Abstract
Gastric ulcer is a very common gastrointestinal disease that may lead to dangerous complications and even death. This study was conducted to evaluate the prophylactic effect of nebivolol against indomethacin [INDO]-induced gastric ulcer. Male Wistar rats were divided into four groups: normal control, ulcer control (INDO only), omeprazole before INDO and nebivolol before INDO. Each rat to receive nebivolol and omeprazole was given the agent orally (by gavage) daily for 10 days prior to induction of ulcer by oral dosing with INDO. Four hours after INDO treatment, all rats were euthanized and their stomachs obtained for measures of gastric acidity, oxidative stress and inflammatory markers, as well as cytoprotective mediators. The results showed that a single oral dose of INDO (100 mg/kg) induced gastric acidity, an ulcer index of 2900 and significantly increased levels of gastric tumor necrosis factor (TNF)-α and malondialdehyde (MDA) and significantly decreased levels of gastric prostaglandin E2 (PGE2), glutathione (GSH) and nitric oxide (NO), compared to in normal control counterpart stomachs. Giving nebivolol before INDO corrected the gastric acidity and resulted in a significant increase in GSH, PGE2 and NO and a significant decrease in TNFα and MDA gastric levels, compared to ulcer control values. Results obtained with nebivolol were comparable to those with omeprazole; the preventive index in the nebivolol group was 90.7% compared to 94.5% in rats in the omeprazole group. These studies showed that nebivolol provided a valuable role in preventing gastric ulcers induced by INDO and provided a promise for potentially protecting hypertensive patients from experiencing gastric ulcer. Thus, it is possible that, pending further studies, nebivolol could be used for pre-exposure prophylaxis from gastric ulcer in these patients.
Introduction
Gastric ulcer is one of the most common disorders considering the gastrointestinal tract, it affects 5% of the population around the world, so its prevention and management are considered very important challenges (Boligon et al. Citation2014). Researchers have revealed several causes of gastric ulcer; these include an imbalance between aggressive and intrinsic defensive factors (Boligon et al. Citation2014). The aggressive factors include non-steroidal anti-inflammatory drugs (NSAID), alcohol, psychological stress and Helicobacter pylori infection; cytoprotective intrinsic factors include mucosal blood flow, bicarbonate, mucus, cell renewal, growth factors, NO and prostaglandins (Abbas & Sakr Citation2013).
NSAID-induced gastric damage is known to be the most common and dangerous side-effect of these drugs and accounts for 25% of gastric ulcer cases (Adhikary et al. Citation2011). Indomethacin (INDO) is considered to be the most common NSAID known to induce experimental gastric ulcer and has been documented to have a higher potential to cause gastric injury than other commonly-used NSAIDs (Suleyman et al. Citation2010).
Although gastric ulcer pathogenesis includes many factors, gastric acid secretion is considered a main component of this disorder and controlling it has been the main goal of therapy for years. To that end, H2-receptor blockers (like ranitidine) or proton pump inhibitors (PPIs, like omeprazole) have been employed (Araujo et al. Citation2011). Overall, the PPI are known to have more potent inhibitory effects on gastric acid secretion than H2-receptor blockers (Mejia & Kraft Citation2009). Presently, these therapies have a major disadvantage in that they cause many side-effects (Araujo et al. Citation2011).
Interestingly, it has been noted that patients with cardiovascular diseases have high gastric ulcer incidence rates (Morsy et al. Citation2012). Moreover, it was documented that adults with hypertension often suffer from peptic ulcer disease and are more likely to receive drugs for hypertension and peptic ulcer (Patil et al. Citation2012). Telmisartan, which is known as an anti-hypertensive drug, was documented to decrease gastric ulcer risk (Morsy et al. Citation2009). Considering this evidence, choosing an anti-hypertensive drug may be beneficial in the prophylaxis of gastric ulcer in hypertensive patients.
Nebivolol is an anti-hypertensive drug that is classified as a third-generation selective β1-adrenoreceptor antagonist and β3-adrenoreceptor agonist. It has been shown to stimulate the production of nitric oxide (NO) through stimulation of endothelial nitric oxide synthase, resulting in vasodilatation. Nitric oxide also plays an important role in gastro-protection in that it helps to maintain adequate blood flow in mucosal tissues (Morsy et al. Citation2012).
The current study was conducted to highlight the role of nebivolol as a gastro-protective agent (pre-exposure prophylactic) against INDO-induced gastric ulcer in male rats. In addition, the study also examined underlying biochemical pathways that were related to oxidative stress, inflammation and cyto-protection in the gut.
Materials and methods
Drugs
Indomethacin (INDO) was obtained as a gift from Kahira Pharmaceuticals and Chemical Industries Co. (Cairo, Egypt). Omeprazole was purchased from Carbosynth LLC (San Diego, CA), while nebivolol was a gift from 4A Pharma for Pharmaceutical Industries (Cairo, Egypt).
