CD3, CD4, CD8, CD14, CD16, CD20, CD21, CD27, CD44, CD45, and CD69 (depending on study, see ), along with the appropriate isotype controls. Additionally, in the 4-week study, IgD and IgM expression on CD20+ or CD40+ B cells were analyzed. Specific information on the manufacturer, clone, and fluorochromes used can be found in the Supplemental Table.
Leukocytes were isolated by whole blood lysis using FACS Lyse (Becton Dickinson, San Jose, CA) solution prior to flow cytometry. Samples were analyzed using a FACSCalibur flow cytometer with CellQuest software (Becton Dickinson). Cells of interest were identified by measuring relative percentages of fluorochrome positive cells in the combined lymphocyte and monocyte light scatter gate (4- and 6-week studies) or a CD45+ vs side scatter lymphocyte only gate (6-month study). Absolute cell counts were calculated using absolute lymphocyte counts from the hematology analyzer and multiplication by the relative percentage of each lymphocyte marker.
Tetanus toxoid and keyhole limpet hemocyanin antibody detection
T-cell-dependent antibody (IgG and IgM) responses were measured using tetanus toxoid and keyhole limpet hemocyanin (KLH). Animals from the 4- and 6-week studies were immunized via intramuscular (IM) injection with a 0.5 ml dose of tetanus toxoid (Calbiochem/Novabiochem, San Diego, CA) that was reconstituted to a concentration of 100 mg/ml in 50% Imject alum adjuvant (Pierce Protein Biology Products, Rockford, IL) in sterile phosphate-buffered saline (PBS). Animals from the 6-week and 6-month studies were immunized with either a 1.0 ml dose of 3.0 mg/ml (6-week) or a 0.5 ml dose of 500 µg/animal of KLH. In the 6-week study, lyophilized KLH (Sigma Chemical Co., St. Louis, MO) was reconstituted with PBS (Spectrum Chemicals, New Brunswick, NJ) to a concentration of 3 mg/ml. For the 6-month study, KLH (Imject Mariculture KLH, Pierce Protein) was emulsified in Freund’s Incomplete Adjuvant (Pierce Protein) to a final concentration of 1 mg KLH/ml. Freund’s Incomplete Adjuvant was used in an attempt to increase the KLH antibody response and to reduce the animal-to-animal variability. Immunizations were administered and blood samples collected for antibody analysis at timepoints noted in . Serum anti-tetanus and anti-KLH IgM and IgG responses were determined by enzyme-linked immunosorbent assays (ELISA).
Toxicokinetics
The serum exposure of tabalumab was determined in all studies but will be reported from the 6-week and 6-month studies to focus on the relevant doses of tabalumab. Blood samples were collected at the times specified in and were centrifuged within 1 h of collection; serum was harvested and stored at −70 °C until shipped for analysis. Concentrations of tabalumab were determined by antigen-capture ELISA methods; these values were subsequently used to determine serum exposure. Toxicokinetic parameters included area under the concentration-time curve (AUC), peak concentration (Cmax), and elimination half-life (t1/2).
Anti-tabalumab antibody detection
Anti-tabalumab antibodies (ATA) were determined from serum samples in the 6-week and 6-month studies. Samples were collected pre-treatment and on Days 57, 99, and 155 in the 6-week study from all animals, and pre-treatment and on Days 238 and 294 from recovery phase animals only (2/sex/group) in the 6-month study (). Serum samples were stored at −70 °C until shipped on dry ice to Eli Lilly and Co. (Greenfield, IN; 6-week study) or Midwest BioResearch (Skokie, IL; 6-month study). For the 6-week study, the ATA method used a ‘bridging’ ELISA format where 96-well microtiter plates were coated with tabalumab and blocked with a gelatin-based protein buffer prior to sample addition. Samples diluted 1:25 (assay minimum required dilution) in blocking buffer and further diluted in 4% base control cynomolgus monkey serum were incubated on the plate, washed, and bound ATA detected with horseradish peroxidase (HRP)-labeled tabalumab. Following another wash step, addition of a tetramethylbenzidine substrate solution produced a color reaction and the resulting absorbance was read at 450 nm using a microtiter plate reader. Samples positive for tabalumab-reactive antibodies were titered until a negative signal was achieved. Antibody positive samples were determined by comparing the sample optical density (OD) with a cut-point OD (non-specific binding of a normal cynomolgus monkey serum pool plus the cut-point constant established during assay validation). Assay sensitivity was estimated at 40 ng/ml; however, the assay exhibited drug interference if a sample contained > 0.1 µg tabalumab/ml.
To improve the drug tolerance of ATA assay, a new method was developed and validated for the 6-month study. This assay utilized a biotin extraction with acid disassociation sample pre-treatment in which anti-tabalumab antibodies in a sample were extracted from serum using biotinylated tabalumab and the extracted antibody biotin complexes were captured on the solid surface of a streptavidin coated 96-well microtiter plate. The antibodies were then dissociated from the capture complexes by the addition of acetic acid, neutralized in buffer, and transferred to a high binding 96-well microtiter plate. After blocking, HRP-labeled tabalumab was added to detect bound ATA. Similar steps as described for the bridging ELISA method were used to complete the enzymatic reaction, measure sample optical densities, and determine antibody positive samples. For this method, the assay sensitivity was estimated at 0.869 µg/ml and drug tolerance 500× higher at 50 µg/ml.
Necropsy and pathology
On the day of euthanization, terminal body weights and organ weights were recorded and protocol-specified tissues from all animals were collected, processed, examined grossly, and sectioned for routine histologic examination following formalin-fixation and paraffin embedding. Relative organ weights were calculated as percentages of final body weight and brain weight. Immunohistochemical (IHC) analysis was performed in the 4-week (control animals and 15.0 mg/kg dose animals only) and 6-month (all animals) studies. On the scheduled day of sacrifice, portions of tissues were collected in 10% neutral buffered formalin (NBF) for 24–72 h, then transferred to 70% ethyl alcohol after which they were processed to paraffin blocks. In the 4-week study, tissue sections of the spleen, mandibular and mesenteric lymph nodes, and femoral bone marrow were processed for CD3 and CD20 antibody labeling; in the 6-month study, tissue sections of the spleen were processed for CD3, CD20, and CD21 antibody labeling.
