Abstract
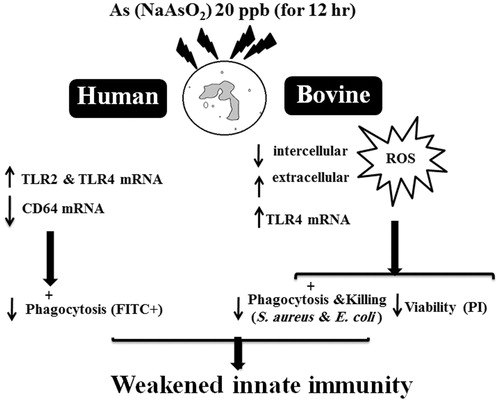
An important outcome arising out of occupational/environmental exposure to arsenic (As) is immunotoxicity. To determine the impact of inorganic As on innate immune cells, effects of a low dose of NaAsO2 (i.e. 20 ng As/ml) on select parameters associated with human and bovine neutrophils (PMN) were evaluated in vitro. PMN isolated from the blood of healthy individuals and cows (n = 8/treatment) were pre-incubated with NaAsO2 for 12 h before effects on PMN phagocytosis, transcription of TLR2, TLR4 and CD64 in human PMN – as well as on phagocytosis-dependent/-independent cell chemiluminescence (CL), phagocytosis and killing of Staphylococcus aureus and Escherichia coli, PMN H2O2 production and necrosis and TLR4 transcription in bovine PMN – were assessed. Relative to control (no As) PMN, treatment with As significantly decreased phagocytic capacity and CD64 mRNA, but increased TLR2 and TLR4 mRNA, in human PMN. In bovine PMN, while As also led to increased TLR4 mRNA abundance, it resulted in decreases in phagocytosis-dependent and -independent CL, PMN H2O2 production, PMN phagocytosis and killing of both E. coli and S. aureus by PMN. Considering the broad roles of PMN in immunology, the results of these studies increase our understanding of functional consequences of As exposure in inducing immunotoxicity and increasing susceptibility to (infectious) diseases in mammals.
Introduction
Arsenic (As) can be found in different chemical forms and oxidation states in the macro/micro-environment. Its origin is both “mostly” geogenic and anthropogenic worldwide (Cohen Citation2004; Wang & Mulligan Citation2006; Andrew et al. Citation2008; Taheri et al. Citation2016). Humans and food-producing animals are unintentionally exposed to As particularly through ingestion of contaminated drinking water and foodstuffs. This poses a huge risk to public health in some parts of the world. While the International Agency for Research on Cancer has classified As as a Group 1 carcinogen, it also damages hemato-/immunological systems (Taheri et al. Citation2016). Accordingly, As exposure may enhance host susceptibility to many types of infectious diseases (Kozul et al. Citation2009a) and contribute to the worsening of even non-infectious pathologies (Kozul et al. Citation2009b; Fie et al. Citation2010).
Many immunotoxicology studies have been performed on As effects on humans and animals (Schulz et al. Citation2002; Argos et al. Citation2006; Ghosh et al. Citation2006; Andrew et al. Citation2008; Banerjee et al. Citation2009; Khan et al. Citation2012). Commonly, the results emphasized the notion that As exposure disrupts the immune system, causes neutropenia and leads to recurrent infections and worsening of cancers and anemia (Biswas et al. Citation2008; Kozul et al. Citation2009a,Citationb; Taheri et al. Citation2016). One of the underlying reasons for the broad toxicity of As is its structural and chemical similarities to inorganic phosphate (Pi; PO43−). As such, phosphorous (P) in Pi-dependent enzymes is simply replaced by As, thereby blocking enzyme function (Biswas et al. Citation2008). The toxicity of As is generally associated with an ability to disable several sulfhydryl-containing biomolecules, and As-bounded compounds are more unstable than Pi compounds (Csanaky & Gregus Citation2001; Gregus & Nemeti Citation2005). Strong As affinity for sulfhydryl (SH) groups in cell and mitochondrial membranes causes mitochondrial damage through activation of cytochrome c, and caspases 9, 3 and 8, and so, induction of apoptosis. As also facilitates apoptosis induction though other mechanisms including a down-regulation of anti-apoptotic molecules, BCL2 (Li and Broome Citation1999; Carre et al. Citation2002; Rojewski et al. Citation2004; Binet et al. Citation2005, Citation2007; Khan et al. Citation2012).
In vitro As-exposed PMN show increased evidence of apoptosis/necrosis mainly through effects on mitogen-activated protein kinases (Morzadec et al. Citation2012). In several studies, As leads to increased formation of reactive oxygen species (ROS) in different cells (Carpenter et al. Citation2011; Wang et al. Citation2013; Li et al. Citation2014). Though still mechanistically inconclusive, As-induced PMN apoptosis is caused not only by increased [mitochondrial] ROS production independent of NADPH activity, but also via synthesis and activation of caspases (Binet et al. Citation2005).
PMN are the key cells in the innate immune system, and their phagocytosis and ROS production capacity are vital for host immunocompetence (Mehrzad et al. Citation2001, Citation2005, Citation2009, Citation2011). With a high affinity for Ig molecules (Hoffmann Citation2009), CD64 (FcγRI) is a key opsonin receptor on PMN needed for receptor-mediated phagocytosis (Wagner et al. Citation2000; Gerrits et al. Citation2013). In addition, pattern recognition receptors (PRR) that are highly expressed on mammalian PMN are Toll-like receptors (TLR) that can contribute to many cell signaling and various cell-related phenomena (Takeuchi & Akira Citation2010; Prince et al. Citation2011; Ma et al. Citation2013; Mohammadi et al. Citation2014). The key transmembrane TLR, i.e., TLR2 and TLR4, are active in mediating responses against Gram-positive and -negative bacteria, respectively (Kawai & Akira, Citation2010; Takeuchi & Akira Citation2010; Prince et al. Citation2011; Ma et al. Citation2013). Any unneeded stimulation via, for example As, of these and similar receptors could promote secretion of many damaging molecules and pro-inflammatory cytokines and, eventually, modulation of host immune responses (Mohammadi et al. Citation2014; Mehrzad et al. Citation2015).
