Abstract
T-cell-dependent antibody responses (TDAR) are suppressed in female C57BL/6N mice exposed to ≥3.75 mg/kg of perfluorooctanoic acid (PFOA) for 15 days. To determine if suppression of humoral immunity by PFOA is peroxisome proliferator activated receptor alpha (PPARα)-dependent and if suppression is associated with specific targeting of T- or B-cells, three separate experiments were conducted: (1) female PPARα constitutive knockout (PPARα KO; B6.129S4-Ppartm1GonzN12) and wild-type controls (WT; C57BL/6-Tac) exposed to 0, 7.5, or 30 mg PFOA/kg for 15 days were immunized on Day 11 with a T-cell-dependent antigen and sera then collected for measures of antigen-specific IgM titers (TDAR) 5 days later; (2) female C57BL/6N WT mice exposed to 0, 0.94, 1.88, 3.75, or 7.5 mg PFOA/kg for 15 days were immunized with a T-cell-independent antigen on Day 11 and sera were then collected for analyses of antigen-specific IgM titers (TIAR) 7 days later; and (3) splenic lymphocyte phenotypes were assessed in unimmunized female C57BL/6N WT mice exposed to 0, 3.75, or 7.5 mg PFOA/kg for 10 days to investigate effects of PFOA in the absence of specific immunization. Separate groups of mice were immunized with a T-cell-dependent antigen after 11 days of exposure and splenic lymphocyte sub-populations were assessed after 13 or 15 days of exposure to assess numbers of stimulated cells. The results indicated that exposure to ≥1.88 mg PFOA/kg suppressed the TIAR; exposure to 30 mg PFOA/kg suppressed the TDAR in both PPARα KO and WT mice. The percentage of splenic B-cells was unchanged. Results obtained in the PPARα KO mice indicated that PPARα suppression of TDAR was independent of PPARα involvement. Suppression of the TIAR and the TDAR with minimal lymphocyte sub-population effects suggested that effects on humoral immunity are likely mediated by disruption of B-cell/plasma cell function.
Introduction
Perfluoroalkyl and polyfluoroalkyl substances (PFAS) are stable, synthetic, fluorinated chemicals used in numerous industrial and consumer products, including protective coatings for packaging, textiles, paper, and carpeting, non-stick agents, and fluoropolymers for other product applications. Chemical stability also means that many PFAS do not easily biodegrade and are detectable as environmental contaminants in air, soil, dust, surface and drinking water, sediment, biota, including humans, and in some commercially prepared foods (due to packaging). In response, the US Environmental Protection Agency (USEPA) and several US manufacturers of these compounds developed a voluntary environmental stewardship program designed to reduce emissions and product content levels of a specific PFAS, i.e. perfluorooctanoic acid (PFOA), precursor chemicals that can breakdown to PFOA, and higher homolog chemicals (USEPA, Citation2013). The USEPA published a draft risk assessment of PFOA in 2005, and released an updated draft in 2014; the latter document is undergoing revisions after a public comment period and external peer review (USEPA, Citation2014).
In a review of the 2005 USEPA draft risk assessment, the USEPA Science Advisory Board highlighted immunotoxicity as an endpoint of concern. This focus was based on a set of papers by Yang et al. (Citation2000, Citation2001, Citation2002b) that indicated reductions in relative spleen and thymus weights and suppression of the T-cell-dependant antibody response (TDAR). Succeeding studies of PFOA and related PFAS (PFOA, PFOS, and ammonium perfluorooctanoate [APFO, a precursor compound to PFOA]) also demonstrated suppression of the TDAR (DeWitt et al., Citation2008; Loveless et al., Citation2008) and that exposure to PFOS also affected the T-cell-independent antibody response (TIAR; Peden-Adams et al., Citation2008). Suppression of both the TDAR and TIAR suggests that B-cells are targeted by PFAS. Additionally, numerous studies have indicated that many toxicological effects induced by PFOA exposure are linked to the ability of PFOA to activate the peroxisome proliferator activated receptor (PPAR)-α, a ligand-activated transcription factor involved in lipid metabolism, energy homeostasis, and inflammatory responses (van den Huevel, Citation2007). Several studies also have suggested that PPARα activation by PFOA is, in part, associated with the immunotoxicologic effects of PFOA in mice (Yang et al., Citation2002b; Corsini et al., Citation2011, Citation2012).
