Abstract
Background: This is the 27th Annual Report of the American Association of Poison Control Centers' (AAPCC) National Poison Data System (NPDS). As of 1 July 2009, 60 of the nation's 60 US poison centers (PCs) uploaded case data automatically. The upload time was 19.9 [9.7, 58.7] (median [25%, 75%]) minutes, creating a near real-time national exposure and information database and surveillance system.
Methodology: We analyzed the case data tabulating specific indices from NPDS. The methodology was similar to that of previous years. Where changes were introduced, the differences are identified. Poison center cases with medical outcomes of death were evaluated by a team of 29 medical and clinical toxicologist reviewers using an ordinal scale of 1-6 to determine Relative Contribution to Fatality (RCF) of the exposure to the death.
Results: In 2009, 4,280,391 calls were captured by NPDS: 2,479,355 closed human exposures, 116,408 animal exposures, 1,677,403 information calls, 6,882 human confirmed nonexposures, and 343 animal confirmed nonexposures. The top 5 substance classes most frequently involved in all human exposures were analgesics (11.7%), cosmetics/personal care products (7.7%), household cleaning substances (7.4%), sedatives/hypnotics/antipsychotics (5.8%), and foreign bodies/toys/miscellaneous (4.3%). Analgesic exposures as a class increased the most rapidly (12,494 calls per year) over the last decade. The top 5 most common exposures in children age 5 or less were cosmetics/personal care products (13.0%), analgesics (9.7%), household cleaning substances (9.3%), foreign bodies/toys/miscellaneous (7.0%), and topical preparations (6.8%). Drug identification requests comprised 63.0% of all information calls. NPDS documented 1,544 human exposures resulting in death with 1,158 human fatalities judged related with an RCF of 1-Undoubtedly responsible, 2-Probably responsible, or 3-Contributory.
Conclusions: Unintentional and intentional exposures continue to be a significant cause of morbidity and mortality in the US. The near real-time, always current status of NPDS represents a national public health resource to collect and monitor US exposure cases and information calls. The continuing mission of NPDS is to provide a nationwide infrastructure for public health surveillance for all types of exposures, public health event identification, resilience response and situational awareness tracking. NPDS is a model system for the nation and global public health.
Introduction
This is the 27th Annual Report of the American Association of Poison Control Centers' (AAPCC; http://www.aapcc.org) National Poison Data System (NPDS). Citation1 On 1 January 2009, Sixty-one regional Poison Centers (PCs) serving the entire population of the 50 United States, American Samoa, District of Columbia, Federated States of Micronesia, Guam, Puerto Rico, and the US Virgin Islands submitted information and exposure case data collected during the course of providing telephonic patient tailored exposure management. On 30 June 2009, The DeVos Children's Hospital Regional Poison Center, Grand Rapids, Michigan, serving upper Michigan ceased operation. Michigan is now served by one poison center. During this transition national coverage remained seamless. Data is compiled by the American Association of Poison Control Centers' (AAPCC) National Poison Data System (NPDS). PCs place emphasis on exposure management, accurate data collection and coding, and the continuing need for poison related public and professional education. The centers' health care professionals are available free of charge to all, 24-hours a day, every day of the year. PCs respond to questions from the public, health care professionals, and public health agencies. The continuous staff dedication at the regional PCs is manifest as the number of exposure and information calls exceeds 4.2 million annually. Calls to PCs either involve an exposed human or animal (EXPOSURE CALL) or a request for information (INFORMATION CALL) with no exposed person or animal.
WARNING: Comparison of exposure or outcome data from previous AAPCC Annual Reports is problematic. In particular, the identification of fatalities (attribution of a death to the exposure) differed from pre-2006 Annual Reports (see Fatality Case Review – Methods). Poison center death cases are described as all cases resulting in death and those determined to be exposure-related fatalities. Likewise, (Exposure Cases by Generic Category) since year 2006 restricts the breakdown including deaths to single-substance cases to improve precision and avoid misinterpretation.
What's New in NPDS and the Annual Report
Several enhancements were made to the tables and figures in this 27th Annual Report. Continuing goals of the writing team have been to remove inconsistencies, improve the reader's ability to clearly understand the data, and provide additional data where appropriate. Achievement of these goals has been exemplified by the new version of that was introduced with the 2006 Annual Report and last year's corrections for clarity to the age labels in all tables (described below). The improvements made to the tables and figures are listed below:
Tables
Five new , , , , and ) have been added to provide additional information on decontamination trends, change of substance exposure rates over time, pediatric exposures, information calls, and deaths. - Decontamination Trends: Total Human and Pediatric Exposures ≤5 Years (2009) provides decontamination trends for 2009 for all exposures and pediatric exposures. , and expand the series. - Substance Categories with the Greatest Rate of Exposure Increase (Top 25), identifies changes in the year over year rate of substances involved in exposures. - Substance Categories Most Frequently Involved in Pediatric (≤5 years) Deaths, provides information related to substances associated with pediatric deaths. - Substance Categories Most Frequently Identified in Drug Identification Calls (Top 25), provides information related to the substances that triggered drug identification (Drug ID) calls to the centers. - Comparisons of Death Data (2006-2009), provides a breakdown of deaths, suicides and pediatric deaths based on the source of the case, either death or death, indirect report. (Death cases that are directly reported to PCs are classified as direct cases and cases that are identified through other sources such as news feeds or medical examiner data, but did not manage nor answer any questions about are classified as death, indirect report.)
For 2009, the population served has been corrected for 2009 in - Growth of the AAPCC Population Served and Exposure Reporting (1983-2009) to include all 50 United States, American Samoa, District of Columbia, Federated States of Micronesia, Guam, Puerto Rico, and the US Virgin Islands. Citation2–6.
Table 1A. Growth of the AAPCC Population Served and Exposure Reporting (1983–2009)
This year, further enhancements for clarity were made to the age labels in the summary tables. All major age brackets were conformed to the following major categories: ≤5 years, 6 – 12 years, 13 – 19 years, ≥ 20 years, Unknown Adult, Unknown Child and Unknown Age. Last year the ≥ 20 years age bracket included ≥ 20 years and Unknown Adult and the Unknown Age bracket included Unknown Child and Unknown Age. Starting this year, these age brackets were reported separately for , , , , , and . Separation of the age brackets did not affect the total data reported. Also, it is important to note that NPDS age values may only be integers. So recorded age cannot be 5½ or 20½, they are recorded as 5 or 20 respectively.
Additional information has been added to 10 existing tables. Raw data counts were added to the existing percentages for and . For each route on a percentage of cases was provided in addition to the previously provided data. which displays the number of substances involved in human exposure cases has been extended to include the number of substances for fatality cases. The series has historically provided information related to the number of substances reported. A case may involve more than one substance and may, therefore, contribute to more than one count in the series tables reported previously. This year the series tables contain additional information related to those cases that involve only one substance. Both the raw counts for single-substance cases and the percentage of single-substance cases have been added to , , and .
This year, lists human exposures with medical outcome of death or death, indirect report regardless of RCF score. Cases are denoted by their Annual Report ID for ease of case retrieval. Pregnancy status is clearly indicated in the report layout although there were no reports of death involving a pregnant patient this year. The Fatality Review process continued with the new system introduced in 2007. However, the fatality case selection process for narrative publication excluded cases with medical outcome of death, indirect report for the last 2 years.
Additionally, the calculations and column headings in several tables have been enhanced this year. These table columns are defined as follows:
This table counts the number of human exposure cases that resulted in the outcome of death or death, indirect. This report indicates that there were 21 pediatric fatality cases where the substances were determined to have contributed to the death where the patient was ≤5 yrs old.
, , ,
All substances column – This column displays the number of substances that were related to any human exposure case regardless of the Relative Contribution to Fatality value. This was the only column in previous reports.
Single substance exposures column – This column, added this year, displays the number of human exposure cases that had one substance (one case, one substance) regardless of Relative Contribution to Fatality.
We have replaced the term All Mentions with the more descriptive term, All Substances, meaning each total category count includes every substance from every exposure that belongs in that category. Thus all substances are counted from every exposure in the 2009 database.
&
(new this year) reports the 25 categories which were increasing the most rapidly over the last 10 years. shows the linear regressions for the top 4 categories. As 17B provides the complimentary data to , we numbered it and renumbered 17B-D as 17C-E.
(new this year) reports the 25 most frequent categories of drugs identified in 2009.
All substances column - This column displays the number of substances that were related to any human exposure case that was determined to be undoubtedly responsible, probably responsible or contributory by the AAPCC review team. This was the only column in previous reports.
Single substance exposures column – This column (new this year) displays the number of human exposure cases that had one substance (one case, one substance) AND was determined to be undoubtedly responsible, probably responsible or contributory by the AAPCC fatality review team.
Although calculations have not changed since 2006, the column headings have changed:
Column 1: All Exposures displays the number of times the specific generic code was reported in all human exposure cases. If a human exposure case has multiple instances of a specific generic code it is only counted once.
Column 2: Single substance exposures column – This column was previously named ‘No. of Single Exposures’ and was renamed this year for clarity displays the number of human exposure cases that had one substance (one case, one substance). The succeeding columns (Age, Reason, Treatment Site, and Outcome) show selected detail from these single-substance exposure cases. Death cases are only those that had a relative contribution to fatality of 1-undoubtedly responsible, 2-probably responsible or 3-contributory.
Figures
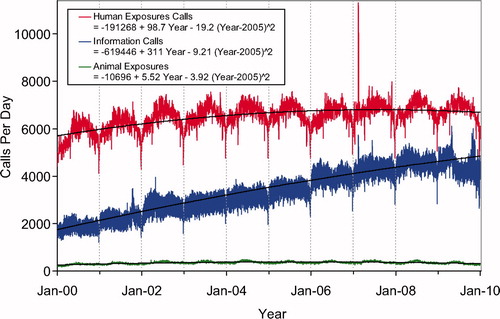
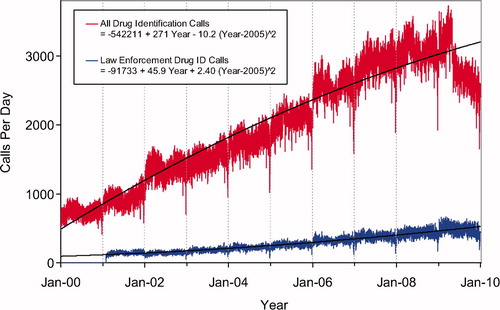
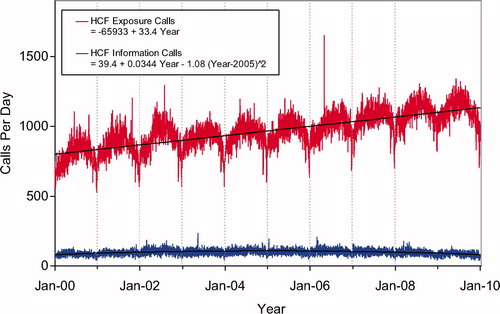
Second order (quadratic) least squares regression for 2000-2009 has been included and shows a statistically significant departure from linearity (declining rate of increase) for Human Exposure Calls, Information Calls, Animal Exposure Calls. All Drug ID Calls and Law Enforcement Drug ID Calls are increasing at an increasing rate ( and ). This year we added , a graphic summary and analyses of Health Care Facility (HCF) Exposure Calls (increasing linearly) and HCF Information Calls (rate is declining since 2005).
The NPDS Application
In 2009, numerous enhancements were introduced in the NPDS web-based application. Many of these focused on surveillance and are described in the Surveillance Section. Others were primarily focused on extending the enterprise reporting tools available for extracting and analyzing the data. Fourteen reports that provide system usage information were added. These reports identify a variety of information about the user community such as identifying the most popular enterprise reports.
The Case Log Reports were extended to include additional parameters including Clinical Effects. Results can now be displayed as aggregated counts, time series graphs (line and bar), US Map, or the traditional case listing. The user may also select the specific data that is included for each case in the usual case listing. Previously the NPDS reports were restricted to a single calendar year. Twenty-eight reports were modified to allow the user to run the report for any time period between 1 January 2000 and the current date. In order to assist the user with selecting the appropriate generic codes and/or product codes for a specific report, 2 new functions were added to the NPDS system. These functions display the generic codes and product codes in a classification tree hierarchy with major and minor generic code categories. This eliminates the need for the user to manually enter a list of generic or product codes and reduces typographical errors. Finally, a new data quality report was introduced that can be performed for an individual PC or nationally. The results of this report provide insight into how NPDS data quality can be maintained and improved. Additional information is also provided by the 2 new Case Log Counts reports. These reports allow the user to extract distributions for a user defined data item such as medical outcome. The user is allowed to define up to 16 different parameters to filter the cases for the result stratification desired. For example, a user may request case counts by medical outcome for exposure cases that involved female patients in their 30s.
NPDS aggregate and case detail web services operate continuously, allowing external systems or viewers to analyze NPDS data in ways not otherwise possible in the NPDS application. The aggregate web service provides total call volume, human exposure call volume, or clinical effects counts allowing an external system such as RODS (Real-time Outbreak and Disease Surveillance, University of Pittsburgh, Department of Biomedical Informatics) to create time-series. Unique to NPDS, the aggregate case count web service is not only accessible by external computer systems but also directly by system users to create their own time series without the need for external system software. Two state health departments utilize the case detail web service to analyze data from their PCs. Four state health departments access the aggregate count web service for data. The web services allow NPDS data to be provisioned in a federated manner where the data is always current in NPDS and can be readily accessed as needed without the need for costly cloning and warehousing.Citation7
Limitations and Plans
As outlined above, the exposure reports and information questions which comprise NPDS are collected from the spontaneous, self-reported calls made to the US PCs. These reflect the limitations of this type of reporting system (see DISCLAIMER). The current AAPCC generic code system categorizes combination products in most cases by their active ingredients and tables are ordered by these groupings. Thus our current review and reporting methods do not distinguish between the individual components of a combination product.
Nonetheless the scope and immediacy of these data have much to offer. In particular the 27 year history offers a unique opportunity to assess the long term (secular) trends in exposures and information calls.
There are a number of plans to improve the data system and reporting for 2009 and beyond including:
Enhancements to NPDS Real-time geographic information system (GIS) with more data display options for appropriate data analyses;
Improvements in data quality edits;
Security paradigm enhancements to support product specific product access for reports and surveillance;
Aggregate enterprise report modifications to span multiple years or parts of years;
Enterprise report enhancements;
New auto-upload requirements and improved solution;
Lexicon based analysis of the current generic code system to better meet current exposure tracking and surveillance needs.
These and other initiatives are under continuous review by the AAPCC Board, NPDS Steering Committee, and CDC.
Methods
Characterization of Participating Poison Centers (PCs)
61 participating centers submitted data to AAPCC through 30 June 2009, with the total center count decreasing to 60 for the remainder of 2009. Fifty-seven centers (95%) were accredited by AAPCC as of 1 July 2009. The entire population of the 50 states, American Samoa, the District of Columbia, Federated States of Micronesia, Guam, Puerto Rico, and the US Virgin Islands was served by the US PC network in 2009.
The average number of human exposure cases managed per day by all US PCs was 6,793. Similar to other years, higher volumes were observed in the warmer months, with a mean of 7,118 cases per day in June compared with 6,584 per day in January. On average, US PCs received a call about a suspected or actual human exposure every 12.7 seconds.
Call Management – Specialized Poison Exposure Emergency Providers
Most PC operations management, clinical education, and instruction are directed by Managing Directors (most are PharmDs and RNs with American Board of Applied Toxicology [ABAT] board certification). Medical direction is provided by Medical Directors who are board-certified physician medical toxicologists. At some PCs, the Managing and Medical Director positions are held by the same person.
Calls received at US PCs are managed by healthcare professionals who have received specialized training in toxicology and managing exposure emergencies. These providers include medical and clinical toxicologists, registered nurses, doctors of pharmacy, pharmacists, chemists, hazardous materials specialists, and epidemiologists. Specialists in Poison Information (SPIs) are primarily registered nurses, PharmDs, and pharmacists. They work under the supervision of a Certified Specialist in Poison Information (CSPI). SPIs must log a minimum of 2,000 calls over a 12 month period to become eligible to take the CSPI examination for certification in poison information. Poison Information Providers (PIPs) are allied healthcare professionals. They manage information-type and low acuity (non-hospital) calls and work under the supervision of a CSPI. Of note is the fact that no nursing or pharmacy school offers a toxicology curriculum designed for PC work and SPIs must be trained in programs offered by their respective PC. Centers are accredited by the AAPCC meeting strict standards and must be reaccredited every 5 years.
NPDS – Near Real-time Data Capture
Launched on 12 April 2006, NPDS is the data repository for all of the US regional PCs. In 2009, 61 of the 61 US PCs uploaded case data automatically to NPDS through 30 June 2009. The center count decreased to 60 as of 30 June 2009. All centers submitted data in near real-time making NPDS one of the few operational systems of its kind. PC staff record calls contemporaneously in 1 of 4 case management systems. Each center uploads case data periodically as it is entered. The time to upload data for all PCs is 19.9 [9.7, 58.7] (median [25%, 75%]) minutes creating a real-time national exposure database and surveillance system.
The web-based NPDS software facilitates querying, reporting and a myriad of surveillance uses allowing AAPCC, its member centers and public health agencies to utilize US exposure data. Users are able to access local and regional data for their own areas and view national aggregate data. The application allows for increased “drill-down” capability and mapping via a geographic information system (GIS). Custom surveillance definitions are available along with ad hoc reporting tools. Information in the NPDS database is dynamic. Each year the database is locked prior to extraction of annual report data to prevent inadvertent changes and ensure consistent, reproducible reports. The 2009 database was locked on 1 October 2010 at 1700 hours EDT.
Annual Report Case Inclusion Criteria
The information in this report reflects only those cases that are not duplicates and classified by the regional PC as CLOSED. A case is closed when the PC has determined that no further follow-up/recommendations are required or no further information is available. Exposure cases are followed to obtain the most precise medical outcome possible. Depending on the case specifics, most calls are “closed” within the first hours of the initial call. Some calls regarding complex hospitalized patients or cases resulting in death may remain open for weeks or months while data continues to be collected. Follow-up calls provide a proven mechanism for monitoring the appropriateness of management recommendations, augmenting patient guidelines and providing poison prevention education, enabling continual updates of case information as well as obtaining final/known medical outcome status to make the data collected as accurate and complete as possible.
Statistical Methods
All tables except the new were generated directly by the NPDS web-based application and can thus be reproduced by any AAPCC member. The Figures and statistics were created using SAS JMP version 6.0.0 (SAS Institute, Cary, NC).
NPDS Surveillance
As previously noted, all of the active US PCs upload case data automatically to NPDS. This unique near real-time upload is the foundation of the NPDS surveillance system. This makes possible both spatial and temporal case volume and case based surveillance. NPDS software allows creation of volume and case based definitions. Definitions can be applied to national, regional, state, or ZIP code coverage areas. Geocentric definitions can also be created. This functionality is available not only to the AAPCC surveillance team, but to every regional PC. PCs also have the ability to share NPDS real-time surveillance technology with external organizations such as their state and local health departments or other regulatory agencies. Another NPDS feature is the ability to generate system alerts on adverse drug events and other products of public health interest like contaminated food or product recalls. NPDS can thus provide real-time adverse event monitoring and surveillance for resilience response and situational awareness.
Surveillance definitions can be created to monitor a variety of volume parameters, any desired substance or commercial product in the product database (Micromedex® Healthcare Series [Internet database]. Greenwood Village, CO: Thomson Reuters [Healthcare] Inc.). The database contains over 360,000 entries, or case based definitions using a variety of mathematical options and historical baseline periods from 1 to 10 years. NPDS surveillance tools include:
Volume Alerts Surveillance Definitions
Total Call Volume
Human Exposure Call Volume
Clinical Effects Volume (signs and symptoms, or laboratory abnormalities)
Case Based Surveillance Definitions
Substance
Clinical Effects
Various NPDS data fields linked in Boolean expressions
Incoming data is monitored continuously and anomalous signals generate an automated email alert to the AAPCC's surveillance team or designated regional PC or public health agency. These anomaly alerts are reviewed daily by the AAPCC surveillance team and/or the regional PC that created the surveillance definition. When reports of potential public health significance are detected, additional information is obtained via e-mail or phone from reporting PCs. The regional PC then alerts their respective affected state or local health departments. Public health issues are brought to the attention of the National Center for Environmental Health – Health Studies Branch at the Centers for Disease Control and Prevention (CDC). This unique near real-time tracking ability is a unique feature offered by NPDS and the regional PCs.
AAPCC Surveillance Team clinical and medical toxicologists review surveillance definitions on a regular basis to fine-tune the queries. CDC, as well as State and local health departments with NPDS access as granted by their respective regional PCs, also have the ability to create surveillance definitions to respond to emerging public health events.
Fatality Case Review and Abstract Selection
NPDS fatality cases can be recorded as either DEATH or DEATH, INDIRECT REPORT. Medical outcome of death is by direct report. Death, indirect report are deaths that the PC acquired from medical examiners or media, but did not manage nor answer any questions about the death.
Although PCs may report DEATH as an outcome, the death may not be the direct result of the exposure. We define exposure-related fatality as a death judged by the AAPCC Fatality Review Team to be at least contributory to the exposure. The definitions used for the Relative Contribution to Fatality (RCF) classification are defined in Appendix B and the methods to select abstracts for publications is described in Appendix C. For details of the AAPCC fatality review process, see the 2008 annual report.1
Results
2009 Case Summary
In 2009, the participating PCs logged 4,280,391 total cases including 2,479,355 closed human exposure cases (), 116,408 animal exposures (), 1,677,403 information calls (), 6,882 human confirmed nonexposures, and 343 animal confirmed nonexposures. An additional 1,990 calls were still open at the time of database lock. The cumulative AAPCC database now contains nearly 51 million human exposure case records (). A total of 13,010,466 information calls have been logged by NPDS since the year 2000.
shows the human exposures, information calls and animal exposures by day since 2000. Second order (quadratic) least squares regression for 2000-2009 has shown a statistically significant departure from linearity (declining rate of calls since mid-2007) for Human Exposure Calls. Information Calls are showing a declining rate of increase and Animal Exposure Calls have been declining since mid-2005.
Table 1B. Non-Human Exposures by Animal Type
Table 1C. Distribution of Information Calls
A hallmark of PC case management is the use of follow-up calls to monitor case progress and medical outcome. US PCs made 2,814,502 follow-up calls in 2009. Follow-up calls were done in 44.4% of human exposure cases. One follow-up call was made in 21.7% of human exposure cases, and multiple follow-up calls (range 2-128) were placed in 22.7% of cases.
Information Calls to Poison Centers (PCs)
Data from 1,677,403 information calls to PCs in 2009 () was transmitted to NPDS, including calls in optional reporting categories such as prevention/safety/education (33,989), administrative (24,508) and caller referral (72,323).
Second order (quadratic) least squares regression for All Drug ID Calls also showed a declining rate of increase for these calls, whereas, Law Enforcement Drug ID Calls are increasing at an increasing rate (). The most frequent information call was for Drug ID, comprising 1,057,632 calls to PCs during the year. Of these, 126,869 (12.0%) could not be identified over the telephone. The majority of the Drug ID calls were received from the public, followed by law enforcement and health professionals. Most of the Drug ID requests from the public and law enforcement were regarding drugs sometimes involved in abuse; however, these cases were categorized based on the drug's abuse potential without knowledge of whether abuse was actually intended.
Drug information calls increased 4.3% from 2008 (230,084 calls) to 2009 (239,943) and comprised 14.3% of all information request calls. Of these, the most common requests were in regards to therapeutic use and indications, followed by drug-drug interactions, questions about dosage and inquiries of adverse effects. Environmental inquiries comprised 1.6% of all information calls. Of these environmental inquiries, questions related to cleanup of mercury (thermometers and other) remains the most common followed by questions involving pesticides.
Of all the information calls, poison information comprised 4.6% of the requests with inquiries involving general toxicity the most common followed by questions involving food preparation practices, plant toxicity and safe use of household products.
Exposure Calls to Poison Centers (PCs)
shows a graphic summary and analyses of Health Care Facility (HCF) Exposure and HCF Information calls. HCF Exposure Calls did not depart from linearity (continued to increase at a steady rate) while the rate of HCF Information Calls has been declining since early 2005. This linearly increasing use of the PCs for the more serious exposures (HCF calls) is important in the face of the declining growth of all exposure and information calls. The 2 May 2006, exposure data spike on the figure was the result of 602 children in a Midwest school reporting a noxious odor which caused anxiety, but resolved without sequalae.