Animals
Wistar rats (male, 180–200 g, 12-weeks-of-age) were obtained from the National Research Center (Giza, Egypt), where they were raised under veterinary care and certified as pathogen-free (confirmed by histopathologic evaluation through the pilot study). The rats were housed in wire cages in a pathogen-free facility maintained at 24 ± 2 °C; 60–70% relative humidity and a 12-h light:dark cycle. All rats had ad libitum access to standard rodent chow and filtered tap water. All rats were acclimatized for 2 weeks before the experiments. This study was conducted in accordance with guidelines for the care and use of laboratory animals and approved by the Research Ethics Committee of Tanta University (Tanta, Egypt).
For the study design, the rats were randomly divided into four groups: Group 1 (control group, n = 20), Group 2 (ulcer group, n = 20), Group 3 (omeprazole group, n = 30) and Group 4 (nebivolol group, n = 20). The number of rats in Group 3 was higher (relative to that in other groups) as omeprazole affected gastric secretion to some extent; thus, the number of rats was increased to accommodate this effect. Note: From among the 20–30 rats/group, the stomachs of 2–4/group were randomly selected for histopathologic studies. Of the remaining rats in each group, the amount of tissue from a single stomach was not enough for chemical measures and so pooling of tissues from two rats each time (within each group) was required. Thus, the number of rats here used in statistical analyses of the non-histopathology end-points below yielded an n = 8/group (for Group 3 rats, tissues of 3–4 rats were randomly pooled to yield the same n = 8 value).
For induction of ulcer, INDO was given by a single oral gavage of 100 mg INDO/kg (Araujo et al. Citation2011). For rats in the drug pre-treatment groups, omeprazole (5 mg/kg) (Izzettin et al. Citation2012) or nebivolol (10 mg/kg) (Sorrentino et al. Citation2011) was given orally (gavage) daily for 10 consecutive days; on the final day, these rats were given INDO (by gavage) 1 h after the omeprazole or nebivolol dosing. Rats in Groups 1 and 2 (that had been given vehicle in place of either drugs for the 10 days) were dosed with, respectively, vehicle or INDO in parallel. All rats were fasted 24 h prior to INDO oral gavage; over this period, rats were kept in wide wire mesh-bottom cages to avoid coprophagy; also, water access was prevented for 2 h prior to the INDO dosing. Four hours after the INDO/vehicle oral gavage, rats were euthanized via ether asphyxiation and their stomachs excised at necropsy.
Measurement of gastric pH
Each stomach obtained was opened along the greater curvature and the gastric content was drained into a centrifuge tube, then centrifuged at 1000 rpm for 10 min (4 °C), the clear supernatant was recovered (Sivaraman & Muralidharan Citation2011) and used for pH measurement using a pH 211 meter (Hanna Instruments, Bucharest, Romania).
Measurement of ulcer index
After gastric contents were removed, a board-certified pathologist then evaluated the total stomach under magnification (in a blinded manner). Based on these evaluations, the ulcer score for each group was calculated as the mean number of ulcers/stomach/rat in each group. From these values, an Ulcer Index (UI) was calculated by multiplying each group’s ulcer score × 100. Subsequently, the net preventive index was calculated as 100% × (UI of ulcer only group − UI of treated group)/UI of ulcerated only group (Dawud et al. Citation2014). After these analyses, the tissues were then processed for use in the various assays outlined below.
Preparation of stomach tissues for analysis
Tissue preparation for assaying gastric levels of glutathione (GSH) and malondialdehyde (MDA), 250 mg tissue was homogenized in 2.5 ml potassium phosphate buffer (pH 7.5) using a Polytron homogenizer (PT 3100, Kinematica, Luzern, Switzerland) then tissue was centrifuged at 4000 rpm for 15 min at 4 °C. Tissue preparation for assaying stomach nitric oxide (NO) levels, 250 mg tissue was homogenized in 2.5 ml ice-cold normal (0.9%) saline. Afterwards, 1 ml absolute ethanol was added to 0.5 ml homogenate to precipitate the proteins and the samples were then centrifuged at 3000 rpm for 10 min at 4 °C. To prepare tissues for assaying gastric PGE2 levels, 100 mg tissue was homogenized in 1 ml of phosphate-buffered saline (PBS, pH 7.4) using the Polytron homogenizer and then left overnight at −20 °C. The homogenate was then centrifuged (5 min, 5000 x g, 2–8 °C) and the supernatant isolated for later assay. Lastly, to prepare tissues for assaying gastric TNFα levels, 100 mg tissue was homogenized in 1 ml PBS using the Polytron homogenizer, the product was then centrifuged (20 min, 2500 rpm, 2–8 °C) and the supernatant isolated for later assay.