Recently, several epidemiological studies have indicated that antibody production in humans also may be susceptible to PFAS exposure. Two separate studies examined the association between exposure to PFAS and responses to vaccinations in relatively large populations of children. These studies were among the first to evaluate the ability of PFAS to modulate immune function in humans. In one study of children from the Faroe Islands, a population that relies heavily on marine mammals contaminated with a variety of persistent organic pollutants, including PCBs and perfluorinated compounds, Grandjean et al. (Citation2012) reported that pre-natal and post-natal serum concentrations of both PFOA and PFOS were negatively associated with responses to diphtheria and tetanus vaccination in 5–7 year old children, although different patterns for the two PFASs did exist across contaminants, vaccines, and ages. In a separate study based on the Norwegian Mother and Child Cohort Study, Granum et al. (Citation2013) reported a negative association between maternal serum concentrations of PFOA and PFOS and the response to rubella immunization in 3-year-old children. The data from these epidemiological studies and from mouse studies suggest that young humans and mice are susceptible to suppression of humoral immunity by exposure to PFASs.
Suppression of antigen-specific antibody responses is considered predictive of immunotoxicity and decreased resistance to infection in rodents (Luster et al., Citation1992, Citation1993; Selgrade, Citation1999); such alterations in murine immune function can be suggestive of health risks in similarly exposed humans (Selgrade, Citation2007). We previously reported that exposure to ≥3.75 mg PFOA/kg/day for 15 days suppressed the TDAR in C57BL/6N mice. The current studies were done to determine if suppression of the TDAR is dependent on activation of the PPARα and to investigate potential targeting of T- or B-cells as an underlying cause of suppressed antibody production in mice exposed to PFOA. The latter may have additional health implications as natural T-cell-independent antibodies contribute significantly to the initial control of respiratory pathogens.
Materials and methods
Study design/dose selection
We previously reported that the primary (IgM) antibody response to sheep red blood cells (SRBC) was suppressed in female C57Bl/6N mice by exposure to doses ranging from 3.75–30 mg PFOA/kg/day (DeWitt et al., Citation2008). To determine if suppression of this response occurs in mice lacking a functional PPARα, doses of PFOA (0, 7.5 and 30 mg/kg/day) were chosen to maintain continuity with these previous studies (DeWitt et al., Citation2008) and those of Yang et al. (2002b) in their studies of PPARα knockout (KO) mice immunized with horse red blood cells. Lower doses and an extended dose range (0, 0.94, 1.88, 3.75, and 7.5 mg PFOA/kg/day) were chosen for TIAR studies to include doses previously determined to suppress the TDAR (3.75 and 7.5 mg/kg/day; DeWitt et al., Citation2008), and to extend the dose–response curve below our calculated benchmark dose of 3 mg/kg/day with a lower bound (95% confidence limit) of 1.75 mg/kg/day (DeWitt et al., Citation2008) in an attempt to identify lowest observed and no observed adverse effect levels for this endpoint. Immunophenotyping studies were conducted at lower doses (0, 3.75, and 7.5 mg/kg/day) to coincide with doses that were immunosuppressive but did not reduce spleen, thymus, or body weights (DeWitt et al., Citation2008), and to avoid potential overt toxicity and its potential confounding effects on lymphoid organ cellularity.
Animals
To assess the role of the PPARα in the humoral response to a T-cell-dependent antigen, B6.129S4-Ppartm1GonzN12 (PPARα KO) and background-matched wild-type (WT) C57BL/6-Tac female mice were purchased from Taconic Farms (Rensselaer, NY). The vendor website (http://www.taconic.com/cs/Satellite?blobcol = urldocument&blobkey = id&blobtable=TA_Document&blobwhere = 1347208841885&ssbinary = true) includes a detailed description of the KO strain. To evaluate immunophenotype in animals immunized with a T-cell-dependent antigen, C57BL/6N female mice were purchased from Charles River Laboratories (Raleigh, NC), the same source and strain of mouse used in our previous work (DeWitt et al., Citation2008).
All animals were delivered to animal facilities at the USEPA that are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). All animals were 6–7-weeks-of-age at delivery. Once at the USEPA, the animals were housed in poly-carbonate cages with hardwood chip bedding (Beta Chip) in groups of six (PPARα KO and WT) or four (C57BL/6N) and allowed to acclimate for at least 10 days before dosing began. They were provided a 12-h light:dark cycle (light, 06:00–18:00 hours; dark, 18:00–06:00 hours), maintained at 22.3 [± 1.1]°C and 40–60% relative humidity, and given ad libitum access to both food (5P00 Prolab RMH 3000, PMI Nutrition International, Richmond, IN) and water. The Institutional Animal Care and Use Committee of the National Health and Environmental Effects Research Laboratory, US EPA, approved all procedures in advance.
To evaluate T- or B-cell targeting by PFOA, C57BL/6N female mice were purchased from Charles River Laboratories and delivered to the East Carolina University (ECU) Brody School of Medicine (BSOM) animal facility (accredited by AAALAC). All animals were 6–7 weeks-of-age at delivery. Once at the ECU animal facility, animals were housed in polycarbonate cages with corncob bedding (Bed O’Cobs) in groups of eight and allowed to acclimate for at least 10 days before dosing began. The mice were provided a 12-h light:dark cycle (light, 06:00–18:00 hours; dark, 18:00–06:00 hours), maintained at 22.8 [± 3] °C and 30–70% relative humidity, and given ad libitum access to both food (5P00 Prolab RMH 3000) and water.
