Abstract
Background: This is the 26th Annual Report of the American Association of Poison Control Centers (AAPCC; http://www. aapcc.org) National Poison Data System (NPDS). During 2008, 60 of the nation's 61 US poison centers uploaded case data automatically. The median upload time was 24 [7.2, 112] (median [25%, 75%]) minutes creating a real-time national exposure and information database and surveillance system.
Methodology: We analyzed the case data tabulating specific indices from NPDS. The methodology was similar to that of previous years. Where changes were introduced, the differences are identified. Poison center cases with medical outcomes of death were evaluated by a team of 28 medical and clinical toxicologist reviewers using an ordinal scale of 1–6 to determine Relative Contribution to Fatality (RCF) from the exposure to the death.
Results: In 2008, 4,333,012 calls were captured by NPDS: 2,491,049 closed human exposure cases, 130,495 animal exposures, 1,703,762 information calls, 7,336 human confirmed nonexposures, and 370 animal confirmed nonexposures. The top five substances most frequently involved in all human exposures were analgesics (13.3%), cosmetics/personal care products (9.0%), household cleaning substances (8.6%), sedatives/hypnotics/antipsychotics (6.6%), and foreign bodies/toys/miscellaneous (5.2%). The top five most common exposures in children age 5 or less were cosmetics/personal care products (13.5%), analgesics (9.7%), household cleaning substances (9.7%), foreign bodies/toys/miscellaneous (7.5%), and topical preparations (6.9%). Drug identification requests comprised 66.8% of all information calls. NPDS documented 1,756 human exposures resulting in death with 1,315 human fatalities deemed related with an RCF of at least contributory (1, 2, or 3).
Conclusions: Poisoning continues to be a significant cause of morbidity and mortality in the US. The near real-time, always current status of NPDS represents a national resource to collect and monitor US poisoning exposure cases and information calls. NPDS continues its mission as one of the few real-time national surveillance systems in existence, providing a model public health surveillance system for all types of exposures, public health event identification, resilience response and situational awareness tracking.
WARNING: Comparison of exposure or outcome data from previous AAPCC Annual Reports is problematic. In particular, the identification of fatalities (attribution of a death to the exposure) differed from pre-2006 Annual Reports (see Fatality Case Review – Methods). Poison center death cases are described as all cases resulting in death and those determined to be poison related fatalities. Likewise, Table 22 (Exposure Cases by Generic Category) since year 2006 restricts the breakdown including deaths to single-substance cases to improve precision and avoid misinterpretation.
Introduction
Sixty-one regional Poison Centers (PCs) serving the entire population of the 50 United States, American Samoa, District of Columbia, Federated States of Micronesia, Guam, Puerto Rico, and the US Virgin Islands submit information calls and exposure case data collected during the course of providing patient tailored exposure management to callers. This data is compiled by the American Association of Poison Control Centers (AAPCC) into its National Poison Data System (NPDS). PCs place emphasis on exposure management, accurate data collection and coding, and the continuing need for poison related public and professional education. The centers' health care professionals are available free of charge to callers 24-hours a day every day of the year. PCs respond to questions from both the public and health care professionals. The continuous staff dedication at regional poison centers is manifest as the number of exposure and information calls continues to rise (). Calls to PCs either involve an exposed human or animal (EXPOSURE CALL) or a request for information (INFORMATION CALL) with no person or animal exposed to a substance.
Table 1A. Growth of the AAPCC population served and exposure reporting (1983–2008)
What's New in NPDS and the Annual Report
Several enhancements were made to the tables and figures in this 26th Annual Report. One of the goals of the writing team has been to remove inconsistencies and improve the reader's ability to clearly understand the data. Our first foray into this area was the new version of introduced with the 2006 Annual Report. This year corrections for clarity were made to the age labels in all tables. NPDS age values may only be integers. So one can not be age 5½ or 20½, they are either 5 or 20 respectively. All major age brackets were conformed to the following major categories: ≤ 5 years, 6–12 years, 13–19 years, ≥ 20 years, and unknown. Only the headers were changed for clarity, this did not effect how the data were summarized. As in previous years, the complete report with all tables has been included in the print and online versions of the report. To better assist readers and are also provided via separate electronic links in the internet version of this report.
Linear least squares regression lines were added to the 5 call frequencies reported ( and ). Doubling times and confidence intervals (CI) provide a simple measure of relative rate of increase. The data array attached to shows the Mean ± SD [90% CI] by day of the week for each of the call types in and . The doubling time for human exposure calls was 38.4 [90% CI:36.4, 40.5] years compared to only 6.5 [90% CI:6.4, 6.7] years for information calls and 3.98 [90% CI: 3.94, 4.02] for all drug identification calls. Clearly the slope for drug identification calls is steeper than human exposures and all information calls. With the current economic crisis and associated impact on PC funding, some PCs have elected to terminate drug identification services. This clearly demonstrated ever increasing demand for drug identification assistance will need to be addressed as it impacts PC available resources and staffing. In addition, drug identification request data adds perspective on prescribing usage and diversion patterns.
Fig. 1. Human Exposure Calls, Information Calls and Animal Exposure Calls by Day since January 1, 2000.
Line shows least-squares linear regression, DT = doubling time from the slope of the linear regression of the log-calls/day and 90% confidence interval.
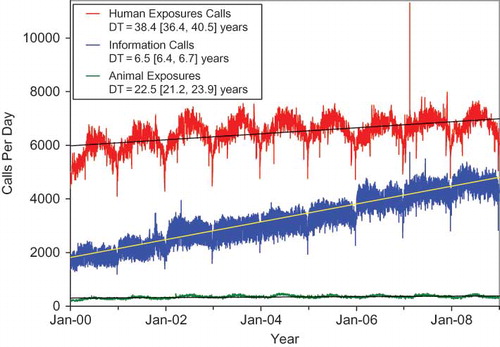
Fig. 2. Drug Identification and Law Enforcement Drug Identification Calls by Day since January 1, 2000.
Line shows least-squares linear regression, DT = doubling time from the slope of the linear regression of the log-calls/day and 90% confidence interval.
