Abstract
Emodin, an anthraquinone derivative, was investigated for potential anti-inflammatory and anti-proliferative effects in vitro. The potential to induce these outcomes was assessed using concanavalin A (ConA)-stimulated mouse splenocytes. Dose–response studies showed that emodin at 100 µM was not cytotoxic to naive cells, and that the same dose caused proliferation to be significantly reduced in ConA-stimulated cells. In addition, emodin significantly reduced ConA-induced nitric oxide (NO) production and the formation/release of TH1 (IL-2, IFNγ, TNFα) and TH17 (IL-6 and IL-17) cell cytokines, but induced those of TH2 (IL-4) and Treg (IL-10) cells. From the results, it is concluded that earlier-reported immunomodulatory effects imparted by emodin may have been attributable, in part, to anti-proliferative effects on lymphocytes, as well as a shift within the TH1/TH2 and TH17/Treg balance (towards TH2 and Treg). These findings, while providing evidence of mechanisms of emodin immunomodulation, are also potentially important for sparking studies that ultimately may result in the potential use of this agent in preventive and/or corrective strategies against autoimmune and other inflammatory diseases.
Introduction
Inflammation is a major concern in the development/pathology of many diseases. T-cell activation is involved in the onset/pathogenesis of several autoimmune diseases, inflammation-related diseases, and transplant rejection (Zeng et al., Citation2012). During inflammation, levels of many soluble factors like nitric oxide (NO) and select cytokines are often increased (Feghali & Wright, Citation1997; Sharma et al., Citation2007), with macrophages being the major source of these agents (Choi et al., Citation2008). The use of induced immunosuppression is state-of-the-art therapy for treatment of autoimmune diseases as well to avert, minimize, or reduce the rejection of allografts. It seems that all immunosuppressive drugs available have the capacity to induce unwanted side-effects (like infections and malignancies); however, frequency/intensity of these effects depend on the drug and dosage used. Therefore, there is an ongoing need to selectively down-regulate such responses (including over-activation of T-cells and macrophages) while trying to attain an ideal state of immune suppression in the afflicted host.
Plant-derived natural products occupy an important position in novel drug development (Lucas et al., Citation2010). The numbers of plant-based drugs under study for potential treatment of some immune-based diseases (as well as cancers and CNS diseases) are increasing. Many herbal preparations have also been reported to impart effects against aberrant immunologic conditions, e.g. Flavocoxid (extract from Scutellaria baicalensis), Grazax (extract from Phleum pretense), PMI-001 (extract of Tripterygium wilfordii Hook F), and PYN-17 (extract of four plants). These latter sets of botanical drugs evinced anti-inflammatory/immunosuppressive activities through unique modes of action; currently, each is in a different phase of clinical trials (Patwardhan & Gautam, Citation2005; Plaeger, Citation2003; Saklani & Kutty, Citation2008). With regard to cancer, some of the most promising drugs used in treatment are derived from plant sources, including Taxol (from Taxus brevifolia), camptothecin (from Camptotheca acuminate), combre-statin (from Combretum caffrum), epipodophyllotoxin (from Podophyllum peltatum), and several Vinca alkaloids. Many plant-based preparations like ZT-1 (DEBIO-9902) (from Huperzia serrata), THC-CBD (combination of Δ 9-tetrahydrocannabinol [THC] and cannabidiol [CBD]), phenserine (derivative of a Physostigma venenosum phytostigmine), and RU 47213 (a pro-alkaloid from Areca catechu L.) are in clinical trial for treatment of pain and some neurologic diseases.
Diets rich in polyphenols have also been found to result in beneficial effects. Several phenolics have been shown to impart a range of effects including an ability to act as anti-oxidant and -inflammatory agents, enzyme activity modulators, and regulators of gene expression (Shema-Didi et al., Citation2013; Simao et al., Citation2013). Some polyphenols have been shown to have potential use as immunosuppressants/anti-inflammatory agents (Feng et al., Citation2013; Ge et al., Citation2010). Among these, emodin (1,2,8-trihydroxy-6-methyl anthraquinone polyphenol) - a component of the root and rhizome of Rheum palmatum () - is a phytoestrogen with an affinity for human estrogen receptors (Brennan et al., Citation2013). Emodin is also known to impart anti-oxidant, -bacterial, -inflammatory, and -cancer effects in experimental model systems (Sharma & Tiku, Citation2014). Other studies have reported immunosuppressive and anti-proliferative properties of emodin in in vitro and in vivo systems (Gao et al., Citation2014; Liu et al., Citation2009). However, to date, mechanisms associated with the anti-inflammatory and immunomodulatory effects of emodin are not completely understood.
