Abstract
This study sought to analyze structures of lymphatic tissues in two commercial fish species, e.g. Sole (Euryglossa orientalis) and Yellowfin Seabream (Acanthopagus latus), collected from five stations with varying levels of pollution in the Musa Creek near the Persian Gulf, e.g. Petro-chemical, Gaafari, Majidieh, Ghazaleh and Zangi Stations. Samples from Genaveh Station located outside Musa Creek were collected as controls. To correlate findings of changes in the studied tissues with local pollution status, levels of Hg, Pb, Zn, Cu and Cd were measured in sediments and water at each station. Fish were caught from the sampling stations; the spleen and head kidney were collected and sections prepared to permit histologic evaluation. The results indicated that, in both species, the most common changes were observed in fish collected near the Petrochemical station and included an increase in melano-macrophage aggregates, hemorrhage and damaged/dead red blood cells in the spleen; in the head kidney, the major findings were melano-macrophage aggregation, hemorrhage and lifting of the tubular basement membrane. No pathological alternations were noted in the spleen and head kidney of fish from the Zangi station. Samples of A. latus collected from Gaafari station and of E. orientalis from Majidieh station also had pathological changes. No significant differences were found in the tissue structures of fish recovered from the Zangi and Genaveh control stations. The concentrations for nearly all of the studied metals in sediment and water samples collected from the different stations followed the pattern: Petrochemical station ≈ Majidieh ≈ Gaafari >> Ghazaleh > Zangi Stations. From the data, it was concluded that changes in lymphoid tissues of the fish studied here “correlated” with geographical conditions and sources of pollution at the different test stations. What these changes mean to the long-term health of both species remains to be determined in ongoing studies.
Introduction
The innate immune system in fish, as in mammals, is a first defense line that consists of non-specific cellular (e.g. phagocytosis) and humoral (e.g. complement system, lysozyme activity) parameters that act against microorganisms, even in a host with no prior exposure to a given pathogen (Halliwell and Gutteridge Citation1999). The spleen and head kidney are the main immune organs in the fish. While the spleen acts primarily as a blood filter, in bony fish it consists primarily of red pulp and is normally a somewhat elongated organ that lies inside the serosal lining of the intestine (Romer and Parsons Citation1977). The kidneys of fish are typically narrow elongated organs occupying a significant portion of the trunk. These organs are similar to the mesonephros found in higher vertebrates. The posterior part of the kidneys contains clusters of nephrons serviced by collecting ducts that usually drain into a mesonephric duct. The anterior part of the kidney is considered the head kidney and is analogous to mammalian bone marrow as the primary site of hematopoiesis (Romer and Parsons Citation1977).
Like in mammals, the immune system in aquatic animals involves resistance against pathogens and maintenance of internal homeostasis. As such, a change in immune status could lead to adverse impacts on these functions (Carlson et al. Citation2002). Several studies on mammals have shown that xenobiotics could affect immune status (Hamers et al. Citation2006; Kakuschke and Prange Citation2007). Field studies have revealed that the immune system of aquatic animals collected from contaminated areas was more affected than systems in those collected from less or non-polluted regions (Frouin et al. Citation2007). This was possibly due to dose-related increases in adverse effects of the pollutants. For example, exposure of fish to increasing amounts of organic pollutants led to greater formation of reactive oxygen species (ROS) that affected their immune system due to lipid peroxidation and membrane destabilization (Di Guilio et al. Citation1989).
According to Khanyian et al. (Citation2014a), exposure of Epinephlus coioides to different concentrations of benzo(α)pyrene (BaP) caused significant decreases in circulating red and white blood cells and in macrophage lysosomal membrane stability, lysozyme activity and phagocytic activity. Khanyian et al. (Citation2014b) also reported dose-related alterations in tissue structures of the spleen and head kidney in the BaP-treated E. coioides (including hemorrhage, necrosis and leukocyte infiltration). Osman et al. (Citation2009) noted increases in lymphoid follicles, melano-macrophage centers and focal necrosis in Oreochromis niloticus exposed to copper (Osman et al. Citation2009). Although numerous studies have been conducted on environmental pollutant immunotoxicity in mammals, a survey of the literature showed that few studies have been undertaken so far with regard to effects of such pollutants on the immune system of aquatic hosts, i.e. fish.
Many water pollutants can be dissolved in the water column and adsorbed by particles in the water or enter the sediments (Holladay et al. Citation1998). As pyrogenic and petrogenic sources of different pollutants are continuously increasing worldwide, marine sediments potentially are a major reservoir for these compounds (Frouin et al. Citation2007). Musa Creek, located in the northwest Persian Gulf in southern Iran, is considered a good place for many aquatic species to pass through larval stages and for spawning. In the last decades, Musa Creek has received a great deal of attention due to a large amount of contaminants (such as heavy metals and oil compounds) that have been dumped into it by surrounding cities and industries (Dehghan Madiseh et al. Citation2009; Safahieh et al. Citation2011). Different studies have confirmed that effluent discharge from surrounding cities, oil jetties and petrochemical/alkali industries are the main sources of water contamination of Musa Creek (Dehghan Madiseh et al. Citation2009; Safahieh et al. Citation2011, Citation2013).