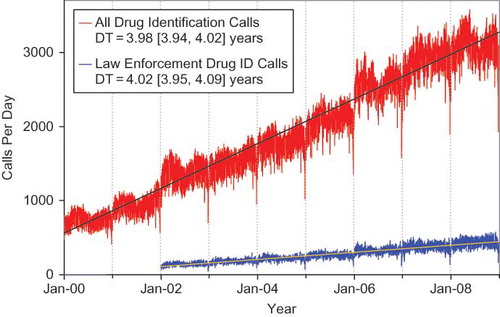
Fig. 3. Information Calls (total) for 2008 by Day of Week.
Means and SEM (diamonds) and SDs (horizontal lines) by Oneway ANOVA, F(6, 1) = 78.3, p<0.0001, Rsquare = 0.566, N = 366. Fewest calls (N = 2825) was Thursday December 25, 2008. Data array shows the Mean ± SD [90% confidence interval] by day of the week for each of the call types in and .
![Fig. 3. Information Calls (total) for 2008 by Day of Week.Means and SEM (diamonds) and SDs (horizontal lines) by Oneway ANOVA, F(6, 1) = 78.3, p<0.0001, Rsquare = 0.566, N = 366. Fewest calls (N = 2825) was Thursday December 25, 2008. Data array shows the Mean ± SD [90% confidence interval] by day of the week for each of the call types in Figures 1 and 2.](/cms/asset/4e656af1-ce39-4805-96ac-5721a33bec5f/ictx_a_444217_f0003_b.jpg)
and its associated table quantifies call rates by “day of week” for human and animal exposures, all information calls, and the subsets all drug identification and law enforcement only requests. All group patterns are statistically significant with the largest absolute volume decrease being all information calls for Saturdays and Sundays. presents a statistical/graphical summary for information calls. Detailed analyses of this type can be used to assist PCs to maintain optimal staffing. In this example, based on information calls alone, centers could down-staff for management of information calls on Saturdays and Sundays. Application of these techniques could help PCs save valuable budget dollars. Curiously, the fewest number of information calls was Christmas day.
Previous versions of , Comparisons of Death Data (1985–2008), mixed deaths associated with exposures and poison related fatalities as categorized by at least 3 different fatality scoring systems. Death case data was meticulously reviewed back to 1985 to establish that most years reported absolute counts of cases with medical outcomes of death or death, indirect report. now reports all years this way. We believe that this change will make it easier to compare absolute death counts from year to year. can still be created using the NPDS application and including the desired Relative Contribution to Fatality (RCF) score. There have been several positive changes to as well. A standardized set of alternate names and a standardized set of analyte names were created. Both names are lower case and uniformly applied throughout the table.
This year, lists human exposures with medical outcome of death or death, indirect report regardless of RCF score. Deaths that were pregnant have also been flagged to allow for rapid identification. The Fatality Review process continued with the new system introduced in 2007. However, the fatality case selection process for narrative publication excluded cases with medical outcome of death, indirect report. Two pediatrician medical toxicologist Fatality Reviewers volunteered to review exclusively the 53 pediatric deaths for 2008. This was undertaken to provide a more consistent and enhanced review of these important cases.
In 2008, numerous enhancements were introduced in the NPDS application. Many of these focused on surveillance and are detailed in the Surveillance section. Transcending all of the improvements were the two NPDS web services: aggregate case counts (total and human exposure call volumes, and clinical effects volume by geographical and time period of interest) and the full case information web service. These web services provide data to external systems or viewers to analyze NPDS data in ways not currently possible in the NPDS application. The aggregate web service provides total call volume, human exposure call volume, or clinical effects counts linked by AND or OR allowing an external system such as RODS (Real-time Outbreak and Disease Surveillance, University of Pittsburgh, Department of Biomedical Informatics) to create time-series. Unique to NPDS, the aggregate case count web service is not only accessible by external computer systems but also directly by system users to create their own time series without the need for external system software. The full case web service provides entire cases or portions of cases to authorized users. Queries for both web services may be done for any date range and for any time period of interest (down to the hour) and by Center, State or ZIP code. Time series technology was not an initial NPDS function but the web service allows for time series creation. The web services also allow for NPDS data to be provisioned in a federated manner where the data is always current in NPDS and can be readily accessed as needed without the need for costly cloning and warehousing.
The communications modules were also completed for the NPDS fatality and surveillance teams allowing for all correspondence to be stored within a fatality case or surveillance anomaly. Finally a new security model was introduced realizing the vision of the individual regional poison center controlling access to its data for its own users, sharing data with other centers, or allowing access to data by respective external organizations like State and local health departments.
Methods
Characterization of participating Poison Centers
All 61 participating centers submitted data to AAPCC for 2008. Fifty-eight centers (95%) were certified by AAPCC at the end of 2008. The entire population of the 50 states, American Samoa, the District of Columbia, Federated States of Micronesia, Guam, Puerto Rico, and the US Virgin Islands was served by PCs in 2008.
The average number of human exposure cases managed per day by all US poison centers was 6,825. Similar to other years, higher volumes were observed in the warmer months, with a mean of 6,121 cases per day in June compared with 5,073 per day in January. On average, ignoring the time of day and seasonal fluctuations, US PCs received one call concerning a suspected or actual human exposure every 12.7 seconds. The median time to upload data is 24 [7.2, 112] (median [25%, 75%]) minutes creating a real-time national exposure database and surveillance system.
Call management – specialized poison exposure emergency providers
Poison center operation as well as clinical education and instruction are directed by Managing Directors (most are PharmDs or RNs with American Board of Applied Toxicology [ABAT] board certification). Medical direction is provided by Medical Directors who are board-certified physician medical toxicologists. At some poison centers, the Managing and Medical Director positions are held by the same person.
Calls received at US PCs are managed by healthcare professionals who have received specialized training in toxicology and managing exposure emergencies. These providers include medical and clinical toxicologists, registered nurses, doctors of pharmacy, pharmacists, chemists, hazardous materials specialists, and epidemiologists. Specialists in Poison Information (SPIs) are primarily registered nurses, PharmDs, and pharmacists. They work under the supervision of a Certified Specialist in Poison Information (CSPI). SPIs must log a minimum of 2,000 calls over a 12 month period to become eligible to take the CSPI examination for specialists in poison information. Poison Information Providers (PIPs) are allied healthcare professionals. They manage information-type and low acuity (non-hospital) calls and work under the supervision of a CSPI. Of note is the fact that no nursing or pharmacy school offers a toxicology curriculum designed for poison center work and SPIs must be trained in programs offered by their respective PC.
