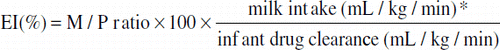Abstract
Over the last few decades, the rate of breastfeeding has increased steadily in the developed countries of the world. During this time, opioid use in the general population has steadily increased as well. Despite this, clinicians remain unclear whether opioid use is safe during breastfeeding. While the vast majority of medications used during breastfeeding occur without incident, case reports and studies have reported possible opioid toxicity in breast-fed infants. Multiple enzymes are involved in the metabolism of opioids. CYP2D6 catabolizes O-demethylation of codeine, tramadol, oxycodone, and hydrocodone to more potent metabolites. CYP3A4 inactivates methadone, meperidine, and buprenorphine. Glucoronide conjugation by the UGT enzyme family inactivates morphine and hydromorphone. Genetic polymorphisms and interfering medications affect the maternal metabolism, which in turn determines the exposure and risk to the breast-fed neonate. We review the production of breast milk, the transfer of xenobiotics from blood to milk, the characteristics that alter xenobiotic breast-milk concentrations, and we review the evidence of specific common opioids and infant toxicity. The short-term maternal use of prescription opioids is usually safe and infrequently presents a hazard to the newborn.
Introduction
Breastfeeding has numerous health, social, and psychological benefits to both mother and newborn.Citation1,Citation2 Since the 1970s, increasing the rate of breastfeeding has been a major public health goal,Citation3 and the initiation of breastfeeding in industrialized countries has increased from less than 50% to 60–90%.Citation2,Citation4,Citation5
Meanwhile, the use of opioid pain medications and the number of fatalities from accidental overuse of opioids has increased dramatically over the last decade.Citation6–8 In the USA, over 201.9 million prescriptions for opioids were filled in 2009,Citation9 and hydrocodone has now become the most prescribed medication in the country.Citation8,Citation10
Although many women take some form of medication in the early post-partum period,Citation11–13 the exact number of breastfeeding mothers who use opioids is currently not well established.
Unfortunately, confusion remains among practitioners and medical trainees on topics related to breastfeeding.Citation14 The transfer of medications into human milk is a complex process and, although the vast majority of opioid use while breastfeeding occurs without incident,Citation3,Citation15,Citation16 several cases of severe toxicity and death have been reported.Citation17–27
The medical toxicologist must understand this complex process in order to assist in determining high-risk scenarios and in determining the cause of newborn and infant toxicity.
In order to gain a greater understanding of the potential transfer of opioids into human milk, we will review the production of human milk, the factors that affect the transfer of xenobiotics into human milk, and the factors that affect infant toxicity. We then review the most common opioids, their potential presence in human milk, and finally we summarize cases of reported toxicity of infants from opioids in human milk.
Human milk production and physiology
Xenobiotic transfer into human milk is a complex process. Initially, we will review the production of human milk because the processes involved are important in understanding xenobiotic transfer. Later, we will review the issues that are specific to opioids.
Lactogenesis
The parenchyma of the human breast consists of approximately 10–15 lobules. Each lobule contains many mammary alveolar complexes that produce milk, which is transported into larger ducts that coalesce into singular lobular mammary ducts, ending in the lactiferous sinuses beneath the areola.Citation28
The mammary alveolar membrane () is responsible for milk synthesis and secretion. It consists of a single row of epithelial cells attached by tight junctions. The apical membrane borders the alveoli and the human milk. The basal membrane is anchored to the basement membrane and borders the capillaries.
Fig. 1. The mammary alveolar membrane and xenobiotic transfer. ME – myoepithelial cell; RER – rough endoplasmic reticulum; SER – smooth endoplasmic reticulum.
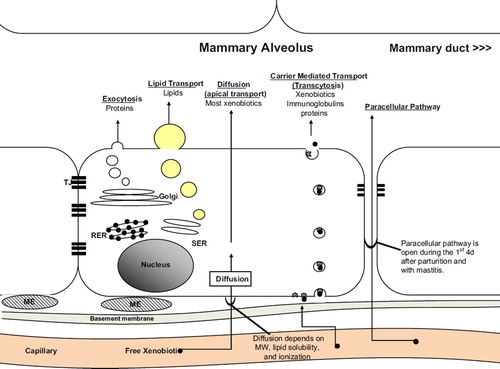
The production of milk occurs via five mechanisms: exocytosis, lipid secretion, diffusion (or apical transport), carrier-mediated transfer (or transcytosis), and paracellular pathways ().
Most small molecules and most xenobiotics are transferred into human milk by simple diffusion (apical transport) across the basal membrane and then across the apical membrane into the alveolus.Citation29–31 Small water-soluble xenobiotics, such as lithium and ethanol, may diffuse through transmembrane channels, whereas larger molecules diffuse based on their lipid solubility and ionization.Citation29
Some xenobiotics, immunoglobulins, and proteins may cross into the alveolar ducts via carrier-mediated transfer, or transcytosis. During this process, proteins bind to receptors on the basal membrane, are endocytosed, transferred across the cell to the apical membrane and exocytosed into the alveolar duct.
Exocytosis and lipid secretion are used to produce proteins and lipids, respectively, within the cell, which are then exocytosed into the alveoli. Human milk contains approximately 1% protein and 4% fats,Citation32 although these values fluctuate during a single feed and change with the age of the child. These mechanisms are not used for xenobiotic transfer.
Finally, a paracellular transfer pathway of xenobiotics may occur under specific circumstances. During pregnancy and the first few days after parturition, the tight junctions between the mammary alveolar cells fail to impede transfer from the interstitial space into the alveolar ducts (). Several days after parturition, the tight junctions seal this membrane and this transfer is not possible during lactation. However, tight junctions may also fail in cases of mastitis, allowing paracellular transfer.Citation33
Factors that affect xenobiotic transfer into human milk
Many factors determine the efficiency and the amount of xenobiotic transfer into human milk.Citation34 We will discuss these factors, including xenobiotic factors, maternal factors, human milk factors, and factors related to the newborn or infant.
Xenobiotic determinants of diffusion
For a xenobiotic to transfer into human milk via diffusion, it must be available in its free form in the blood and pass from the blood to the mammary alveolar membrane.Citation29,Citation31 The characteristics of a xenobiotic to transfer efficiently into human milk are similar to those that allow diffusion through other membranes, such as the placenta and the dialysis membranes – molecular weight, protein binding, ionization, and lipid solubility.Citation29
In general, diffusion is increased in xenobiotics that have smaller molecular weights, low protein binding, are weak bases, and are lipid soluble.
Opioids have a fairly small molecular weight, are weak bases (), and readily diffuse across the alveolar membrane. Protein binding differs among opioids; hydrocodone has minimal (2%) protein binding while methadone (80–90%) and buprenorphine (96%) are largely protein bound. Opioids have volumes of distribution (Vd) that range from 1.8 to 5 (). In aggregate, these data suggest that opioids are available in low free serum concentrations (moderate Vd and protein binding) with moderate diffusion capabilities, but with variability among specific opioids.
Table 1. Common opioids, metabolism, potential induction, active metabolites, and pharmacokinetic parameters.Citation35,Citation67
Maternal determinants of diffusion
Maternal pharmacokinetics may determine much of the availability of the xenobiotic in the blood and, therefore, its availability to the mammary alveolar membrane and human milk.
Maternal genetic polymorphism or the induced activity of hepatic enzymes may affect the analgesic or CNS depressive effects of opioids by influencing the amount of an active parent drug or by increasing metabolism to an active metabolite. Oxycodone, hydrocodone, codeine, methadone, and tramadol are metabolized by CYP2D6 and CYP3A4. Morphine, hydromorphone, and oxymorphone are not metabolized by phase I (CYP) oxidative metabolism, but instead undergo glucuronidation ().Citation35 The CYP or UGT enzymes may be pharmacologically inhibited and lead to an increase in the serum concentration of the active parent drug, for example, with morphine or methadone. Alternatively, increased enzyme activity may produce more of an active metabolite and increase the concentrations of that active metabolite in human milk, as may be the case with hydrocodone, oxycodone, tramadol, and codeine. An elevation of the maternal concentrations of the active drug may lead to increased exposure of the infant to the active drug via human milk.