Statistical analyses
Statistical analyses and determination of significant differences between dose groups were performed by SNBL Japan for the 4- and 6-week studies and by Covance for the 6-month study. Quantitative results were analyzed using one-factor analysis of variance (ANOVA) with an overall F-test at a 0.01 significance level for treatment effects. In the 4- and 6-week studies, monotonicity of dose responses was examined by a sequential trend test of linear treatment contrasts based on ordinal spacing of dose levels. The sequential trend test was performed at a 0.05 significance level. When the trend test was not significant, while the overall ANOVA was significant, a Dunnett’s test (Dunnett, Citation1964) was performed at a 0.05 significance level to evaluate non-monotonic dose response relationships. A Shapiro-Wilk’s test (Shapiro & Wilk, Citation1965) for normality and a Levene’s test (Levene, Citation1960) for homogeneity of variances was performed at the 0.001 significance level in the 4- and 6-week studies and at the 0.05 level for the 6-month study to assist in interpretation of treatment effects. For flow cytometry phenotypic markers, absolute counts and the square root of relative percentages were analyzed using the methods described above.
In the 4-week study, for analysis of tetanus toxoid antibody formation, one-factor analysis of variance and pairwise difference tests were used to compare group means for the Day 1 ‘baseline’ antibody values (µg/ml). Examination of recovery period data included repeated measures ANOVA and pairwise difference tests to compare group means for antibody values (µg/ml) on Days 8, 15, 22, and 29. Results of statistical analyses of anti-KLH and anti-tetanus toxoid antibodies in Study 2 were analyzed in two separate runs: one for Groups 1 and 2 and one for Groups 2–5. For Groups 1 and 2, the effect of immunization on anti-KLH and anti-tetanus toxoid antibody response was evaluated for each sex and at each timepoint. The contrast of the means for these two groups was evaluated using a t-test at a 0.05 significance level in one-factor analysis of variance (Winer, Citation1971) model. Shapiro-Wilk and Levene tests were performed as described above and logarithmic or rank transformations were applied accordingly for anti-tetanus toxoid and anti-KLH antibody responses. For Groups 2–5, quantitative results for each sex and time point were analyzed using a one-factor analysis of variance with an overall F-test at a 0.01 significance level for treatment effects. Monotonicity of dose responses was examined by a sequential trend test of linear treatment contrasts based ordinal spacing of dose levels (Tukey et al., Citation1985). No statistical analysis was performed on the anti-tabalumab antibody data.
Results
Survival, clinical observations, and hematology
Animals in all studies survived until euthanized, except for one female in the 0.1 mg/kg group in the 6-month study that was euthanized on Day 182 with a probable dislocated shoulder that was not considered treatment-related. Overall, in all three studies, there were no treatment-related effects on body weight, food consumption, or clinical examinations (physical, neurological, ophthalmic). Any statistically significant differences in ECG results reflected normal variation and did not appear dose-responsive. Changes in ECG parameters that may have been treatment-related were within normal range. Tabalumab treatment produced no important effects on any hematological parameters for any of the studies.
Effects of tabalumab on lymphocyte subsets
CD20+ B cells
Tabalumab treatment modulated both the relative percentage and absolute counts of peripheral blood CD20+ B cells. Relative percentages of CD20+ B cells were normalized to the average baseline levels; the percentage of baseline is presented in . In the 4-week study, with doses up to 15 mg/kg administered on Days 1 and 8, an initial increase in B cells was noted for the 1 and 15 mg/kg doses on Day 8 (). After Day 8, B-cell values returned to baseline and then began to drop below baseline levels in a dose-dependent manner. The mean reduction of CD20+ B cells in animals receiving 0.3 mg/kg reached ≈64% of baseline levels by Day 60, while animals dosed with 15 mg/kg reached a maximum reduction of 54% of baseline on Day 193. B-cell recovery to baseline was observed by Day 172 in animals receiving < 5 mg/kg, while the 5- and 15-mg/kg groups remained decreased at levels of 30 and 46% reductions, respectively, at the final timepoint on Day 193.
Figure 1. Relative percentages of peripheral CD20+ B cells normalized to baseline in cynomolgus monkeys treated with tabalumab. (a) 4-week study (Study 1): baseline average at Day 0 represents mean of Day −1 and Day 1; arrows represent dosing days. (b) 6-week study (Study 2): baseline average at Day 0 represents mean of Day −5 and Day 1; arrows represent dosing days. *Non-immunized, all other groups immunized for tetanus toxoid and KLH. (c) 6-month study (Study 3).
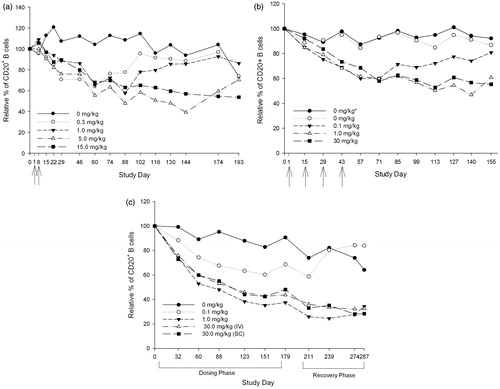
Since the initial B-cell increase was characterized in the 4-week study, an early timepoint was not evaluated in studies of longer exposure. In the 6-week study, tabalumab treatment maximally reduced the mean absolute counts of CD20+ cells by 35, 53, and 55% in, respectively, the 0.1, 1.0, and 30 mg/kg treatment groups (). The maximum effect of tabalumab treatment on suppression of CD20+ cells was reached by Day 57 in the 0.1 mg/kg dosing group, by Day 140 for the 1 mg/kg dosing group, and by Day 155 for the 30 mg/kg group. The decrease in CD20+ cells showed a nadir between ≈2 and 4 weeks (between Day 57 and 71) after the end of the 6-week dosing period in the 0.1 mg/kg group. Values were returning towards control level by the end of the 16-week recovery phase in the 0.1 mg/kg group, as evidenced by the fact that mean absolute counts of CD20+ cells were reduced 21% when compared to baseline values on Day 155. Return to control values was not clearly established by 16 weeks after the end of the dosing (Day 155) for the 1 and 30 mg/kg groups; there was a continued decrease of 47 and 55%, respectively, in mean absolute counts compared to baseline. Changes in the mean relative percentage of CD20+ values mirrored the extent of the mean absolute count changes (data not shown).