The present study was initiated to clarify mechanisms so as to allow for a determination to be made as to the practicality of any potential wider use of emodin as an anti-inflammatory agent and/or immunosuppressant. Specifically, the study evaluated for the first time the in vitro effects of emodin on mitogen (i.e. concanavalin A [ConA])-induced proliferation among mouse splenocytes. The in vitro effects of emodin on the release of T-helper (TH)-1, -2, and -17 cell-associated cytokines, NO formation/release as well as any changes in the TH17/regulatory T-cell (Treg) balance - endpoints that each have an important role in immune responses and disease outcomes in animal models and in human autoimmune and/or inflammatory diseases - were also evaluated.
Materials and methods
Chemicals
ConA (Concanavalin A), propidium iodide (PI), and MTT [3-(4, 5-di-methylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide] were each purchased from Sigma (St. Louis, MO). RPMI 1640, fetal bovine serum (FBS), and antibiotic solution were obtained from Himedia India (Mumbai, India). Murine TH1/TH2/TH17 cytometric beads array (CBA) kits were bought from BD Biosciences (San Jose, CA). RNase A, DMSO, and Emodin (cat# 324694; purity by HPLC: 96.1%) were purchased from Merck (Darmstadt, Germany). All other chemicals used were of analytical grade from local firms.
Preparation of emodin and ConA
A 10 mg/ml stock solution of emodin was prepared in DMSO. This material was further diluted to required concentrations using phosphate-buffered saline (PBS, pH 7.4). In no case did the final concentration of DMSO in any test solution exceed 0.2%. ConA (1 mg/ml) solution was prepared fresh for use in PBS.
Isolation and culture of splenocytes
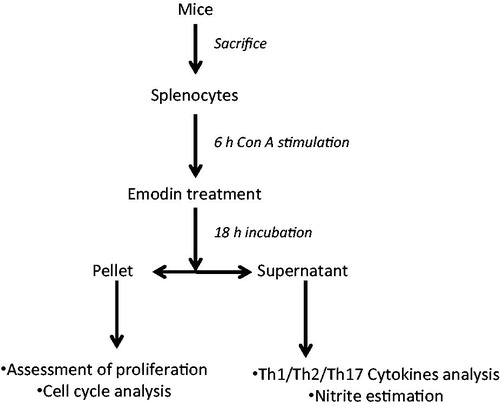
These studies were conducted according to the guidelines of the Committee for Control and Supervision of Experiments on Animals, Government of India, on the use of animals for scientific research. For the study, naïve male Swiss mice were obtained from the Animal House Facility of Jawaharlal Nehru University (New Delhi, India) and then euthanized by cervical dislocation. At necropsy, the spleen of each mouse was aseptically removed and washed in 0.9% saline before being ground between two frosted slides and rinsed into a tube containing PBS. The single-cell suspension was then treated with 0.84% [v/v] ammonium chloride to rupture any erythrocytes present. The remaining splenocytes were then washed twice (in PBS) by centrifugation at 400 × g for 5 min at 4 °C. This final pellet was then re-suspended in RPMI 1640 supplemented with 10% FBS and 1% antibiotic-antimycotic solution (i.e. Complete RPMI), and then counted in a hemocytometer. The final cell concentration was adjusted to 2 × 106 cells/ml medium for subsequent use in the experiments outlined below. All treatments were based on the cells being cultured in a 5% CO2 atmosphere at 37 °C in a humidified incubator () with the various indicated reagents (alone or in combination). Each data point represents the spleens that were pooled from three mice. Each observation is mean of four-to-five replicates/set. Each experiment was repeated 3-times.
Cell viability
Trypan blue dye exclusion was used to determine the number of viable splenocytes still present after treatment with the test reagents. In this test, 10 µl of cell suspension was mixed with equal volume of trypan dye (0.1% in PBS) and then examined under a microscope. Different concentrations of emodin were tested for cytotoxicity against splenocytes in a pilot study and a representative dose range (10, 20, 40, 80, 100, 200, and 400 µM) was chosen for use in the present study based on those outcomes. Nonetheless, splenocyte viability was routinely analyzed after 18 h of emodin treatment. For all studies, the viability of the control group was set to 100%.
Cell proliferation
Murine splenocyte proliferation was assessed using the MTT reduction assay of Mossman (Citation1983) with some modification (Wang et al., Citation2006). Briefly, for the various treatments, splenocytes were placed into wells of a 96-well culture plate (5 × 104 cells/200 µl/well) and then incubated at 37 °C for either 18 h in the emodin only dose–response study or for 24 h with ConA only or ConA + emodin in the co-treatment study. The cells were then removed from each well, washed by centrifugation, and re-suspended (and re-plated) in fresh media. Thereafter, 20 µl MTT solution (5 mg/ml in PBS) was added to each well and the plate incubated for a further 4 h. After this period, DMSO was added to each well (200 µl/well) to dissolve formazan crystals that formed in the viable cells present. The absorbance in each well was then assessed at 570 nm (with 690 nm reference wavelength) in a Spectramax microplate reader (MTX Lab Systems Inc. Vienna, VA).