All treated animals received PFOA in deionized water for 15 days. Dosing water was changed and water consumption per cage (based on water bottle weights) was recorded twice weekly. Controls received deionized water for the same duration as treated animals. At the end of the dosing period, all water bottles were changed to control water.
Dosing solutions
PFOA was purchased from Sigma-Aldrich (St. Louis, MO) as its ammonium salt (≥98% purity, lot 1349401-14907133). PFOA drinking water dosing solutions were prepared based on total compound weight, as described by DeWitt et al. (Citation2008) at concentrations of 6.25, 12.5, 25, 50, or 200 mg/L, to provide doses of 0.94, 1.88, 3.75, 7.5, or 30 mg/kg/day, respectively, based on average daily water consumption and animal body weights.
Body weights and lymphoid organ weights
Immediately prior to euthanasia, terminal body weights were collected from all animals. After euthanasia, the spleen and thymus were removed from animals immunized with sheep red blood cells (SRBC, Rockland Immunochemicals, Gilbersville, PA) or dinitrophenyl-ficoll (DNP, Sigma) (see below) and then weighed. Relative lymphoid organ weights were determined by adjusting the organ weight to body weight. Lymphoid organ weights were not collected from mice slated for use in the flow cytometry studies.
Immunizations and IgM antibody responses
Experiment 1 (the TDAR)
WT and PPARα KO mice were immunized on the 11th day of dosing (0, 7.5, or 30 mg PFOA/kg/day) by intravenous injection of 4.0 × 107 SRBC in 0.2 ml of sterile saline. Five days later (the peak of IgM response to SRBC in C57BL/6 mice) and 1 day after dosing ended, the mice were euthanized by CO2 inhalation and exsanguinated by neck vein transection. Blood was collected, held at room temperature for 30 min, and then centrifuged at 4 °C to separate serum. The sera were then frozen at −80 °C until analysis of IgM antibody titers.
For those analyses, flat bottom 96-well Immunolon-2 ELISA microtiter plates (Dynatech Labs, Chantilly, VA) were coated with 125 μl of 2 μg/ml of SRBC membrane [1.46 mg/ml stock solution diluted in phosphate-buffered saline (PBS); prepared according to Temple et al. (1995)] and then incubated at 4 °C for at least 16 h. Each plate included 20 wells coated with pooled serum collected from healthy mice 5 days after primary immunization with SRBC, and 16 wells contained 100 μl PBS as blanks. After washing, blocking of non-specific binding, and addition of serum samples (serially diluted 1:2, starting at 1:32), secondary antibody (goat anti-mouse IgM horseradish peroxidase; Accurate Chemical and Scientific Corp., Westbury, NY) was added to the wells. Following three washes and addition of substrate [10 mg 2,2′-azino-di-(3 ethylbenzthiazoline sulfonic acid, ABTS, Sigma) added to 50 ml phosphate-citrate buffer with one tablet of urea hydroxide peroxide (Sigma) in 100 ml distilled water, 0.05 M final solution], plates were incubated for 45 min at room temperature and then the absorbance in each well evaluated at 410 nm on a BioTek Synergy HT plate reader (BioTek Instruments, Inc., Winooski, VT). IgM antibody titers were processed using SOFTmax Pro software (Molecular Devices, LLC, Sunnyvale, CA) to determine the log2 serum titers for an optical density of 0.5 U from the log–log curve of optical density versus dilution, as described by Temple et al. (1995).
Experiment 2 (the TIAR)
To investigate the effects of PFOA exposure on the TIAR in the same strain of mice used in our original studies, C57BL/6 N mice were immunized on the 11th day of dosing (0, 0.94, 1.88, 3.75, or 7.5 mg PFOA/kg/day) by intravenous injection of 1 µg of the Type 2 T-cell-independent antigen DNP in 0.2 ml of sterile saline. Seven days later (which we determined to be the peak of antibody production in pilot studies with this strain of mice) and 2 days after dosing ended, the mice were euthanized by CO2 inhalation and exsanguinated by neck vein transection. Blood was collected and held at room temperature for 30 min and then centrifuged at 4 °C to separate serum. The sera were then frozen at −80 °C and stored until analysis of IgM antibody titers. Here, the flat bottom 96-well Immunolon-2 ELISA microtiter plates were coated with 125 μl of 1 mg/ml of DNP-bovine serum albumin (BSA) solution and then incubated at 4 °C for at least 16 h. Each plate included 16 wells containing 100 μl PBS as blanks. All subsequent steps were the same as described above for the T-cell-dependent antibody response. Experiments to evaluate the effects of PFOA exposure on the TIAR were conducted twice, in two separate sets of animals.
