To the Editor:
Despite being a metal, the radiopacity of lithium tablets is not fully determined. We found only two previously published reports investigating this, both in vitro studies. One study found two lithium carbonate preparations to be moderately detectable by plain film radiography.Citation1 The other study, using computed tomography (CT), found one preparation of lithium carbonate to be weakly to moderately detectable, while a lithium carbonate preparation from another manufacturer was not detectable at all.Citation2 The only lithium tablet currently approved in Sweden is a sustained release preparation containing lithium sulphate. According to the literature, whole bowel irrigation (WBI) and gastric lavage may be indicated in cases of massive acute overdose of such tablets if the patient presents very early.Citation3,Citation4
We performed a prospective survey during the year 2010 of all inquiries to the Swedish poison centre concerning acute lithium poisoning. All patients who presented with a suspected intake of 20 or more lithium tablets within 6 hours before the call were included. In addition to other appropriate management advice, we recommended acute abdominal imaging, but let the clinician decide whether to obtain plain film radiography or abdominal CT.
A total of 17 cases were enrolled. Three cases were excluded, either because of co-ingestion of iron tablets or because of lack of reliable clinical data. The remaining 14 cases constituted the final study population. According to the history, they had ingested between 25 and 130 lithium tablets within 6 hours before the call. Eleven of the 14 patients had ingested the tablets within 3 hours before their arrival in the hospital. Three of the patients had ingested other medications together with lithium, but none of the co-ingested drugs are known to be radiopaque.Citation1,Citation2 In two patients, no emergency x-ray was performed. In six patients, plain film radiography was performed, all with a negative result. These patients had ingested an average of 56 lithium tablets. The remaining six patients had ingested an average of 67 lithium tablets and were examined by abdominal CT. In five of these latter patients, tablets were clearly visible in the gastrointestinal tract. All the 14 patients developed toxic serum lithium concentrations during the hospital course (maximum S-Li 1.7–5.9 mmol/L; therapeutic level: 0.4–1.0 mmol/L) and all the patients survived.
One of the included cases is summarized as follows. A 56-year-old man on lithium treatment ingested 70 lithium sulphate tablets, but no other medication. A CT scan approximately 2 hours after ingestion showed numerous tablets in the stomach and small intestine (). Gastric lavage was performed and WBI was started three and a half hours after ingestion. The serum lithium concentration reached a maximum of 5.0 mmol/L. The patient eventually received treatment with haemodialysis.
Fig. 1. A computed tomography of the abdomen performed about 2 hours after a large lithium sulphate overdose and showing numerous tablets in the stomach and small intestine.
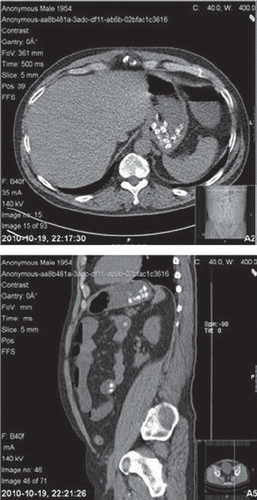
A limitation needs to be mentioned. The sulphate content of the ingested tablets may have been of some importance for their radiopacity, wherefore the findings of this study may not be applicable for lithium carbonate preparations.
Based on these findings, ingested sustained release lithium sulphate tablets may be visible on abdominal CT. The use of plain film radiography seems less sensitive. In cases with suspected recent ingestion of a large lithium sulphate dose, performance of abdominal CT may help the decision whether to perform gastrointestinal decontamination or not.
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- O'Brian RP, McGeehan PA, Helmeczi AW, Dula DJ. Detectability of drug tablets and capsules by plain radiography. Am J Emerg Med 1986; 4:302–312.
- Savitt DL, Hawkins HH, Roberts JR. The radiopacity of ingested medications. Ann Emerg Med 1987; 16:331–339.
- American Academy of Clinical Toxicology, European Association of Poison Centresand Clinical Toxicologists. Position paper: whole bowel irrigation. J Toxicol Clin Toxicol 2004; 42:843–854.
- Vale JA, Kulig K.American Academy of Clinical Toxicology, European Association of Poison Centres and Clinical Toxicologists. Position paper: gastric lavage. J Toxicol Clin Toxicol 2004; 42: 933–943.