Abstract
The objective of this study was to characterize the variability of rat lymphoid organ weights and morphology following treatment with a known immunotoxicant, with a focus on the usefulness of evaluating popliteal lymph node weight and histology. Cyclophosphamide was administered to male Sprague-Dawley rats by oral gavage at doses of 2, 7 or 12 mg/kg/day for 10 consecutive days. Left and right popliteal lymph nodes (PLN), spleen and thymus were collected at necropsy, weighed, fixed and processed for histopathology. Femoral bone marrow was also collected, fixed and processed for histology. Organ weight variability was greater for PLN than for either spleen or thymus in control animals. There was a significant but weak correlation between paired left and right PLN weights (p < 0.005; r2 = 0.2774). Significant treatment-related decreases in lymphoid organ weights were observed in spleen and thymus at ≥ 7 mg/kg/day (p < 0.01), whereas in PLN a significant decrease (p < 0.05) was noted only at 12 mg/kg/day. The inclusion of PLN did not enhance the sensitivity of detection of systemic treatment-related changes in lymphoid organs in a rat cyclophosphamide model.
Introduction
Assessment of potential adverse effects of human pharmaceuticals on the immune system is an important component of the overall evaluation of safety evaluation in drug development (U.S. Food and Drug Administration Citation2002). Evaluation of the lymphoid system is part of the standard safety assessment of new chemical entities and alterations in immune system organ weights combined with histopathology are considered to be reliable indicators of immunotoxicity in standard non-clinical toxicology studies (Hastings Citation2002). Internationally harmonized guidelines outlined for immunotoxicity studies of human pharmaceuticals and adopted by the regulatory bodies of the European Union, Japan and the US suggest that alterations in immune system organ weights and/or histopathological evaluation of the thymus, spleen, lymph nodes and/or bone marrow be evaluated for signs of immunotoxicity during standard toxicity studies, with lymph node weights optional (International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use: Harmonized Tripartite Guideline Citation2005). Similarly, published best practice guidelines and reviews by the Society of Toxicologic Pathology recommend weighing of spleen and thymus and microscopic examination of spleen, thymus, bone marrow and lymph node draining the site of administration, as standard approaches for routine examination of the immune system (Haley et al. Citation2005; Michael et al. Citation2007; Sellers et al. Citation2007; Everds et al. Citation2013).
Because of limitations and variability that may be associated with collection, processing and examination of lymph nodes, evaluation of peripheral lymph nodes that do not drain the site of xenobiotic application is not recommended for routine assessment of systemic immunotoxicity (Haley et al. Citation2005). Although weighing of lymph nodes has proven to be a useful parameter in validation studies with immunomodulatory compounds, artifacts resulting from imprecise separation of the lymph node from the surrounding fat and connective tissue may have a negative impact on evaluation and may induce large individual variations (Ruehl-Fehlert et al. Citation2005).
Peripheral lymph nodes in rodents, especially when quiescent, may be difficult to locate because of their small size and obtaining accurate weights on such small lymph nodes is often problematic due to intimately associated adipose and/or connective tissue (Haley Citation2003). In addition, the variability of baseline morphology due to variations in tissue orientation during embedding and sectioning can limit the usefulness of small lymph nodes in immunotoxicity assessment. Compared to peripheral lymph nodes, alterations in spleen and thymus weights and histology are generally considered to be more sensitive and reliable indicators of systemic immunotoxicity, as they are less subject to the confounding factors described above (Haley et al. Citation2005). However, these considerations are mostly based on anecdotal observations and to our knowledge have not previously been investigated in an immunotoxicity model. Therefore, the present study was undertaken to compare the variability and reliability of spleen, thymus and peripheral lymph node weights and histology in cyclophosphamide-induced immunotoxicity in rats.