Immunophenotyping
Experiment 3 (splenic lymphocyte phenotype)
Four C57BL/6 N mice from each dose were euthanized after 10, 13, or 15 days of dosing and spleens were harvested. Animals euthanized after 10 days of exposure were used to determine the effects of PFOA exposure at immunological steady state; i.e. in animals that were exposed to PFOA but not immunized. The remaining mice were immunized with SRBC on Day 11 to stimulate a primary immune response and euthanized on Days 13 and 15 to assess the effects of immunization on lymphocyte sub-set distributions over time and to determine if PFOA exposure had an impact on sub-sets. Spleens from the mice were rapidly removed after euthanasia and immediately placed into 4 ml cold Hanks Balanced Salt Solution (HBSS without CaCl2, MgSO4, or phenol red) and processed into single cell suspensions using a Stomacher 80 blender. Red blood cells were lysed with 0.84% [w/v] ammonium chloride, cells were washed twice with HBSS, and then resuspended in cold PBS containing 1% BSA with 0.1% sodium azide (flow cytometry staining buffer). The total number of nucleated lymphocytes was determined on a Beckman Coulter Counter (Beckman Coulter, Indianapolis, IN) and cell viability was determined by trypan blue exclusion. Cells were adjusted to 107/ml in flow cytometry staining buffer and kept on ice until immunofluorescent labeling.
Single-cell suspensions were pre-incubated for 10 min on ice with Fc-receptor blocking antibody to reduce non-specific binding of immunoglobulins, followed by incubation on ice for 30 min with the appropriate concentration of monoclonal antibody diluted in staining buffer. The following monoclonal antibodies were used: Purified rat anti-mouse CD16/CD32 (Fc block), fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD4, phycoerythrin (PE)-anti-mouse NK1.1, PE-Cy7-anti-mouse CD8a, allophycocyanin (APC)-anti-mouse CD3ɛ, APC-Cy7-anti-mouse CD45R. Isotype controls were prepared by labeling cells at the same protein concentration as the test antibody. Isotype control antibodies included: FITC rat IgG2a, APC-Cy7 rat IgG2a, R-PE mouse IgG2a, APC hamster IgG1, and PE-Cy7 rat IgG2a. All antibodies were purchased from BD Biosciences (San Jose, CA); PE-Cy7 Rat IgG2a was from eBioscience (San Diego, CA). After three washes, cells were fixed with 0.05% formaldehyde in PBS prior to analysis. Cells were analyzed in the following ways: (1) Pan T-cells: single parameter histogram of CD3 only, for CD3+ T-cells; (2) B-cells: 2-parameter histogram of 90° angle side scatter versus CD45R/B220-APC-Cy7 for CD45R/B220+ B-cells; (3) T-cell sub-sets: 2-parameter histogram of CD4− FITC versus CD8a-PE-Cy7 for CD4+CD8a− T-helper cells and CD4−CD8a+ T-suppressor cells; and (4) NK cells: 2-paramter histogram of CD3−APC versus NK-1.1-PE for CDe-NK−1.1+ cells.
Cells were analyzed with a LSR II flow cytometer (BD Biosciences) using FACS Diva software (BD Biosciences). A minimum of 10,000 events per sample was collected. Dead cells and debris were excluded from analysis by using forward scatter and 90° light scatter to establish a gate around the viable lymphocyte population. Non-stained cells, isotype controls, and fluorescence minus one (FMO) controls were used to distinguish the negative populations from positive populations for B-cells, T-cells, and NK cell sub-populations. Cells were gated based on CD3 expression for the subsequent analysis of CD4/CD8 T-sub-populations. Analysis of the data was performed using FlowJo software (TreeStar, Inc., Ashland, OR).
Statistical analysis
All data are presented as mean ± standard deviation. Experiments for TIAR were performed twice. Statistical analyses were performed with the SAS System (SAS Institute, Cary, NC). A one-way analysis of variance (ANOVA) was run on IgM antibody titers by dose for TIAR. A two-way ANOVA was run on immunophenotype by dose and by day and in TDAR by dose and by type of mouse (i.e. WT or PPARα KO). When ANOVA indicated a statistically significant effect within the model, individual post-hoc comparisons using one-way ANOVA, Tukey’s test, or an LSMeans t-test were run, as appropriate. Statistical significance was determined using an α = 0.05.