Fig. 3. Health Care Facility (HCF) Exposure Calls and HCF Information Calls by Day since 1 January 2000. Black lines show least-squares first and second order regressions – linear regression for HCF Exposure Calls (second order term was not statistically significant) and second order regression for HCF Information Calls. All terms shown were statistically significant for each of the 2 regressions.
(Nonpharmaceuticals) and (Pharmaceuticals) provide summary demographic data on patient age, reason for exposure, medical outcome, and use of a health care facility for all 2,479,355 human exposure cases, presented by substance categories.
Column 1: All Exposures displays the number of times the specific generic code was reported in all human exposure cases. If a human exposure case has multiple instances of a specific generic code it is only counted once.
Column 2: Single substance exposures column – This column was previously named ‘No. of Single Exposures’ and was renamed this year for clarity displays the number of human exposure cases that had one substance (one case, one substance). The succeeding columns (Age, Reason, Treatment Site, and Outcome) show selected detail from these single-substance exposure cases. Death cases are only those that had a relative contribution to fatality of 1-undoubtedly responsible, 2-probably responsible or 3-contributory.
and restrict the breakdown columns to single-substance cases. Prior to 2007, when multi-substance exposures were included, a relatively innocuous substance was mentioned in a death column when, for example, the death was attributed to an antidepressant, opioid, or cyanide. This subtlety was not always appreciated by the user of this table. The restriction of the breakdowns to single-substance exposures should increase precision and reduce misrepresentation of the results in this unique by-substance table. Single substance cases reflect the majority (90.4%) of all exposures ().
and tabulate 2,849,086 substance-exposures, of which 2,241,191 were single-substance exposures including 1,176,304 (52.5%) nonpharmaceuticals and 1,064,887 (47.5%) pharmaceuticals.
In 16.8% of single-substance exposures that involved pharmaceutical substances, the reason for exposure was intentional, compared to only 3.2% when the exposure involved a nonpharmaceutical substance. Correspondingly, treatment in a health care facility was provided in a higher percentage of exposures that involved pharmaceutical substances (26.5%) compared with nonpharmaceutical substances (14.0%). Exposures to pharmaceuticals also had more severe outcomes. Of single-substance exposure-related fatal cases, 497 were pharmaceuticals compared with 221 nonpharmaceuticals.
Age and Gender Distributions
The age and gender distribution of human exposures is outlined in . Children younger than 3 years were involved in 38.9% of exposures and children younger than 6 years accounted for just over half of all human exposures (51.9%). A male predominance is found among cases involving children younger than 13 years, but this gender distribution is reversed in teenagers and adults, with females comprising the majority of reported exposures.
Caller Site and Exposure Site
As shown in , of the 2,479,355 human exposures reported, 75.8% of calls originated from a residence (own or other) but 93.8% actually occurred at a residence (own or other). Another 16.4% of calls were made from a health care facility. Exposures occurred in the workplace in 1.5% of cases, schools (1.3%), health care facilities (0.3%), and restaurants or food services (0.2%).
Table 2. Site of Call and Site of Exposure, Human Exposure Cases
Table 3. Age and Gender Distribution of Human Exposures
Exposures in Pregnancy
Exposure during pregnancy occurred in 8,005 women (0.32% of all human exposures). Of those with known pregnancy duration (n = 7,302), 31.5% occurred in the first trimester, 38.0% in the second trimester, and 30.4% in the third trimester. Most (72.6%) were unintentional exposures and 20.3% were intentional exposures. There were no deaths involving pregnant females in 2009.
Multiple Patients
In 2009, 9.5% (234,811) of human exposures involved multiple patients. Examples of these include siblings sharing found medication, multiple victims of carbon monoxide exposure such as a family, or multiple patients inhaling vapors at a hazardous material spill.
Chronicity
Most human exposures, 2,239,950 (90.3%) were acute cases (single, repeated or continuous exposure occurring over 8 hours or less) compared to 801 acute cases of 1,544 fatalities (51.9%). Chronic exposures (continuous or repeated exposures occurring over > 8 hours) comprised 1.9% (47,118) of all human exposures. Acute-on-chronic exposures (single exposure that was preceded by a continuous, repeated, or intermittent exposure occurring over a period greater than eight hours) numbered 167,047 (6.7%).
Reason for Exposure
The reason for most human exposures was unintentional (82.4%); unintentional general reason code was reported in 59% of all exposures (). Unintentional misuse comprised 5.1% of all exposures. Therapeutic errors accounted for 11.2% of exposures. Of the total 276,694 therapeutic errors, the most common scenarios for all ages included: inadvertent double-dosing in (31.4%) cases, wrong medication taken or give (14.7%), other incorrect dose (13.7%), doses given/taken too close together (9.6%) and inadvertent exposure to someone else's medication (9.0%). The types of therapeutic errors observed are different for each age group and are summarized in .
Table 4. Distribution of Agea and Gender for Fatalitiesb
Table 5. Number of Substances Involved in Human Exposure Cases
Table 6A. Reason for Human Exposure Cases
Table 6B. Scenarios for Therapeutic Errors by Agea
Intentional exposures accounted for 13.9% of human exposures. Suicidal intent was suspected in 8.9% of cases, intentional misuse in 2.3% and intentional abuse in 1.9%. Unintentional exposures outnumbered intentional exposures in all age groups with the exception of age 13-19 years (). Intentional exposures were reported as frequently as unintentional exposures in patients aged 13-19 years. In contrast, of the 1,158 reported fatalities, the majority reason reported for children ≤5 years was unintentional while most fatalities in adults (≥ 20 years) were intentional ().
Table 7. Distribution of Reason for Exposure by Age
Table 8. Distribution of Reason for Exposure and Age for Fatalitiesa
Table 9. Route of Exposure for Human Exposure Cases
Route of Exposure
Ingestion was the route of exposure in 83.9% of cases (Table 9), followed in frequency by dermal (7.25%), inhalation/nasal (5.35%), and ocular routes (4.5%). For the 1,158 exposure-related fatalities, ingestion (85.1%), inhalation/nasal (8.8%), and parenteral (3.5%) were the predominant exposure routes.
Clinical Effects
The NPDS database allows for the coding of up to 131 different clinical effects (signs, symptoms, or laboratory abnormalities) for each case. Each clinical effect can be further defined as related, not related, or unknown if related. Clinical effects were coded in 849,516 (34.3%) cases. (17.6% had 1 effect, 8.9% had 2 effects, 4.6% had 3 effects, 1.8% had 4 effects, 0.7% had 5 effects, and 0.7% had >5 effects coded). Of clinical effects coded, 79.7% were deemed related to the exposure(s), 9.1% were considered not related, and 11.2% were coded as unknown if related.
The duration of effect is required for all cases that report at least one clinical effect and have a medical outcome of minor, moderate or major effect (n = 455,084). demonstrates an increasing duration of the clinical effects observed with more severe outcomes.
Case Management Site
The majority of cases reported to PCs were managed in a non–health care facility (72.5%), usually at the site of exposure, primarily the patient's own residence (). Another 1.8% of cases were referred to a health care facility but refused to go. Treatment in a health care facility was rendered in 24.1% of cases.
Table 10. Management Site of Human Exposures
Of the 597,787 cases managed in a health care facility, 291,545 (48.8%) were treated and released, 95,429 (16.0%) were admitted for critical care, and 60,122 (10.1%) were admitted for noncritical care.
The percentage of patients treated in a health care facility varied considerably with age. Only 11.2% of children ≤5 years or younger and only 13.2% of children between 6 and 12 years were managed in a health care facility compared with 22.1% of teenagers (13-19 years) and 40.2% of adults (age ≥20 years).
Medical Outcome
displays the medical outcome of the human exposure cases distributed by age, showing a greater incidence of severe outcomes in the older age groups. compares medical outcome and reason for exposure and shows a greater frequency of serious outcomes in intentional exposures.
Table 11. Medical Outcome of Human Exposure Cases by Patient Agea
Table 12. Medical Outcome by Reason: Human Exposuresa
Decontamination Procedures and Specific Antidotes
and outline the use of decontamination procedures, specific antidotes, and measures to enhance elimination in the treatment of patients reported in the NPSD database. These must be interpreted as minimum frequencies because of the limitations of telephone data gathering.
Ipecac-induced emesis for poisoning continues to decline as shown in and . Ipecac was administered in only 330 (<0.01%) pediatric exposures in 2009. The continued decrease in ipecac syrup use in the last decade was likely a result of ipecac use guidelines issued in 1997 by the American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists and updated in 2004.Citation8 , Citation9 In a separate report, the American Academy of Pediatrics concluded not only that ipecac should no longer be used routinely as a home treatment strategy, but also recommended disposal of ipecac currently in homes.Citation10
Table 13. Duration of Clinical Effects by Medical Outcome
Table 14. Decontamination and Therapeutic Interventions
Table 15. Therapy Provided in Human Exposures by Age
Table 16A. Decontamination Trends (1985–2009)
Table 16B. Decontamination Trends: Total Human and Pediatric Exposures ≤5 Years (2009)a
Top Substances in Human Exposures
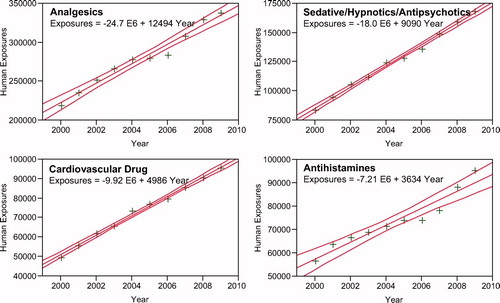
presents the most common 25 substance categories, listed by frequency of human exposure. This ranking provides an indication where we might focus public health resources on prevention, as well as the types of exposures PCs frequently manage. It is relevant to know whether exposures to these substances are increasing or decreasing. We examined exposures per year over the last decade for the change over time for each of the 67 major generic categories via least squares linear regression. The calls per year were increasing for 42 and decreasing for 25 of the 67 categories. The change over time for the 10 yearly values was statistically significant (p < 0.05) for 52 of the 67 categories. shows the 25 categories which were increasing the most rapidly. Statistical significance of the 25 regressions can be verified by noting the 95% confidence interval on the rate of increase excludes zero. shows the linear regressions for the top 4 increasing categories in .
and present exposure results for children and adults, respectively, and show the differences between substance categories involved in pediatric and adult exposures.
(new this year) reports the 25 categories of substances most frequently involved in pediatric (≤5 years) fatalities in 2009.
(new this year) reports the 25 Drug ID categories most frequently identified in 2009. The most often identified drug category is miscellaneous and unknown – this category includes medications which could not be identified and for which no answer recorded (null response). The null response is permitted because the product identified is not a required field for Information Calls. Drug ID information is of value to AAPCC, public health, public safety, and regulatory agencies. Internet based resources do not allow data capture nor do they afford the caller the ability to speak with a specialist in poison information if the inquiry is more than a drug identification question. Proper resources to continue this vital public service are essential, especially since the top 10 substance categories include antibiotics as well as drugs with widespread use and abuse potential such as opioids and benzodiazepines.
Distribution of Suicides
shows the modest variation in the distribution of suicides over the past 2 decades as reported to the NPDS national database (45-68%). Since 1985, the percent of fatal cases has increased from 0.036% to 0.070% and the percent of pediatric cases has decreased from 6.1% to 2.4%.
Plant Exposures
provides the number of times the specific plant occurred in NPDS. The 25 most commonly involved plant species and categories account for 24,344 of a total number of plants that were reported (58,933). The top 3 categories in the table are essentially synonymous for unknown plant and comprise 14.3% (8,427/58,933) of all plant exposures. For a variety of reasons it was not possible to make a precise identification in these 3 groups. The top 5 most frequent plant exposures where a positive plant identification was made were (descending order): Spathiphyllum spp. Not otherwise specified (NOS), Phytolacca americana (L.), Toxicodendron radicans (L.), Philodendron spp. (NOS), and Ilex spp. (NOS).
Table 17A. Substance Categories Most Frequently Involved in Human Exposures (Top 25)
Table 17B. Substance Categories with the Greatest Rate of Exposure Increase (Top 25)
Table 17C. Substance Categories Most Frequently Involved in Pediatric (≤5 years) Exposures (Top 25)a
Table 17D. Substance Categories Most Frequently Involved in Adult (≥20 years) Exposures (Top 25)a
Table 17E. Substance Categories Most Frequently Involved in Pediatric (≤5 years) Deathsa
Table 17F. Substance Categories Most Frequently Identified in Drug Identification Calls (Top 25)
Table 18. Categories Associated with Largest Number of Fatalities (Top 25)a
Table 19A. Comparisons of Death Data (1985–2009)a
Table 19B. Comparisons of Death Data (2006–2009)a
Table 20. Frequency of Plant Exposures (Top 25)
Table 21. Listing of fatal nonpharmaceutical and pharmaceutical exposures
Table 22A. Demographic profile of SINGLE-SUBSTANCE nonpharmaceuticals exposure cases by generic category
Table 22B. Demographic profile of Single-Substance pharmaceuticals exposure cases by generic category
Deaths and Exposure-related Fatalities
A listing of cases () and summary of cases (, , , , , , , , and ) are provided for fatal cases for which there exists reasonable confidence that the death was a result of that exposure (exposure-related fatalities). Of the 1,544 cases initially reviewed, 1,158 were judged exposure-related fatalities (Relative Contribution to Fatality Category = 1-Undoubtedly responsible, 2-Probably responsible, or 3-Contributory). The remaining 386 cases were judged as follows: 62: RCF = 4-Probably not responsible, 45: 5-Clearly not responsible, and 279: RCF = 6-Unknown.
Deaths are sorted in according to the category, patient age and substance deemed most likely responsible for the death (Substance Rank). The Cause Rank permits the PC to judge 2 or more substances as indistinguishable in terms of cause, e.g., 2 substances which appear equally likely to have caused the death could have Substance Rank of 1,2 and Cause Rank of 1,1. Additional agents implicated are listed below the primary agent in the order of their contribution to the fatality.
As shown in , a single substance was implicated in 90.4% of reported human exposures, and 9.6% of patients were exposed to 2 or more drugs or products. The exposure-related fatalities involved a single substance in 491 cases (42.4%), 2 substances in 275 cases (23.7%), 3 in 167 cases (14.4%), and 4 or more in the balance of the cases (). The cross-references at the end of each major category section list all cases that identify this substance as other than the primary substance.
The Annual Report ID number [bracketed] indicates that the abstract for that case is included in Appendix C. The letters following the Annual Report ID number include: i = death, indirect report after the fatality occurred in 46 cases (4.0%), p = prehospital cardiac and/or respiratory arrest in 402 (34.7%), h = hospital records reviewed in 223 cases (19.3%), a = autopsy report reviewed in 325 cases (28.1%).
The distribution of NPDS RCF was: 1 = Undoubtedly responsible in 518 cases (44.7%), 2 = Probably responsible in 461 cases (39.8%), 3 = Contributory in 179 cases (15.5%).
All fatalities – all ages
presents the age and gender distribution for these 1,158 exposure-related fatalities. The age distribution of reported fatalities is similar to that in past years with 93.2% (1,079 of 1,158) of fatal cases occurring in adults (age > 19 years). Although children younger than 6 years were involved in the majority of exposures, the 21 fatalities comprised just 1.8% of the exposure-related fatalities. Most (70.9%) of the fatalities occurred in 20-to 59-year-old individuals.
lists each of the 1,158 human fatalities along with all of the substances involved. Please note: the Substance listed in column 3 of was chosen to be the most specific generic name based upon the substances entered for that case. This Substance name may not agree with the AAPCC generic categories used in the summary tables (including ).
lists the top 25 substance categories associated with reported fatalities and the number of single substance exposure fatalities for that category – sedative/hypnotics/antipsychotics, cardiovascular drugs, opioids, and acetaminophen in combination products, lead this list followed by antidepressants, acetaminophen alone, alcohols, and stimulants/street drugs. Although sedative/hypnotics/antipsychotics ranks 4th and antidepressants 7th among the most frequent exposures (), there is otherwise little concordance between the frequency of exposures to a substance and the number of fatalities. Note that summarizes all substances to which a patient was exposed (i.e., a patient exposed to an opioid may have also been exposed to 1 or more other products).
The first ranked substance was a pharmaceutical in 925 of the 1,158 fatalities (79.9%). These 925 first ranked pharmaceuticals included:
441 analgesics (100 acetaminophen, 62 acetaminophen/hydrocodone, 49 methadone, 37 salicylate);
123 cardiovascular drugs (18 cardiac glycoside, 18 verapamil, 14 amlodipine, 12 diltiazem, 11 metoprolol, 8 diltiazem (extended release));
91 antidepressants (20 amitriptyline, 8 doxepin, 7 bupropion, 7 bupropion (extended release);
79 sedative/hypnotic/antipsychotics (20 quetiapine, 11 alprazolam, 6 diazepam, 6 zolpidem);
65 stimulants/street drugs (22 heroin, 16 cocaine, 12 methamphetamine, 4 MDMA)
The exposure was acute in 621 (53.7%), A/C = acute on chronic in 250 (21.6%), C = chronic exposure in 77 (6.6%) and U = unknown in 210 (18.1%).
A total of 3,375 tissue concentrations for 1 or more related analytes were reported in 457 cases (39.5%).
Route of exposure was: Ingestion in 985 cases (79.0%), inhalation/nasal in 102 cases (7.9%), parenteral in 24 cases (2.1%). ()
Intentional exposure reasons: Suspected suicide in 652 cases (56.3%), Intentional-Abuse in 97 cases (17.4%), Intentional-Misuse in 38 cases (3.3%).
Unintentional exposure reasons: Environmental in 37 cases (3.2%), Therapeutic error in 24 cases (2.1%), Misuse in 14 cases 1.2%). ()
Acetaminophen/Propoxyphene Fatalities
The current AAPCC generic code system categorizes combination products in most cases by their active ingredients and tables ordered by these groupings. Our current review and reporting methods do not distinguish between the individual components of a combination product. To better understand this issue, an independent team of fatality medical toxicologists reviewed one group of fatality cases (acetaminophen/propoxyphene combination products) as a pilot.
Eight fatalities involved acetaminophen/propoxyphene only – age ranged from 19 to 74 (median 43) year, 5 were female, 6 of the calls were from health care facilities, relative contribution to fatality (RCF) determined by the initial contributor was 1 for 3 cases, 2 for 3 cases, and 3 for 2 cases. An independent team of medical toxicologists (DH Jang and LS Nelson) reviewed these cases for the relative contribution of acetaminophen and propoxyphene to the fatality. Their determination of the relative contribution to fatality (RCF) based on the reports was 1 for 1 case, 2 for 1 case, and 3 for 2 cases, 4 or 6 for 4 cases. In none of the cases was acetaminophen clearly the cause of death. In 6 of 8 cases other causes were judged more likely than propoxyphene to have caused the death (propoxyphene was contributory or not involved).
Pediatric fatalities – age ≤5 years
Although children younger than 6 years were involved in the majority of exposures, they comprised 37 of 1,158 (2.4%) of fatalities. These numbers are similar to those reported since 1985 (). The percentage fatalities in children ≤5 years related to total pediatric exposures was 21/1,290,784 = 0.00163%. By comparison, 1,076/853,039 = 0.126% of all adult exposures involved a fatality. Of these 21 pediatric fatalities, 18 (85.7%) were reported as unintentional and 1 (4.7%) were coded as resulting from malicious intent (). These 21 cases included 7 pharmaceuticals and 14 nonpharmaceuticals. The first ranked substances associated with these fatalities included: disc battery (4 cases); methadone and smoke (3 cases each); kerosene (2 cases) and 10 other substances (1 each).
Pediatric fatalities – ages 6-12 years
In the age range 6 to 12 years, there were 10 reported fatalities (), 6 of which were unintentional exposures and 2 intentional exposures. These 10 cases included 3 pharmaceuticals and 7 nonpharmaceuticals. The first ranked substances associated with these fatalities included: carbon monoxide or smoke inhalation (5 cases); air freshener aerosol, benzonatate, isopropranol, quetiapine, senna (1 each).
Adolescent fatalities – ages 13-19 years
In the age range 13 to 19 years, there were 48 reported fatalities (). These 48 cases included 42 pharmaceuticals and 6 nonpharmaceuticals. The first ranked pharmaceuticals associated with these fatalities included: methadone (5 cases); acetaminophen and acetaminophen/diphenhydramine (3 cases each); carbon monoxide, heroin, MDMA, opioid, oxycodone, and unknown drug (2 each) and 23 other substances (1 each).
Of the 48 reported fatalities for adolescents, 32 (66.7%) were suspected suicides, and 7 (14.6%) were intentional abuse exposures (). The suspected suicide percentage is higher than in recent years. The percentage of intentional abuse cases is lower than in recent years (25.8% in 2006 to 35.7% in 2008). As in the past years, only a small number (2 of 48) of adolescent fatalities were coded as being unintentional.
Pregnancy and fatalities
A total of 26 deaths of pregnant women have been reported from the years 2000 through 2008. An average of 2 deaths per year was recorded from 2000 through 2004. Since 2005, the average number of deaths in pregnant women reported to NPDS doubled to 4 per year. The majority (20 of 26) were intentional exposures of misuse, abuse and suspected suicide. There were no deaths in pregnant women reported to NPDS in 2009.
AAPCC Surveillance Results
A key component of the NPDS surveillance system is the variety of monitoring tools available to the NPDS user community. In addition to AAPCC national surveillance definitions, 37 regional PCs utilize NPDS as part of their surveillance programs. Eleven state health departments have been given NPDS access by their regional centers. Since Surveillance Anomaly 1, generated at 2:00 pm EDT on 17 September 2006, over 151,000 anomalies have been detected. More than 600 were confirmed as being of public health significance with regional PCs working collaboratively with their local and state health departments and in some instances CDC on the public health issues identified.
At the time of this report, 378 surveillance definitions run continuously, monitoring case and clinical effects volume and a variety of case based definitions from food poisoning to nerve agents. These definitions represent the surveillance work by many regional PCs, state health departments, the AAPCC, and Health Studies Branch, Division of Environmental Hazards and Health Effects, National Center for Environmental Health, Centers for Disease Control and Prevention.
In 2009, the NPDS surveillance application module underwent incremental improvements. These included analysis process enhancements such as clinical effect analysis notes simplification. Surveillance reporting enhancements were also implemented in 2009. More enhancements were scheduled for 2010 including: information call surveillance, animal exposure call surveillance, updates to anomaly status tracking and case based time series reports.
Automated surveillance continues to remain controversial as a viable methodology to detect the index case of a public health event. Uniform evaluation algorithms are not available to determine the optimal methodologies.Citation11 Less controversial is the benefit to situational awareness that NPDS can provide. Typical NPDS surveillance data detects a response to an event rather than event prediction. This aids in situational awareness and resilience during and after a public health event.
On Saturday, 25 April 2009, the Director-General of WHO declared the 2009 H1N1 outbreak a Public Health Emergency of International Concern and recommended that countries intensify surveillance for unusual outbreaks of influenza-like illness and severe pneumonia. Cases were suspected in New York City, Ohio and Kansas.Citation12 The US followed with declaration of a nationwide public health emergency on 26 April 2009. Calls were also coming into US PCs. Several centers had activated Public Health Emergency lines to specifically manage questions from the public. On 1 May 2009 and again on 20 August 2009, AAPCC and Micromedex® activated two H1N1 vaccine product codes (Inactivated and Live, attenuated) in the Poisindex® products database. Earlier in 2009, on 27 April a general product code for the H1N1 virus was activated. These codes allowed PCs to track exposure and medical information calls related to the Pandemic.