Breast-milk determinants of diffusion
Diffusion of xenobiotics may be influenced by both the pH and the lipid content of human milk.
Human milk has a pH of approximately 7.0, whereas the serum pH ranges from 7.35 to 7.45. The lower pH of human milk allows for some ion trapping of weak bases in the human milk. Medications that are weak bases, such as the opioids, may accumulate in human milk at concentrations that are higher than serum, despite following passive diffusion.
Human milk also has a higher lipid content than blood (4% vs. < 1%). This may allow for ‘lipid trapping’ within the milk and may allow for higher milk to plasma (M/P) concentration ratios for some highly lipid-soluble xenobiotics, despite following passive diffusion into the milk.
Mechanisms of infant exposure related to the infant
Once a xenobiotic reaches human milk, several additional factors determine if the xenobiotic will affect the infant – absorption, metabolism, and body composition.
Xenobiotics present in human milk are absorbed by the infant. In some cases, the published oral bioavailability of the xenobiotic may be helpful. However, the oral bioavailability may additionally depend on the gastric pH and the presence of intestinal flora and absorptive elements, which may be different in the newborn or infant. In general, the absorption of xenobiotics is slower in neonates than in adults.Citation36
Xenobiotic metabolism and elimination may play a role in the accumulation of xenobiotics in neonates and the development of toxicity from exposure in human milk.Citation37
In some cases, the hepatic metabolism is not mature at the time of birth, and this may be particularly important in the case of premature newborns. CYP2D6 and 2E1 expression increases rapidly after birth, but CYP3A4 and 1A2 activity only slowly increases over several months.Citation36,Citation38–42 Other hepatic enzymes, including the enzymes that metabolize morphine (UGT2B7), have reduced activity in the initial few months of life.Citation43
The renal excretion of xenobiotics may additionally be impaired in the neonate.Citation36 A full-term newborn has a glomerular filtration rate (GFR) of only 25% of an adult by mass. The adult rate is attained by about 3–5 months.Citation44,Citation45 This renal impairment may lead to the accumulation of the pharmacologically active morphine-6-glucuronideCitation46 and tramadol.Citation47 Severe renal impairment may lead to the accumulation of norbuprenorphine in adults taking buprenorphine,Citation47 but there is no evidence of its accumulation in neonates.Citation48,Citation49
The metabolism and the expected maturity of specific enzymes should be considered when evaluating for toxicity. During pregnancy, the fetus exchanges xenobiotics with the mother via the placenta and these xenobiotics may be metabolized and excreted by the mother. In addition, the maternal placenta contains CYP enzymes and may contribute to metabolism.Citation50 Once born, the newborn's limited metabolic capacity may lead to prolonged half-lives and the accumulation of the xenobiotic, leading to toxicity.
The body composition of an infant should be considered as a source of potential toxicity, particularly when considering premature infants. The fat content of a newborn ranges from 3% in a 1-kg premature infant to 12% in a 3.5-kg full-term infant. Administration of a highly fat-soluble xenobiotic to an infant with a lower body fat composition may allow more of the xenobiotic to accumulate in the brain and lead to more CNS symptoms per dose. This effect is in addition to the effect of body weight.
Effect of post-natal time (age) on breast milk
Human milk changes in volume and composition with post-natal time (). In infants who are continually exposed to a xenobiotic, these changes occur gradually and will not likely affect the infants’ exposure. However, in the case of transient dosing of xenobiotics, composition and volume alterations may influence the potential for toxicity.
Table 2. Characteristics of human milk with age.Citation28
Once lactogenesis begins, the volume of human milk increases to a mean of 600 mL/day at about the fourth day post-partum. The volume produced slowly increases to 800–900 mL/day at 6 months post-partum and then slowly decreases as food is typically introduced, but remains > 750 mL/day at 12 months post-partum ().Citation32,Citation51 The mature infant takes about five to seven feeds per day, which equates to about 75–125 mL/feed (2.5–4 oz/feed); however, this is highly variable.Citation52 A standard simplified value for the volume of human milk consumption is 150 mL/kg/day, or 30 mL/kg/feed.Citation29,Citation31 These volumes should be used in considering the total dose to an infant; however, each individual maternal-infant unit is unique and the total volume of human milk production may vary by individual.
The majority (63%) of medication-related adverse events occur within the first month of life.Citation16 There are several factors that put neonates at highest risk for these events: 1. Neonates ingest a larger volume of milk per kilogram. Whereas milk production increases from 600 to 900 mL/day in the first 6 months post-partum, the average infant increases in weight from 3.5 to 8 kg. This may expose the newborn to higher xenobiotic doses than the infant, despite stable xenobiotic concentrations in human milk. 2. During the initial week post-partum, tight junctions inefficiently seal the interstitial space from the mammary alveolus and the paracellular pathway may allow for more xenobiotic transport. 3. Several neonatal hepatic enzymes are immature in the first month of life. 4. Renal function is immature in the first months of life. 5. Newborns have a lower fat content and may be prone to larger distributions of fat-soluble drugs into the CNS. 6. Neonates may be more sensitive to medications due to an immature blood–brain barrier.
The fat content of human milk may increase significantly in mothers who are breastfeeding for more than 1 year.Citation52 Human milk contains a mean of about 25 g/L of lipids in the first month post-partum. In women who continue to breastfeed after 1 year, the human milk lipid content may be as high as 48–62 g/L ().Citation51–53 Although this may theoretically allow for more lipid-soluble xenobiotics to accumulate in the milk, the exposure risk remains small because of the infant's size.
Determinants of infant drug exposure
There are many methods to estimate the potential for infant toxicity of a xenobiotic in human milk. All of these methods have limitations, and a thoughtful approach considering the xenobiotic characteristics and the pharmacokinetics of both mother and child is necessary to determine if a particular xenobiotic is a risk to a breastfeeding child in a particular instance. A useful reference for practitioners is the US National Library of Medicine's LactMed database (http://toxnet.nlm.nih.gov).
Milk to plasma ratio (M:P ratio)
The M/P ratio is defined as the concentration of a xenobiotic in human milk divided by the concentration of a xenobiotic in plasma. The ratio defines whether the xenobiotic is poorly transferred into human milk (M/P < 1) or accumulates in human milk (M/P > 1). Unfortunately, the M/P ratio requires that there is accurate published information on plasma and human milk concentrations, but these data are available for a surprisingly small number of xenobiotics. In addition, the M/P ratio only provides a ratio of the human milk concentration to the concentration in the maternal plasma. In many cases, the plasma concentration does not correlate with toxicity, particularly with xenobiotics with high volumes of distribution, and this may limit its usefulness. Finally, the M/P ratio may fluctuate depending on the maternal pharmacokinetics and human milk characteristics. In general, the M/P ratio is a simple tool that is fairly inaccurate.
Relative infant dose (RID) percentage
The RID percentage is calculated by dividing the infant's weight-based daily dose via human milk by the mother's weight-based daily dose and multiplying by 100. This percentage is intended to put the infant's dose in perspective to the mother's dose in order to predict symptoms. It has been recommended that a xenobiotic with an RID > 10% should be used with caution and that a medication with an RID > 25% is not acceptable for maternal use.Citation5 For the vast majority of xenobiotics, the RID is < 1%.Citation29 This method is also limited in that it requires human milk concentrations; however, it may be used in investigating the toxicity of an infant if this information is available.
Exposure index
The exposure index (EI) is a tool to take into account the M/P ratio as well as the milk intake and the drug clearance of the infant.
*600 mL/kg/day intake = 0.42 mL/kg/min.
The EI may be useful in assessing the risk of xenobiotics in human milk; however, it is limited by the insufficient information on infant drug clearance with many xenobiotics.Citation2
American Academy of Pediatrics (AAP)
The AAP Committee on Drugs published their first statement on drugs and breastfeeding in 1983 and later published several revisions.Citation3 However, this policy was officially retired on 1 August 2010; therefore, there is no current AAP policy on the safety of medications in breast milk.Citation54
Opioid toxicity in infants due to human milk exposure
Despite the well-accepted safety of breastfeeding, there have been several cases of severe toxicity due to opioids, presumably from human milk. Some of these reports are not completely documented and the exposure in human milk has been assumed. In future publications, a thorough documentation of opioid concentrations in the mother, the infant, and the human milk would be preferred.