Results
Water consumption and body weights
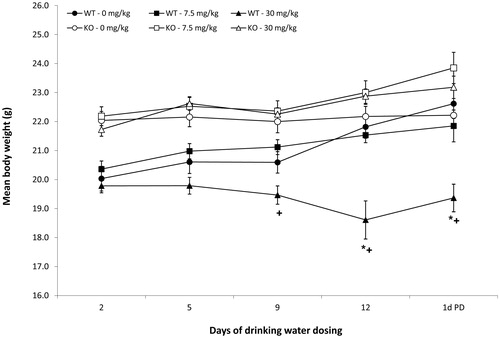
Water consumption did not differ by dose for any of the three experiments (data not shown). Consistent with our previous studies of PFOA exposure in this strain of mouse, exposure to 30 mg PFOA/kg statistically reduced body weights of WT mice relative to body weights of WT mice exposed to 0 mg PFOA/kg (). After 9 days of exposure, body weights of WT mice exposed to 30 mg PFOA/kg were 14% lower relative to the body weights in the similarly exposed PPARα KO group. After 12 days of exposure and 1 day after exposure ended, body weights of the WT mice exposed to 30 mg PFOA/kg were 15–20% lower than both the 0 mg PFOA/kg WT group and the PPARα KO group exposed to 30 mg PFOA/kg. Body weights of C57BL/6N mice exposed to 0.94–7.5 mg PFOA/kg did not vary statistically by dose (data not shown), which is consistent with our previous studies of PFOA in this strain of mouse (DeWitt et al., Citation2008).
Figure 1. Body weights of mice exposed to PFOA via drinking water for 15 days. C57BL/6N mice (n = 8/dose). Data represent mean ± SD. *Statistical (p < 0.05) difference between treated group and appropriate 0 mg PFOA/kg group. +Statistical (p < 0.05) difference between WT and PPARα KO group at same PFOA dose. Note that the interval between weight collection days is not evenly spaced, as depicted by the graph.
IgM antibody responses
Experiment 1 (The TDAR)
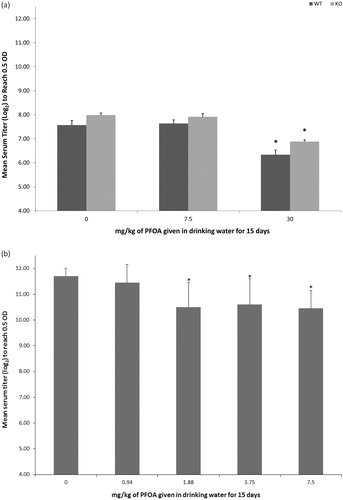
Lymphoid organ weights of PPARα KO mice were not changed by exposure to PFOA. Consistent with our previous studies of PFOA exposure in this strain of mouse, compared to weights in the 0 mg PFOA/kg WT group, relative spleen weights were statistically reduced by 29.8% after exposure to 30 mg PFOA/kg () and relative thymus weights were statistically reduced by 55.4% after exposure to 7.5 mg PFOA/kg (). Exposure to 30 mg PFOA/kg for 15 days reduced SRBC-specific IgM antibody responses in both PPARα KO and WT mice (). Relative to the responses in respective 0 mg PFOA/kg groups, responses were reduced by 14% in PPARα KO mice and by 16% in WT mice. Exposure to 7.5 mg PFOA/kg had no statistical effect on antibody responses. Additionally, antibody responses between WT and PPARα KO groups did not differ at any dose.
Figure 2. Lymphoid organ weights of mice exposed to PFOA via drinking water. Mice were exposed for 15 days, and organs collected 1 day after exposure ended. Data represent mean ± standard deviation. (a) Relative spleen weights of wild-type C57BL/6-Tac (WT) or PPARα KO B6.129S4-Ppartm1GonzN12 mice (n = 6/strain/dose). (b) Relative thymus weights of wild-type C57BL/6-Tac (WT) or PPARα KO B6.129S4-Ppartm1GonzN12 mice. At each dose level, lymphoid organ weights did not differ between WT or PPARα KO mice (n = 6/strain/dose). *Statistical (p < 0.05) difference between treated group and appropriate 0 mg PFOA/kg group.
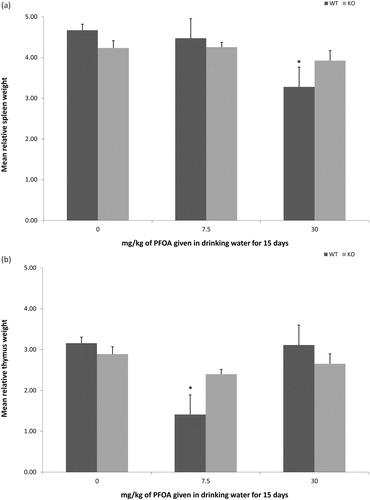
Figure 3. T-cell-dependent (TDAR) or T-cell-independent (TIAR) IgM antibody responses. Responses of mice exposed to PFOA via drinking water for 15 days, evaluated in sera collected 1 day (TDAR) or 2 days (TIAR) after exposure ended. Data represent mean ± SD. (a) The TDAR of wild-type C57BL/6-Tac (WT) or PPARα knockout (KO) B6.129S4-Ppartm1GonzN12 mice (n = 6/strain/dose). The TDAR did not differ between WT or PPARα KO mice at any dose. (b) The TIAR of C57BL/6N mice (n = 8/dose). *Statistical (p < 0.05) difference between treated group and corresponding 0 mg PFOA/kg group.
