To the Editor:
Intravenous lipid emulsion (ILE) preparations have recently emerged as novel antidotes for poisonings with highly lipid soluble drugs. Both pharmacokinetic and pharmacodynamic mechanisms have been proposed as explanations for the beneficial effects observed following ILE administration. The predominant pharmacokinetic hypothesis purports sequestration of lipophilic toxins away from target organs and into a newly created circulating lipid phase – the so called “lipid sink”.Citation1 It has nevertheless been postulated that administration of ILE early in the course of enteric overdose may lead to more rapid toxin absorption from gastrointestinal (GI) tract as enteric perfusion with blood of increased affinity for lipophilic substances comes in contact with luminal contents of similar lipophilicity.Citation2,Citation3 Increased toxin absorption may thereby speed up the development of systemic toxicity.
Octreotide is a long-acting synthetic somatostatin analogue with an established utility in reducing the frequency of recurring hypoglycaemia following sulfonylurea poisoning.Citation4 In addition to suppression of neuroendocrine and exocrine intestinal secretion, octreotide is known to reduce splanchnic blood flow and to increase intestinal transit time.Citation5 Although the benefit of octreotide administration in sulphonylurea overdose has largely been attributed to an effect on inhibition of pancreatic insulin secretion, additional benefits may occur through delayed drug absorption secondary to these later effects.Citation6 Furthermore, the potential for octreotide to modulate absorption of alternate ingested xenobiotics through reduction in enteric blood flow remains unexplored.
We were interested to examine the effects of these two antidotes on the speed of developed toxicity for an enterically administered lipophillic toxin in an intact animal model. Specifically, we determined to document survival following both ILE and octreotide, administration in enteric thiopentone overdose in whole rats. Death by respiratory depression (as a marker of central nervous system [CNS] thiopentone toxicity) was utilised as the primary outcome variable. Thiopentone was elected as CNS toxin of interest given its high lipid solubility and predictable redistribution kineticsCitation7 with established modulation following ILE administration.Citation8
With institutional ethics committee approval of the Ruakura Animal Research Centre, Hamilton, New Zealand, 15 male Sprague Dawley rats weighing between 210 and 314 g were studied. On the day of study, animals underwent sedation with ketamine at 50 mg/kg (Mayne Pharma Ltd, Auckland, New Zealand) and xylazine at 4 mg/kg (Bayer HealthCare, Leverkusen, Germany) via intraperitoneal injection. Cannulation of a tail vein was performed and animals were placed supine on a warmed surgical board. Study agents were administered intravenously as pre-treatment according to random number generation at time zero. Control group animals received 0.9% saline solution at 3 ml.kg− 1; ILE group animals received 20% lipid emulsion (Intralipid 20%, Pharmatel Fresenius Kabi Pty Ltd, NSW, Australia) at 3 ml.kg− 1; and octreotide group animals received octreotide (Novartis, Auckland, New Zealand) at 2 mg.kg− 1 in 3 ml.kg− 1 0.9% saline. All rats then underwent rectal administration of 300 mg.kg− 1 thiopentone (Link Pharmaceuticals Ltd, West Sussex, UK [100 mg.ml− 1]) at time equals one minute, followed by 50 mg.kg− 1 at time equals 25 minutes and repeated every five minutes thereafter until animal death.
Time to respiratory arrest (defined by the absence of any respiratory activity) was recorded. Cardiac puncture was performed following death with blood sampled for determination of serum thiopentone concentration according to the method described by Russo et al.Citation9, and triglyceride levels. Statistical analysis was conducted using GraphPad Prism (version 5.0, GraphPad Software Inc, La Jolla, USA). Kruskal-Wallis ANOVA and Mann-Whitney U testing were utilised in appropriate places to compare differences between groups. All data were presented as median and interquartile range (IQR). A p value of less than 0.05 was considered statistically significant.
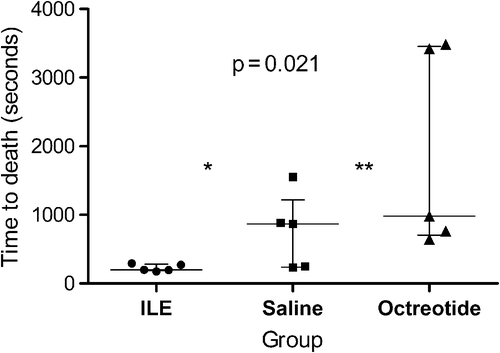
No difference in baseline physiologic characteristics (age [p = 0.28], weight [p = 0.32], respiratory rate [p = 0.24]) was observed between groups. All animals died of respiratory arrest. Time to death was 198 seconds (IQR 185-281) in the lipid emulsion group, 868 seconds (IQR 189-1217) in the saline group and 982 seconds (IQR 704-3454) in the octreotide group (P = 0.021); ().