Various fish species live in Musa Creek, many of which are very popular as food in the south of Iran. Yellowfin seabream (Acanthopagrus latus) and sole (Euryglossa orientalis) are two such commercial species. These fish species can serve as particularly reliable indicators (i.e. sentinels) of changes in the local environment as they are native species that are geographically widely distributed. To date, few studies have focused on alterations in tissue structure (especially immune system tissues) induced in fish by environmental pollutants found along Musa Creek. Therefore, in the present study, histopathological changes in lymphoid organs (i.e. spleen and head kidney) were studied in both A. latus and E. orientalis that were collected at various sites along Musa Creek to provide an assessment of overall health of the creek ecosystem.
Materials and methods
Sampling
In the present study, 150 mature male and female yellowfin sea bream (Acanthopagrus latus) (36 [±0.4] g weight, 22 [±0.32] cm body length) and sole (Euryglossa orientalis) (25 [±0.3] g weight and 26 [±0.42] cm body length) were collected from five sampling sites (15 fish/species/site) along the Musa Creek during October 2013. The sites were: (A) Petrochemical Station, (B) Majidieh Station, (C) Ghazaleh Station, (D) Gaafari Station and (E) Zangi Station (). Another set of 30 A. latus and E. orientalis were caught at Genaveh Station (located outside Musa Creek); this is considered to be a reference (control) site here due to its relatively clean water. Physicochemical parameters associated with the waters collected at the different stations are presented in . Samples of water (and sediment) at all sites were also analyzed for pollutant metal content. Sediment samples were collected (in three replicates) using a Van Veen Grab sampler like that commonly used in hydrology studies.
Table 1. Physicochemical parameters associated with water samples from the different stations.
All fish were anesthetized with 2-phenoxy-ethanol (0.1 g/L) and then euthanized by cervical dislocation. The fish were then dissected and samples taken from the spleen and head kidney were then fixed in Bouin’s fluid for 48 h prior to histologic processing.
Histologic analyses
Each tissue sample was dehydrated in ascending concentrations of ethanol and then embedded in paraffin using a tissue processor (Tissue Tek Rotary, Rx-11B). Thereafter, 5-µm thick sections were prepared using a RMZZ45 rotary microtome (Leica, Wetzlar, Germany) and then stained with hematoxylin and eosin (H&E; Bancroft and Gamble Citation2002). The sections were then evaluated by light microscopy and digital images were taken using Dino Capture software (FDP2, Taipei, Taiwan).
Degree of tissue change (DTC) in head kidney and spleen
The degree of tissue changes (DTC) is a scoring parameter based on the severity of lesions and possibility of recovery according to Poleksic and Mitrovic-Tutundzic (Citation1994). The pathological changes noted in the head kidney and spleen in both fish species were classified into three progressive stages ( and ): Stage I = slight changes that do not alter function of the organs and repair is possible with improvement of environmental conditions; Stage II = moderate changes that affect normal function of these lymphoid organs – these alterations may be repaired, but they can lead to severe alterations in the case of chronic pollution; and Stage III = severe changes that cause irreversible damage from which recovery is not possible, even with improved water quality. For each form of damage, the numbers of fish at a given site positive for the particular damage was determined from among all the fish collected (data not shown). From within each of these populations, the number of the specific microscopic findings in each fish was then evaluated; thereafter, the mean incidence of each type of pathological alteration within the total population of A. latus or E. orientalis caught at that site was calculated ( and ).
Table 2. Histopathologic changes (mean incidence of each form of specific microscopic finding) in spleen of fish collected from different polluted sites.
Table 3. Histopathologic changes (mean incidence of each form of specific microscopic findings) in head kidney of fish collected from different sites.
Based on those evaluations, the DTC was then calculated for each fish as: DTC = (1 × SI) + (10 × SII) + (100 × SIII), wherein I, II and III relate to the number of fish in each set having the types of alterations indicated in Stages 1, 2 and 3, respectively. DTC values between: 0–10 show normal function of the organ; 11–20 indicate slight damage to the organ; 21–50 indicate moderate changes; 50–100 indicate severe lesions; and >100 indicate irreversible damage. As an example of how this calculation worked, the average (±SE) DTC values for the spleen and head kidney of A. latus at the Petrochemical Station site were calculated to be, respectively, 162.0 [±3.3] and 132 [±2.5].