Determination of stomach reduced glutathione (GSH)
The concentration of reduced GSH in the stomach tissue homogenate was determined colorimetrically using a kit obtained from Biodiagnostics (Cairo, Egypt), according to manufacturer instructions. Reduction of 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) by GSH yields a yellow product which was measured at 405 nm in a spectrophotometer (Unico 2100, Dayton, NJ). Values were then calculated using the kit-provided formula and presented as mg GSH/g starting tissue.
Determination of stomach malondialdehyde (MDA)
The gastric concentration of MDA was determined colorimetrically using a kit from Biodiagnostics. According to the protocol, thiobarbituric acid (TBA) reacts with MDA present in the sample (in acidic medium, at 95 °C for 30 min) to form TBA-reactive products (TBARS). The absorbance of these resultant pink products was then measured at 534 nm in the spectrophotometer. Values were then calculated using a kit-provided formula and presented as nmol MDA/g starting tissue.
Determination of nitric oxide (NO) content
The nitric oxide content in the stomach tissue was determined by measuring its nitrite (an indicator of original NO present). This method depends on reduction of nitrate to nitrite by vanadium trichloride (VCl3), which was followed by addition of Griess reagent (Miranda et al. Citation2001). In brief, homogenate supernatant (500 μl) was mixed with an equal volume of VCl3 (400 mg dissolved in 50 ml 1 M HCl) and Griess reagent (0.1 g naphthylethylenediamine in 100 ml distilled water and 2 g sulphanilamide in 100 ml 5% HCl). After incubation at 37 °C for 30 min, the absorbance was measured at 540 nm in the spectrophotometer. Values were compared with those from sodium nitrite standards that were assessed in parallel and plotted in standard curve and the nitrite concentration in each sample was calculated. Results were presented as nmol NO/g starting tissue.
Determination of prostaglandin E2 (PGE2) and tumor necrosis factor (TNF)-α
Levels of PGE2 and TNFα in stomach tissue homogenates (supernatants) were measured using ELISA kits obtained from Cusabio Biotech Co., Ltd (Wuhan, China) and Glory Science Co., Ltd. (Hangzhou, China), respectively, according to manufacturer protocols.
Histopathology
Stomach sections were fixed in 10% formalin and then embedded in paraffin to form blocks. The samples were then serially-sectioned (5-μm thick) using a Leica RM2135 microtome (Leica, Berlin, Germany), mounted on glass slides and then stained using hematoxylin and eosin solution. A board-certified pathologist then evaluated the materials under a light microscope (in a blinded manner).
Statistical analysis
Analysis of data was performed using SPSS software (v.17.0, Armonk, NY, USA). All data are presented as mean ± SEM. Statistical comparisons among groups were performed using a one-way analysis of variance (ANOVA). Statistical significance was set at p < 0.05.
Results
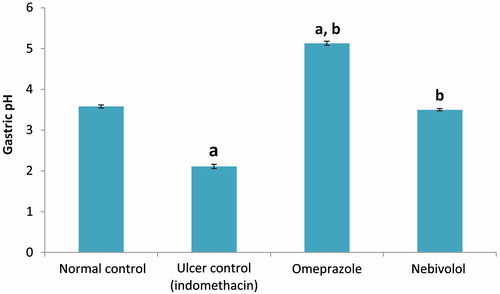
Effect on gastric pH
Administration of INDO caused a significant decrease in gastric pH (41.1%) as compared to the value in fluids from normal control rats (). Values dropped from 3.58 ± 0.04 to 2.11 ± 0.05. Pre-treatment with nebivolol preserved gastric pH at the level of the control group (i.e. 3.50 ± 0.03), whereas pre-treatment with omeprazole significantly increased the gastric pH above the control value (to 5.13 ± 0.05).
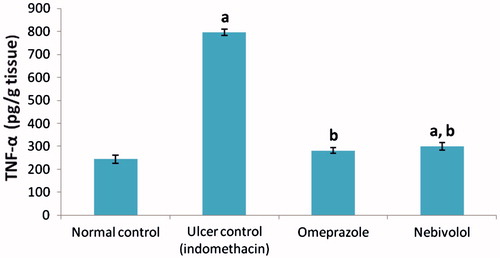
Effect on gastric TNFα
INDO alone caused a significant increase in gastric TNFα levels (3.26-fold increase; 796.5 ± 13.7 vs 244.0 ± 17.8) compared to values in samples from the normal control rats (). Omeprazole or nebivolol pre-treatment significantly decreased gastric TNFα levels (by 64.7% and 62.5%, respectively, i.e. to 281.0 ± 12.2 and 299.0 ± 16.4) compared to levels seen in the ulcer (INDO only) rats ().