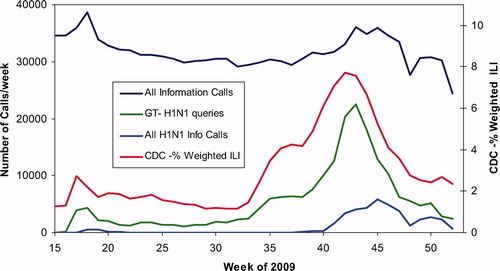
shows a by-week graph of All Information Call data for weeks 15-52 of 2009 showing an unusual “dip” between week 18 and week 43. The bottom line shows All H1N1 Info Calls, i.e., calls where the product was H1N1, the live H1N1 Vaccine, or the H1N1. The red curve shows the weighted % of cases of Influenza Like Illness (ILI) data from CDC.12 The green curve Google Trends for H1N1 over the same interval (relative scale only).Citation13 The similarity in these profiles illustrates the importance of public access to PCs in times of public health events. US PCs represent the only 24/7 365 system always open where anyone can speak to a health care professional at no charge. This data demonstrates how the public utilizes PCs in times of crisis. This unique system can be supported and enhanced to serve as a national public health hotline providing information and management beyond traditional poison exposure calls.Citation12
Fig. 4. All Information Calls, H1N1 Information Calls, CDC Influenza-Like Illness, Google H1N1 queries by Week since 6-Apr-2009. All Information Calls and All H1N1 Calls/week are shown on the left axis, patients with influenza-like illness (ILI) reported to CDC are shown on the right axis. Google Trends for H1N1 is not a scaled variable and is shown at a convenience scale.
Discussion
The exposure cases and information requests reported by PCs in 2009 do not reflect the full extent of PC efforts which also include activities such as poison prevention and education.
NPDS exposure data may be considered as providing “numerator data”, but we lack a true denominator, that is, we do not know the number of actual exposures that occur in the population. NPDS data covers only those exposures which are reported to PCs.
Depending on the source, the numbers may be different, but NPDS regression analyses confirms that all analgesic exposures including opioids and sedatives are increasing year after year. This trend is shown in and . NPDS data mirrors CDC data that demonstrates similar findings.Citation14 Thus NPDS provides a real-time view of these public health issues without the need for data source extrapolations.
Fig. 5. Human Exposure Calls By Year 2000–2009 – Top 4 Categories. Solid lines show least-squares linear regressions for the Human Exposure Calls per year for that category (+). Broken lines show 95% confidence interval on the regression.
One of the limitations of NPDS data has been the perceived lack of fatality case volume compared to other reporting sources. However, when change over time is studied, NPDS is clearly consistent with other public health analyses. One of the issues leading to this problem is the fact that medical record systems seldom have common output streams. This is particularly apparent with the various electronic medical record systems available. It is important to build a federated approach similar to the one modeled by NDPS to allow data sharing, for example, between hospital emergency departments and other medical record systems including medical examiner offices nationwide. Enhancements to NPDS can promote interoperability between NPDS and electronic medical records systems to better trend poison-related morbidity and mortality in the US and internationally.
Summary
Salient findings from this 27th Annual Report include:
The number of human exposure calls in 2009 was less than 2008 and the second order least squares regression for 2000-2009 calls by day departed from linearity (declining rate since mid 2007);
Both total information calls and drug information calls in 2009 were greater than 2008, but the second order least squares regression for 2000-2009 calls by day departed from linearity (declining rate of increase);
Drug identification calls show a distinct drop in late 2009 and the most frequently identified drug is miscellaneous and unknown – it is important to AAPCC to continue providing and improving this important public health service;
HCF information calls have been declining since 2005, but HCF exposure calls are continuing to increase linearly since 2000, suggesting PC use in the more severe cases continues to increase.
Unintentional and intentional exposures continue to be a significant cause of morbidity and mortality in the US. The near real-time, always current status of NPDS represents a national public health resource to collect and monitor US exposure cases and information calls. The continuing mission of NPDS is to provide a nationwide infrastructure for public health surveillance for all types of exposures, public health event identification, resilience response and situational awareness tracking. NPDS is a model system for the nation and global public health.
Disclaimer
The American Association of Poison Control Centers (AAPCC; http://www.aapcc.org) maintains the national database of information logged by the country's regional Poison Centers (PCs) serving all 50 United States, Puerto Rico and the District of Columbia. Case records in this database are from self-reported calls: they reflect only information providedwhen the public or healthcare professionals report an actual or potential exposure to a substance (e.g., an ingestion, inhalation, or topical exposure, etc.), or request information/educational materials. Exposures do not necessarily represent a poisoning or overdose. The AAPCC is not able to completely verify the accuracy of every report made to member centers. Additional exposures may go unreported to PCs and data referenced from the AAPCC should not be construed to represent the complete incidence of national exposures to any substance(s).
References
- National Poison Data System: Annual reports 1983-2008 [Internet]. Alexandria (VA): American Association of Poison Control Centers; [cited 2010 Oct 1]. Available from: http://www.aapcc.org/dnn/NPDSPoisonData/AnnualReports/tabid/125/Default.aspx
- Annual Estimates of the Resident Population for the United States, Regions, States, and Puerto Rico: April 1, 2000 to July 1, 2009 (NST-EST 2009-01). Source: U.S. Census Bureau, Population Division, Release Date: December 2009.
- US Census Bureau: International Data Base (IDB) 2009 Midyear population estimate American Samoa [Internet]. Washington (DC): US Census Bureau; [cited 2010 Oct 20] Available from: http://www.census.gov/ipc/www/idb/country.php
- US Census Bureau: International Data Base (IDB) 2009 Midyear population estimate Guam [Internet]. Washington (DC): US Census Bureau; [cited 2010 Oct 20] Available from: http://www.census.gov/ipc/www/idb/country.php
- US Census Bureau: International Data Base (IDB) 2009 Midyear population estimate Micronesia, Federated States of [Internet]. Washington (DC): US Census Bureau; [cited 2010 Oct 20] Available from: http://www.census.gov/ipc/www/idb/country.php
- US Census Bureau: International Data Base (IDB) 2009 Midyear population estimate Virgin Islands, US. [Internet]. Washington (DC): US Census Bureau; [cited 2010 Oct 20] Available from: http://www.census.gov/ipc/www/idb/country.php
- Savel TG, Bronstein A, Duck, M, Rhodes MB, Lee, B, Stinn J, Worthen, K. Using Secure Web Services to Visualize Poison Center Data for Nationwide Biosurveillance: A Case Study [Internet]. Online Journal of Public Health Informatics 2010; 2: 1–9; [cited 2010 Nov 1]. Available from: http://ojphi.org/htbin/cgiwrap/bin/ojs/index.php/ojphi/article/view/2920/2505
- Position statement: ipecac syrup. American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. J Toxicol Clin Toxicol. 1997; 35:699–709.
- American Academy of Clinical Toxicology European Association of Poisons Centres and Clinical Toxicologists. Position Paper: Ipecac Syrup. J Toxicol Clin Toxicol 2004; 42: 133–143.
- American Academy of Pediatrics Policy Statement. Poison treatment in the home. Pediatrics 2003; 112:1182–1185.
- Sosin DM, DeThomasis J. Evaluation challenges for syndromic surveillance–making incremental progress. MMWR Morb Mortal Wkly Rep. 2004; 53 Suppl: 125–129.
- Centers for Disease Control and Prevention: 2009 H1N1 Flu U.S. Situation Update: percentage of visits for influenza-like-illness reported by sentinel providers 2009-2010 [Internet]. Atlanta (GA): Centers for Disease Control and Prevention; [cited 2010 Oct 28]. Available from: http://www.cdc.gov/flu/weekly/weeklyarchives2009-2010/data/senAllregt52.htm
- Google trends: H1N1 2009 [Internet]. Mountain View (CA): Google, Inc.; [cited 2010 Oct 25] Available from: http://www.google.com/trends?q = h1n1&ctab = 0&geo = us&geor = all&date = 2009
- Centers for Disease Control and Prevention. QuickStats: Number of Poisoning Deaths* Involving Opioid Analgesics and Other Drugs or Substances — United States, 1999—2007. MMWR 2010; 59:1026.
- McGraw-Hill's AccessMedicine, Laboratory Values of Clinical Importance (Appendix), Harrison's Principles of Internal Medicine 17e. McGraw-Hill Professional; 2008[cited 2010 Nov 1]. Available from: http://www.accessmedicine.com/
- Goldfrank's Toxicologic Emergencies, Eighth Edition>, McGraw-Hill Companies, 2006.
- Dart RC, editor. Medical Toxicology, Third Edition, Philadelphia, Lippincott, Williams & Wilkins, 2004.
Appendix A – Acknowledgments
The compilation of the data presented in this report was supported in part through the U.S. Centers for Disease Control AAPCC Contract 200-2007-19121.
The authors wish to express their appreciation to the following individuals who assisted in the preparation of the manuscript: Kathy W. Worthen, Laura J. Rivers, Carol L. Hesse, and Denise A. Martinez.
The authors express their sincere gratitude to Jim Hirt, AAPCC Executive Director and the staff at the AAPCC Central Office for their support during the preparation of the manuscript.
Thanks to David H. Jang, MD, and Lewis S. Nelson, MD, New York University School of Medicine, New York, NY for the thoughtful review of the acetaminophen/propoxyphene fatalities.
Poison Centers (PCs)
We gratefully acknowledge the extensive contributions of each participating PC and the assistance of the many health care providers who provided comprehensive data to the PCs for inclusion in this database. We especially acknowledge the dedicated efforts of the Specialists in Poison Information (SPIs) who meticulously coded 4,280,391 calls made to US PCs in 2009.
As in previous years, the initial review of reported fatalities and development of the abstracts was the responsibility of the staff of the participating PCs. These PCs and individuals are listed at the beginning of this report.
Many individuals at each center participate in the review of their centers fatality cases. The following toxicology professionals summarized and prepared their center's fatality data for NPDS:
Alabama Poison Center
Perry Lovely, MD, ACMT
John Fisher, PharmD, DABAT, FAACT
Lois Dorough BSN, RN, CSPI
Arizona Poison and Drug Information Center
Keith Boesen, PharmD, CSPI
F. Mazda Shirazi, MS, MD, PhD, FACEP
Jude McNally RPh, DABAT
Leslie Boyer MD, FACMT
Arkansas Poison & Drug Information Center
Henry F. Simmons, Jr., MD
Pamala R. Rossi, PharmD
Howell Foster, PharmD
Banner Good Samaritan Poison and Drug Information Center
Daniel Brooks, MD
Mary-Ann Calley, RN, CSPI
Jane Klemens, RN, CSPI
Sharyn Welch, RN
Blue Ridge Poison Center
Christopher Holstege, MD
Nathan Charlton, MD
David Lawrence, MD
Brigid Wonderly, RN
Stephen Dobmeier, RN
California Poison Control System – Fresno/Madera Division
Richard J. Geller, MD, MPH
California Poison Control System – Sacramento Division
Timothy Albertson, MD, PhD
Judith Alsop, PharmD
California Poison Control System – San Diego Division
Richard F. Clark, MD
Lee Cantrell, PharmD
Shaun Carstairs, MD
Sean Nordt, MD, PharmD
Allyson Kreshak, MD
Alicia Minns, MD
Joshua Nogar, MD
California Poison Control System – San Francisco
Kent R. Olson, MD
Thanjira Jiranantakan, MD
Susan Kim, PharmD
Alan Tani, PharmD
Raymond Ho, PharmD
Kathryn Meier, PharmD
Sandra Hayashi, PharmD
Tanya Mamantov, MD
Derrick Lung, MD
Patil Armenian, MD
Carolinas Poison Center
Michael C. Beuhler, MD
Marsha Ford, MD
William Kerns II, MD
Anna Rouse Dulaney, PharmD
Central Ohio Poison Center
Marcel J. Casavant, MD, FACEP, FACMT
Heath Jolliff, DO, FACEP, FAAEM
S. David Baker, PharmD, DABAT
Julee Fuller-Pyle
Julie Lecky, BA
Central Texas Poison Center
Ryan Morrissey, MD
Vikhyat Bebarta, MD
Douglas J. Borys, PharmD, DABAT
Children's Hospital of MI Regional Poison Center
Cynthia Aaron, MD
Lydia Baltarowich, MD
Keenan Bora, MD
Bram Dolcourt, MD
Matthew Hedge, MD, ACMT
Susan C. Smolinske, PharmD
Suzanne R. White, MD
Cincinnati Drug and Poison Information Center
G. Randall Bond, MD
Rachel Sweeney, RN
Connecticut Poison Center
Charles McKay, MD
Kathy Hart MD
Jarrett Lefberg, DO
Kevin O'Toole, MD
Bernard C. Sangalli, MS
Florida/USVI Poison Information Center – Jacksonville
Thomas Kunisaki, MD, FACEP, ACMT
Florida Poison Information Center – Miami
Jeffrey N. Bernstein, MD
Richard S. Weisman, PharmD
Florida Poison Information Center – Tampa
Cynthia R. Lewis-Younger, MD, MPH
Pam Eubank, RN, CSPI
Judy Gussett, RN, CSPI
Judy Turner, RN, CSPI
Georgia Poison Center
Robert Geller, MD
Brent W. Morgan, MD
Arthur Chang, MD
Ziad Kazzi, MD
Gaylord P. Lopez, PharmD
Stephanie Hon, PharmD
Andre Matthews, MD
Soumya Pandalai, MD
SaraJane Reedy, MD
Rizwan Riyaz, MD
Hennepin Regional Poison Center
David J. Roberts MD
Elisabeth F. Bilden MD
Deborah L. Anderson PharmD
Samuel J. Stellpflug, MD
Jon B. Cole, MD
Illinois Poison Center
Michael Wahl, MD
Sean Bryant, MD
Indiana Poison Center
James B. Mowry, PharmD
R. Brent Furbee, MD
Iowa Statewide Poison Control Center
Sue Ringling, RN
Edward Bottei, MD
Kentucky Regional Poison Center
George M. Bosse, MD
Henry A. Spiller, MS, RN, DABAT, FAACT
Long Island Regional Poison and Drug Information Center
Thomas R. Caraccio, PharmD, DABAT
Michael McGuigan, MDCM, MBA
Louisiana Poison Center
Mark Ryan, PharmD
Thomas Arnold, MD
Maryland Poison Center
Suzanne Doyon, MD, FACMT
Patrick Dougherty, PharmD
Mississippi Poison Control Center
Robert Cox MD, PhD, DABT, FACMT
Tanya Calcote, RN, CSPI
Missouri Poison Center at SSM Cardinal Glennon
Children's Medical Center
Anthony Scalzo, MD
Shelly Enders, PharmD, CSPI
National Capital Poison Center
Cathleen Clancy, MD, FACMT
Nicole Whitaker, RN, BA, BSN, MEd, CSPI
Nebraska Regional Poison Center
Claudia Barthold, MD
Ronald I. Kirschner, MD
New Jersey Poison Information and Education System
John Kashani, DO
Steven M. Marcus, MD
New Mexico Poison and Drug Information Center
Steven A. Seifert, MD, FAACT, FACMT
New York City Poison Control Center
Maria Mercurio-Zappala, MS, RPh
Robert S. Hoffman, MD
Lewis Nelson, MD
Alex Manini, MD
Silas Smith, MD
Oladapo Odujebe, MD
Jen Prosser, MD
Brenna Farmer, MD
Samara Soghoian, MD
North Texas Poison Center
Brett Roth MD, ACMT, FACMT
Northern Ohio Poison Center
Lawrence S. Quang, MD
Alfred Aleguas, PharmD
Gerald Maloney, MD
Northern New England Poison Center
Theresa Maher, MFA
Tamas Peredy, MD
Oklahoma Poison Control Center
William Banner, Jr., MD, PhD, ABMT
Lee McGoodwin, PharmD, MS, DABAT
Oregon Poison Center
Zane Horowitz, MD
Sandra L. Giffin, RN, MS
Palmetto Poison Center
William H. Richardson, MD
Jill E. Michels, PharmD
Pittsburgh Poison Center
Kenneth D. Katz, MD
Rita Mrvos, BSN
Edward P. Krenzelok, PharmD
Puerto Rico Poison Center
José Eric Díaz-Alcalá, MD
Regional Center for Poison Control and Prevention
Serving Massachusetts and Rhode Island
Michele Burns Ewald, MD
David Kemmerer, RN
Nilam Patil, MD
Kishan Kapadia, MD
Alfred Aleguas, PharmD
Regional Poison Control Center – Children's Hospital of Alabama
Diane Smith, RN, CSPI
Valoree Stanley, RN, CSPI
Erica Liebelt, MD, FACMT
Michele Nichols, MD
Rocky Mountain Poison & Drug Center
Carol L. Hesse, RN, CSPI
Alvin C. Bronstein, MD, FACEP, FACMT
Ryan Chuang, MD
Andrew Monte, MD
Shan Yin, MD, MPH
Amy Zosel, MD
Denise Martinez
South Texas Poison Center
Douglas Cobb, RPh
Cynthia Abbott-Teter, PharmD
Miguel C. Fernandez, MD
George Layton, MD
C. Lizette Villarreal, MA
Southeast Texas Poison Center
Wayne R. Snodgrass, MD, PhD, FACMT
Jon D. Thompson, MS, DABAT
Jean L. Cleary, PharmD, CSPI
Tennessee Poison Center
John G. Benitez, MD, MPH
Saralyn Williams, MD
Donna Seger, MD
Texas Panhandle Poison Center
Shu Shum, MD
Jeanie E. Jaramillo, PharmD
Cristie Johnston, RN, CSPI
The Poison Control Center at the Children's Hospital of Philadelphia
Allison A. Muller, PharmD
Kevin Osterhoudt, MD
The Ruth A. Lawrence Poison and Drug Information Center
Ruth A. Lawrence, MD
Norma Barton, BS Pharm, CSPI
Timothy J. Wiegand, MD
University of Kansas Hospital Poison Control Center
Tama Sawyer, PharmD
Upstate NY Poison Center
Jeanna M. Marraffa, PharmD
Jamie Nelsen, PharmD
Christine M. Stork, PharmD
Utah Poison Control Center
Martin Caravati, MD, MPH
Virginia Poison Center
Rutherfoord Rose, PharmD
Kirk Cumpston, DO
Brandon Wills, DO
Washington Poison Center
William T. Hurley, MD, FACEP
David Serafin, CPIP
West Texas Regional Poison Center
John F. Haynes, Jr., MD, ABEM, ABMT
Stephen W. Borron, MD, MS, FACEP,
FACMT Leo Artalejo III, PharmD
Hector L. Rivera, RPh
West Virginia Poison Center
Lynn F. Durback-Morris RN, BSN, MBA, DABAT
(Posthumous Recognition)
Anthony F. Pizon, MD, ABMT
Western New York Poison Center
Prashant Joshi, MD
Ashley N. Webb, PharmD, MSc
Wisconsin Poison Center
David D. Gummin, MD
Lori Rohde, SPI, Poison Center Supervisor
Fatality Review Team
The Lead and Peer review of the 2009 fatalities was carried out by the 29 individuals listed here. The authors and the AAPCC wish to express our appreciation for their volunteerism, dedication, hard work and good will in completing this task in a limited time.
Alfred Aleguas Jr., PharmD, DABAT, Managing Director, Northern Ohio Poison Center, Cleveland, OH
Anna M. Rouse*, PharmD, DABAT, Assistant Director, Education, Carolinas Poison Center
Ann-Jeannette Geib, MD, Assistant Professor of Emergency Medicine, UMDNJ-Robert Wood Johnson Medical School, New Brunswick NJ
Bernard C. Sangalli*, MS, DABAT, Director, Connecticut Poison Center
Charles McKay, MD, Associate Medical Director, Connecticut Poison Control Center, University of Connecticut School of Medicine
Cynthia R. Lewis-Younger, MD, MPH, Managing/Medical Director, Florida Poison Information Center-Tampa
Daniel E. Brooks*, MD, Department of Medical Toxicology, Banner Good Samaritan Medical Center, Phoenix
David Gummin, MD, Wisconsin Poison Center, Milwaukee, WI
Elizabeth J. Scharman, PharmD, DABAT, BCPS, FAACT, Director West Virginia Poison Center
Heath Jolliff, DO, Central Ohio Poison Center, Columbus, Ohio
Henry Spiller, MS, DABAT, FAACT, Kentucky Regional Poison Center, Louisville, KY
Howell Foster, PharmD, DABAT, Arkansas Poison & Drug Information Center
Jill E. Michels, PharmD, DABAT, Managing Director, Palmetto Poison Center, SC
Judith A. Alsop, PharmD, DABAT, California Poison Control System - Sacramento Division
Karen E. Simone, PharmD, DABAT, Director, Northern New England Poison Center, Maine Medical Center
Kathryn Meier, PharmD, DABAT, Senior Toxicology Management Specialist, California Poison Control System, San Francisco Division
Lewis Nelson, MD, New York City Poison Center, New York University School of Medicine
Luke Yip*, MD, FACMT, FACEM, FACEP, Rocky Mountain Poison and Drug Center, Denver, CO
Lynn Durback-Morris**, RN, MBA, DABAT, CSPI, Supervisor of Operations, West Virginia Poison Center
Maria Mercurio-Zappala, RPh, MS, DABAT, FAACT Managing Director, NYC Poison Control Center
Mark Su, MD, FACEP, FACMT, Assistant Professor of Emergency Medicine, Director, Fellowship in Medical Toxicology, Department of Emergency Medicine, North Shore University Hospital, Manhasset, NY
Michael Levine, MD, Department of Medical Toxicology, Banner Good Samaritan Medical Center, Phoenix
Richard J. Geller*, MD, MPH, FACP, Medical and Managing Director, California Poison Control System, Fresno/Madera
Robert B. Palmer, PhD, DABAT, Toxicology Associates, Denver, CO; Rocky Mountain Poison and Drug Center, Denver, CO
S. David Baker, PharmD, DABAT, Managing Director, Central Ohio Poison Center
Susan C. Smolinske, PharmD, DABAT, Children's Hospital of Michigan Regional Poison Control Center, Detroit
Tammi Schaeffer, DO, Rocky Mountain Poison & Drug Center, Denver, CO
Tim Wiegand, MD; Director, Ruth A. Lawrence -Finger Lakes Poison and Drug Information Center, Rochester, NY
William T. Hurley, MD, Medical Director, Washington Poison Center
*These reviewers further volunteered to read the top ranked 200 abstracts and judged to publish or omit.
**Ms. Lynn F. Durback-Morris passed away in October 2010. The Fatality Review Team and the AAPCC recognize Ms. Durback-Morris for her years of selfless service to the fatality review process and to poison centers and clinical toxicology.
AAPCC Surveillance Team
NPDS surveillance anomalies are analyzed daily by a team of 10 medical and clinical toxicologists working across the country in a distributed system. These dedicated professionals interface with the National Center for Environmental Health – Health Studies Branch at the Centers for Disease Control and Prevention (CDC) and the PCs on a regular basis to identify anomalies of public health significance and improve NPDS surveillance systems:
Alfred Aleguas, Pharm D, DABAT
S. David Baker, PharmD, DABAT
Alvin C. Bronstein, MD, FACEP, FACMT
Blaine (Jess) Benson, PharmD, DABAT
Douglas J. Borys, PharmD, DABAT
John Fisher, PharmD, DABAT, FAACT
Jeanna M. Marraffa, PharmD, DABAT
Maria Mercurio-Zappala, RPH, MS, DABAT, FAACT
Henry A. Spiller, MS, DABAT, FAACT
Richard G. Thomas, Pharm D, DABAT
Regional Poison Center (PC) Fatality Awards
Each year the AAPCC and the Fatality Review team recognized several regional PCs for their extra effort in their preparation of fatality reports and prompt responses to reviewer queries during the review process. The awards were presented at the October 2010, North American Congress of Clinical Toxicology meeting in Denver, CO
First Center to Complete all Cases, 1-Dec-2009 South Texas Poison Center (San Antonio)
Highest Percentage with Autopsy Reports, 100% of 2 cases Western New York Poison Center (Buffalo),
Largest Number Autopsy Reports, 31 autopsies of 43 fatalities Kentucky Regional Poison Center (Louisville)
Highest Overall Quality of Reports, 11.0 of possible 14 for their 2 fatalities Western New York Poison Center (Buffalo)
Most Abstracts Published in 2008 Annual report – a tie with 9 each California Poison Control System (San Francisco) Carolinas Poison Center (Charlotte)
Outstanding Case Preparation New York City Poison Control Center (New York)
Most Helpful Regional Poison Center Staff – a tie Hennepin Regional Poison Center (Minneapolis) Children's Hospital of Michigan Regional Poison Control Center (Detroit)
APPENDIX B – Data Definitions
Reason for Exposure
NPDS classifies all calls as either EXPOSURE (concern about an exposure to a substance) or INFORMATION (no exposed human or animal). A call may provide information about one or more exposed person or animal (receptors).
Specialists in Poison Information (SPIs) coded the reasons for exposure reported by callers to PCs according to the following definitions:
Unintentional general: All unintentional exposures not otherwise defined below.
Environmental: Any passive, non-occupational exposure that results from contamination of air, water, or soil. Environmental exposures are usually caused by manmade contaminants.