In the study reported here, in vitro effects of As were evaluated on phagocytosis and bactericidal activity, necrosis, ROS production and TLR4 mRNA expression in bovine PMN. In addition, effects of As on expression of TLR2 and TLR4 mRNA expression in, and on phagocytosis by, PMN isolated from healthy humans were also evaluated. Even though the dose of As selected for use in this study was low and based on our previous studies (Taheri et al. Citation2016) [note: even less than levels that have been observed in human and animal hosts (Wu et al. Citation2001; Hall et al. Citation2006)], it was expected that data from the studies could provide insight into the granulotoxic properties of As in PMN and additional information about potential immunotoxic effects of As in mammals.
Materials and methods
Blood PMN preparation, in vitro exposures and experimentation
A group of eight healthy pregnant lactating Holstein cows (age 35.0 ± 0.5 months) and eight young healthy male humans (age 23 ± 2 years) were used as sources of PMN for the assays herein. Blood samples from the humans and cows were aseptically collected from the external saphenous and external jugular veins, respectively, into heparinized Vacutainer tubes. After first differentiating the relative levels (%) of nucleated blood cells from blood smears (Mehrzad et al. Citation2001, Citation2005), PMN were isolated from each sample by an initial hypotonic lysis of erythrocytes present, followed by gradient centrifugation (Mehrzad et al. Citation2001). From the resulting cell pellet, a total number of isolated PMN was quantified using an MEK 6450K Coulter counter (Nihon Kohden Celltac, Tokyo, Japan). These procedures yielded >98% granulocytes (PMN + eosinophil; predominantly PMN [>87%]) with >98% viability (Mehrzad et al. Citation2001, Citation2005, Citation2011). To ensure reproducibility between days and humans/cows, each functional assay was performed with the same number of calculated viable PMN. For use in each study, PMN suspensions were adjusted to 5 × 106 viable PMN/ml with Dulbecco’s phosphate-buffered saline (DPBS; Sigma, Deisenhofen, Germany). Use of the animals and humans in these non-clinical studies (i.e. blood sampling for in vitro cell culture assays) was in accordance with local human and animal welfare regulations. The Ethics Committee of Ferdowsi University of Mashhad approved all of these studies.
As (as NaAsO2) was obtained from Sigma (Taufkirchen, Germany); for these studies, further dilutions of the As were made with DPBS. All human and bovine PMN were maintained in complete RPMI 1640 medium (Biochrom, Berlin, Germany), i.e. RPMI 1640 supplemented with 2% human albumin (AB) serum (BioWhittaker, Walkersville, MD), 2 mM L-glutamine (Life Technologies, Carlsbad, CA), 50 U penicillin/ml and 50 μg streptomycin/ml, 1% fetal calf serum (FCS), 25 μg kanamycin/ml, 50 μg gentamycin/ml, 1 mg non-essential amino acid/ml, 1 mM sodium pyruvate and 50 μM β-mercaptoethanol (2-ME) (all Sigma).
In all of the studies below, isolated PMN were washed with culture media without phenol red, seeded at 3 × 106 cells/ml in culture plates, and treated with 20 ng As/ml for 12 h (37 °C, 5% CO2, 95% humidity); phenol red-free media were used to mitigate any possible As-phenol red interaction during the 12-h exposure and any potential auto-luminescence/fluorescence from phenol red during luminometric and flow cytometric assays. This As concentration was chosen based on a previous study (Taheri et al. Citation2016). As one could question the relevance/justification of this dose, in countries where there is As contamination, blood levels of As have been measured at >60 ng/ml (Hall et al. Citation2006) in humans and at >600 ng/ml in key biomarkers (i.e. urine, a preferred specimen for assessment of As exposure) of exposed animals. Blood concentrations of As are elevated for a short time after exposure, and then rapidly the metal is dispersed into tissues and/or excreted via the urine (Li et al. Citation2016). Accordingly, to reflect what could occur in exposed human/bovine hosts, the dose of 20 ng As/ml was used in these studies. Further, the use of the single 12 h exposure period was meant to reflect an acute, not chronic, effect of As on key PMN functions/molecules. More work using a wider range of timepoints would be valuable and will be undertaken based on the studies here.
Effect of As on bovine PMN phagocytosis-dependent and -independent chemiluminescence
Isolated bovine PMN were exposed to As (20 ng/ml in RPMI 1640 + 10% FCS, 2 mM L-glutamine, 100 U penicillin/ml, 100 μg streptomycin/ml [all Sigma]) in 12-well culture plates for 12 h in a humidified 37 °C atmosphere. Wells without As served as controls. All chemiluminescence (CL) assays were performed in 96-well white flat-bottom microtiter culture plates (Nunc, Wiesbaden, Germany) with each well containing 4 × 105 PMN/200 μl. For non-phagocytosis-dependent CL assays, phorbol-12-myristate-13-acetate (PMA, Sigma) at a final concentration of 200 ng/ml was used as activator. For phagocytosis-dependent CL assays, either polystyrene beads (0.76 μm diameter, 4 × 1011 particles/ml; Sigma) at 130/PMN, or opsonized Staphylococcus aureus (Pansorbin, Calbiochem, Carlsbad, CA) at 25/PMN were added to dedicated wells. In all cases, immediately after addition of the PMN activator, luminol (at 0.3 mM final concentration; Sigma) was added into each well (60 μl/well) and measures of CL were then performed continuously over a period of 30 min in a Mithras LB 940 luminometer (MicroWin, Bad Wildbad, Germany). The area under the curve (AUC) was calculated for registered impulse rates (counts/min or relative light units (RLU)/sec) over the entire period. Tmax, expressed as time when peak intracellular ROS generation happened during 30 min, was also quantified during the assays. All CL measures were carried out at pH 7.2 and 37 °C. Ultimately, each CL response was adjusted in terms of 1000 viable PMN in each sample.