Effect on ulcer index and preventive index
The ulcerated (INDO only) group had an ulcer score of 29.0 ± 1.7/stomach and a group UI of 2900 (). Rats that had been treated with nebivolol or omeprazole prior to INDO showed significant improvements (i.e. decreases) in ulcer scores; rats in the nebivolol group had a mean score of 2.7 ± 0.3/stomach, while those in the omeprazole group had a value of 1.6 ± 0.2/stomach. Based on the calculated preventive index, omeprazole appeared to have imparted a greater gastroprotective effect, with these rats having a group UI of 160 and a net preventive index of 94.5%. In comparison, rats in the nebivolol regimen had a group UI of 270 and a net preventive index of 90.7%.
Table 1. Ulcer scores, indices and effects of drugs (preventive indices).
Histopathology
In line with the findings about the UI (and preventive indices), compared to the sections obtained from normal control rats (), stomach sections from INDO only-treated rats showed gastric erosions with decreased gastric mucosa thickness, disruption of gastric mucosa and inflammatory cell infiltration (). Sections from omeprazole and nebivolol pre-treated rats groups showed intact gastric mucosa and only slight congestion ( and ).
Figure 3. Sections of gastric mucosa of normal control rats. (a) Normal mucosal thickness with surface mucus layer covering gastric pits with intact mucosa and more gastric glands at the bottom (Magnification = 100×). (b) Parietal cells with central rounded nuclei are dispersed throughout the glands (Magnification = 400×). H&E stain. Representative images are shown.
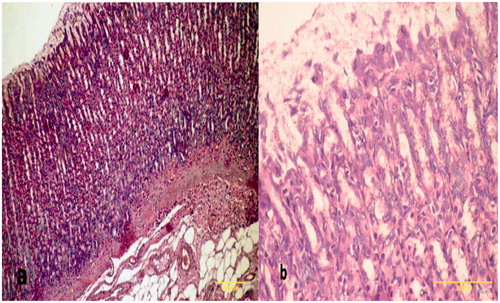
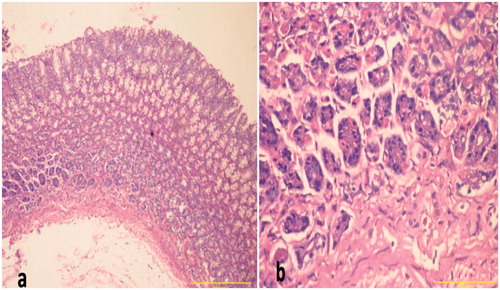
Figure 4. Sections of gastric mucosa of indomethacin-treated rats. (a) Section indicates areas of tissue damage, loss of the epithelial layer and gastric pits with decrease in mucosal thickness and distorted arrangement of glands in addition to neutrophil infiltrates in the mucosa and submucosa (Magnification = 100×). (b) Section indicates inflammatory cells (neutrophils) infiltration of the lamina propria with a loss of normal architecture of glandular cells (Magnification = 400×). H&E stain. Representative images are shown; focus was on particular lesion.
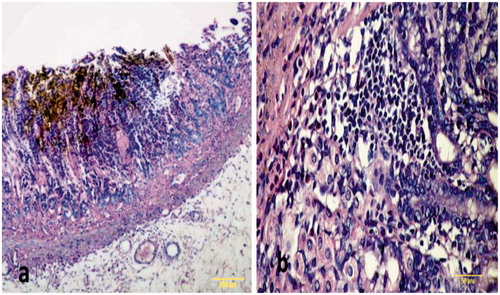
Figure 5. Sections of gastric mucosa of omeprazole-pre-treated rats. (a) Section indicates intact gastric mucosa (Magnification = 100×). (b) Gastric mucosa like normal and gastric glands with slight dilatation (Magnification = 400×). H&E stain. Representative images are shown. All of these tissues (and others from the group) were used by the pathologist to generate ulcer scores for each rat and, subsequently, the net protective index value for each group.
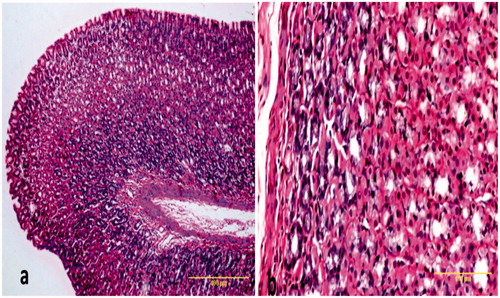
Figure 6. Sections of gastric mucosa of nebivolol pre-treated rats. (a) The section indicates ameliorated epithelial erosions by nebivolol and increase in the thickness of the mucosa compared to ulcer group with slight disturbance in the surface (Magnification = 100×). (b) The section indicates gastric mucosa with mild congestion in the connective tissue in the lamina propria (Magnification = 400×). H&E stain. Representative images are shown. All of these tissues (and others from the group) were used by the pathologist to generate ulcer scores for each rat and, subsequently, the net protective index value for each group.