In the 6-month study, decreases in CD20+ B cells were noted at Days 32–179 of the treatment period for all dose levels, ranging from 31–52% reductions from pre-treatment baseline to Day 179 (). Mean group reductions were generally similar in both the 1 and 30 mg/kg dose groups. CD20+ B cells continued to decrease for the higher dose levels into the recovery period. These decreases were partially or completely recovered for animals given 0.1 mg/kg by Day 55 of the recovery phase (study Day 239), but persisted for the remaining treated animals through the end of the 16-week recovery phase.
B-cell subsets
To gain better insight into any effects on B-cell sub-populations during the recovery period, B-cell subsets were analyzed at two timepoints in the 4-week study, i.e. on Days 172 and 193. The following subsets were analyzed: IgD and IgM expression on CD20+ B cells or CD40+ B cells, as well as mature B cells (CD20 + CD21+), and memory B cells (CD20 + CD27+). Consistent with the CD20+ B-cell reductions noted in the 15 mg/kg group on Day 172 (), mature B cells expressing CD20 and CD21 were also reduced (data not shown). There were no reductions noted in the other dose groups. Additionally, the 15 mg/kg group showed reductions from control group levels in immunoglobulin-bearing B-cell subsets, including those for CD40 + IgM+, IgD + IgM+, CD20 + IgD+, CD20 + IgM+, and CD40 + IgD+ cells; these normalized to control levels by Day 193. Memory B cells (CD20 + CD27+) were not affected at Days 172 and 193 (data not shown).
Similar findings on selected B-cell subsets were observed in the 6-month study for all dose groups, where naïve (CD20 + CD21 + CD27−), CD40+, CD40 + CD20+, and CD20 + CD21+ B cells were also reduced during the treatment period (). The 0.1 mg/kg group showed clear recovery of the B-cell subsets by Day 239 of the recovery period that persisted through the end of the recovery period on Day 287. Slight reductions from vehicle control were noted in memory B cells (CD20 + CD21 + CD27+) for the 1 and 30 mg/kg groups (). It is unclear why vehicle control animals showed persistent reductions from baseline in memory B cells.
Table 3. Mean relative percentages and percentages of baseline of selected B-cell subsets in the 6-month study animals.
CD3+ T-cells/T-cell subsets
Total T-cells and T-cell subsets were assessed through measures of CD3+, CD3 + CD4+, and CD3 + CD8+ cells. In the 4-week study group, mean relative percentages and absolute counts of CD3+ cells increased in a dose-dependent fashion on Day 60 in the 1.0, 5.0, and 15.0 mg/kg dose groups (data not shown). Except for the highest dose group (15.0 mg/kg), these changes were reversible and complete recovery was observed at the end of the study (Day 193). Despite the observed changes in CD3+ cells, there was no tabalumab-related effect on the ratio of T-lymphocyte subsets, CD3 + CD4+, and CD3 + CD8+ cells (data not shown). In studies of longer exposure duration out to 26 weeks, tabalumab treatment had no effect on T-cells or the major T-cell subsets assessed using CD3+, CD3 + CD4+, and CD3 + CD8+ markers (data not shown). No distinct differences between hosts given 30 mg/kg intravenously or subcutaneously were noted.
CD16+ natural killer cells and CD14+ monocytes
Natural killer cells were identified as CD16 + CD20− cells and monocytes as CD14 + CD20− cells. Tabalumab had no effect on monocytes in the 4-week study, and during studies with exposure up to 6 months, tabalumab treatment had no effect on NK cell numbers (data not shown).
Activation markers on T and NK cells
In the 4-week study, activation of T-cells was evaluated by measures of co-expression of CD4 or CD8 with CD44 or CD69. NK cell activation was studied using co-expression of CD16 and CD69. Tabalumab treatment did not demonstrate changes in the relative percentages of activated CD4+, CD8+, or NK cells (data not shown).
Effects of tabalumab on total IgG, IgA and IgM
Total IgG, IgA and IgM levels were assessed in the 4-week study. There were no significant changes in any of these immunoglobulin classes out to Day 193 of study (data not shown).
Effects of tabalumab on T-cell-dependent antibody responses to tetanus toxoid and keyhole limpet hemocyanin
Tetanus
Anti-tetanus IgG antibody development following tetanus toxoid immunization was assessed in the 4- and 6-week studies. Prior to toxoid immunization, tetanus-reactive IgG was detected in 19/20 monkeys in the 4-week study; no positive anti-tetanus toxoid antibody titers were detected in any animals in the 6-week study prior to immunization. All immunized animals in both studies developed positive or increased anti-tetanus IgG titers following immunization. In both studies, there were no significant differences in levels of anti-tetanus IgG antibodies in tabalumab-treated monkeys compared to control animals. and present the kinetics of the anti-tetanus IgG antibody responses in the 4- and 6-week studies.
Figure 2. Study 1 (4-week): Kinetics of human immune response to tetanus toxoid in cynomolgus monkeys treated with tabalumab. Animals were given weekly doses of 0, 0.3, 1.0, 5.0, or 15.0 mg/kg tabalumab for 2 weeks. Monkeys were immunized with tetanus toxoid on Day 1 of tabalumab dosing, and a secondary challenge of tetanus toxoid was administered on Day 11. Serum was collected over a 193-day period to assess primary and secondary IgG response to tetanus toxoid.