Occupational: An exposure that occurs as a direct result of the person being on the job or in the workplace.
Therapeutic error: An unintentional deviation from a proper therapeutic regimen that results in the wrong dose, incorrect route of administration, administration to the wrong person, or administration of the wrong substance. Only exposures to medications or products used as medications are included. Drug interactions resulting from unintentional administration of drugs or foods which are known to interact are also included.
Unintentional misuse: Unintentional improper or incorrect use of a nonpharmaceutical substance. Unintentional misuse differs from intentional misuse in that the exposure was unplanned or not foreseen by the patient.
Bite/sting: All animal bites and stings, with or without envenomation, are included.
Food poisoning: Suspected or confirmed food poisoning; ingestion of food contaminated with microorganisms is included.
Unintentional unknown: An exposure determined to be unintentional, but the exact reason is unknown.
Suspected suicidal: An exposure resulting from the inappropriate use of a substance for reasons that are suspected to be self-destructive or manipulative.
Intentional misuse: An exposure resulting from the intentional improper or incorrect use.
Medical Outcome
No effect: The patient did not develop any signs or symptoms as a result of the exposure.
Minor effect: The patient developed some signs or symptoms as a result of the exposure, but they were minimally bothersome and generally resolved rapidly with no residual disability or disfigurement. A minor effect is often limited to the skin or mucus membranes (e.g., self-limited gastrointestinal symptoms, drowsiness, skin irritation, first-degree dermal burn, sinus tachycardia without hypotension, and transient cough).
Moderate effect: The patient exhibited signs or symptoms as a result of the exposure that were more pronounced, more prolonged, or more systemic in nature than minor symptoms. Usually, some form of treatment is indicated. Symptoms were not life-threatening, and the patient had no residual disability or disfigurement (e.g., corneal abrasion, acid-base disturbance, high fever, disorientation, hypotension that is rapidly responsive to treatment, and isolated brief seizures that respond readily to treatment).
Major effect: The patient exhibited signs or symptoms as a result of the exposure that were life-threatening or resulted in significant residual disability or disfigurement (e.g., repeated seizures or status epilepticus, respiratory compromise requiring intubation, ventricular tachycardia with hypotension, cardiac or respiratory arrest, esophageal stricture, and disseminated intravascular coagulation).
Death: The patient died as a result of the exposure or as a direct complication of the exposure.
Not followed, judged as nontoxic exposure: No follow-up calls were made to determine the outcome of the exposure because the substance implicated was nontoxic, the amount implicated was insignificant, or the route of exposure was unlikely to result in a clinical effect.
Not followed, minimal clinical effects possible: No follow-up calls were made to determine the patient's outcome because the exposure was likely to result in only minimal toxicity of a trivial nature. (The patient was expected to experience no more than a minor effect.).
Unable to follow, judged as a potentially toxic exposure: The patient was lost to follow-up, refused follow-up, or was not followed, but the exposure was significant and may have resulted in a moderate, major, or fatal outcome. Unrelated effect: The exposure was probably not responsible for the effect.
Confirmed nonexposure: This outcome option was coded to designate cases where there was reliable and objective evidence that an exposure initially believed to have occurred actually never occurred (e.g., all missing pills are later located). All cases coded as confirmed nonexposure are excluded from this report.
Death, indirect report: Death, indirect report are deaths that the poison center acquired from medical examiner or media, but did not manage nor answer any questions about the death.
Relative Contribution to Fatality (RCF)
The definitions used for the Relative Contribution to Fatality (RCF) classification were as follows:
Undoubtedly responsible - In the opinion of the CRT the Clinical Case Evidence establishes beyond a reasonable doubt that the SUBSTANCES actually caused the death.
Probably responsible - In the opinion of the CRT the Clinical Case Evidence suggests that the SUBSTANCES caused the death, but some reasonable doubt remained.
Contributory – In the opinion of the CRT the Clinical Case Evidence establishes that the SUBSTANCES contributed to the death, but did not solely cause the death. That is, the SUBSTANCES alone would not have caused the death, but combined with other factors, were partially responsible for the death.
Probably not responsible - In the opinion of the CRT the Clinical Case Evidence establishes to a reasonable probability, but not conclusively, that the SUBSTANCES associated with the death did not cause the death
Clearly not responsible - In the opinion of the CRT the Clinical Case Evidence establishes beyond a reasonable doubt that the SUBSTANCES did not cause this death.
Unknown - In the opinion of the CRT the Clinical Case Evidence is insufficient to impute or refute a causative relationship for the SUBSTANCES in this death.
Appendix C – Abstracts of Selected Cases
Selection of Abstracts for Publication
The abstracts included in Appendix C were selected for publication in a 3-stage process consisting of qualifying, ranking and reading. Qualifying was based on the RCF – only RCF = 1-Undoubtedly Responsible, 2-Probably Responsible or 3-Contributory were eligible for publication. Fatalities by Indirect report were excluded beginning with the 2008 annual report. Ranking was based on the number of substances (1/N) and weighted case score. The case weighting factors were the averages chosen based on review team recommendations in 2006. Each case score was multiplied by the respective factors to obtain a weighted publication score: Hospital records *4.4 + Postmortem *7.6 + Blood levels *6.9 + Quality/Completeness *6.4 + Novelty/Educational value * 6.0. Scores were normalized (z-score) within each reviewer before the final weighting: 33% for 1/N and 67% for weighted case scores.
The top ranked abstracts (200 + ties) were each read by individual reviewers (See Appendix A) and the 2 managers (Cantilena and Spyker). Each reader judged each abstract as “publish” or “omit” and all abstracts receiving 4 or more publish votes were selected, further edited and cross-reviewed by the 2 managers.
Abstracts
Abstracts of the cases were selected (see Selection of Abstracts for Publication, above) from the human fatalities judged related to be an exposure as reported to US PCs in 2009. A structured format for abstracts was required in the PC preparation of the abstracts and was used in the abstracts presented. Abbreviations, units and normal ranges omitted from the abstracts are given at the end of this appendix
Case 13. Acute methanol ingestion: undoubtedly responsible.
Scenario/Substances: A 44 y/o female drank windshield wiper fluid containing methanol, told her parents she was not feeling well, and had a witnessed seizure and cardiac arrest, and presented to the ED
Past Medical History: depression, ethanol abuse including non-beverage alcohol, pancreatitis.
Physical Exam: Unresponsive, pupils fixed and dilated, BP 92/45, HR 93, RR 26, T 32.6°C.
Laboratory Data: ABG-pH 6.78/pCO2 41/pO2 308/HCO36
WBC 16.2, Hgb 11.1, Platelets 231, AST 200, ALT 116, Alk phos 128 IU/L, bilirubin: 0.2, INR 1.1, PTT 70.4, fibrinogen 250, lactate 14.8 mmol/L, troponin 0.37, UDS positive for hydroxyzine and cetirizine. Methanol 269 mg/dl; ethanol, ethylene glycol, propylene glycol, acetaminophen and salicylate were not detected.
Clinical Course: The patient was initially in asystole, but was resuscitated after ∼20 min of CPR. IV fomepizole was administered. She was given sodium bicarbonate and started on a dopamine drip. The patient was then transferred to a tertiary facility for hemodialysis. On arrival her methanol level was 269 mg/dL, she was started on dialysis and her methanol level gradually decreased to 14 mg/dL over 24 hr. IV fomepizole was administered every 4 hr while on dialysis. She received therapeutic hypothermia. After re-warming, she was determined to be brain dead. Comfort measures were instituted and she expired 48 hr post-admission.
Autopsy Findings: No autopsy performed.
Case 17. Chronic methanol ingestion: undoubtedly responsible.
Scenario/Substances: A 47 y/o male drank methanol that he obtained from work, became ataxic and confused before going to sleep. He was found comatose and transported to the ED
Past Medical History: Alcoholism
Physical Exam: Altered mental status, intubated patient.
Laboratory Data: ABG-pH 6.72/pCO2 165/pO2 58.
3 ½ hr later: ABG-pH 7.13/pCO2 68/p O2 56. SGOT 89, SGPT 72, lactic acid 10.6 mg/dL, ammonia 501 mcg/dL. Methanol > 200 mg/dL (initial); then 66 mg/dL post dialysis.
Clinical Course: The patient arrived intubated, was given fomepizole and leucovorin. Dialysis initiated within a few hours of arrival; lactulose was given for hyperammonemia. Pressors administered for hypotension. Day 2 pupils were fixed and dilated; fomepizole, leucovorin and lactulose continued. Day 3 CT scan showed cerebellar and cerebral edema with global hypoxia. He expired on Day 4.
Autopsy Findings: Not available.
Case 53. Acute ethylene glycol ingestion: undoubtedly responsible.Scenario/Substances: A 53 y/o male found unresponsive in a hotel room with a suicide note and a ¾ full bottle of antifreeze beside him. EMS found the patient unresponsive with cold and mottled extremities. Intubation performed in the field.
Past Medical History: Not provided.
Physical Exam: In ED, BP 224/101, HR 101, 35.1°C, pupils were normal.
Laboratory Data: Ethylene glycol 37.4 mg/dL, anion gap 37,
![]()
osmolar gap 73, UDS negative, WBC 38.5; ethanol and acetaminophen not detected.
Clinical Course: Fomepizole 15 mg/kg, thiamine and sodium bicarbonate were given and hemodialysis initiated within 2.5 hr of ED arrival; post hemodialysis pH 7.28, HCO3 15. Norepinephrine was given for hypotension. Fomepizole and hemodialysis continued for 2 days. An ethylene glycol level at the end of the 3rd 4-hr hemodialysis run was 18 mg/dL. Day 4, he remained unresponsive, a head CT done at the time revealed extensive injury to the brainstem, basal ganglia, and left occipital lobe. Comfort measures were instituted and he expired on Day 6.
Autopsy Findings: Not available.
Case 54. Ethylene glycol, methadone, and alprazolam ingestion: undoubtedly responsibleScenario/Substances: A 55 y/o female was heard making sonorous respirations while sleeping the night before being discovered in cardiopulmonary arrest.
Past Medical History: Diabetes mellitus, drug abuse (methamphetamine and methadone).
Physical Exam: Cardiopulmonary arrest; Post-resuscitation: Anisocoria, unresponsive, BP 113/60, HR 108.
Laboratory Data: ABG-pH 6.8/pCO2 66/p O2 517, Ca 9,
lactic acid 10.6 mmol/L, ethylene glycol 50 mg/dL; methanol, acetaminophen and salicylate not detected. UDS positive for methadone and benzodiazepines.
Clinical Course: The patient received fomepizole for suspected toxic alcohol ingestion which was discontinued on Day 2 when ethylene glycol was no longer detected. On Day 3, no neurological improvement was seen, brain death was determined and the patient was taken to the operating room for organ harvesting.
Autopsy Findings: Death was attributed to complication of a polysubstance overdose with ethylene glycol, methadone and alprazolam as the major contributors. Postmortem analysis of serum obtained at hospital admission showed methadone 134 ng/mL and alprazolam 40.8 ng/mL.
Case 60. Acute disc battery ingestion: undoubtedly responsible.Scenario/Substances: A 2 ½ y/o male ingested a watch battery and had a week of daily, nonbloody, nonbilious emesis after eating solid food but tolerating liquids. The last day the emesis was bloody with clots and the child's father found the battery in the emesis.
Physical Exam: No acute distress, GCS 15, BP 111/50, P 135, R 29, T 36°C. Physical exam was unremarkable.
Laboratory Data: Hgb 9.6, coagulation panel normal, abdominal and CxR were normal.
Clinical Course: No further episodes of emesis noted in the ED and patient transferred to a tertiary hospital for GI endoscopy. 90 min after transfer, he had a possible seizure episode with bradycardia (HR 50s) which resolved with oxygen. His level of responsiveness declined; he passed a large melenotic stool; Hgb 7.9. Packed RBCs were given. 2 hours later, he deteriorated, was intubated and given more packed RBCs for profound hypotension. Hgb was 3l; platelets 4,000. Approximately 500 ml of bright red blood returned from an NG tube. He developed bradycardia with hypotension for which ACLS was instituted. Treatment with epinephrine, NaCO3, calcium, transfusion, FFP and glucose-insulin for hyperkalemia were given. An upper GI endoscopy was unsuccessful due to blood obscuring the visualization of a possible source. A Sengstaken-Blakemore tube was inserted orally and the upper balloon inflated, but this failed to stabilize the patient. The resuscitation was unsuccessful and the patient expired.
Autopsy Findings: Anatomic findings on autopsy were foreign body aspiration, anamnestic (watch battery) and esophageal 2 cm ulceration with erosion into an anomalous right subclavian artery. The surrounding tissue was fibrotic and exhibited diffuse insinuating hemorrhage. Cause of death was acute hemorrhage due to esophageal ulceration. Manner of death was accidental.
Case 69. Acute lithium dermal exposure: undoubtedly responsible.Scenario/Substances: A 23 y/o female working in a university chemistry laboratory sustained burns to 45% of her body because of an explosion involving t-butyl lithium.
Physical Exam: 45% body surface area burns.
Clinical Course: The patient was aggressively debrided, admitted to a burn unit, and given burn and aggressive supportive care for 18 days. Day 13 she produced 8,000 ml of urine and was diagnosed with diabetes insipidus. Day 13 blood lithium was 0.3 mEq/L. She died of sepsis and respiratory failure on Day 19.
Autopsy Findings: 43% BSA burns, mostly third degree.
Case 74. Acute ammonia exposure (inhalation, ocular, dermal): undoubtedly responsible.Scenario/Substances: A 31 y/o male was exposed to anhydrous ammonia while transporting it under pressure. The patient was pronounced dead at the scene.
Past Medical History: Cardiomegaly, hepatic steatosis, morbid obesity (BMI 48.8)
Physical Exam: 43% body-surface area superficial and partial-thickness burns, tongue burns noted
Laboratory Data: UDS negative, urine ethanol 30 mg/dL, blood drug screen negative; blood acetone, isopropanol, and methanol not detected
Autopsy Findings: Pulmonary congestion and hemorrhage, mild cerebral edema, skin and airway burns due to anhydrous ammonia exposure (∼43% total body surface area burns, airway mucosal damage)
Case 79. Acute cyanide inhalation: undoubtedly responsible.Scenario/Substances: A 45 y/o male jeweler was found down after mixing an electroplating solution with a second chemical. EMS administered CPR until the cyanide hazard was appreciated. He was pronounced dead at the scene.
Laboratory Data: Not available.
Autopsy Findings: Spotty cherry red discoloration, moderate atherosclerotic coronary vascular disease, serum cyanide 18 mcg/mL.
Case 80. Acute methyl bromide inhalation: undoubtedly responsible.Scenario/Substances: A 45 y/o female set off a fogger containing methyl bromide in her home, reentered without airing out the home and went to sleep. She was found outside her home drooling, with diarrhea, with a nose bleed. EMS found her O2 sat 80%, administered atropine and oxygen.
Past Medical History: Diabetes mellitus.
Physical Exam: Awake, oriented but lethargic. BP 124/51, HR 124, RR “rapid and shallow”, no muscle fasciculations noted.
Laboratory Data: ABG-pH 7.22, Hct 48, K 3.3, BUN 14, Cr 2.1, glucose 382, AST 426, ALT 183.
Clinical Course: In the ED 6 hr after exposure, she became agitated and confused, exhibited myoclonic jerking with a picking behavior. IV fluids and norepinephrine were administered for hypotension. Decontamination was performed prior to intubation and transfer to another hospital for intensive care. She became unresponsive and hypothermic requiring a warming blanket. Pralidoxime was administered without improvement. Pupils became fixed and dilated; pH 7.08, O2 sat 86% on O2. BP declined despite pressors and a fatal cardiopulmonary arrest occurred on Day 2.
Autopsy Findings: No significant external findings. Lungs were dark red and mottled with anthracotic pigment, congestion and edema. Microscopic examination showed congestion of the lungs, with a few extravasated blood cells in the alveoli and focal atelectais. The liver showed congestion and mild lobular inflammation of the liver. Kidney showed congestion. Femoral blood bromide level 9.2 mg/dL (normal <0.5 mg/dL, toxic after methyl bromide exposure > 15 mg/dL). The medical examiner concluded that the patient died an accidental death from complications of methyl bromide toxicity.
Case 85. Acute ammonia exposure (inhalation, ocular, dermal): undoubtedly responsible.Scenario/Substances: A 53 y/o male, transporting anhydrous ammonia under pressure at work, was exposed when the chemical vessel leaked. Rescuers at the factory doused the patient with water and EMS placed a King Airway. The patient's HR ranged from 20 to 40 throughout transport to a local level 1 trauma center with a burn unit.
Physical Exam: Intubated and unresponsive, partial thickness burns to the face that involved the nares and oral mucosa, diffuse chemosis of the eyes with corneal and conjunctival burns, anterior and lateral partial thickness burns to the neck, and superficial burns of the chest.
Laboratory Data: WBC 26.2, Hgb 15.7, platelets 281, INR 1.3, PTT 39.3, ABG-pH 7.26/pCO2 37/p O2 65/HCO3 17
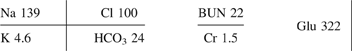
CK 229, troponin <0.030, ethanol not detected, UDS negative.
Clinical Course: In the ED the King Airway was replaced with an endotracheal tube. An orogastric tube returned gross blood. Chest x-ray showed bilateral perihilar infiltrates. A central line was placed and vasopressors were started. The patient's eyes were kept moist with saline and antibiotics. Pro-Kera amniotic rings were placed in the eyes to attempt to re-grow the conjunctivae. The patient progressed to acute renal failure and intermittent hemodialysis was initiated, and progressed to continuous renal replacement therapy. The patient's acute lung injury progressed to ARDS. The initial head CT was concerning for neurologic injury and an EEG confirmed global encephalopathy. The grafts sloughed shortly after placement secondary to the patient's multi-organ failure. Given the patient's anoxic brain injury, severe chemical eye burns, respiratory failure, renal failure and need for dialysis, the patient's family elected institution of comfort measures and he expired 14 days after admission.
Autopsy Findings: Not performed.
Case 95. Acute sodium hydroxide ingestion: undoubtedly responsible.Scenario/Substances: A 75 y/o male ingested an unknown amount of a drain cleaner containing sodium hydroxide in a suicide attempt.
Past Medical History: depression, chronic obstructive pulmonary disease, diabetes mellitus, hypertension, arthritis, and hyperlipidemia.
Physical Exam: Awake, alert and oriented, responding appropriately to questions. Erythema of the oral cavity and coffee ground emesis noted. BP 190/88, HR 100, RR 12, O2 sat 100% on room air.
Laboratory Data: ABG-pH 7.30/pCO2 57/pO2 187. WBC 20.2, Hgb 14.3, Hct 44, INR 0.88. Acetaminophen and salicylates were not detected.
Clinical Course: After several attempts, he was intubated and placed on a ventilator. NG tube was placed and returned bloody drainage. IV fluids and antibiotics were given. BP declined to 80/50. Emergent bronchoscopy was done to evaluate blood in the endotracheal tube. Because of the severity of the burn and the poor prognosis, the decision for comfort measures was made and the patient expired 8 hours after ingestion.
Autopsy Findings: Internal examination showed a scant amount of bloody liquid in the stomach. The lower 1/3 of the esophagus and the gastroesophageal junction was a red-black to gray-black, eroded and thin. Ninety percent of the gastric mucosa was markedly congested with superficial mucosal ulceration. The duodenum and jejunum had marked mucosal congestion. The liver was noted to have macrovesicular steatosis, focal hepatocellular necrosis and mild chronic triaditis. The esophagus showed coagulative necrosis with transmural mixed inflammatory infiltrate. The stomach showed focal transmural necrosis with submucosal edema, fibrin and mixed inflammatory infiltrate.
Case 101. Acute drain cleaner (acid) ingestion: undoubtedly responsible.Scenario/Substances: A 34 y/o female drank 16 ounces of a drain cleaner containing 93% sulfuric acid, was found vomiting with burns of her mouth and lips. EMS noted burns of the epiglottis, intubated, and transported her to the ED.
Past Medical History: Medications: lithium and trazodone for an unspecified psychiatric disorder.
Laboratory Data: 10–12 hr post ingestion: WBC 43.5, INR 1.39, ABG-pH 6.8/pCO2 44

CK, 628, AST 56, ALT 17, lactate 0.9 mmol/L
Clinical Course: In the ED, lethargic, BP 135/90, HR 80. She developed hypoventilation (O2 sat 67%) with evidence of pulmonary edema, hypotension (BP 71/40) with maximum doses of norepinephrine and IV fluids, T 38.9° C, pupils 8 mm and nonreactive. Her stomach became distended and she died on Day 2.
Autopsy Findings: Chemical burns on lips, oral mucosa, and tongue; esophageal burns with perforation of the proximal esophagus; chemical burns of the stomach with multiple perforations; caustic fluid in the pleural cavity and abdomen; chemical burns in the lung, spleen, liver, and intestines.
Case 105. Acute ammonia based cleaner and bleach ingestion: undoubtedly responsible.Scenario/Substances: A 60 y/o female drank unknown quantities of ammonia based cleaner and bleach. The patient reported vomiting and diarrhea.
Past Medical History: Depression and diabetes.
Clinical Course: About 1 hr after arriving at the hospital
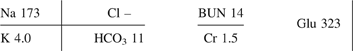
Acetaminophen and salicylate not detected, AST 11, ALT 37, Alk phos 76. At 3 hr after arrival in the ED, the patient had a seizure, was intubated, ventilated, and given oxygen. Fomepizole was administered empirically. She received vasopressors and IV fluids for hypotension. An arterial line and dialysis catheter were placed. The patient's BP was 130/70 on vasopressors and dialysis was performed. Day 2 methanol and ethylene glycol were not detected (sampled 15 hr after admission), Hgb 2, Na 147, ALT 6, AST 86, CK 251, troponin 0.06 ng/mL, INR 4.6. The patient began bleeding from all orifices and lines. She received FFP, packed RBCs, and cryoprecipitate. The patient was dialyzed again on Day 2; urine output was 30–60 mL/hr. Vasopressors were discontinued, systolic BP was 160–170 and HR 71. On Day 3, the patient had a massive GI bleed, developed cerebral edema and a herniated brain stem and expired.
Autopsy Findings: Gastrointestinal erosions, cause of death due to adverse effects of bleach and window cleaner ingestion. Post mortem midazolam 0.07 mg/L and lidocaine detected.
Case 106. Acute drain cleaner ingestion: undoubtedly responsible.Scenario/Substances: A 62 y/o male intentionally ingested 4–6 ounces of drain cleaner. EMS found a HR 140. He complained of abdominal pain and was transported to the ED.
Past Medical History: Atrial fibrillation.
Clinical Course: From the ED he was immediately taken to surgery which showed burns to the esophagus and the stomach and evidence of a perforated bowel. He was hypotensive with a BP 90/60 on phenylephrine. He was intubated, sedated, and ventilated. About 9 hr later, he was taken back to surgery and his entire stomach was removed because of extensive necrosis. They were unable to assess the esophagus because of intubation. The patient remained sedated with propofol, but when sedation was lightened, the patient responded appropriately to questions. On Day 3, the patient was transferred to an advanced care facility. He was hemodynamically unstable requiring high doses of vasopressors and developed multi-system organ failure. Further surgery was not possible because the patient was too unstable and he expired.
Autopsy Findings: No autopsy performed.
Case 108. Acute hydrofluoric acid ingestion: undoubtedly responsible.Scenario/Substance: A 70 y/o female told her husband she ingested 8 ounces of rust remover 4 hours earlier. She complained to EMS personnel of chest tightness while enroute to the ED.
Past Medical History: diabetes, anxiety, and depression with multiple prior suicide attempts.
Physical Exam: Awake, alert and oriented; appeared depressed and was acting inappropriately. Chest pain subsided to 0/10.
Clinical Course: During ED triage, she stated “I don't feel good”;11 min after ED arrival, she became unresponsive, and had a ventricular fibrillation arrest. Resuscitation was unsuccessful and she expired 27 minutes after arrival to the ED.
Autopsy Findings: Gross internal and external findings were unremarkable except for arteriosclerotic heart disease. Hypocalcemia (0.8 mg/dL) and hypomagnesemia (0.4meq/L) were noted on a metabolic panel. Uncertain if the blood sample was pre or post mortem. UDS positive for citalopram. The pathologist concluded that death was caused by ventricular fibrillation due to the effects of hydrofluoric acid poisoning and the manner of death was suicide.
