Abstract
Objective. There is limited information on mirtazapine overdose, but cases of severe effects (seizures, serotonin toxicity and coma) have been reported. We aimed to investigate the clinical effects and complications of mirtazapine overdose. Methods. This was an observational case series of mirtazapine overdoses (> 120 mg) identified from admissions to a toxicology unit between January 1987 and August 2013. Demographic information, details of ingestion, clinical effects, ECG parameters (HR, QT and QRS), and length of stay were extracted from a clinical database. Results. From 267 mirtazapine overdoses, there were 89 single-agent mirtazapine ingestions and 178 cases where mirtazapine was taken with at least one other drug. The median age of the 89 single-agent mirtazapine ingestions was 36 years [interquartile range (IQR): 26–49 years; Range: 15–81 years]; 45 were female (51%). The median ingested dose was 420 mg (IQR: 270–750 mg; Range: 150–1350 mg) and 41 patients (46%) had a Glasgow coma score (GCS) < 15, but the minimum GCS was 10. There were no seizures, serotonin toxicity or delirium. Tachycardia occurred in 29 patients (33%) and hypertension in 32 patients (36%). The median QRS was 80 ms (Range: 80–120 ms) and there were no cases with QT prolongation. There were no arrhythmias and no deaths. The median length of stay was 14 h (IQR: 8.8–18.2 h; Range:2.2–75 h). No single-agent mirtazapine patient was admitted to intensive care. The 178 patients taking co-ingestants had more severe toxicity depending on the co-ingested drug. Conclusion. Mirtazapine appears to be relatively benign in overdose, associated with tachycardia, mild hypertension and mild CNS depression not requiring intervention.
Introduction
Background
Mirtazapine is a commonly prescribed antidepressant used in major depression and other psychiatric disorders. It is unlike other commonly prescribed antidepressants although it is structurally related to mianserin, a tetracyclic antidepressant.Citation1 It is classified as a noradrenergic and specific serotonergic antidepressant as it promotes release of noradrenaline and serotonin. It antagonises 5-HT2 and 5-HT3 receptors thereby resulting in greater stimulation of the 5-HT1 receptor by serotonin.Citation2,Citation3 It also acts as a potent presynaptic antagonist of central alpha adrenoreceptors.Citation2
There is limited and conflicting information on the effect of mirtazapine in overdose, including case studies suggesting that it causes ‘serotonin syndrome’, suggestions of an association with rhabdomyolysis and a handful of post-mortem cases of suicides positive for high concentrations of mirtazapine in bodily fluids.Citation4–7 In contrast, other studies have suggested it is relatively non-toxic.Citation2,Citation8–13
Importance
Reporting the relative safety of a drug in overdose is important to help guide clinicians in making correct risk assessments and treatment decisions regarding overdose patients. Reports of single cases, usually of unusual or large ingestions, may be useful in identifying important and uncommon effects of a particular drug in overdose. However, it is more important for larger series of cases to be reported to define the spectrum of effects and determine if the majority of cases cause either serious toxicity or only minor effects.
Goals of this investigation
This study aimed to investigate the spectrum of clinical features, electrocardiogram (ECG) parameters, significant complications (e.g. seizures, delirium, cardiac arrhythmias, hypotension, coma and serotonin toxicity), required interventions (intubation and ventilator support), and length of stay for mirtazapine overdoses.
Methods
Study design and setting
This study was an observational case series of all mirtazapine overdose presentations to a regional toxicology treatment unit, which is the primary referral centre for over 500,000 people. Clinical and demographic information is obtained on all patients upon admission to the toxicology service. A clinical data collection form is used at the time of presentation by emergency staff. The information is then entered into a relational database by two blinded research assistants. Exemption for use of the database and medical records as an audit has been previously approved by the Hunter and New England Research Ethics Committee.
Selection of participants
All presentations to the toxicology service between January 1987 and August 2013 were reviewed, and mirtazapine was first taken as an overdose in 2001. Any admission in which the patient ingested more than two times the maximum recommended dose (120 mg) of mirtazapine was included. Confirmation of mirtazapine ingestion was ascertained from patient history taken at least twice, as well as collateral history from family, emergency medical services, other health care providers and empty medication packets.
