Abstract
Cytokines are considered important factors in the modulation of various immune responses. Among them, interleukin (IL)-21 is one of the major immune modulators, adjusting various immune responses by affecting various immune cells. It has been suggested that IL-21 may enhance autoimmunity through different mechanisms, such as development and activation of helper T (TH)-17 and follicular helper T (TFH) cells, activation of natural killer (NK) cells, enhancing B-cell differentiation and antibody secretion and suppression of regulatory T (Treg) cells. Moreover, IL-21 has also been suggested to be an inducer of autoimmunity when following treatment of MS patients with some therapeutics such as alemtuzumab. This review will seek to clarify the precise role of IL-21/IL-21R in the pathogenesis of MS and, in its animal model, experimental autoimmune encephalomyelitis (EAE).
Introduction
Cytokines play an important role in the modulation of several immune and non-immune related processes. Moreover, some cytokines have critical roles in the induction, initiation, progression and attenuation of a variety of diseases (Ghalamfarsa et al. Citation2013; Tangye Citation2015). Thus, it seems that identification of the precise mechanism(s) by which cytokines modulate disease status may help design new therapeutic methods of treatment.
Interleukin-21 (IL-21), a member of the common γ-chain cytokine family, has attracted much attention among investigators because of its complex structure and multi-functionality. Several cell types, such as helper T (TH)-17, follicular helper T (TFH), Th2 and natural killer T (NKT) cells, can produce IL-21. Moreover, a wide variety of immune and non-immune cells express the IL-21 receptor (IL-21R) on their surface (Nurieva et al. Citation2007; Zhou et al. Citation2007; King et al. Citation2008; Suto et al. Citation2008). Thus, it seems that IL-21 may play a critical role in the immuno-pathogenesis of several types of cancers and autoimmune diseases.
Multiple sclerosis (MS), a central nervous system (CNS)-related chronic inflammatory demyelinating disease, is characterized by re-occurring episodes of demyelination and axonal lesion induced by TH (particularly TH1 and TH17) cells, macrophages and soluble inflammatory mediators (Jadidi-Niaragh and Mirshafiey Citation2010, Citation2011b, Citation2011c). There is also evidence implying the role of other immune cells such as TFH, NK and B-cells in the immunopathogenesis of MS (Chanvillard et al. Citation2013; Plantone et al. Citation2013; Romme Christensen et al. Citation2013; Haugen et al. Citation2014). Although the precise etiology of MS is unknown, it has been is suggested that genetic and environmental factors have great influences on the onset and progression of MS (Mirshafiey and Jadidi-Niaragh Citation2010a, Citationb).
Increased levels of IL-21 have been observed during the progression of several auto-immune diseases; this implies a pathologic function for this cytokine in autoimmunity (Zanin-Zhorov et al. Citation2014). Moreover, it has been reported that IL-21 acts as a pro-inflammatory agent during MS progression and so it has been suggested that IL-21 may promote disease advancement (Vollmer et al. Citation2005). Since TH17 cells play an important role in the neuro-inflammatory process of MS, with regards to the stimulatory effect of IL-21 on development and expansion of TH17 cells, it has been proposed that IL-21 and IL-21R may be considered key targets in the treatment of MS. This review intends to discuss the precise roles for IL-21/IL-21R in the immunopathogenesis/treatment of MS and in its animal model, experimental autoimmune encephalomyelitis (EAE).
Multiple sclerosis
MS is a complex heterogenic autoimmune disease of the CNS in which the myelin sheaths of the neurons are relatively destroyed by self-reactive immune responses (Mirshafiey et al. Citation2014). MS patients may express several manifestations such as paresthesia, diplopia, visual impairment, numbness or weakness of the limbs, bowel or bladder dysfunction, spasticity, ataxia, fatigue and altered mental functions (Gaby Citation2013). Although the precise etiology of MS is unknown, genetic and environmental factors play key roles in its etiology (Hoglund and Maghazachi Citation2014). There are four main phenotypes of MS including relapsing remitting (RR), primary progressive (PP), secondary progressive (SP) and progressive relapsing (PR) (Gaby Citation2013). It seems that destruction of the blood–brain barrier (BBB) and migration of autoreactive T-cells from the blood to the CNS are the main processes involved in the development of MS (Cheng and Chen Citation2014). Following the breakdown of the BBB (due to several triggers such as oxidative stress), peripheral activated lymphocytes infiltrate the CNS and induce local immune responses, ultimately destroying the myelin and even the axons themselves (Ortiz et al. Citation2014). Injured cells in the BBB secrete various inflammatory cytokines and chemokines that play an important role in the recruitment of inflammatory cells to the CNS (Cheng and Chen Citation2014). It seems that autoreactive lymphocytes are initially activated in the systemic lymphoid organs by various mechanisms like molecular mimicry and bystander activation. Subsequently, they transmigrate to the CNS where they are re-activated following the encounter of their specific antigen, which eventually leads to demyelination and axonal damage (Hartung et al. Citation2014).
Although autoreactive T (particularly TH1 and TH17)-cells are the main players in the immunopathogenesis of MS, innate immune responses are also involved in its initiation and progression (Roozbeh et al. Citation2014). In addition to TH cells, CD8+ T-cells also contribute to the development of the disease and clonally expanded CD8+ T-cells are observed within the MS lesions (Denic et al. Citation2013). The invariant natural killer T (iNKT)-cells are another lymphocyte sub-type which can secrete various cytokines (such as interferon [IFN]-γ), interleukin (IL)-10, IL-4) and may be involved in disease progression (Roozbeh et al. Citation2014). Regulatory T (Treg) cells play an important role in the maintenance of self-tolerance in the immune system. It has been reported that, although the frequency of Treg cells is intact in MS patients, their functionality is rather perturbed (Jadidi-Niaragh and Mirshafiey Citation2011a). A skewed balance between Treg and TH17 cells during MS progression has also been demonstrated (Jadidi-Niaragh and Mirshafiey Citation2012).
In addition to T-cells, B-cells are also supposed to have a critical effect in the immunopathogenesis of MS. B-cells secrete various antibodies and cytokines which can enhance disease furtherance. They can also act as antigen presenting cells for T-cells. Moreover, identification of regulatory B-cells with anti-inflammatory function further complicates the identification of the precise role of B-cells in MS development (Krumbholz and Meinl Citation2014).
Immunobiology of IL-21
IL-21 is a member of the Class I cytokine family, which includes IL-2, -4, -7, -9 and -15 (Spolski and Leonard Citation2008). The IL-21R exhibits homology with the β-chain of IL-2/IL-15Rs and constitutes a complex with the common γ-chain (Collins et al. Citation2003; Parrish-Novak et al. Citation2000). Several types of immune cells, such as activated TH, NKT, TFH and TH17 cells, can produce IL-21 (Parrish-Novak et al. Citation2000; Mehta et al. Citation2004; Leonard and Spolski Citation2005; Spolski and Leonard Citation2008). This cytokine regulates the differentiation and function of various immune cells. Interaction of IL-21 with its receptor leads to regulation of activation, proliferation and survival of both TH and B-cells. It can also inhibit the differentiation and function of inducible Treg cells (Monteleone et al. Citation2009). Moreover, IL-21 plays an important role in the development of TFH (Shekhar and Yang Citation2012), B-cell somatic hypermutation and immunoglobulin class switching (Eto et al. Citation2011), TH2 cell function (Pesce et al. Citation2006), expansion and activity of CD8+ T-cells (Zeng et al. Citation2005), cytotoxic activity of NK cells (Strengell et al. Citation2003) and the differentiation and function of NKT cells (Smyth et al. Citation2005; Coquet et al. Citation2007). It is reported that, while the IL-21 enhances the cytotoxic function and IFNγ secretion in murine-derived activated NK cells, it inhibits IL-15-mediated expansion of resting NK cells (Kasaian et al. Citation2002). IL-21 also promotes the terminal differentiation of mouse NK cells (Brady et al. Citation2004; Li et al. Citation2015). Further, IL-21 enhances the proliferation, IFNγ generation and cytotoxic activity of CD8+ effector T-cells in allogeneic mixed-lymphocyte responses (MLR) (Kasaian et al., Citation2002).