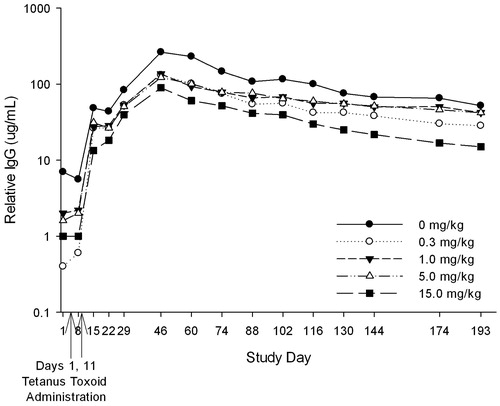
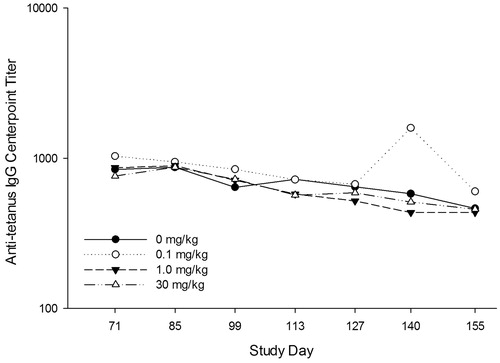
Figure 3. Study 2 (6-weeks): Tetanus toxoid-reactive IgG antibody titers in cynomolgus monkeys treated with tabalumab. Animals were treated with tabalumab at 2-week intervals for 6 weeks at dose levels of 0, 0.1, 1.0, or 30.0 mg/kg. Monkeys were immunized on Day 57 of the study with tetanus toxoid; serum was collected over a 155-day period to assess the primary IgG response to tetanus toxoid. Levels of tetanus-toxoid specific IgG in serum at Days 1 and 57 were below the detectable threshold and are not shown.
KLH
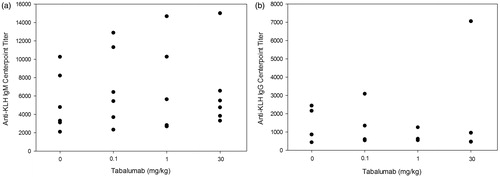
Responses to KLH were determined in the 6-week and 6-month studies. In the 6-week study, one female in the 0.1 mg/kg treatment group had measurable anti-KLH titer before immunization (Days 1 and 140). Positive titers of KLH-reactive IgM were detectable in 23/24 immunized animals on Day 148 (7 days after KLH administration; ). By Day 155, IgM titers remained positive in all but the same animal that did not respond on Day 148 (female in 1 mg/kg treatment group). The Day 155 IgM response appeared to be declining in magnitude relative to the Day 148 response, with mean and median center-point titers ranging from 50–77% lower than those observed on Day 148 (data not shown). Anti-KLH IgG was detected 7 days following immunization on Day 148 in 9/24 animals in the treated groups. By Day 155, the incidence of responders increased to 18/24 treated animals (); one of the IgG non-responders was the same female in the 1.0 mg/kg group that did not develop an IgM titer. There was no apparent difference in titer responses among any of the treated groups compared with controls.
Figure 4. Study 2 (6-weeks): KLH-reactive IgM and IgG antibody titers in cynomolgus monkeys treated with tabalumab. Monkeys were immunized on Day 141 with KLH and serum was collected on Days 1, 140 (pre-immunization), 148, and 155 to assess the primary IgM and IgG responses to KLH. (a) The IgM response to KLH at Day 148; (b) the IgG response to KLH at Day 155.
Three animals in the 6-month study had measurable anti-KLH IgM titers prior to immunization (two females in the 1 mg/kg group and one female in the 30 mg/kg intravenous group, all dosing phase). Positive titers of KLH-reactive IgM were detectable in 56/60 animals 10 days after KLH immunization (). Two males (one in the control group and one in the 30 mg/kg subcutaneous, both in dosing phase) and two females (both in the 30 mg/kg subcutaneous group, one in the dosing phase and one in the recovery phase) did not have detectable IgM. By post-immunization Day 17, only two animals continued to have IgM that were not measurable (one male in the 30 mg/kg subcutaneous group, in dosing phase; and one female in the 30 mg/kg subcutaneous group, in recovery phase). From 10–17 days after KLH immunization, mean interpolated IgM titers generally decreased or maintained similar values for all groups. Administration of tabalumab did not produce any statistically remarkable changes for mean interpolated anti-KLH IgM values.
Figure 5. Study 3 (6-months): KLH-reactive IgG and IgM antibody titers in cynomolgus monkeys treated with tabalumab. Dosing phase animals were immunized on Day 161 and recovery phase animals were immunized on Day 273 with KLH and serum was collected on Days 1, 171, 178, and 185 from dosing and Days 1, 283, 290, and 297 from recovery phase animals to assess the primary IgG and IgM responses to KLH. (a) The IgM response to KLH at Day 171 for dosing and Day 283 for recovery phase animals; (b) the IgG response to KLH at Day 178 for dosing and Day 290 for recovery phase animals.
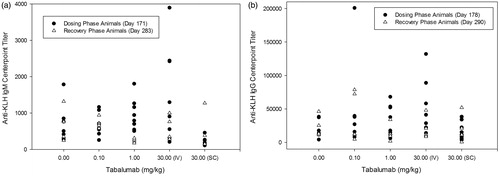
For anti-KLH IgG, 14/60 animals in the 6-month study had measurable titers before immunization. Immunization with KLH produced measurable anti-KLH IgG responses in all animals (60/60) at both timepoints post-immunization (). In most animals (50/60), mean interpolated IgG titers generally increased above pre-treatment values or maintained similar values for all groups 17–24 days post-KLH injection. Administration of tabalumab did not produce any statistically remarkable changes for mean interpolated anti-KLH IgG titers.