The medical literature describes scant evidence of opioid toxicity in breast-fed infants (). One death has been described that may have been caused by a mother's rapid metabolism of codeine to morphine with transfer of the morphine via human milk to the child.Citation22,Citation55 An additional death has been reported that was most likely from a non-human milk source of oxycodone.Citation23 Significant toxicity has been described with hydrocodone, codeine, and methadone and these are listed in . Symptoms in these cases have been similar to the expected symptomatology in older children and adults with CNS and respiratory depression. Symptoms may be less specific in newborns with opioid exposure and may lead to poor feeding, hypersomnolence, or hypotonia.
Table 3. Reported cases of opioid-related severe adverse events potentially related to infant exposure through human milk.
Although mild symptoms, including minor alterations in wakefulness, may occur, cases of severe toxicity are rare. There are several common themes in the reported cases of severe toxicity; the majority of the cases involved codeine and most cases of toxicity occurred in children less than 2 months old. The remaining cases involved large doses of opioids or suspect histories.Citation23,Citation26
Individual opioids
We shall discuss several individual opioids with reference to the reported toxicity and measured milk concentrations. These data are summarized in .
Table 4. Concentrations of opioids in human milk and in infants exposed to human milk.
For each opioid, a search was performed in both MEDLINE and OLDMEDLINE for English language papers from 1946 to 2011. Search terms included the opioid's name or names (e.g. “morphine”, “pethidine,” or “meperidine”) and (milk, human, or breast feeding or poisoning or overdose). All abstracts were reviewed independently by the authors and a list of pertinent articles generated by consensus.
Morphine
Morphine is the prototypical opioid medication available in solution, concentrate, tablet, capsule, extended release tablet, and suppository. Morphine is metabolized in small quantities (10%) by UGT2B7 to morphine-6-glucuronide, which has a mild analgesic activity.Citation47 There is a small amount of UGT2B7 genetic variability with at least two rare variants leading to a reduced metabolism of morphine.Citation56,Citation57 Morphine may also be metabolized by UGT1A3 to morphine-3-glucuronide,Citation58 which is thought to lack analgesic activityCitation59 and may be responsible for CNS excitatory effects.Citation60
Data on morphine concentrations in human milk are limited to only 18 patients. Robieux et al. reported on a single 15-day post-partum woman who was treated with morphine (5 mg every 6 h) and had milk morphine concentrations of 100 ng/mL before feeding, 10 ng/mL at the end of feeding, and 12 ng/mL 30 min after maternal dose.Citation61 The infant's serum morphine concentration of 4 ng/mL was measured 4 h after maternal dose and 1 h after breastfeeding. Feilberg et al. described five women > 1 month post-partum, who were given either epidural morphine (4 and 8 mg [4 mg × 2 doses]) or intravenous and intramuscular morphine (doses ranged from 10 to 25 mg).Citation62 Milk morphine concentrations were highest 30 min after maternal dose and peak concentrations were 82 ng/mL in the epidural group and 500 ng/mL in the intravenous and intramuscular (total dose 15 mg) group. The mean M/P ratio was 2.45.
Wittels et al. compared five post-partum women who were treated with IV PCA morphine followed by oral morphine (5–30 mg every 2–3 h as needed) after a cesarean section.Citation63 The maximum morphine breast-milk concentration was 64 ng/mL 24 h after the initiation of pain control.
Oberlander et al. described one woman who had an intrathecal morphine pump for complex regional pain syndrome and analyzed breast-milk samples from birth to 7 weeks post-partum.Citation64 In the first 5 weeks post-partum, the weekly morphine dose was 59.9 mg, which was reduced to 28.6 mg/week until week 7 for decreased pain medication requirements. All samples were either not detected (i.e. < 8 ng/mL) or not quantifiable (8–25 ng/mL) and no symptoms were reported in the infant from birth to 7 months.
Baka et al. described seven post-partum patients who were treated with IV morphine (PCA, 1 mg q10 min with 20 mg max over 4 h).Citation65 The mean dose was 0.58 mg/kg (± 0.07) over the initial 24 h, then 0.17 mg/kg (± 0.06) during the next 24-h period. Morphine was detected in the breast milk of three of the seven patients with a range of < 1–37 ng/mL in the first 24 h. Morphine-6-glucuronide concentrations in breast milk ranged from < 5 to 1084 ng/mL in the first 24-h period.
We are not aware of any reports of suspected infant toxicity from maternal morphine use while breastfeeding.
Oxycodone
Oxycodone is a semi-synthetic opioid derived from thebaine. It is available as a solution, concentrate, tablet, capsule, and extended release tablet. Oxycodone is metabolized mostly by CYP3A4 to non-toxic metabolites and in small quantities (15%) by CYP2D6 to oxymorphone, which is more potent than oxycodone.Citation66
Ultra-rapid CYP2D6 metabolizers exist and may produce increased concentrations of oxymorphone and may have increased sedation and analgesia.Citation67,Citation68 Poor CYP2D6 metabolizers may have decreased clearance of the active parent compound, oxycodone.Citation69
Two published studies have reported concentrations of oxycodone in breast milk. In the first study, published as an abstract, six post-partum women administered 5–10 mg of oxycodone every 4–7 h had maternal plasma concentrations of 14–35 ng/mL with milk concentrations that ranged from < 5 to 226 ng/mL. The mean M/P ratio was 3.4; however, there were large variations in the ratio.Citation70 In the second study, 50 women taking 10 mg of oxycodone every 2 h as needed had breast-milk oxycodone concentrations ranging from < 2 to 168 ng/mL.Citation71 The median milk concentrations were 58 ng/mL (42–73 ng/mL, 95% CI) in the first 24 h, 49 ng/mL (35–62 ng/mL, 95% CI) over the second 24-h period, and 35 ng/mL (15–55 ng/mL, 95% CI) over the third 24-h period. Of 41 neonates who were tested, only one had a detectable serum concentration of 7.4 ng/mL. The median M/P ratio was 3.2 (2.6–4.3 IQR) in the first 24 h and 3.4 (2.4–4.5 IQR) in the next 24 h.
One retrospective cohort study evaluated CNS depression in the children of breastfeeding mothers who were treated with oxycodone, codeine, and acetaminophen.Citation72 The study noted that children of mothers taking oxycodone or codeine had similar rates of symptoms of CNS depression (20.1% for oxycodone; 16.7% for codeine) and higher rates of CNS depression than the acetaminophen-only group (0.5%). None of the children of mothers who were taking oxycodone required medical evaluation. Of the mothers of symptomatic children, 93% had maternal CNS depression themselves, and 97% noted that the children's symptoms abated on stopping the oxycodone. Symptomatic children of mothers taking oxycodone were taking significantly higher doses of oxycodone (median 0.4 mg/kg/d; range 0.03–4.06 mg/kg/d) than those children who were asymptomatic (median 0.15 mg/kg/d; range 0.02–2.30 mg/kg/d).
Only one case of severe toxicity from oxycodone during breastfeeding was found in the literature and this was not likely due to exposure through human milk.Citation23 A 10-month-old male who suffered cardiac arrest had detectable post-mortem concentrations of 600 ng/mL in the whole blood and 1.6 mg/kg in the liver. The mother was reported to be feeding the infant only three times a day with supplementation of solid food. The mother had claimed to not have ingested any oxycodone that day, but reported three doses of 60 mg oxycodone and carisoprodol (650 mg) over 24 h prior to the infant's death, with only two doses of hydrocodone with acetaminophen that day. Samples of human milk were not available. The authors concluded that given the history of medications ingested, the lack of other medications found on autopsy, and the amount of milk that the infant would be ingesting at only three times a day, it was unlikely that the infant could have such a high blood concentration through breast milk alone and was likely given oxycodone orally.Citation23
Hydrocodone
Hydrocodone is a semi-synthetic opioid derived from codeine and thebaine. It is available in tablet, capsule, and syrup form, most often compounded with acetaminophen, less commonly ibuprofen, and anti-histamines. Hydrocodone is metabolized in small quantities (5%) by CYP2D6 to hydromorphone, which is more potent than hydrocodone.Citation47,Citation73
Ultra-rapid CYP2D6 metabolizers exist and may produce increased concentrations of hydromorphoneCitation67,Citation74 and may have enhanced analgesic effects.Citation74
Data on the hydrocodone content of human milk are available for 32 patients. Anderson et al. reported two post-partum women (7 and 16 days) and calculated an RID of 3.1 and 3.7% and neonatal dosages of 3.07–8.58 mcg/kg/d.Citation75 Sauberan et al. reported 30 post-partum (3–11 days) patients using 44.3–423.2 mcg/kg/d of hydrocodone, who produced a mean hydrocodone milk concentration of 1.6–99.6 ng/mL. Only 12/30 patients had detectable hydromorphone milk concentrations ranging from 0.2 to 86.7 ng/mL. The RID was reported as 2.4%.Citation76 Neither paper tested the maternal blood concentrations, so an M/P ratio could not be calculated.