IL-21 can potently affect the B-cell proliferation, survival, differentiation and immuno-globulin responses (Ghalamfarsa et al. Citation2013, Citation2015; Spolski and Leonard Citation2014) While the IL-21 induces apoptosis of B-cells, it enhances IL-4 mediated B-cell proliferation and differentiation toward memory or plasma cells (Ettinger et al. Citation2005). These contradictory functions of IL-21 on B-cells depend on the interplay with co-stimulatory signals and the developmental stage of B-cells (Konforte et al. Citation2009). IL-21 also stimulates antibody production and, thereby, enhances host defense against various malignances and infectious diseases (Ettinger et al. Citation2008).
IL-21 also increases resistance of effector T-cells against the suppressive function of Treg cells (Peluso et al. Citation2007). It can also inhibit granulocyte–macrophage colony-stimulating factor (GM-CSF)-mediated dendritic cell (DC) maturation. Thus, IL-21 shifts DC toward immature phenotype, which inhibits T-cell responses and induces Treg cells (Brandt et al. Citation2003). In addition, IL-21 decreases the expression of MHC Class II, CD80, CD86 and CCR7 on the surface of DC and inhibits the secretion of IL-6, IL-12, IL-1β and tumor necrosis factor (TNF)-α cytokines by DC (Brandt et al. Citation2003; Strengell et al. Citation2006). It can also induce apoptosis of conventional DC in a STAT3- and BIM-dependent manner (Wan et al. Citation2013).
TH17 cells generate IL-21 in a STAT3-dependent manner and IL-21 induces expression of RAR-related orphan receptor γ-t (RORγt), IL-17A and IL-17F in a positive autocrine loop that leads to expansion of TH17 cells (Wei et al. Citation2007). IL-21 can promote differentiation of TH17 cells, even in the absence of IL-6 (Deenick and Tangye Citation2007). Moreover, IL-21 skews the balance between TH17 and Treg cells toward TH17 cells through STAT3 and RORγt transcription factors (Deenick and Tangye Citation2007; Korn et al. Citation2007; Nurieva et al. Citation2007). It was also demonstrated that the frequency of TH17 cells was significantly reduced when the IL-21/IL-21R signaling was blocked (Deenick and Tangye Citation2007; Korn et al. Citation2007; Nurieva et al. Citation2007). Secretion of IL-21 and IL-17 from T-cells can be controlled via ROCK2 through STAT3, IRF-4 and RORγt. It is reported that inhibition of ROCK2 increases the suppressive function of Treg cells via up-regulation of STAT5 phosphorylation. Thus, the use of ROCK2 antagonist shifts the TH17/Treg balance toward the Treg phenotype (Zanin-Zhorov et al. Citation2014).
TFH cells express B-cell CLL/lymphoma-6 (BCL6) transcription factor and can produce high levels of IL-21 (Chtanova et al. Citation2004). IL-21 enhances the secretion of TNFα by conventional T-cells, which leads to up-regulation of CXCL9 on DC in the draining lymph nodes and facilitates TFH differentiation (Yoo and Braciale Citation2014). As IL-21 affects the differentiation, function and survival of a wide variety of immune cells, it may be considered as a worthy target for treatment of several diseases.
IL-21 and IL-21R in CNS-related disorders
There is evidence implying IL-21 modulates the self-reactive immune responses in various CNS-related autoimmune diseases (Petrelli et al. Citation2011; Yoshizaki et al. Citation2012; Linhares et al. Citation2013; Liu et al., Citation2015). Geri et al. (Citation2011) have reported that IL-21 promotes inflammatory lesions in Behçet disease (BD) through the expansion of TH17 and suppression of Treg cells. BD is chronic systemic inflammatory disease that can also involve the CNS. They showed that, while the serum of patients with active BD enhances the production of IL-17 in purified CD4+ T-cells of healthy donors, it reduces the expression of forkhead box protein 3 (FoxP3) in vitro. Moreover, they could detect IL-21- and IL-17A-producing T-cells within cerebrospinal fluid, brain parenchyma inflammatory infiltrates and intra-cerebral blood vessels of patients with active BD with CNS involvement. Further, treatment of isolated CD4+ T-cells with IL-21 led to the up-regulation of TH17 and TH1 and down-regulation of Treg cells. On the other hand, blocking IL-21 via an IL-21R-Fc restored the TH17 and Treg cell balance in BD patients by suppressing IL-17A production and increasing FoxP3 expression (Geri et al. Citation2011). Thus, it was proposed that IL-21 enhanced disease progression, in part, through promotion of inflammatory responses by expansion of TH17 cells and also interfered with self-tolerance by down-regulation of Treg cells.
In another study, Linhares et al. (Citation2013) investigated the function of T-cells in patients with neuromyelitis optica (NMO), which is a type of CNS-related autoimmune disease. Those authors showed that, while TH1 cytokines were decreased in cultured T-cells isolated from NMO patients, TH17 and related cytokines (i.e. IL-23 and IL-6) were significantly increased. Moreover, polyclonal stimulation of CD4+ T-cells led to the secretion of IL-21 and IL-6 that was positively correlated to neurological disability and was resistant to glucocorticoid inhibition in NMO patients. Based on that previous study, it seemed that the presence of a positive autocrine loop between TH17 and IL-21 might play a pivotal role in the immunopathogenesis of CNS-related autoimmunity.
There is also evidence which indicates an important role of IL-21 in CD8+ T-cell survival in the CNS during acute mortal JHMV and West Nile (WNV) viral infections (Stohlman et al. Citation1998; Janssen et al. Citation2005; Sitati and Diamond Citation2006; Fröhlich et al. Citation2009; Barker et al. Citation2010). It is suggested that CD4+ T-cells are the main source of IL-21 in the CNS, since it was observed that its mRNA level was significantly reduced in the CNS of CD4+ T-cell-depleted mice (Phares et al. Citation2011). It is also suggested that IL-21 producing CD4+ T-cells promote the peripheral activation of CD8+ T-cells and increase their antiviral function within the CNS (Phares et al. Citation2012). Moreover, IL-21R deficiency was associated with the down-regulation of IFNγ and IL-10 in CNS-derived CD4+ T-cells and disturbed peripheral B-cell activation and impaired CNS humoral responses (Phares et al. Citation2013). Thus, IL-21 secreting T-cells may enhance antibody production by B-cells, which may promote autoimmune responses (Metcalf et al. Citation2013). Similar results regarding the stimulatory and pathologic effects of IL-21, on both T- and B-cells in the CNS, have been reached using toxoplasma gondii-infected IL-21-deficient mice (Stumhofer et al. Citation2013).