Experiment 2 (The TIAR)
Lymphoid organ weights of C57BL/6N mice were statistically reduced by exposure to 7.5 mg PFOA/kg for 15 days. Relative to weights in the 0 mg PFOA/kg group, relative spleen weights were reduced by 17% and relative thymus weights were reduced by 14%; in previous studies with this strain of mouse, only spleen weights were reduced at this concentration. Exposure to 1.88, 3.75, and 7.5 mg PFOA/kg for 15 days reduced DNP-specific IgM antibody responses in C57BL/6N mice (). Relative to the response in the 0 mg PFOA/kg group, PFOA reduced DNP-specific IgM antibody responses by 10.3%, 9.4%, and 10.7%, for the 1.88, 3.75, and 7.5 mg PFOA/kg dose groups, respectively. Exposure to 0.94 mg PFOA/kg did not change antibody responses relative to the response in the 0 mg PFOA/kg group.
Immunophenotype
PFOA had no statistical impact on splenic lymphocyte sub-populations after 10 days of exposure (). After 13 days of exposure and 2 days after immunization with SRBC, exposure to 7.5 mg PFOA/kg statistically increased the percentage of CD4−/CD8+ T-cell sub-populations relative to percentages in the 0 mg PFOA/kg group. The percentage of CD4−/CD8− T-cell sub-populations were statistically decreased in groups exposed to 3.75 or 7.5 mg PFOA/kg, respectively, relative to the 0 mg PFOA/kg group. After 15 days of exposure and 5 days after immunization, which also is the peak of IgM antibody production in mice after immunization with a T-cell-dependent antigen, the sub-populations of CD4+/CD8− T-cells were statistically increased, the CD4−/CD8+ T-cells were statistically decreased, and NK cells were statistically decreased in the group exposed to 3.75 mg PFOA/kg relative to values for the 0 mg PFOA/kg group. However, sub-population distributions were similar in the 0 and 7.5 mg PFOA/kg groups.
Table 1. Splenic lymphocyte sub-populations in adult C57BL/6N female mice orally exposed to PFOA for 10, 13, or 15 days.
Discussion
The current studies were conducted to determine if signaling via activation of the PPARα is required for suppression of the TDAR and if the TIAR also is affected by exposure to PFOA in a murine model. PFOA has been reported to up-regulate human and mouse PPARα activity in vitro (Takacs and Abbott, Citation2007), while others have reported that PFOA does not activate humanized PPARα expressed in mice (Nakamura et al., Citation2009). The role of PPARα ligation in rodent carcinogenesis is relatively well accepted, as are the differences between rodent and human PPARα biology and function, but PPARα ligation as a mode of action for suppression of antibody synthesis has yet to be established in experimental rodent models. Although Yang et al. (Citation2002b) reported that effects of PFOA and the specific PPARα agonist WY-14,643 on lymphoid organ weights, lymphocyte sub-population distributions and cell cycling were blunted in PPARα KO mice, the TDAR to immunization was not assessed in their study. To better understand the role of PPARα in suppression of the TDAR in mice and to improve the rodent data for applicability to potential immunotoxicological risk for humans exposed to PFOA, experiments were conducted to determine if suppression of the TDAR in rodents is dependent on PPARα ligation. Effects of PFOA exposure on the TIAR were also investigated because the structurally-related compound perfluorooctane sulfonate (PFOS) was reported to inhibit antibody responses by targeting B-cells (Peden-Adams et al., Citation2008). The T-helper cell-independent (i.e. innate) antibodies are critical to surviving bacterial pneumonia (Haas et al., Citation2005) and influenza (Baumgarth et al., Citation2000). These somatically encoded, naturally occurring antibodies are constitutively expressed and are active early in infection, before the adaptive humoral response to T-cell-dependent pathogen-associated antigens are synthesized in protective quantities.
In the current study, PPARα KO mice exposed to 30 mg PFOA/kg did not experience the loss of body weight observed in our strain-matched C57BL/6-Tac WT controls, in agreement with an earlier observation (Yang et al., Citation2002b) that weight loss is PPARα-dependent. Additionally, those authors reported that increased liver weight, an endpoint in experimental animal models that is highly sensitive to PFOA exposure, was increased in PPARα KO mice, indicating that PPARα sensitivity may be organ, system, or cell-type dependent. Spleen and thymus weights were not affected in PPARα KO mice exposed to 30 mg PFOA/kg, in agreement with data published by Yang et al. (Citation2002b) who compared effects in PPARα−/− SV129 knockouts and C57BL/6 control mice. Relative spleen and thymus weights were reduced in our WT controls exposed to 30 mg PFOA/kg (spleen) or 7.5 mg PFOA/kg (thymus). The reduction in spleen weight () was consistent with our previous study (DeWitt et al., Citation2008) and studies by others (Yang et al., Citation2002a; Loveless et al., Citation2008) that demonstrated reductions in spleen weights in C57BL/6 mice given 30 mg PFOA/kg. However, the reduction in the relative thymus weight () associated with the 7.5 mg PFOA/kg was not consistent with our previous studies with C57BL/6N mice (DeWitt et al., Citation2008), which demonstrated a 10% reduction in relative thymus weight relative to the vehicle group that was not statistically significant. Two of the animals in this 7.5 mg PFOA/kg dose group had extremely low thymus weights (< 10 mg), but, even when excluded, absolute and relative thymus weight of this group was still suppressed relative to the 0 mg PFOA/kg group. Regardless, in this strain of mice, this decrease in thymus weight at this dose was not associated with a decrease in the TDAR and a lack of decrease in thymus weight at 30 mg PFOA/kg was associated with a decrease in the TDAR. These results suggest that lymphoid organ weights and measures of immune function are not equally sensitive to PFOA exposure across all strains of mice, although the basis for this difference in sensitivity is unknown.