Effect of As on flow cytometry-based H2O2 production by and necrosis among bovine PMN
Modified H2O2 production tests were performed in 96-well round-bottom microtiter plates (Corning, NY). Each well was pre-filled with 100 μl isotonic Percoll (Pharmacia, Freiburg, Germany) to avoid adherence and loss of activated bovine PMN. For the detection of H2O2 produced, non-fluorescent dihydrorhodamine (DHR 123, Mobitec, Goettingen, Germany) dye at a final concentration of 750 ng/ml was added to each Percoll cushion. Here, PMN MPO (myeloperoxidase) catalyzes the oxidation of DHR 123 to fluorescent rhodamine 123. In brief, bovine PMN (50 μl of 107/ml, 200 μl total) suspensions were overlaid atop Percoll cushions in 96-well plates; thereafter, 20 μl As solution (at 20 ng/ml) or medium was added to specified wells. After 12 h incubation at 37 °C, the cells were recovered and transferred to flow cytometer tubes. To specified tubes within each treatment group, 100 nM PMA (or vehicle) was added and the relative amounts of H2O2 generated by the activated PMN then measured by flow cytometry (FACScan, Becton Dickinson, Heidelberg, Germany). For this, determination of relative (mean) green fluorescence intensity of gated PMN populations was done after acquisition of 10,000 events/sample.
For determination of necrosis, isolated PMN (2 × 105/well of 96 round-bottom microtiter plate) were incubated for 12 h at 37 °C in the presence of 20 ng As/ml in culture medium. After flow cytometric acquisition of the PMN incubated with/without As, absolute numbers of viable PMN were determined by a standard cell dilution assay (Mehrzad et al. Citation2011). Reduced proportions of viable PMN compared to levels in control wells indirectly indicated necrotic PMN.
Effect of As on human PMN phagocytosis
To determine the effect of As on PMN functional activity, 107 fluorescein isothiocyanate (FITC)-loaded polystyrene microparticles (1.0 μm, 20 beads/PMN; Sigma) were added to 106 human PMN that had been pre-incubated with/without 20 ng As/ml for 12 h at 37 °C in 6-well plates. After 3 h of culture at 37 °C, the bead-treated PMN were harvested on ice, washed with ice-cold DPBS, and particle internalization then assessed by flow cytometry [minimum event count/sample = 10,000]. Results were recorded as mean fluorescence intensity (MFI, intensity of phagocytosed FITC-labeled microparticles); the number of FITC+ PMN reflected the number of PMN that could phagocytize microparticles and so reflected phagocytic ability of the PMN.
Effect of As on bovine PMN-pathogen interactions and microbicidal activity
Phagocytosis and bactericidal activity against Escherichia coli (P4:032) and Staphylococcus aureus Newbold 305 (both obtained from clinical cases of mastitis [Ghent, Belgium]) by the PMN were assessed in a bactericidal assay described in (Mehrzad et al. Citation2005,Citation2009). In brief, bovine PMN were exposed to 20 ng As/ml or medium only for 12 h. In preparation for use in these assays, E. coli and S. aureus were each cultured in nutrient broth at 37 °C for 18 h. Levels of bacteria in each culture were then estimated by measures of turbidity [optical density >1.0]; actual concentrations were assessed via plating (in triplicate) on Columbia sheep blood agar to provide accurate estimates of infecting levels used in the uptake/killing assays. To prevent further organism growth prior to their use, each media containing the specific bacteria was placed at 4 °C for 24 h until used in the protocol. Samples of each media were re-plated to confirm bacterial levels had not changed significantly from the day before.
For the PMN-bacteria co-incubations, into 1.5 ml microtubes (in final volume of 1 ml) was placed 100 μl S. aureus or E. coli (5 × 107 cfu/ml), 500 μl As-exposed (or non-exposed) PMN (5 × 106 viable cells in HBSS) and 400 μl pooled heat-inactivated bovine serum (5% [v/v]). Control tubes (C0, C60; expressed as CFU/ml) contained bacteria, HBSS, and serum without PMN. The microtubes were then rotated end-over-end at 37 °C for 60 min. Samples (25 μl) were taken from the mixture assay (Ma0, Ma60; expressed as CFU/ml) at 0 and 60 min, diluted in 1 ml ddH2O and kept at 0 °C for 3 h to disrupt the PMN. Extracellular (EC; expressed as CFU/ml) bacteria were separated from the PMN by centrifugation (100 × g, 1 min, 4 °C). A 25-μl sample of the supernatant from Ma60 was taken and diluted as for the mixture assay samples.
Ten-fold serial dilutions of C0, C60, EC, Ma0 and Ma60 were performed and the last dilutions spread onto Columbia sheep blood agar. After overnight incubation at 37 °C, bacterial colonies were counted and the net change relative to the original bacterial suspension levels calculated. Results from the bactericidal assay were expressed as % phagocytosis and killing of bacteria using the reported formulas (Mehrzad et al. Citation2009, Citation2011).
qPCR analyses of PMN mRNA
Total RNA from As-treated (12 h)/control human and bovine PMN, as well as from human and bovine PMN that had been treated with 10 ng LPS/ml (lipopolysaccharide – Type 0111:B4 from Escherichia coli [Sigma, St. Louis, MO]; TLR4 agonist, positive control) for 12 h, was isolated. In each case, the PMN suspensions were centrifuged (350 × g, 5 min, 4 °C) and cell pellets stored at −80 °C for later use in real-time quantitative PCR (qPCR) analyses (TLR2 and CD64 for human PMN only; TLR4 for both bovine and human PMN).
For the analyses, RNA was extracted from each PMN sample using TriPure isolation reagent (Roche Diagnostics, Indianapolis, IN); 1 μg RNA was then used for first strand cDNA synthesis (using oligo-dT primers). Exon junction or intron-spanning primers () were designed for human TLR2, TLR4, CD64, GAPDH and bovine TLR4 and GAPDH. “5x HOT FIREPol® EvaGreen®qPCR Mix Plus” (Solis BioDyne, Tartu, Estonia) was used to perform the qPCR, according to manufacturer protocols. Each qPCR reaction was done in a 20 μl final volume containing 10 pM of specific forward and reverse primers, 4 μl EvaGreen master mix and 1 μl cDNA template. After optimization, the qPCR conditions for all genes were carried out (in duplicate) using a Rotter gen 6000 light-cycler system (QIAGEN, Venlo, the Netherlands) with a cycling program including holding for 15 min at 95 °C, followed by cycling 45 times at 94 °C, specific annealing temperatures for each gene (), and 72 °C with melting curve analyses (included ramping from 50 to 99 °C, rising 0.5 °C/step and waiting 10 s for each step afterward; a single peak was obtained in each qPCR product reaction) accompanied by agarose gel electrophoresis to ascertain the absence of non-specific qPCR products.