Analysis of cell cycle status
The cells from the ConA ± emodin groups were washed with PBS and then fixed by drop-wise addition of ice-cold (70%) ethanol before being stored overnight at 4 °C. The fixed cells were then harvested, washed with PBS, suspended in 250 µl PBS, and then incubated with 0.2 mg RNase A/ml for 1 h. After this period, 10 µl of 0.5 mg PI/ml solution was added to each sample. Data acquisition was then performed using a BD FACS Calibur system (BD Biosciences) using Cell Quest software (BD Biosciences); analysis of different phases of the cell cycle was done using Modfit software (Verity Software House, Topsham, ME). The percentage of cells in G1, S, and G2 phases was estimated by assessing the PI fluorescence using an FL-2 filter (585 nm). For each sample, a minimum of 10,000 events was acquired.
Nitric oxide estimation
Nitric oxide (NO) formation was measured as nitrite (a primary stable breakdown product of NO) in a Griess assay. In brief, 100 μl of media supernatant from each test well was collected at the end of the 24 h treatment period (outlined above) and transferred to a 96-well flat-bottomed plate. An equal volume of freshly-prepared Griess reagent (1% sulfanilamide and 0.1% naphthalene ethylene diamine dihydrochloride [in 5% o-phosphoric acid]) was then immediately added. After 20 min incubation at 37 °C, the absorbance in each well was assessed at 540 nm in the microplate reader system. Nitrite content in each sample was calculated by extrapolation from a standard sodium nitrite curve prepared/assayed in parallel, and was expressed in µM.
Cytokine analysis
Splenocytes were added to the wells of microtiter plates at 2 × 106 cells/ml (200 µl/well) and then stimulated with 10 µg ConA/ml for 6 h (at 37 °C). Thereafter, dedicated wells received emodin (at 100 µM final concentration/well); controls received vehicle only. After a further 18 h incubation at 37 °C, supernatant from each well (normal, ConA-activated, Con A + emodin) was collected and stored at −20 °C until used for cytokine analysis. Cytokine levels in the supernatant were ultimately analyzed (according to manufacturer protocol) using a CBA murine TH1/TH2/TH17 cytometric beads array kit designed to simultaneously measure levels of tumor necrosis factor (TNF)-α, interleukin (IL)-4, -10, -6, -17, and -2, and interferon (IFN)-γ. Mean fluorescence intensity for each sample was quantified using the BD FACS Calibur system. All data were analyzed using FCAP Array software (Soft Flow, Inc., St. Louis Park, MN). All samples were analyzed in triplicate. The limit of sensitivity of the kit was 0.9, 0.03, 16.8, 1.4, 0.8, 0.1, and 0.5 pg/ml for, respectively, TNFα, IL-4, IL-10, IL-6, IL-17, IL-2, and IFNγ.
Statistical analysis
All results are reported as mean ± SD. A Student’s t-test (Microsoft Excel, 2007; Redmond, WA) was used to analyze all the data. In all cases, a p value < 0.05 was considered as significant.
Results
Effect of emodin on viability of unstimulated splenocytes
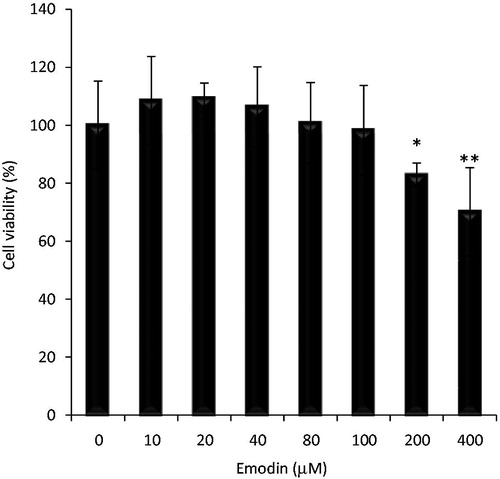
To determine whether emodin caused toxicity to normal mouse splenocytes, cell viability was assessed after an 18 h exposure to various concentrations of emodin (0–400 µM). The results indicated that emodin at ≤ 100 µM had no major effect on splenocyte viability (98.4%) when compared with untreated control (100.0%). At higher concentrations of 200 and 400 µM, viability was reduced to 83.0% (p < 0.05) and 70.2% (p < 0.01), respectively (). Based on these outcomes, a 100 µM concentration of emodin was selected for use in the remainder of the proposed experiments.
