Abstract
The complex immune system displays a coordinated transcriptional response to xenobiotic exposure by altering expression of designated transcription factors that, in turn, trigger immune responses. Despite the identification of several transcription factors that contribute to regulatory response, very little is known about the specific role of factors that are triggered due to exposure to obnoxious pesticides. Here, for the first time, alterations in human peripheral blood lymphocyte expression of transcriptional factors – thrombospondin-1 (THBS-1), secretory phospho-protein-1 (SPP-1), glycoprotein non-metastatic-β (GPNMB) and fasciculation and elongation factor ζ-1 (FEZ-1), due to in vitro exposure to the crop protection chemicals cypermethrin and mancozeb are reported. Results revealed significant changes in expression profiles due to mancozeb exposure, supporting its immune dysfunction potential; in contrast, cypermethrin exposure did not cause significant changes. Based on these effects on gene expression across the doses tested, it was likely key components of immune mechanisms such as proliferation, cell adhesion, apoptosis and cell activation in human PBMC were affected. Although these data are from in vitro experiments, the results point out the potential role for changes in these factors in the etiology of defective T-cell immune function seen in humans occupationally exposed to crop protection chemicals like mancozeb. These studies suggest the involvement of transcription factors in regulation of pesticide-induced immune dysfunction; these studies also represent a novel approach for identifying potential immune-related dysfunctions due to exposure to pesticides. Further studies are needed to better understand the functional significance of these in vitro findings.
Introduction
Pesticide-induced immune dysfunction is a major concern, eventually having an impact on life-threatening immune-related diseases, such as cancer, hypersensitivity and autoimmunity (Corsini et al. Citation2008). Nevertheless, epidemiological associations between exposure and the myriad complex immune-related functions in a host are often challenging and there are often no sound conclusions reached about causal relationships between pesticide exposure and such effects (Colosio et al. Citation2013). Apart from standard paradigms that form the basis of common assays of immunotoxicity, gene–environment interactions also might contribute to immune dysfunction in response to toxicants, including pesticides. Thus, information on expression profile changes in specific transcription factors due to pesticide exposure might help improve the overall scientific knowledge about, and risk assessment strategies related to, these agents (Corvi et al. Citation2006).
Molecular genetics has a pivotal role in the response to xenobiotics by the immune system of man and other vertebrates. Global gene expression studies in the draining lymph nodes after a single topical exposure to the contact allergen dinitrofluorobenzene (DNFB) had shown significant changes on GlyCAM-1 (glycosylation-dependent cell adhesion molecule 1), guanylate binding protein 2 and onzin (Betts et al. Citation2003). Similarly, occupational exposure to benzene identified CXCL16, ZNF331, JUN and PF4, as potential biomarkers of early responses to benzene in peripheral blood mononuclear cells (PBMC) of six exposed-control pairs (Forrest et al. Citation2005). In vitro exposure to TBTO (bis-[tri-n-butyltin] oxide) by the Jurkat human T-lymphocyte cell line (Katika et al. Citation2011) and mouse thymocytes (van Kol et al. Citation2012) showed significant changes in Atf4, Atf6, Hspa5, Park7 (Dj-1) and Atox1, that are involved in endoplasmic reticulum stress, and in expression of Bax and Bcl2l11 (Bim) associated with apoptosis.
Several studies have successfully demonstrated expression changes and their casual relation with representative responses in identifying immunotoxicity potentials of various agents (Zeytun et al. Citation2002; Kinser et al. Citation2004; Baken et al. Citation2006, Citation2007a,Citationb, Citation2008). Similarly, gene expression profiling studies conducted in tissues/cells such as the liver, spleen, thymus, lymph nodes and peripheral blood lymphocytes also proved useful in predictive risk assessment (Bulera et al. Citation2001; Thomas et al. Citation2001; Waring et al. Citation2001; Hamadeh et al. Citation2002a,Citationb; Steiner et al. Citation2004). Therefore, gene expression profiling is a promising tool in toxicity assessment and can be useful for developing new biomarkers (van Leeuwen et al. Citation2005, Citation2008; Baken et al. Citation2007a,Citationb; Hochstenbach et al. Citation2010).
Modern trends in genomic research have made it possible to generate a hypothesis based on available data that saves the time and resources. Indeed, these approaches address the major goal of identifying key genes and their products that show significant changes in expression profile in a diseased state or due to chemical exposure (Hamadeh et al. Citation2002a,Citationb; Zhang et al. Citation2002). This approach is also a promising tool for the generation and testing of toxicity hypotheses (Donald et al. Citation2002; Zhang et al. Citation2002) or the identification of perturbed pathways (Borisy et al. Citation2003) and, thus, plays a crucial role in risk assessment.