NPDS – near real-time data capture
Launched on 12 April 2006, NPDS is the data repository for the 61 regional poison centers. In 2008, 60 of the 61 US PCs uploaded case data automatically to NPDS with one center uploading data periodically (beginning on 22 January 2009, all centers submitted data near real-time). The web-based NPDS software facilitates querying, reporting and a myriad of surveillance uses allowing AAPCC, its member centers and public health agencies to utilize US poisoning exposure data. Users are able to access local and regional data for their own areas and view national aggregate data. The application allows for increased “drill-down” capability and mapping via a geographic information system (GIS). Custom surveillance definitions are available along with ad hoc reporting tools. Information in the NPDS database is dynamic. Each year the database is locked prior to extraction of annual report data to prevent inadvertent changes and ensure consistent, reproducible reports. The 2008 database was locked on 9 October 2009 at 1905 hours EDT.
Annual report case inclusion criteria
The information in this report reflects only those cases that are not duplicates and classified as CLOSED. A case is closed when the PC has determined that no further follow-up/recommendations are required or no further information is available. Cases are followed as to precise a medical outcome as possible. Depending on the case specifics, most calls are “closed” within the first hours of the initial call. Some calls regarding complex hospitalized patients or cases resulting in death may remain open for weeks or months while data continues to be collected. Follow-up calls provide a proven mechanism for monitoring the appropriateness of management recommendations, augmenting patient guidelines and providing poison prevention education, enabling continual updates of case information as well as obtaining final/known medical outcome status to make the data collected as accurate and complete as possible.
NPDS surveillance
As previously noted, 60 of the 61 US PCs upload case data automatically to NPDS. This unique near real-time upload is the foundation of the NPDS surveillance system permitting both spatial and temporal case volume and case based surveillance. NPDS software allows creation of volume and case based definitions at will. Definitions can then be applied to national, regional, state, or ZIP code coverage areas. Geocentric definitions can also be created. This functionality is available not only to the AAPCC surveillance team, but to every regional poison center. PCs also have the ability to share NPDS real-time surveillance technology with external organizations such as their state and local health departments or other regulatory agencies. Another unique NPDS feature is the ability to generate system alerts on adverse drug events and other products of public health interest like contaminated food or product recalls. NPDS can thus provide real-time adverse event monitoring and surveillance for resilience response and situational awareness.
Surveillance definitions can be created to monitor a variety of volume parameters using a multiplicity of mathematical options with historical baseline periods from 1 to 8 years. Case based definitions for any desired substance or commercial product in the product database of over 350,000 entries are also available (Micromedex® Healthcare Series [Internet database]. Greenwood Village, CO: Thomson Reuters [Healthcare] Inc.). NPDS surveillance tools include:
Volume alerts
Total Call Volume
Human Exposure Call Volume
Clinical Effects Volume (signs and symptoms or laboratory abnormalities)
Case Based Surveillance Definitions
Substance
Clinical Effects
Various NPDS data fields
Boolean field expressions
Incoming data is monitored continuously and any anomalous signal detected generates an automated email alert to the AAPCC's surveillance team or designated regional PC or public health agency. These anomaly alerts are reviewed by the AAPCC surveillance team and/or the regional PC that created the surveillance definition. When reports of potential public health significance are detected, additional information is obtained via e-mail or phone from reporting PCs. The regional PC then alerts their respective affected state or local health departments. Public health issues are brought to the attention of the Health Studies Branch, Division of Environmental Hazards and Health Effects, National Center for Environmental Health, Centers for Disease Control and Prevention (CDC). This unique near real-time trace back ability is a unique feature offered by NPDS and the regional PCs.
AAPCC Surveillance team toxicologists review surveillance definitions on a regular basis to fine-tune the queries. CDC as well as state and local health departments with NPDS access as granted by their respective regional poison centers also have the ability to create surveillance definitions to respond to emerging public health events.
Fatality case review
NPDS fatality cases can be recorded as either DEATH or DEATH, INDIRECT REPORT. Medical outcome of death is by direct report. A reported fatality is coded as “indirect” if the PC was, in most cases, not directly contacted by the medical team, coroner, or family member, or post mortem reports were sent by the coroner or medical examiner for PC review, or the PC summarized a news account. However, a coroner or medical examiner or family member calling the PC with a question about the cause of death or a family member requesting interpretation of a toxicology laboratory result from a case not previously discussed with the PC is coded as a Death rather than Death, indirect report.
Although poison centers may report DEATH as an outcome, the death may not be the direct result of the poisoning exposure. We define Poison-related fatality as a death that was judged by the Fatality Review Team to be at least contributory to the exposure.
During the year, each death case is abstracted by the reporting regional PC center and verified for accuracy. These cases are then systematically reviewed at year end by Fatality Case Review Teams (CRT). Each CRT consisted of the following members:
Author – the PC medical director or their designee responsible for the case data entered, the abstract, and the initial choices of RCF and Substances;
Lead Reviewer – Medical Director or Managing Director (assigned from a PC other than the center from which the individual case originated using pseudorandom numbers) to provide the primary review of the case;
Peer Reviewer – Managing Director (if the lead reviewer was a Medical Director) or Medical Director (if the lead reviewer was a Managing Director) assigned (using pseudorandom numbers) to provide the second (complementary) review of the case;
Manager – Louis Cantilena MD (east coast) or Daniel A. Spyker MD (west coast) assigned by PC ZIP code.
The fundamental classification for the NPDS fatalities reporting is whether the exposure caused the death. The review teams assessed the following parameters for each fatality case:
Relative contribution of the toxic exposure to the death, RCF (see grading system below);
Cause Rank of the substances involved as described below;
Abstract scoring (see scoring system below);
Degree of agreement between the Abstract and the NPDS database entries for that case;
Degree of agreement and if resolution was required between determinations made by members of the CRT.
Cause Rank is a separate field associated with each substance to address the circumstance where 2 or more substances were judged causative, but we lack evidence to distinguish between them. Cause Rank defaults to the same number as the Substance Rank, 1, 2, 3, . . . so it does not require additional data entry in the usual single substance or clear ranking circumstances. Changing Cause Rank permits assignment of equivalence in the event the reviewer cannot distinguish between causative substances, e.g., they may rank substances as 1, 1, 3 instead of 1,2,3 and so forth.