Figure 3. Effect of emodin on splenocyte viability. Mouse splenocytes were incubated in the presence of emodin (0–400 µM). Viability was then determined using trypan blue. Experiments were performed in triplicate. Data shown are mean ± SD. Significant differences from ‘0’ control (*p < 0.05, **p < 0.01).
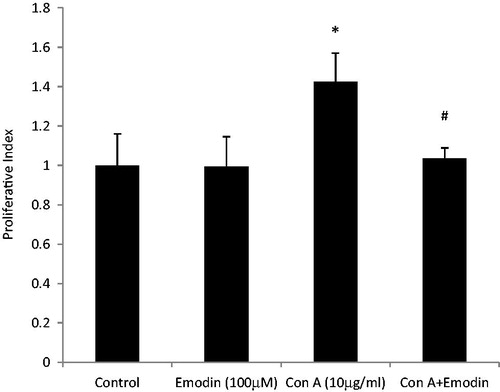
Effect of emodin on ConA-induced proliferation
Among the naïve mice splenocytes stimulated with various concentrations of ConA (0–100 µg/ml) for 24 h, a dose-related increase in proliferative index was observed (data not shown). Based on these responses, 10 µg ConA/ml was selected for use in all subsequent studies. ConA treatment increased proliferation up to 143.3% (p < 0.05) with respect to control non-stimulated cell responses (100%). Co-treatment with 100 µM emodin starting 6 h after the initial stimulation with ConA resulted in a reduction in expected proliferation to 103.5% (p < 0.001) versus the control value ().
Figure 4. Effect of emodin on ConA-induced splenocyte proliferation. Splenocytes were cultured with ConA (10 µg/ml), emodin (100 µM), or ConA + emodin and then proliferation assessed using MTT assay. Data shown are mean ± SD of six separate observations performed in triplicate. Significant differences *p < 0.001 versus control group. #p < 0.001 versus ConA control.
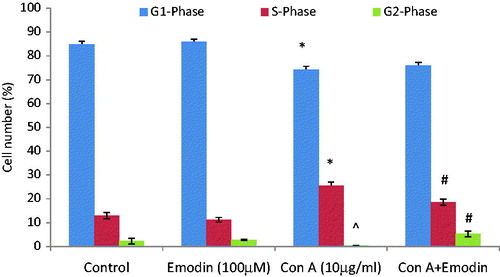
Emodin modulates ConA-induced cell cycle progression
As shown in , unstimulated splenocytes largely remained in the G1/G0 phase. Emodin (100 µM) alone did not significantly affect the progression of cells to different phases of cell cycle. In contrast, ConA stimulation caused an increased entry of cells into the S phase (25.5%) by the end of the 24 h culture; untreated controls only had 13% of cells in that phase. When emodin was co-present with ConA, this led to a significant reduction in progression of the cells to the S phase (18.7%).
Figure 5. Effect of emodin on cell cycle progression. Splenocytes were cultured with ConA (10 µg/ml), emodin (100 µM), or ConA + emodin and stained with propidium iodide for cell cycle analysis. Bar diagram shows percentage of cells in indicated phase of cell cycle. The frequency (%) for different phases of cell cycle was generated from three experiments and expressed as average percentage change from untreated control ± SD. In ConA-alone set; ^p < 0.05, *p < 0.001 versus control. In ConA + emodin set; #p < 0.01 versus control.
Emodin modulates cytokine response/nitrite release in ConA-treated splenocytes
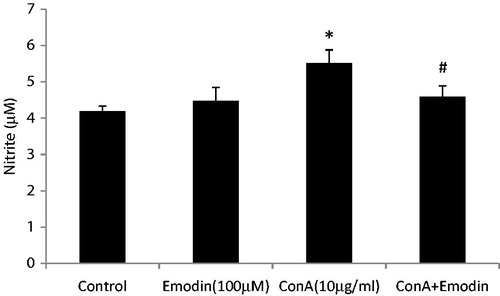
To evaluate the effect of emodin on cytokine secretion in ConA-stimulated splenocytes, supernatants from unstimulated normal, Con A activated, and ConA activated + emodin treated splenocyte cultures were collected and analyzed for secretion of seven cytokines (e.g. IL-2, IL-4, IL-6, IL-10, TNFα, IFNγ, and IL-17A). illustrates how ConA treatment resulted in significant increases in cytokine production compared to that by unstimulated control splenocyte cultures. Emodin + ConA co-treatment suppressed formation/release of IL-2, TNFα, and IFNγ (inhibition was, respectively, 2.7-, 3.6-, and 5.0-fold) as compared to that seen with ConA-only controls. Emodin co-treatment also resulted in inhibition of ConA-induced IL-17 and IL-6 formation/release by 4.7- and 1.9-fold, respectively. At the same time, the levels of two major anti-inflammatory cytokines, i.e. IL-4 and IL-10, were significantly higher in the ConA + emodin-treated cultures (1.5- and 4.9-fold greater than in ConA-only cultures). In the present study, ConA treatment also significantly increased production of NO (p < 0.01) by the splenocytes. However, emodin + ConA co-treatment reduced NO levels from 5.5 µM to 4.6 µM (p < 0.01) ().