An increased frequency of IL-21- and IL-9-producing CD4+ T-cells has also been noted in Alzheimer’s disease (Saresella et al. Citation2011). The extent of brain atrophy was also highly correlated with IL-21 producing CD4+ T-cells (Baglio et al. Citation2013). Moreover, using IL-21R-deficient mice with experimental autoimmune uveitis, it was demonstrated that blocking IL-21 signaling could lead to attenuation of CNS auto-inflammatory diseases (Wang et al. Citation2011b). It was also reported that IL-21 was increased in the injured mouse brain after cerebral ischemia. Moreover, IL-21-deficient mice exhibited smaller infarcts, better neurological function and decreased lymphocyte infiltration into the brain. The brain infiltrated CD4+ T-cells were the main source of IL-21. Administration of IL-21 receptor Fc fusion protein to these mice protected them from re-perfusion injury. Further, IL-21 was identified as a mediator of brain injury in post-mortem human brain tissue, since IL-21 was detected in perivascular CD4+ T-cells in the area surrounding acute stroke lesions (Clarkson et al. Citation2014). Lastly, Daga et al. (Citation2007) was able to successfully eradicate glioblastoma (a tumor of the CNS) in mice through local delivery of IL-21 by IL-21 gene-transduced cells or using recombinant IL-21. These investigators showed that IL-21 exerted its anti-tumor effects mainly through the activation of anti-tumor antibody-secreting B-cells. That study aimed to prove the ability of IL-21 in the activation of B-cells in the CNS, which has been little researched until now.
Thus, it seems IL-21 is an important factor for the modulation of immune responses and inflammatory reaction in the CNS. In some disease such as BD and brain stroke, inhibition of IL-21 led to disease attenuation. On the other hand, IL-21 protected hosts against viral encephalitis and glioblastoma through stimulation of anti-viral and anti-tumor immune responses. The above-mentioned studies demonstrated that IL-21 exerted its effects, in part, through effects on the expansion of TH17 and TH1 cells, suppression of Treg cells and modulation of humoral immune responses. The skewed balance between TH17 and Treg cells has frequently been noted in various cancers and autoimmune diseases and known to be an important factor in the immuno-pathogenesis of several diseases (Gol-Ara et al. Citation2012; Azizi et al. Citation2013; Jadidi-Niaragh and Mirshafiey Citation2012; Jadidi-Niaragh et al. Citation2013a, Citationb, Citationc). Thus, it is obvious to us that IL-21 was an essential immune factor for the modulation of neuro-inflammatory reactions in CNS-related disorders.
Expression of IL-21 and IL-21R in multiple sclerosis
It has been suggested that the expression of IL-21/IL-21R has high associations with different autoimmune diseases (Collins et al. Citation2003; Vollmer et al. Citation2005). Moreover, the polymorphism of IL-21 showed a high association rate with the susceptibility to autoimmune disease in Caucasians (Liu et al., Citation2015). Consistently, the gene and surface expressions of IL-21 and IL-21R were significantly increased in the CD4+ T-cells from patients with progressive MS (Romme Christensen et al. Citation2013). On the other hand, it has been reported that IL-21 was only over-expressed on infiltrating CD4+ T-cells in the acute and chronic white matter of MS lesions, whereas IL-21R was mainly expressed on CD4, CD19 and CD8 lymphocytes, but not MHC II-expressing macrophages/microglia. Both of the IL-21 and IL-21R were also expressed in neurons in the cortical area (Tzartos et al. Citation2011). There are similar results regarding the association of IL-21R gene polymorphism and MS disease (Nohra et al. Citation2010). Further, increased expression of IL-21 mRNA was detected during MS relapses (Tegla et al. Citation2013). Oddly, there was no significant change in the cerebrospinal fluid (CSF) concentration of IL-21 in MS patients (Wu et al. Citation2012). In addition, it was reported that IL-21 was not considered a major risk factor for MS in Spanish and Swedish populations (Fedetz et al. Citation2009; Linden et al. Citation2011). Another study demonstrated there were no significant differences in serum levels of IL-21 between MS patients and normal subjects (Wang et al. Citation2011a). These discrepancies (as shown in
Table 1. Studies related to expression of IL-21/IL-21R in MS patients.
IL-21/IL-21R in EAE
Several studies have been demonstrated that prove IL-21/IL-21R can exacerbate EAE in part through the development of TH17 cells (
Table 2. Studies related to the role of IL-21/IL-21R in EAE.
Li et al. (Citation2009) suggested that the expression of epidermal fatty acid-binding protein (E-FABP) on T-cells could enhance development of TH17 and suppress that of Treg cells. Those investigators showed that the myelin oligodendrocyte glycol-protein peptide (MOG)-mediated induction of EAE in E-FABP-deficient mice was associated with down-regulation of TH17 and up-regulation of Treg cells, an outcome that was due in part to significant decreases in IL-21 levels. It has also been shown that tolerogenic DC can suppress EAE development, in part through down-regulation of RORγt, IL-17A, IL-17F, IL-21 and IL-22 (Zhou et al. Citation2014). It has also been reported that treatment of EAE mice with salmon cartilage proteoglycans could lead to down-regulated IL-6, IL-21, IL-23R and RORγt, and up-regulated Foxp3 in both draining lymph nodes and spinal cords (Sashinami et al. Citation2012).
NR4A2 (nuclear receptor sub-family 4, group A, member 2) plays an important role in the differentiation of TH17 cells and production of IL-21 and IL-17 cytokines. Consistently, in vivo inhibition of NR4A2 expression reduced TH17 and IL-21 levels and protected mice against EAE induction (Raveney et al. Citation2013). Moreover, it has been demonstrated that the frequencies of CNS-infiltrating TH1, TH17, IFNγ+IL-17A+ and IL-21+IL-17A+ CD4+ T-cells were significantly increased in the brain and spinal cord of B-lymphocyte-induced maturation protein-1 (BLIMP-1)-deficient NOD mice (Lin et al. Citation2014).
Contrary to the studies previously mentioned, there is evidence to indicate the IL-21/IL-21R axis has no effect on development of TH17 cells and that IL-21/IL-21R-deficient mice are also susceptible to EAE progression (Coquet et al. Citation2008; Sonderegger et al. Citation2008). Moreover, increased infiltration of pro-inflammatory cells into the CNS has also been seen when IL-21 was suppressed (Piao et al. Citation2008). Another study showed the frequency of Treg cells and expression of FoxP3 were significantly decreased in both blocked IL-21 and IL-21R deficient mice with EAE (Liu et al. Citation2008; Piao et al. Citation2008). Interestingly, it was seen that IL-21R-deficient mice developed EAE faster and in a more severe form when compared to control mice. This faster initiation was also associated with defective Treg and FoxP3 expression and intact TH17 development. Moreover, recovery among IL-21R-deficient mice was associated with expansion of Treg cell levels and organ-specific redistribution of NK cells (Liu et al. Citation2008).
Recent studies have shown that the new sub-sets of B-cells that produce IL-10 can negatively regulate immune responses; therefore, these cells are known as regulatory B-cells (B10 or Breg cells) (Yanaba et al. Citation2008; Yoshizaki et al. Citation2012). Generation of in vivo effector B10 cells depends on the presences of IL-21 and its receptor (Tedder and Leonard Citation2014). Therefore, B10 cells regulate immune responses by IL-10 production in neuro-inflammatory processes such as MS (Tedder and Leonard Citation2014). A study on purified spleen CD19+ B-cells showed that IL-21 significantly increased the production of IL-10 without the need of phorbol esters and ionomycin for stimulation. The effector function of B10 cells in EAE was dependent to the existence of IL-21R, MHC-II and CD40 (Yanaba et al. Citation2009; Yoshizaki et al. Citation2012). Moreover, initiation of EAE was significantly reduced in mice treated by adoptive transfer of antigen-specific (MOG-sensitized) B10 cells; it seems this function was related to CD4+ T-cell IL-21 production. Thus, it seems B10 cells could naturally regulate acute immune responses in EAE in an IL-21-dependent manner.