Table 1. Nucleotide sequences of designed primers for genes TLR2, TLR4, CD64 and GAPDH with junction temperature and Amplicon size.
Normalization and analyses of qPCR data were calculated using GenEX Version 5 software (MultiD, Göteborg, Sweden) and Relative Expression Software Tool (REST; QIAGEN, Hilden, Germany). In each qPCR reaction, the cycle number at which the fluorescence rose appropriately above background was determined as the crossing point (CP). Cycle threshold (Ct) values were means of duplicates in each qPCR run and eight biological repeats after interplate calibration. Optimization experiments were also performed to ensure that the efficiency of the target and the reference genes was approximately equal. A Pfaffl equation (Pfaffl Citation2001) was first used to calculate relative gene expression ratio, i.e. the change in each target gene expression divided by the change in glyceraldehyde phosphate dehydrogenase (GAPDH) expression. A slope was determined from the exponential phase. Amplification efficiency (E) was calculated based on slope, where E = 10[−1/slope]. The expression of each target gene was calibrated to that of GAPDH using the formula: Relative fold change (RCF) = [Etarget gene × (Control CPtarget gene – Treated CPtarget gene)]/[EACTB × (Control CPACTB – Treated CPACTB)].
Statistical analyses
SAS Version 9.1 (SAS Institute, Cary, NC) was used to analyze As effects on the measured parameters. All results were presented as mean ± SEM. Each bovine and human was considered as a random factor. After performing tests of normality, comparisons of means between As-treated and control groups was performed using an independent sample t-test. A p-value ≤ 0.05 was accepted as significant.
Results
Effect of As on bovine PMN phagocytosis-dependent and -independent chemiluminescence
The bovine PMN phagocytosis-(in)dependent CL/(non)particle-stimulated CL results showed a significant decrease in phagocytic activity by As-exposed PMN (, left panel). Similar patterns of decrease were noted in the PMA-stimulated CL assay. Among the bovine PMN stimulated with PMA, latex beads or Pansorbin, the AUC values for As-exposed PMN were, respectively, 79, 44 and 58% lower than observed in respective counterpart control cells. Further, the Tmax values for the As-treated PMN stimulated with PMA, latex or Pansorbin were decreased by 8.4, 8.9 and 9.1%, respectively (), relative to the values for the controls.
Figure 1. Effects of arsenic (As) on in vitro PMN phagocytosis and killing activity. Left panel, luminol-dependent chemiluminescence (CL) in bovine blood PMN exposed for 12 h with or without As; PMN were then immediately stimulated with PMA (black bars), latex beads (gray bars) or opsonized Pansorbin (white bars). Values are expressed as area under the curve (AUC) of relative photon/light units of 1000 viable PMN during 30 min. Middle panel, phagocytosis/killing of S. aureus and E. coli after incubation (1 h) with isolated bovine PMN exposed to As or medium for 12 h. Right panel: phagocytosis of 1-μm fluorescent microparticles after 3 h incubation by human PMN that had been treated with/without As for 12 h. In all panels, data shown are means ± SE (n = 8). Data significantly different (*p < 0.05, **p < 0.01 or ***p < 0.001) versus corresponding control.
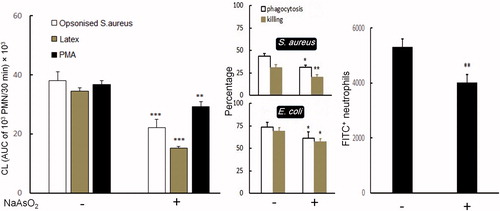
Table 2. Tmax of luminol-dependent chemiluminescence (CL) in stimulated bovine PMN.
Effects on PMN phagocytic and killing capacity
The FACS-based PMN phagocytosis assays revealed a significant decrease in phagocytosis of FITC-labeled microparticles by post-As-treated human PMN (, right panel). This decrease in function was consistent with results of the bovine PMN–pathogen interaction assays (, middle panel). Indeed, As caused PMN to be significantly less efficient/potent at killing pathogens S. aureus and E. coli. Rate of killing was greatly lower (∼17 and 29% in phagocytosis and ∼18 and 36% in killing activity versus E. coli and S. aureus, respectively) by the As-exposed PMN relative to the values in/by respective control PMN.
Effects on bovine PMN H2O2 production and necrosis
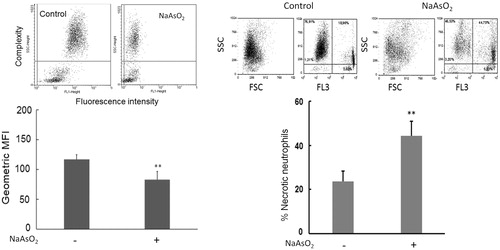
The flow-based measures of H2O2 production by bovine PMN pre-exposed to As revealed a lower percentage of DHR 123-labeled PMN (i.e., PMN responded with clear shift in fluorescence intensity, indicating diminished respiratory burst activity and/or decreased intracellular H2O2 production) among As-exposed PMN (, left panel). In contrast, in the FACS assay to determine percentage PI+ PMN (i.e. PMN necrosis), a remarkably increased percentage of necrosis was noted among the As-exposed PMN (, right panel).
Figure 2. Representative results of flow cytometric determinations of bovine PMN H2O2 production (left upper portion) and necrosis (right upper portion). Left half of figure presents cells treated for 12 h with or without As. PMN H2O2 production reported in terms of mean green fluorescence intensity (MFI). Dihydrorhodamine added to show ROS production. Right half of figure presents results of overall necrotic neutrophils (%) due to treatments (lower panel). In all cases, data shown are means ± SE (n = 8). Data significantly different (**p < 0.01) versus corresponding control.