Case 109. Acute household cleaner ingestion: probably responsible.Scenario/Substances: A 70 y/o female nursing home resident drank an unknown amount of a household all purpose cleaner. She began to vomit and developed wheezing. She was transported to the ED by EMS.
Past Medical History: Dementia.
Physical Exam: Respirations very shallow with wheezing.
Clinical Course: She had a “not to be resuscitated advanced directive” and was pronounced dead shortly after arriving in the ED. The tracheobronchial tree contained a slight amount of frothy fluid, and the stomach contained 720 cc of yellowish/tan liquid mixed with food contents that smelled like oranges.
Autopsy Findings: Final necropsy diagnosis included: 1) h/o dementia 2) h/o drinking some “all-purpose” cleaner 3) nodular fibrosis of mitral valve leaflets 4) toxicology results included therapeutic levels of diphenhydramine 0.05 mg/L, chlordiazepoxide 0.37 mg/L, nordiazepam 0.32 mg/L, oxazepam 0.04 mg/L, citalopram 0.52 mg/L, and norcitalopram 0.18 mg/L 5). Gastric samples were not tested quantitatively but qualitatively results listed as compounds detected consistent with those found in the all purpose cleaning product. Cause of death listed as metabolic abnormalities due to ingestion of commercial cleaning fluids. Blood and ocular fluid samples measured with GC/MS for ethanol, methanol, acetone, and isopropanol levels were negative. ELISA and FPIA testing was positive for benzodiazepines.
Case 115. Acute alkaline degreaser ingestion: undoubtedly responsible.Scenario/substance: An 88 y/o female nursing home resident accidentally ingested a “taste” of a grease cutter that was on a cart in a food preparation area. Within 1 hour of exposure, she complained of oral irritation, throat and chest discomfort and was taken to a nearby hospital.
Past Medical History: Dementia.
Physical Exam: Remarkable for lip and tongue swelling and erythema.
Laboratories: Chest CT revealed esophageal perforation extending into trachea.
Clinical Course: The patient underwent endotracheal intubation and was transferred to a tertiary care hospital. Clinical evidence of esophageal perforation with subsequent mediastinitis developed; Antibiotics were administered. After consultation with the family comfort care only was provided and she died on Day 7.
Autopsy Findings: Medical examiner's cause of death: complications from liquid alkaline degreaser ingestion with contribution from dementia.
Case 122. Acute dry fire extinguisher ingestion: undoubtedly responsible.Scenario/Substances: A 34 y/o male ingested the contents of a powdered fire extinguisher.
Past Medical History: Schizophrenia, retardation, and inappropriate ingestions. In the week before this ingestion he was seen twice in an ED for attempting to inhale the same powdered fire extinguisher.
Physical Exam: Unconscious, powder about his nose and mouth, and in respiratory distress.
Clinical Course: During EMS transport he was suctioned and intubation attempted. He expired shortly after ED arrival.
Autopsy Findings: Absence of powder in the airway or of signs of airway obstruction. A large amount of powder was found in the stomach. Toxicology screening was negative. Coroner ascribed death to: “airway compromise with chemical exposure”.
Case 132. Acute hydrogen sulfide, inhalation: undoubtedly responsible.Scenario/Substances: An 18 y/o male researched hydrogen sulfide online before purchasing an unknown animal fungicide and a lye-based drain cleaner in order to make hydrogen sulfide. He was heard to scream and was found unresponsive supine on floor with blankets and towels around his head in his bedroom. A large cooking pot containing an unknown fluid was on the floor and an unknown odor was in the air. His mother called 911. EMS found the patient in cardiac and respiratory arrest, he was intubated, given epinephrine and atropine. The fire department later reported that patient's clothing tested positive for hydrogen sulfide.
Past Medical History: Apparent depression, but not formally diagnosed. No prior suicidal threats or attempts.
Physical Exam: He arrived in ED intubated and was placed on a propofol drip. BP 140/55, HR 152, RR 23, O2 sat 99% on 100% fiO2, T 36.4°C. Pupils fixed and dilated. No gag or corneal reflexes. Spontaneous upper and lower extremity muscle activity present. Skin on face, left hand and both feet was red.
Laboratory Data: COHgb 2.2, UDS negative
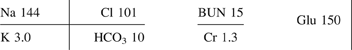
ABG (6 hr after admission) pH 7.31/pCO2 35/pO2 379.
Clinical Course: Chest x-ray showed opaque area in right upper lung. Head CT showed severe cerebral edema with possible tonsillar herniation. Day 2 neurological exam showed no change in corneals/gag reflex and no spontaneous respirations. Comfort measures were instituted and he expired on Day 2.
Autopsy Findings: Upper and lower airways contained abundant yellow mucoid fluid. Cut surfaces of pulmonary parenchyma exuded profound amounts of blood and frothy edema fluid. Multiple lung sections showed profound alveolar edema, extravasated RBC and a diffuse acute inflammatory infiltrate. Cerebral hemispheres were profoundly edematous with prominent cerebellar notching and necrosis, consistent with herniation. Sections of hippocampus showed marked diffuse acute neuronal injury. Urine Cr 243 mg/L. Cause of death: complications of cerebral edema due to anoxic brain injury due to inhalation of hydrogen sulfide gas.
Case 134. Acute carbon monoxide inhalation. undoubtedly responsible.Scenario/Substances: A 20 y/o female was asleep in her house when it was set aflame in an arson fire. She was asleep for ∼30 min before she was removed and brought to the ED.
Past Medical History: Asthma and anxiety.
Physical Exam: BP 113/69, HR 119, RR 24, able to breathe on her own, and would slightly move her extremities; Pupils 2 to 3 mm and responsive to light, extensive carbonaceous material on her tongue and the roof of the mouth, secretions thick in the back of her throat and had gurgling respirations. Soot was caked in her nostrils; coarse rhonchorous breath sounds. No burns to the skin
Laboratory Data: COHb 41.1%, O2 sat 74% on 100% O2.
![]()
ABG-pH 6.92/pCO2 57/pO2 386; acetaminophen and salicylate were not detected, UDS positive for benzodiazepines and cannabinoids.
Clinical Course: She was intubated on arrival to the ED, treated with hyperbaric oxygen. In the burn ICU, she was found to have PVCs and a mild troponin elevation. Echocardiogram was unremarkable. Head CT and EEGs showed no neurological recovery. The patient's family elected the institution of comfort measures and she expired on Day 4.
Autopsy Findings: Lungs showed pneumonia and some remaining soot particles. Post-mortem toxicology screen negative.
Case 141. Acute aluminum phosphide ingestion: undoubtedly responsible.Scenario/Substances: A 29 y/o male intentionally ingested 4 tablets of aluminum phosphide pesticide he had purchased in India.
Past Medical History: Not provided.
Physical Exam: His O2 sat was 83% on room air. He had one episode of urinary incontinence.
Laboratory Data: pH 6.99, bicarbonate 8 mEq/L.
Clinical Course: In the ED, the patient complained of abdominal pain and drowsiness. Over the next hour he became hypotensive, hypoxic, and acidotic. Within 3 HR of presenting to the ED, the patient died.
Autopsy Findings: Cause of death was the adverse effects of phosphine.
Case 147. Carbon monoxide inhalation: undoubtedly responsible.Scenario/Substances: A 41 y/o male was found unconscious in a camper which was being heated by a generator along with another male who was pronounced dead at the scene. Ambient CO level was 12 ppm 30 minutes after the doors to camper were opened.
Past Medical History: Heroin abuse.
Physical Exam: BP 81/53, HR 102, RR 20. Pupils were 2 mm bilaterally and unreactive. Coarse breath sounds were heard on both lung fields. GCS 4 on no sedation with minimal withdrawal to pain.
Laboratory Data: ABG-pH 7.39/CO230/pO2 342
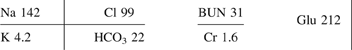
Lactate 5.3 mmol/L, COHb >70% by transcutaneous co-oximeter.
Clinical Course: Hypotension (60 systolic) was treated with dopamine. Hyperbaric oxygen (HBO) was started but discontinued after 60 minutes due to BP 240/120 that persisted despite stopping dopamine. COHb was 9% after HBO therapy. ICU care included intermittent norepinephrine for continuing hypotension. Day 2: Echocardiogram revealed severely reduced global left ventricular systolic function (EF 20%). Day 3: MRI of the brain with and without contrast showed extensive bilaterally symmetrical T2 signal hyperintensities in the basal ganglia and in the periventricular white matter typical for severe carbon monoxide poisoning. Septic shock occurred requiring antibiotics. On Day 5 he was transferred to hospice where he died on Day 8.
Autopsy Findings: No autopsy was done. Cause of death was carbon monoxide poisoning. Manner of death was accidental.
Case 155. Acute carbon monoxide inhalation: undoubtedly responsible.Scenario/Substances: A 42 y/o female was found in full cardiopulmonary arrest in her bedroom. Her parents were found deceased in the garage, next to the generator being used by the family during an ice storm/power outage.
Past Medical History: Down syndrome.
Physical Exam: The patient had been deceased for an unknown duration,
Clinical Course: No resuscitation was attempted and the patient was declared dead at the scene.
Autopsy Findings: Death was attributed to carbon monoxide intoxication, secondary to generator exhaust fumes. Post mortem COHb42%.
Case 157. Acute hydrogen sulfide inhalation: undoubtedly responsible. Scenario/Substances: A 23 y/o male worker lost his footing and fell into an 18 foot deep by 3 foot wide cesspool while trying to unstop the drain with a long pipe plunger. The father, a 49 y/o male, went down the hole to rescue his son. When the 2 failed to respond to cries from their employees, a third employee, a 52 y/o male went into the hole also.
Physical Exam: All 3 patients expired at the scene
Overcome by fumes. When first responders arrived at the scene, they retrieved the bodies of all 3 men. They were found face down at the bottom of the ladder. The hydrogen sulfide gas level at the bottom of the cesspool was 200 parts per million.
Autopsy Findings: none
Case 158. Acute inhalation: undoubtedly responsible.Scenario/Substances: A 23 y/o male worker lost his footing and fell into an 18 foot deep by 3 foot wide cesspool while trying to unstop the drain with a long pipe plunger. The father, a 49 y/o male, went down the hole to rescue his son. When the 2 failed to respond to cries from their employees, a third employee, a 52 y/o male went into the hole also and was overcome by fumes.
Clinical Course: When first responders found all 3 face down at the bottom of the ladder. All 3 patients expired at the scene. Hydrogen sulfide gas concentration at the bottom of the cesspool was 200 ppm.
Autopsy Findings: No autopsy was performed.
Case 160. Acute carbon monoxide inhalation: undoubtedly responsible.Scenario/Substances: A 54 y/o female found in the garage next to a generator used by the family during an ice storm/power outage and on top of the body of her deceased husband. Her body was found wedged between that of her husband and the generator. Her clothing had caught on fire.
Past Medical History: Permanent tracheostomy, s/p lap-band procedure.
Physical Exam: The patient had been deceased for an unknown duration and had 45% body surface area burns.
Clinical Course: No resuscitation was attempted and the patient was declared dead at the scene.
Autopsy Findings: Death was attributed to carbon monoxide intoxication, secondary to generator exhaust fumes. Postmortem blood analyses (site unspecified) revealed COHb50.4% and oxycodone 41.7 ng/mL.
Case 167. Acute carbon monoxide inhalation: undoubtedly responsible.Scenario/Substances: A 63 y/o male was found in full cardiopulmonary arrest of unknown duration in the garage next to the generator used by the family during an ice storm/power outage and beneath the body of his deceased wife. A flashlight was found underneath the decedent and the coroner believed the descendent had been working on the generator in the dark and was overcome by the fumes.
Past Medical History: Not available.
Physical Exam: The patient had been deceased for an unknown duration,
Clinical Course: No resuscitation was attempted and the patient was declared dead at the scene.
Autopsy Findings: Death was attributed to carbon monoxide intoxication, secondary to generator exhaust fumes. Postmortem COHb73.3%, pseudoephedrine 870 ng/mL and codeine 33.2 ng/mL.
Case 192. Acute elemental mercury ingestion/aspiration: undoubtedly responsible.Scenario/Substance: A 23 y/o male intentionally drank an unknown quantity of elemental mercury which he had obtained by “recycling computers” at his home. He vomited after the ingestion. There was no evidence that the mercury was heated in his home.
Past Medical History: Depression, anxiety, h/o appendectomy.
Physical Examination: ED VS: BP 116/65, HR 145, RR 14, T 37.7°C, O2 Sat 96% on room air.
Laboratory Data: Serum and urine toxicological screens were positive for opiates; acetaminophen and ethanol were not detected. K 3.4, Cr 1.32, random urine mercury 9,980 mcg/L.
Clinical Course: He presented to the ED with nausea, vomiting, abdominal pain, headache, body aches and a cough ∼3 hr after ingesting the mercury. He was admitted and started on succimer chelation therapy. He developed respiratory distress with RR in the 40s. He was started on bilevel positive airway pressure, developed O2 Sat in the 80s, and was intubated. Serial chest and abdominal X-rays showed radio opaque substance in the bilateral lung bases and throughout the small intestine. Whole bowel irrigation with polyethylene O2glycol solution was unable to clear the mercury from his bowel. He developed worsening ARDS and could not tolerate bronchoscopy with attempted lavage of the mercury. On Day 12, despite being in a rotor-bed and being on FiO2 of 100%, the patient's O2 sat dropped into the 40s%. He required multiple vasopressors, he was unable to maintain a viable BP and expired from acute respiratory failure.
Autopsy Findings: Immediate cause of death: Mercury intoxication and complications (external viewing only).
Post-mortem toxicology: negative screen for ethanol, acetone, isopropanol, and methanol. Negative prescription drug panel screen.
Case 193. Acute thallium, ethanol ingestion: undoubtedly responsible.Scenario/Substance: A 36 y/o male ingested grains of thallium sulphate from an old bottle of rodent and ant poison in an apparent suicide attempt.
Past Medical History: Depression.
Physician Exam: Extremely agitated and confused. BP 141/60, HR 146, RR 20, O2 sat 96% on room air. The remainder of the examination was unremarkable.
Laboratory Data: Bun 32, Cr 3.7, 5 hr post ingestion serum thallium >1000 mcg/L (reference range <5); urine spot thallium >2000 mcg/L (reference range 0.4 mcg/g Cr), 24 hour urine thallium for the first 48 hr post exposure (2 separate 24 hr urine collections): >2000 mcg/L (reference range less than 2 mcg/L).
Clinical Course: Chemical and physical restraints were employed with lorazepam infusion; IV fluids and multi-dose activated charcoal were given; Prussian blue (200 mg/kg/day) was given via NG tube 18 hours after presentation when it became available. Activated charcoal was discontinued when Prussian blue was started. Day 2 the patient was intubated for airway protection; Tachycardia and hypertension continued for 48 hours with renal insufficiency. Day 3 pressors were required for hypotension and he was transferred to a tertiary care center for CRRT. The patient expired shortly after transfer to the second hospital.
Autopsy Results: Not provided.
Case 194. Acute copper sulfate pentahydrate ingestion: undoubtedly responsible.Scenario/Substances: A 41 y/o female ingested 21 ounces of a 99% crystalline copper sulfate pentahydrate solution used as a root killer.
Past Medical History: Not obtainable
Physical Exam: Agitated and following commands on arrival but rapidly became obtunded. VS: BP 131/106, HR 110, T 37.1°C, RR 30. She had oropharyngeal erythema and edema with blue stained lips and vomitus, Pupils 5 mm and reactive. Extremities were cool and blue; circumoral and acral cyanosis noted.
Laboratory Data: Glucose 159, Ca 8.8, ionized Ca 3.9, albumin 6.1, AST 1753, ALT 480, alk phos 138, total bilirubin 1.5, lipase 1382, WBC 18.1, Hgb 17.8, Hct 54.7, platelets 326.
UDS negative; acetaminophen, salicylate, and ethanol were not detected. Urine 2+ ketones, comprehensive toxicology showed diphenhydramine. ECG: sinus HR 95 with unusual P axis, tall peaked T waves in all leads, PR 158 msec, QRS 108 msec, QTc 434 msec. Chest X-ray showed no infiltrate.
Clinical Course: Immediately after arrival in the ED (∼5 hr after ingestion) an NG tube was placed and drained a large amount of blue liquid. 50 gm of activated charcoal was instilled. She rapidly became unresponsive and was intubated. O2 sat 80%, HR 180, QRS widened with tall peaked T waves. Hyperkalemia (K 7.8) treated with IV calcium chloride + sodium bicarbonate + insulin + glucose. She received BAL IM and IV N-acetylcysteine. She developed an anion gap metabolic acidosis, hepatorenal insufficiency and pancreatitis. She was noted to be blue and cold. Upon transfer to the ICU, she became hypotensive, initially responded to IV fluids. BP remained labile requiring norepinephrine, phenylephrine, and vasopressin. O2 sat 80s on 100% FiO2 + 5 PEEP. At 5 hr after arrival her pH 6.79, metHgb level of 20%, platelets 107, Hgb 13.2, Hct 46 with metamyelocytes and nucleated RBCs and evidence of hemolysis. Hemodialysis was precluded by hemodynamic instability. Her K has corrected to 5, but HCO3 dropped to 14, Cr 2.41, glu 68, total Ca 5.8, albumin 1.3, CK 786, troponin I 0.35. She showed evidence of bleeding. A 2-D echocardiogram showed decreased LV chamber size with moderate LV concentric hypertrophy, and severely decreased left ventricular systolic function. She remained blue. At 10 hr post-arrival, she had no palpable BP and a sinus rhythm between 80–90. Her skin and lips were slate-blue colored and her nailbeds were grey. She was 34°C axillary on a warming blanket. She was flaccid with no reflexes, pupils were 6–8 mm and unresponsive to light. She became bradycardic, CPR was started and atropine and methylene blue were given IV. She could not be revived and was pronounced dead 11 hr after arrival.
Autopsy Findings: Autopsy showed green discoloration of the lips and nail of hands, feet, brain, and internal organs; pericentral necrosis and micro-vesicular steatosis of the liver; mild intra-alveolar edema, and ascites. Toxicology analysis detected no drugs.
Case 200. Acute kerosene ingestion/aspiration: undoubtedly responsible.
Scenario/Substances: A 62 y/o male ingested an unknown amount of kerosene and spilled kerosene onto his skin. He had access to 1 quart of kerosene. He was found within minutes by a caretaker who noted him to be hallucinating and coughing. He was transported to the ED.
Past Medical History: dementia secondary to tertiary syphilis, seizure disorder, hypertension, depression, anemia, thrombocytopenia. Medications included phenytoin and lisinopril.
Physical Exam: The smell of kerosene was noted on the patient, BP 109/76, HR 104, RR 17 (ventilator) and O2 sat 99% (FiO2 not provided).
Laboratory Data: ABG-pH 7.28–7.4/pCO2 30–36/pO2 72–92, HCO3 11, Na 138, K 3.8. UDS positive for barbiturates and benzodiazepines.
Clinical Course: He was decontaminated, sedated with propofol, intubated, placed on a ventilator, and given IV fluids and antibiotics in the ICU. He was noted to have loose, oily stools and developed bilateral pulmonary infiltrates with metabolic acidosis. Sodium bicarbonate infusion was initiated. Hypotension required treatment with pressors; he exhibited skin sloughing and rising Cr. His respiratory status declined and he remained unresponsive off sedation. He exuded the smell of kerosene throughout the hospitalization. He expired on Day 7.
Autopsy Findings: External examination and medical records were reviewed. The pathologist concluded that the patient died of complications of an accidental kerosene ingestion.
Case 203. Acute kerosene ingestion/aspiration: undoubtedly responsible.Scenario/Substances: A 14-month-old male ingested a “gulp” of kerosene, coughed and choked. Respiratory symptoms persisted, and he was transported by EMS to the ED. En route, his mental status deteriorated.
Physical Exam: In the ED he was unresponsive, in respiratory distress, systolic BP 30–40, HR 150, pupils 6 mm and sluggish bilaterally, O2 sat 50% with bag-valve mask.
Laboratory Data: Chest x-ray showed diffuse interstitial infiltrates.
Clinical Course: He was intubated the ED and was transferred to a tertiary care hospital for pediatric intensive care. He remained hypotensive during flight and was given several IV crystalloid boluses. On arrival to the pediatric ICU, his endotracheal tube was changed from an uncuffed to a cuffed endotracheal tube because of high airway pressure and persistent air leak. He was started on norepinephrine and dopamine infusions, was switched to bag ventilation, but had persistent anion-gap negative metabolic acidosis and progressive pulmonary dysfunction. He was changed over to a respiratory oscillator, started on an epinephrine infusion and was then flown to another center for extracorporeal membrane oxygenation (ECMO). Perfusion pressure, oxygenation, and acid-base status improved with ECMO, but he developed an intra-abdominal compartment syndrome and laparotomy demonstrated diffusely ischemic bowel. His grave prognosis was discussed with his family, they elected the institution of comfort measures, and he expired 23 hr after the ingestion/aspiration.
Autopsy Findings: Final cause of death was “metabolic acidosis due to hypoxia, and shock, due to ingestion and inhalation of kerosene. Laparotomy “showed ischemic bowel.”
Case 204. Acute Amanita phalloides ingestion: undoubtedly responsible.Substances/Scenario: A 31 y/o female ingested sautéed wild mushrooms with her husband and cousin after picking them at an unknown location. The mushrooms were yellowish in color and looked similar to mushrooms that patient's family grew on their farm. She awoke 9 hr after ingestion with acute abdominal pain, nausea, and vomiting every 15 – 20 min followed by watery diarrhea.
Physical Exam: Awake, alert. BP 106/75, HR 124, RR 22, T 36.3 C, O2 sat 99% on room air. No asterixis. Chest was clear to auscultation. Normal heart sounds, regular rate and rhythm. Abdomen was soft, mildly tender, no ascites, with active bowel sounds. Skin was nonicteric.
Laboratory Data: ABG-pH 7.22/pCO2 30/pO2 103
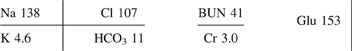
Ca 8.8, WBC 18.6, Hgb 16.1, Hct 48.0, platelets 443; albumin 5.0, total protein 9.0, total bilirubin 0.7, INR 1.07, AST 115, ALT 86
Nine hr later: Albumin 3.9, amylase 168, lipase 748
AST 751, ALT 816, total protein 7.5, total bilirubin 2.1, alk phos 88, WBC 16.9, Hgb 17.2, platelets 434
Day 3: AST 3995, ALT 4166, total bilirubin 3.6, PT 37.4, INR 3.4
Clinical Course: Multiple dose activated charcoal 25 gm every 4 hrs, IV N-acetylcysteine, vitamin K 10 mg, and penicillin G every 6 hours were started. A mycologist identified the mushroom specimen as Amanita phalloides. She was given vitamin K 10 mg subcutaneously and a sodium bicarbonate drip was started prior to transfer to a tertiary care center for possible liver transplantation. IV carnitine and silybinin were administered. IV Pen G was discontinued due to severe thrombocytopenia. Coagulopathy with low measured factor V, gastrointestinal bleeding, massive diarrhea with bloody output developed; blood products and FFP continuous infusion were given during entire hospitalization. Mental status deteriorated, elevated ammonia levels and eventual coma with nonconjugate gaze were seen. Seizure activity began late in the hospitalization and worsened cardiovascular status despite multiple pressors and aggressive blood product support. Renal failure was identified on Day 4; the patient expired on Day 13 from fulminant hepatic failure secondary to Amanita toxicity.
Autopsy findings: Not available.
Case 205. Acute cyclopeptide mushroom ingestion: undoubtedly responsible.Scenario/Substances: A 52 y/o male picked mushrooms in Maryland, froze them then later defrosted one and ate it in his soup. He noticed the first GI symptoms ∼8 hr later. He continued to have nausea, vomiting, diarrhea and abdominal pain and presented to the ED 2 days after ingestion with hematochezia.
Past Medical History: Hypertension
Physical Exam: BP 62/42, HR 113, T 33.4°C, tachypnea, respiratory distress, but still able to speak with difficulty.
Laboratory Data: ABG-pH 6.87/pCO2 19.4/pO2 152 /, HCO3 4.1, O2 sat 98 % on room air, Cr 3.8, HCO3 7.5, AST <10, ALT <5, Alp phos 132, bilirubin (total) 4.3, amylase 642 IU/dL, lipase 416 IU/L, INR 12.5, PTT 76.