Metal analysis
Sediment samples were each desiccated at 105°C and then ground using a mortar and sieved. Samples (1 g) of each material were then digested in a mixture of H2O2, HCl and HNO3 according to the Manual of Oceanographic Observations and Pollutant Analysis Methods (MOOPAM) (ROPME, Citation1999). The resulting solutions were then analyzed for Hg, Pb, Zn, Cu and Cd using an AA-670 Flame Atomic Absorption Spectrophotometer (Shimadzu, Kyoto, Japan). As with the final sediment solutions, all water samples were analyzed neat [for the same metals]. The limit of detection for each metal as measured in this system is reported in . Detection limits were determined using instrumental parameters optimized for the individual element, including use of System 2 electrode-less discharge lamps when available. All values for sediment and water metal contents were reported in µg/ml.
Table 4. Limit of detection of metals measured by AA-670 flame atomic absorption spectroscopy.
Statistics
All data were presented as mean ± SE and analyzed using SPSS software (v16; Chicago, IL). The normality of data was firstly checked using a Shapiro–Wilk test. Parametric tests (Student’s t-test, one-way analysis of variance [ANOVA], Tukey post-hoc test) were performed to determine any significant differences in content of metals between both fish species sampled at the different stations. Similarly, the level of each tissue alteration was compared within each fish species collected at the different sites (using ANOVA) and between the two fish species at each station (using t-test).
Results
Physicochemical parameters of water samples from different stations
The results of physicochemical characteristics of water in different sampling stations are presented in . While there were clear differences in absolute values for pH and dissolved oxygen in the various sites’ samples, these differences were not significant.
Splenic alternations
Spleen with normal tissue structure was found in A. latus and E. orientalis collected from Genaveh Station (control site). As expected, the splenic parenchyma of these fish consisted of normal red and white pulp, stroma and blood vessels ( and ). For the analyses of the incidence of pathologies, the data in indicate variations in occurrence of several types of damage. Clearly, any value >0 would be indication of a level of damage significantly different compared to occurrence of the same in Genaveh control fish (for either species) – accordingly, this table does not report values “significantly different from control”.
Figure 2. Representative photomicrographs of A. latus spleen tissue structures. (A) Control site with normal Red pulp and white pulp. (B) Petrochemical Station: Significant increase in melano-macrophage aggregations (black arrow). (C) Gaafari station, (D) Majidieh Station, (E) Ghazaleh Station and (F) Zangi Station: Melano-macrophage aggregations (black arrows). All samples; H&E-stained, magnification = ×725).
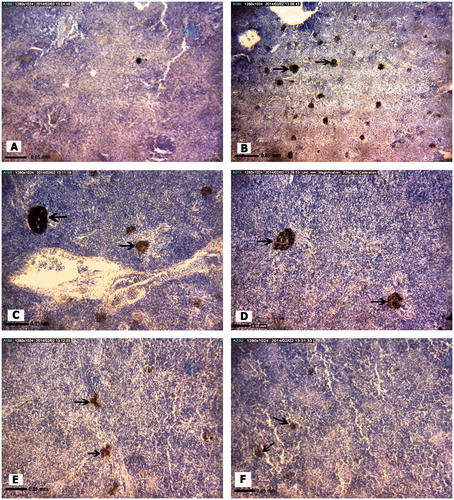
Figure 3. Representative photomicrographs of E. orientalis spleen tissue structures. (A) Control site: Red and white pulp, splenic capsule (arrow). (B) Petrochemical Station: Hemorrhage (black ). (C) Majidieh Station: Hemorrhages (black ). (D) Ghazaleh Station: Melano-macrophage aggregations (black arrows). (E) Gaafari Station: Relatively normal tissue structure. (F) Zangi Station: Normal tissue structure: Red pulp (white ), white pulp (black ). All samples; H&E-stained, magnification = ×725).
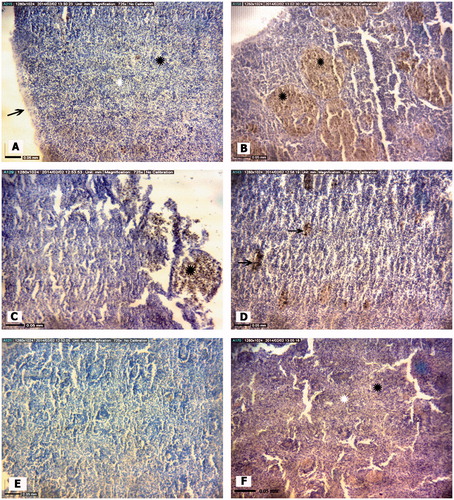
Among the Stage I forms of pathologies, a slight increase in melano-macrophage (M-M) aggregation was the only histological alternation found in the spleens of A. latus caught at Zangi Station. A similar patter of only M-M aggregation in the spleens of A. latus was obtained at the Gaafari and Ghazaleh Stations, although the trend was toward an increasing incidence of these aggregations (compared to Zangi fish levels). The greatest incidence of M-M aggregations was evident in fish harvested from the Majideh and Petrochemical Stations. Ultimately, the levels in the fish from the Petrochemical Station were significantly greater than values in fish from all the other sites (except from Majidieh). Moreover, only in the fish at Petrochemical Station was there also evidence of significant sinusoid dilation.