Methods and measurements
The following data were extracted from the database by GKI using standard database queries: patient demographic information (age and sex), details of ingestion (time of ingestion, time of presentation, estimated ingested dose [mg] and co-ingestants), clinical effects (heart rate [HR], blood pressure [BP], Glasgow coma score [GCS], seizures, signs and symptoms of serotonin toxicity and delirium), ECG characteristics (HR, QRS and QT), laboratory investigations (creatine kinase [CK]), intensive care admission, length of stay and treatment given (decontamination and ventilatory support). Further detailed information on the time between ingestion and minimum GCS was obtained from the medical record by one of the authors (IB). The QT and QRS intervals were measured manually by trained personnel in lead II of each ECG using a magnifying ruler. The HR was taken from the ECG. The QT–HR pair was plotted on the QT nomogram. In patients with more than one ECG, the ECG with the most abnormal finding was used. Hypotension was defined as a systolic BP less than 90 mmHg, hypertension as a systolic greater than 140 mmHg and tachycardia as a HR greater than or equal to 100 beats per minute (bpm).
Patients were admitted to ICU for ongoing airway support (intubation and ventilation), a decreased level of consciousness (GCS < 9), inotropic support (haemodynamic monitoring) or multi-organ failure. Discharge criteria were pre-determined by the toxicology unit, in conjunction with the liaison psychiatry service and could occur 24 h a day. However admission to a psychiatric hospital if required was dependant on bed availability and could result in an extended toxicology admission.
Outcomes
The following outcomes were measured: decreased level of consciousness measured as a GCS of < 15 and more severe central nervous system (CNS) depression as a GCS < 9; requirement for ICU admission; seizure activity; delirium, serotonin toxicity; an abnormal QT defined as the QT–HR pair being above the abnormal line on the QT nomogram; and hospital LOS.
Analysis
All continuous variables are reported as medians and interquartile ranges (IQRs) because some of the data was not normally distributed (e.g. length of stay) and it is easier to interpret if all continuous variable are reported with the same summary statistics. All graphical analyses were done in GraphPad Prism version 5.03 for Windows, GraphPad Software, San Diego California USA, www.graphpad.com.
Results
Characteristics of study subjects
There were 269 mirtazapine overdose presentations between August 2001 and August 2013, where more than 120 mg was ingested. Two patients with staggered ingestions were excluded, leaving 267 patients.
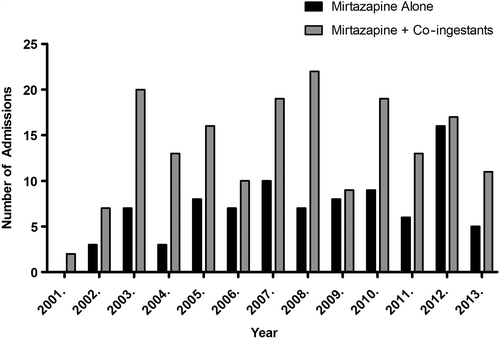
Patients were grouped according to the ingestion of mirtazapine (plus or minus alcohol) only and ingestion of mirtazapine and co-ingestants. Eighty-nine (33%) patients ingested mirtazapine as a single agent and the remaining 178 patients ingested at least one other drug in overdose. shows the frequency of mirtazapine overdoses over the time period studied.
Of the 89 single-agent mirtazapine overdoses there were 84 patients, which included 80 patients presenting once, three patients presenting twice and one patient presenting thrice. The median age was 36 years (IQR: 26–49 years, Range: 15–81 years) and 45 were female (51%). The median ingested dose of mirtazapine was 420 mg (IQR: 270–750 mg, Range: 150–1350 mg). Characteristics for the single-agent mirtazapine overdose group are listed in . The median length of stay for patients with single-agent mirtazapine ingestions was 14 h (IQR: 8.8–18.2 h; range: 2.2–75 h). The patient staying for 75 h satisfied discharge criteria from toxicology within 15 h but required a mental health bed that was unavailable. No patient in the single agent mirtazapine group was admitted to ICU or required ventilatory support. Only three patients were given activated charcoal. There were no deaths.
Table 1. Characteristics of mirtazapine overdose patients, divided into isolated ingestion of tablets with or without alcohol and co-ingestant groups.