Immune functions are complex responses coordinated among various cell types by several transcription factors such as thrombospondin-1 (THBS-1), secretory phospho-protein-1 (SPP-1), glycoprotein non-metastatic-β (GPNMB) and fasciculation and elongation factor ζ-1 (FEZ-1) that have recently been identified as highly and differentially expressed in human lymphocytes upon exposure to exogenous chemicals (Hochstenbach et al. Citation2010). These key transcription factors (alone or in combination) play significant roles in several key immune functions such as cell adhesion, proliferation, motility, survival (Kyriakides and MacLauchlan Citation2009; Zhang et al. Citation2013) and mediating the clearance of apoptotic cells by phagocytes (Tabib et al. Citation2009) and cell activation (Poole et al. Citation2013). Similarly, important roles in cytokine production, promotion of cell survival by regulating apoptosis (Wang and Denhardt Citation2008), neutrophil migration (Apte et al. Citation2005) and mast cell migration/degranulation (Nagasaka et al. Citation2008) have also been noted for these factors.
Cypermethrin and mancozeb are widely used in agriculture, households and industry due to their “low” toxicity in mammals and short environmental persistence (Hayes and Laws Citation1990; Lorgue and Lechenet Citation1996; Descotes Citation2004; Hasan Citation2010). Nevertheless, studies have noted immunomodulatory effects from exposure to cypermethrin (Dési et al. 1985, Citation1986; WHO Citation1989; Liu et al. Citation2006) or mancozeb (Colosio et al. Citation1996; Corsini et al. Citation2005; Mandarapu and Prakhya Citation2015). Accordingly, the present in vitro study using human PBMC cultures was undertaken to assess immunomodulatory potentials of the pesticides based on changes induced in expression of some key transcriptional factors (i.e. THBS1, SPP1, GPNMB, and FEZ1) upon exposure to the pesticides at non-cytotoxic concentrations. To provide relative context about the potential utility of examining these specific factors here, analyses were performed in parallel using both a known immunotoxicant (i.e. benzo(a)pyrene; positive control) and non-immunotoxicant (i.e. urethane; negative control).
Materials and methods
Chemicals
Cypermethrin (> 99%), mancozeb (> 95%), benzo(a)pyrene (> 96%), and urethane (> 99%) were purchased from Sigma (St. Louis, MO). Stock solutions of these chemicals were made in dimethyl sulfoxide (DMSO; Sigma) and stored at −80 °C until further dilution in culture medium at desired concentration(s). The final concentrations of DMSO never exceeded 0.1%.
Peripheral blood mononuclear cells
Upon obtaining informed consent, peripheral blood was collected from individual male volunteers (26–35 years-of-age). The 3rd Institutional Ethics Committee of the International Institute of Biotechnology and Toxicology approved all experiments. Each blood sample was collected into a heparinized tube and mononuclear cells subsequently isolated using Ficoll-Hypaque (ρ = 1.077 g/ml) density gradient centrifugation (400 × g, 30 min). The buffy coat containing mono-nuclear cells was isolated, transferred to a fresh centrifuge tube and washed twice with phosphate-buffered saline (PBS, pH 7.4), using ≈3 vol of collected buffy coat each time. The final cell pellet containing peripheral blood mononuclear cells (PBMC) was re-suspended to a final level of 1–2 × 106 cells/ml in RPMI 1640 medium supplemented with L-glutamine, 10% heat-inactivated fetal bovine serum (FBS), 100 U penicillin/ml and 0.1 mg streptomycin/ml (all Gibco, Paisley, UK).
Cytotoxicity assessment
Preliminary cytotoxicity studies were performed using PBMC from two donors/test agents to assess biovariance. PBMC (105 cells/well, 24-well plate) were exposed for 24 h to serial doses of cypermethrin or mancozeb in the presence of a metabolic activator (rat liver S9 fraction; Moltox, Boone, NC). Immediately before use, 10% S9-mix containing 15% S9 fraction was added to the reaction medium. Based on trypan blue (Gibco) dye exclusion; doses that caused > 10% cytotoxicity were excluded from further analysis.
Culture set-up/PBMC exposure
To assess the biological variance, assays were performed using PBMC from three donors with each chemical separately. Cultures of PBMC (105 cells/well, 24-well plate) were exposed to non-cytotoxic concentrations of cypermethrin (7.50, 15 or 30 μM) or mancozeb (1.87, 3.75 or 7.50 μM) or benzo(a)pyrene (2.5, 5 or 10 μM; positive control) or urethane (250, 500 or 1000 μM; negative control) or to medium containing solvent (DMSO) or medium alone for 6 h in the presence of freshly-prepared S9 mix at 37 °C under 5% CO2 and 95% humidity. At the end of the exposure, cells are washed with fresh culture medium and then RNA was isolated.
RNA isolation and qRT-PCR
Total RNA from cells was isolated using RNeasy Mini kit (Qiagen, Hilden, Germany) according to manufacturer protocols. Real-time quantitative PCR assay was performed with a Verso SYBR green 1-step qRT-PCR kit (ThermoFisher Scientific, Chennai, India) using Qiagen RotorGene Q plus with a 1-step protocol. In brief, 30 min at 50 °C (one cycle) for reverse transcription, 10 min at 95 °C to fully activate the Taq DNA polymerase (one cycle), 30 s in 35–40 cycles of 95 °C to denaturate DNA, 1 min in 60 °C followed by 30 s at 72 °C to anneal and extend the primers (data acquisition). Specificity of amplification was controlled by melt-curve analysis (dissociation program) and gel electrophoresis. The amplified product was incubated at 95 °C for 1 min, ramping down to 55 °C at a rate of 2 °C/s. The melt curve analysis was begun at 55 °C and ended at 95 °C by increasing 0.5 °C/cycle. RT-PCR results were analyzed with the ΔΔ threshold cycle method, using β-actin as an internal standard to normalize mRNA amounts.