Clinical Course: He was transferred to a tertiary center based on his renal failure and coagulopathy. He was intubated the next day, received a sodium bicarbonate drip, vasopressors, FFP, CVVHD, IV N-acetylcysteine, IV penicillin G, and antibiotics for sepsis. He was not felt to be a transplant candidate due to his multisystem organ failure. Three days after ingestion troponin was 2.29, ALT 2,203, AST 2,165. He showed some improvement in mental status and vasopressors were discontinued as hypotension resolved but his extremities became ischemic and gangrenous 12 days after ingestion; pupils became fixed and dilated. The patient's family elected the institution of comfort measures and he expired 13 days after the ingestion. The assessment was fatal cyclopeptide mushroom toxicity.
Autopsy Findings: ME agreed that the death was from acute mushroom toxicity.
Case 215. Acute endosulfan ingestion: undoubtedly responsible.Scenario/Substances: A 2 y/o 15 kg female ingested an unknown amount of what was thought to be a pesticide and was given milk to drink by a parent shortly afterwards. She was found by EMS in status epilepticus. Diazepam (1.25 mg) and midazolam (4 mg) were given in the field.
Physical Exam: HR 190 – 200; L pupil 2 mm, R pupil 3 mm. Lung exam described as “wet”. The patient did not have a chemical odor.
Laboratory Data: ABG-pH 6.7/pCO2 113/pO2 49/HCO3 14.
Clinical Course: Seizures continued despite additional doses of benzodiazepines and multiple 1 mg doses of atropine and IV sodium bicarbonate. Pralidoxime was considered, but was not available. The ingested chemical was brought to the ED in an unlabeled water bottle and reportedly smelled like paint remover. The product was identified as endosulfan 4 hr post ingestion when a family member retrieved the product label. The patient was sedated with pentobarbital and given cholestyramine (80 mg per kilogram 3 times a day) and started on N-acetylcysteine. On Day 3, hemodynamic instability developed requiring pressor support. Pupils were reported as dilated. Day 5 head CT revealed cerebral edema; hemodynamic instability continued through Day 8 when an EEG and brain flow studies revealed absence of higher brain function. Comfort measures were instituted and she expired on Day 9.
Autopsy Findings: not available
Case 216. Acute pesticide, ethanol, xylene ingestion: probably responsible.Scenario/Substances: A 23 y/o male was found unresponsive on the floor of his residence. EMS noted emesis, coma, pinpoint pupils, shallow respirations, ronchi on lung exam, and urinary/fecal incontinence. He was intubated and transported to the ED.
Past Medical History: Chronic ethanol abuse.
Physical Exam: BP 70/50, HR 62, RR 12, T 88 F. Pupils pinpoint, nonreactive. Abdomen flat and without bowel sounds.
Laboratory Data:
ABG-pH 6.9/pCO2 54/pO2 555 (on high flow oxygen)
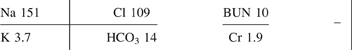
WBC 19,800, Hct 34.2; INR 1.2, Ca 7.3, Mg 2.5, CPK 3,946, CKMB 86.7, AST 77, ALT 25, amylase 201, lactic acid 15.2 mmol/L. ECG: ST depression in inferior and lateral leads. UDS negative.
Clinical Course: A central line was placed for IV fluids administration. BP 90 systolic with dopamine and norepinephrine infusion. Bradycardia to HR 40 then 20s prior to an asystolic arrest. Despite full resuscitation attempts, he expired.
Autopsy Findings: During the postmortem exam a strong chemical odor was noted forcing the pathologist to leave the room and call a hazmat team, who tested liquid material found in the stomach and advised it was a volatile organic compound. Premortem blood was analyzed for solvents and found to contain: ethanol 66 mg/dL, ethyl acetate (not quantified). o-xylene 2.8 mcg/mL, m-xylene 6.8 mcg/mL, p-xylene 2.8 mcg/mL. Subsequent laboratory testing of gastric contents and postmortem blood found thiabendazole (not quantified).
Case 220. Acute strychnine ingestion: undoubtedly responsible.Scenario/Substances: A 43 y/o male was observed by a co-worker to ingest 3 handfuls of gopher getter pellets (1.8% strychnine). He rapidly collapsed and EMS was called.
Past Medical History: depression with prior suicide attempts. Medications included: zolpidem 10 mg, duloxetine 60 mg, trazodone 50 mg, alprazolam, and methylin.
Physical Exam: Comatose, apneic patient in cardiac arrest; cyanotic, CPR in progress.
Laboratory Data: Cardiac monitor revealed asystole.
Clinical Course: Resuscitation efforts were unsuccessful and he was pronounced dead in the ED.
Autopsy Findings: Cause of death: Acute strychnine poisoning. Pulmonary edema and congestion; diffuse cerebral extracellular edema; dilated cardiomyopathy. Femoral blood strychnine 22.6 mg/L, trazodone 0.25 mg/L (therapeutic).
Case 225. Acute methyl bromide dermal/inhalation: undoubtedly responsible.Scenario/Substances: A 56 y/o pesticide applicator was working with a canister of soil fumigant when it exploded. The fumigant contained 67% methyl bromide, and 33% chloropicrin.
Past Medical History: Diabetes mellitus, hypertension, coronary artery disease (s/p CABG x 4 vessels).
Physical Exam: Awake, alert complaining of burning in his lungs. BP 110/64, HR 80, RR 14–16. Superficial burns noted on his lower extremities and back.
Clinical Course: The patient was intubated and ventilated. BP was supported with IV fluids and pressors. Rapidly progressive multi-organ system (pulmonary and cardiovascular) failure occurred – intractable hypotension preceded terminal cardiac arrest 14 hours after exposure.
Autopsy Findings: Trachea showed areas of denuded mucosa. Both lungs were congested and weighed 970 grams (left) and 1130 grams (right). Death was attributed to “sequelae of inhalation of methyl bromide”.
Case 231. Chronic brodifacoum exposure: undoubtedly responsible.Scenario/Substances: A 35 y/o female who ultimately succumbed to coagulopathy associated with brodifacoum exposure from an unclear source.
Past Medical History: The patient developed pulmonary embolus 15 months PTA and was treated initially with heparin and then warfarin for 6 months. She reported anemia 7 months PTA, easy bruising 3 months PTA and current persistent dizziness and malaise. Colonoscopy and esophageal-gastric endoscopy showed hemorrhagic gastritis treated with esomeprazole. Her hematologist followed her severe intermittent iron deficiency anemia (Hgb 3 – 4), INR 6–7, persistently low protein C and S and low vitamin K-dependent factors. One month PTA she received FFP and vitamin K (10 mg daily 3 times a week) which did not reverse the coagulopathy. A blood test was positive for brodifacoum. Neither the patient nor her family could offer any explanation for the brodifacoum in her blood. She received factor VII concentrate as an outpatient. But she became dizzy, developed a swollen leg and was taken to the ED.
Clinical Course: At the time of her initial (and subsequent) ED admissions she exhibited bruising, and ecchymoses. She was found to have a severe anemia and a severe bleed into her legs. She received high dose vitamin K and therapeutic levels were confirmed by blood vitamin K concentrations. She presented to the ED 8 months later with a brain stem hemorrhage and was declared brain dead the following day. The family donated her organs.
Autopsy Findings: No autopsy was performed.
Case 246. Acute multiple drug ingestion: undoubtedly responsible.Scenario/Substances: A 17 y/o male ingested an unknown quantity of his methadone, propranolol, gabapentin, fexofenadine, and lovastatin. He was found unresponsive and apneic. EMS found him in PEA and transported him to the ED.
Clinical Course: He was successfully resuscitated in the ED and admitted to the pediatric ICU. On arrival to the PICU, he suffered a cardiac arrest from which he could not be resuscitated.
Autopsy Findings: Postmortem serum: methadone 267 ng/mL, EDDP 35.5 ng/mL, gabapentin 31.1 ng/mL, venlafaxine 118 ng/mL, and propranolol 140 ng/mL. Cause of death was multiple drug toxicity.
Case 260. Acute morphine ingestion: undoubtedly responsible.Scenario/Substances: A 20 y/o male ingested 10 tablets of 100 mg sustained release morphine in an attempt to harm himself. He received naloxone by EMS although at that time he did not have respiratory depression. He was transported to the ED.
Past Medical History: Asthma, previous drug addiction
Physical Exam: On arrival to the ED, ∼1 hr after ingestion, the patient was awake and alert, physical exam was unremarkable. Approximately 12 hr later, he was unresponsive in respiratory and cardiac arrest. At that time, pupils were 1–2 mm and minimally reactive, hypoactive bowel sounds, VS: (after resuscitation by ACLS protocol), HR 112, systolic BP 80–90.
Laboratory Data: Pre-arrest: acetaminophen, salicylate, and ethanol were not detected, AST 21, ALT 32,
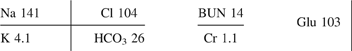
Clinical Course: While in the ED, he had a normal mental status, did not have respiratory depression and remained hemodynamically stable. He was transferred to the inpatient psychiatry ward ∼5 hr after exposure where he was noted to be lethargic but had good vital signs and RR. Nearly 12 hr after exposure, the patient was found to have snoring respirations and shortly thereafter, had apnea and asystole. He was intubated and received multiple doses of atropine, epinephrine and bicarbonate. He remained asystolic for ∼15 min and was successfully resuscitated. After resuscitation
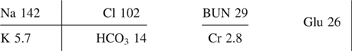
Gluc rose to 98 mg/dL after 50 g of D50), anion gap 26, ABG-pH 7.22/pCO2 31/ pO2 451, WBC 19.7, Hgb 13.3, Hct 38.5, platelets 53, CK 18,659, CKMB 105. He required vasopressor support for hypotension (dopamine, norepinephrine, and dobutamine). Approximately 2.5 hr after the initial cardiac arrest, he arrested again with PEA. After successful resuscitation (duration ∼7 min), he had a prolonged ICU course including hemodynamic instability, renal failure requiring CVVHD and hemodialysis and seizure activity. He never regained consciousness and head CT Hand EEG was consistent with severe anoxic brain injury. He was made comfort care only on Day 14 and he expired on Day 15.
Autopsy Findings: Preliminary autopsy report: cause of death was terminal bronchopneumonia, manner of death was suicide.
Case 272. Acute oxycontin ingestion: undoubtedly responsible.Scenario/Substances: A 21 y/o male with chronic pain and depression was found lethargic by family members after he texted one of them that he no longer wanted to live and was taking pills.
Past Medical History: Depression, chronic pain. Medications included oxycodone, alprazolam, and carisoprodol.
Laboratory Data: UDS positive for opiates, benzodiazepines, and tricyclic antidepressants. Serum acetaminophen and salicylate were not detected; ethanol was 10 mg/dL; ECG showed normal QRS and QT intervals.
Clinical Course: He arrived at the ED lethargic with miotic pupils and responded to naloxone. A naloxone drip 0.4 mg/hr was begun and he was admitted. He was discharged the following day. He appeared depressed to family members but he ate a regular dinner before going to bed. The next morning he was found dead.
Autopsy Findings: Pills and pill particles were found in the stomach. There was dried yellow/orange substance inside and outside the mouth. Evidence of pulmonary aspiration of gastric contents was found. The cause of death was determined to be multidrug toxicity and respiratory arrest. Premortem blood: ethanol 0.01 g%, alprazolam 0.018 mg/L, carisoprodol 13 mg/L, meprobamate 7.8 mg/L, diazepam 0.02 mg/L, oxycodone 0.13 mg/L, quetiapine 0.004 mg/L, cannabinoids positive by immunoassay. Postmortem subclavian blood: ethanol blood 0.03 g% (vitreous not detected), alprazolam 0.007 mg/L, carisoprodol 20.2 mg/L, meprobamate 5.4 mg/L, paroxetine 0.11 mg/L, oxycodone 6.33 mg/L, cannabinoids positive by immunoassay.
Case 304. Acute parenteral methadone exposure: undoubtedly responsible.
Scenario/Substances: A 26 y/o male was found unresponsive by a roommate. A syringe containing pink tinged fluid was found at the scene and later confirmed to contain methadone. CPR was initiated. When EMS arrived, the patient was endotracheally intubated and defibrillated for a wide complex tachycardia. Atropine, epinephrine, vasopressin, and amiodarone were administered. The patient developed a perfusing rhythm and was transported to the ED.
Past Medical History: Depression, anxiety and drug abuse. Sertraline and alprazolam were recently prescribed.
Physical Exam: Unresponsive, dilated and non-reactive pupils, pale and clammy skin. Systolic BP 60, HR 70–80, T 32.6° C.
Laboratory Data: ECG: HR 81, QRS 146 msec, QTc 569 msec. Glucose 25, electrolytes unremarkable except HCO3 19, CK 367, troponin 0.19, AST 1489, ALT 3075, INR 1.3, UDS positive for methadone and benzodiazepines ethanol 79.8 mg/dL.
Clinical Course: On arrival to the ED, the patient became pulseless and resuscitation continued with atropine, epinephrine, sodium bicarbonate, and high dose naloxone. He developed a perfusing rhythm but remained hypotensive. He received IV crystalloid and multiple vasopressors for persistent hypotension. D50 IV bolus was given for the hypoglycemia. Head CT was consistent with diffuse cerebral edema. Multiple blood products were given to treat the DIC that developed. On Day 2, the clinical exam and cerebral perfusion study were consistent with brain death and medical support was withdrawn. Death was judged due to prolonged cardiac arrest with severe anoxic injury.
Autopsy Findings: Cerebral edema, pulmonary edema, hepatic parenchymal damage, and an ulceration of the cecum all presumed to be due to an anoxic episode. Toxicology: vitreous ethanol not detected, UDS positive for benzodiazepine and methadone. Blood methadone 0.08 mg/L. Contents of the syringe found at the prehospital scene identified as methadone.
Case 385. Acute-on-chronic citalopram, amlodipine, lisinopril, colchicine, allopurinol, and acetaminophen ingestion: undoubtedly responsible.Scenario/Substances: A 35 y/o male was found sitting in his car with empty bottles of his medications on his lap (citalopram, amlodipine, lisinopril, colchicine, allopurinol, and acetaminophen) and transported to the ED.
Past Medical History: Hypertension, depression with suicidal ideation, obesity, obstructive sleep apnea, gout, and back pain. He was changing from fluoxetine to citalopram.
Clinical Course: In the ED complaining of recent onset severe back pain, BP 66/43, HR 104, afebrile, O2 sat 90% on 100% oxygen. He had episodes of apnea O2 sat 80%. Glucose 199, Cr 2, BUN 17, acetaminophen 122 mcg/mL (2 hr post ingestion), salicylate not detected. AST was 34 initially and peaked at 742, ALT was 33 and peaked at 218. He received glucagon and calcium chloride with minimal effect then given pressors to maintain his BP and admitted to the ICU. The patient was put on a fentanyl patch for pain management. He remained hypotensive, developed bradycardia, went into shock with hypoxemia. The patient required an intra-aortic balloon pump, insulin for hyperglycemia, dialysis for renal failure and continued with IV infusion of n-acetylcycsteine. The patient became unresponsive, hyperkalemic (initially 4.8 peaking at 7.2) with heart block and wide complex QRS. He developed rhabodmyolysis, remained unconscious, with a poor prognosis. The family elected to give comfort measures only and he expired on Day 13.
Autopsy Findings: Cause of death was citalopram, acetaminophen and poly-prescription medication overdose. Manner of death was suicide. Hospital first blood: acetaminophen 1,207 mg/L, citalopram 24.3 mg/L, fentanyl 80 ng/mL, hydromorphone 28.6 ng/mL and unquantitated norfluoxetine and norcitalopram.
Case 424. Acute-on-chronic colchicine ingestion: undoubtedly responsible.Scenario/Substances: A 39 y/o male was found by his landlord with superficial slits to his wrists. Upon arrival to ED, he reported that he took 100 tablets of his medications the morning of his presentation. It was initially unclear which of his medications he had taken.
Past Medical History: Gout and hypertension. Mediations: colchicine, amlodipine/benazepril, aspirin, and allopurinol.
Physical Exam: Mild abdominal pain, normal vital signs, and superficial wrist lacerations.
Laboratory Data: Salicylate 21 mg/dL WBC 25, lactate 9.3 mmol/L, bicarbonate 16.3.
Clinical Course: Shortly after arrival to the ED he developed vomiting and profuse diarrhea but remained hemodynamically stable. Supportive care was instituted and ∼24 hr after arrival, he had a sudden cardiovascular collapse and was unable to be resuscitated.
Autopsy Findings: The postmortem colchicine level was 68 ng/mL. The medical examiner concluded that the death was acute suicidal overdose secondary to colchicine intoxication.
Case 433. Acute acetaminophen and diphenhydramine ingestion: undoubtedly responsible.Scenario/Substances: A 40 y/o male took an overdose of diphenhydramine and acetaminophen.
Past Medical History: Bipolar affective disorder; previous suicide attempts.
Physical Exam: Combative with dry mucous membranes and an altered sensorium. BP 97/50, HR 120, RR 42 - 48. Laboratory Data:

Lactate > 12 mg/dL, INR 6.89; PTT 52, ALT 6,692, AST > 9,000, lipase 1,662 U/mL, ammonia 273 mcg/dL, total bilirubin 8.4, direct 6.7, PO4 10.0 mg/dL, salicylates 2 mg/dL, acetaminophen 198 mg/dL, diphenhydramine 0.11 mg/L; Li, valproic acid, methanol, and ethylene glycol not detected. Head CT was unremarkable.
Clinical Course: The patient was treated as a late presenting acetaminophen overdose, with diphendyramine and possibly other co-ingestants. Fomepizole was started until toxic alcohols were ruled out. After sedation with benzodiazepines and fentanyl, IV N-acetylcysteine was given along with 3 units of FFP and CVVHD was started. He was resuscitated from a cardiopulmonary arrest shortly after arrival, but later became hypotensive, requiring multiple pressors and sodium bicarbonate for progressive metabolic acidosis. On Day 2 the family requested that comfort care only be given and the patient expired.
Autopsy Findings: Centrilobular necrosis of the liver with hepatomegaly was noted. The cause of death was attributed to acetaminophen and diphenhydramine toxicity.
Case 454. Acute acetaminophen and diphenhydramine ingestion: undoubtedly responsible.Scenario/Substances: A 42 y/o female was found unresponsive at home by family members with 2 empty bottles of over-the-counter acetaminophen with diphenhydramine at her bedside and evidence of vomiting. EMS transported the patient to the ED; Naloxone 4 mg, was given enroute with unknown effect.
Past Medical History: Lupus erythematosis, fibromyalgia.
Physical Exam: Unresponsive, with fixed and dilated pupils. BP 65, HR 111, O2 sat 100% (FiO2 unknown), T 32.7°C. Bilateral Babinski sign noted, absent corneal reflexes. ECG: HR 95 with slight QT/QTc prolongation at 450/565.
Laboratory Data: ABG-pH 7.238/pC02 18.9/p02 286. WBC 7.1, Hgb13.9, Hct 42.5, platelets 204.
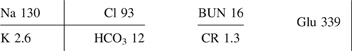
Ca 8.0, AST 40, ALT 39, INR 1.16, PTT 29. APAP 761 mcg/mL, salicylate 6.5 mg/dL, alcohol not detected. UDS positive for benzodiazepines and tricyclics antidepressants.
Clinical Course: The patient was intubated, placed on a ventilator, and given IV fluids and IV N-acetylcysteine. CxR and CT of the head were normal. In ICU she remained unresponsive with decerebrate posturing. She was alkalinized and given pressors to maintain BP. Antibiotics, vitamin K, valproic acid and supplemental Mg and Ca were given. She was placed on warming blanket initially for hypothermia, then cooling blankets for increased T (39.6°C). Hypotension continued with acidosis (pH 7.11) despite HCO3 infusions. Oxygenation decreased to 80% O2 sat on 100% FIO2. Hepatic function increased: AST 4,429, ALT 1,934 U/L: Cr rose to 2.8, INR 9.7. Troponin 29.71. The family changed the patient's status to comfort measures and she expired on Day 3.
Autopsy Findings: Gross exam noted bilateral pleural effusions. Microscopic findings included congestion of the lungs, and hepatic necrosis with loss of normal hepatic architecture and necrosis of hepatocytes and portal triad. Acetaminophen 780 mcg/mL, diphenhydramine 6,600 ng/mL. Cause of death was complications of multidrug overdose and the manner of death was suicide.
Case 576. Acetaminophen ingestion: undoubtedly responsible.Scenario/Substances: A 54 y/o male called EMS who found him vomiting, lethargic and then unresponsive. Naloxone was administered with no response
Past Medical History: Hypertension and depression.
Laboratory Data: Initial acetaminophen 1,134 mcg/mL, salicylate and ethanol not detected, ammonia,31umol/L, AST 17, ALT 15, bilirubin 0.3, lactate 4.2 mmol/L, ABG-pH 7.36/pCO2 38/pO2 92/HCO3 21, INR 1.2, ECG unremarkable. Toxicology screens for cocaine, tricyclic antidepressants, opiates, and phencycladine were negative.
Clinical Course: ED VS: HR 90, BP 93/47, RR 28, afebrile. Acetaminophen concentrations were 633 mcg/mL @ 11.7 HR 120 mcg/mL @ 11.7 HR and 13 mcg/mL @ ∼55 hr after the initial sample. He was intubated due to worsening altered mental status, 21 hr IV N-acetylcysteine was initiated and the patient was admitted to the ICU. Maximum hepatic enzymes were reported on Day 4 were AST 3641, ALT 7783. On Day 6, bilirubin was 4.0, his hepatic enzymes began to decrease, he was extubated and placed on bilevel positive airway pressure. On Day 7 he developed respiratory failure and was reintubated, but he developed intractable metabolic acidosis and hypotension and expired.
Autopsy Findings: Microscopic hepatic necrosis. Cause of death: acetaminophen ingestion, sepsis, DIC, and pneumonia.
Case 596. Chronic acetaminophen ingestion: undoubtedly responsible.Scenario/Substances: A 56 y/o female ingested 8 g of acetaminophen daily (2 g every 6 hrs) for 3 days because she could no longer get narcotic pain medications from her physicians.
Past Medical History: Pancreatitis, congestive heart failure, recurrent urinary tract infections, opiate abuse, previous methadone and dilaudid use for chronic pain. Medications included montelukast, methadone, esomeprazole, gemfibrozil, carisoprodol, diazepam and pyridium.
Physical Exam: Lethargic female.
Laboratory Data: AST 1843, ALT 698, acetaminophen 8.3 mg/L. Day 2: AST 3766, ALT 1515, acetaminophen 12.1 mg/L, INR 7.0. Day 3: AST 3645, ALT 2122, INR 12.0, bilirubin 5.8, alk phos 131.
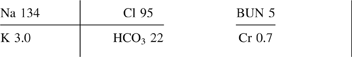
Bilirubin peaked at 17.8. Day 7: AST 109 and ALT 606.
Clinical Course: Oral N-acetylcysteine was started. The patient transferred to a tertiary care HCF for potential liver transplant. Enroute to the second HCF, she became obtunded and was intubated during transfer and placed on a ventilator at accepting HCF. Metabolic acidosis (pH 6.9) was treated with IV fluids and bicarbonate; vasopressin, CVVHD, FFP and Factor VII were also given. She became less responsive and mannitol was administered. Head CT showed no cerebral edema or bleeding. She was not considered a viable liver transplant candidate due to her deteriorating mental status and history of narcotic drug abuse. N-acetylcysteine was changed to continuous IV dosing. Day 10 she became septic was given antibiotics and pressors to support BP. Comfort measures were instituted and she expired on Day 10.
Autopsy Findings: Not performed.
Case 643. Acetaminophen, ethanol ingestion: undoubtedly responsible.Scenario/Substances: A 70 y/o male visited a funeral home, planned his own service, and purchased multiple bottles of acetaminophen. He was found by his family confused and wandering in his back yard and brought to the ED. The acetaminophen bottles were later found by family members.
Past Medical History: Depression, bipolar disorder, hyperthyroidism and dyslipidemia. Medications included perphenazine, levothyroxine, quetiapine, fluoxetine, pravastatin, and aspirin.
Physical Exam: The patient was confused and lethargic.
Laboratory Data: Acetaminophen 347 mg/L; AST 128, ALT 156, INR 1.5. Urine drug screen was negative, salicylate was not detected.