The primary basis of the case classification and abstract evaluations were the:
Clinical Case Evidence – includes a variety of information such as the data entered into NPDS and, when available, the medical examiner's report.
Medical Examiner's Report – the postmortem examination results, autopsy report or the coroner's report were always sought and, when available, became an important part of fatality case review.
Relative Contribution to Fatality
The definitions used for the Relative Contribution to Fatality (RCF) classification were as follows:
Undoubtedly responsible – In the opinion of the CRT the Clinical Case Evidence establishes beyond a reasonable doubt that the SUBSTANCES actually caused the death.
Probably responsible – In the opinion of the CRT the Clinical Case Evidence suggests that the SUBSTANCES caused the death, but some reasonable doubt remained.
Contributory – In the opinion of the CRT the Clinical Case Evidence establishes that the SUBSTANCES contributed to the death, but did not solely cause the death. That is, the SUBSTANCES alone would not have caused the death, but combined with other factors, were partially responsible for the death.
Probably not responsible – In the opinion of the CRT the Clinical Case Evidence establishes to a reasonable probability, but not conclusively, that the SUBSTANCES associated with the death did not cause the death
Clearly not responsible – In the opinion of the CRT the Clinical Case Evidence establishes beyond a reasonable doubt that the SUBSTANCES did not cause this death.
Unknown – In the opinion of the CRT the Clinical Case Evidence is insufficient to impute or refute a causative relationship for the SUBSTANCES in this death.
Review team procedure
A total of 14 review teams (28 individuals) volunteered to participate in the review. Reviewers were Medical Toxicologists (N = 15) or Clinical Toxicologists (N = 13). This year, a separate team of two Medical Toxicologists focused on the 53 pediatric fatalities. Names and affiliations of the reviewers are listed in Appendix A. Training and communication included weekly teleconferences, written guidance documents, spreadsheets (for assignment and reporting), the NPDS-Fatality Module (NPDS-FM) and individual telephone contacts. The initial 1,756 fatalities were randomly assigned such that each of the 28 individuals served as Lead Reviewer on 65–67 adult cases or 26–27 pediatric cases. Each individual peer reviewed another similar number of cases. For each fatality assigned, the Lead Reviewer:
Recorded their independent assessment of the RCF;
Recorded their assessment of the author's listing and ranking of the substance(s), and edited the case abstract as needed;
Scored the fatality case in regard to quality/completeness and novelty/educational value;
Evaluated the degree of agreement between the abstract and the NPDS database entries for that case;
Led the resolution of any questions with the CRT and Manager as required.
For each fatality assigned, the Peer Reviewer:
Recorded the agreement between the abstract and the NPDS database as described above for the Lead reviewer;
Reviewed the decisions of the Lead Reviewer (steps 1–4) and recorded their agreement with the Lead Reviewer.
Final decisions as to the fatality category and involved substances and sequence were derived from the Clinical Case Evidence. In most cases, the 3 members of the CRT were able to reach consensus. Decisions, which could not be resolved within the CRT, were queried to the responsible Manager for review and discussion.
Selection of abstracts for publication
The 89 abstracts included in Appendix C were selected for publication in a 3-stage process consisting of qualifying, ranking and reading. Qualifying was based on the RCF. Project reviewers recommended qualifying only RCF = 1, 2 or 3 (Undoubtedly Responsible, Probably Responsible or Contributory) as eligible for publication. Fatalities by Indirect report were not eligible for 2008. Qualifying cases thus numbered 1136. Ranking was based on the number of substances (33%) and weighted abstract scores (67%). The weightings were the averages chosen by the review team recommendations in 2006. Each was multiplied by the respective factors to obtain a weighted publication score: Hospital records * 4.4 + Postmortem * 7.6 + Blood levels * 6.9 + Quality/Completeness * 6.4 + Novelty/Educational value * 6.0.
The top ranked 200 abstracts were each read by 5 of the individual reviewers (Durback-Morris, Geller, Sangalli, Simone, Wiegand) and the 2 managers (Cantilena and Spyker). Each reader judged each abstract as “publish” or “omit” and all abstracts receiving 4 or more publish votes were selected, further edited and cross-reviewed by the 2 managers and a third writer (Rumack).
Results
2008 case summary
In 2008, the 61 participating PCs logged 4,333,012 total cases including 2,491,049 closed human exposure cases (), 130,495 animal exposures (), 1,703,762 information calls (), 7,336 human confirmed nonexposures, and 370 animal confirmed nonexposures. An additional 1,296 calls were still open at the time of database lock. The cumulative AAPCC database now contains close to 48.5 million human exposure case records (). A total of 11,333,063 information calls (as described below) have been logged by NPDS since the year 2000. shows the human exposures, information calls and animal exposures by day since 2000. Linear regression and the least squares regression lines were added to the call frequencies reported. All were highly statistically significant (p < 0.001, N = 3288). Regressions of the natural log of the same data permitted calculation of a doubling time = ln(2)/slope (just as is done to calculate a disappearance half-life). We report these doubling times and 90% confidence intervals as a measure of relative rate of increase. The 90% CI was chosen as it gives the 5% lower bound and 95% upper bound.
Table 1B. Non-human exposures by animal type
Table 1C. Distribution of information calls
PCs made 2,897,491 follow-up calls (or a series of calls) in 2008. Follow-up calls were done in 45.1% of human exposure cases. One follow-up call was made in 22.2% of human exposure cases, and multiple follow-up calls (range 2–128) were placed in 22.9% of cases.
Information calls to Poison Centers
Data from 1,703,762 information calls to PCs in 2008 () was transmitted to NPDS, including calls in optional reporting categories such as prevention/safety/education (36,804), administrative (27,727) and caller referral (64,081). Overall, the volume of information calls handled by US PCs increased by 6.3% over the 1,602,489 calls handled in 2007.Citation1
The most frequent information call was for drug identification, comprising 1,138,254 calls to PCs during the year (). Of these, 147,144 (12.9%) could not be identified over the telephone. We examined call rates for 2008 by “day of week” using Analysis of Variance (ANOVA) with day as a nominal variable. shows a graphical summary for information calls where ANOVA F(6, 1) = 78.3, p < 0.0001, Rsquare = 0.566, N = 366. The majority of the drug identification calls were received from the public, followed by law enforcement and health professionals. Most of the drug identification requests were regarding drugs sometimes involved in abuse; however, these cases were categorized based on the drug's abuse potential without knowledge of whether abuse was actually intended.