The primer sequences used were: β-actin: 5′-CCTGGCACCCAGCACAAT-3′ (forward) and 5′-GCC-GATCCACACGGAGTACT-3′ (reverse); THBS1: 5′-CATGCCACGGCCAACAA-3′ (forward) and 5′-TGGCCCAGGTAGTTGCACTT-3′ (reverse); SPP1: 5′-CAGCCACAAGCA-GTCCAGATTATA-3′ (forward) and 5′-CCTGACTATCAATCACATCGGAAT-3′ (reverse); GPNMB: 5′-TCTAAGATCATGTTCCAAGCTAACTGA-3′ (forward) and 5′-GGCTTGGGCCT-GTTATTGTTC-3′ (reverse); FEZ1: 5′-ACAAACATTCTCTTTGCCATGAAG-3′ (forward) and 5′-GTTAGGTAGGGCAGAGCACTTTTAA-3′ (reverse). All primers were obtained from VBC Biotech (Vienna, Austria).
RNA yield was assessed spectrophotometrically and quality determined and considered sufficient by having absorbance values (OD 260/280) that fell between 1.9–2.1. RNA from control samples served as a calibrator (reference standard). Each PCR analysis was run in parallel with a known positive template control (PTC) containing quantified RNA and a known negative template control (NTC) containing water to test the reaction solution.
Data analysis
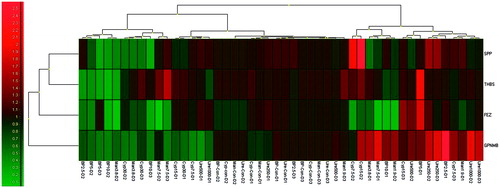
The fluorescence data acquired on the RotorGene Q plus was analyzed using Rotor Gene Q2plex software (v.1.74; Qiagen) using standard curves specific for each transcription factor (THBS1, SPP1, GPNMB and FEZ1), including the housekeeping β-actin gene and recorded as Ct values (2−ΔCT and 2−ΔΔCT). Relative expression changes were determined based on Ct values obtained from the samples for each transcription factor and for the reference β-actin gene using the Relative Expression Software Tool (REST 2009; v2.0.13; Qiagen) which includes normalization and pair-wise fixed reallocation randomization. The program makes no assumptions about distribution of observations in populations and is more flexible than non-parametric tests based on ranks (i.e. Mann–Whitney, Kruskal-Wallis, etc.) and does not suffer a reduction in power relative to parametric tests (i.e. t-tests, ANOVA, etc.) (Pfaffl et al. Citation2002) with a p = 0.05. Quantification results were expressed in terms of fold-change with respect to the corresponding control samples (calibrator). Different sample groups were obtained by clustering using Euclidean Distance as the distance metric and complete linkage clustering procedure using GenEx software (v6.0; multiD Analyses, Göteborg, Sweden) ().
Results
Quality control of samples
Sample quality and the assay procedures were assessed at different stages. Prior to chemical exposure cell viability was checked and those with > 90% were allowed for exposure. Extracted RNA from the cells was assessed for its quantity and integrity. The average quantity of RNA present in the samples was 5.2 (4.1–6.3) μg and the spectrophotometric readings for quality were between 1.9–2.1. Melt-curve analysis after PCR cycles confirmed primer specificity and identity; all samples met these criteria. The efficiency of the real-time PCR experiment derived from the standard curve was 0.98–1.00, with slopes of −3.38 to −3.31 and linear regressions (R2) of 0.98–1.00 among all the samples. NTC (no template controls) showed no amplification in any run validating the experiments.
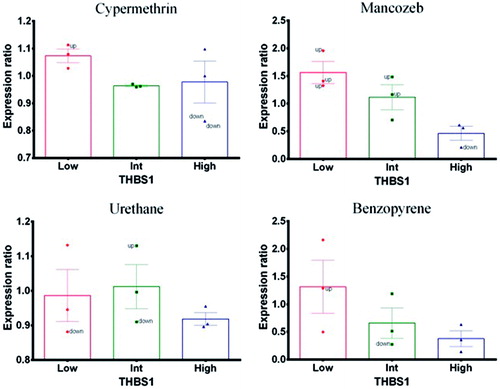
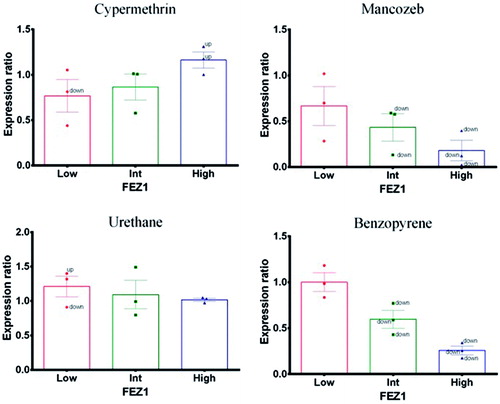
Effect on thrombospondin-1 (THBS-1)
Mancozeb and benzopyrene (positive control) caused a dramatic effect on THBS1 expression profiles. At lower concentrations, expression was up-regulated with respect to the reference gene ACTB (); however, at high doses, there was a down-regulation. Nevertheless, when expression profiles were assessed together, there appeared to be a concentration-related decrease indicative of a treatment-related effect. In contrast, cypermethrin and urethane (negative control) did not cause significant changes in expression profiles related to treatment.