Effects on expression of human TLR2, TLR4, and CD64 and bovine TLR4 mRNA
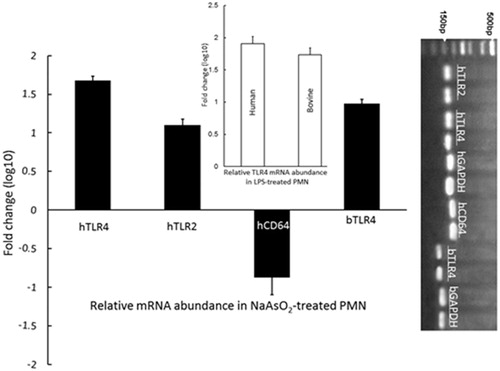
The 12-h As treatment caused up-regulated expression of human TLR2 and human and bovine TLR4, but down-regulated expression of human CD64 transcripts, in neutrophils. To optimize the PCR assays, specific single PCR products were determined for TLR4, TLR2, CD64 and GAPDH (, right panel). The qPCR data revealed that expression levels of both TLR2 and TLR4 transcripts in As-treated PMN were significantly higher than in non-treated cells (p < 0.05); the increased transcription for TLR2 and TLR4 in human PMN was 1.1 log10-fold and 1.68 log10-fold higher, respectively, than in control counterpart cells, (). TLR4 transcript levels in human and bovine LPS-treated PMN were respectively 1.91 and 1.74 log10-fold higher than in control PMN counterparts (, insert) (p < 0.05). Conversely, CD64 transcript levels in human As-treated PMN were 0.89 log10-fold lower than in control PMN () (p < 0.05).
Figure 3. Expression of TLR2 and TLR4 mRNA transcripts in human and/or bovine PMN, and CD64 mRNA transcripts in human PMN, treated 12 h with/without As. Compared to in untreated controls, relative gene expression (fold-change, “logarithmic, log10, scale”) for both TLR2 and TLR4 in As-treated PMN were significantly higher (p < 0.05). Human and bovine PMN were also treated with 10 ng LPS/ml for 12 h as positive controls for TLR4 (insert); TLR4 transcript levels in human and bovine LPS-treated PMN were, respectively, 1.91 and 1.74 log10-fold higher than in control PMN counterparts (p < 0.05). Values shown are means (± SE) from PMN isolated from 8 subjects/8 cows. (Right panel) Specific single qPCR products for TLR4, TLR2, CD64 and GAPDH in PMN – a representative set of products is shown; h (human), b (bovine).
Discussion
The main rationale for evaluating arsenic (As) in the in vitro tests here was an ongoing concern about the presence of As in the drinking water in parts of Iran (Chelpu and Bijar) where residents and animals appear to suffer from various non-infectious/infectious diseases (Taheri et al. Citation2016). In a normal host, neutrophils (PMN) are pivotal circulating phagocytes in the innate immune system, and their respiratory burst activity is essential to host ability to kill microbial pathogens. Changes in respiratory burst activity and other key functional parameters in PMN (and many other immune cell types) are often used to assess immunotoxic effects of environmental toxicants, including metals (Hermann & Kim, Citation2005). In the context of As, an array of mixed effects of As on PMN respiratory burst activity has been reported in various animal studies (Nayak et al. Citation2007; Binet & Girard Citation2008; Bourdonnay et al. Citation2011; Li et al. Citation2014).
In general, PMN produce high levels of ROS against invading pathogens. Here, overall ROS production in PMN was inhibited by the applied As dose. Biochemically, intra-phagosomal PMN ROS production yields a chemiluminescence (CL) [or photon emission] that is quantifiable (Mehrzad et al. Citation2001,Citation2009,Citation2011). As expected, here, stimulated PMN (with all three forms of stimulators, i.e. PMA, latex beads or opsonized S. aureus) produced high levels of free radicals. This study showed there was a significant decrease in respiratory burst activity in As-exposed PMN (re: CL data). After ingesting pathogens, PMN are able to kill and destroy pathogens through oxygen-(in)dependent pathways (Reeves et al. Citation2002; Mehrzad et al. Citation2001, Citation2009, Citation2011). It was also noted that Tmax values tended to be decreased in the As-exposed PMN in comparison to in control cells, meaning that ROS production reached its highest value in a shorter time, an outcome that would likely be harmful to a cell. It was also noted that H2O2 production was decreased in these As-exposed PMN; this could potentially be due to inhibitory effects of As on enzymes associated with the respiratory burst.
Measuring intracellular ROS generation in PMN using CL (ROS via luminol) and flow cytometry (DHR, exclusively sensing H2O2, and phagocytosis) methods revealed the inhibiting effects of As on this particular activity, i.e. the PMN respiratory burst pivotal for killing activity after phagocytosis. Arsenic is known to induce a direct inhibitory effect on respiratory burst activities in part through induced disruptions in (de)phosphorylation events in cells (Binet et al. 2004; Li et al. Citation2014). It is also known that As inhibits interferon (IFN)-mediated activities (including subsequent augmented cyto-/chemokine secretion) in exposed cells (Wijeweera et al. Citation2001; Chen et al. Citation2003; Cheng et al. Citation2004; Hermann & Kim Citation2005; Nayaka et al. Citation2007; Binet & Girard Citation2008). Unfortunately, this study did not measure culture levels of key cytokines such as interleukin (IL)-1β or tumor necrosis factor (TNF)-α or chemokines (CXCL6, CXCL7, CXCL8, etc.) that help activate protein kinase C-dependent processes and respiratory burst activity in PMN (and other phagocytes) (Dewas et al. Citation2003; Hermann & Kim Citation2005). Future studies will analyze the As-treated samples to ascertain if indeed, the induced changes in ROS formation/respiratory burst activity among the PMN was due, in part, to alterations in cytokine production/release.
Normally, increased necrosis in a population of As-exposed cells could be due to increases in the presence of extracellular ROS. However, as noted above, the As-treated cells formed less ROS than expected. Instead, induced changes in cytoskeletal (especially microtubule) structures are another potential important mechanism underlying necrosis in cells. Microtubule components possess abundantly high levels of sulfhydryl groups; a strong affinity of As for these results in As accumulation inside the PMN cytoskeleton (Li & Broome Citation1999; Binet et al. Citation2005). Accordingly, with increased cellular levels of As, it is possible that the increases in necrosis observed within 12 h was likely due to alterations in cell structure rather than due to ROS-related events. This reduced PMN viability due to enhanced necrosis could be a key underlying reason for the higher growth of E. coli and S. aureus seen here in the cultures of As-exposed PMN. Indeed, all of the measured in phagocytosis, bacterial killing and ROS production induced by the 12-h As treatment might not likely due only to adverse effects on these specific activities within the PMN per se but also due to the increases in necrotic PMN and thus reduced numbers of PMN to kill bacteria, phagocytize, produce ROS, etc.