Significant suppression of the TDAR was observed in both WT and PPARα KO strains exposed to 30 mg PFOA/kg, indicating that PPARα ligation is not integral to suppression of the TDAR. However, in our previous studies, we detected suppression of TDAR at ≥3.75 mg PFOA/kg given for 15 days (DeWitt et al., Citation2008) in C57BL/6N mice, suggesting that the C57BL/6-Tac strain used in the PPARα KO studies may be less sensitive to suppression of the TDAR than other strains as 7.5 mg PFOA/kg was not sufficient to suppress TDAR in this strain. It is possible that inconsistent effects on spleen and thymus weights and reduced sensitivity of the TDAR to lower doses of PFOA are causally related; further studies must be done to determine if that is the case.
Suppression of the TDAR that we previously reported in C57BL/6N mice (DeWitt et al., Citation2008) occurred at doses (i.e., 3.75 and 7.5 mg PFOA/kg) that had little to no effect on splenic lymphocyte sub-population distributions in these mice and no statistical effect on the overall number of cells/spleen. While immunized mice exposed to 3.75 or 7.5 mg PFOA/kg had significant reductions (∼30–40%) in CD4−/CD8− T-cell sub-populations after 13 days of PFOA exposure, these sub-populations had returned to control levels after 15 days of PFOA exposure. However, the impact of this sub-population reduction on the TDAR is unclear as changes in other T-cell sub-populations were observed after 13 or 15 days of exposure, but not across both doses. Exposure to PFOA during antigen introduction and antibody production can modulate splenic lymphocyte sub-populations, but not in a consistent or dose-responsive manner that would explain reductions in the TDAR. Although immunophenotype was not determined in the same C57BL/6N mice used to assess the TIAR, suppression of TIAR occurred at doses lower than (i.e. 1.88 mg/kg) or equivalent to doses required to suppress the TDAR (i.e. 3.75 and 7.5 mg/kg) without consistent and concomitant changes in splenic lymphocyte sub-populations in the C57BL/6N mice. While it could be argued that doses of 15 and 30 mg PFOA/kg, which suppress TDAR in C57BL/6N mice, could change splenic lymphocyte sub-populations and suppress TIAR, we did not evaluate these doses because they were associated with systemic toxicity (DeWitt et al., Citation2008). Therefore, any changes to splenic lymphocyte sub-populations at these doses would not be immediately relevant to understanding how PFOA is immunotoxic. It is, therefore, unlikely that suppression of the TIAR was accompanied by reductions in splenic lymphocyte sub-populations at these lower doses. These results indicate that suppression of the TDAR and the TIAR was caused by changes in cellular function, rather than direct lymphocytotoxicity. These results also indicate that the LOAEL for these endpoints was very similar, likely between 1.88–3.75 mg/kg.