Figure 6. Emodin-related regulation of cellular production of cytokines. The levels of TH1/TH2/TH17 cytokines (in pg/ml; IL-2, IL-4, IL-6 TNF-α, IL-10, IL-17, and IFN-γ) were analyzed in medium recovered from cultures of the splenocytes in the presence of emodin and ConA. Cytokine levels were quantified by FACS. Experiments were performed in triplicate. Data shown are mean ± SD. Significantly different from ConA (10 µg/ml) group at *p < 0.001.
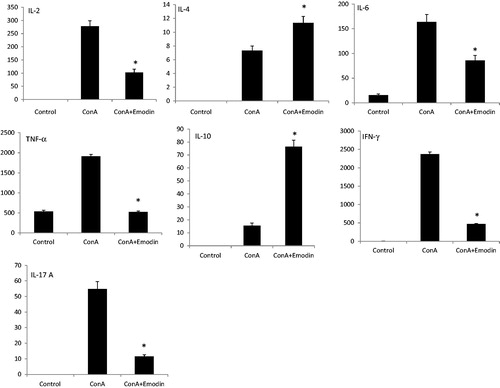
Figure 7. Effect of emodin on nitrite release in mouse splenocytes. Splenocytes were cultured with ConA (10 µg/ml), emodin (100 µM), or ConA + emodin. Nitrite release was assessed using Griess regent. Data shown are mean ± SD of six separate observations performed in triplicate. Significant differences from control; *p < 0.001 versus control group. #p < 0.001 versus ConA alone control.
Discussion
T-cell activation is a complex process, involving induction of gene transcription, cytokine secretion, and cell proliferation, which plays an important role in the onset and pathogenesis of several inflammatory diseases (Mantovani et al., Citation2008; Song et al., Citation2013). As ConA acts as a mitogen to stimulate T-lymphocyte proliferation (Gao et al., Citation2014; Tatsuno et al., Citation1991), ConA-stimulated splenocytes were used as an in vitro model system to assess potential effects of the anthraquinone emodin on T-cell proliferation and cytokine secretion.
The present study showed that emodin had an inhibitory effect on ConA-induced proliferation. Since emodin is known for growth inhibitory effects in many cell types (Srinivas et al., Citation2007) and inhibits angiotensin II-induced proliferation of rat vascular smooth muscle and renal tubular cells (Wang et al., Citation2008; Zheng, Citation1993), it was not surprising that it inhibited ConA-induced proliferation of the splenocytes. More importantly, emodin at the same concentration did not affect viability of the unstimulated splenocytes, indicating that anti-proliferative effect was not due to cytotoxicity. Recently it was shown that emodin at a concentration of 6–100 μM (dose-relatedly) had an anti-proliferative effect on ERα-over-expressing breast cancer cells and no effect on normal breast cells (Huang et al., Citation2013). Non-toxic effects for emodin have been previously reported in human vascular endothelial cells (Meng et al., Citation2012), and emodin did not cause a change in the growth of normal human peripheral blood mononuclear cells or in normal mice bone marrow cells (Muto et al., Citation2007).
To further examine the relationship between the inhibitory effects of emodin on ConA-induced proliferation, this study evaluated its impact on cell cycle progression. T-cell proliferation was largely affected by emodin in the S + G2/M phase. Anti-proliferative activity of emodin has been attributed to its effects on the progress through the cell cycle for different cell types including human fibroblasts and smooth muscle cells (Srinivas et al., Citation2007).
In addition to proliferation, T-cells can also be activated to produce cytokines, multi-functional soluble factors with pro- and anti-inflammatory activities (Feghali & Wright, Citation1997; Feng et al., Citation2013). A diverse number of stimuli such as ConA, lipopolysaccharide (LPS), and phytohemagglutinin (PHA) (Cross & Gill, Citation1999; Dąbrowski & Stankiewicz, Citation2011) can be used to induce cytokine release. Classically, TH1 cells regulate cellular immunity via production of IFNγ, TNFα, and IL-2, whereas TH2 cells regulate humoral immunity via production of IL-4, IL-5, and IL-13 (Fiorentino et al., Citation1989; Mosmann et al., Citation1986; Szabo et al., Citation2000). By producing IL-10 and IL-35, regulatory T (Treg)-cells (a third type of CD4+ cells) suppress effector T-cell responses (Vignali et al., Citation2008). Recently, TH17 cells have been identified as regulators of chronic inflammation and autoimmune responses via their production of IL-17, IL-6, and GM-CSF, etc. (Korn et al., Citation2009).