With regard to Stage II forms of pathologies (12 types assessed), fish from the Petrochemical Station had significant expression of six pathologies, fish from Gaafari Station had five, and fish from Majidieh Station had four (). Only for the category “Increased M-M aggregation” was damage clearly evident in fish isolated from the five test sites. Interestingly, except for the incidence of hemorrhage and hematopoietic cell proliferation, the mean incidence of four other types of damage, i.e. presence of dead/destroyed erythrocytes, red pulp congestion, increased M-M aggregation, and white pulp hyperplasia, was highest among all fish examined for (albeit not significantly different between) those fish recovered from the Petrochemical and Gaafari Stations. With regard to Stage III pathologies (five types assessed), the only significant damage found was “cell degeneration” among fish harvested from the Petrochemical Station.
In the analyses of the E. orientalis collected at the various sites, the trends noted above with A. latus were for the most part reproduced (except that fish from Ghazaleh site now had lowest levels of this damage among the five sites) (). Nevertheless, there were some notable differences from outcomes with A. latis. For example, E. orientalis collected at Gaafari Station also had significant increases in the occurrence of Stage I sinusoid dilation. Among the Stage II pathologies, this species also had notable increases in the occurrence of hemorrhage in fish collected at Ghazaleh Station, but not in those from Gaafari Station. Similarly, fish collected at Ghazaleh and Majidieh Stations had significant increases in incidence of white pulp hyperplasia, but this was not seen in fish from the Gaafari site. As with the A. latus, of the five Stage III pathologies, the most significant damage found was “cell degeneration” among fish harvested from the Petrochemical Station. However, these same hosts also had significant increases in the incidence of splenic necrosis.
Thus, by comparing all the data shown in and illustrated in the representative histology photographs provided in and , it is clear there were site-related associations for the type/extent of pathologies induced in the spleens of both the A. latus and E. orientalis. However, it is also clear that, at any one given site, there were also species-related associations for type/extent of these inducible pathologies.
Head kidney alterations
No pathologic alternations were found in the head kidney of both fish species recovered from Genaveh Station. The head kidney of these hosts consisted of normal lymphoid tissue, some renal tubules, and mature and immature blood cells ( and ). In contrast, to varying degrees related to collection site, empty spaces between cells, melano-macrophage aggregations, leukocyte hyperplasia, hematopoietic precursor cell swelling and an incidence of eccentric nuclei, disrupted tissue integrity, thickening of basal endothelial membrane, vacuolation and degeneration of renal tubular epithelial cells, deposition of hemosiderin pigments and/or a lifting of tubular basement membranes were (cyto)pathologies observed in both the A. latus () and E. oreintalis hosts ().
Figure 4. Representative photomicrographs of A. latus head kidney tissue structures. (A) Control site: Lymphoid tissue (), renal tubules (black arrow). (B) Petrochemical Station: Hemorrhage (black ). (C) Gaafari Station and (D) Majidieh Station: Melano-macrophage aggregations (black arrow), Empty spaces between cells (white arrows), Hemorrhage (black ). (E) Ghazaleh Station and (F) Zangi Station: Lifting of tubular basement membrane (black chevrons). All samples; H&E-stained, magnification = ×725).
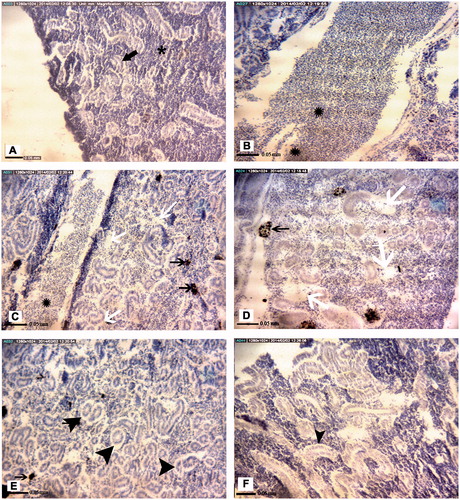
Figure 5. Representative photomicrographs of E. orientalis head kidney tissue structures. (A) Control site: Lymphoid tissue (black ), renal tubules (arrow). (B) Petrochemical Station: Hemorrhage (black ). (C) Majidieh Station: Empty spaces between cells (black arrows). (D) Ghazaleh Station: Melano-macrophage aggregations (black arrows). (E) Gaafari Station: vacuolation of renal tubular epithelial cells (black arrow). (F) Zangi Station: Lifting of tubular basement membrane (black arrow). All samples; H&E-stained, magnification = ×725).