Effect on gastric MDA and GSH
INDO caused a significant increase in gastric tissue MDA levels compared to values in tissues from the normal control rats (2.2-fold increase) (). Pre-treatment with omeprazole or nebivolol led to significant decreases in gastric MDA (50.5% and 48.2%, respectively) relative to that in the INDO only rats. On the other hand, GSH levels were significantly decreased by treatment with INDO (62.6% compared to control group). Pre-treatment with omeprazole or nebivolol partially corrected the gastric GSH levels (values were increased 2.48- and 2.29-fold, respectively) compared to levels in the INDO only ulcer group.
Table 2. Effects of drugs on stomach MDA and GSH levels.
Effect on cytoprotective mediators
INDO produced a significant 57.9% decrease in gastric nitric oxide (NO) content relative to levels seen in tissues from the normal control rats (). Pre-treatment with omeprazole or nebivolol increased gastric NO levels significantly (2.35- and 2.34-fold, respectively) compared to values in the INDO only ulcer rats. INDO also caused a significant decrease in gastric PGE2 levels (44.1%) compared to values in the normal controls. Pre-treatment with omeprazole or nebivolol resulted in a significant increase in gastric PGE2 level (1.71- and 1.69-fold increase, respectively) relative to the INDO only group value.
Table 3. Effect of treatments on stomach cytoprotective mediators.
Discussion
Gastric ulcers often co-exist with hypertension and both diseases share some etiological factors (Morsy et al. Citation2012; Patil et al. Citation2012). An imbalance between endogenous gastroprotective and aggressive factors in the gastric mucosa leads to gastric injury and ulceration (Raji & Oloyede Citation2011). Endogenous gastroprotective factors include nitric oxide (NO) and prostaglandins (PGs), each of which is considered an important regulator of blood flow and mucus secretion (Suleyman et al. Citation2010; Morsy et al. Citation2012). Since NO, PGs and oxidative stress have been implicated in both hypertension and gastric ulcer (Abdel-Raheem Citation2010; Giles et al. Citation2012), one could hypothesize that use of an anti-hypertensive drug with anti-oxidant effects might be helpful in maintaining the normal balance in the gastric mucosa and preventing ulcer development. The research here sought to evaluate the ability of the anti-hypertensive drug, nebivolol, to protect against development of gastric ulcer pathogenesis as a pre-exposure prophylactic drug and to investigate potential underlying mechanisms of any such effects.
In the present work, gastric ulcer was induced in rats by indomethacin (INDO) and was evidenced by the histopathological examination and by a very high ulcer score/index value. The gastric tissues obtained from INDO only rats provided evidence of mucosal injury, loss of epithelial layers, decreased mucosal thickness, distortion of the mucosa and mucosal glands and inflammatory cell infiltration. These results also indicated that INDO-induced ulcers were associated with significant increases in gastric acidity. Increased gastric acid secretion plays an important role in gastric ulcer induction and is involved in its etiology as it decreases the process of restitution and ulcer healing via altering angiogenesis (Musumba et al. Citation2009).
The INDO only rats here also showed a significant decrease of gastric PGE2 and NO. These results supported that of previous researchers (Abdallah et al. Citation2011; Abbas & Sakr Citation2013), who noted that gastric ulcers induced by INDO were accompanied by significant increases in gastric acidity and significant decreases in gastric PGE2 and NO levels. This INDO effect on NO could be explained by an ability to up-regulate endothelin-1, a factor that leads to decreased release of endothelial NO, leading to an eventual loss of mucosal integrity (an event that is normally maintained by a presence of NO; Abdel-Raheem Citation2010).
The results of the present study also showed that nebivolol pre-treatment, before INDO, normalized gastric acidity, led to reductions in UI values and yielded a high preventive index. These outcomes could be explained, in part, by the ability of nebivolol to increase PGE2 and NO levels since it is known that PGE2 and NO increase mucus secretion and regulate gastric acidity via reduction in gastric acid secretion (Suleyman et al. Citation2010; Morsy et al. Citation2012; Rahim et al. Citation2014). This observed effect of nebivolol was significantly lower than that exerted by the proton pump inhibitor omeprazole (which had the greater preventive index value) that increased the pH value to one greater than normal. The results here indicated that gastric NO and PGE2 levels were increased significantly in the nebivolol group when compared to the INDO only group. Nebivolol increases NO through activation of a β3-adrenoreceptor that activates the NO synthase pathway (Aragón et al. Citation2011; Morsy et al. Citation2012). Although NO is considered a form of reactive oxygen species, any oxidative stress related to its presence should not be involved in the process of gastric tissue injury. COX-1 derived PGE2 was shown to regulate gastric pH, while COX-2 has an important role in the mucosal defense via increasing proliferation of epithelial cells and re-construction of glands during the repair process. Thus, both COX enzymes can contribute to gastric mucosal defense (Morsy et al. Citation2012). Some reports illustrated that there was positive mutual interaction between NO and COX enzymes, which together act side-by-side in protecting the gastric tissue from injury (Mollace et al. Citation2005; Heeba et al. Citation2009; Usman et al. Citation2014).