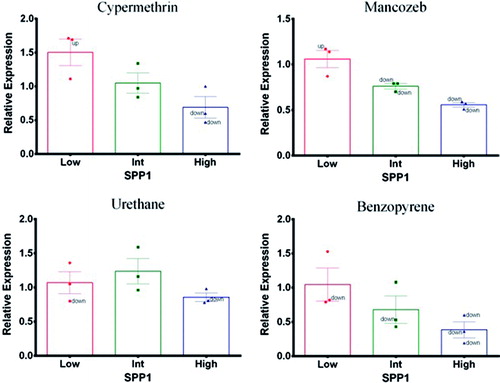
Effect on secretary phospho protein-1 (SPP-1)
Mancozeb and benzopyrene (positive control) had shown a concentration-related decrease in expression profiles of SPP1 with corresponding endogenous reference gene (ACTB), similar to THBS1 (). On the other hand, cypermethrin caused a decreased expression profile only at high concentration. Urethane (negative control) exposure did not cause significant changes that could be attributable to treatment at any concentration.
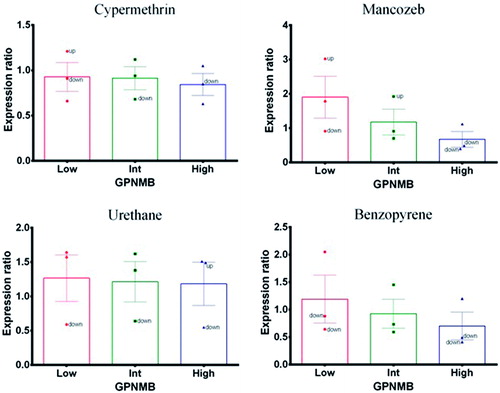
Effect on glycoprotein non-metastatic-β (GPNMB)
Mancozeb and benzopyrene (positive control) caused expression changes in GPNMB that were similar to THBS1. At lower concentrations, expression showed up-regulation with respect to the reference gene (ACTB) and at high doses there was a down-regulation. Nevertheless, when expression profiles were assessed together from all three experiments, there was a concentration-related decrease – thus indicating treatment-related effects (). Cypermethrin and urethane (negative control) did not cause any significant changes in expression profile that could be related to treatment.
Effect on fasciculation and elongation factor ζ-1 (FEZ-1)
At lower concentrations, both mancozeb and benzopyrene (positive control) did not cause any expression changes (). However, at intermediate and high doses, FEZ-1 expression was down-regulated with respect to the endogenous ACTB reference gene. In contrast, cypermethrin caused up-regulation of FEZ-1 expression in a concentration-related fashion. Urethane (negative control) did not cause any expression changes.
Biological interpretation
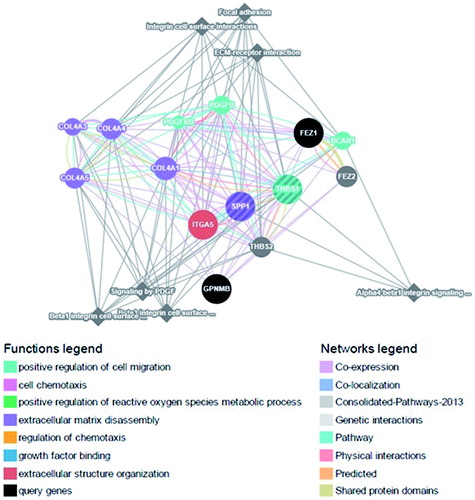
An attempt was made to identify various biological functions related to the immune system that might be affected by the observed expression changes among the factors analyzed. This approach used gene ontology-based weighing for identifying the biological functions related to the selected query genes (THBS1, SPP1, FEZ1, GPNMB) in association with 10 more closely related genes with/without 10 attributes using GeneMania software (). The query genes were involved in several biological functions directly or in combination with other genes that are related to the immune system. Interestingly, several of these biological functions were closely associated with xenobiotic exposure, such as: chemotaxis of leukocytes, granulocytes, and neutrophils; migration of leukocytes, granulocytes, and neutrophils; regulation of cell adhesion; positive regulation of cell motility; regulation of leukocyte activation; macrophage differentiation; positive regulation of T-cell/lymphocyte activation; positive regulation of cell migration; platelet activation; and apoptotic cell clearance.
THBS1 in combination with other genes was involved in most of the functions, followed by SPP1 > FEZ1 > GPNMB. Therefore, any down-regulated expression due to mancozeb or benzopyrene exposure might indicate likely immunomodulatory effects at the doses tested.