Toxicokinetics
Toxicokinetic data were assessed from the 6-week and 6-month studies. In the 6-week study, mean trough serum concentrations showed a linear increase of ≈2-fold from Day 15 to a peak at Day 57 (); the latest Day with detectable concentration for the 0.1 mg/kg group was Day 113, with the other doses having their lowest detectable doses on Day 155 (). Mean serum half-life was 13.49 and 22.57 days in the 1.0 and 30.0 mg/kg groups, respectively. Serum half-life was not calculated for the 0.1 mg/kg group, due to an insufficient number of samples with quantifiable concentrations after the final dose. There were no obvious differences in exposure between male and female monkeys for trough concentrations or half-life.
Table 4. Mean serum concentration of tabalumab in 6-week study animals (Study 2).
In the 6-month study, tabalumab serum levels were detected above the lower limit of quantitation (> 0.025 μg/ml) in 11/355 control group samples. Most of these were only slightly above the limit of quantitation and considerably lower than the mean value at the corresponding time point in the 0.1 mg/kg group, indicating no mis-dosing of control group animals, but rather possible assay variability in serum background absorbance values between different animals. AUC(0–336h) and Cmax increased in relation to dose on Day 1 compared to Day 169 (). The increases were approximately dose-proportional between the 1 and 30 mg/kg groups, but not between the 0.1 and 1 mg/kg groups. Day 169 AUC were ≈2-fold higher than Day 1 for the 1 and 30 mg/kg groups, indicating accumulation of tabalumab in the serum after 6 months of dosing; this was not observed for the 0.1 mg/kg group. Bioavailability for tabalumab from the subcutaneous site was ≈72%. In the recovery phase, the 0.1 mg/kg group had measurable serum levels through Day 214. The half-life of elimination for the 1 and 30 mg/kg groups ranged from ≈11–27 days and there was no difference between the intravenous or subcutaneous routes of administration. Toxicokinetic parameters were not calculated for the 0.1 mg/kg group due to insufficient numbers of timepoints with quantifiable concentrations after the final tabalumab dose. No obvious sex differences in exposure between males and females were observed.
Table 5. Toxicokinetics in the 6-month study animals (Study 3).
Anti-tabalumab antibodies (immunogenicity)
The immunogenicity of tabalumab was evaluated in the 6-week and 6-month studies. In the 6-week study, tabalumab-reactive antibody analysis was not performed in the 0.1, 1.0, or 30.0 mg/kg groups on Day 57 or in the 1.0 and 30.0 mg/kg groups on Day 99 due to expected assay interference that would result from a presence of circulating tabalumab concentrations of > 0.1 µg/ml. On Day 155, positive anti-tabalumab antibodies were present in one of six animals in all groups, with the exception of the 30 mg/kg group (data not shown). However, since the incidence for tabalumab-treated groups was no different than that of control groups, it was concluded that no substantial anti-drug antibody response occurred. For the 6-month study, because of the known issue of drug interference in the immunogenicity-screening assay, only serum samples from pre-treatment and recovery animals were evaluated. No detectable tabalumab reactive antibody response was seen in tabalumab-treated animals 2 or 4 months after the last administered dose, although a presence of circulating tabalumab in some animals may have interfered with accuracy of the anti-tabalumab analyses (data not shown).
Necropsy and pathology
In the 4- and 6-week studies, there were no treatment-related changes for gross pathology, organ weights, or histopathology (data not shown). In the 6-month study, a decrease in spleen weight for all animals in the dosing phase of both 30 mg/kg groups was considered compound-related and correlated microscopically with a decrease in the size and/or number of white pulp germinal centers. In all dosing groups in both the dosing phase and recovery phase, compound-related microscopic decreases in the size and/or number of white pulp germinal centers occurred in the spleen and mesenteric and mandibular lymph nodes. These changes were generally minimal-to-moderate, except for one female in the 0.1 mg/kg group with a severe decrease in germinal centers of the spleen. No organ weight changes in recovery phase animals were compound-related. No macroscopic findings were considered compound-related and there were no morphologic effects on T-cell dependent zones. Histopathology data were consistent with the immunophenotyping data.
Immunohistochemistry
Immunohistochemistry was performed in the 4-week and 6-month studies. In the 4-week study, there were no treatment-related effects on anti-CD20 and anti-CD3 immunoreactivity in mesenteric lymph node, mandibular lymph node, spleen and right femoral bone marrow tissue sections in the control or 15 mg/kg groups (only groups analyzed). In contrast, in the 6-month study, there was decreased white pulp CD20 and CD21 staining in the spleen of all treated animals (data not shown). The IHC staining pattern generally correlated with the lymphoid changes seen with the routine hematoxylin and eosin (H&E)-stained tissues; however, two females in the 30 mg/kg intravenous group had a relative decrease in CD21 staining without a noticeable decrease in the examined H&E spleen section germinal centers. In recovery phase animals, there was decreased splenic white pulp CD20 and CD21 staining in all animals in both 30 mg/kg groups (data not shown). There were no clear differences between dose groups for CD3 staining in any animals in either the dosing or recovery phases. The reductions in B cells (CD20 and CD21) and microscopic changes in the spleen and lymph nodes were not considered adverse.
Discussion
The immunotoxicity potential of tabalumab treatment was investigated as part of three pre-clinical safety studies. The functional and histological immune parameters assessed were chosen based on similar tests that could be used in clinical trials to monitor overall immunocompetence. Both immunized and non-immunized control animal study groups were included in the 6-week study because at the time this study was conducted, it was not known if immunization with either KLH or tetanus toxoid would significantly affect the immune or histologic endpoints being evaluated. No apparent differences related to immunization were observed, so subsequent studies did not include non-immunized control groups.