Total plasma thiopentone levels were 139 (IQR 57-149) umolL− 1 in the ILE group, 181 (IQR 108-245.5) umolL− 1 in the saline group and 286 (IQR 220-313.5) umolL− 1 in the octreotide group; p = 0.0178. Plasma triglyceride levels were 2.45 (IQR 1.72-3.00) mmolL− 1 in the octreotide group, 3.34 (IQR 2.77-3.91) mmolL− 1 in the saline group and 7.48 (IQR 6.87- 11.55) mmolL− 1 in the ILE group; p = 0.0059.
Thiopentone is a lipophilic barbiturate anaesthetic with logP (log octanol–water partition co-efficient) of 2.78.Citation6 Respiratory depression is a recognised complication following administration. In this pilot study, intravenous lipid emulsion administered prior to enteric thiopentone overdose resulted in a trend towards more rapid onset of respiratory arrest. That CNS toxicity might develop earlier, despite lesser measured thiopentone concentration, suggests a role for increased plasma lipaemia in augmenting translocation of toxin from the GI tract lumen to target organ (brain). Although not the subject of this study, efficiencies in cerebral toxin accumulation with increased blood lipophilicity may have ensued through increased GI absorption – as newly lipaemic blood with greater lipophilic toxin carrying capacity comes into contact with highly lipid soluble toxin across mucosal capillaries. Plasma lipaemia may have additionally contributed to enhanced blood carriage of lipophilic thiopentone and/or more efficient thiopentone uptake by CNS tissues of similarly high lipophilicity.
Pharmacokinetic modulation following early ILE administration has been previously reported with increased depth of sedation observed following ILE co-administration with intravenous thiopentone in rabbits.Citation7 The authors postulated increased cerebral recirculation of lipid-sequestered thiopentone, and lesser initial inter-compartmental clearance, as potential mechanisms for such an effect. Furthermore, five minutes after oral parathion exposure, ILE administration resulted in a non-significant trend towards earlier respiratory arrest compared with control animals in the work of Dunn et al.Citation10 Conversely, when administered at 20 minutes ILE prolonged survival. An increase in early parathion absorption mediated by ILE may in part explain these observed differences in survival.
While apparently bi-modal in distribution, our results following octreotide are suggestive of increased survival in some animals. Albeit this experiment was not designed to explore mechanistically the potential reasons for such a finding, they are consistent with a lesser rate of enteric thiopentone absorption secondary to octreotide-induced reduction in splanchnic blood flow.
This study is subject to a number of limitations precluding clinical extrapolation of the presented findings. By very nature, as a pilot study it involves small animal numbers and as such is underpowered to detect anything other than gross differences in key study metrics. Additionally the protocol entails rectal drug delivery, rather than the more commonly encountered oral route of toxin administration. Therapies were furthermore administered as pre-treatment rather than following development of manifest toxicity, thereby negating direct application of any findings to commonly encountered clinical poisoning scenarios. Finally, the study was not intended to explore in detail the potential mechanisms of any inter-group outcome differences.
We plan further work exploring the early effects of ILE in enteric lipophillic toxin overdose, and the intriguing possibility that octreotide may modulate the development of toxicity in enteric toxin poisoning.
Declaration of interest The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Weinberg G. Lipid resuscitation from local anaesthetic cardiac toxicity. Toxicol Rev 2006; 25:139–145.
- Turner-Lawrence D, Kerns W. Intravenous fat emulsion: a potential novel antidote. J Med Toxicol 2008; 4:109–114.
- Harvey M, Cave G. Case report: successful lipid resuscitation in multi-drug overdose with predominant tricyclic antidepressant toxidrome. Int J Emerg Med 2012; 5:8.
- Dougherty P, Klein-Schwartz W. Octreotides role in the management of sulfonylurea-induced hypoglycaemia. J Med Toxicol 2010; 6:199–206.
- Harris A. Somatostatin and somatostatin analogues: pharmacokinetics and pharmacodynamic effects. Gut 1994;supplement 3:S1–S4.
- Cooper A, Braatvedt G, Qamar M, Brown H, Thomas DM, Halliwell M, .Fasting and post-prandial splanchnic blood flow is reduced by a somatostatin analogue (octreotide) in man. Clin Sci (Lond) 1991; 81:169–175.
- Price H. A dynamic concept of the distribution of thiopental in the human body. Anesthesiology 1960; 21:40–45.
- Kazemi A, Harvey M, Cave G, Lahner D. The effect of lipid emulsion on depth of anaesthesia following thiopental administration to rabbits. Anaesthesia 2011; 66:373–378.
- Russo H, Allaz J, Bressolle F. High-performance liquid chromatographic assay for thiopental in human plasma. Application to pharmacokinetic studies. J Chromatogr B Biomed Sci Appl 1997; 694:239–245.
- Dunn C, Bird S, Gaspari R. Intralipid fat emulsion decreases respiratory failure in a rat model of parathion exposure. Acad Emerg Med 2012; 19:504–509.