Looker et al. (Citation2014) concluded that elevated serum concentrations of PFOA in a highly exposed human population were associated with reduced antibody responses to a widely used multivalent influenza vaccine, although no differences in the incidence of self-reported influenza or common colds were detected in the study population. While the margin of exposure between this study and our previous study of the TDAR in mice (DeWitt et al., Citation2008) is four orders of magnitude, these human data indicate that the human immune system is sensitive to PFOA exposure. Therefore, our results, combined with these human data, suggest that assumed differences in human and rodent PPARα biology may not protect humans from suppression of humoral immunity induced by exposure to PFOA.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Acknowledgments
We would like to thank Dr Deborah Keil and Dr David Lehmann for reviewing the manuscript for scientic accuracy and grammatical clarity. N. J. Creech was supported, in part, by an Undergraduate Research and Creative Activity Award from the ECU Office of Undergraduate Research and through the ECU Summer Biomedical Research Program (SBRP). This report has been reviewed by the Environmental Protection Agency’s Office of Research and Development, and approved for publication. Approval does not signify that the contents necessarily reflect the views and policies of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
References
- Baumgarth, N., Herman, O.C., Jager, G.C., et al. 2000. B-1 and B-2 cell-derived immuno-globulin M antibodies are non-redundant components of the protective response to influenza virus infection. J. Exp. Med. 192:271–280
- Corsini, E., Avogadro, A., Galbiati, V., et al. 2011. In vitro evaluation of the immunotoxic potential of perfluorinated compounds (PFCs). Toxicol. Appl. Pharmacol. 250:108–116
- Corsini, E., Sangiovanni, E., Avogadro, A., et al. 2012. In vitro characterization of the immune-toxic potential of several perfluorinated compounds (PFC). Toxicol. Appl. Pharmacol. 258:248–255
- DeWitt, J. C., Copeland, C. B., Strynar, M. J., and Luebke, R. W. 2008. Perfluorooctanoic acid-induced immunomodulation in adult C57BL/6J or C57BL/6N female mice. Environ. Health Perspect. 116:644–650
- Grandjean, P., Andersen, E. W., Budtz-Jørgensen, E., et al. 2012. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA 307:391–397
- Granum, B., Haug, L. S., Namork, E., et al. 2013. Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J. Immunotoxicol. 10:373–379
- Haas, K. M., Poe, J. C., Steeber, D. A., and Tedder, T. F. 2005. B-1a and B-1B-cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity 23:7–18
- Looker, C., Luster, M. I., Calafat, A. M., et al. 2014. Influenza vaccine response in adults exposed to perfluorooctanoate and perfluorooctanesulfonate. Toxicol. Sci. 138:76–88
- Loveless, S. E., Hoban, D., Sykes, G., et al. 2008. Evaluation of the immune system in rats and mice administered linear ammonium perfluorooctanoate. Toxicol. Sci. 105:86–96
- Luster, M. I., Portier, C., Pait, D.G., et al. 1992. Risk assessment in immunotoxicology. I. Sensitivity and predictability of immune tests. Fundam. Appl. Toxicol. 18:200–210
- Luster, M. I., Portier, C., Pait, D. G., et al. 1993. Risk assessment in immunotoxicology. II. Relationships between immune and host resistance tests. Fundam. Appl. Toxicol. 21:71–82
- Nakamura, T., Ito, Y., Yanagiba, Y., et al. 2009. Microgram-order ammonium perfluorooctanoate may activate mouse PPARα, but not human PPARα. Toxicology 265:27–33
- Peden-Adams, M. M., Keller, J. M., Eudaly, J. G., et al. 2008. Suppression of humoral immunity in mice following exposure to perfluorooctane sulfonate. Toxicol. Sci. 104:44–154
- Selgrade, M. K. 1999. Use of immunotoxicity data in health risk assessments: Uncertainties and research to improve the process. Toxicology 133:59–72
- Selgrade, M. K. 2007. Immunotoxicity: The risk is real. Toxicol. Sci. 100:328–332
- Takacs, M. L., and Abbott, B. D. 2007. Activation of mouse and human peroxisome proliferator-activated receptors (alpha, beta/delta, gamma) by perfluorooctanoic acid and perfluorooctane sulfonate. Toxicol. Sci. 95:108–117
- Temple, L., Butterworth T. T., Kawabata, A. E., et al. 1995. ELISA to measure SRBC specific serum IgM: method and data evaluation. In: Methods in Immunology (Burleson, G. R., Dean, J. H., and Munson, A. E., Eds.). New York: Wiley-Liss, Inc., pp. 137–157
- USEPA. 2013. 2010/2015 PFOA Stewardship Program. www.epa.gov/oppt.pfoa/pubs/stewardship [last accessed 21 October 2014]
- USEPA. 2014. External peer review of EPA’s draft health effects documents for perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS). Peerreview.versar.com/epa/pfoa [last accessed 21 October 2014]
- van den Heuvel, J. P. 2007. The PPAR resource page. Biochim. Biophys. Acta. 1771:1108–1112
- Yang, Q., Abedi-Valugerdi, M., Xie, Y., et al. 2002a. Potent suppression of the adaptive immune response in mice upon dietary exposure to the potent peroxisome proliferator, perfluorooctanoic acid. Int. Immunopharmacol. 2:389–397
- Yang, Q., Xie, Y., Alexson, S. E., et al. 2002b. Involvement of the PPARα in the immunomodulation caused by peroxisome proliferators in mice. Biochem. Pharmacol. 63:1893–1900
- Yang, Q., Xie, Y., and Depierre, J. W. 2000. Effects of peroxisome proliferators on the thymus and spleen of mice. Clin. Exp. Immunol. 122:219–226
- Yang, Q., Xie, Y., Eriksson, A. M., et al. 2001. Further evidence for the involvement of inhibition of cell proliferation and development in thymic and splenic atrophy induced by the peroxisome proliferator perfluoroctanoic acid in mice. Biochem. Pharmacol. 62:1133–1140