In the studies presented herein, the predominant immunopharmacologic effect of tabalumab treatment was modulation of peripheral B-cell populations. In the 4-week study, following two doses of tabalumab, a slight increase in the relative percentage of circulating B cells was noted at Day 8 in the 1 and 15 mg/kg doses followed by a progressive decline in B cells that persisted into the recovery period. The observed B-cell nadir typically occurred first for the lowest dose (Day 60), with a progressively longer time to nadir as the dose increased. With the middle dose evaluated in all studies, 15 mg/kg, the B-cell nadir occurred at Day 173 and did not reduce further at the last recovery time point evaluated (Day 193). This finding is consistent with the pharmacokinetic analyses where detectable levels of tabalumab were still noted in the serum on Day 144 and the half-life of tabalumab was calculated to be ≈20 days for the 15 mg/kg group. With longer treatment duration (6 months), B-cell reductions continued well into the recovery period where at Day 270, ≈3 months following the final dose, B-cell reductions up to 68% from baseline were observed. At the final recovery timepoint, B cells appeared stable and did not show additional decreases. B cell changes were also noted in lymphoid organs, where decreases in germinal center size and/or number was noted in the spleen, and in mesenteric and mandibular lymph nodes. These effects are similar to those observed in a cynomolgus monkey study with belimumab, where treatment reduced splenic and mesenteric follicular B-lymphocytes in addition to a corresponding decrease in circulating B cells (Halpern et al., Citation2006). Tabalumab had no effect on circulating T-cells/T-cell subsets (CD3 + CD4+, CD3 + CD8+), monocytes or NK cells; tabalumab treatment also did not change the number of circulating activated T or NK cells, or induce a measurable anti-drug antibody response.
BAFF is important in the development and maintenance of T-dependent humoral immunity because BAFF plays a role in T-cell co-stimulation and activation (Huard et al., Citation2001; Ng et al., Citation2004). Mice treated with a BAFF/APRIL antagonist (soluble B-cell maturation factor-Fc) show a loss of B cells and inhibition of specific antibody production to recombinant oligodendrocyte glycoprotein (MOG), a T-dependent antigen that suppresses both onset and progression of autoimmune encephalomyelitis disease (Huntington et al., Citation2006).
Monkeys in the 4-week study were found to be previously immunized with tetanus and had positive tetanus-reactive IgG titers. In contrast, in the 6-week study, animals with pre-existing anti-tetanus toxoid antibodies at pre-screening were not randomized to study. This difference in tetanus immune status between the two studies then provided for the assessment of tabalumab treatment on a primary and secondary immune response to tetanus challenge in the 6-week and 4-week studies, respectively. Tetanus and KLH specific antibody responses were unaffected by tabalumab treatment. Tetanus vaccine was administered when peripheral blood lymphocytes were near their nadir in all tabalumab treatment groups, while KLH immunizations occurred when peripheral B-cell numbers were recovering in the low dose group but were still at nadir levels for the mid- and high-dose groups. Throughout the study and specifically at the times of immunization, absolute lymphocyte counts in tabalumab-treated animals were not remarkably different compared to controls, nor did they change appreciably from pre-treatment levels. Although the majority of immunized animals developed a KLH-reactive IgG response by Day 155, it is likely that the peak extent of the IgG response to KLH may not have been attained by the final study assessment (14 days post-immunization), given that the peak response of tetanus-reactive IgG occurred in these animals 14–28 days after immunization. This response confirms that tabalumab treatment, despite causing a significant reduction in peripheral blood CD20 levels, did not prevent the production of either a primary or secondary antibody response by cynomolgus monkeys to T-cell-dependent antigens.
The published literature provides additional information on the assessment of immune parameters with other biologic entities that inhibit BAFF. Monkeys exposed to belimumab, currently approved for the treatment of SLE, had significant decreases in peripheral blood B-lymphocytes by 13 weeks of exposure, continuing into the recovery period, despite total lymphocyte counts similar to the controls. There were concomitant decreases in spleen and lymph node B-lymphocyte representation after 13 or 26 weeks of treatment with belimumab (Baker et al., Citation2003; Halpern et al., Citation2006). Microscopically, monkeys treated with belimumab for 13 or 26 weeks had decreases in the number and size of lymphoid follicles in the white pulp of the spleen. All findings were generally reversible within a 34-week recovery period. Similar reductions in levels of CD20+ peripheral blood, follicular. and marginal zone B cells in the spleen and lymph nodes occurred in cynomolgus monkeys treated weekly for 18 weeks with BR3-Fc, a molecule that blocks BAFF activity in vivo (Vugmeyster et al., Citation2006).
Inhibition of BAFF activity in vivo was also studied in adult male C57/BL6 mice treated with rhTACI-Ig, which inhibits binding of both BAFF and TACI to BAFF receptors and contains the extracellular ligand-binding portion of TACI and the Fc portion of human immunoglobulin G (TACI-Ig) (Wang et al., Citation2011). This immune function study explored the treatment effect of rhTACI-Ig on activated T-cells that were obtained from mice immunized with KLH. There was a significant positive relationship between KLH-induced BAFF levels in serum and the extent of spleen histopathology. Serum concentration of BAFF, APRIL, KLH IgM and IgG antibodies, and interleukin (IL)-4 were increased, but IL-2 synthesis was decreased, resulting in a down-regulation of the IL-2:IL-4 ratio. Antigen-specific T-cell proliferation, IL-5 production, the percentage of T-helper (TH) and activated T-cells were significantly up-regulated; however, IL-2 secretion and the percentage of naïve T-cells was down-regulated in vitro. Overall, the administration of rhTACI-Ig significantly inhibited T-cell proliferation, cytokine production, and T-cell differentiation, in line with the ability of rhTACI-Ig to neutralize both exogenous and endogenetic BAFF and APRIL (Wang et al., Citation2011).
Similar to effects observed in the pre-clinical studies presented herein, tabalumab treatment in patients with rheumatoid arthritis in a Phase 2 clinical trial produced an initial increase in absolute B-cell counts at Week 1 (at all dose levels), which subsequently decreased back to baseline or below beginning at Week 4 and continuing through Week 24 (Genovese et al., Citation2013). Mature, naïve (CD19 + IgD + CD27−) B-cell counts showed a similar pattern while switched (CD19 + IgD − CD27+) and non-switched (CD19 + IgD + CD27+) memory B-cell counts increased from baseline to the end of the treatment period. Mean IgM and IgA levels tended to be lower than baseline at the end of the treatment period, while IgG levels remained constant. Infections occurred more frequently in patients exposed to tabalumab and, while serious infections were only reported in tabalumab-treated patients, the number was small (n = 3, 2.5%) and there was no association between changes in serum immunoglobulin levels and occurrence of infection or other adverse events. There was no correlation between clinical response, B-cell counts, or serum immunoglobulin levels.