Two reports in the literature have reported possible adverse reactions to human milk exposure to hydrocodone. In the first, an 18-day-old infant became “groggy” and “slept for most of the day” after his mother was taking a 20-mg oral hydrocodone and 1300-mg acetaminophen combination product every 4 h along with fluconazole for nipple candidiasis and mastitis. She decreased her dose by half, with a reported improvement in symptoms.Citation24 In the second case, a 5-week-old breastfeeding infant was found with cyanosis, minimal respiratory effort, and recurrent apnea and was intubated by the EMS. In the ED, naloxone was given with a reported improvement in the respiratory rate. The mother had reported using both methadone and hydrocodone/acetaminophen products for migraine headaches prior to breastfeeding, but doses were not reported. The infant's urine drug screen was positive for opiates. Work-up for his symptoms did not reveal another cause.Citation25
Hydromorphone
Hydromorphone is a semi-synthetic derivative of morphine. It is available as a capsule, tablet, solution, and suppository. Hydromorphone is almost completely metabolized by glucuronidation to a metabolite, hydromorphone-3-glucuronide, which lacks analgesic activity.Citation35
Data on human milk exposure to hydromorphone are limited to a report of eight lactating women who received a single 2-mg intranasal hydromorphone dose and had a calculated RID of 0.67%.Citation77
Methadone
Methadone is a synthetic opioid and is available as a tablet and a solution. Methadone is metabolized by CYP3A4 (as well as CYP2D6, CYP1A2, CYP2B6, and CYP2C8) to inactive metabolites.Citation35,Citation47,Citation78
Inhibitors of CYP3A4 may theoretically cause accumulation of methadone. Inhibitors include azole anti-fungals, calcium channel blockers, macrolides, SSRIs, anti-retroviral drugs, dexamethasone, amiodarone, cimetidine, cyclosporine, and disulfiram.Citation35,Citation67,Citation79,Citation80
Data on methadone in human milk are limited to 60 patients. Begg et al. studied eight lactating women on methadone maintenance therapy with doses ranging from 40 to 105 mg/day.Citation81 The studied milk was tested in the first several days post-partum (immature milk) and again > 15 days post-partum (mature milk). For immature milk (infants < 15 days old) (n = 8), the M/P ratio for R-methadone was 0.68 (0.48–0.89 95% CI) and for S-methadone it was 0.38 (0.26–0.5 95% CI). For mature milk (n = 2), the M/P ratio range for R-methadone was 0.39–0.54 and for S-methadone it was 0.24–0.30. The estimated RIDs of R- and S-methadone via immature milk were 3.5 and 2.1%, respectively.Citation81
In four similar studies on 23 lactating women taking methadone in ranges from 25 to 185 mg/day, milk concentrations ranged from 10 to 570 ng/mL. The M/P ratio ranged from 0.05 to 1.89.Citation82–85
Wojnar-Horton et al. reported 12 patients taking methadone doses of 20–80 mg/day who had milk methadone concentrations from 39 to 232 ng/mL and plasma concentrations of 121–603 ng/mL.Citation86 Eight of the 12 infants had plasma methadone concentrations obtained after feeding, which were undetectable in 7 of the 8 infants and was 6.5 ng/mL in 1 infant. The M/P ratio ranged from 0.13 to 1.19 with a mean of 0.44. The mean infant dose was 17.4 mcg/kg/day, while the mean RID was 2.79 (2.07–3.51%). Despite breastfeeding, 7/12 infants developed neonatal abstinence syndrome (NAS), with 6 requiring treatment.Citation86
Jansson et al. reported two studies on methadone in human milk. In the first study, nine patients taking methadone (40–110 mg qd) had milk concentrations of 21–314 ng/mL.Citation87 The mean infant ingestible dose peaked at 0.084 mg/d and the M/P ratio was 0.4–0.9. In their second study, eight patients taking methadone (50–105 mg/d) had milk methadone concentrations of 21–462 ng/mL.Citation88 Infant serum methadone concentrations were low (2.2–8.1 ng/mL) but detectable and they found no neurobehavioral effects.Citation88
Two cases of infant toxicity of methadone have been found in the literature. A 5-week-old male was found dead by his mother who was on methadone maintenance therapy, approximately 4 h after being put to bed. Although further details of the case, including doses and feeding, were not reported, autopsy methadone concentrations of 400 ng/mL were found in the blood and the child was malnourished.Citation27 The blood concentrations in this case seem inconsistent with human milk as the source of methadone. West and the authors of this review reported a case of a 13-month-old breastfeeding child whose mother ingested two doses of 40-mg methadone tablets for acute pain 2 h prior to breastfeeding.Citation26 The mother became somnolent while breastfeeding. The child became apneic shortly after feeding and responded to naloxone in the pre-hospital setting. The child required multiple boluses of naloxone over a 19-h period. Methadone was qualitatively detected in the child's urine.Citation26 Both the mother and child were methadone naïve.
Codeine
Codeine is a naturally occurring opiate. It is available as a tablet or in syrup form in isolation or in combination with aspirin, acetaminophen, and others. Codeine is a prodrug that must be metabolized to morphine by the CYP2D6 system, a process that may be variable due to high polymorphism by the CYP2D6 gene.Citation89 Approximately 8% of Europeans do not possess any active gene copiesCitation90 and subsequently produce less morphine or have less pain relief from codeine. Alternatively, 20–40% of individuals, depending on ethnic background, are found to have duplications of the CYP2D6 gene, which make them ultra-rapid metabolizers of codeine and these patients may produce more morphine than is predicted.Citation90 Codeine is also metabolized through UGT2B7 to codeine-6-glucuronide and codeine-3-glucuronide.Citation91
Data on human milk codeine and morphine concentrations are available in 13 patients. In 1935, Kwit and Hatcher reported that four post-partum (3–9 days) women who were treated with 65–190 mg of codeine had no detectable codeine in the breast milk.Citation92 However, several factors may make these results difficult to interpret. The study was reported in 1935 when laboratory techniques (colorimetric estimation) and levels of detection were not similar to modern standard. In addition, all samples, except one, were obtained more than 4 h after the last dose of codeine. Using a radioimmunoassay method, Findlay et al. reported that two breastfeeding mothers (7th and 13th week post-partum) who took 60 mg of codeine had a maximum milk concentration of 455 ng/mL at 1 h after their dose and an M/P ratio of 1.3–2.5.Citation93 Meny et al., also using a radioimmunoassay method, studied free codeine and morphine concentrations in the breast milk of 17 samples from 7 mothers and the neonatal plasma of 24 samples from 11 healthy, term neonates. The milk codeine concentrations ranged from 33.8 to 314 ng/mL, 20–240 min after taking codeine; the morphine concentrations ranged from 1.9 to 20.5 ng/mL. The infant plasma codeine concentrations ranged from < 0.8 to 4.5 ng/mL and morphine ranged from < 0.5 to 2.2 ng/mL, 1–4 h after feeding.Citation94
Several cases of potential infant codeine toxicity have been reported. In an abstract, Davis et al. reported on four neonates born between 35 and 40 weeks whose mothers were taking 60 mg of codeine every 4–6 h. These infants showed episodes of short apneic episodes, periodic breathing, with one episode of prolonged apnea (> 20 sec) and bradycardia. Codeine was discontinued in all cases and all episodes resolved in 24–48 h.Citation18
In a cohort study of 838 mother-infant pairs, Ito et al. reviewed the adverse reactions in breastfeeding infants to maternal medications. The author reported five minor adverse reactions of drowsiness in mothers who were using codeine. All infants were under 1 month of age. Also listed were one reaction to irritability and two with constipation. No doses were recorded and no other information was available regarding the reactions.Citation95
Maddadi et al. studied 72 breastfeeding mothers who reported using codeine for pain control.Citation89 Seventeen infants were reported to have some degree of CNS depression while the mothers were using codeine and improved when codeine was discontinued. Mothers using codeine and whose infants exhibited CNS depression, on average used higher total daily amounts (1.62 ± 0.79 vs. 1.02 ± 0.54 mg/kg/day). There was 71% concordance between mother and infant sedation, although there was no significant difference in the total daily amounts that the mother ingested compared to mothers without sedation. Maternal genotype testing was also reported. Two of the mothers with infant CNS depression had a CYP2D6 UM and UGT2B7*2/*2 genotype.