In the present study, ConA activation resulted in the induction of all measured cytokines, i.e. TNFα, IL-4, IFNγ, IL-10, IL-6, IL-17, and IL-2. Over-production of pro-inflammatory cytokines like IFNγ and TNFα are associated with deleterious inflammatory reactions that may cause tissue damage and destruction (Calla-Magariños et al., Citation2013). Emodin appeared to exert suppressive effects on the release of TH1 (TNFα, IFNγ, and IL-2) and TH17 (IL-17 and IL-6) cytokines; however, IL-4 (i.e. a TH2 cytokine) and IL-10 (i.e. a Treg cytokine) were concurrently induced. This is suggestive to us that emodin treatment modulates cytokine responses towards TH2 and Treg cells. Emodin causing a shifting of the TH1/TH2 paradigm towards TH2 is consistent with previous reports (Lin et al., Citation2010; Liu et al., Citation2009; Tong et al., Citation2011). An expansion of TH17 cells and an induced dysfunction/depletion of Treg cells are also closely related to the onset of autoimmune diseases (Bedoya et al., Citation2013; Eisenstein & Williams, Citation2009). Thus, the modulation of the TH17/Treg cell balance towards Treg cells could be associated with suppression of autoimmunity by emodin.
The release of pro-inflammatory cytokines from different cell types is in part dependent upon nitric oxide (NO) pathways (Bécherel et al., Citation1997; Geller et al., Citation1993). The present study also investigated the effects of emodin on NO formation and found treatment reduced the level of production among ConA-stimulated cells. Emodin is known to be a major inhibitor of inducible nitric oxide synthase (iNOS) (Wang et al., Citation2002) via inhibition of NF-κB, leading to decreased accumulation of nitrite in cells (Sharma & Tiku, Citation2014; Shrimali et al., Citation2013).
On the basis of the above, we believe that emodin could eventually have a possible therapeutic role in the treatment of diseases associated with synthesis/release of pro-inflammatory cytokines. As the present study also demonstrated anti-proliferative and immunomodulatory effects upon splenocytes in vitro (at sub-cytotoxic concentrations), it is plausible that emodin - at a concentration of 100 µM - could have potential use as a modulator of inflammation in vivo. A number of in vivo studies in mice have shown that emodin, at a dose of 10–100 mg/kg BW (a concentration of ≈100 µM approximate to a level of 27 µg/ml), imparts a protective effect in various disease states and does not impart any overt adverse effects (Bhadauria, Citation2010; Liu et al, Citation2009; Singh & Trigun, Citation2013) Further, there are studies suggesting that cytokine responses can be similar in in vitro and in vivo. For example, Vella et al. (Citation1998) suggested that IL 4 - one of the TH2-class of cytokines - can promote T-cell survival in vivo as well as in vitro. Thiebaud et al. (Citation1997) also reported consistency among in vitro and in vivo responses in a study of cytokines during acute phase responses associated with exposures to bisphosphonates. Thus, we also conclude that the effects on cytokines seen here with emodin are likely to occur in vivo.
Since emodin is a pleiotropic molecule capable of interacting with several major molecular targets including androgen receptors, further studies are absolutely required to validate any future application of emodin in potential treatment of autoimmune/inflammatory diseases.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgements
ABT is grateful to the University Grants Commission (UGC), India for funding this project (UGC-02100113-501). A grant received from Jawaharlal Nehru University in the form of UGC-Networking and Department of Science and Technology-Purse (DST-08090712-462) is also gratefully acknowledged. RS is thankful to UGC for providing financial support in the form of project fellowship. The authors wish to thank Dr Mitchell D. Cohen for critical reading and editing of the manuscript.