A limitation in our analyses was that no longer-term evaluations of total immunoglobulin levels were performed. Evaluation of total immunoglobulin levels, as an indicator of B-cell function, later in the treatment period are warranted since the half-life of IgG in particular is ≈21 days, and it would take some time to note a reduction in total IgG should it occur related to tabalumab treatment. While no data on plasma cell changes related to tabalumab treatment was collected, sustained reductions in B cells might translate in reductions in immunoglobulin levels. Additionally, to characterize potential tabalumab immunotoxicity in future clinical studies, changes in the major B-cell subset populations should be evaluated early in the course of treatment to better discern the early increase in B cells noted in the cynomolgus monkey at Day 8 together with monitoring the subsets to determine whether specific B-cell populations are affected. Lastly, while we showed no effect on humoral immunity to antigen challenge, we did not assess the effects of tabalumab treatment on susceptibility to opportunistic infections, which can be investigated with experimental immune host resistance models. Such models, if used appropriately, can provide an additional perspective on the results of immune function assays.
Conclusions
As would be expected by an agent that inhibits both membrane and soluble BAFF, tabalumab treatment produced an early increase (Day 8) followed by a progressive and sustained reduction in total mature B cells in three pre-clinical toxicology studies in cynomolgus monkeys. B-cell recovery varied by dose, with only the lowest dose level achieving pre-treatment values by ≈112 days following the final dose. Higher dose levels still resulted in ≈30–55% decreases as compared to pre-treatment values. There was no effect in total IgG, IgA or IgM after 4 weeks of treatment. Following 6 months of dosing, reductions in germinal center size/number were noted in lymphoid tissues and reductions in spleen weight were noted with the 30 mg/kg dose. Despite the circulating B-cell decreases, the humoral immune response remained intact, as evidenced by the development of anti-tetanus IgG antibodies and both IgM and IgG anti-KLH antibodies. There were no changes in T-cell subsets, NK cells, activated T-cells, or activated NK cells that were attributed to the tabalumab treatments. Given the mechanism of action of tabalumab as a B-cell immunomodulator, confirmation of humoral immune function in human clinical trials along with the analysis of infection rates would be useful in understanding the potential immunomodulatory effects of tabalumab in larger clinical populations.
Supplementary material available online
Supplementary material Table 1
Supplemental Material.pdf
Download PDF (35.2 KB)Acknowledgements
The authors would like to acknowledge the staff and investigators of SNBL, Covance Laboratories, Inc., and Alta, Inc., and, in particular, Paul Franklin, Jacob Jabbour, Josh Arrington, and Robert Caldwell for their work in generating the data for these studies. We would also like to thank Jennifer Martin and Victor Wroblewski (Eli Lilly and Company) for their work in analyzing and interpreting the toxicokinetic data, and Helen Nicely, Victoria Crotzer, and Katherine Oneacre (inVentiv Clinical Health) for their technical assistance in preparing this manuscript.
Declaration of interest
WJK and JLB are employed by Eli Lilly and Company and own stock in Eli Lilly; CAH is employed by Eli Lilly and Company; CGW owns Lilly stock; DW is a retiree of Eli Lilly and Company and receives a pension from and owns stock in Eli Lilly and Company.
References
- Abrams, J. R., Lebwohl, M. G., Guzzo, C. A., et al. 1999. CTLA4Ig-mediated blockade of T-cell costimulation in patients with psoriasis vulgaris. J. Clin. Invest. 103:1243–1252
- Baker, K. P., Edwards, B. M., Main, S. H., et al. 2003. Generation and characterization of LymphoStat-B, a human monoclonal antibody that antagonizes the bioactivities of B-lymphocyte stimulator. Arthritis Rheum. 48:3253–3265
- Bearden, C. M., Agarwal, A., Book, B. K., et al. 2005. Rituximab inhibits the in vivo primary and secondary antibody response to a neoantigen, bacteriophage phiX174. Am. J. Transplant. 5:50–57
- Bingham, C. O., Looney, R. J., Deodhar, A., et al. 2010. Immunization responses in rheumatoid arthritis patients treated with rituximab: Results from a controlled clinical trial. Arthritis Rheum. 62:64–74
- Dunnett, C. W. 1964. New tables for multiple comparisons with a control. Biometrics 20:482–491
- Food and Drug Administration. 2012. Tofacitinib (Xeljanz). 2012. 8–18–2014. Available at: http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm327152.htm
- Furie, R. A., Petri, M., Zamani, O., et al. 2011. A Phase III, randomized, placebo-controlled study of belimumab, a monoclonal antibody that inhibits B-lymphocyte stimulator, in patients with systemic lupus erythematosus. Am. Coll. Rheumatol. 63:3918–3930
- Genovese, M. C., Bojin, S., Biagini, I. M., et al. 2013. Tabalumab in rheumatoid arthritis patients with an inadequate response to methotrexate and naive to biologic therapy: A Phase II, randomized, placebo-controlled trial. Arthritis Rheum. 65:880–889
- GlaxoSmithKline. Benlysta Prescribing Information. 2014. Available at: https://www.gsksource.com/gskprm/htdocs/documents/BENLYSTA-PI-MG.PDF