Nevertheless, because little is known as to how As might affect phagosomes/lysosomes where large amounts of oxidants are released into/stored as a result of activation of the MPO–H2O2–HOCl system (Paape et al. Citation2002; Burvenich et al. Citation2003; Mehrzad et al. Citation2001, Citation2005, Citation2009, Citation2011), it is also plausible that the higher growth of E. coli and S. aureus seen in As-treated bovine PMN was also associated with some key enzymatic dysfunctions, including possibly (but not limited to) those of NADPH-oxidase, MPO, and thus the MPO–H2O2–HOCl system. This clearly remains to be resolved in more mechanistic follow-up studies.
This study also evaluated As effects on TLR2, TLR4 and CD64 mRNA abundance in human and bovine PMN. Recent studies noted substantial interference with expression and/or function of TLR4 and TLR2 in bovine (Mehrzad et al. Citation2013), porcine (Mehrzad et al. Citation2015) and human (Malvandi et al. Citation2013; Mohammadi et al. Citation2014) mononuclear cells exposed to toxicants (i.e. aflatoxin) in vitro. Because TLR4 and TLR2 are highly expressed on the PMN surface, changes in their mRNA levels could eventually ultimately give rise to enhanced inflammatory responses. Such an outcome is due, in part, to the fact that TLR2 and TLR4 contribute to many cell signaling and various pathological phenomena. As such, their increased presence/stimulation on cells (including PMN) could promote secretion of many damaging molecules/pro-inflammatory cytokines/chemokines and, eventually, modulation of host immune responses (Kawai & Akira Citation2010; Takeuchi & Akira Citation2010; Ma et al. Citation2013). While the current study clearly showed As exposure led to significant increases in TLR2 and TLR4 mRNA abundance in the in vitro-exposed human (TLR2 and TLR4) and bovine (TLR4) PMN, the molecular pathways underlying these changes remain unclear. Even so, the data are in line with findings by our group and others who noted similar changes in TLR2 and TLR4 and PMN/immune cell activity in studies of the (immuno)toxicity of As (Kozul et al. Citation2009a,Citationb), lead (Liu et al. Citation2015), aflatoxin (Mehrzad et al. Citation2015), opioids (Castelli et al. Citation2105) and aryl hydrocarbons (Quintana et al. Citation2008; Veldhoen et al. Citation2008; Platzer et al. Citation2009).
Unlike with TLR2 and TLR4, there is little research on the effects of As on CD64 – even less so in the context of PMN function. Due to the changes regarding phagocytic functions of the As-treated PMN, these studies also investigated whether the metal was impacting on opsonin receptors on the cells. While direct measures of receptor expression would have been beneficial, that these are internalized/re-circulate would have opened up questions as to timing/kinetics of receptor expression. Instead, to assess if As impacted on a key PMN opsonin receptor, i.e. CD64, mRNA expression levels of this protein (at least in the human PMN) were analyzed. The data clearly showed there were significant reductions in CD64 mRNA in the As-treated human PMN. Such a finding would be in accordance with the reductions in phagocytic activities of these treated cells. Regardless of the lack of data in the literature on As and CD64, the current data are in line with findings by van Grevenynghe et al. (Citation2003) and Platzer et al. (2009) who noted similar changes in CD64 and cell phagocytic activity in studies of the immunotoxicity of aryl hydrocarbon-based toxicants.
Conclusions
In summary, the granulotoxic properties induced by a low dose of As provides another compelling reason to minimize As exposures. The outcomes regarding TLR2 and TLR4 mRNA up-regulation and CD64 mRNA down-regulation in As-exposed PMN open novel doors to understanding molecular mechanisms of effects of As upon phagocytosis, inflammation, and infection in As-exposed animals and humans (). As the effects of As on PMN functions were examined at only one timepoint (12 h) and using only a single low dose (20 ng As/ml), further studies with an array of doses and varying lengths of As exposure would be valuable to further refine the bases for the outcomes observed here.
Disclosure statement
The authors declare no conflicts of interest. The authors alone are responsible for the content of this manuscript.
Funding
This study was supported by the Ferdowsi University of Mashhad and Payamnoor University of Mashhad.
References
- Andrew AS, Jewell DA, Mason RA, Whitfield ML, Moore JH, Karagas MR. 2008. Drinking-water arsenic exposure modulates gene expression in human lymphocytes from a U.S. population. Environ Health Perspect. 116:524–531.
- Argos M, Kibriya MG, Parvez F, Jasmine F, Rakibuz-Zaman M, Ahsan H. 2006. Gene expression profiles in peripheral lymphocytes by arsenic exposure and skin lesion status in a Bangladeshi population. Cancer Epidemiol Biomarkers Prev. 15:1367–1375.
- Banerjee N, Banerjee S, Sen R, Bandyopadhyay A, Sarma N, Majumder P, Das JK, Chatterjee M, Kabir SN, Giri AK. 2009. Chronic arsenic exposure impairs macrophage functions in the exposed individuals. J Clin Immunol. 29:582–594.
- Binet F, Cavalli H, Moisan E, Girard E. 2005. Arsenic trioxide (AT) is a novel human neutrophil pro-apoptotic agent: Effects of catalase on AT-induced apoptosis, degradation of cytoskeletal proteins and de novo protein synthesis. Br J Hematol. 132:349–358.
- Binet F, Chiasson S, Girard D. 2007. Arsenic trioxide induces de novo protein synthesis of annexin-1 in neutrophils: Association with a heat shock-like response and not apoptosis. Br J Hematol. 140:454–463.
- Binet F, Girard D. 2008. Novel human neutrophil agonistic properties of arsenic trioxide: Involvement of p38 mitogen-activated protein kinase and/or c-jun NH2-terminal MAPK but not extracellular signal-regulated kinases-1/2. J Leukocyte Biol. 84:1613–1622.