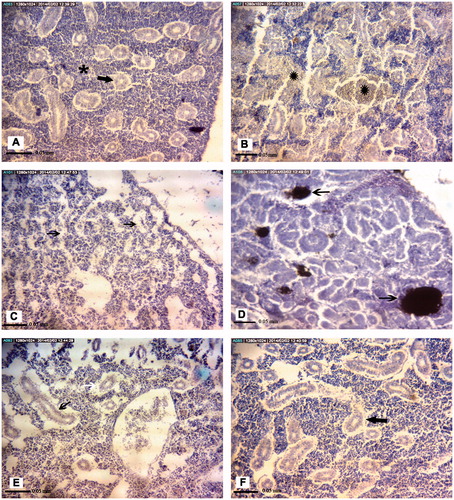
Still, there were some cases of species-specific damage to the head kidney observed here (). For example, no pathological alternations were detected in the organs recovered from either species of fish recovered at Zangi Station. Similarly, leukocyte hyperplasia, thickening of vein walls and hemorrhage were each observed in organs from both species when they were collected at Gaafari Station. In addition, hypertrophy and vacuolization of hematopoietic pre-cursors, leukocytes hyperplasia, thickening of the vein walls and hemorrhage were also seen in samples from A. latus and E. orientalis caught at the Majidieh Station. On the other hand, hypertrophy and vacuolization of hematopoietic precursors were found only in samples from E. oreintalis caught at the Ghazaleh Station. In addition, only samples from A. latus collected at Gaafari Station had clear increases in incidence of dead/destroyed blood cells, hemosiderin pigments and lifting of tubular basement membrane.
Based on these results overall, a generalized non-statistical ranking of histologic damage in the spleen and head kidney of E. orientalis and A. latus obtained from the different sampling sites was generated. For the E. orientalis, the outcomes followed the pattern: Petrochemical > Majidieh > Ghazaleh > Gaafari > Zangi Station. For A. latus, the pattern was: Petrochemical > Gaafari > Majidieh > Ghazaleh > Zangi Station.
Degree of tissue changes (DTC) of the head kidney and spleen
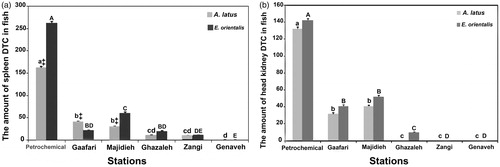
As seen in and , higher frequencies of the most severe damage, i.e. those that would fall into the categories of Stages II and III, were seen in the spleen and head kidney of fish caught at the Petrochemical Station (). The average (±SE) mean DTC values for the spleen and head kidney of A. latus at this site were, respectively, 162.0 ± 3.3 and 132 ± 2.5. For E. orientalis, values were, respectively, 262.0 ± 3.7 and 142.0 ± 2.5. In contrast, fish of both species caught at the Genaveh Station (control site) had DTC values of 0. Based on the DTC results, this site might be considered as having the best environmental quality. There was no significant difference between DTC mean values for the head kidney and spleens of E. orientalis and A. latus caught at Gaafari Station (range from 22.0 [±0.6] to 41.0 [±1.4]). Also, no significant difference was detected between DTC values for the head kidney of E. orientalis and A. latus caught at Majideh Station (range from 41.0 [±1.0] to 52.0 [±1.5]). In contrast, spleen DTC values did appear to be greater in the E. orientalis caught at this site (61 vs 31 for A. latus). The lowest DTC values for the spleen were found in both fish species caught at Ghazaleh and Zangi stations; head kidney samples from these hosts indicated little damage at all. This set of outcomes was suggestive of a determination that these sites had some-what better environmental quality than the others tested here ().
Figure 6. Average DTC values for the spleen and head kidney in A. latus and E. orientalis isolated from the different exposure sites. Values shown are mean ± SE (n = 15/species/site). For both A. latus (A. l.) and E. orientalis (E. o.), values with differing superscript lowercase letters indicate significant difference as a function of exposure site. ‡For a given site, DTC value for A. latus significantly differ from that for E. orientalis.
Heavy metals in water and sediment
The mean concentrations of select metals (e.g. Hg, Pb, Cu, Zn, Cd) in the water and sediment of different sampling sites are presented in . The highest and lowest amounts of all metals were measured in samples collected, respectively, from the Petrochemical and Zangi Station sites (). Among the various stations, levels of the metals were almost uniformly highest in the Petrochemical, Majidieh and Gaafari Stations water and sediments, with the levels of specific elements varying to some degree from site-to-site among the three sites. In all cases, levels at those sites were always greater than at the Ghazaleh and Zangi Stations. It was interesting to note that, among the five metals analyzed, Zn and Cu tended to be encountered at the highest levels in the sediments and waters recovered from each test site.
Table 5. Levels of metals (Hg, Pb, Zn, Cu, Cd) in the water and sediment at the different stations.
Discussion
The spleen is an immune organ whose function in bony fish (teleosts) includes acting as a site of hematopoiesis, facilitating lymphocyte proliferation and capturing antigens. When impacted by pollutants/infectious pathogens, the spleen undergoes changes in structure/function (Anderson and Zeeman Citation1995). The analyses performed in the present study showed there were different pathological alternations (in type and magnitude) in the spleen [and head kidney] of both A. latus and E. orientalis collected from different sampling stations along the Musa Creek.