Discussion
The present study analyzed the expression profiles of key transcriptional factors THBS1, SPP1, GPNMB and FEZ1 in human PBMC cultures after exposure to cypermethrin, mancozeb, benzopyrene or urethane. These factors might operate as key modulators of immune function under basic conditions or in the case of xenobiotic exposure and could be helpful in identifying the candidate marker genes of toxicity. This study showed that these transcription factors expressed a balanced profile due to exposure of chemicals and their changes were correlatable with the known immunomodulatory properties of the two test pesticides, mancozeb and benzopyrene (positive control). To our knowledge, this is the first study to examine the expression profiles of these transcription factors in cultured human PBMC after exposures to cypermethrin or mancozeb.
Adhesion and migration are two key mechanisms in regulation of lymphocyte behavior and function and are sensitive to signals delivered through antigen recognition. THBS1 is one of the key transcriptional factors in regulating T-cell adhesion and migration (Forslöw et al. Citation2007), inflammation and tissue damage or stress (Kyriakides and MacLauchlan Citation2009; Zhang et al. Citation2013). The predicted biological functions based on gene ontology also indicate the role of THBS1 in several immunological functions. In the present study, the observed down-regulation of THBS1 due to mancozeb or benzopyrene (positive control) exposure (in dose-related fashion) suggests that THBS1 could be a promising candidate biomarker in assessing inducible changes in immune function.
Secretary phosphoprotein-1 (SPP1, also called osteopontin) has both inflammatory and anti-inflammatory actions, the capability to modify gene expression and an ability to promote migration of monocytes/macrophages (Denhardt et al. Citation2001). The expression changes reported here showed an up-regulation due to cypermethrin exposure and a down-regulation due to mancozeb (or benzopyrene [positive control]) in a dose-related fashion. The network predictions based on biological functions also indicated a role for SPP1 in modulating the immune functions such as T-cell chemotaxis, adhesion and proliferation as a result of toxicant exposure. Thus, SPP1 could be another sensitive marker for assessing immunomodulation due to toxicant/pesticide exposure.
GPNMB is expressed on antigen-presenting cells (APC) that attenuate T-cell activation by binding to Syndecan-4 (SD-4) on activated T-cells (Chung and Pyo Citation2005; Chung et al. Citation2007a,Citationb; Tomihari et al. Citation2010). This factor is also expressed constitutively at high levels by many other APC subsets and at lower levels by some non-lymphoid cells (Weterman et al. Citation1995; Shikano et al. Citation2001). Key immunological functions mediated by GPNMB include macrophage differentiation and serving as a negative regulator of macrophage inflammatory responses (Ripoll et al. Citation2007). In the current study, decreased expression of GPNMB due to mancozeb or benzopyrene (positive control) exposure was observed. Ultimately, these changes could manifest as an inhibition of macrophage differentiation after exposure to these chemicals.
Unlike the other three factors, the role of FEZ1 is not clearly defined in immune-related functions. Nevertheless, FEZ1 was shown to have a differential expression in a recent study conducted in human bone marrow stromal cells (BMSC; Ren et al. Citation2011). It has been suggested FEZ1 may actually play a role in cell adhesion (Bloom and Horvitz Citation1997) and interactions with various intracellular partners, such as signaling, motor and structural proteins, to function as an anti-viral factor (Maturana et al. Citation2010). Here, FEZ1 expression increased due to cypermethrin and decreased due to mancozeb or benzopyrene (positive control). However, the analyses here were unable to define any immune system-related biological functions that were mediated through FEZ1 alone or in combination with other genes, based on network predictions using Genemania.
Down-regulation of some of these transcription factors due to mancozeb or benzopyrene (positive control) exposure was likely a basis for previous observations showing there were significant changes in proliferation and TH1/TH2 cytokine (TNFα, IFNγ, IL-2, IL-4, IL-6, IL-10) secretion by human peripheral blood lymphocytes (Mandarapu et al. Citation2014). Further, the functional significance of these altered gene expressions may contribute to a multitude of immune cell-related functions/processes, including chemotaxis, migration, cell adhesion and motility, lymphocyte activation, immune cell migration, macrophage differentiation, platelet activation and apoptotic cell clearance/cell pathways/processes, due to mancozeb or benzopyrene (positive control) exposure.
As genomic-based approaches are gaining more attention in toxicity assessment, the current approach using selected transcription factors based on various methods might help in improving the current testing strategies. Further, the approach here reflects the potential utility of including these novel protocols in assessing changes in immune function that reflect toxicities of these specific and other commonly-encountered pesticides/environmental agents.
Conclusions
The present in vitro study demonstrated the utility of transcription factors in enhancing researchers’ understanding of potential immunomodulatory effects due to pesticide exposure. Furthermore, the results obtained also indicated there was now also an opportunity for understanding the functional immunotoxicologic significance of alterations in gene expressions in PMBC due to pesticide exposure. We wish to note for the readers, however, that a fuller validation of the outcomes here requires an even more robust approach. While one might view the current work as mainly preliminary, we strongly believe the current experimental set-up with three different donors was sufficient enough to generate an understanding of the utility of the approach. This then paved the way for future investigations with a far more expansive pool of donors, etc. to more fully understand the functional immunotoxicologic significance of alterations in gene expressions in PMBC due to pesticide exposure.