Moreover, omeprazole caused a significant increase in gastric NO levels compared to INDO only group values. This outcome was in agreement with previous reports (Rouhollahi et al. Citation2014) that omeprazole increased NO levels and provided a gastroprotective effect against ethanol-induced gastric ulcers in rats. Both omeprazole and nebivolol here caused comparable results and helped rats maintain gastric NO and PGE2 to near-normal control values.
Oxidative stress is implicated in induction of gastric ulcers and is considered one of the mechanisms involved in INDO-induced ulcers (Adhikary et al. Citation2011). The present results supported this hypothesis as MDA levels were significantly elevated while GSH levels were significantly decreased in gastric tissues of the INDO only group when compared to normal values. Oxidative stress is produced as a result of increasing levels of reactive oxygen species (ROS) in gastric tissues; these ROS cause injury to the gastric tissue by damaging membranes and cellular biomolecules such as proteins, DNA and lipids (Suzuki et al. Citation2012; Badr & Al-Mulhim Citation2014). Further, increase in ROS levels could lead to uncoupling of (eNOS) and, thus, decreased NO synthesis (Landmesser et al. Citation2003; Kato et al. Citation2009). Hence, using drugs with anti-oxidant properties that decrease ROS formation and increase NO levels could protect gastric tissues from oxidant-mediated damage and be beneficial against ulcer formation/progression.
In our work, pre-treatment with nebivolol before INDO resulted in significant decreases in gastric MDA levels and significant increases in gastric GSH levels relative to those in the INDO only rats. Nebivolol is thought to possess anti-oxidant activity, possibly by inhibiting NADPH oxidase-induced free radical formation (Morsy et al. Citation2012), direct scavenging of ROS (Goel et al. Citation2009), decreasing free radicals pool (Gideroglu et al. Citation2008) and/or up-regulating hemoxygenase-1 (HO-1) enzyme expression (which augments GSH levels) (Morsy et al. Citation2012). Omeprazole pre-treatment, before INDO, here had the same impact as the nebivolol on MDA and GSH. This effect was in line with that noted in earlier reports (Swamy et al. Citation2011) that illustrated the omeprazole could restore GSH levels in models with dexamethasone-induced ulcers. The omeprazole anti-oxidant activity could be explained by its ability to react with the oxidant hypochlorous acid (HOCl), scavenge free radicals, restore endogenous anti-oxidant levels (Biswas et al. Citation2003; Abdul-Aziz Citation2011; Ittiyavirah & Shenika Citation2014) and up-regulate HO-1 (Becker et al. Citation2006). While both drugs had the same net effect/trend on MDA and GSH in the stomach tissues, omeprazole herein returned both to near-normal levels, whereas nebivolol only corrected the GSH and MDA levels to levels that still differed from normal.
Involvement of inflammatory mediators in INDO-induced gastric ulcer was previously reported, i.e. INDO was shown to up-regulate biosynthesis of TNFα (Abdallah et al. Citation2011). The present results confirmed the contribution of TNFα to the development of gastric ulcers induced by INDO. Platelet activating factor and TNFα are known to play the key roles in gastric injury induced by NSAIDs; these mediators induce inflammation and tissue damage through activation of adhering molecules, thus resulted in recruiting leukocytes. Neutrophil infiltration can cause injury by causing enhanced release of ROS that, in turn, damage gastric tissue (Musumba et al. Citation2009). In the current work, pre-treatment with nebivolol or omeprazole before INDO decreased gastric TNFα levels significantly compared to that in rats that received INDO only. It has been shown previously that nebivolol had the ability to decrease TNFα gene expression (Garbin et al. Citation2008). Further, omeprazole decreased serum TNFα levels and was protective against ethanol-induced gastric ulcers (Abood et al. Citation2014). Lastly, PGE2 mitigates effects of TNFα and has been documented as a potent inhibitor of TNFα (Abood et al. Citation2014). Thus, it is plausible that each agent could have caused significant decreases in gastric TNFα via induced improvements in gastric PGE2 levels.
Conclusion
The β3-agonist nebivolol imparted a gastroprotective role (pre-exposure prophylactic) against INDO-induced gastric ulcer; the effect appeared to be mediated, in part, by reductions in oxidative stress and a preservation of gastroprotective mechanisms. Thus, nebivolol could possibly be used to maintain the required balance between aggressive and defensive forces and could be used as prophylaxis for patients suffering from hypertension who have a high tendency to develop gastric ulcer and/or who are hypertensive NSAIDs consuming patients.