Many histology studies have been conducted on such changes (Garcia-Abiado et al. Citation2004). Oliveira Ribeiro et al. (Citation2002) posited that pathological damage to the spleen of fish exposed to environmental pollutants (such as those observed in the present study) had a direct correlate with levels of pollutants present and/or length of host exposure to the toxicants. Agius and Roberts (Citation2003) reported that the histological changes in immune tissues of fish could serve as indicators of environmental stress. Suresh (Citation2009) suggested that changes in melano-macrophage centers in the spleen could be used as an indicator of stress induced by various pollutants in aquatic environments. The increase in number of melano-macrophage centers is likely due to changes in innate as well as adaptive immunity mechanisms that lead to increases in phagocytic/chemotactic activities – and thus migration of lymphocytes to the spleen – creating lymphocyte accumulation (Kakkar Citation2011). In a study of the effects of water pollution on splenic tissue structure in Orechromis niloticus, Osman et al. (Citation2009) reported that the more fish were exposed to copper, the higher were the splenic levels of melano-macrophage accumulation.
The head kidney is another hematopoietic lymphoid organ in fish whose function is to serve as a site for the production of erythroid as well as lymphoid cells (Romano et al. Citation2002). In the head kidney of the fish studied here, lesions were observed – including separation of the renal tubular epithelium from basement membranes and renal tubular epithelium vacuolization. These changes have been deemed in some studies as non-specific pathologic lesions in various parts of the kidney (including the head) that were seen in fish exposed to various types of pollutants including organochlorines, organophosphates, pesticides and heavy metals (Banerjee and Bhattacharya Citation1994; Global Tox Citation1997). In the present study, additional histopathologic outcomes such as bleeding, increased numbers of melano-macrophage centers, as well as increased deposition of hemosiderin pigment in the head kidney of the fish were observed.
Many studies have reported an accumulation of melano-macrophages in fish exposed to high doses of toxic chemicals in their aquatic environments (Dezfuli et al. Citation2006; Giari et al. Citation2008). Apart from functioning in the removal of pathogens and triggering immune responses to pathogenic agents, Hapaaranta et al. (Citation1996) reported that melano-macrophage centers also have a role in neutralizing free radicals in tissues that undergo oxidative damage. In studying histopathological changes in the head kidney of Channa Panctatus that had been exposed to fossil fuels, Kakkar (Citation2011) suggested that increases in melano-macrophage centers was likely due to metabolic disorders induced in response to the pollutants. On the other hand, those authors also associated tissue bleeding with pollutant-induced vascular disorders and severe physical damage. Agius and Roberts (Citation2003) reported accumulation of hemosiderin along with an increase in melano-macrophage centers in the head kidney of fish exposed to pollutants.
The results of the histopathology analyses of the head kidney and spleen of E. orientalis showed that fish caught at the Zangi and Ghazaleh Stations had the lowest levels of histopatho-logical changes (and DTC scores) among all non-control station fish analyzed. It also happened that these two sites also contained the lowest levels of the studied metals (e.g. Hg, Pb, Zn, Cu and Cd) compared to the other test sites here. Both the Zangi and Ghazaleh Stations receive the least polluted effluents from pollution sources; Dehghan Madiseh et al. (Citation2009) noted there was no meaningful difference in levels of metals in water at these sites. Safahieh et al. (Citation2011) also reported that levels of specific pollutants like mercury in the sediments/water at these sites were routinely significantly less than at the other test stations investigated in the current study.
The most severe lesions in both tissues of both fish species belonged to those who lived at/near the Petrochemical Station. Numerous studies blame the discharge of untreated wastewater from a petrochemical complex and a chlor-alkali plant for the severe pollution at the Petrochemical Station (Dehghan Madiseh et al. Citation2009; Safahieh et al. Citation2011, Citation2013). The results of the present study also showed that the high levels of the studied metals (Hg, Pb, Zn, Cu and Cd) were found at this site. These results were in keeping with those of Salamat et al. (Citation2013), who also reported severe tissue lesions in the organs of Liza abu captured near this station. According to Safahieh et al. (Citation2013), sediments/water at this site contained very high levels of metals, especially mercury. Those measures paralleled our own in the present study. Levels of damage to organs, as well as of pollutants in the water/sediment, were then high – albeit much less than that associated with the Petrochemical Station samples – in fish isolated at the Gaafari and Majidieh Stations. In the case of the latter, the presence of an oil pier in the vicinity of the station as well as restricted water exchange with open water [due to the site being too far from the sea compared to the distance faced by the Ghazalen and Zangi Stations] allowed significant amounts of time for contaminants (organics [PAHs and PCBs] and heavy metals) from the oil docks to settle and accumulate in the local sediments. The current study also showed that the sediments at the sites contained high levels of Hg, Pb, Zn, Cu and Cd. Those findings are in keeping with others that reported pollutant accumulation in the sediments of this estuary due to oil exports from Majidieh (Safahieh et al. Citation2011, Citation2013).