Lam et al. reported a retrospective cohort study evaluating CNS depression in the children of breastfeeding mothers who were treated with codeine, oxycodone, or acetaminophen.Citation71 They noted that the children of mothers taking codeine had similar rates of symptoms of CNS depression as those taking oxycodone (16.7% in codeine group; 20.1% in oxycodone group) and higher rates of CNS depression than the acetaminophen-only group (0.5%). Of the 35 symptomatic infants of mothers taking codeine, 4 (11%) were evaluated in an emergency department for CNS depression, with one death as described below. A total of 93% of the mothers of symptomatic children had maternal CNS depression themselves, and 86% noted that the children's symptoms abated on stopping the codeine. The symptomatic children of mothers taking codeine were taking significantly higher doses (median 1.4 mg/kg/d; range 0.7–10.5 mg/kg/d) than those children who were asymptomatic (median 0.9 mg/kg/d; range 0.18–5.8 mg/kg/d).
There is one reported death of a 13-day-old breast-fed infant whose mother was using a combination codeine 30 mg and acetaminophen 500 mg product (initially two tablets every 12 h but decreased to half the dose after day 2 secondary to maternal somnolence and constipation). The mother had stored breast milk on day 10, which was assayed and found to contain morphine concentrations of 87 ng/mL, with typical concentrations of 1.9–20.5 ng/mL at doses of 60 mg every 6 h. Autopsy blood (source not identified) concentrations were reported at 70 ng/mL. Genotype analysis found the mother to be an ultra-rapid metabolizer of codeine (heterozygous for CYP2D6*2A allele with CYP2D6#2 × 2 gene duplication), which may explain the high concentrations of morphine found in the child and the breast milk.Citation22 Although some authors have suggested that neonatal morphine concentrations in this case were too high to have been caused only by ingestion of breast milk,Citation55 a pharmacokinetic model has suggested that a combination of ultra-rapid CYP2D6 activity and low neonatal clearance of morphine may have produced this highly elevated concentration.Citation37
Both the US Food and Drug Administration and Health Canada have issued public health advisories warning health care professionals and patients about the risks associated with codeine in breast milk.Citation96,Citation97
Meperidine (Pethidine)
Meperidine is a synthetic opioid that is available in tablet, solution, or syrup form. Meperidine is metabolized by several CYP enzymes (2B6, 3A4, 2C19) to metabolites that have no analgesic activity (e.g. normeperidine).Citation47
Data on meperidine's presence in human milk have been described in 20 patients. Nine women who were treated with a single dose of 50 mg had peak milk meperidine concentrations of 130 ng/mL after 2 h, and 20 ng/mL after 24 h, with an M/P ratio reported as > 1.Citation98 Quinn et al. reported two women who took 75–150 mg in a single dose and had milk meperidine concentrations of 209 and 275 ng/mL (8–12 h after the dose), respectively. The M/P ratio was reported as 0.84–1.59.Citation99 Borgatta et al. measured breast-milk samples in nine women after IV meperidine during general anesthesia. In eight women treated with 25-mg IV meperidine, the milk meperidine concentrations were 134–244 ng/mL after 1–2 h, 76–318 ng/mL after 2–4 h, and undetectable in seven of the eight women at 8–10 h. One woman had a milk meperidine concentration of 80 ng/mL after 8–10 h. In one patient treated with 75-mg meperidine, milk meperidine concentrations were 571 ng/mL (at 4 h), 224 ng/mL (at 8 h), and undetectable at 24 h.Citation100 The M/P ratio was 2.3 and the EI was estimated to be 1.2–3.5%.
Wittels et al. reported five nursing mothers who received intravenous meperidine in patient-controlled analgesia (PCA) after a cesarean section and were then transitioned to oral meperidine as needed. When comparing to infants whose mothers received intravenous morphine by PCA, infants on the third day of life whose mothers received meperidine had significantly higher neurobehavioral depression.Citation101
Tramadol
Tramadol is a synthetic analgesic with weak binding to the mu receptor. Tramadol is largely a prodrug and is metabolized by CYP2D6 to O-desmethyltramadol (M1), which more potently binds to mu receptors.Citation35,Citation47
Genetically extensive CYP2D6 metabolizers exist and may produce increased concentrations of M1Citation102 and may have enhanced analgesic effects.Citation103
Information on human milk concentrations are limited to one study of 75 women who were 2–4 days post-caesarian section and were given 100 mg of tramadol every 6 h, with breast-milk samples obtained after four or more doses. The M/P ratio was reported as 2.2. The infant dose was 112 mcg/kg/day with an RID of 2.24%. No adverse reactions were noted in the infants.Citation104
Buprenorphine
Buprenorphine is a semi-synthetic opioid that is unique to the above-mentioned opioids because it is a partial opioid agonist. It is available as a tablet or a solution. Buprenorphine is metabolized by CYP3A4 to the poorly active metabolite norbuprenorphine.Citation105
Inhibitors of CYP3A4 may theoretically cause accumulation of buprenorphine. CYP3A4 inhibitors include azole anti-fungals, calcium channel blockers, macrolides, SSRIs, anti-retroviral drugs, dexamethasone, amiodarone, cimetidine, cyclosporine, and disulfiram.Citation35,Citation67
Data on buprenorphine concentrations in human milk are limited to nine patients. Grimm et al. reported on milk concentrations in a breastfeeding mother 8 days post-partum receiving a common therapeutic dose of 8 mg daily sublingually.Citation106 Buprenorphine and norbuprenorphine concentrations ranged from 1.0 to 14.7 ng/mL and 0.6–6.3 ng/mL, respectively. The estimation of infant dose from peak concentrations was 1.47 mcg per 100 mL of breast milk with an ingested 24-h dose of less than 10 mcg for an infant weighing 4 kg.Citation106 Lindemalm et al. reported on seven breastfeeding mothers who were receiving an average of 0.32 mg/kg/day of buprenorphine with the calculated RID of < 1%.Citation48 Marquet reported on a single mother taking 4 mg/day of buprenoprhine. At 4 weeks, the total amount of buprenorphine and norbuprenorphine ingested by the infant was 3.28 mcg of buprenorphine and 0.33 mcg of norbuprenorpine over the 24 h of testing.Citation107
How should a physician advise a nursing mother?
Medical toxicologists may be called on to (1) assess for the potential toxicity to infants from maternal medications and (2) assess infants with opioid toxicity and attempt to determine whether the source was human milk or the ingestion of maternal pills.
When considering the initiation of xenobiotics in a breastfeeding woman, the benefits of the medication should be weighed heavily against not only the potential effect on the nursing infants by the xenobiotic, but also the negative effects of the mother decreasing or stopping breastfeeding altogether.Citation1,Citation2,Citation5 In one study, one-fifth of lactating women stopped breastfeeding when they initiated an antibiotic that was known to be safe.Citation108
When most opioids are initiated in a breastfeeding mother, there is little need to alter breastfeeding in any way. In the vast majority of cases, mothers should be encouraged to continue to breastfeed while using medically appropriate opioids. In some cases, mothers may note mild symptoms, such as minor alterations in wakefulness. However, of the millions of breastfeeding mothers who are taking opioids, there are a very small number of cases of significant toxicity in the literature.