Drug information calls increased 30% from 2007 (177,436 calls) to 2008 (230,084 calls) and comprised 13.5% of all information request calls. Of these, the most common requests were in regards to therapeutic use and indications, followed by drug-drug interactions and questions about dosage. Environmental inquiries comprised 1.7% of all information calls. Of these environmental inquiries, questions related to cleanup of mercury thermometers were most common followed by questions involving pesticides.
Of all the information calls, poison information comprised 5.0% of the requests., with inquiries involving general toxicity the most common followed by questions involving food poisoning or food preparation practices and plant toxicity.
Exposure calls to poison centers
Tables 22A (Nonpharmaceuticals) and 22B (Pharmaceuticals) provide summary demographic data on patient age and gender, reason for exposure, medical outcome, and use of a health care facility for all 2,491,049 human exposure cases, presented by substance categories.
Column 1 (grey shading) lists the number of exposures to the substance in the total number of cases including multiple exposures (as in previous years) and is sorted in the table under the name of the substance ranked first by the regional PC. This column counts all exposures to that substance.
Column 2 (and the breakdowns by Age, Treatment Site, Reason, and Outcome) report single substance exposures only, i.e., excludes cases with a multiple substance exposure. Subtracting column 2 from column 1 provides the number of cases where there were multiple substance exposures.
and restrict the breakdown columns to single-substance cases to improve precision (avoid misrepresentation). Prior to 2007, when multi-substance exposures were included, a relatively innocuous substance was mentioned in a death column when, for example, the death was attributed to an antidepressant, opioid, or cyanide. This subtlety was not always appreciated by the uninformed user of the information. The restriction of the breakdowns to single-substance exposures should increase precision and reduce misrepresentation of the results in this unique by-substance table. Single substance cases reflect the majority (90.5%) of all exposures ().
and tabulate 2,858,250 substance-exposures mentions, of which 2,254,267 were single-substance exposures including 1,204,673 (53.4%) nonpharmaceuticals and 1,049,594 (46.6%) pharmaceuticals.
In 16.8% of exposures that involved pharmaceutical substances the reason for exposure was intentional, compared to only 2.9% when the exposure involved a nonpharmaceutical substance. Correspondingly, treatment in a health care facility was provided in a higher percentage of exposures that involved pharmaceutical substances (26.4%) compared with nonpharmaceutical substances (14.1%). Exposures to pharmaceuticals also had more severe outcomes. Of single-substance poison-related fatal cases, 350 were pharmaceuticals compared with 178 nonpharmaceuticals.
Age and gender distribution
The age and gender distribution of human poison exposure victims is outlined in . Children younger than 3 years were involved in 38.7% of exposures and children younger than 6 years accounted for half of all human exposures. A male predominance is found among recorded cases involving children younger than 13 years, but this gender distribution is reversed in teenagers and adults, with females comprising the majority of reported poison exposure victims.
Caller site and exposure site
As shown in , of the 2,491,049 human exposures reported, 76.0% of calls originated from a residence (own or other) but 93.4% actually occurred at a residence (Own or Other). Another 16.1% of calls were made from a health care facility. Exposures occurred in the workplace in 1.75% of cases, schools (1.3%), health care facilities (0.3%), and restaurants or food services (0.3%).
Table 2. Site of call and site of exposure, human exposure cases
Exposures in pregnancy
Exposure during pregnancy occurred in 8,477 women (0.34% of all human exposures). Of those with known pregnancy duration (n = 7,817), 31.4% occurred in the first trimester, 37.6% in the second trimester, and 31.0% in the third trimester. Most (72.6%) were unintentional exposures and 20.4% were intentional exposures.
Multiple patients
In 2008, 9.7% (241,258) of human exposures involved multiple patients. Examples of these include siblings sharing found medication, multiple victims of carbon monoxide exposure such as a family, or multiple patients inhaling vapors at a hazardous material spill.
Chronicity
The overwhelming majority of human exposures, 2,261,862 (90.8%) were acute cases (single, repeated or continuous exposure occurring over 8 hours or less) compared to 830 acute cases of 1,756 fatalities (47.3%). Chronic exposures (continuous or repeated exposures occurring over > 8 hours) comprised 1.9% (46,917) of all human exposures. Acute-on-chronic exposures (single exposure that was preceded by a continuous, repeated, or intermittent exposure occurring over a period greater than eight hours) numbered 158,490 (6.4%).
Reason for exposure
Most (82.8%) poison exposures were unintentional; suicidal intent was suspected in 8.7% of cases (). Therapeutic errors accounted for 10.6% of exposures (263,942 cases), with unintentional misuse comprising 4.3% of exposures. Of the 263,942 therapeutic errors, the most common scenarios for all ages included: inadvertent double-dosing in 84,814 (34.0%) cases, wrong medication taken or give (15.3%), other incorrect dose (14.8%), doses given/taken too close together (9.5%) and inadvertent exposure to someone else's medication (9.4%). The types of therapeutic errors observed are different for each age group and are summarized in .
Table 3. Age and gender distribution of human exposures
Table 5. Number of substances involved in human exposure cases
Table 6A. Reason for human exposure cases
Table 6B. Scenarios for therapeutic errors by age
The reason for most exposures was unintentional (82.8%) of all PC calls. Unintentional exposures outnumbered intentional poisonings in all age groups with the exception of age 13–19 years (). Intentional exposures were reported as frequently as unintentional exposures in patients age 13–19 years. In contrast, of the 1,315 of the reported human poisoning fatalities, the majority of the fatalities in children less than or equal to age 5 years was unintentional while most fatalities in adults (≥ 20 years) were intentional ().
Table 7. Distribution of reason for exposure by age
Route of exposure
Ingestion was the route of exposure in 79.3% of cases (), followed in frequency by dermal (7.2%), inhalation/nasal (5.2%), and ocular routes (4.5%). For the 1,315 fatalities, ingestion (77.7%), inhalation/nasal (7.8%), and parenteral (3.6%) were the predominant exposure routes.