Neurological effects
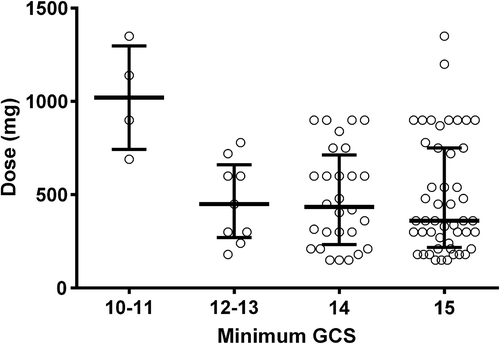
In the single-agent mirtazapine ingestions, 41 patients (46%) had a GCS < 15 at least once during their admission. Of these, all 41 had a GCS between 9 and 14, and none had a GCS < 9. The median time to minimum GCS for these 41 patients was 3 h (IQR: 2–4 h, range; 0.5–9 h). All patients who had a GCS < 14 had this recorded within 4.5 h except one patient with a GCS of 11 at 7 h post-ingestion. Patients with a GCS < 12 had ingested larger amounts (), with doses in excess of 1000 mg more likely to cause a decreased GCS. Both patients ingesting greater than 1000 mg in the GCS=15 group presented more than 15 h after ingestion. No patient had a CK above the normal range. No patient ingesting mirtazapine alone had a seizure, developed serotonin toxicity or developed a delirium.
Cardiovascular effects
All 89 single-agent mirtazapine overdoses had documented BP and HR. The median maximum systolic BP was 130 mmHg (IQR: 122–147 mmHg, Range: 100–215 mmHg), with 32 (35%) developing hypertension. The median maximum HR was 92 bpm (IQR: 81–101 bpm, Range: 50–175 bpm), with 29 (33%) patients developing a tachycardia. The median minimum BP was 112 mmHg (IQR: 101–127 mmHg, Range: 86–161 mmHg). Only two patients had hypotension by definition with systolic BPs of 86 mmHg and no patients required inotropic support. The median minimum heart rate recorded was 75 bpm (IQR: 66–86 bpm; Range: 50–139 bpm).
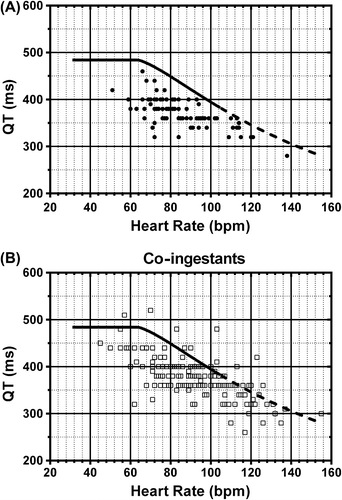
ECGs were available for 81 of the 89 cases. No arrhythmias occurred. The median QRS was 80 ms (IQR: 80–90 ms; Range: 80–120 ms). The median QT was 360 ms (IQR 360–400 ms; Range: 280–460 ms). The QT–HR pairs for the 81 patients with an ECG are plotted in , and there were no abnormal QT intervals.
Co-ingestant group
The co-ingestant group is compared to single-agent mirtazapine overdoses in . Twenty-nine patients in the co-ingestant group required admission to ICU including 20 that were intubated, compared to none in the mirtazapine-alone group. Nine patients developed delirium and 26 had a GCS < 9. Two patients developed serotonin toxicity, having co-ingested 900 mg sertraline and 560 mg citalopram. There were 21 abnormal QT intervals in the 162 patients in the co-ingestant group with an ECG, and in the majority of these a co-ingested drug (citalopram, escitalopram, methadone and oxycodone) was the most likely cause ().Citation14–17
Discussion
This study shows that mirtazapine alone in overdose is relatively benign, and is associated with tachycardia, mild hypertension and drowsiness. No patient ingesting mirtazapine alone had a seizure, developed delirium or serotonin toxicity, required respiratory support, or had an abnormal QT. This is consistent with previous studies of mirtazapine overdose.Citation12,Citation13,Citation18,Citation19 In the few cases of large single-agent mirtazapine ingestions (> 1000 mg) there appeared to be an association with reduced GCS, but none of these cases required any intervention (). Comparison between single-agent mirtazapine ingestions and mirtazapine with co-ingestants showed that the co-ingested drug is often more important and this latter group had more serious outcomes ().