There are some steps that seem reasonable when considering the treatment of lactating women with opioids and these may further reduce the very low potential for toxicity:
Consider if pain medication is necessary. If it is, then use the safest drug available (e.g. acetaminophen or NSAIDs) as first-line agents for pain in breastfeeding women.Citation3,Citation71
Avoiding codeine for long-term therapyCitation108 seems reasonable as it has been associated with one death, produced multiple cases of toxicity, has a highly variable metabolism, and there are more effective opioid choices.
Avoid dosing breastfeeding women with high-dose opioids or opioids that cause maternal sedation.Citation27,Citation71,Citation109,Citation110 Using the lowest effective dose of opioid is prudent.
The majority of adverse events occur in the first few weeks of life. Particular caution should be taken in this time period.Citation16,Citation109
In the vast majority of cases of maternal opioid use, no adverse events occur. However, in many cases, the lactating mother is instructed to discard human milk (“pump and dump”). Recommending the discarding of human milk should involve weighing the risk of the opioid to the infant versus the risk that a physician's advice to discard human milk will lead to the mother decreasing or stopping breastfeeding. Discarding human milk is not necessary in almost all circumstances where the mother is taking an opioid.
There are certainly non-opioid xenobiotics that may expose the infant to potential harm and the decision to discard human milk is made. In the case of opioids, discarding human milk is probably only necessary in the rare instance when the mother becomes symptomatic from the opioid (e.g. CNS depression) or there is a dosing error. If this occurs and the decision to discard human milk is made, then some basic procedures should be followed. The mother should produce a human milk sample and discard it, at a minimum, after peak maternal serum concentrations are reached and any adverse symptoms that the mother experiences (e.g. CNS sedation) have abated.Citation5,Citation110
When assessing a child with opioid toxicity, it is prudent to consider that the source of the opioid may be from the child's ingestion of the maternal medication. When the human milk is deemed a potential source, opioid concentrations in infant blood, maternal blood, and milk should all be collected as soon as possible after toxicity appears.
In the future, advances in pharmacogenomics research may allow physicians to tailor opioid therapy to their patients, taking into account how both the mother and the infant metabolize the medication to further decrease risks.Citation111
Conclusion
Maternal genetic polymorphism in CYP2D6 likely explains most of the observed variation in effect. Mothers who are ultra-rapid metabolizers may have a higher risk of neonatal CNS and respiratory depression with certain opioids. This has only been documented to occur with codeine, which is highly dependent on CYP2D6 in the conversion to morphine, and is the least preferred opioid. This effect is less likely to occur with oxycodone or hydrocodone, which are less dependent on CYP2D6. CYP3A4 is important in the clearance of methadone, meperidine, and buprenorphine, which should be avoided in mothers taking other medications known to inhibit CYP3A4. Morphine and hydromorphone are inactivated by glucuronide conjugation. Polymorphism in the family of UGT enzymes may play a role in the maternal accumulation of morphine or hydromorphone, but this is not well studied in this population. Neonatal metabolic enzymes are immature, and drug accumulation may occur with repeated exposure on successive days.
Serious adverse neonatal events attributable to maternal opioid use are infrequent. Using the lowest effective analgesic dose for the minimum time needed for pain control should minimize the modest risk.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- American Academy of Pediatrics, Work Group on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics 1997; 100: 1035–1039.
- Ito S, Lee A. Drug excretion into breast milk – overview. Adv Drug Deliv Rev 2003; 55:617–627.
- American Academy of Pediatrics, Committee on Drugs. The transfer of drugs and other chemicals into human milk. Pediatrics 2001; 108:776–789.
- Ryan AS, Wenjun Z, Acosta A. Breastfeeding continues to increase into the new millennium. Pediatrics 2002; 110:1103–1109.
- Wight NE. Maternal medications and breastfeeding. Cal J Health-Sys Pharm 2007; 19:5–17.
- Bohnert ASB, Valenstein M, Bair MJ, Ganoczy D, McCarthy JF, Ilgen MA, Blow FC. Association between opioid prescribing patterns and opioid overdose-related deaths. J Am Med Assoc 2011; 305:1315–21.
- Kuehn BM. Safety plan for opioids meets resistance: opioid-linked deaths continue to soar. J Am Med Assoc 2010; 303:495–497.
- Kuehn BM. Opioid prescriptions soar: increase in legitimate use as well as abuse. J Am Med Assoc 2007; 297:249–251.
- Volkow ND, McLellan TA, Cotto JH, Karithanom M, Weiss SR. Characteristics of opioid prescriptions in 2009. J Am Med Assoc 2011; 305:1299–1301.
- The Use of Medicines in the United States: review of 2010. IMS Institute for Healthcare Informatics. http://www.imshealth.com/deployedfiles/imshealth/Global/Content/IMS%20Institute/Static%20File/IHII_UseOfMed_report.pdf. Accessed 23 April 2011.
- Matheson I. Drugs taken by mothers in the puerperium. Br Med J (Clin Res Ed) 1985; 290:1588–1589.
- Passmore CM, McElnay JC, D’Arcy PF. Drugs taken by mothers in the puerperium: inpatient survey in Northern Ireland. Br Med J 1984; 289:1593–1596.
- Collaborative group on drug use in pregnancy. Medication during pregnancy: an intercontinental cooperative study. Int J Gynecol Obstet 1992; 39:185–196.
- Freed GL, Clark SJ, Sorenson J, Lohr JA, Cefalo R, Curtis P. National assessment of physicians’ breast-feeding knowledge, attitudes, training, and experience. J Am Med Assoc 1995; 273:472–476.
- Ito S. Drug therapy for breast-feeding women. New Engl J Med 2000; 343:118–126.
- Anderson PO, Pochop SL, Manoguerra AS. Adverse drug reactions in breastfed infants: less than imagined. Clin Pediatr 2003; 42:325–340.
- Smith JW. Codeine-induced bradycardia in a breast-fed infant [abstract]. Clin Res 1982; 30:259a.
- Davis JM, Bhutani VK. Neonatal apnea and maternal codeine use [abstract]. Pediatr Res 1985; 19(4 pt 2):170a.
- Naumburg EG, Meny RG. Breast milk opioids and neonatal apnea. Am J Dis Child 1988; 142:11–12.
- Ito S, Koren G, Einarson TR. Maternal non-compliance with antibiotics during breastfeeding. Ann Pharmacother 1993; 27:40–42.
- Madadi P, Shirazi F, Walter FG, Koren G. Establishing causality of CNS depression in breastfed infants following maternal codeine use. Pediatr Drugs 2008; 10:399–404.
- Koren G, Cairns J, Chitayat G, Leeder SJ. Pharmacogenetics of morphine poisoning in a breast fed neonate of a codeine-prescribed mother. Lancet 2006; 368:704.
- Levine B, Moore KA, Aronica-Pollak P, Fowler DF. Oxycodone intoxication in an infant: accidental or intentional exposures? J Forensic Sci 2004; 49:1358–1360.
- Bodley V, Powers D. Long-term treatment of a breastfeeding mother with fluconazole-resolved nipple pain caused by yeast: a case study. J Hum Lact 1997; 13:307–311.
- Meyer D, Tobias JD. Adverse effects following the inadvertent administration of opioids to infants and children. Clin Pediatr 2005; 44:499–503.
- West PL, McKeown NJ, Hendrickson RG. Methadone overdose in a breast-feeding toddler. Clin Toxicol 2009; 47:721.
- Smialek JE, Monforte JR, Aronow R, Spitz WU. Methadone deaths in children. A continuing problem. J Am Med Assoc 1977; 238:2516–2517.
- Lawrence RA, Lawrence RM. Breastfeeding. A Guide for the Medical Profession. 7th ed. St. Louis, MI: Elsevier Mosby; 2011: 40–61, 98–116, 860–866.
- Berlin CM, Briggs GC. Drugs and chemicals in human milk. Sem Fet Neonat Med 2005; 10:149–159.
- Fleisheker JC, McNamara PJ. In vivo evaluation in the lactating rabbit of a model for xenobiotic distribution into breast milk. J Pharmacol Exp Ther 1988; 244:919–924.
- Spigset O, Hagg S. Analgesics and breast-feeding. Safety considerations. Paediatr Drugs 2000; 2:223–238.