Clinical Course: He was intubated, started on N-acetylcysteine and transferred on Day 2 to a tertiary healthcare facility. Day 2 BP 70/60 despite vasopressors, HR 120's, acetaminophen 558 mg/L, AST 4851, ALT 6310, INR 4.4; later that day acetaminophen 598 mg/L; pH 6.5, HCO3 11, lactate 14.5, Cr 5, K 5. Fomepizole was initiated; norepinephrine and bicarbonate infusion continued. The patient became anuric with fixed and dilated pupils, absent gag reflex, and was not breathing over the ventilator. FFP was given; Repeat AST 11,312, ALT 12,482, alk phos 157, ammonia 345 mmol/L, INR 4.3. Ethylene glycol, methanol, and isopropyl levels were not detected. Due to multisystem organ failure, comfort measures were instituted and he expired on Day 3.
Autopsy Findings: No autopsy or post mortem toxicology was performed. Cause of death was determined to be acetaminophen toxicity due to ingestion.
Case 697. Acute-on-chronic valproic acid and gabapentin ingestion: undoubtedly responsible.Scenario/Substances: A 41 y/o male intentionally ingested an estimated 180 tablets of valproic acid and 10 gabapentin doses about 3.5 hr prior to ED arrival.
Past Medical History: Hepatitis C, bipolar disorder, seizure disorder
Physical Exam: In the ED he was alert and oriented, HR 122, BP unremarkable, normal O2 sat.
Laboratory Data: Valproic acid 867 mg/L, Hepatic panel normal, ammonia 55 mcg/dL, WBC 2400, platelets 119; acetaminophen and salicylate not detected.
Clinical Course: He had a steady decline in mental status within the first 2 hr and required intubation. A repeat valproic acid level before dialysis was 1060 mg/L. Following dialysis on Day 1, the patient's repeat vital signs were reported as stable and a repeat valproic acid level was 301 mg/L. On Day 2 the valproic acid level was 517 mg/L then 541 mg/L. L-carnitine was administered via NGT along with a 50 gram repeat dose of activated charcoal. A repeat valproic acid was 585 mg/L. On Day 3 the valproic acid was 300 mg/L and the ammonia 115 mcg/dL. The patient was not dialyzed again but did continue to receive L-carnitine and lactulose while remaining intubated and sedated. Further valproic acid levels were 146 mg/L then 94.1 mg/L. On Day 5, the patient developed a wide QRS complex rhythm (QRS reported 150 ms) with hr in the 72–100 range and BPs in the systolic 90–100 range and expired later that day.
Autopsy Findings: Valproic acid overdose with mild myocarditis and marked bilateral pulmonary edema and congestion. The cause of death was cardiac arrhythmia induced by valproic acid overdose. Manner of death listed as suicide. The report also mentions “rising troponin levels and no significant atherosclerotic changes. The report concludes noting that cardiac arrhythmias, although not the most frequent complication, are documented in medical literature in association with valproic acid intoxication (of note, the arrhythmia occurred on Day 5 after the valproic acid level was down to 94.1 mg/L).
Case 700. Acute valproic acid (extended release) ingestion: undoubtedly responsible.Scenario/Substances: A 53 y/o male ingested an unknown amount of valproic acid in a suicide attempt and was brought to the ED.
Laboratory Data: Upon arrival at the ED, acetaminophen and salicylate were not detected, valproic acid was 615 mcg/mL.
Clinical Course: The patient was lethargic, non-verbal, arousable to painful stimuli only with a systolic BP 98. The patient was admitted to the ICU. Naloxone was administered, multiple dose activated charcoal was begun, and a head CT scan was performed. The patient was intubated and ventilated and lorazepam 2 mg was given for sedation. On Day 2 BP 218/88, QTc 480 msec; dialysis was performed and the patient received L-carnitine. Several hr later, dialysis was repeated. The patient received neosynephrine for hypotension. On Day 3, BP 94/38 and HR 96 on neosynephrine, RR 12 (ventilated), O2 sat 93%. The patient developed a fever to 39.2° C. Valproic acid 665 mcg/mL, ammonia 1249 mcg/mL, AST 22, ALT 23, Alk phos 111, BUN 20, Cr, 2.7, 8 hr later valproic acid 676 mcg/mL, ammonia 1626 mcg/mL. A seizure occurred; corneal reflexes were absent. Lactulose was administered. Ammonia 1697 mcg/mL, valproic acid 521 mcg/mL. After dialysis valproic acid in the 300s mcg/mL, ammonia 1700 mcg/mL. About 12 hr later, valproic acid 399 mcg/mL, ammonia 2200 mcg/mL; the patient's pupils were fixed and dilated. The patient remained profoundly hypotensive despite intensive vasopressors. Antibiotics were initiated for hyperthermia. The patient could not tolerate CVVHD, comfort measures were instituted, and he expired.
Autopsy Findings: Not performed.
Case 703. Acute-on-chronic valproic acid (extended release), ethanol ingestion: undoubtedly responsible.Scenario/Substances: A 55 y/o female ingested 100 of her 500 mg valproic acid tablets in an apparent suicide attempt. EMS transported her to the ED ∼1 hr after ingestion.
Past Medical History: Bi-polar disorder, depression, hypertension, alcoholism and multiple arrests for drug violations.
Physical Exam: Agitated. BP 96/50, HR 100, RR 20s, T 34°C.
Laboratory Data: ABG-pH 7.04/pCO2 52/pO2 56, O2 sat 73%. HCO3 13. Valproic acid levels (1 hr post ingestion) 667 mg/L, 7 hr post ingestion 1336 mg/L and 12 hr post ingestion 1457 mg/L. Ammonia 246 mcg/dL.
Clinical Course: She was intubated for airway protection, received 25 g charcoal and started on whole bowel irrigation. BP dropped to 60/30. She was given 4 boluses of bicarbonate and IV norepinephrine, dopamine and phenylephrine. CRRT was considered, but not started as the patient had fixed and dilated pupils, continued hypotension. Comfort measures were instituted and she expired on Day 2.
Autopsy Findings: Copious liquid charcoal in mouth, esophagus, and in the airways extending into the right lung
Aspiration pneumonia of charcoal in posterior right upper lobe of lung and right lower lobe of lung; s/p bilateral chest tube placement with hemothorax of both lungs; anasarca; pulmonary and cerebral edema; atheromatous changes to mitral valve, arterionephrosclerosis. Ethanol 0.29 grams%, valproic acid 415 mg/L. Cause of death: Valproic acid overdose with acute alcohol intoxication.
Case 762. Acute-on-chronic desipramine ingestion: undoubtedly responsible.Scenario/Substances: A 50 y/o female emailed a suicide note to her friend and was found unresponsive in her house with myoclonic movements and 2 nearby empty bottles of a recently filled desipramine prescription.
Past Medical History: Depression. Medications: lamictal, risperdal, and clonazepam.
Physical Exam: Unresponsive patient. BP 89/72, HR 91. Pupils reactive at 4 mm, dry mucous members, myoclonic movements.
Laboratory Data: ABG-pH 7.46/pCO2 38/pO2 480 (intubated); WBC 6.4, Hgb 9, Hct 27.5, platelets 339,
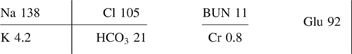
lactate 1.8, ALT 12, AST 22, CPK 89, UDS negative except for benzodiazepines which were likely hospital administered.
Clinical Course: The patient was intubated in ED and sedated with propofol. ECG: QRS 160 msec with prominent R wave in lead aVR; 2 boluses of sodium bicarbonate given, BP increased to 126/87 and QRS decreased to 130 msec. Within a few hours, hypotension and myclonis returned, treated with sodium bicarbonate infusion, norepinephrine then propofol and midazolam with increase in BP noted. On Day 3 she became febrile and aspiration pneumonia was considered. On Day 4 the sodium bicarbonate was discontinued with QRS at 120 msec. On Day 5 he developed a wide complex tachycardia with hypotension on pressors. Intralipid were administered without effect. Aystole ensued and the patient expired shortly thereafter. An ECG performed after the sodium bicarbonate was discontinued showed QRS >160 msec. Day 5 desipramine level taken pre-intralipid: 1949 mcg/L; post-intralipid 2343 mcg/L.
Autopsy Findings: No autopsy performed.
Case 776. Acute-on-chronic paroxetine (extended release) ingestion: undoubtedly responsible.Scenario/Substances: A 56 y/o male told his wife he took 180 25 mg paroxetine tablets over the preceding 12 hrs.
Past Medical History: Depression and attention-deficit/hyperactivity disorder. Medications: paroxetine, atomoxetine and bupropion.
Physical Exam: Drowsy but responsive, with tremors and diaphoresis. BP 160/95; P 92; RR 30; 36.7°C; ECG: sinus rhythm, first degree AV block.
Laboratory Data: Na 131, glucose 152; APAP, salicylate and ethanol not detected;.
Clinical Course: VS (9 hr after hospital arrival): BP 151/71, P 81, T 37.1°F. Clonus, lower extremity hyperreflexia, muscle rigidity and altered mental status developed at 13 hrs. Lorazepam (2 mg) and IV fluids were administered. ECG showed QTc 520 msec. Serotonin syndrome was diagnosed and cyproheptadine 4 mg every 8 hours was started. Day 2, BP 143/92, P 75, RR, 20, T 36.1°C. Tremors, confusion and skin flushing continued. Laboratory Na 140, K 3.6, Cl 110, HCO3 24, BUN 15, Cr 0.8, Ca 8.9, glucose 91, alk phos 74, AST 21, ALT 18, CPK reported as normal; QTc 515 msec. Lorazepam and cyproheptadine were continued. Day 4 agitation and diaphoresis increased, restraints were applied and lorazepam dosage increased. CK peaked at 4000; Urine output was normal. Day 7: the patient was intubated, sedated and ventilated; Cyproheptadine was discontinued. Day 8: clinical improvement, patient extubated but agitation persisted. Day 9: Respiration became agonal and the patient expired.
Autopsy Findings: Medical examiner's stated probable cause of death was serotonin syndrome due to paroxetine overdose. Post-mortem blood paroxetine 5.6 mg/L.
Case 800. Acute bupropion (extended release) ingestion: undoubtedly responsible.Scenario/Substances: A 12 m/o female ingested up to 12 tablets of bupropion 300 mg extended release from her mother's purse. Mother brought child to ED immediately.
Physical Exam: Playful. No vitals signs available.
Clinical Course: She received 1 dose of activated charcoal before the first seizure (4 hours post ingestion) treated with midazolam. She was transferred to a tertiary care center pediatric ICU where she experienced 2 more seizures at 6 hours post ingestion then status epilepticus at 12 hr post ingestion. She received benzodiazepines, barbiturates and propofol which did not terminate the seizures. Continuous EEG monitoring was started and intralipid 1.5 mL/kg IV bolus then 0.25 mL/kg/min with the intent of 1 hr administration. Seizures stopped 5 minutes after the bolus of intralipid. Severe hypotension occurred every time the infusion of intralipid was stopped. Intralipid 0.25 mL/kg/min was given intermittently over 5 hrs. in an off and on manner for the next 5 hours. Her CBC and serum electrolytes were un-measurable because of hyperlipidemia from the intralipid infusion. She developed massive cerebral edema and expired 24 hr after presentation.
Autopsy Findings: Ten partially dissolved tablets were recovered from the large intestine. The cerebral cortex and cerebellum were pink, the brainstem was intact. The heart was intact. Acute lung injury with hyaline membrane perforation, pulmonary edema and multifocal bronchopneumonia and intravascular lipid droplets were reported. Post-mortem liver bupropion concentration was 4.0 mg/kg. The cause of death was bupropion overdose and the manner of death was accidental.
Case 802. Acute-on-chronic amitriptyline ingestion: undoubtedly responsible.Scenario/Substances: A 80 y/o female took an intentional overdose of amitriptyline, called 911, was found minimally responsive by EMS, she was endotracheally intubated and fluid resuscitation was begun enroute to the ED.
Past Medical History: Her medications included amitriptyline 5mg, ∼90 tablets were missing from the bottle.
Physical Exam: Vital signs on admission: HR 80, BP 60/P, T 36.2° C, RR 12–14 (ventilated), O2 Sat 99% with FiO2 100%. She was unresponsive and noted to have intermittent 10 sec tonic-clonic seizures.
Laboratory Data: ABG-pH 7.56/pCO2 48/pO2 315/HCO3 23; acetaminophen, salicylate, and ethanol not detected; electrolytes and hepatic enzymes were unremarkable, ECG: HR 80, QRS 200 ms.
Clinical Course: On arrival, the patient was placed on a ventilator, a nasogastric tube was placed, and activated charcoal given. Sodium bicarbonate (6–7 amps) was given IV and fluid resuscitation was continued. Norepinephrine was given for BP support, lorazepam for seizure activity and the patient was admitted to the ICU. Her hemodynamic status continued to worsen, ∼5 hr after admission she sustained a cardiopulmonary arrest and resuscitation was unsuccessful.
Autopsy Findings: Atherosclerotic cardiovascular disease without acute infarction and multifocal consolidations of both lungs. Post-mortem amitriptyline 14.5 mg/L, nortriptyline 4.77 mg/L, fluoxetine 1.21 mg/L, norfluoxetine 2.27 mg/L (all in cardiac blood), codeine: 0.03 mg/L (vitreous). While the tricyclic antidepressant measurements are well above those known to be associated with fatalities, the fluoxetine, ratio of fluoxetine/norfluoxetine, and codeine concentrations were consistent with therapeutic exposure.
Case 817. Acute vincristine infusion: undoubtedly responsible.Scenario/Substances: A 52 y/o female with a CNS lymphoma presented for outpatient infusions of IV vincristine and intracerebroventricular methotrexate via an Ommaya reservoir. The vincristine (2 mg), which was intended for IV administration, was also infused into the Ommaya reservoir.
Past Medical History: CNS lymphoma.
Physical Exam: Her initial vitals signs and physical examination findings were unremarkable.
Clinical Course: Approximately 15 min after the injection of vincristine into the Ommaya reservoir the error was realized and 30 mL of CSF was aspirated from the reservoir. The patient was transferred to the neurosurgical ICU for CSF exchange and ventriculolumbar lavage. FFP was added to the perfusate and dexamethasone, glutamic acid, pyridoxine and folinic acid were administered intravenously. The patient was unable to tolerate ventriculolumbar lavage reporting a severe headache when the rate was increased and the intracranial pressure increased resulted in bradycardia. The patient had no complaints or neurological deficits until Day 3, when she developed hearing loss, headache, and left lower extremity weakness. Her symptoms progressed rapidly with ascending paralysis, autonomic dysfunction, respiratory failure, and coma. Electroencephalography on Day 6 was consistent with brain death and she expired on Day 12. Death was attributed to progressive ascending myeloencephalopathy, autonomic dysfunction and hypotension.
Autopsy Findings: No autopsy was performed.
Case 818. Acute vincristine CNS infusion: undoubtedly responsible. Scenario/Substances: A 63 y/o male seen in an oncology clinic for 6th cycle of methotrexate for HIV-related Burkett's lymphoma to be administered via his Ommaya reservoir. The patient inadvertently was given approximately 15ml (2mg) of vincristine instead of methotrexate via the reservoir. The infusion was stopped immediately and ∼14 ml of fluid aspirated, the Ommaya reservoir was flushed with normal saline multiple times. The patient was asymptomatic during the reservoir irrigation but later developed nausea and vomiting prior to transfer to the ED.
Past Medical History: HIV, Burkett's lymphoma, R hip Herpes Zoster. Medications: antiretroviral therapies for HIV.
Physical Exam: Awake, alert and oriented ill appearing patient complaining of generalized weakness and nausea without pain or other complaints. Afebrile, HEENT, abdomen, Neuro exam intact cranial nerves, motor normal except 4/5 lower extremity strength; absent clonus, Babinski and tremor.
Laboratory Data: WBC 2.6, electrolytes normal. CxR increased bronchovascular markings, CT head normal except for catheter in place.
Clinical Course: Interventional radiology placed drain in lumbar-sacral spine and irrigated via a butterfly needle in the Ommaya reservoir at 10 ml/hr for approximately 10 hrs. The drain clogged 24 hours post exposure and the left upper extremity was weaker. Day 2 noted decreased mental status with inconsistent responses to verbal cues with anisocoria and worsened upper and lower extremity weakness. Day 4 the patient did not follow verbal commands and appeared quadriplegic. Palliative care was instituted on Day 7; the patient expired on Day 10.
Autopsy Findings: Not available.
Case 826. Acute propafenone ingestion: undoubtedly responsible.Scenario/Substances: An 18 y/o female ingested 50 of her grandmother's 150 mg propafenone tablets.
Past Medical History: Previous suicide attempts, schizophrenia, substance abuse.
Physical Exam: Unresponsive with multiple prior self inflicted injuries noted on her arms.
Laboratory Data: pH 7.4 after resuscitation.
Clinical Course: She developed a widened QRS upon arrival to the ED and had a brady/asystolic arrest ∼3.5 hr after ingestion. After multiple cardiac arrests she developed a wide complex tachycardia. Norepinephrine and bicarbonate infusions were administered along with intermittent bicarbonate boluses. She expired 7 hours after ingestion after the wide QRS no longer responded to bicarbonate boluses.
Autopsy Findings: Pulmonary congestion and edema. Post mortem aortic blood: acetone 7 mg/dL, clozapine 0.15 mg/L, diazepam 0.025 mg/L, norsertraline 2.1 mg/L, propafenone 11 mg/L, and sertraline 0.72 mg/L; Iliac vein blood: norsertraline 0.99 mg/L, sertraline 0.61 mg/L: Liver tissue: norsertraline 61 mg/kg, sertraline 6.9 mg/kg. Medical examiner cause of death: propafenone overdose.
Case 829. Acute metoprolol, amlodipine/benazepril, citalopram ingestion: undoubtedly responsible.Scenario/Substances: A 21 y/o male ingested citalopram, metoprolol and amlodipine/benazepril. EMS found a HR 34 with an initial irregular rhythm, but enroute he became asystolic and they started CPR.
Clinical Course: In the ED he was unresponsive with a HR 51 and a prolonged PR interval. He received calcium chloride sodium bicarbonate, and glucagon with a brief return of pulse. Resuscitation continued with maximum doses of norepinephrine and then dopamine, calcium chloride + glucagon + insulin infusions with BP 60/30, HR 44, O2 sat 94% on ventilator. He was transferred to the ICU on dopamine and vasopressin infusions, placed on a bicarbonate drip for a pH of 6.9 and continued on glucagon + calcium chloride infusions. Insulin infusion was stopped for a glucose of 64. HR 78 and BP 79/44 by arterial line. Intraplipid was started. About 7 hr later, the patient was receiving maximum dose epinephrine, norepinephrine and levophed infusions, insulin and 10% dextrose infusions and midazolam. He developed ARDS and shock liver with hepatic enzymes in the 3000's. Urine output declined, he had evidence of poor peripheral perfusion, and he died.
Autopsy Findings: Cause of death: intoxication with metoprolol, manner of death: suicide by ingestion of prescription medication. Autopsy: coronaries free of atherosclerosis; concentric hypertrophy of left ventricle without dilation; trachea filled with serosanguinous fluid; symmetrical moderately swollen cerebral hemispheres, flattened gyri and narrowed sulci, uncal lobes were notched but without definite herniation, cerebellar tonsils were not herniated, compression of the lateral ventricles. Post-mortem toxicology: ethanol, 38 mg/dL, acetaminophen 8.7 mcg/mL, clonazepam 4.8 ng/mL, 7-aminoclonazepam 46 ng/mL, diazepam 60 ng/mL, metoprolol 7500 ng/mL, atropine 8.6 ng/mL, citalopram/escitalopram 120 ng/mL, caffeine + in blood. Urine positive for benzodiazepines and cannabinoids.
Case 845. Acute propafenone ingestion: undoubtedly responsible.Scenario/Substances: A 40 y/o male took an overdose of lisinopril and propafenone in a suicide attempt and EMS transported him to the ED.
Physical Exam: BP 116/65, HR 145, RR 14, O2 sat 96% on room air, slurred speech, able to answer questions, exam otherwise unremarkable.
Laboratory Data: ECG: HR 71, left axis deviation, PR 238 ms, QRS 170 ms, QTc 545 ms.

CBC unremarkable, calcium 9.0, CK 20, troponin I <0.04 ng/mL, ethanol 284 mg/dL, acetaminophen not detected, salicylate 4.4 mg/dL, UDS negative.
Clinical Course: Gastric lavage was performed in the ED with return of orange pill fragments. Charcoal was given through the orogastric tube. Chest and abdominal x-rays and chest CT showed radiopaque substance dispersed in bilateral lung bases and throughout small intestine. Patient became unresponsive and was intubated. He became hypotensive with systolic BP in the 50s, received normal saline boluses, sodium bicarbonate, calcium, atropine, and a norepinephrine infusion was started. Several seizures were noted. Repeat ECG showed further QRS widening and sodium bicarbonate were given. The patient became pulseless and CPR was initiated. The patient died before planned lipid emulsion could be given.
Autopsy Findings: Mild narrowing (0–25%) of the left anterior descending and right coronary arteries, pulmonary congestion, and congestion of abdominal viscera. Blood ethanol level 260 mg/dL. Amphetamines, phencyclidine, methadone, cannabinoids, propoxyphene, barbiturates, cocaine metabolites, benzodiazepines, and opiates not detected. No lisinopril level is reported. Propafenone (cardiac blood) 8.3 mcg/mL, with reference trough propafenone levels listed as 0.2–1.5 mcg/mL.
Case 847. Acute-on-chronic verapamil, quetiapine, and ethanol ingestion: probably responsible.Scenario/Substances: A 42 y/o female ingested 20 quetiapine, 30 verapamil and unknown quantity of ethanol ∼3 hr prior to arrival at the ED.
Past Medical History: Bipolar disorder, parenteral drug abuse, hepatitis C positive, chronic pain, headaches and seizures associated with a brain tumor diagnosed 6 years earlier for which she was receiving chemotherapy. Other medications included clonazepam, quetiapine, verapamil, amitriptyline, dihydromorphinone, and butalbital.
Physical Exam: In the ED BP 94/51, HR 133, pupils 1–2 mm, drowsy, but arousable.
Laboratory Data: Ethanol 131 mg/dL, UDS positive for opiates; acetaminophen and salicylate not detected.
Clinical Course: Whole bowel irrigation was initiated without an NG tube, but she became obtunded and ∼4 hr later an NG tube was placed and whole bowel irrigation restarted. At ∼11 hr after ED admission she had a cardiorespiratory arrest, ACLS resuscitation was initiated, a temporary pacer was placed, but resuscitation was unsuccessful and she expired.
Autopsy Findings: Antemortem blood: quetiapine 0.55 mg/L, verapamil 0.02 mg/L, morphone 0.008 mg/L. Post mortem blood: ethanol 93 mg/dL, morphine 0.0101 mg/L, quetiapine 2.2 mg/L (therapeutic 0.04–0.4 mg/L), verapamil 2.9 mg/L (therapeutic 0.07–0.35 mg/L)
Case 853. Acute metoprolol, metformin, amlodipine, hydrochlorothiazide, zolpidem ingestion: undoubtedly responsible.Scenario/ Substances: A 45 y/o female found somnolent and incontinent of stool with slurred speech and a suicide note approximately 9 hr after taking a large overdose of multiple medications belonging to a friend. Based on pill counts, the exposure was estimated as 10 metoprolol 25 mg tablets, 9 metformin 1000 mg tablets, up to 30 amlodipine 10 mg tablets, and unknown amounts of hydrochlorothiazide, irbesartan, sitagliptin, fenofibric acid and zolpidem.
Past Medical History: Diabetes and hepatitis C.
Physical Exam: Lethargic, but able to follow commands. BP 60/20, HR 50, RR 20–30 and T 33.1°C with O2 sat 100% on room air.
Laboratory Data: ABG-pH 6.91 pCO2 32 pO2 66.
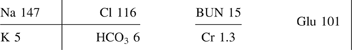
Lactate 18.1 mmol/L; acetaminophen and salicylate not detected; ethanol 80 mg/dL. ECG: SR at 50, narrow complex, 1st degree AV block.