- Biswas D, Banerjee M, Sen G, Das JK, Banerjee A, Sau TJ, Pandit S, Giri AK, Biswas T. 2008. Mechanism of erythrocyte death in human population exposed to arsenic through drinking water. Toxicol Appl Pharmacol. 230:57–66.
- Bourdonnay E, Morzadec C, Fardel O, Vernhet L. 2011. Arsenic increases LPS-dependent expression of IL-8 gene by stimulating a redox-sensitive pathway that strengthens p38-kinase activation. Mol Immunol. 48:2069–2078.
- Burvenich C, van Merris V, Mehrzad J, Diez-Fraile A, Duchateau L. 2003. Severity of E. coli mastitis is mainly determined by cow factors. Vet Res. 34:521–562.
- Carpenter RL, Jiang Y, Jing Y, He J, Rojanasakul Y, Liu LZ, Jiang BH. 2011. Arsenite induces cell transformation by reactive oxygen species, AKT, ERK1/2, and p70S6K1. Biochem Biophys Res Commun. 414:533–538.
- Carre M, Carles G, Andre N, Douillard S, Ciccolini J, Briand C, Braguer D. 2002. Involvement of microtubules and mitochondria in antagonism of arsenic trioxide on paclitaxel-induced apoptosis. Biochem Pharmacol. 63:1831–1842.
- Castelli M, Panerai A, Sacerdote P, Franchi S. 2015. Measurement of macrophage TLR4 expression after morphine treatment. Methods Mol Biol. 1230:263–271.
- Chen Q, Powell DW, Rane MJ, Singh S, Butt W, Klein JB, McLeish KR. 2003. Akt phosphorylates p47phox and mediates respiratory burst activity in human neutrophils. J Immunol. 170:5302–5308.
- Cheng HY, Li P, David M, Smithgall TE, Feng L, Lieberman MW. 2004. Arsenic inhibition of the JAK-STAT pathway. Oncogene. 23:3603–3612.
- Cohen MD. 2004. Pulmonary immunotoxicology of select metals: Aluminum, arsenic, cadmium, chromium, copper, manganese, nickel, vanadium, and zinc. J Immunotoxicol. 1:39–69.
- Csanaky I, Gregus Z. 2001. Effect of phosphate transporter and methylation inhibitor drugs on the disposition of arsenate and arsenite in rats. Toxicol Sci. 63:29–36.
- Dewas C, Dang PM, Gougerot-Pocidalo MA, El-Benna J. 2003. TNFα induces phosphorylation of p47 (phox) in human neutrophils: Partial phosphorylation of p47phox is a common event of priming of human neutrophils by TNFα and granulocyte-macrophage colony-stimulating factor. J Immunol. 171:4392–4398.
- Fei DL, Li H, Kozul CD, Black KE, Singh S, Gosse A., DiRenzo J, Martin KA, Wang B, Hamilton JW, et al. 2010. Activation of hedgehog signaling by environmental toxicant arsenic may contribute to etiology of arsenic-induced tumors. Cancer Res. 70:1981–1988.
- Gerrits JH, McLaughlin PM, Nienhuis BN, Smit JW, Loef B. 2013. Polymorphic mononuclear neutrophils CD64 index for diagnosis of sepsis in post-operative surgical patients and critically ill patients. Clin Chem Lab Med. 51:897–905.
- Ghosh D, Bhattacharya S, Mazumder S. 2006. Perturbations in the catfish immune responses by arsenic: Organ- and cell-specific effects. Comp Biochem Physiol C. 143:455–463.
- Gregus Z, Nemeti B. 2005. Glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase works as arsenate reductase in human red blood cells and rat liver cytosol. Toxicol Sci. 85:859–869.
- Hall M, Chen Y, Ahsan H, Slavkovich V, van Geen A, Parvez F. 2006. Blood arsenic as a biomarker of arsenic exposure: Results from a prospective study. Toxicology. 225:225–233.
- Hermann AC, Kim CH. 2005. Effects of arsenic on zebrafish innate immune system. Marine Biotechnol. 7:494–505.
- Hoffmann JJ. 2009. Neutrophil CD64: A diagnostic marker for infection and sepsis. Clin Chem Lab Med. 47:903–916.
- Kawai T, Akira S. 2010. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat Immunol. 11:373–384.
- Khan S, Vala JA, Nabi SU, Gupta G, Kumar D, Telang AG, Malik JK. 2012. Protective effect of curcumin against arsenic-induced apoptosis in murine splenocytes in vitro. J Immunotoxicol. 9:148–159.
- Kozul CD, Ely KH, Enelow RI, Hamilton JW. 2009a. Low-dose arsenic compromises the immune response to influenza A infection in vivo. Environ Health Perspect. 117:1141–1147.
- Kozul CD, Hampton TH, Davey JC, Gosse JA, Nomikos AP, Eisenhauer PL, Weiss DJ, Thorpe JE, Ihnat MA, Hamilton JW. 2009b. Chronic exposure to arsenic in the drinking water alters expression of immune response genes in mouse lung. Environ Health Perspect. 117:1108–1115.
- Li J, Li C, Sun HJ, Juhasz AL, Luo J, Li HB, Ma LQ. 2016. Arsenic relative bioavailability in contaminated soils: Comparison of animal models, dosing schemes, and biological endpoints. Environ Sci Technol. 50:453–461.
- Li YM, Broome JD. 1999. Arsenic targets tubulins to induce apoptosis in myeloid leukemia cells. Cancer Res. 59:776–780.
- Li YN, Xi MM, Gue Y, Hai CX, Yang WL, Qin XJ. 2014. NADPH oxidase-mitochondria axis-derived ROS mediate arsenite-induced HIF-1α stabilization by inhibiting prolyl hydroxylase activity. Toxicol Lett. 224:165–174.
- Liu JT, Chen BY, Zhang JQ, Kuang F, Chen LW. 2015. Lead exposure-induced microgliosis and astrogliosis in hippocampus of young mice potentially by triggering TLR4-MyD88-NF-κB signaling cascades. Toxicol Lett. 239:97–107.