- Ferris AM, Jensen RG. Lipids in human milk: a review. J Pediatr Gastroenterol Nutr 1984; 3:108–122.
- Prentice A, Prentice AM, Lamb WH. Mastitis in rural Gambian mothers and the protection of the breast by milk antimicrobial factors. Trans R Soc Trop Med Hyg 1985; 79:90–95.
- Anderson PO. Drug use during breast-feeding. Clin Pharm 1991; 10:594–624.
- Smith HS. Opioid metabolism. Mayo Clin Proc 2009; 84:613–624.
- Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology – drug disposition, action, and therapy in infants and children. N Engl J Med 2003; 349:1157–1167.
- Willman S, Edginton AN, Coboeken K, Ahr G, Lippert J. Risk to the breast-fed neonate from codeine treatment to the mother: a quantitative mechanistic modeling study. Clin Pharmacol Ther 2009; 86:634–643.
- Lacroix D, Sonnier M, Moncion A, Cheron G, Cresteil T. Expression of CYP3A in the human liver—evidence that the shift between CYP3A7 and CYP3A4 occurs immediately after birth. Eur J Biochem 1997; 247:625–634.
- Sonnier M, Cresteil T. Delayed ontogenesis of CYP1A2 in the human liver. Eur J Biochem 1998; 251:893–898.
- Treluyer JM, Jacqz-Aigrain E, Alvarez F, Cresteil T. Expression of CYP2D6 in developing human liver. Eur J Biochem 1991; 202: 583–588.
- Vieira I, Sonnier M, Cresteil T. Developmental expression of CYP2E1 in the human liver. Hypermethylation control of gene expression during the neonatal period. Eur J Biochem 1996; 238:476–483.
- Blake MJ, Gaedigk A, Pearce RE, Bomgaars LR, Christensen ML, Stowe C, . Ontogeny of dextromethorphan O- and N-demethylation in the first year of life. Clin Pharmacol Ther 2007; 81(4):510–516.
- McRorie TI, Lynn AM, Nespeca MK, Opheim KE, Slattery JT. The maturation of morphine clearance and metabolism. Am J Dis Child 1992; 146:972–976.
- Arant BS Jr. Developmental patterns of renal functional maturation compared in the human neonate. J Pediatr 1978; 92:705–712.
- Leake RD, Trygstad CW. Glomerular filtration rate during the period of adaptation to extrauterine life. Pediatr Res 1977; 11:959–962.
- Choonara I, Lawrence A, Michalkiewicz A, Bowha A, Ratcliffe J. Morphine metabolism in neonates and infants. Br J Clin Pharmacol 1992; 34:434–437.
- Coller JK, Christrup LL, Somogyi AA. Role of active metabolites in the use of opioids. Eur J Clin Pharmacol 2009; 65:121–139.
- Lindemalm S, Nydert P, Svensson JO, Stahle L, Sarman I. Transfer of buprenorphine into breast milk and calculation of infant drug dose. J Human Lact 2009; 25:199–205.
- Kraft WK, Gibson E, Dysart K, Damle VS, Larusso JL, Greenspan JS, . Sublingual buprenorphine for treatment of neonatal abstinence syndrome: a randomized trial. Pediatrics 2008; 122:e601–e607.
- Concheiro M, Jones HE, Johnson RE, Choo R, Shakleya DM, Huestis MA. Maternal buprenorphine dose, placenta buprenorphine, and metabolite concentrations and neonatal outcomes. Ther Drug Monit 2010; 32:206–215.
- Dewey KG, Finley DA, Lonnerdal B. Breast milk volume and composition during late lactation (7–20 months). J Pediatr Gastroenterol Nutr 1984; 3:713–720.
- Mandel D, Lubetzky R, Dollberg S, Barak S, Mimouni FB. Fat and energy contents of expressed human breast milk in prolonged lactation. Pediatrics 2005; 116:e432–e435.
- Larnkjaer A, Schack-Nielsen L, Michaelsen KF. Fat content in human milk according to duration and lactation. Pediatrics 2006; 117:988–989.
- AAP publications reaffirmed and retired. Pediatrics 2010; 126:404.
- Bateman DN, Eddleston M, Sandilands E. Codeine and breastfeeding. Lancet 2008; 372(9639):625.
- Darbari DS, van Schaik RH, Capparelli EV, Rana S, McCarter R, van den Anker J. UGT2B7 promoter variant −840G > A contributes to the variability in hepatic clearance of morphine in patients with sickle cell disease. Am J Hematol 2008; 83:200–202.
- Duguay Y, Baar C, Skorpen F, Guillemette C. A novel functional polymorphism in the uridine diphosphate-glucuronosyltransferase 2B7 promoter with significant impact on promoter activity. Clin Pharmacol Ther 2004; 75:223–233.
- Green MD, King CD, Mojarrabi B, Mackenzie PI, Tephly TR. Glucuronidation of amines and other xenobiotics catalyzed by expressed human UDP-glucuronosyltransferease 1A3. Drug Metab Dispos 1998; 26:507–512.
- Penson RT, Joel SP, Clark S, Gloyne A, Slevin ML. Limited phase I study of morphine-3-glucuronide. J Pharm Sci 2001; 90:1810–1816.
- Smith MT. Neurotoxicity effects of morphine and hydromorphone: evidence implicating the 3-glucuronide metabolites. Clin Exp Pharmacol Physiol 2000; 27:524–528.
- Robieux I, Koren G, Vandenbergh H, Schneiderman J. Morphine excretion in breast milk and resultant exposure of a nursing infant. J Toxicol Clin Toxicol 1990; 28:365–370.
- Feilberg VL, Rosenborg D, Broen Christensen C, Mogensen JV. Excretion of morphine in human breast milk. Acta Anaesth Scand 1989; 33:426–428.
- Wittels B, Scott DT, Sinatra RS. Exogenous opioids in human breast milk and acute neonatal neurobehavior: a preliminary study. Anesthesiology 1990; 73:864–869.
- Oberlander TF, Robeson P, Ward V, Huckin RS, Kamani A, Harpur A, McDonald W. Prenatal and breast milk morphine exposure following maternal intrathecal morphine treatment. J Human Lact 2000; 16:137–142.
- Baka NE, Bayoumeu F, Boutroy MJ, Laxenaire MC. Colostrum morphine concentrations during postcesarean intravenous patient-controlled analgesia. Anesth Analg 2002; 94:184–187.
- Lemberg KK, Kontinen VK, Siiskonen AO, Vijakka KM, Yli-Kauhaluoma JT, Korpi ER, Kalso EA. Antinociception by spinal and systemic oxycodone: why does the route make a difference? Anesthesiology 2006; 105:801–812.
- Pelkonen O, Turpeinen M, Hakkola J, Honkakoski P, Hukkanen J, Raunio H. Inhibition and induction of human cytochrome P450 enzymes: current status. Arch Toxicol 2008; 82:667–715.
- Samer CF, Daali Y, Wagner M, Hopfgartner G, Eap CB, Rebsamen MC, . Genetic polymorphisms and drug interactions modulating CYP2D6 and CYP3A activities have a major effect on oxycodone analgesic efficacy and safety. Br J Pharmacol 2010; 160:919–930.
- Foster A, Mobley E, Wang Z. Complicated pain management in a CYP450 2D6 poor metabolizer. Pain Pract 2007; 7:352–356.
- Marx CM, Pucino F, Carlson JD, Driscoll JW, Ruddock V. Oxycodone excretion in human milk in the puerperium [abstract]. Drug Intell Clin Pharm 1986; 20:474.
- Seaton S, Reeves M, McLean S. Oxycodone as a component of multimodal analgesia for lactating mothers after Caesarean section: relationships between maternal plasma, breast milk and neonatal plasma levels. Aust N Z J Obstet Gynaecol 2007; 47:181–185.
- Lam J, Kelly L, Ciszkowski C, LandsmeerMLA, NautaM, CarletonBC, Central nervous system depression of neonates breastfed by mothers receiving oxycodone for postpartum analgesia. J Pediatr 2011; DOI 10.1016/j.jpeds.2011.06.050 (in press).
- Chen ZR, Irvine RJ, Somogyi AA, Bochner F. Mu receptor binding of some commonly used opioids and their metabolites. Life Sci 1991; 48:2165–2171.