Clinical Course: The patient was intubated and given IV fluids and multiple doses of glucagon and a glucagon drip, IV calcium, and multiple vasopressors, ultimately requiring maximum doses of dopamine, norepinephrine, phenylephrine, epinephrine and vasopressin without improvement of hemodynamic status. Day 1. Na 143; K 3.6; Cl 104; HCO3 6; Glu 293; BUN 10; Cr 1.2; Anion gap 33; lactate 24.5 mmol/L; WBC 34.2; Hgb 7.1; Ethanol 53 mg/dL; AST 541; ALT 134. She was hemodialyzed on Day 1 and Day 2 for lactic acidosis. Day 3 ABG pH 7.17/pCO2 60/pO2 41; Na 118, K 5.1, BUN 22, Cr 1.9; Lactate 10.2 mmol/L. The patient remained unresponsive with fixed and dilated pupils, and continued hypotension and difficulty oxygenating. The patient's family elected the institution of comfort measures and she expired on Day 4.
Autopsy Findings: No autopsy was performed. Postmortem peripheral blood: amlodipine 360 ng/mL, metformin 59 mcg/mL, hydrochlorothiazide 0.34 mcg/mL, metoprolol 150 ng/mL, midazolam 40 ng/mL, zolpidem 19 ng/mL, ethanol 35mg/dL. Urine analysis (time unknown): nicotine 12 ng/mL, cotinine 12 ng/mL and eszopiclone/zopiclone 29 ng/mL.
Case 856. Acute diltiazem (extended release) ingestion: undoubtedly responsible.Scenario/Substances: A 46 y/o female found unresponsive 5–6 hours after taking 120 of a friend's 60 mg sustained release diltiazem tablets.
Physical Exam: Unresponsive, BP 41/25, HR 59.
Laboratory Data: ECG: 3rd degree heart block.
Clinical Course: The patient was intubated, received 2 liters IV fluids, 2 amps calcium gluconate, 4 mg glucagon, 2 amps atropine, and norepinephrine infusion. 90 minutes after arrival insulin infusion (75 units) was started while pacemaker placement initiated. The patient expired before the pacemaker was placed.
Autopsy Findings: A thick gray liquid was noted in the stomach, admixed with a soft mass of material that included small granules consistent with the medication. Diltiazem in postmortem blood 10.1 mg/L; gastric contents 4.2 g; and liver 58.4 mg/kg. Death was determined to be the result of an acute large ingestion of diltiazem.
Case 863. Acute diltiazem, benzodiazepine ingestion: undoubtedly responsible.Scenario/Substances: A 38 y/o female ingested 240 mg sustained release diltiazem tablets and possibly benzodiazepines in an apparent suicide attempt.
Past Medical History: Depression, anxiety, bipolar disorder, seizure disorder, asthma, peptic ulcer disease and prior suicide attempts.
Physical Exam: Intubated and sedated; bradycardic and hypotensive; lower extremity edema. Pupils 6 mm and nonreactive, facial grimace with pain only but no withdrawal to pain.
Laboratory Data: Ca 7.1, Mg 1.3.

Clinical Course: Asystole occurred requiring calcium chloride IV, atropine, dopamine, norepinephrine, epinephrine, sodium bicarbonate and a temporary pacemaker. Due to hypotension, she was given glucagon 5 mg/hr, CaCl, atropine, insulin (35 U/hr) and glucose, norepinephrine 60 mcg/min, dopamine 50 mcg/min, vasopressin 0.4 mcg/min and IV lipid emulsion after which she stabilized. She later developed ARDS and multisystem organ failure. The family decided to withdraw support and she expired on Day 3.
Autopsy Findings: No autopsy was performed. Cause of death was listed as multiple drug intoxication. Manner of death was suicide.
Case 869. Acute-on-chronic diltiazem extended release ingestion: undoubtedly responsible.Scenario/Substances: A 49 y/o male ingested several handfuls of his extended release diltiazem in a suicide attempt at about midnight. The empty bottle had contained 90 240 mg tablets. He was transported to the ED.
Past Medical History: Hypertension, atrial fibrillation, pacemaker.
Laboratory Data: A volatile alcohol screen was negative.
Clinical Course: In the ED he was alert with BP 71/51, ECG showed a paced rhythm at 60 with underlying atrial fibrillation and frequent premature ventricular beats. He received IV crystalloid, an oral dose of activated charcoal, 3 amps of calcium gluconate, and was admitted to the ICU. A central line was placed and a norepinephrine drip maintained his systolic BP in the range of 80. At 10 hr post-ingestion: he became diaphoretic, his mental status deteriorated, he required intubation, was placed on a ventilator; dopamine was added with no response in BP. He was started on high dose insulin and vasopressin infusions, and 3 grams of IV calcium chloride were administered. Continued deterioration prompted epinephrine and phenylephrine infusions. An intra-aortic balloon pump was placed but he developed progressive metabolic acidosis, refractory hypotension and oliguric renal failure. IV fat emulsion was administered without improvement. He was started on continuous renal replacement therapy, but acidosis and hypotension progressed. Comfort measures were instituted and he expired 33 hr after ingestion.
Autopsy Findings: Autopsy was not performed. Cause of death was diltiazem overdose. Ante mortem blood diltiazem 9 hr post-ingestion was 0.63 mcg/mL.
Case 884. Acute-on-chronic nifedipine ingestion: undoubtedly responsible.Scenario/Substances: A 56 y/o male ingested 2.7 grams of nifedipine.
Past Medical History: End-stage renal disease.
Physical Exam: Systolic BP 65–80, HR 120.
Laboratory Data: Glucose 55, Ca 8.8 while on a calcium drip.
Clinical Course: Calcium gluconate, D50W, and glucagon were administered intravenously. The patient was admitted to the ICU and whole bowel irrigation was instituted as well as vasopressor therapy. 9 hr later, the patient decompensated and expired.
Autopsy Findings: Femoral blood nifedipine 9.8 mg/L. Cause of death: intoxication due to effect of nifedipine.
Case 892. Acute-on-chronic metoprolol, cocaine, ethanol ingestion: undoubtedly responsible.Scenario/Substance: A 59 y/o male took a bottle of his BP medication in a suicide attempt. EMS found him intoxicated and transported him to the ED.
Past Medical History: Atrial fibrillation, stroke, hypertension, and coronary artery disease. Family reported he had stopped taking his prescribed warfarin.
Physical Exam: After arrival he became unresponsive, P 20, hypotensive. Pupils poorly responsive, lungs with bilateral crackles noted.
Laboratory Data: ABG-pH 7.1/pC02 28/p02 102, O2 sat 94%; WBC 11.5, Hgb15, Hct 45, platelets 281,
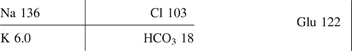
Ca 4.1, AST 125, ALT 134, INR 1.11, PTT 20.0, troponin <0.05 ng/mL, myoglobin 416 ng/mL; acetaminophen and salicylates not detected, blood alcohol 119, UDS positive for cocaine. ECG: atrial fibrillation with slow ventricular rate and diffuse ST-T changes.
Clinical Course: Despite glucagon, bicarbonate, norepinephrine, and dopamine infusions at maximum doses followed by neosynephrine, his condition worsened and asystole occurred. Resuscitation efforts were unsuccessful and the patient died on Day 1.
Autopsy Findings: No significant external or internal findings; antemortem blood metoprolol 1800 ng/mL, benzoylecgonine 490 ng/mL, and ethanol at 0.102 g/dL. Cause of Death was Metroprolol Overdose Manner of Death: Suicide.
Case 956. Acute sodium bicarbonate ingestion: undoubtedly responsible.Scenario/Substances: A 97 y/o male was found unresponsive surrounded by remains of an empty box of baking soda. He was transported to the ED.
Past Medical History: Implanted pacemaker/defribillator
Clinical Course: Soon after arrival in the ED he experienced a generalized seizure. His initial BP 141/70, HR 108, O2 sat 100%. He had a normal paced rhythm. The seizure responded to lorazepam and he was intubated and placed on a ventilator. His initial electrolytes: Na 158, HCO3 40, BUN 25, Cr 1.5. Repeat Na 180 lactate 4.5 ionized Ca 1.04. His vital signs deteriorated to BP 88/50, HR 100. Head CT scan and CxR were unremarkable. He was started on phenytoin. His implanted defibrillator had cycled multiple times and the patient had a cardiac arrest and was resuscitated. Na 178, HCO3 > 45. Hemodialysis was started, but he did not regain consciousness and a brain blood flow study revealed no perfusion. Comfort measures were instituted and he expired on Day 4.
Autopsy Findings: No autopsy was performed.
Case 958. Acute metformin ingestion: probably responsible.Scenario/Substances: A 4 y/o female went to bed at and awoke and vomited 4 hr later, complained of thirst and was given milk to drink. She awoke twice more complaining of thirst and was given something to drink. She was then found unresponsive 13 hr after going to bed. EMS found low glucose, gave IV glucose prior to transport. It was later discovered that the child's grandfather who visited 2 wks earlier, was taking metformin.
Past Medical History: Normal healthy child
Physical Exam: Responsive only to painful stimuli. BP 98/60, HR 157, O2 sat 98%.
Laboratory Data: ABG-pH 6.84/pO2 54/pCO2 21, lactate > 20 mmol/L; glucose 47, then > 150 on dextrose infusion. UDS negative.
Clinical Course: She was started on D5W, then a 12.5 gram bolus of dextrose, and then D10W. Acidosis was treated with sodium bicarbonate IV. Repeat glucose 435 mg/dL. Disconjugate gaze developed with a normal head CT scan. She experienced hypotension, anuria, acidosis, bloody emesis and rising troponin measurements. Day 3, shortly after intubation, she developed bradycardia unresponsive to resuscitation efforts and she expired. A urine sample came back positive for metformin confirmed by GC/MS. Serum tox screen was negative.
Autopsy Findings: Autopsy results showed generalized visceral congestion and secondary ischemic necrosis. The coroner found that blood (sample timing unknown) contained a “small amount” of metformin. Postmortem toxicology showed acetaminophen 12.8 mg/L and was negative for other prescription drugs including metformin, ethanol, and illicit drugs. The cause of death was reported as hypoglycemic shock due to metformin toxicity.
Case 964. Acute insulin injection: undoubtedly responsible.Scenario/Substances: A 34 y/o medical assistant lived and worked at a substance abuse rehabilitation facility where he had previously been a patient. He told his mother he was going to steal insulin from the medication room because it could kill him. Two weeks later he was found down in his room surrounded by vomitus; his glucose was <20. A fellow employee later reported seeing empty insulin glargine vials in his room along with a bag of what was presumed to be heroin.
Past Medical History: Heroin and cocaine abuse, Hepatitis C, depression with a prior suicide attempt.
Physical Exam: Comatose with decorticate posturing, coarse breath sounds; T 34.5°C. Hands mildly edematous with multiple injection sites noted.
Laboratory Data: In ED, electrolytes normal, glucose <20. Acetaminophen, ethanol, and salicylate not detected; UDS positive for benzodiazepines and tramadol; Electrolytes normal; lactate 1.8, AST 38, ALT 64.
Clinical Course: The patient was intubated, given dextrose 50% by bolus and as a 10% infusion, dexamethasone and 100 mcg of octreotide. CT scan showed diffuse cerebral edema. He was started on antibiotics for possible aspiration pneumonia; glucose was continually low for 24 hours; his dextrose infusion was increased to 40% and his glucose stabilized. The gag and corneal reflexes were absent and a repeat CT showed no change in cerebral edema. Day 2 insulin level was 70.9 uIU/mL (normal 0.0 – 29.1); and a C-peptide level was 0.3 ng/mL (normal 1.1 – 5). Comfort measures were instituted and he expired on Day 4.
Autopsy Findings: No autopsy was performed. Medical examiner ruled the cause of death to be anoxic injury secondary to profound hypoglycemia due to an insulin overdose.
Case 981. Acute hydrocodone, carisoprodol, meprobamate, methamphetamine, marijuana, acetaminophen, and N-acetylcysteine exposure: undoubtedly responsible.Scenario/Substances: A 53 y/o male arrived at the ED an unknown time after apparently taking a number of drugs.
Past Medical History: Chronic obstructive pulmonary disease, cardiomegaly, polysubstance abuse, chronic alcoholism.
Physical Exam: Systolic BP 110.
Laboratory Data: Acetaminophen 48 mcg/mL, UDS positive for opioids, methamphetamine and THC, ABG-pH 7.19/ pCO2 47, troponin 658, hepatic enzymes unremarkable.
Clinical Course: He was admitted to the hospital and IV N-acetylcysteine was started. The initial bag of IV contained 126 grams instead of 12.6 grams. After receiving ∼100 grams of N-acetylcysteine, he developed a rash, angioedema, hypotension and bradycardia. He was intubated and placed on a ventilator and CPR was started. He was placed on norepinephrine and phenylephrine drips and a dose of diphenhydramine. He was resuscitated, but died later that day.
Autopsy Findings: Cause of death: multiorgan system failure due to acute acetaminophen poisoning. Other significant conditions include: acute myocardial infarct, probably allergic reaction to acetylcysteine, chronic hepatitis B, chronic hepatitis C, History of polysubstance abuse, chronic alcoholism, chronic obstructive pulmonary disease, cardiomegaly, recent marijuana use. Hospital blood: lidocaine 1.02 mg/L, hydrocodone 0.12 mg/L, carisoprodol 2.41 mg/L, meprobamate 16.7 mg/L, delta 9 thc-cooh 12 ng/mL, acetaminophen 59 mcg/mL; positive for diphenhydramine, cannabinoids, methamphetamine, morphine, and codeine.
Case 1000. Acute quetiapine ingestion: undoubtedly responsible.Scenario/Substances: A 14 y/o male ingested quetiapine, dose and time of ingestion unknown.
Physical Exam: Patient had a tonic-clonic seizure. Post seizure BP 100/40, HR 160.
Laboratory Data: HCO3 21, pH 7.3; UDS pos for tricyclic antidepressants; ECG QRS duration 100 msec, QT 513 msec.
Clinical Course: The patient was intubated placed on a ventilator. VS declined to BP 70/20, HR 15. IV fluids and dopamine were administered. QRS 110 msec, QT 513 msec. Sodium bicarbonate given by bolus. Hypotension persisted, norepinephrine was started; BP responded to 80/40. Sodium bicarbonate at 150 mEq/hr, then increased to 300 mEq/hr. The patient remained unresponsive and developed a wide complex ventricular rhythm prior to a fatal ventricular fibrillation cardiac arrest.
Autopsy Findings: Pulmonary edema and congestion; quetiapine blood concentration 18 mg/L. Conclusion: Cause of death: acute quetiapine toxicity.
Case 1061. Acute-on-chronic quetiapine ingestion: probably responsible.Scenario/Substances: A 72 y/o female was found unresponsive in her home after ingesting 180 tablets of 200 mg quetiapine. EMS found her unresponsive with respiratory depression and systolic BP in the 60's. She had a complex generalized seizure lasting 25–30 sec. She was intubated, received IV fluids, started on dopamine, and transported to the ED.
Past Medical History: Prior suicide attempts.
Physical Exam: Unresponsive, hypotensive.
Laboratory Data: Toxicology screen showed salicylate, acetaminophen, and ethanol were not detected.
Clinical Course: The patient was admitted to the ICU and remained hypotensive despite IV fluids and pressor support. An IV drip of sodium bicarbonate was initiated for severe acidosis. The patient was initially tachycardic but subsequently developed heart block which deteriorated to PEA. ACLS resuscitative measures were unsuccessful and she died 4 hr after admission.
Autopsy Findings: Occlusive coronary artery disease with 80–90% occlusion of the left anterior descending artery. Cause of Death: Drug Intoxication-quetiapine. Stomach contained gray sludge-like material. Postmortem blood quetiapine 17μg/mL.
Case 1073. Acute MDMA, amphetamine, methamphetamine, cocaine, acetaminophen and hydrocodone ingestion: undoubtedly responsible.Scenario/Substances: A 16 y/o male was seen taking 5 methylenedioxymethamphetamine (MDMA) pills and 30 min later was found down with a large bottle of acetaminophen and hydrocodone at his side. EMS was called, the patient was given midazolam, succinylcholine, intubated, and transported to the ED.
Past Medical History: Marijuana use.
Physical Exam: In the ED the patient was tremulous with rigid extremities, pupils were 6 mm and nonreactive bilaterally, BP 60/38, HR 151, T 41.7°C, RR 17, O2 sat 84%.
Laboratory Data: Urine was positive for amphetamines, methamphetamines, cocaine, opiates, and THC. Acetaminophen, salicylates and ethanol were not detected.
Clinical Course: In the ED he was placed on dantrolene. Renal insufficiency developed with K 8.9, Cr 2.3, and required hemodialysis. Rhabdomyolysis ensued with CPK 290,430, he developed profound shock and DIC. AST 12,134, ALT 7,298, and liver function continued to worsen, he received 22 units of FFP and 8 units of packed RBC, multiple platelet units and cryoprecipitate, but the coagulopathy could not be controlled. Hypotension was treated with pressors and epinephrine. The patient remained unresponsive, an EEG showed extensive high voltage delta waves consistent with encephalopathy. The patient's family elected the institution of comfort measures and he expired on Day 2. Final diagnosis was multidrug overdose including MDMA, profound shock with multiple organ system failure, malignant hyperthermia, serotonin syndrome, rhabdomyolysis, acute hepatorenal failure, DIC, and hypoglycemia with hypoxic ischemic encephalopathy.
Autopsy Findings: Manner of death accidental. Toxicology: MDMA 1.3 mg/L, diazepam 0.11 mg/L, midazolam 0.01 mg/L, unquantitated cocaine, opiates, amphetamines and cannabinoids..
Case 1076. Acute hallucinogenic amphetamine ingestion: probably responsible.Scenario/Substances: A 19 y/o male ingested 10 mg of Bromo DragonFly with his older brother and a female friend at his home. He developed seizures and then a cardiac arrest. The companions were agitated, “out of it,” with tachycardia but recovered and were discharged.
Past Medical History: Suicide attempt with acetaminophen one year prior.
Physical Exam: Arrived in asystole. No other information available.
Laboratory Data: Not available.
Clinical Course: Resuscitation efforts were not successful.
Autopsy Findings: Postmortem peripheral blood was negative for 2C-B-Fly, but did contain a related drug, Bromo Dragon Fly (1-(8-bromobenzo[1,2-b;4,5-b']difuran-4-yl) −2-aminopropane) at a concentration of 22 ng/mL as well as phentermine 160 ng/mL, nicotine, cotinine, and THC. The cause of death was reported as Bromo DragonFly intoxication. A detailed description of the incident was posted on Erowid: http://www.erowid.org/chemicals/bromo_dragonfly/bromo_dragonfly.shtml
Case 1077. Acute MDMA ingestion: undoubtedly responsible.Scenario/Substances: A 20 y/o male ingested 29 tabs of ecstasy in a suicide attempt. He complained of calf cramping and was transported to the ED.
Past Medical History: Depression with suicidal ideation. Recreational use of marijuana and ecstasy.
Physical Exam: Awake, agitated, diaphoretic, with mydriasis and a clenched jaw. BP 128/78, HR 160, RR 44.
Clinical Course: Severe tremor and agitation persisted in the ED. The patient was paralyzed, intubated and ventilated; he underwent gastric lavage, which yielded many pill fragments. Aggressive supportive care was given. T increased to 42.2°C prior to death which occurred within 10 hours of presentation.
Autopsy Findings: Post-mortem heart blood: methylenedioxymethamphetamine (MDMA) 15 mcg/mL, MDA 0.37 mcg/mL and methamphetamine 0.40 mcg/mL. Femoral blood: MDMA 4.3 mcg/mL, MDA 0.09 mcg/mL and methamphetamine 0.12 mcg/mL.
Case 1100. Acute MDMA ingestion: undoubtedly responsible.Scenario/Substance: A 31 y/o male ingested 2 yellow “street” pills about 6 hr prior to presentation; his level of consciousness rapidly deteriorated, he experienced apnea, hyperthermia (T 42.8 C), was intubated and ventilated.
Physical Exam: Agitated, diaphoretic, flushed appearing patient with tremors. BP 151/110, HR 105–112, T 35°C.
Laboratory Data: ABG-pH 7.14/pCO2 68

Cr kinase1368, bilirubin total 0.8 mg/dL, AST 89, ALT 85, Alk phos 81, CK 281. UDS positive for amine group and THC; PCP not detected. Fibrin split products 320 mcg/mL, lactate 5.4 mg/dL, WBC 25.8.
Clinical Course: HR 131, T 105 F. Dantrolene 1mg/kg and IV fluids were administered. Renal function declined; urine was milky in appearance. Epistaxis was noted. Approximately 20 hr after presentation, the patient expired due to multiple organ system failure.
Autopsy Findings: Probable Cause of Death: methylenedioxymethamphetamine (MDMA), Manner of Death: Accidental.
Case 1112. Acute phencyclidine exposure: probably responsible.Scenario/Substances: A 38 y/o male was observed to be running through traffic, “jousting with vehicles and presenting a combative demeanor”. He suffered a fall, struck his head on the curb, and did not get up. EMS described altered mental status. He had a cardiac arrest, was intubated, resuscitated and transported to the ED.
Past Medical History: Hypertension.
Physical Exam: During transport he was unresponsive, BP 70/30, HR 115–120,
Laboratory Data: UDS positive for phencyclidine and THC.
Clinical Course: He remained intubated and required pressors to maintain BP support. He had another cardiac arrest ∼4 hr after admission and could not be resuscitated.
Autopsy Findings: No evidence of traumatic brain injury or other trauma. Myocardial contraction band necrosis. Heart blood: carboxy-THC 15 ng/mL, THC not detected. Femoral blood: phencyclidine 647 ng/mL. Coroner ascribed death to “sequelae of phencyclidine-associated agitated delirium.”
Case 1118. Acute amphetamine/dextroamphetamine ingestion: contributory.Scenario/Substance: A 42 y/o male took up to 104 amphetamine/dextroamphetamine 20 mg tablets, not his prescribed medication.
Physical Exam: Disoriented, diaphoretic, BP 151/110, HR 105–112, T 35°C.
Clinical Course: A few minutes after arriving in the ED the patient screamed with head pain, developed bradycardia and respiratory arrest. He was intubated and ventilated, BP 95/78, HR 106, and transported to a tertiary care center ICU. His neurological status remained unchanged, and he continued to require ventilation. An IV nicardipine drip was ordered, but it could not be confirmed if it was given, BP 120/60, HR 110. On Day 2, imaging showed a broad-based dissected aneurysm at the base of the skull. The patient's family was informed of the grim prognosis, elected the institution of comfort measures, and he expired on Day 5.
Autopsy Findings: Not available.
Case 1119. Acute dextroamphetamine ingestion: undoubtedly related.Scenario/Substances: A 43 y/o male, after a domestic dispute, ingested 15 to 20 of his son's 10 mg dextroamphetamine tablets, called EMS and stated he had taken an overdose. Upon arrival of EMS the patient was alert and ambulatory, but deteriorated with loss of consciousness during transport.
Past Medical History: previous suicide attempts.
Physical Exam: Diaphoretic, unresponsive with posturing, no gag reflex. BP 210/110, HR 70, RR 6.
Laboratory Data: UDS positive for amphetamines; acetaminophen and salicylate were not detected.
Clinical Course: The patient was intubated and placed on mechanical ventilation. A CT scan showed large left frontal parietal bleed. The patient was transported to a tertiary healthcare facility where he did not show any neurological response for 48 hours. With the family's consent, an apnea test was performed and brain death was declared. The patient was taken to the OR for organ donation.
Autopsy Findings: No autopsy was performed. Blood drawn on arrival in the ED, (analyzed postmortem), showed amphetamine level of 338 ng/mL.
Case 1121. Methamphetamine exposure: undoubtedly responsible.Scenario/Substances: A 46 y/o male reportedly had seizures 45 min prior to collapsing. EMS gave epinephrine and atropine and was brought to the ED in cardiac arrest.
Physical Exam: Pulseless, T 41.7° C, no muscle rigidity.
Clinical Course: Cardiac monitor in ED revealed PEA. He was pronounced dead.
Autopsy Findings: Pulmonary edema. Cause of Death: Acute methamphetamine toxicity. Blood methamphetamine 10 mg/L, amphetamine 0.39 mg/L. Urine methamphetamine 66 mg/L, amphetamine 13 mg/L.
Abbreviations & Normal ranges for Abstracts
Disclaimer – all laboratories are different and provide their own normal ranges. Units and normal ranges are provided here for general guidance only. These values were taken from Harrison'sCitation15, GoldfrankCitation16 or Dart.Citation17
Serum electrolyte summary table


serum electrolytes have units of mEq/L = mmol/L
∼ = approximately
ABG-pH/pCO2/pO2/HCO3/BE