Table 9. Route of exposure for human exposure cases
Clinical effects
The AAPCC database allows for the coding of up to 131 different clinical effects (signs, symptoms, or laboratory abnormalities) for each case. Each clinical effect can be further defined as related, not related, or unknown if related. Clinical effects were coded in 707,555 (28.4%) cases. (14.9% had 1 effect, 7.5% had 2 effects, 3.7% had 3 effects, 1.3% had 4 effects, 0.5% had 5 effects, and 0.5% had >5 effects coded). Of clinical effects coded, 79.2% were deemed related to the exposure(s), 9.2% were considered not related, and 11.6% were coded as unknown if related.
The duration of effect is required for all cases that report at least one clinical effect and have a medical outcome of minor, moderate or major effect (n = 493,386). demonstrates an increasing duration of the clinical effects observed with more severe outcomes.
Case management site
The majority of cases reported to PCs were managed in a non–health care facility (72.6%), usually at the site of exposure, primarily the patient's own residence (). Another 1.8% of cases were referred to a health care facility but refused to go. Treatment in a health care facility was rendered in 24.0% of cases.
Table 10. Management site of human exposures
Of the 598,048 cases managed in a health care facility, 295,834 (49.5%) were treated and released without admission, 93,096 (15.6%) were admitted for critical care, and 55,878 (9.3%) were admitted for noncritical care.
The percentage of patients treated in a health care facility varied considerably with age. Only 9.9% of children younger 5 years or younger and only 11.1% of children between 6 and 12 years were managed in a health care facility compared with 42.8% of teenagers (13–19 y) and 35.8% of adults (age ≥20 y).
Medical outcome
displays the medical outcome of the human poison exposure cases distributed by age, showing a greater incidence of severe outcomes in the older age groups. compares medical outcome and reason for exposure and shows a greater frequency of serious outcomes in intentional exposures.
Table 11. Medical outcome of human exposure cases by patient age
Table 12. Medical outcome by reason for exposure in human exposures
Decontamination procedures and specific antidotes
and outline the use of decontamination procedures, specific antidotes, and measures to enhance elimination in the treatment of patients reported in this database. These must be interpreted as minimum frequencies because of the limitations of telephone data gathering.
Table 13. Duration of clinical effects by medical outcome
Table 14. Decontamination and therapeutic interventions
Table 15. Therapy provided in human exposures by age
demonstrates the continuing decline in the use of ipecac-induced emesis in the treatment of poisoning. Ipecac was administered in only 641 (<0.01%) pediatric human poison exposures in 2008. The continued decrease in ipecac syrup use in the last decade was likely a result of ipecac use guidelines issued in 1997 by the American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists and updated in 2004.Citation2,Citation3 In a separate report, the American Academy of Pediatrics concluded not only that ipecac should no longer be used routinely as a home treatment strategy, but also recommended disposal of ipecac currently in homes.Citation4
Table 16. Decontamination trends (1985–2008)
Top substances in human exposures
presents the most common 25 substance categories involved in human exposures, listed by frequency of exposure. and present similar data for children and adults, respectively, and show the differences between pediatric and adult poison exposures.
Table 17A. Substance categories most frequently involved in human exposures (Top 25)
Table 17B. Substance categories most frequently involved in pediatric (≤ 5 y) exposures (Top 25)
Table 17C. Substance categories most frequently involved in adult (≥20 y) exposures (Top 25)
Distribution of suicides
shows the modest variation in the distribution of suicides over the past 2 decades as reported to the NPDS's national database (45–68%). Since 1985, the percent of fatal cases has increased from 0.036% to 0.070% and the percent of pediatric cases has decreased from 6.1% to 2.2%. (See ).
Table 18. Categories associated with largest number of fatalities (Top 25)
Table 19. Comparisons of death data (1985–2008)
Plant exposures
provides a summary of plant exposures for those species and categories most commonly involved (top 25 of 63,362 total plant exposures). The top three table categories are essentially synonymous for unknown plant and comprise 14.1% (8,918/63,362) or all plant exposures. For a variety of reasons it was not possible to make a precise identification in these three groups. The top five most frequent plant exposures where a positive plant identification was made were descending order): Spathiphyllum species, Phytolacca americana (L.) – pokeweed, Euphorbia pulcherrima (Wild)), Philodendron (Species unspecified), and Toxicodendron radicans (L.).
Table 20. Frequency of plant exposures (Top 25)
Deaths and poison-related fatalities
A listing of cases () and summary of cases (, , , and ) are provided for fatal cases for which there exists reasonable confidence that the death was a result of that exposure. Of the 1,769 cases initially referred, 13 were miscoded (animal death, not a death, or not primary center). Of the 1756 finally classified, the CRT judged 1,315 poison related fatalities (RCF category = 1-Undoubtedly responsible, 2-Probably responsible, or 3-Contributory). The remaining 441 cases were judged as follows: 91 as RCF = 4-Probably not responsible, 57 as 5-Clearly not responsible, and 293 as RCF = 6-Unknown.
Table 21. Listing of fatal nonpharmaceutical and pharmaceutical exposures (this table can be viewed separately online at www.informahealthcare.com/ctx)
Deaths are sorted in this listing according to the category, patient age and substance deemed most likely responsible for the death. Please note: the Substance listed in column 3 of was chosen to be the most specific based upon all of the clinical information including the substances entered for that case. This substance may not agree with the categories used in the summary tables (including ). Additional agents implicated are listed below the primary agent in the order of their contribution to the fatality. The exposure-related fatalities involved a single substance in 528 cases (40.2%), 2 substances in 316 cases (24.0%), 3 in 220 cases (16.7%), and 4 or more in the balance of the cases. The cross-references at the end of each major category section list all cases that identify this substance as other than the primary substance.
Table 22A. Demographic profile of SINGLE-SUBSTANCE nonpharmaceuticals exposure cases by generic category (this table can be viewed separately online at www.informahealthcare.com/ctx)
Table 22B. Demographic profile of SINGLE-SUBSTANCE pharmaceuticals exposure cases by generic category (this table can be viewed separately online at www.informahealthcare.com/ctx)
The Case number is [bracketed] to indicate that the abstract for that case is included in Appendix C. The letters following the Case number include: i = reported to poison center indirectly (by coroner, medical examiner, or other) after the fatality occurred in 179 cases (13.6%), p = prehospital cardiac and/or respiratory arrest in 567 (43.1%), h = hospital records reviewed in 176 cases (143.4%), a = autopsy report reviewed in 359 cases (27.3%).