Materials and methods
Animals
Forty male Sprague-Dawley rats (Crl:CD®(SD)IGS BR), ≈8–12 weeks of age and 176–200 g upon arrival, were obtained from Charles River Laboratories (Kingston, NY). The rats were housed in a pathogen-free facility maintained at 20–26 °C with 30–70% relative humidity and a 12-h light:dark cycle. A standard ground rodent diet (PMI 5002, PMI Feeds, St. Louis, MO) and water were provided ad libitum for the duration of the study. The rats were acclimated to research facilities for 1 week prior to the initiation of dosing. All animals were housed individually in stainless steel wire mesh cages and standard procedures/conditions were employed for animal care, feeding and maintenance of cages, animal rooms and environment. All procedures performed on animals in this study were in accordance with established guidelines and regulations and were reviewed and approved by the Pfizer Institutional Animal Care and Use Committee. Pfizer animal care facilities that supported this work are fully accredited by AAALAC International.
Treatments
Animals were assigned to four dose groups (n = 10 per group) using a computer-assisted randomization procedure. Treated animals were dosed once daily in the morning by oral gavage at doses of 2, 7 or 12 mg/kg/day of cyclophosphamide (Sigma, St. Louis, MO) for 10 consecutive days. Cyclophosphamide was administered in a dose volume of 10 ml/kg/dose and dosing solutions were prepared daily immediately prior to dosing by diluting with distilled water. Control animals received distilled water alone. The required volume of cyclophosphamide for each animal was based on the most recent individual body weight. Animals were weighed individually every 3 days beginning on Day 1 prior to dosing. Dosing was initiated on Day 1 and all animals were necropsied on Day 11.
Parameters
At the conclusion of the study, all animals were euthanized by carbon dioxide asphyxiation followed immediately by exsanguination via the abdominal vena cava. The brain, thymus, spleen, left and right popliteal lymph nodes (PLN), a mesenteric lymph node (MLN) and left femur containing bone marrow were then collected at necropsy. Adipose and connective tissue surrounding or adherent to the thymus, spleen and lymph nodes was carefully trimmed away and discarded. Thymus, spleen and PLN were weighed individually, then immersion-fixed for 24 h in 10% neutral buffered formalin. The mesenteric lymph node was not weighed since these lymph nodes often occur in groups or short chains in the mesentery, thus precluding accurate assessment of weights. The left femur containing bone marrow was immersion-fixed for 24 h in 10% neutral buffered formalin and then decalcified in formic acid (Immunocal™, Decal Chemical Corporation, Tallman, NY) for 24 h prior to processing for microscopic examination. Tissues were dehydrated, cleared, infiltrated with paraffin, embedded, sectioned at 5-μm, mounted onto glass slides, stained with hematoxylin and eosin and examined by light microscopy by a board-certified veterinary pathologist blinded to treatment (JML). The histopathology results were peer-reviewed by a second board-certified veterinary pathologist (RAV).
Statistical analysis
Descriptive statistics (e.g. mean, standard error, standard deviation, coefficient of variation, range and ratio) were determined for lymphoid organ weights relative to brain weights. The correlation between paired left and right popliteal lymph node weights was determined by linear regression and calculation of the correlation coefficient and coefficient of determination (r2). A one-way analysis of variance (ANOVA) was used to compare relative lymphoid organs weights among treatment groups. When ANOVA showed a significant difference, a Dunnett’s multiple comparisons test was used for between-group comparisons. For all statistical analyses, the threshold value for statistical significance was set at p < 0.05.
Results
There was a significant dose-related decrease in mean terminal body weights in cyclophosphamide-treated animals at 7 mg/kg (0.92 × control mean; p < 0.01) and at 12 mg/kg (0.87 × control mean; p < 0.01) compared to vehicle control animals (data not shown). There were no significant treatment-related differences in terminal brain weights in any of the dose groups of treated animals compared to vehicle control animals (data not shown). Lymphoid organ weights were compared for variability in control animals (). There was greater variability in PLN weights (relative to brain weight), as defined by the coefficient of variation (CV), than in spleen or thymus weights. To provide another estimate of variability, the ratio between highest and lowest weights was calculated for each lymphoid organ. This ratio was higher for PLN, either weighed separately or together, than for spleen and thymus (). There was a significant, but weak, correlation (p < 0.0005, r2 = 0.2774) between paired left and right PLN weights in individual control and treated animals ().