Acknowledgments
The authors gratefully acknowledge Professor Dr Mona A. Yehia, at the Medical Research Center in Alexandria, Egypt, for conducting and interpreting the histopathology investigations.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abbas AM, Sakr HF. 2013. Effect of selenium and grape seed extract on indomethacin-induced gastric ulcers in rats. J Physiol Biochem. 69:527–537.
- Abdallah IZ, Khattab HA, Heeba GH. 2011. Gastroprotective effect of Cordia myxa L. fruit extract against indomethacin-induced gastric ulceration in rats. Life Sci J. 8:433–445.
- Abdel-Raheem IT. 2010. Gastroprotective effect of rutin against indomethacin-induced ulcers in rats. Basic Clin Pharmacol Toxicol. 107:742–750.
- Abdul-Aziz KK. 2011. Comparative evaluation of anti-ulcer activity of curcumin and omeprazole during the acute phase of gastric ulcer. Food Nut Sci. 2:628–640.
- Abood WN, Abdulla MA, Ismail S. 2014. Involvement of inflammatory mediators in the gastroprotective action of Phaleria macrocarpa against ethanol-induced gastric ulcer. World Appl Sci J. 30:344–350.
- Adhikary B, Yadav SK, Roy K, Bandyopadhyay SK, Chattopadhyay S. 2011. Black tea and theaflavins assist healing of indomethacin-induced gastric ulceration in mice by anti-oxidative action. Evid-Based Compl Alt Med. 8:1–11.
- Aragón JP, Condit ME, Bhushan S. 2011. β3-Adrenoreceptor stimulation ameliorates myocardial ischemia-reperfusion injury via endothelial nitric oxide synthase and neuronal nitric oxide synthase activation. J Am Coll Cardiol. 58:2683–2691.
- Araujo DA, Araujo V, Takayama C, de-Faria FM, Socca EA, Dunder RJ. 2011. Gastro-protective effects of essential oil from Protium heptaphyllum on experimental gastric ulcer models in rats. Rev Braz Farmacogn. 21:721–729.
- Badr GM, Al-Mulhim JA. 2014. The protective effect of aged garlic extract on non-steroidal anti-inflammatory drug-induced gastric inflammation in male albino rats. Evid- Based Compl Alt Med. doi: 10.1155/2014/759642.
- Becker JC, Grosser N, Waltke C, Schulz S, Erdmann K, Domschke W, Schröder H, Pohle T. 2006. Beyond gastric acid reduction: Proton pump inhibitors induce heme oxygenase-1 in gastric and endothelial cells. Biochem Biophys Res Commun. 345:1014–1021.
- Biswas K, Bandyopadhyay U, Chattopadhyay I, Varadaraj A, Ali E, Banerjee RK. 2003. A novel anti-oxidant and anti-apoptotic role of omeprazole to block gastric ulcer through scavenging of hydroxyl radical. J Biol Chem. 278:10993–11001
- Boligon AA, de Freitas RB, de Brum TF, Waczuk EP, Klimaczewski CV, de Ávila DS. 2014. Anti-ulcerogenic activity of Scutia buxifolia on gastric ulcers induced by ethanol in rats. Acta Pharm Sin B. 4:358–367.
- Dawud FA, Mabrouk MA, Mohammed A, Umar IA. 2014. Effect of Vitamins C and E on aspirin-induced gastric mucosal damage and oxidative stress. Curr Res J Biol Sci. 6:36–41.
- Garbin U, Fratta Pasini A, Stranieri C, Manfro S, Mozzini C, Boccioletti V, Pasini A, Cominacini M, Evangelista S, Cominacini L. 2008. Effects of nebivolol on endothelial gene expression during oxidative stress in human umbilical vein endothelial cells. Med Inflamm. doi: 10.1155/2008/367590.
- Gideroglu K, Alagoz S, Uygur F, Evinc R, Celikoz B, Bugdayci G. 2008. Effects of nebivolol on skin flap survival: A randomized experimental study in rats. Curr Ther Res Clin Exp. 69:449–458.
- Giles TD, Sander GE, Nossaman BD, Kadowitz PJ. 2012. Impaired vasodilation in the pathogenesis of hypertension: Focus on nitric oxide, endothelial-derived hyper-polarizing factors, and prostaglandins. J Clin Hypertens (Greenwich). 14:198–205.
- Goel R, Goel A, Manocha A, Pillai KK, Srivastava RS. 2009. Influence of nebivolol on anti-convulsant effect of lamotrigine. Indian J Pharmacol. 41:41–46.