Acknowledgements
This work is a part of the PhD Thesis of Rajesh Mandarapu, approved by the University of Madras, and was undertaken at the International Institute of Biotechnology and Toxicology (IIBAT). The clinical support of Dr Srivatsa Prakhya (SRL, Indore, India) is appreciated.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Apte UM, Banerjee A, McRee R, Wellberg E, Ramaiah SK. 2005. Role of osteopontin in hepatic neutrophil infiltration during alcoholic steatohepatitis. Toxicol Appl Pharmacol. 207:25–38.
- Baken KA, Arkusz J, Pennings JL, Vandebriel RJ, van Loveren H. 2007a. In vitro immunotoxicity of bis-(tri-n-butyltin)oxide (TBTO) studied by toxicogenomics. Toxicology. 237:35–48.
- Baken KA, Pennings JLA, Jonker MJ, Schaap MM, de Vries A, van Steeg H, Breit TM, van Loveren H. 2008. Overlapping gene expression profiles of model compounds provide opportunities for immunotoxicity screening. Toxicol Appl Pharmacol. 226:46–59.
- Baken KA, van Loveren H, Pennings JLA, de Vries A, Breit TM, van Steeg H. 2006. Gene expression profiling of bis-(tri-n-butyltin) oxide (TBTO)-induced immunotoxicity in mice and rats. J Immunotoxicol. 3:227–244.
- Baken KA, Vandebriel RJ, Pennings JL, Kleinjans JC, van Loveren H. 2007b. Toxicogenomics in the assessment of immunotoxicity. Methods. 41:132–141.
- Betts CJ, Moggs JG, Caddick HT, Cumberbatch M, Orphanides G, Dearman RJ, Ryan CA, Hulette BC, Gerberick GF, Kimber I. 2003. Assessment of glycosylation-dependent cell adhesion molecule-1 as a correlate of allergen-stimulated lymph node activation. Toxicology. 185:103–117.
- Bloom L, Horvitz HR. 1997. The Caenorhabditis elegans gene UNC-76 and its human homologs define a new gene family involved in axonal outgrowth and fasciculation. Proc Natl Acad Sci USA. 94:3414–3419
- Borisy AA, Elliott PJ, Hurst NW, Lee MS, Lehar J, Price ER, Serbedzija G, Zimmermann GR, Foley MA, Stockwell BR, et al. 2003. Systematic discovery of multi-component therapeutics. Proc Natl Acad Sci USA. 100:7977–7982.
- Bulera SJ, Eddy SM, Ferguson E, Jatkoe TA, Reindel JF, Bleavins MR, De La Iglesia FA. 2001. RNA expression in the early characterization of hepatotoxicants in Wistar rats by high-density DNA microarrays. Hepatology. 33:1239–1258.
- Chung AH, Pyo MY. 2005. Effects of mancozeb on the activities of murine peritoneal macrophages in vitro and ex vivo. Arch Pharm Res. 28:100–105.
- Chung JS, Dougherty I, Cruz PD, Ariizumi K. 2007a. Syndecan-4 mediates co-inhibitory function of DC-HIL on T-cell activation. J Immunol. 179:5778–5784.
- Chung JS, Sato K, Dougherty II, Cruz PD, Ariizumi K. 2007b. DC-HIL is a negative regulator of T-lymphocyte activation. Blood. 109:4320–4327.
- Colosio C, Alegakis AK, Tsatsakis AM. 2013. Emerging health issues from chronic pesticide exposure: Innovative methodologies and effects on molecular cell and tissue level. Toxicology. 307:1–2.
- Colosio C, Barcellini W, Maroni M, Alcini D, Bersani M, Cavallo D, Galli A, Meroni P, Pastorelli R, Rizzardi GP, et al. 1996. Immunomodulatory effects of occupational exposure to mancozeb. Arch Environ Health. 51:445–451.
- Corsini E, Birindelli S, Fustinoni S, De Paschale G, Mammone T, Visentin S, Galli CL, Marinovich M, Colosio C. 2005. Immunomodulatory effects of the fungicide mancozeb in agricultural workers. Toxicol Appl Pharmacol. 208:178–185.
- Corsini E, Liesivuori J, Vergieva T, van Loveren H, Colosio C. 2008. Effects of pesticide exposure on the human immune system. Human Exp Toxicol. 27:671–680.
- Corvi R, Ahr H, Albertini S, Blakey DH, Clerici L, Coecke S, Douglas GR, Gribaldo L, Groten JP, Haase B, et al. 2006. Meeting Report: Validation of toxicogenomics-based test systems: ECVAM – ICCVAM/NICEATM considerations for regulatory use. Environ Health Perspect. 114:420–429.
- Denhardt DT, Noda M, O’Regan AW, Pavlin D, Berman JS. 2001. Osteopontin as a means to cope with environmental insults: Regulation of inflammation, tissue remodeling, and cell survival. J Clin Invest. 107:1055–1061.