- Ma D, Jin S, Li E, Doi Y, Parajuli B, Noda M, Sonobe Y, Mizuno T, Suzumura A. 2013. The neurotoxic effect of astrocytes activated with toll-like receptor ligands. J Neuroimmunol. 254:10–18.
- Malvandi AM, Mehrzad J, Saleh-Moghaddam S. 2013. Biologically-relevant doses of mixed afla-toxins B and G up-regulate MyD88, TLR2, TLR4 and CD14 transcripts in human PBMC. Immunopharmacol Immunotoxicol. 35:528–532.
- Mehrzad J, Dosogne H, Meyer E, Heyneman R, Burvenich C. 2001. Respiratory burst activity of blood and milk neutrophils in dairy cows during different stages of lactation. J Dairy Res. 68:399–415
- Mehrzad J, Duchateau L, Burvenich C. 2005. High milk neutrophil chemiluminescence limits the severity of bovine coliform mastitis. Vet Res. 36:101–116.
- Mehrzad J, Duchateau L, Burvenich C. 2009. Phagocytic and bactericidal activity of blood and milk-resident neutrophils against Staphylococcus aureus in primiparous and multiparous cows during early lactation. Vet Microbiol. 134:106–112.
- Mehrzad J, Klein G, Kamphues J, Wolf P, Grabowski N, Schuberth HJ. 2011. In vitro effects of very low levels of aflatoxin B1 on free radical production and bactericidal activity of bovine blood neutrophils. Vet Immunol Immunopathol. 141:16–25.
- Mehrzad J, Milani M, Mahmoodi M. 2013. Naturally occurring level of mixed aflatoxins B and G stimulate toll-like receptor-4 in bovine mononuclear cells. Vet Quart. 33:186–190.
- Mehrzad J, Devriendt B, Baert K, Cox E. 2015. Aflatoxins type B and G affect porcine dendritic cell maturation in vitro. J Immunotoxicol. 12:194–198.
- Mohammadi A, Mehrzad J, Mahmudi M, Schneider M. 2014. Environmentally-relevant level of aflatoxin B1 dysregulates human dendritic cells through signaling. Int J Toxicol. 33:175–186.
- Morzadec C, Bouezzedine F, Macoch M, Fardel O, Vernhet L. 2012. Inorganic arsenic impairs proliferation and cytokine expression in human primary T-lymphocytes. Toxicology. 300:46–56.
- Nayak AS, Lage CR, Kim CH. 2007. Effects of low concentrations of arsenic on the innate immune system of the zebrafish (Danio rerio). Toxicol Sci. 98:118–124.
- Paape MJ, Mehrzad J, Zhao X, Detilleux J, Burvenich C. 2002. Defense of bovine mammary gland by polymorphonuclear neutrophil leukocytes. J Mammary Gland Biol Neoplasia. 7:109–121.
- Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45.
- Platzer B, Richter S, Kneidinger D, Waltenberger D, Woisetschläger M, Strobl H. 2009. Aryl hydrocarbon receptor activation inhibits in vitro differentiation of human monocytes and Langerhans dendritic cells. J Immunol. 183:66–74.
- Prince LR, Whyte MK, Sabroe I, Parker LC. 2011. The role of TLRs in neutrophil activation. Curr Opin Pharmacol. 11:397–403.
- Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. 2008. Control of Treg and TH17 cell differentiation by the aryl hydrocarbon receptor. Nature. 453:65–71.
- Reeves EP, Lu H, Jacobs HL, Messina CG, Bolsover S, Gabella G, Potma EO, Warley A, Roes J, Segal AW. 2002. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 416:291–297.
- Rojewski MT, Korper S, Thiel E, Schrezenmeier H. 2004. Arsenic trioxide-induced apoptosis is independent of CD95 in lymphatic cell lines. Oncol Rep. 11:509–513.
- Schulz H, Nagymajtenyi L, Institoris L, Papp A, Siroki O. 2002. A study on behavioral, neurotoxicological, and immunotoxicological effects of subchronic arsenic treatment in rats. J Toxicol Environ Health. 65:1181–1193.
- Taheri M, Mehrzad J, Mahmudy Gharaie MH, Afshari R, Dadsetan A, Hami S. 2016. High soil and groundwater arsenic levels induce high body arsenic loads, health risk and potential anemia for inhabitants of northeastern Iran. Environ Geochem Health 38:469–482.
- Takeuchi O, Akira S. 2010. Pattern recognition receptors and inflammation. Cell. 140:805–820.
- van Grevenynghe J, Rion S, Le Ferrec E, Le Vee M, Amiot L, Fauchet R, Fardel O. 2003. Polycyclic aromatic hydrocarbons inhibit differentiation of human monocytes into macrophages. J Immunol. 170:2374–2381.
- Veldhoen M, Hirota K, Westendorf AM, Buer J, Dumoutier L, Renauld JC, Stockinger B. 2008. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature. 453:106–109.
- Wagner C, Pioch M, Meyer C, Iking-Konert C, Andrassy K, Hänsch GM. 2000. Differentiation of polymorphonuclear in patients with systemic infections and chronic in inflammatory diseases: Evidence of prolonged life span and de novo synthesis of fibronectin. J Mol Med (Berlin). 78:337–345.
- Wang S, Mulligan C. 2006. Occurrence of arsenic contamination in Canada: Sources behavior and distribution. Sci Total Environ. 366:701–721.
- Wang F, Zhou X, Liu W, Sun X, Chen C, Hudson LG, Jian K. 2013. Arsenite-induced ROS/RNS generation causes zinc loss and inhibits activity of poly (ADP-ribose)-polymerase-1. Free Radic Biol Med. 61C:249–256.
- Wijeweera JB, Gandolfi AJ, Parrish A, Lantz RC. 2001. Sodium arsenite enhances AP-1 and NF-κB DNA binding and induces stress protein expression in precision-cut rat lung slices. Toxicol Sci. 61:283–294.
- Wu MM, Chiou HY, Wang TW, Hsueh YM, Wang IH, Chen CJ, Lee TC. 2001. Association of blood arsenic levels with increased reactive oxidants and decreased anti-oxidant capacity in a human population of northeastern Taiwan. Environ Health Perspect. 109:1011–1017.