- Heeba GH, Hassan MK, Amin RS. 2009. Gastroprotective effect of simvastatin against indomethacin-induced gastric ulcer in rats: Role of nitric oxide and prostaglandins. Eur J Pharmacol. 607:188–193.
- Ittiyavirah SP, Shenika MS. 2014. Evaluation of anti-oxidant and anti-inflammatory activity of omeprazole against experimentally-induced colitis. JSIR. 3:352–356.
- Izzettin FV, Sancar M, Okuyan B, Apikoglu-Rabus S, Cevikbas U. 2012. Comparison of the protective effects of various anti-ulcer agents alone or in combination on indomethacin-induced gastric ulcers in rats. Exp Toxicol Pathol. 64:339–343.
- Kato S, Ohkawa F, Ito Y, Amagase K, Takeuchi K. 2009. Role of endothelial nitric oxide synthase in aggravation of indomethacin-induced gastric damage in adjuvant arthritic rats. J Physiol Pharmacol. 60:147–155.
- Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM. 2003. Oxidation of tetra-hydrobiopterin leads to uncoupling of endothelial cell NO synthase in hypertension. J Clin Invest. 111:1201–1209.
- Mejia A, Kraft WK. 2009. Acid peptic diseases: pharmacological approach to treatment. Expert Rev Clin Pharmacol. 2:295–314.
- Miranda K, Espey MG, Wink DA. 2001. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 5:62–71.
- Mollace V, Muscoli C, Masini E, Cuzzocrea S, Salvemini D. 2005. Modulation of prostaglandin biosynthesis by NO and NO donors. Pharmacol Rev. 57:217–252.
- Morsy M, Ashour O, Amin E, Rofaeil R. 2009. Gastroprotective effects of telmisartan on experimentally-induced gastric ulcers in rats. Pharmazie. 64:590–594.
- Morsy MA, Heeba GH, Abdelwahab SA, Rofaeil RR. 2012. Protective effects of nebivolol against cold restraint stress induced gastric ulcer in rats: Role of NO, HO-1 and COX-1,2. Nitric Oxide. 27:117–122.
- Musumba C, Pritchard DM, Pirmohamed M. 2009. Cellular and molecular mechanisms of NSAID-induced peptic ulcers. Aliment Pharmacol Ther. 30:517–531.
- Patil AN, Advani MG, Mali SN, Pawar S, Raut SB. 2012. Evaluation of anti-ulcer effect of amlodipine in gastric ulcer models in rats. Indian J Pharmacol. 44:387–409.
- Rahim NA, Hassandarvish P, Golbabapour S, Ismail S, Tayyab S, Abdulla MA. 2014. Gastro-protective effect of ethanolic extract of Curcuma xanthorrhiza leaf against ethanol-induced gastric mucosal lesions in Sprague-Dawley rats. Biomed Res Int. 2:1–10.
- Raji Y, Oloyede GK. 2011. Anti-ulcerogenic effects and possible mechanism of action of Quassia amara (L. Simaroubaceae) extract and its bioactive principles in rats. Afr J Tradit Complement Alt Med. 9:112–119.
- Rouhollahi E, Moghadamtousi S, Hamdi O, Fadaeinasab M, Hajrezaie M, Awang K. 2014. Evaluation of acute toxicity and gastroprotective activity of Curcuma purpurascens BI. rhizome against ethanol–induced gastric mucosal injury in rats. BMC Compl Alt Med. 14:378.
- Sivaraman D, Muralidharan P. 2011. Cytoprotective effect of Morinda tinctoria Roxb. against surgical and chemical factor induced gastric and duodenal ulcers in rats. Ulcers. 20:1–9
- Sorrentino SA, Doerries C, Manes C, Speer T, Dessy C, Lobysheva I. 2011. Nebivolol exerts beneficial effects on endothelial function, early endothelial progenitor cells, myocardial neovascularization, and left ventricular dysfunction early after myocardial infarction beyond conventional β1-blockade. J Am Coll Cardiol. 57:601–611.
- Suleyman H, Albayrak A, Bilici M, Cadirci E, Halici Z. 2010. Different mechanisms in formation and prevention of indomethacin-induced gastric ulcers. Inflammation 33:224–234.
- Suzuki H, Nishizawa T, Tsugawa H, Mogami S, Hibi T. 2012. Roles of oxidative stress in stomach disorders. J Clin Biochem Nutr. 50:35–39.
- Swamy AH, Sajjan M, Thippeswamy AH, Koti BC, Sadiq AJ. 2011. Influence of proton pump inhibitors on dexamethasone-induced gastric mucosal damage in rats. Indian J Pharm Sci. 73:193–198.
- Usman D, Sunday OF, Gabriel AT. 2014. Effects of L-arginine and L-citrulline on indomethacin-induced gastric ulceration and gastric pH in male albino rats. Eur J Med Plants. 4:623–640.