- Descotes J, editor. 2004. Regulatory aspects of immunotoxicity evaluation. Immunotoxicology of drugs and chemicals. An experimental and clinical approach. Vol. 1. Principles and methods of immunotoxicology. Amsterdam: Elsevier Science. p. 257–268.
- Dési I, Dobronyi I, Varga L. 1986. Immuno-, neuro-, and general toxicologic animal studies on a synthetic pyrethroid: Cypermethrin. Ecotoxicol Environ Safety. 12:220–232.
- Desi I, Varga L, Dobronyi I, Szklenarik G. 1985. Immunotoxicological investigation of the effect of a pesticide; cypermethrin. Arch Toxicol. 8:305–309.
- Donald S, Verschoyle RD, Edwards R, Judah DJ, Davies R, Riley J, Dinsdale D, Lazaro LL, Smith AG, Gant TW, et al. 2002. Hepatobiliary damage and changes in hepatic gene expression caused by the anti-tumor drug ecteinascidin-743 (ET-743) in the female rat. Cancer Res. 62:4256–4262.
- Forrest MS, Lan Q, Hubbard AE, Zhang L, Vermeulen R, Zhao X, Li G, Wu YY, Shen M, Yin S, et al. 2005. Discovery of novel biomarkers by microarray analysis of peripheral blood mononuclear cell gene expression in benzene-exposed workers. Environ Health Perspect. 113:801–807.
- Forslöw A, Liu Z, Sundqvist KG. 2007. Receptor communication within the lymphocyte plasma membrane: A role for the thrombospondin family of matricellular proteins. Cell Mol Life Sci. 64:66–76.
- Hamadeh HK, Bushel PR, Jayadev S, Martin K, DiSorbo O, Sieber S, Bennett L, Tennant R, Stoll R, Barrett JC, et al. 2002a. Gene expression analysis reveals chemical-specific profiles. Toxicol Sci. 67:219–231.
- Hamadeh HK, Bushel PR, Jayadev S, DiSorbo O, Bennett L, Li L, Tennant R, Stoll R, Barrett JC, Paules RS, et al. 2002b. Prediction of compound signature using high density gene expression profiling. Toxicol Sci. 67:232–240.
- Hasan HO. 2010. Fungicides and their effects on animals, fungicides. In: Carisse O, editor. ISBN: 978-953-307-266-1. London: InTech.
- Hayes WJ, Laws ER, editors. 1990. Handbook of pesticide toxicology. Vol. 3. Classes of pesticides. New York: Academic Press, Inc.
- Hochstenbach K, van Leeuwen DM, Gmuender H, Stølevik SB, Nygaard UC, Løvik M, Granum B, Namork E, Van Delft JHM, van Loveren H. 2010. Transcriptomic profile indicative of immunotoxic exposure: In vitro studies in peripheral blood mononuclear cells. Toxicol Sci. 118:19–30.
- Katika MR, Hendriksen PJ, van Loveren H, Peijnenburg A. 2011. Exposure of Jurkat cells to bis-(tri-n-butyltin) oxide (TBTO) induces transcriptomics changes indicative for ER- and oxidative stress, T-cell activation and apoptosis. Toxicol Appl Pharmacol. 254:311–322.
- Kinser S, Jia Q, Li M, Laughter A, Cornwell P, Corton JC, Pestka J. 2004. Gene expression profiling in spleens of deoxynivalenol-exposed mice: Immediate early genes as primary targets. J Toxicol Environ Health. 67:1423–1441.
- Kyriakides TR, MacLauchlan S. 2009. Role of thrombospondins in wound healing, ischemia, and foreign body reaction. J Cell Commun Signal. 3:215–225.
- Liu P, Song X, Yuan W, Wen W, Wu X, Li J, Chen X. 2006. Effects of cypermethrin and methyl parathion mixtures on hormone levels and immune functions in Wistar rats. Arch Toxicol. 80:449–457.
- Lorgue G, Lechenet J, editors. 1996. Clinical veterinary toxicology. London: Blackwell Science.
- Mandarapu R, Ajumeera R, Venkatesan V, Prakhya BM. 2014. Proliferation and TH1/TH2 cytokine production in human peripheral blood mononuclear cells after treatment with cypermethrin and mancozeb in vitro. J Toxicol. Jan-Mar;12(1):48-55. doi: 10.1155/2014/308286.
- Mandarapu R, Prakhya BM. 2015. In vitro myelotoxic effects of cypermethrin and manc-ozeb on human hematopoietic progenitor cells. J Immunotoxicol. 12:48–55.
- Maturana AD, Fujita T, Kuroda S. 2010. Functions of fasciculation and elongation protein zeta-1 (FEZ1) in the brain. Sci World J. 10:1646–1654.
- Nagasaka A, Matsue H, Matsushima H, Aoki R, Nakamura Y, Kambe N, Kon S, Uede T, Shimada S. 2008. Osteopontin is produced by mast cells and affects IgE-mediated degranulation and migration of mast cells. Eur J Immunol. 38:489–499.