Figure 1. Correlation of left vs right popliteal lymph node (PLN) weights for all treated and control animals (n = 40). Lymph node weights are expressed as a percentage of brain weights. Coefficient of determination (r2) = 0.2774; p < 0.0005.
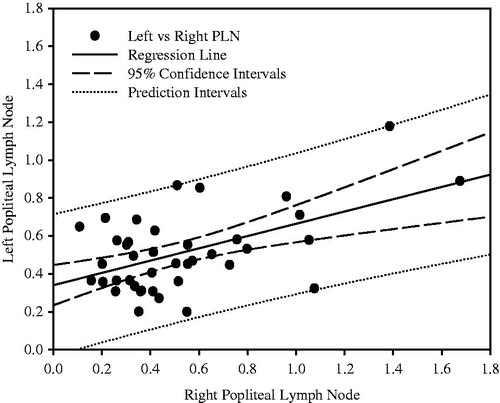
Table 1. Lymphoid organ weights in control animals.
Significant differences in organ weights between treated and control group were observed for spleen (p < 0.01) and thymus (p < 0.01) at 7 and 12 mg/kg/day (). Differences were also observed in left + right PLN weight and in left PLN weight alone at 12 mg/kg (p < 0.05), but not in right PLN weight ().
Table 2. Effects of cyclophosphamide on lymphoid organs weights.
Microscopic examination did not reveal any treatment-related lymphoid changes at the lowest (2 mg/kg/day) cyclophosphamide dose (). At the mid dose (7 mg/kg/day), treatment-related changes were observed in the spleen, PLN and MLN of all animals and in the thymus of five of 10 animals. At the high dose (12 mg/kg), changes were observed in all lymphoid organs of all animals. The severity of changes in lymphoid organs and bone marrow were generally dose-related, with minimal-to-mild changes observed at 7 mg/kg and mild-to-moderate changes at 12 mg/kg. Morphologic changes observed in the spleen consisted of depletion of red pulp hematopoietic cells and depletion of white pulp lymphocytes. Changes in the thymus consisted of a decrease in the ratio of the cortex to medulla as a result of cortical lymphocyte depletion. Changes in the PLN and MLN were similar, with depletion of lymphocytes in the cortex, paracortex and medulla and lack of distinct lymphoid follicles. Changes in the bone marrow included a decrease in cellularity of hematopoietic cells and hemorrhage.
Table 3. Incidence of cyclophosphamide-induced microscopic tissue changes.
Discussion
In this study, cyclophosphamide was used as a model immunotoxicant, as its effects on lymphoid tissues are well characterized (Smialowicz et al. Citation1985). The treated animals showed the tissue changes expected with this agent, with severity varying in a dose-dependent manner. The results of the study indicate that alterations in thymus and spleen weights, in conjunction with morphological evaluation of spleen, thymus and mesenteric lymph nodes (MLN), constitute a robust and sensitive indicator of orally administered xenobiotic immunotoxicity and that addition of peripheral popliteal lymph node (PLN) weighing and histology did not increase the sensitivity of immunotoxicity detection. The weak correlation between paired left and right PLN indicate that unilateral sampling would be insufficiently representative. Even with bilateral sampling, there was greater variability in total PLN weight than for spleen and thymus in control animals and the sensitivity of detection of statistically significant treatment-related weight changes was lower for PLN than for spleen and thymus. Although histology of PLN was a sensitive indicator of cyclophosphamide-induced immunotoxicity, it did not add sensitivity or additional information to the standard evaluation of the spleen, thymus and lymph node draining the site of xenobiotic administration (i.e. mesenteric). These results support the recommendations of an international collaborative immunotoxicity study (ICICIS) that evaluated direct immunotoxicity of immunosuppressive compounds in the rat (ICICIS Group Investigators Citation1998).