As noted earlier, there are conflicts between different studies regarding the role of IL-21/IL-21R in the immunopathogenesis of EAE (). However, these discrepancies may be due to genetic backgrounds of mice, different EAE-inducing protocols (using various doses of antigen for EAE induction [i.e. 100 μg (Bauquet et al. Citation2009), 150 μg (Coquet et al. Citation2008) or 200 μg (Vollmer et al. Citation2005)]) and the fact IL-21 is highly pleiotropic – affecting many immune and non-immune components of the body which have not been fully addressed until recently (Spolski and Leonard Citation2014; Croce et al. Citation2015). Further investigations with the same disease induction protocols (in large scale) and optimal doses of antigens are required to clarify the precise roles for the IL-21/IL-21R axis in EAE.
Role of IL-21/IL-21R in immunopathogenesis of MS
Since TH17 cells play an important role in the neuro-inflammatory process of MS and IL-21 significantly promotes the development of TH17, it seems that IL-21 may enhance MS progression. It has been reported that simvastatin reduces neuro-inflammatory CNS lesion formation in RR-MS patients (Giovannoni et al. Citation2014). Zhang et al. (Citation2011) reported that the mechanism by which simvastatin exerted anti-inflammatory effects on RR-MS patients was due in part to inhibition of TH17 and its secreted cytokines, particularly IL-21. Those authors demonstrated that simvastatin blocked differentiation of TH17 cells and generation of TH17-derived cytokines (IL-17A, IL-17F, IL-21 and IL-22) in in vitro-differentiated naive TH cells.
IL-21 not only inhibits the development of Treg cells (Deenick and Tangye Citation2007; Petrelli et al. Citation2011), but also converts Treg to TH17 (Yang et al. Citation2008) and increases the resistance of conventional T-cells against the suppressive function of Treg cells (Peluso et al. Citation2007). Therefore, it seems that IL-21 enhances the development of MS, in part, through the suppression of Treg cells. It has been reported that treatment of MS patients with UVB phototherapy was associated with down-regulation of IL-21 and up-regulation of Treg cells and tolerogenic DC, resulting in disease attenuation (Breuer et al. Citation2014). It was also demonstrated that the frequency of TFH cells was significantly increased in the peripheral blood and cerebrospinal fluid of RR-MS and SP-MS patients. Moreover, increased expression of IL-21, IL-21R and ICOS was noted in CD4+ T-cells obtained from progressive MS patients. Treatment of such patients with mitoxantrone was associated with the down-regulation of TFH and IL-21, outcomes that led to disease alleviation (Romme Christensen et al. Citation2013). Further, accumulated populations of IL-21R+ and IL-21+CD4+ T-cells were observed in both active and chronic MS lesions (Tzartos et al. Citation2011; Romme Christensen et al. Citation2013). Although most studies have shown that IL-21 indirectly plays an important role in the pathogenesis of MS (
Table 3. Studies related to the role of IL-21/IL-21R in MS.
Alemtuzumab is a humanized monoclonal antibody targeting the campath-1 antigen (CD52) that is broadly expressed on immune cells. This antibody quickly eliminates CD52-expressing immune cells from the circulation. It has been suggested this antibody could also exert neuroprotective effects, presumably by inducing production of neurotrophic factors in autoreactive T-cells (Klotz et al. Citation2012; Fernandez Citation2014). Alemtuzmab can significantly reduce the rate of relapse in RR-MS patients (Brown and Coles Citation2013). However, treatment of MS patients with alemtuzumab may also be associated with new secondary autoimmune disorders, such as auto-immune thyroiditis or idiopathic thrombocytopaenic purpura (Jones et al. Citation2009; Klotz et al. Citation2012; Fernandez Citation2014).
Treatment of MS patients with alemtuzumab also led to decreased IL-21 levels (Zhang et al. Citation2013). Secondary autoimmunity following treatment with alemtuzumab usually occurs and can be predicted by the high baseline levels of IL-21 in the MS sera (Costelloe et al. Citation2012). Evaluation of IL-21 in the serum of MS patients prior to treatment with alemtuzumab showed that groups of patients who exhibited secondary autoimmunity had higher levels of IL-21 compared to non-autoimmune hosts (Jones et al. Citation2009; Costelloe et al. Citation2012). IFNβ has usually been used as a first-line treatment for RR-MS patients and can decrease production of IL-21 from CD4+ T-cells (Tao et al. Citation2014). Thus, it would seem that documented therapeutic effects of IFNβ are due in part to a down-regulation of IL-21.
As discussed earlier, little is known regarding the immunopathologic potential of IL-21/IL-21R in MS patients. Thus, it is difficult to reach a conclusive result based on previous studies about the precise role of IL-21/IL-21R in the immune status of MS patients. With that in mind, it appears IL-21 contributes to MS progression partly through up-regulation of TH17 and down-regulation of Treg cells. TH17 cells, which are the main pathogenic cells in MS, produce IL-21 that can lead to the expansion of the same and other TH17 cells in a positive autocrine loop. Moreover, there is no comprehensive data regarding precise effects of IL-21 on other immune cells involved in MS such as B-cells, macrophages/microglia and DC. Moreover, since IL-21 can affect several immune and non-immune components of the body, it is difficult to make a direct correlation between the therapeutic mechanisms of some agents and IL-21.
As discussed, there were several therapeutic agents that could affect the production of different cytokines (particularly IL-21) in the MS patients and EAE animal models. It is not rational to assume, just because IL-21 is included in affected cytokines, the major effect must come from it rather than from a combination of cytokines. Therefore, further comprehensive studies are needed to discriminate the precise role of IL-21 in the immunopathogenesis of MS between all affected cytokines.
Conclusion
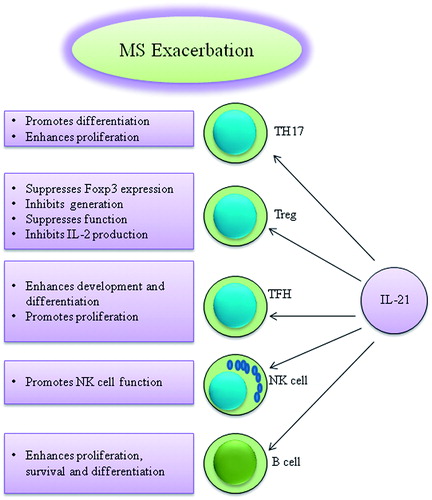
IL-21 affects the development and function of various immune cells, including TH17, Treg, TFH, NK and B-cells. Since these cells play an important role in the immune responses, IL-21 may be involved in the immunopathogenesis of several diseases, such as MS (). Moreover, there is evidence supporting the theory that IL-21 is in fact involved in the development of MS. However, reports regarding the role of IL-21/IL-21R in EAE animal models remain controversial. One item that needs to be clarified is the role of IL-21 in the development and function of B10 cells in EAE and MS. This issue will determine whether IL-21 can be considered as having a protective role in MS or not. On the other hand, little is known regarding the precise role of the cytokine in the immunopathogenesis of MS. As IL-21 promotes development of effector cells involved in the neuro-inflammatory process associated with MS such as TH17 and directly inhibits induction of Treg cells, therefore inhibition of IL-21 may be considered a worthy target for MS therapy. However, it should be noted that Treg and TH17 cells are in the reciprocal regulation and development of each cell is associated with expansion of another one. Further investigations regarding the immunobiology of IL-21 are required to enable the designing of novel therapeutics based on IL-21 targeting.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Andersson A, Isaksson M, Wefer J, Norling A, Flores-Morales A, Rorsman F, Kämpe O, Harris RA, Lobell A. 2008. Impaired autoimmune TH17 cell responses following DNA vaccination against rat experimental autoimmune encephalomyelitis. PLoS One 3:e3682.