- Otton SV, Schadel M, Cheung SW, Kaplan HL, Busto UE, Sellers EM. CYP2D6 phenotype determines the metabolic conversion of hydrocodone to hydromorphone. Clin Pharmacol Ther 1993; 54: 463–472.
- Anderson PO, Sauberan J, Lane JR, Rossi SS. Hydrocodone excretion into breastmilk: the first two reported cases. Breastfeed Med 2007; 2:10–14.
- Sauberan JB, Anderson PO, Lane JR, Rafie S, Nguyen N, Rossi SS, Stellwagen LM. Breast milk hydrocodone and hydromorphone levels in mothers using hydrocodone for postpartum pain. Obstet Gynecol 2011; 117:611–617.
- Edwards JE, Rudy AC, Wermeling DP, Desai N, McNamara PJ. Hydromorphone transfer into breast milk after intranasal administration. Pharmacotherapy 2003; 23:153–158.
- Wang JS, DeVane CL. Involvement of CYP3A4, CYP2C8, and CYP2D6 in the metabolism of ®- and (S)-methadone in vitro. Drug Metab Disp 2003; 31:742–747.
- Chabra S, Bull J. Methadone. Am J Hosp Pall Med 2008; 25:146–150.
- Ferrari A, Rosario Coccia CP, Bertolini A, Sternieri E. Methadone-metabolism, pharmacokinetics and interactions. Pharmacol Res 2004; 50:551–559.
- Begg EJ, Malpas TJ, Hackett LP, Ilett KF. Distribution of R- and S-methadone into human milk during multiple, medium to high oral dosing. Br J Clin Pharmacol 2001; 52:681–685.
- McCarthy JJ, Posey BL. Methadone levels in human milk. J Hum Lact 2000; 16:115–120.
- Kreek MJ, Schecter A, Gutjahr CL, Bowen D, Field F, Queenan J, Merkatz I. Analyses of methadone and other drugs in maternal and neonatal body fluids: use in evaluation of symptoms in a neonate of mother. Am J Drug Alcohol Abuse 1974; 1:409–419.
- Blinick G, Inturrissi CE, Jerez E, Wallach RC. Methadone assays in pregnant women and progeny. Am J Obstet Gynecol 1975; 121: 617–621.
- Pond SM, Kreek MJ, Tong TG, Raghunath J, Benowitz NL. Altered methadone pharmacokinetics in methadone-maintained pregnant women. J Pharmacol Exp Ther 1985; 233:1–6.
- Wojnar-Horton RE, Kristensen JH, Yapp P, Ilett KF, Dusci LJ, Hackett LP. Methadone distribution and excretion into breast milk of clients in a methadone maintenance programme. Br J Clin Pharmacol 1997; 44:543–547.
- Jansson LM, Choo RE, Harrow C, Velez M, Schroeder JR, Lowe R, Huestis MA. Concentrations of methadone in breast milk and plasma in the immediate perinatal period. J Hum Lact 2007; 23:184–190.
- Jansson LM, Choo R, Velez ML, Lowe R, Huestis MA. Methadone maintenance and breastfeeding in the neonatal period. Pediatrics 2008; 121:106–114.
- Madadi P, Ross CJD, Hayden MR, Carleton BC, Gaedigk A, Leeder JS, Koren G. Pharmacogenetics of neonatal opioid toxicity following maternal use of codeine during breastfeeding: a case-control study. Clin Pharmacol Ther 2009; 85:31–35.
- Sistonen J, Sajantilla A, Lao O, Corander J, Barbujani G, Fuselli S. CYP2D6 worldwide genetic variation shows high frequency of altered activity variants and no continental structure. Pharmacogenet Genomics 2007; 17:93–101.
- Vree TB, Verwey-van Wissen CP. Pharmacokinetics and metabolism of codeine in humans. Biopharm Drug Dispos 1992; 13:445–460.
- Kwit NT, Hatcher RA. Excretion of drugs in milk. Am J Dis Child 1935; 49:900–904.
- Findlay JW, DeAngelis RL, Welch RM, Findlay JM. Analgesic drugs in breast milk and plasma. Clin Pharmacol Ther 1981; 29:625–633.
- Meny RG, Naumburg EG, Alger LS, Vrill-Miller JL, Brown S. Codeine and the breastfed neonate. J Hum Lact 1994; 9:237–240.
- Ito S, Blajchman A, Stepheson M, Eliopoulos C, Koren G. Prospective follow-up of adverse reactions in breast-fed infants exposed to maternal medication. Am J Obstet Gynecol 1993; 168:1393–1399.
- http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm118113.htm. Accessed 13 September 2011.
- http://www.hc-sc.gc.ca/ahc-asc/media/advisories-avis/_2008/2008_164-eng.php. Accessed 13 September 2011.
- Peiker G, Muller B, Ihn W, Noschel H. Excretion of pethidine in mother's milk. Zentralbl Gynakol 1980; 102:537–541.
- Quinn PG, Kuhner BR, Kaine CJ, Syracuse CD. Measurement of meperidine and normpeperidine in human breast milk by selected ion monitoring. Biomed Environ Mass Spectrom 1986; 13:133–135.
- Borgatta L, Jenny RW, Gruss L, Ong C, Barad D. Clinical significance of methohexital, meperidine, and diazepam in breast milk. J Clin Pharmacol 1997; 37:186–192.
- Wittels B, Glosten B, Faure EA, Moawad AH, Ismail M, Hibbard J, . Postcesarean analgesia with both epidural morphine and intravenous patient-controlled analgesia: neurobehavioral outcomes among nursing neonates. Anesth Analg 1997; 85:600–606.
- Fliegert F, Kurth B, Gohler K. The effects of tramadol on static and dynamic pupillometry in health subjects—the relationship between pharmacodynamics, pharmacokinetics, and CYP2D6 metaboliser status. Eur J Pharmacol 2005; 61:257–266.
- Poulsen L, Arendt-Nielsen L, Vrosen K, Sindrup SH. The hypoalgesic effect of tramadol in relation to CYP2D6. Clin Pharmacol Ther 1996; 60:636–644.
- Ilett KF, Paech MJ, Page-Sharp M, Sy SK, Kristensen JH, Goy R, . Use of a sparse sampling study design to assess transfer of tramadol and its O-desmethyl metabolite into transitional breast milk. Br J Clin Pharmacol 2008; 65:661–666.
- Ohtani M, Kotaki H, Sawada Y, Iga T. Comparative analysis of buprenorphine and norbuprenorphine-induced analgesic effects based on pharmacokinetic-pharmacodynamic modeling. J Pharmacol Exp Ther 1995; 272:505–510.
- Grimm D, Pauly E, Poschl J, Linderkamp O, Skopp G. Buprenorphine and norbuprenorphine concentrations in human breast milk samples determined by liquid chromatography-tandem mass specometry. Ther Drug Monit 2005; 27:526–530.
- Marquet P, Chevrel J, Lavignasse P, Merle L, Lachatre G. Buprenorpine withdrawal syndrome in a newborn. Clin Pharmacol Ther 1997; 62:569–571.
- Madadi P, Moretti M, Djokanovic N, Bozzo P, Nulman I, Ito S, Koren G. Guidelines for maternal codeine use during breastfeeding. Can Fam Phys 2009; 55:1077–1078.
- Berlin CM, Paul IM, Vesell ES. Safety issues of maternal drug therapy during breastfeeding. Clin Pharmacol Ther 2009; 85:20–22.
- Anderson PO. Drug use during breast-feeding. Clin Pharm 1991; 10:594–624.
- Blumenfeld YJ, Reynolds-May MF, Altman RB, El-Sayed YY. Maternal-fetal and neonatal pharmacogenomics: a review of the current literature. J Perinatol 2010; 30:571–579.
- Osborne R, Thompson P, Joel S, Trew D, Patel N, Slevin M. The analgesic activity of morphine-6-glucuronide. Br J Clin Pharmacol 1992; 34:130–138.
- Lalovic B, Phillips B, Risler LL, Howald W, Shen DD. Quantitative contribution of CYP2D6 and CYP3A to oxycodone metabolism in human liver and intestinal microsomes. Drug Metab Disp 2004; 32:447–454.