- Gonzalez-Stawinski, G. V., Yu, P. B., Love, S. D., et al. 2001. Hapten-induced primary and memory humoral responses are inhibited by the infusion of anti-CD20 monoclonal antibody (IDEC-C2B8, Rituximab). Clin. Immunol. 98:175–179
- Halpern, W. G., Lappin, P., Zanardi, T., et al. 2006. Chronic administration of belimumab, a BLyS antagonist, decreases tissue and peripheral blood B-lymphocyte populations in cynomolgus monkeys: Pharmacokinetic, pharmacodynamic, and toxicologic effects. Toxicol. Sci. 91:586–599
- Huard, B., Schneider, P., Mauri, D., et al. 2001. T-Cell co-stimulation by the TNF Ligand. J. Immunol. 167:6225–6231
- Huntington, N. D., Tomioka, R., Clavarino, C., et al. 2006. A BAFF antagonist suppresses experimental autoimmune encephalomyelitis by targeting cell-mediated and humoral immune responses. Intl. Immunol. 18:1473–1485
- Kalled, S. L. 2006. Impact of the BAFF/BR3 axis on B-cell survival, germinal center maintenance and antibody production. Semin. Immunol. 18:290–296
- Levene, H. (Ed.). 1960. Robust tests for equality of variances. In: Contributions to Probability and Statistics. Palo Alto, CA: Stanford University Press, pp. 278–292
- Mackay, F., and Schneider, P. 2009. Cracking the BAFF code. Nat. Rev. Immunol. 9:491–502
- Mackay, F., Schneider, P., Rennert, P., and Browning, J. 2003. BAFF AND APRIL: A tutorial on B-cell survival. Annu. Rev. Immunol. 21:231–264
- Mackay, F., Sierro, F., Grey, S. T., and Gordan, T. P. (Eds.). 2005. The BAFF/APRIL system: An important player in systematic rheumatic diseases. In: B-Cell Trophic Factors and B-Cell Antagonism in Autoimmune Disease. Curr Dir Autoimmun. Basel, Switzerland: Karger, Vol 8, pp. 243–265
- Manetta, J., Bina, H., Ryan, P., et al. 2014. Generation and characterization of tabalumab, a human monoclonal antibody that neutralizes both soluble and membrane-bound B-cell activating factor (BAFF). J. Inflamm. Res. 7:121–131
- Martin, P. L., and Bugelski, P. J. 2012. Concordance of preclinical and clinical pharmacology and toxicology of monoclonal antibodies and fusion proteins: Soluble targets. Br. J. Pharmacol. 166:806–822
- Martin, P. L., Oneda, S., and Treacy, G. 2007. Effects of an anti-TNFα monoclonal antibody, administered throughout pregnancy and lactation, on development of macaque immune system. Am. J. Reprod. Immunol. 58:138–149
- Mysler, E. F., Spindler, A. J., Guzman, R., et al. 2013. Efficacy and safety of ocrelizumab in active proliferative lupus nephritis: Results from a randomized, double-blind, Phase III study. Arthritis Rheum. 65:2368–2379
- Ng, L. G., Sutherland, A. P., Newton, R., et al. 2004. B-Cell-activating factor belonging to the TNF family (BAFF)-R is the principal BAFF receptor facilitating BAFF co-stimulation of circulating T- and B cells. J. Immunol. 173:807–817
- Ribeiro, A. C., Laurindo, I. M., Guedes, L. K., et al. 2013. Abatacept and reduced immune response to pandemic 2009 influenza A/H1N1 vaccination in patients with rheumatoid arthritis. Arthritis Care Res. (Hoboken) 65:476–480
- Ronchese, F., Hausmann, B., Hubele, S., and Lane, P. 1994. Mice transgenic for a soluble form of murine CTLA-4 show enhanced expansion of antigen-specific CD4+ T-cells and defective antibody production in vivo. J. Exp. Med. 179:809–817
- Sabahi, R., and Anolik, J. H. 2006. B-Cell-targeted therapy for systemic lupus erythematosus. Drugs 66:1933–1948
- Schmitz, J. E., Kuroda, M. J., Santra, S., et al. 2003. Effect of humoral immune responses on controlling viremia during primary infection of rhesus monkeys with simian immunodeficiency virus. J. Virol. 77:2165–2173
- Scholz, J. L., Crowley, J. E., Tomayko, M. M., et al. 2008. BLyS inhibition eliminates primary B cells but leaves natural and acquired humoral immunity intact. Proc. Natl. Acad. Sci. USA 105:15517–15522
- Shapiro, S. S., and Wilk, M. B. 1965. An analysis of variance test for normality (complete samples). Biometrika 52:591–611
- Stohl, W., Hiepe, F., Latinis, K. M., et al. 2012. Belimumab reduces autoantibodies, normalizes low complement levels, and reduces select B-cell populations in patients with systemic lupus erythematosus. Arthritis Rheum. 64:2328–2337
- Tukey, J. W., Ciminera, J. L., and Heyse, J. F. 1985. Testing the statistical certainty of a response to increasing doses of a drug. Biometrics 41:295–301
- van Assen, S., Holvast, A., Benne, C. A., et al. 2010. Humoral responses after influenza vaccination are severely reduced in patients with rheumatoid arthritis treated with rituximab. Arthritis Rheum. 62:75–81
- Vincent, F. B., Saulep-Easton, D., Figgett, W. A., et al. 2013. The BAFF/APRIL system: Emerging functions beyond B-cell biology and autoimmunity. Cytokine Growth Factor Rev. 24:203–215
- Vugmeyster, Y., Seshasayee, D., Chang, W., et al. 2006. A soluble BAFF antagonist, BR3-Fc, decreases peripheral blood B cells and lymphoid tissue marginal zone and follicular B cells in cynomolgus monkeys. Am. J. Pathol. 168:476–489
- Wang, Q. T., Ma, Y. K., Huang, B., et al. 2011. Effect of rhTACI-Ig fusion protein on antigen-specific T cell responses from keyhole limpet haemocyanin challenged mice. Mol. Immunol. 49:380–386
- Winer, B. J. (Ed.). 1971. Statistical Principles in Experimental Design. New York: McGraw-Hill
- Winthrop, K. L., Neal, J., Hrycaj, P., et al. 2013. Evaluation of influenza and pneumococcal vaccine responses in rheumatoid arthritis patients using tofacitinib. Ann. Rheum. Dis. 72:(Suppl 3)107