- Azizi G, Jadidi‐Niaragh F, Mirshafiey A. 2013. TH17 cells in immunopathogenesis and treatment of rheumatoid arthritis. Intl J Rheum Dis. 16:243–253.
- Baglio F, Saresella M, Preti MG, Cabinio M, Griffanti L, Marventano I, Piancone F, Calabrese E, Nemni R, Clerici M. 2013. Neuro-inflammation and brain functional disconnection in Alzheimer's disease. Front Aging Neurosci. 5:81.
- Barker BR, Gladstone MN, Gillard GO, Panas MW, Letvin NL. 2010. Critical role for IL-21 in both primary and memory anti-viral CD8+ T-cell responses. Eur J Immunol. 40:3085–3096.
- Bauquet AT, Jin H, Paterson AM, Mitsdoerffer M, Ho IC, Sharpe AH, Kuchroo VK. 2009. The costimulatory molecule ICOS regulates the expression of c-Maf and IL-21 in the development of follicular T-helper cells and TH17 cells. Nat Immunol. 10:167–175.
- Brady J, Hayakawa Y, Smyth MJ, Nutt SL. 2004. IL-21 induces the functional maturation of murine NK cells. J Immunol. 172:2048–2058.
- Brandt K, Bulfone-Paus S, Jenckel A, Foster DC, Paus R, Rückert R. 2003. IL-21 inhibits dendritic cell-mediated T-cell activation and induction of contact hypersensitivity in vivo. J Invest Dermatol. 121:1379–1382.
- Breuer J, Schwab N, Schneider-Hohendorf T, Marziniak M, Mohan H, Bhatia U, Gross CC, Clausen BE, Weishaupt C, Luger TA, et al. 2014. Ultraviolet B light attenuates the systemic immune response in central nervous system autoimmunity. Ann Neurol. 75:739–758.
- Brown JW, Coles AJ. 2013. Alemtuzumab: Evidence for its potential in relapsing-remitting multiple sclerosis. Drug Design Devel Ther. 7:131–138.
- Chanvillard C, Jacolik RF, Infante-Duarte C, Nayak RC. 2013. The role of natural killer cells in multiple sclerosis and their therapeutic implications. Front Immunol. 4:63.
- Chen M, Chen G, Nie H, Zhang X, Niu X, Zang YC, Skinner SM, Zhang JZ, Killian JM, Hong J. 2009. Regulatory effects of IFNβ on production of osteopontin and IL-17 by CD4+ T-Cells in MS. Eur J Immunol. 39:2525–2536.
- Cheng W, Chen G. 2014. Chemokines and chemokine receptors in multiple sclerosis. Med Inflamm. 2014:659206.
- Chtanova T, Tangye SG, Newton R, Frank N, Hodge MR, Rolph MS, Mackay CR. 2004. T-Follicular helper cells express a distinctive transcriptional profile, reflecting their role as non-TH1/TH2 effector cells that provide help for B-cells. J Immunol. 173:68–78.
- Clarkson BD, Ling C, Shi Y, Harris MG, Rayasam A, Sun D, Salamat MS, Kuchroo V, Lambris JD, Sandor M, et al. 2014. T-Cell-derived IL-21 promotes brain injury following stroke in mice. J Exp Med. 211:595–604.
- Collins M, Whitters MJ, Young DA. 2003. IL-21 and IL-21 receptor: A new cytokine pathway modulates innate and adaptive immunity. Immunol Res. 28:131–140.
- Coquet JM, Chakravarti S, Smyth MJ, Godfrey DI. 2008. Cutting edge: IL-21 is not essential for TH17 differentiation or experimental autoimmune encephalomyelitis. J Immunol. 180:7097–7101.
- Coquet JM, Kyparissoudis K, Pellicci DG, Besra G, Berzins SP, Smyth MJ, Godfrey DI. 2007. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol. 178:2827–2834.
- Costelloe L, Jones J, Coles A. 2012. Secondary autoimmune diseases following alemtu-zumab therapy for multiple sclerosis. Expert Rev Neurother. 12:335–341.
- Croce M, Rigo V, Ferrini S 2015. IL-21: A pleiotropic cytokine with potential applications in oncology. J Immunol Res. 2015:696578.
- Daga A, Orengo AM, Gangemi RM, Marubbi D, Perera M, Comes A, Ferrini S, Corte G. 2007. Glioma immunotherapy by IL-21 gene-modified cells or by recombinant IL-21 involves antibody responses. Intl J Cancer 121:1756–1763.
- Deenick EK, Tangye SG. 2007. Autoimmunity: IL-21: A new player in TH17-cell differentiation. Immunol Cell Biol. 85:503–505.
- Denic A, Wootla B, Rodriguez M. 2013. CD8+ T-cells in multiple sclerosis. Expert Opin Ther Targets 17:1053–1066.
- Eto D, Lao C, DiToro D, Barnett B, Escobar TC, Kageyama R, Yusuf I, Crotty S. 2011. IL-21 and IL-6 are critical for different aspects of B-cell immunity and redundantly induce optimal follicular helper CD4 T-cell (TFH) differentiation. PLoS One 6:e17739.
- Ettinger R, Kuchen S, Lipsky PE. 2008. The role of IL-21 in regulating B-cell function in health and disease. Immunol Rev. 223:60–86.
- Ettinger R, Sims GP, Fairhurst AM, Robbins R, da Silva YS, Spolski R, Leonard WJ, Lipsky PE. 2005. IL-21 induces differentiation of human naive and memory B-cells into antibody-secreting plasma cells. J Immunol. 175:7867–7879.
- Fedetz M, Ndagire D, Fernandez O, Leyva L, Guerrero M, Arnal C, Lucas M, Izquierdo G, Delgado C, Alcina A, et al. 2009. Multiple sclerosis association study with the TENR-IL-2-IL-21 region in a Spanish population. Tissue Antigens 74:244–247.
- Fernandez O. 2014. Alemtuzumab in the treatment of multiple sclerosis. J Inflamm Res. 7:19–27.
- Frühlich A, Kisielow J, Schmitz I, Freigang S, Shamshiev AT, Weber J, Marsland BJ, Oxenius A, Kopf M. 2009. IL-21R on T-cells is critical for sustained functionality and control of chronic viral infection. Science 324:1576–1580.
- Gaby A. 2013. Multiple sclerosis. Global Adv Health Med. 2:50–56.
- Geri G, Terrier B, Rosenzwajg M, Wechsler B, Touzot M, Seilhean D, Tran TA, Bodaghi B, Musset L, Soumelis V, et al. 2011. Critical role of IL-21 in modulating TH17 and regulatory T-cells in Behçet disease. J Allergy Clin Immunol. 128:655–664.