- Pfaffl MW, Horgan GW, Dempfle L. 2002. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucl Acids Res. 30:9–15.
- Poole DH, Ndiaye K, Pate JL. 2013. Expression and regulation of secreted phosphoprotein-1 in bovine corpus luteum and effects on T-lymphocyte chemotaxis. Reproduction. 146:527–537.
- Ren J, Jin P, Sabatino M, Balakumaran A, Feng J, Kuznetsov SA, Klein HG, Robey PG, Stroncek DF. 2011. Global transcriptome analysis of human bone marrow stromal cells (BMSC) reveals proliferative, mobile and interactive cells that produce abundant extracellular matrix proteins, some of which may affect BMSC potency. Cytotherapy. 13:661–674.
- Ripoll VM, Irvine KM, Ravasi T, Sweet MJ, Hume DA, Ripoll VM, Irvine KM, Ravasi T, Sweet MJ, Hume DA. 2007. GPNMB is induced in macrophages by IFNγ and lipopolysaccharide and acts as a feedback regulator of pro-inflammatory responses. J Immunol. 178:6557–6566.
- Shikano S, Bonkobara M, Zukas PK, Ariizumi K. 2001. Molecular cloning of a dendritic cell-associated transmembrane protein [DC-HIL] that promotes RGD-dependent adhesion of endothelial cells through recognition of heparan sulfate proteoglycans. J Biol Chem. 276:8125–8134.
- Steiner G, Suter L, Boess F, Gasser R, de Vera MC, Albertini S, Ruepp S, Vera, M.C. De, Albertini, S., and Ruepp, S., 2004. Discriminating different classes of toxicants by transcript profiling. Environ Health Perspect. 112:1236–1248.
- Tabib A, Krispin A, Trahtemberg U, Verbovetski I, Lebendiker M, Danieli T, Mevorach D. 2009. Thrombospondin-1-N-terminal domain induces a phagocytic state and thrombospondin-1-C-terminal domain induces a tolerizing phenotype in dendritic cells. PLoS ONE. 4:1–7.
- Thomas RS, Rank DR, Penn SG, Zastrow GM, Hayes KR, Pande K, Glover E, Silander T, Craven MW, Reddy JK, et al. 2001. Identification of toxicologically-predictive gene sets using cDNA microarrays. Mol Pharmacol. 60:1189–1194.
- Tomihari M, Chung JS, Akiyoshi H, Cruz PD, Ariizumi K. 2010. DC-HIL/glyco-protein NMB promotes growth of melanoma in mice by inhibiting the activation of tumor-reactive T-cells. Cancer Res. 70:5778–5787.
- van Kol SW, Hendriksen PJ., van Loveren H, Peijnenburg A. 2012. Transcriptomics analysis of primary mouse thymocytes exposed to bis-(tri-n-butyltin)dioxide (TBTO). Toxicology. 296:37–47.
- van Leeuwen DM, Gottschalk RWH, Schoeters G, van Larebeke NA, Nelen V, Baeyens WF, Kleinjans JCS, van Delft JHM. 2008. Transcriptome analysis in peripheral blood of humans exposed to environmental carcinogens: A promising new biomarker in environmental health studies. Environ Health Perspect. 116:1519–1525.
- van Leeuwen DM, Gottschalk RW, van Herwijnen MH, Moonen EJ, Kleinjans JC, van Delft JH. 2005. Differential gene expression in human peripheral blood mononuclear cells induced by cigarette smoke and its constituents. Toxicol Sci. 86:200–210.
- Wang KX, Denhardt DT. 2008. Osteopontin: Role in immune regulation and stress responses. Cytokine Growth Factor Rev. 19:333–345.
- Waring JF, Jolly RA, Ciurlionis R, Lum PY, Praestgaard JT, Morfitt DC, Buratto B, Roberts C, Schadt E, Ulrich RG. 2001. Clustering of hepatotoxins based on mechanism of toxicity using gene expression profiles. Toxicol Appl Pharmacol. 175:28–42.
- Weterman MA, Ajubi N, van Dinter IM, Degen WG, van Muijen GN, Ruitter DJ, Bloemers HP. 1995. NMB, a novel gene, is expressed in low-metastatic human melanoma cell lines and xenografts. Intl J Cancer. 60:73–81.
- WHO (World Health Organization). 1989. International Program on Chemical Safety: WHO Task Group Meeting on Environmental Health Criteria for Cypermethrin. Cypermethrin. Environ. Health Criteria 82. Geneva: WHO.
- Zeytun A, McKallip RJ, Fisher M, Camacho I, Nagarkatti M, Nagarkatti PS. 2002. Analysis of 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced gene expression profile in vivo using pathway-specific cDNA arrays. Toxicology. 178:241–260.
- Zhang H, Wu Y, Liu J, Jiang J, Geng X. 2013. TSP1-producing B-cells show immune regulatory property and suppress allergy-related mucosal inflammation. Sci Reports. 3:3345.
- Zhang W, Wang H, Song SW, Fuller GN. 2002. Insulin-like growth factor binding protein 2: Gene expression microarrays and the hypothesis-generation paradigm. Brain Pathol. 12:87–94.