The distribution of RCF is as follows: 1 = Undoubtedly responsible in 670 cases (50.1%), 2 = Probably responsible in 466 cases (35.4%), 3 = Contributory in 179 cases (13.6%).
All fatalities – all ages
presents the age and gender distribution for these 1,315 poison-related fatalities. The age distribution of reported fatalities is similar to that in past years with 74.8% (983 of 1,315) of fatal cases occurring in adults (age > 19 years) (). Although children younger than 6 years were involved in the majority of exposures, they comprised just 2.0% of the exposure-related fatalities. Most (76.2%) of the poisoning fatalities occurred in 20-to 59-year-old individuals.
lists each of the 1,315 human fatalities along with all of the substances involved. Please note: the Substance listed in column 3 of was chosen to be the most specific generic name based upon the substances entered for that case. This substance may not agree with the categories used in the summary tables (including ).
lists the top 25 substance categories associated with reported fatalities and the number of single exposure fatalities for that category – sedative/hypnotics/antipsychotics, opioids, and antidepressants lead this list followed by cardiovascular drugs, acetaminophen in combination, alcohols, and stimulants/street drugs. Although sedative/hypnotics/antipsychotics ranks 4th and antidepressants 7th among the most frequent exposures (), there is otherwise little concordance between the frequency of exposures to a substance and the number of fatalities. Note that this Table tabulates all substances to which a patient was exposed (i.e., a patient exposed to an opioid may have also been exposed to 1 or more other products).
A single substance was implicated in 90.5% of reported human exposures, and 9.5% of patients were exposed to 2 or more drugs or products (). The exposure-related fatalities () involved a single substance in 528 cases (40.2%) and 2 or more substances in 787 cases (59.8%). The first ranked substance was a pharmaceutical in 1,066 of the 1,315 fatalities (81.2%). These 1,066 pharmaceuticals included (first ranked substance):
544 analgesics (110 acetaminophen, 73 acetaminophen/ hydrocodone, 71 methadone, 62 oxycodone, 48 salicylate, 34 morphine, 32 fentanyl transdermal, 25 acetaminophen/diphenhydramine, 16 acetaminophen/oxycodone, 15 acetaminophen/propoxyphene)
113 cardiovascular drugs (17 verapamil, 15 cardiac glycoside, 13 beta blockers, 12 diltiazem (extended release), 8 metoprolol, and 6 amlodipine)
111 antidepressants (16 amitriptyline, 13 lithium, 10 bupropion, 11,)
87 stimulants/street drugs (45 cocaine, 16 heroin, 8 amphetamine, 8 methamphetamine, and 6 MDMA)
The exposure was acute in 692 (52.6%), A/C = acute on chronic in 241 (18.3%), C = chronic exposure in 87 (6.6%) and U = unknown in 295 (22.4%).
Tissue Concentrations for 1 or more related analytes were reported in 597 cases (45.4%).
Route of exposure was: Ingestion in 1,101 cases (77.7%), Inhalation/nasal in 110 cases (7.8%), Parenteral in 51 cases (3.6%). ()
Intentional exposure reasons: Suspected suicide in 663 cases (50.4%), Intentional-Abuse in 229 cases (17.4%), Intentional-Misuse in 39 cases (3.0%). Unintentional exposure reasons: Environmental in 41 cases (3.1%), Therapeutic error in 36 cases (2.7%), Misuse in 10 cases 0.8%). ()
Pediatric fatalities – age less than 6 years
Although children younger than 6 years were involved in the majority of exposures, they comprised just 34 of 1,315 (2.0%) of fatalities. These numbers are similar to those reported since 1985 (). The percentage fatalities in children younger than 6 years related to total pediatric exposures was 26/1,292,754 = 0.00201%. By comparison, 1,201/856,908 = 0.140% of all adult exposures involved a fatality. Of these 26, 17 (65.4%) were reported as unintentional and 3 (11.5%) were coded as resulting from malicious intent (). These 26 cases included 16 pharmaceuticals and 10 nonpharmaceuticals. The substances associated with these fatalities included: carbon monoxide/smoke inhalation (4 cases), oxycodone (3 cases), sodium bicarbonate (2 cases) and 17 other substances (1 each).
Pediatric fatalities – ages 6–12 years
In the age range 6 to 12 years, there were 8 reported fatalities (), 4 of which were unintentional exposures and 2 intentional exposures. These 12 cases included 3 pharmaceuticals and 5 nonpharmaceuticals. The substances associated with these fatalities included: carbon monoxide/smoke inhalation (3 cases), bupropion, methadone, tramadol, hair spray and paraquat (1 each).
Adolescent fatalities – ages 13–19 years
In the age range 13 to 19 years, there were 74 reported fatalities (). These 74 cases included 60 pharmaceuticals and 14 nonpharmaceuticals, similar to the numbers reported in this age group reported annually since 1999. The first ranked pharmaceuticals associated with these fatalities included: methadone and oxycodone (6 cases each), acetaminophen, bupropion and quetiapine (4 each), acetaminophen/oxycodone, MDMA, opioid, and salicylate (3 each), and amphetamine, and lithium (2 each) and 26 other substances (1 each).
Of the 74 reported fatalities for adolescents, 28 (37.8%) were presumed suicides, and 26 (35.1%) were intentional abuse exposures (). The suspected suicide percentage is similar to recent years. The percentage of intentional abuse cases increased from 25.8% in 2006 to 35.7% in 2008. As in the past years, only a small number (8 of 74) of adolescent fatalities were coded as being unintentional.
Pregnancy and fatalities
A total of 26 deaths of pregnant women have been reported from the years 2000 through 2008. An average of 2 deaths per year was recorded from 2000 through 2004. Since 2005, the average number of deaths in pregnant women reported to NPDS doubled to four per year. The majority (20 of 26) were intentional exposures of misuse, abuse and suspected suicide. These deaths are flagged in as of this year's report.