- Ghalamfarsa G, Jadidi-Niaragh F, Amiri MM, Razavi SM, Saboor-Yaraghi AA, Shokri F. 2015. All-trans-retinoic Acid differentially regulates proliferation of normal and leukemic B-cells from different subsets of chronic lymphocytic leukemia. Nutr Cancer 67:285–291.
- Ghalamfarsa G, Jadidi-Niaragh F, Hojjat-Farsangi M, Asgarian-Omran H, Yousefi M, Tahmasebi F, Khoshnoodi J, Razavi SM, Saboor-Yaraghi AA, Rabbani H, et al. 2013. Differential regulation of B-cell proliferation by IL-21 in different subsets of chronic lymphocytic leukemia. Cytokine 62:439–445.
- Giovannoni G, Baker D, Schmierer K. 2014. Simvastatin in patients with progressive multiple sclerosis. Lancet 384:952.
- Gol-Ara M, Jadidi-Niaragh F, Sadria R, Azizi G, Mirshafiey A. 2012. The role of different subsets of regulatory T-cells in immunopathogenesis of rheumatoid arthritis. Arthritis. 2012:805875.
- Hartung HP, Aktas O, Menge T, Kieseier BC. 2014. Immune regulation of multiple sclerosis. Handbook Clin Neurol. 122:3–14.
- Haugen M, Frederiksen JL, Degn M. 2014. B-Cell follicle-like structures in multiple sclerosis - with focus on the role of B-cell activating factor. J Neuroimmunol. 273:1–7.
- Hoglund RA, Maghazachi AA. 2014. Multiple sclerosis and the role of immune cells. World J Exp Med. 4:27–37.
- Jadidi-Niaragh F, Mirshafiey A. 2010. Histamine and histamine receptors in pathogenesis and treatment of multiple sclerosis. Neuropharmacology 59:180–189.
- Jadidi-Niaragh F, Mirshafiey A. 2011a. Regulatory T-cell as orchestra leader in immunosuppression process of multiple sclerosis. Immunopharmacol Immunotoxicol. 33:545–567.
- Jadidi-Niaragh F, Mirshafiey A. 2011b. TH17 cell, the new player of neuro-inflammatory process in multiple sclerosis. Scand J Immunol. 74:1–13.
- Jadidi-Niaragh F, Mirshafiey A. 2011c. Therapeutic approach to multiple sclerosis by novel oral drug. Recent Pat Inflamm Allergy Drug Discov. 5:66–80.
- Jadidi-Niaragh F, Mirshafiey A. 2012. The deviated balance between regulatory T-cell and TH17 in autoimmunity. Immunopharmacol Immunotoxicol. 34:727–739.
- Jadidi-Niaragh F, Ghalamfarsa G, Memarian A, Asgarian-Omran H, Razavi SM, Sarrafnejad A, Shokri F. 2013a. Down-regulation of IL-17-producing T-cells is associated with regulatory T-cell expansion and disease progression in chronic lymphocytic leukemia. Tumor Biol. 34:929–940.
- Jadidi-Niaragh F, Ghalamfarsa G, Yousefi M, Tabrizi MH, Shokri F. 2013b. Regulatory T-cells in chronic lymphocytic leukemia: Implication for immunotherapeutic interventions. Tumor Biol. 34:2031–2039.
- Jadidi-Niaragh F, Yousefi M, Memarian A, Hojjat-Farsangi M, Khoshnoodi J, Razavi SM, Jeddi-Tehrani M, Shokri F. 2013c. Increased frequency of CD8+ and CD4+ regulatory T-cells in chronic lymphocytic leukemia: Association with disease progression. Cancer Invest. 31:121–131.
- Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP. 2005. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature 434:88–93.
- Jones JL, Phuah CL, Cox AL, Thompson SA, Ban M, Shawcross J, Walton A, Sawcer SJ, Compston A, Coles AJ. 2009. IL-21 drives secondary autoimmunity in patients with multiple sclerosis, following therapeutic lymphocyte depletion with alemtuzumab (Campath-1H). J Clin Invest. 119:2052–2061.
- Kasaian MT, Whitters MJ, Carter LL, Lowe LD, Jussif JM, Deng B, Johnson KA, Witek JS, Senices M, Konz RF, et al. 2002. IL-21 limits NK cell responses and promotes antigen-specific T-cell activation: A mediator of the transition from innate to adaptive immunity. Immunity 16:559–569.
- King C, Tangye SG, Mackay CR. 2008. T-follicular helper (TFH) cells in normal and dysregulated immune responses. Annu Rev Immunol. 26:7417–7466.
- Klotz L, Meuth SG, Wiendl H. 2012. Immune mechanisms of new therapeutic strategies in multiple sclerosis: A focus on alemtuzumab. Clin Immunol. 142:25–30.
- Konforte D, Simard N, Paige CJ. 2009. IL-21: An executor of B-cell fate. J Immunol. 182:1781–1787.
- Korn T, Bettelli E, Gao W, Awasthi A, Jäger A, Strom TB, Oukka M, Kuchroo VK. 2007. IL-21 initiates an alternative pathway to induce pro-inflammatory TH17 cells. Nature 448:484–487.
- Krumbholz M, Meinl E. 2014. B-Cells in MS and NMO: Pathogenesis and therapy. Semin Immunopathol. 36:339–350.
- Leonard WJ, Spolski R. 2005. IL-21: Modulator of lymphoid proliferation, apoptosis, and differentiation. Nat Rev Immunol. 5:688–698.
- Li B, Reynolds JM, Stout RD, Bernlohr DA, Suttles J. 2009. Regulation of TH17 differentiation by epidermal fatty acid-binding protein. J Immunol. 182:7625–7633.
- Li Q, Ye LJ, Ren HL, Huyan T, Li J, Shi JL, Huang QS. 2015. Multiple effects of IL-21 on human NK cells in ex vivo expansion. Immunobiology 220:876–888.
- Liu J, Cen H, Ni J, Zhang M, Li P, Yang XK, Leng RX, Pan HF, Ye DQ. 2015. Association of IL-21 polymorphisms (rs907715, rs2221903) with susceptibility to multiple autoimmune diseases: A meta-analysis. Autoimmunity 48:108–116.
- Lin MH, Yeh LT, Chen SJ, Chiou HY, Chu CC, Yen LB, Lin KI, Chang DM, Sytwu HK. 2014. T-Cell-specific BLIMP-1 deficiency exacerbates experimental autoimmune encephalomyelitis in nonobese diabetic mice by increasing TH1 and TH17 cells. Clin Immunol. 151:101–113.
- Linden M, Nohra R, Sundqvist E, Khademi M, Hillert J, Alfredsson L, Olsson T, Kockum I. 2011. No evidence of IL-21 association with multiple sclerosis in a Swedish population. Tissue Antigens 78:271–274.
- Linhares UC, Schiavoni PB, Barros PO, Kasahara TM, Teixeira B, Ferreira TB, Alvarenga R, Hygino J, Vieira MM, Bittencourt VC, et al. 2013. The ex vivo production of IL-6 and IL-21 by CD4+ T-cells is directly associated with neurological disability in neuromyelitis optica patients. J Clin Immunol. 33:179–189.
- Liu R, Bai Y, Vollmer TL, Bai XF, Jee Y, Tang YY, Campagnolo DI, Collins M, Young DA, La Cava A, et al. 2008. IL-21 receptor expression determines the temporal phases of experimental autoimmune encephalomyelitis. Exp Neurol. 211:14–24.