It is interesting to note there were often differences in the extent of damage (for samples from a given site) in the same organs across the two fish species. Generally, the levels of metals studied in the present investigation were higher in the sediments than in the water at the different stations. Other researchers reported higher levels of different metals in sediments of different parts of Musa Creek (Dehghan Madiseh et al. Citation2009; Safahieh et al. Citation2011, Citation2013). According to Jezierska and Witeska (Citation2006), different fish species from the same polluted body of water may accumulate vastly different amounts of the same pollutants. Such inter-species differences in pollutant accumulation have a close relationship with each host’s living and feeding habits. As reported by Ney and van Hassel (Citation1983), the potential accumulation of metals was higher in benthic fish than in non-benthics. Similarly, higher concentrations of metals/other pollutants have often been found in predatory as compared to in non-predatory fish (Voigt Citation2004). As E. orientalis is a benthic saprophagous that feeds on benthic invertebrates (Sattari et al. Citation2004), it appears logical to find more tissue damage in this species than in A. latus hosts, even for a given level of pollution at any particular studied station here.
Conclusions
In this study, the highest incidence/severity of lesions were found in the spleen and head kidney tissues of A. Latus and E. Orientalis caught from Petrochemical Station; it is reasonable to assume that these effects could be attributable to the wastewater that is being discharged from a petrochemical complex and chlor-alkali plant near this site. Lesser levels of damage were noted in fish isolated from Majidieh Station: these differences in outcomes compared to fish from the Petrochemical Station are most like due to (1) accumulation of lower levels/different forms of pollutants due to contamination from a near-by oil export pier and (2) geographical features of the station relative to that of some of the other sites examined in the study. In sum, it can be concluded that changes in lymphoid tissues of the fish studied here “correlate” with geographical conditions and sources of pollution entering the different stations. What these changes mean to the long-term health of both species remains to be determined in our follow-on studies.
Acknowledgements
The authors would like to acknowledge the Institute of the Khorramshahr University of Marine Science and Technology for their support of this research.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Agius C, Roberts RJ. 2003. Melano-macrophage centers and their role in fish pathology. J Fish Dis. 26:499–509
- Anderson DP, Zeeman MF. 1995. Immunotoxicology in fish. In: Rand G, editor. Fundamentals of aquatic toxicology: effect, environmental fate, and risk assessment. Washington. Taylor and Francis. p. 371–404
- Bancroft JD, Gamble M, editors. 2002. Theory and practice of histological techniques. 5th ed. Oxford. Elsevier
- Banerjee S, Bhattacharya S. 1994. Histopathology of kidney of Channa punctatus exposed to chronic non-lethal level of mercury and ammonia. Ecotoxicol Environ Safety. 29:265–275
- Carlson EA, Li Y, Zelikof JT. 2002. The Japanese medaka (Oryzias latipes) model applicability for investigating the immunosuppressive effects of aquatic pollutant benzo[α]pyrene (B[α]P). Mar Environ Res. 54:565–568
- Dehghan Madiseh S, Savary A, Parham H, Sabzalizadeh S. 2009. Determination of the level of contamination in Khuzestan coastal waters (Northern Persian Gulf) by using an ecological risk index. Environ Monit Assess. 159:521–530
- Dezfuli BS, Simoni E, Giari L, Manera M. 2006. Effects of experimental terbutylazine exposure on the cells of Dicentrarchus labrax (L.). Chemosphere 64:1684–1694
- Di Guilio RT, Washburn PC, Wenning RJ, Winston GW, Jewell CS. 1989. Biochemical responses in aquatic animals: a review of determinants of oxidative stress. Environ Toxicol Chem. 8:1103–1123
- Frouin H, Pellerin J, Fournier M, Pelletier E, Richard P, Pichaud N, Rouleau C, Garnerot F. 2007. Physiological effect of polycyclic aromatic hydrocarbons on soft-shell clam Mya arenaria. Aquat Toxicol. 82:120–134
- Garcia-Abiado MA, Mbahinzireki G, Rinchard J, Lee KJ, Dabrowski K. 2004. Effect of diets containing gossypol on blood parameters and spleen structure in tilapia, Oreochromis sp., reared in a re-circulating system. J Fish Dis. 27:359–368