References
- Bécherel, P. A., Chosidow, O., Le Goff, L., et al. 1997. Inducible nitric oxide synthase and pro-inflammatory cytokine expression by human keratinocytes during acute urticaria. Mol. Med. 3:686–694
- Bedoya, S. K., Lam, B., Lau, K., and Larkin, J. 2013. TH17 cells in immunity and autoimmunity. Clin. Dev. Immunol. Article ID 986789. doi: 10.1155/2013/986789
- Bhadauria, M. 2010. Dose-dependent hepato-protective effect of emodin against acetaminophen-induced acute damage in rats. Exp. Toxicol. Pathol. 62:627–635
- Brennan, J. C., Denison, M. S., Holstege, D. M., et al. 2013. 2,3-cis-2R, 3R-(-)-epiafzelechin-3-O-p-coumarate, a novel flavan-3-ol isolated from Fallopia convolvulus seed, is an estrogen receptor agonist in human cell lines. BMC Complement Altern. Med. 13:133
- Calla-Magariños, J., Quispe, T., Giménez, A., et al. 2013. Quinolinic alkaloids from Galipea longiflora Krause suppress production of pro-inflammatory cytokines in vitro and control inflammation in vivo upon Leishmania infection in mice. Scand. J. Immunol. 77:30–38
- Choi, H. J., Eun, J. S., Park, Y. R., et al. 2008. Ikarisoside A inhibits inducible nitric oxide synthase in lipopolysaccharide-stimulated RAW 264.7 cells via p38 kinase and NF-κB signaling path-ways. Eur. J. Pharmacol. 601:171–178
- Cross, M. L., and Gill, H. S. 1999. Modulation of immune function by a modified bovine whey protein concentrate. Immunol. Cell Biol. 77:345–350
- Dąbrowski, M. P., and Stankiewicz, W. 2011. Immunostimulatory effects of lipopolysaccharide. Centr. Eur. J. Immunol. 36:85–86
- Eisenstein, E. M., and Williams, C. B. 2009. The Treg/TH17 cell balance: A new paradigm for autoimmunity. Pediatr. Res. 65:26R–31R
- Feghali, C. A., and Wright, T. M. 1997. Cytokines in acute and chronic inflammation. Front. Biosci. 2:d12–d26
- Feng, L. L., Wu, X. F., Liu, H. L., et al. 2013. Vaticaffinol, a resveratrol tetramer, exerts more preferable immunosuppressive activity than its precursor in vitro and in vivo through multiple aspects against activated T-lymphocytes. Toxicol. Appl. Pharmacol. 267:167–173
- Fiorentino, D. F., Bond, M. W., and Mosmann, T. R. 1989. Two types of mouse T-helper cell. IV. TH2 clones secrete a factor that inhibits cytokine production by TH1 clones. J. Exp. Med. 170:2081–2095
- Gao, J., Wang, F., Wang, W., et al. 2014. Emodin suppresses hyperglycemia-induced proliferation and fibronectin expression in mesangial cells via inhibiting cFLIP. PLoS One 9:e93588
- Ge, H. M., Yang, W. H., Shen, Y., et al. 2010. Immunosuppressive resveratrol aneuploids from Hopea chinensis. Chemistry 16:6338–6345
- Geller, D. A., Di Silvio, M., Nussler, A. K., et al. 1993. Nitric oxide synthase expression is induced in hepatocytes in vivo during hepatic inflammation. J. Surg. Res. 55:427–432
- Huang, P. H., Huang, C. Y., Chen, M. C., et al. 2013. Emodin and aloe-emodin suppress breast cancer cell proliferation through ERα inhibition. Evid.-Based Complement Alt. Med. 2013:376123
- Korn, T., Bettelli, E., Oukka, M., and Kuchroo, V. K. 2009. IL-17 and TH17 Cells. Annu. Rev. Immunol. 27:485–517
- Lin, S. Z., Chen, K. J., Tong, H. F., et al. 2010. Emodin attenuates acute rejection of liver allografts by inhibiting hepatocellular apoptosis and modulating the TH1/TH2 balance in rats. Clin. Exp. Pharmacol. Physiol. 37:790–794
- Liu, Y. X., Shen, N. Y., Liu, C., and Lv, Y. 2009. Immunosuppressive effects of emodin: An in vivo and in vitro study. Transplant. Proc. 41:1837–1839
- Lucas, D. M., Still, P. C., Pérez, L. B., et al. 2010. Potential of plant-derived natural products in the treatment of leukemia and lymphoma. Curr. Drug Targets. 11:812–822
- Mantovani, A., Allavena, P., Sica, A., and Balkwill, F. 2008. Cancer-related inflammation. Nature. 454:436–444
- Meng, L., Yan, D., Xu, W., et al. 2012. Emodin inhibits TNFα-induced migration and inflammatory responses in rat aortic smooth muscle cells. Int. J. Mol. Med. 29:999–1006
- Mosmann, T. R., Cherwinski, H., Bond, M. W., et al. 1986. Two types of murine helper T-cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 136:2348–2357