- Mehta DS, Wurster AL, Grusby MJ. 2004. Biology of IL-21 and the IL-21 receptor. Immunol Rev. 202:84–95.
- Metcalf TU, Baxter VK, Nilaratanakul V, Griffin DE. 2013. Recruitment and retention of B-cells in the central nervous system in response to alphavirus encephalomyelitis. J Virol. 87:2420–2429.
- Mirshafiey A, Jadidi-Niaragh F. 2010a. Immunopharmacological role of the leukotriene receptor antagonists and inhibitors of leukotrienes generating enzymes in multiple sclerosis. Immunopharmacol Immunotoxicol. 32:219–227.
- Mirshafiey A, Jadidi-Niaragh F. 2010b. Prostaglandins in pathogenesis and treatment of multiple sclerosis. Immunopharmacol Immunotoxicol. 32:543–554.
- Mirshafiey A, Asghari B, Ghalamfarsa G, Jadidi-Niaragh F, Azizi G. 2014. The significance of matrix metalloproteinases in the immunopathogenesis and treatment of multiple sclerosis. Sultan Qaboos Univ Med J. 14:e13–25.
- Monteleone G, Pallone F, Macdonald TT. 2009. IL-21 as a new therapeutic target for immune-mediated diseases. Trends Pharmacol Sci. 30:441–447.
- Nohra R, Beyeen AD, Guo JP, Khademi M, Sundqvist E, Hedreul MT, Sellebjerg F, Smestad C, Oturai AB, Harbo HF, et al. 2010. RGMA and IL-21R show association with experimental inflammation and multiple sclerosis. Genes Immun. 11:279–293.
- Nurieva R, Yang XO, Martinez G, Zhang Y, Panopoulos AD, Ma L, Schluns K, Tian Q, Watowich SS, Jetten AM, et al. 2007. Essential autocrine regulation by IL-21 in the generation of inflammatory T-cells. Nature 448:480–483.
- Ochoa-Reparaz J, Rynda A, Ascon MA, Yang X, Kochetkova I, Riccardi C, Callis G, Trunkle T, Pascual DW. 2008. IL-13 production by regulatory T-cells protects against experimental autoimmune encephalomyelitis independently of autoantigen. J Immunol. 181:954–968.
- Ortiz GG, Pacheco-Moisés FP, Macías-Islas MÁ, Flores-Alvarado LJ, Mireles-Ramírez MA, González-Renovato ED, Hernández-Navarro VE, Sánchez-López AL, Alatorre-Jiménez MA. 2014. Role of the blood-brain barrier in multiple sclerosis. Arch Med Res. 45:687–697.
- Parrish-Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA, Johnston J, Madden K, Xu W, West J, et al. 2000. IL 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 408:57–63.
- Peluso I, Fantini MC, Fina D, Caruso R, Boirivant M, MacDonald TT, Pallone F, Monteleone G. 2007. IL-21 counteracts the regulatory T-cell-mediated suppression of human CD4+ T-lymphocytes. J Immunol. 178:732–739.
- Pesce J, Kaviratne M, Ramalingam TR, Thompson RW, Urban JF Jr, Cheever AW, Young DA, Collins M, Grusby MJ, Wynn TA. 2006. The IL-21 receptor augments TH2 effector function and alternative macrophage activation. J Clin Invest. 116:2044–2055.
- Petrelli A, Carvello M, Vergani A, Lee KM, Tezza S, Du M, Kleffel S, Chengwen L, Mfarrej BG, Hwu P, et al. 2011. IL-21 is an anti-tolerogenic cytokine of the late-phase alloimmune response. Diabetes 60:3223–3234.
- Phares TW, Disano KD, Hinton DR, Hwang M, Zajac AJ, Stohlman SA, Bergmann CC. 2013. IL-21 optimizes T-cell and humoral responses in the central nervous system during viral encephalitis. J Neuroimmunol. 263:43–54.
- Phares TW, Marques CP, Stohlman SA, Hinton DR, Bergmann CC. 2011. Factors supporting intrathecal humoral responses following viral encephalomyelitis. J Virol. 85:2589–2598.
- Phares TW, Stohlman SA, Hwang M, Min B, Hinton DR, Bergmann CC. 2012. CD4 T-cells promote CD8 T-cell immunity at the priming and effector site during viral encephalitis. J Virol. 86:2416–2427.
- Piao WH, Jee YH, Liu RL, Coons SW, Kala M, Collins M, Young DA, Campagnolo DI, Vollmer TL, Bai XF, et al. 2008. IL-21 modulates CD4+CD25+ regulatory T-cell homeostasis in experimental autoimmune encephalomyelitis. Scand J Immunol. 67:37–46.
- Plantone D, Marti A, Frisullo G, Iorio R, Damato V, Nociti V, Patanella AK, Bianco A, Mirabella M, Batocchi AP. 2013. Circulating CD56dim NK cells expressing perforin are increased in progressive multiple sclerosis. J Neuroimmunol. 265:124–127.
- Raveney BJ, Oki S, Yamamura T. 2013. Nuclear receptor NR4A2 orchestrates TH17 cell-mediated autoimmune inflammation via IL-21 signalling. PLoS One 8:e56595.
- Romme Christensen J, Bornsen L, Ratzer R, Piehl F, Khademi M, Olsson T, Sorensen PS, Sellebjerg F. 2013. Systemic inflammation in progressive multiple sclerosis involves follicular TH, TH17, and activated B-cells and correlates with progression. PLoS One 8:e57820.
- Roozbeh M, Mohammadpour H, Azizi G, Ghobadzadeh S, Mirshafiey A. 2014. The potential role of iNKT cells in experimental allergic encephalitis and multiple sclerosis. Immunopharmacol Immunotoxicol. 36:105–113.
- Saresella M, Calabrese E, Marventano I, Piancone F, Gatti A, Alberoni M, Nemni R, Clerici M. 2011. Increased activity of TH17 and TH9 lymphocytes and a skewing of the post-thymic differentiation pathway are seen in Alzheimer's disease. Brain Behav Immun. 25:539–547.
- Sashinami H, Asano K, Yoshimura S, Mori F, Wakabayashi K, Nakane A. 2012. Salmon proteoglycan suppresses progression of mouse experimental autoimmune encephalo-myelitis via regulation of TH17 and Foxp3+ regulatory T-cells. Life Sci. 91:1263–1269.
- Shekhar S, Yang X. 2012. The darker side of follicular helper T-cells: From autoimmunity to immunodeficiency. Cell Mol Immunol. 9:380–385.
- Sitati EM, Diamond MS. 2006. CD4+ T-cell responses are required for clearance of West Nile virus from the central nervous system. J Virol. 80:12060–12069.
- Smyth MJ, Wallace ME, Nutt SL, Yagita H, Godfrey DI, Hayakawa Y. 2005. Sequential activation of NKT cells and NK cells provides effective innate immunotherapy of cancer. J Exp Med. 201:1973–1985.
- Sonderegger I, Kisielow J, Meier R, King C, Kopf M. 2008. IL-21 and IL-21R are not required for development of TH17 cells and autoimmunity in vivo. Eur J Immunol. 38:1833–1838.
- Spolski R, Leonard WJ. 2008. IL-21: Basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 26:57–79.
- Spolski R, Leonard WJ. 2014. IL-21: A double-edged sword with therapeutic potential. Nat Rev Drug Discov. 13:379–395.