- Giari L, Simoni E, Manera M, Dezfuli BS. 2008. Histo-cytological responses of Dicentrarchus labrax (L.) following mercury exposure. Ecotoxicol Environ Safety 70:400–410
- Global Tox. 1997. Technical evaluation of histopathology as an environmental monitoring tool for the mining industry in Canada. Report prepared for Aquatic Effects Technology Evaluation (AETE) Program, Ottawa. Natural Resources Canada by Global Tox International Consultants Inc., Ottawa
- Halliwell B, Gutteridge JM, editors. 1999. Free radicals in biology and medicine. 3rd ed. Oxford. Clarendon Press
- Hamers T, van den Berg JH, van Gestel CA, van Schooten FJ, Murk AJ. 2006. Risk assessment of metals and organic pollutants for herbivorous and carnivorous small mammal food chains in a polluted floodplain (Biesbosch, the Netherlands). Environ Poll. 144:581–595
- Hapaaranta A, Valtonen ET, Hoffmann R, Holmes J. 1996. Do macrophage centers in freshwater fishes reflect the differences in water quality. Aqua Toxicol. 34:253–272
- Holladay SD, Smith SA, Besteman EG, Deyab AS, Gogal RM, Hrubec T, Robertson JL, Ahmed SA. 1998. Benzo[α]pyrene-induced hypo-cellularity of the pronephros in tilapia (Oreochromis niloticus) is accompanied by alterations in stromal and parenchymal cells and by enhanced immune cell apoptosis. Vet Immunol Immunopathol. 64:69–82
- Jezierska B, Witeska M. 2006. Metal uptake and accumulation in fish living in polluted waters. Soil Water Poll Monit Protect Remed. 69:107–114
- Kakkar KG. 2011. Water soluble fraction of diesel fuel induced histopathological alterations in the liver of Channa punctatus. Fish Shellfish Immunol. 18:14–16
- Kakuschke A, Prange A. 2007. The influence of metal pollution on the immune system: a potential stressor for marine mammals in the North Sea. Int J Comp Psychol. 20:179–193
- Khanyian M, Salamat N, Safahieh A, Movahedinia A. 2014a. Detection of benzo[a]-pyrene-induced immunotoxicity in orange spotted grouper (Epinephelus coioides). Environ Toxicol. [Epub ahead of print]. doi: 10.1002/tox.22047
- Khanyian M, Salamat N, Safahieh A, Movahedinia A. 2014b. Liver pathological alterations in Epinephlus coioides exposed to benzo(a)pyrene. J Mar Sci Technol. 13:1–11
- Ney JJ, van Hassel JH. 1983. Sources of variability in accumulation of heavy metals by fishes in a roadside stream. Arch Environ Contam Toxicol. 12:701–706
- Oliveira Ribeiro CA, Belger L, Pelletier E, Rouleau C. 2002. Histopathological evidence of inorganic mercury and methyl mercury toxicity in the arctic charr (Salvelinus alpines). Environ Res. 90:217–225
- Osman HA, Abbas MM, Ibrahim W, Taghreed B. 2009. An approach to the inter-action between trichodiniasis and pollution with benzo(α)pyrene in catfish (Clarias garie-pinus). World J Fish Mar Sci. 1:283–289
- Poleksic V, Mitrovic-Tutundzic V. 1994. Fish gills as a monitor of sublethal and chronic effects of pollution. In: Muller R, Lloyd R, editors. Sublethal and chronic effects of pollutants on freshwater fish. Oxford. Fishing News Books. p. 339–352
- Romano N, Ceccariglia S, Mastrolia L, Mazzini M. 2002. Cytology of lympho-myeloid head kidney of antarctic fishes Termatomus bernacchii (Nototheniidae) and Chionodraco hamatus (Channicthyidae). Tissue Cell. 17:34–42
- Romer AS, Parsons TS, editors. 1977. The Vertebrate Body. Philadelphia (PA). Holt-Saunders International. p. 161–170
- ROPME (Regional Organization for the Protection of the Marine Environment). 1999. Regional organization for the protection of the marine environment. 3rd ed. Kuwait: ROPME
- Safahieh A, Abdolahpur Monikh F, Savari A, Doraghi A. 2011. Heavy metal concentration in mullet, Liza abu from petrochemical waste receiving creeks, Musa Estuary (Persian Gulf). J Environ Prot. 2:1218–1226
- Safahieh A, Babadi S, Nabavi MB, Ronagh MT, Ghanemi K. 2013. Assessment of mercury intake through consumption of yellowfin seabream (Acanthopagrus latus) from Musa Estuary. J Life Sci Tech. 1:142–146
- Salamat N, Soleimani Z, Safahieh A, Savari A, Ronagh MT. 2013. Using histopathological changes as a biomarker to trace contamination loading of Musa Creeks (Persian Gulf). Toxicol Pathol. 41:913–920
- Sattari M, Shahsavani D, Shafiei S, editor. 2004. Fish biology 2 (Systematic). Amsterdam. Hagh Shenas Publishers. p. 245–246
- Suresh N. 2009. Effect of benzo(α)pyrene on liver, spleen and kidney melano-macrophage centers in Tilapia mossambica. J Environ Biol. 30:505–508
- Voigt HR. 2004. Concentrations of mercury (Hg) and cadmium (Cd), and the condition of some coastal Baltic fishes. Environ Fennica. 21:26