- Mossman T. 1983. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Meth. 65:55–63
- Muto, A., Hori, M., Sasaki, Y., et al. 2007. Emodin has a cytotoxic activity against human multiple myeloma as a Janus-activated kinase 2 inhibitor. Mol. Cancer Ther. 6:987–994
- Patwardhan, B., and Gautam, M. 2005. Botanical immunodrugs: Scope and opportunities. Drug Disc. Today. 10:495–502
- Plaeger, S. F. 2003. Clinical immunology and traditional herbal medicines. Clin. Diagn. Lab. Immunol. 10:337–338
- Saklani, A., and Kutty, S. K. 2008. Plant-derived compounds in clinical trials. Drug Disc. Today. 13:161–171
- Sharma, J. N., Al-Omran, A., and Parvathy, S. S. 2007. Role of nitric oxide in inflammatory diseases. Inflammopharmacology. 15:252–259
- Sharma, R., and Tiku, A. B. 2014. Emodin, an anthraquinone derivative, protects against gamma radiation-induced toxicity by inhibiting DNA damage and oxidative stress. Int. J. Radiat. Biol. 90:275–283
- Shema-Didi, L., Kristal, B., Ore, L., et al. 2013. Pomegranate juice intake attenuates the increase in oxidative stress induced by intravenous iron during hemodialysis. Nutr. Res. 33:442–446
- Shrimali D., Shanmugam, M. K., Kumar, A. P., et al. 2013. Targeted abrogation of diverse signal transduction cascades by emodin for the treatment of inflammatory disorders and cancer. Cancer Lett. 341:139–149
- Simão, T. N., Lozovoy, M. A., Simão, A. N., et al. 2013. Reduced-energy cranberry juice increases folic acid and adiponectin and reduces homocysteine and oxidative stress in patients with the metabolic syndrome. Br. J. Nutr. 110:1885–1894
- Singh, K. B., and Trigun, S. K. 2013. Apoptosis of Dalton's lymphoma due to in vivo treatment with emodin is associated with modulations of hydrogen peroxide metabolizing antioxidant enzymes. Cell Biochem. Biophys. 67:439–449
- Song, B., Huang, G., Tong, C., et al. 2013. Gossypol suppresses mouse T lymphocytes via inhibition of NF-κB, NFAT, and AP-1 pathways. Immunopharmacol. Immunotoxicol. 35:615–621
- Srinivas, G., Babykutty, S., Sathiadevan, P. P., and Srinivas, P. 2007. Molecular mechanism of emodin action: Transition from laxative ingredient to an anti-tumor agent. Med. Res. Rev. 27:591–608
- Szabo, S. J., Kim, S. T., Costa, G. L., et al. 2000. A novel transcription factor, T-bet, directs TH1 lineage commitment. Cell. 100:655–669
- Tatsuno, I., Gottschall, P. E., and Arimura, A. 1991. Inhibition of mitogen-stimulated proliferation of murine splenocytes by a novel neuropeptide, pituitary adenylate cyclase activating polypeptide: A comparative study with vasoactive intestinal peptide. Endocrinology. 128:728–734
- Thiébaud, D., Sauty, A., Burckhardt, P., et al. 1997. An in vitro and in vivo study of cytokines in the acute-phase response associated with bisphosphonates. Calcif. Tissue Int. 61:386–392
- Tong, H., Chen, K., Chen, H., et al. 2011. Emodin prolongs recipient survival time after orthotopic liver transplantation in rats by polarizing the TH1/TH2 paradigm to TH2. Anat. Rec. (Hoboken) 294:445–452
- Vella, A. T., Dow, S., Potter, T. A., et al. 1998. Cytokine-induced survival of activated T-cells in vitro and in vivo. Proc. Natl. Acad. Sci. US. 95:3810–3815
- Vignali, D. A., Collison, L. W., and Workman, C. J. 2008. How regulatory T-cells work. Nat. Rev. Immunol. 8:523–532
- Wang, C. C., Huang, Y. J., Chen, L. G., et al. 2002. Inducible nitric oxide synthase inhibitors of Chinese herbs III. Rheum palmatum. Planta Med. 68:869–874
- Wang, S., Liu, Y., Fan, F., et al. 2008. Inhibitory effects of emodin on proliferation of cultured rat vascular smooth muscle cell-induced by angiotensin II. Phytother. Res. 22:247–251
- Wang, X., Ge, J., Wang, K., et al. 2006. Evaluation of MTT assay for measurement of emodin-induced cytotoxicity. Assay Drug Dev. Technol. 4:203–207
- Zeng, X., Wang, T., Zhu, C., et al. 2012. FTY720 mediates activation suppression and G0/G1 cell cycle arrest in a concanavalin A-induced mouse lymphocyte pan-activation model. Inflamm. Res. 61:623–634
- Zheng, F. 1993. Effect of Rheum officinal on the proliferation of renal tubular cells in vitro. Zhonghua Yi Xue Za Zhi. 73:343–345