Our series does not support the suggestion that mirtazapine causes serotonin toxicity, based on a small number of case reports.Citation4,Citation20–23 Hernandez et al. describe agitation, confusion, gait disturbance, bilateral cogwheel rigidity and a temperature of 37.5°C in a 75-year-old male on 120 mg daily for 8 days of treatment.Citation4 Ubogu and Katirji present a similar case in an 85-year-old female on mirtazapine monotherapy who developed cogwheeling, tremors, mutism with a normal temperature.Citation20 These cases are not consistent with serotonin toxicity, and include extrapyramidal movements and non-specific effects that do not meet the criteria for serotonin toxicity.Citation24 Serotonin toxicity only occurred in patients ingesting known serotonergic drugs, sertraline and citalopram, and did not occur in single-agent mirtazapine overdoses. Mirtazapine is an antagonist at the 5-HT2A receptor and is unlikely to produce serotonin toxicity,Citation1 which is most likely due to high levels of serotonin in the CNS acting at the 5-HT2A receptor.Citation25 There have been case reports suggesting mirtazapine causes significant CNS depression and that it has been associated with rhabdomyolysis.Citation5,Citation26,Citation27 Kuliwaba discusses a 1800-mg mirtazapine overdose in a 40-year-old man, with known cognitive impairment.Citation5 The patient slept for 10 h post-ingestion before presenting to hospital and had a peak CK of 9186 at 24 h. Khandat suggests an interaction between mirtazapine and lisinopril in an elderly 74-year-old male who presented with delirium and a CK of 43,000 IU/L,Citation26 but was treated for a urinary tract infection. In all these cases there are other more likely causes for a reduced level of consciousness or CK elevation than mirtazapine toxicity. No patients in our series had an elevated CK, although this was not routinely tested. In patients ingesting mirtazapine alone the GCS never dropped below 10, and this degree of CNS depression would be unlikely to result in coma-induced rhabomyolysis. We found that a GCS of 10 or 11 was more likely in patients taking larger amounts, over 1000 mg (). And excluding the two patients with a GCS of 15 who ingested over 1000 mg, but presented >15 h post ingestion, doses less than 1000 mg are unlikely to cause significant CNS depression.
A major limitation of the study is the retrospective method of data review. However, the data are entered into the database prospectively and independently of any postulated hypotheses. It is unlikely that this introduces significant bias into the study because the majority of the data is objective measurements made by nursing staff per hospital protocol including HR, BP and GCS. However, the GCS may be problematic with scores between 13 and 15 because of the variability in obtaining the patient's best response. It was evident on review that some staff recorded GCS as 14 when patients were asleep. For this reason we chose two thresholds of a GCS < 14 and GCS < 9.
Mirtazapine ingestion was not confirmed with any laboratory testing and was based entirely on comprehensive history taking. This has been shown to be reliable in populations of overdose patients based on previous pharmacokinetic studies,Citation28–30 where the treating clinicians examine and take a history on multiple occasions. It was difficult to determine the amount of alcohol ingested in patient who co-ingested alcohol. However, this is unlikely to affect the results of the study which show that mirtazapine only cause mild sedation despite a proportion of the mirtazapine alone patients also drinking alcohol.
Only including patients who took twice the maximum recommended dose of mirtazapine was arbitrarily chosen by the investigators prior to extracting the data. This may have meant that adverse effects at lower doses were missed. However, it was felt given the range of therapeutic dosing (15–60 mg daily) a clear inclusion criteria was required to define an overdose.
Conclusion
In summary, mirtazapine overdose is associated with tachycardia, mild hypertension and mild CNS depression. There is no association with QT prolongation, seizure activity, serotonin toxicity, delirium or any need for intervention. Doses less than 1000 mg are unlikely to cause major toxicity.
Declaration of interest
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
Geoff Isbister is funded by an NHMRC Clinical Career Development Award.
References
- de Boer T. The pharmacologic profile of mirtazapine. J Clin Psychiatry 1996; 57:19–25.
- Montgomery SA. Safety of mirtazapine: a review. Int Clin Psychopharmacol 1995; 10:37–45.
- de Boer T. The effects of mirtazapine on central noradrenergic and serotonergic neurotransmission. Int Clin Psychopharmacolo 1995; 10:19–23.
- Hernandez JL, Ramos FJ, Infante J, Rebollo M, González-Macías J. Severe serotonin syndrome induced by mirtazapine monotherapy. Ann Pharmacother 2002; 36:641–643.
- Kuliwaba A. Non-lethal mirtazapine overdose with rhabdomyolysis. Austr N Z J Psychiatry 2005; 39:312–313.