- Stohlman SA, Bergmann CC, Lin MT, Cua DJ, Hinton DR. 1998. CTL effector function within the central nervous system requires CD4+ T-cells. J Immunol. 160:2896–2904.
- Strengell M, Lehtonen A, Matikainen S, Julkunen I. 2006. IL-21 enhances SOCS gene expression and inhibits LPS-induced cytokine production in human monocyte-derived dendritic cells. J Leukocyte Biol. 79:1279–1285.
- Strengell M, Matikainen S, Sirén J, Lehtonen A, Foster D, Julkunen I, Sareneva T. 2003. IL-21 in synergy with IL-15 or IL-18 enhances IFNγ production in human NK and T-cells. J Immunol. 170:5464–5469.
- Stumhofer JS, Silver JS, Hunter CA. 2013. IL-21 is required for optimal antibody production and T-cell responses during chronic Toxoplasma gondii infection. PLoS One 8:e62889.
- Suto A, Kashiwakuma D, Kagami SI, Hirose K, Watanabe N, Yokote K, Saito Y, Nakayama T, Grusby MJ, Iwamoto I. 2008. Development and characterization of IL-21-producing CD4+ T-cells. J Exp Med. 205:1369–1379.
- Tangye SG. 2015. Advances in IL-21 biology - enhancing our understanding of human disease. Curr Opin Immunol. 34:107–115.
- Tao Y, Zhang X, Chopra M, Kim MJ, Buch KR, Kong D, Jin J, Tang Y, Zhu H, Jewells V, et al. 2014. The role of endogenous IFNβ in the regulation of TH17 responses in patients with relapsing-remitting multiple sclerosis. J Immunol. 192:5610–5617.
- Tedder TF, Leonard WJ. 2014. Autoimmunity: Regulatory B-cells - IL-35 and IL-21 regulate the regulators. Nat Rev Rheumatol. 10:452–453.
- Tegla CA, Cudrici CD, Azimzadeh P, Singh AK, Trippe R, Khan A, Chen H, Andrian-Albescu M, Royal W, Bever C, et al. 2013. Dual role of response gene to complement-32 in multiple sclerosis. Exp Mol Pathol. 94:17–28.
- Tzartos JS, Craner MJ, Friese MA, Jakobsen KB, Newcombe J, Esiri MM, Fugger L. 2011. IL-21 and IL-21 receptor expression in lymphocytes and neurons in multiple sclerosis brain. Am J Pathol. 178:794–802.
- Vollmer TL, Liu R, Price M, Rhodes S, La Cava A, Shi FD. 2005. Differential effects of IL-21 during initiation and progression of autoimmunity against neuroantigen. J Immunol. 174:2696–2701.
- Wan CK, Oh J, Li P, West EE, Wong EA, Andraski AB, Spolski R, Yu ZX, He J, Kelsall BL, et al. 2013. The cytokines IL-21 and GM-CSF have opposing regulatory roles in the apoptosis of conventional dendritic cells. Immunity 38:514–527.
- Wang HH, Dai YQ, Qiu W, Lu ZQ, Peng FH, Wang YG, Bao J, Li Y, Hu XQ. 2011a. IL-17-secreting T-cells in neuromyelitis optica and multiple sclerosis during relapse. J Clin Neurosci. 18:1313–1317.
- Wang L, Yu CR, Kim HP, Liao W, Telford WG, Egwuagu CE, Leonard WJ. 2011b. Key role for IL-21 in experimental autoimmune uveitis. Proc Natl Acad Sci USA 108:9542–9547.
- Wei L, Laurence A, Elias KM, O'Shea JJ. 2007. IL-21 is produced by TH17 cells and drives IL-17 production in a STAT3-dependent manner. J Biol Chem. 282:34605–34610.
- Wu A, Zhong X, Wang H, Xu W, Cheng C, Dai Y, Bao J, Qiu W, Lu Z, Hu X. 2012. Cerebrospinal fluid IL-21 levels in neuromyelitis optica and multiple sclerosis. Can J Neurol Sci. 39:813–820.
- Xie L, Li X, Funeshima-Fuji N, Kimura H, Matsumoto Y, Isaka Y, Takahara S. 2009. Amelioration of experimental autoimmune encephalomyelitis by curcumin treatment through inhibition of IL-17 production. Intl Immunopharmacol. 9:575–581.
- Xu J, Wagoner G, Douglas JC, Drew PD. 2009. Liver X receptor agonist regulation of TH17 lymphocyte function in autoimmunity. J Leukocyte Biol. 86:401–409.
- Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. 2008. A regulatory B-cell subset with a unique CD1dhiCD5+ phenotype controls T-cell-dependent inflammatory responses. Immunity 28:639–650.
- Yanaba K, Bouaziz JD, Matsushita T, Tsubata T, Tedder TF. 2009. The development and function of regulatory B-cells expressing IL-10 (B10 cells) requires antigen receptor diversity and TLR signals. J Immunol. 182:7459–7472.
- Yang XO, Nurieva R, Martinez GJ, Kang HS, Chung Y, Pappu BP, Shah B, Chang SH, Schluns KS, Watowich SS, et al. 2008. Molecular antagonism and plasticity of regulatory and inflammatory T-cell programs. Immunity 29:44–56.
- Yoo JK, Braciale TJ. 2014. IL-21 promotes late activator APC-mediated T-follicular helper cell differentiation in experimental pulmonary virus infection. PLoS One 9:e105872.
- Yoshizaki A, Miyagaki T, Dilillo DJ, Matsushita T, Horikawa M, Kountikov EI, Spolski R, Poe JC, Leonard WJ, Tedder TF. 2012. Regulatory B-cells control T-cell autoimmunity through IL-21-dependent cognate interactions. Nature 491:264–268.
- Zanin-Zhorov A, Weiss JM, Nyuydzefe MS, Chen W, Scher JU, Mo R, Depoil D, Rao N, Liu B, Wei J, et al. 2014. Selective oral ROCK2 inhibitor down-regulates IL-21 and IL-17 secretion in human T-cells via STAT3-dependent mechanism. Proc Natl Acad Sci USA 111:16814–16819.
- Zeng R, Spolski R, Finkelstein SE, Oh S, Kovanen PE, Hinrichs CS, Pise-Masison CA, Radonovich MF, Brady JN, Restifo NP, et al. 2005. Synergy of IL-21 and IL-15 in regulating CD8+ T-cell expansion and function. J Exp Med. 201:139–148.
- Zhang X, Tao Y, Chopra M, Ahn M, Marcus KL, Choudhary N, Zhu H, Markovic-Plese S. 2013. Differential reconstitution of T-cell subsets following immunodepleting treatment with alemtuzumab (anti-CD52 monoclonal antibody) in patients with relapsing-remitting multiple sclerosis. J Immunol. 191:5867–5874.
- Zhang X, Tao Y, Troiani L, Markovic-Plese S. 2011. Simvastatin inhibits IFN regulatory factor-4 expression and TH17 cell differentiation in CD4+ T-cells derived from patients with multiple sclerosis. J Immunol. 187:3431–3437.
- Zhou F, Ciric B, Zhang GX, Rostami A. 2014. Immunotherapy using lipopolysaccharide-stimulated bone marrow-derived dendritic cells to treat experimental autoimmune encephalomyelitis. Clin Exp Immunol. 178:447–458.
- Zhou L, Ivanov II, Spolski R, Min R, Shenderov K, Egawa T, Levy DE, Leonard WJ, Littman DR. 2007. IL-6 programs TH17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 8:967–974.