Alterations in relative thymus and spleen weight changes in conjunction with morphological evaluation have been shown to be useful indicators of immune dysfunction or stress in overall immune assessment (Dean et al. Citation1982; Everds et al. Citation2013). Weighing of spleen and thymus within standard toxicity studies is recommended by internationally harmonized guidelines for immunotoxicity studies of human pharmaceuticals, which have been adopted by regulatory bodies of the European Union, Japan and the US (International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use Citation2005). These guidelines also mention that acquisition of lymph node weights is optional. Best practice publications from the Society of Toxicologic Pathology also do not recommend weighing lymph nodes (Haley et al. Citation2005; Sellers et al. Citation2007). In the context of oral cyclophosphamide-induced immunotoxicity, the results of the present study demonstrate the limitations and variability associated with peripheral lymph node parameters and, therefore, support the best practice recommendations above.
Acknowledgments
The authors thank Rosemarie Behan, Cheryl Edmonds, Renee Huynh, Mary Payette and Jamie Whitman for technical support.
Disclosure statement
The authors report no conflicts of interest. At the time this study was conducted, JML, RAV and AMR were employees of Pfizer and PJH was an employee of Incyte. The authors alone are responsible for the content and writing of the paper.
References
- Dean JH, Luster MI, Boorman GA, Lauer LD. 1982. Procedures available to examine the immunotoxicity of chemicals and drugs. Pharmacol Rev. 34:137–148.
- Everds NE, Snyder PW, Bailey KL, Bolon B, Creasy DM, Foley GL, Rosol TJ, Sellers T. 2013. Interpreting stress responses during routine toxicity studies: A review of the biology, impact and assessment. Toxicol Pathol. 41:560–614.
- Haley R. 2003. Species differences in structure and function of the immune system. Toxicology. 188:49–71.
- Haley P, Perry R, Ennulat D, Frame S, Johnson C, Lapointe JM, Nyska A, Snyder P, Walker D, Walter G. (STP Immunotoxicology Working Group). 2005. Society of Toxicologic Pathology Position Paper: Best practice guideline for the routine pathology evaluation of the immune system. Toxicol Pathol. 33:404–407.
- Hastings KL. 2002. Implications of the new FDA/CDER immunotoxicology guidance for drugs. Center for Drug Evaluation and Research, US FDA. Intl Immunopharmacol. 2:1613–1618.
- ICICIS Group Investigators. 1998. Report of validation study of assessment of direct immunotoxicity in the rat. Toxicology. 125:183–201.
- International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. 2005. ICH Harmonized Tripartite Guideline. Immunotoxicity studies for human pharmaceuticals. Available from: http://www.ich.org/products/guidelines/safety/safety-single/article/immunotoxicity-studies-for-human-pharmaceuticals.html.
- Michael B, Yano B, Sellers RS, Perry R, Morton D, Roome N, Johnson JK, Schafer K, Pitsch S. 2007. Evaluation of organ weights for rodent and non-rodent toxicity studies: A review of regulatory guidelines and a survey of best practices. Toxicol Pathol. 35:742–750.
- Ruehl-Fehlert C, Bradley A, George C, Germann PG, Bollinger AP, Schultee A. 2005. Harmonization of immunotoxicity guidelines in the ICH process – pathology considerations from the guideline Committee of the European Society of Toxicological Pathology (ESTP). Exp Toxicol Pathol. 57:1–5.
- Sellers RS, Morton D, Michael B, Roome N, Johnson JK, Yano BL, Perry R, Schafer K. 2007. Society of Toxicologic Pathology Position Paper: Organ weight recommendations for toxicology studies. Toxicol Pathol. 35:751–755.
- Smialowicz RJ, Luebke RW, Riddle MM, Rogers RR, Rowe DG. 1985. Evaluation of immunotoxic potential of chlordecone with comparison to cyclophosphamide. J Toxicol Environ Health 15:561–574.
- U.S. Food and Drug Administration (FDA), Department of Health and Human Services, Center for Drug Evaluation and Research (CDER). 2002. Guidance for industry. Immunotoxicology evaluation of investigational new drugs. Rockville, MD: FDA. Available from: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm079239.pdf.