- Sauvageau A. A suspicious bathtub death in a suicidal patient on mirtazapine. Clin Toxicol 2006; 44:91.
- Salomone A, Di Corcia D, Gerace E, Vincenti M. A fatal case of simultaneous ingestion of mirtazapine, escitalopram, and valproic acid. J Anal Toxicol 2011; 35:519–523.
- Hoes MJ, Zeijpveld JH. First report of mirtazapine overdose. Int Clin Psychopharmacol 1996; 11:147.
- Raja M, Azzoni A. Mirtazapine overdose with benign outcome. Eur Psychiatry 2002; 17:107.
- Garlipp P, Brüggemann BR, Machleidt W. A non-fatal mirtazapine overdose in a suicide attempt.Aust N Z J Psychiatry 2003; 37: 244–245.
- Holzbach R, Jahn H, Pajonk FG, Mähne C. Suicide attempts with mirtazapine overdose without complications. Biol Psychiatry 1998; 44:925–926.
- Waring WS, Good AM, Bateman DN. Lack of significant toxicity after mirtazapine overdose: a five-year review of cases admitted to a regional toxicology unit. Clin Toxicol 2007; 45:45–50.
- LoVecchio F, Riley B, Pizon A, Brown M. Outcomes after isolated mirtazapine (Remeron) supratherapeutic ingestions. J Emerg Med 2008; 34:77–78.
- Berling I, Whyte IM, Isbister GK. Oxycodone overdose causes naloxone responsive coma and QT prolongation. QJM 2013; 106: 35–41.
- Isbister GK, Friberg LE, Stokes B, Buckley NA, Lee C, Gunja N, et al. Activated charcoal decreases the risk of QT prolongation after citalopram overdose. Ann Emerg Med 2007; 50:593–600, 600 e1–46.
- Calver L, Dunlop AJ, Isbister GK. Individual patient assessment of methadone-induced QT prolongation with digital holter recording. J Addict Med 2012; 6:92–93.
- van Gorp F, Whyte IM, Isbister GK. Clinical and ECG effects of escitalopram overdose. Ann Emerg Med 2009; 54:404–408.
- Kelly CA, Dhaun N, Laing WJ, Strachan FE, Good AM, Bateman DN. Comparative toxicity of citalopram and the newer antidepressants after overdose. J Toxicol Clin Toxicol 2004; 42:67–71.
- Waring WS, Graham A, Gray J, Wilson AD, Howell C, Bateman DN. Evaluation of a QT nomogram for risk assessment after antidepressant overdose. Br J Clin Pharmacol 2010; 70:881–855.
- Ubogu EE, Katirji B. Mirtazapine-induced serotonin syndrome. Clin Neuropharmacol 2003; 26:54–57.
- Demers JC, Malone M. Serotonin syndrome induced by fluvoxamine and mirtazapine. Ann Pharmacother 2001; 35:1217–1220.
- Benazzi F. Serotonin syndrome with mirtazapine-fluoxetine combination. Int J Geriatr Psychiatry 1998; 13:495–496.
- Turkel SB, Nadala JG, Wincor MZ. Possible serotonin syndrome in association with 5-HT(3) antagonist agents. Psychosomatics 2001; 42:258–260.
- Dunkley EJ, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM 2003; 96:635–642.
- Isbister GK, Buckley NA. The pathophysiology of serotonin toxicity in animals and humans: implications for diagnosis and treatment. Clin Neuropharmacol 2005; 28:205–214.
- Khandat AB, Nurnberger JI Jr, Shekhar A. Possible mirtazapine-induced rhabdomyolysis. Ann Pharmacother 2004; 38:1321.
- Retz W, Maier S, Maris F, Rösler M. Non-fatal mirtazapine overdose. Int Clin Psychopharmacol 1998; 13:277–279.
- Friberg LE, Isbister GK, Hackett LP, Duffull SB. The population pharmacokinetics of citalopram after deliberate self-poisoning: a Bayesian approach. J Pharmacokinet Pharmacodyn 2005; 32: 571–605.
- Kumar VV, Isbister GK, Duffull SB. The effect of decontamination procedures on the pharmacodynamics of venlafaxine in overdose. Br J Clin Pharmacol 2011; 72:125–132.
- Isbister GK, Friberg LE, Hackett LP, Duffull SB. Pharmacokinetics of quetiapine in overdose and the effect of activated charcoal. Clin Pharmacol Ther 2007; 81:821–827.