AAPCC surveillance system
NPDS surveillance processes, anomaly definitions, and application software continue to be developed, refined, and evaluated. Surveillance Anomaly 1 was generated at 2:00 pm EDT on September 17, 2006. This event marked the transition of AAPCC surveillance to NPDS. Since then more than 136,000 anomalies have been detected. Many individual PCs and CDC have created surveillance case definitions. Case definitions have been authored by many regional poison centers, the AAPCC, and Health Studies Branch, Division of Environmental Hazards and Health Effects, National Center for Environmental Health, Centers for Disease Control and Prevention. The system has been expanded to include new case based definitions, and enhanced surveillance capabilities at the regional PC level. At the time of this report, 370 surveillance definitions run continuously, monitoring case and clinical effects volume and a variety of case based definitions from food poisoning to nerve agents.
Significant NPDS surveillance enhancements were introduced in 2008. Geocentric coverage was added to the volume definitions allowing centers to create definitions for all cases from their geographic coverage area, not just cases taken by their center. Case based definitions were improved with the addition of expanded Boolean expression size to allow more complex case based surveillance definitions to be created. Clinical effect anomaly analysis screen was optimized to allow for analysis per clinical effect. Increased definition creation flexibility was added to volume definitions. This flexibility included the ability to extend active definitions beyond their original expiration date. Anomaly alert email information was expanded to make the alert information richer and definition alerts could be chosen for one-time alerts to give the user the option to use the NPDS surveillance engine as a notification system to detect cases for study rather than a surveillance system. Six new surveillance reports and logging reports were added to the application to allow the user to search for definitions based on a variety of parameters in order to find a definition that pertains to certain characteristics of interest. A communications module was built into NPDS surveillance simplifying the process to store all analysis actions and correspondence with each anomaly.
NPDS implemented an aggregate count web service that allows users to extract case counts based on user defined parameters such as clinical effects and generate time series graphs for analysis. A second web service was added to allow external systems such as BioSense, RODS, ESSENCE, or state based system to extract NPDS data for the purpose of using NPDS full case data with their own surveillance engines and algorithms. Thus external system viewers can be used to augment existing NPDS analysis tools in a federated approach transforming NPDS into a data utility with always the most current data available for analysis. The advent of the NPDS web services marked a new era in NPDS development direction with all external system access under the purview of the responsible regional PC.
External users may now be given NPDS surveillance access by a poison center. NPDS surveillance access is now controlled by each poison center through the NPDS application. A center may grant any user access to their data and they may limit that access by one or more state and/or by one or more ZIP code. Regional Poison Centers remain completely in control of all access to their poison center data from a surveillance perspective.
Each surveillance definition now has a defined organization (owner) who is responsible for monitoring and analyzing the anomalies detected by the definition. NPDS now supports Full and Basic surveillance access. Basic access allows a user to survey the granting organization's data through the creating of surveillance definitions. Full access allows a user to view all definitions and anomalies owned by the granting organization and it allows the user to survey the granting organization's data by creating their own definitions. NPDS provided new functionality to allow user's to update and maintain his/her contact information used for surveillance notifications from NPDS. Surveillance storage was expanded to manage this additional data.
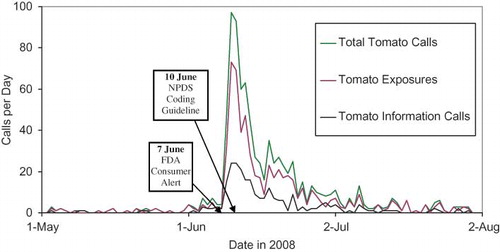
Automated surveillance remains controversial as a system to detect the index case of a public health event. Uniform evaluation algorithms are not available to determine the best and optimize methodologies.Citation5 Less controversial is the benefit to situational awareness that NPDS can provide. Typical NPDS surveillance data detects a response to an event rather than event prediction. The pre-recall tomato cases of the 2008 Tomato recall (see below) demonstrated the potential predictive value of NPDS as described using internet search engines. Recent work from several institutions has focused on the concept that queries to on-line information systems can predict major outbreaks.Citation6,Citation7 Analysis of PC calls analogous to internet search queries may provide predictive data for events of public health significance.
On June 7, 2008, FDA released an alert warning consumers not to eat certain types of raw red tomatoes. Possibly contaminated with Salmonella Saintpaul, an uncommon type of the bacteria.Citation8 Since mid-April 2008, 145 cases if salmonellosis had been reported nationwide. AAPCC issued a tomato recall coding guideline to poison centers on 10 June 2008. A retrospective review of NPDS tomato information calls and exposure calls revealed that prior to the FDA recall, daily calls to poison centers ranged from 0 to 2 calls per day. Beginning on 1 June 2008, calls increased ranging from 1 to 7 per day until 9 June when total tomato calls increased to 46 before peaking on 10–11 June at over 90 calls per day (). Clearly, the initial rise of tomato related calls occurred prior to the FDA release and before the coding guidelines were released to the centers. This marks the first time that such phenomena in NPDS data has been seen although it has been shown in other Internet information systems.Citation9 Food recalls are becoming more common. It is possible that augmenting NPDS with automated time series technology for common food products may provide useful data on outbreaks before they reach mainstream media. Although NPDS did not detect the index case, implementation of refined algorithms and close work with public health agencies shows NPDS promise as part of an early detection system. NPDS case data clearly presaged the FDA alert and the tomato information and exposure call patterns provided situational awareness about the event ().
Discussion
Trends in reported poisonings/exposures
The total of 4,333,012 human exposure cases and information calls reported to PCs in 2008 does not reflect the full extent of poison center efforts which also include activities such as poison prevention and education and poison awareness. Additionally, we do not know the true denominator for exposures and information calls in the United States.
These data () do not directly identify a trend in the overall incidence of poisonings in the US because the percentage of actual exposures and poisonings reported to PCs is unknown. As is explained in this section, NPDS data reports fewer fatalities and more exposures than other CDC databases.
NPDS may be considered as providing “numerator data” since the “denominator” is not accurately determined. Attempts have been made to better define the incidence of poisoning. For example, based on the National Health Interview Survey (NHIS), the estimated number of poisoning episodes in the US for the year 2000 was estimated to be 1,575,000.Citation10 During the same time AAPCC centers received reports of 2.2 million poisoning exposures of which 475,079 were managed in a health care facility.Citation1
The frequency of any injury rests on the definition used. National Center for Health Statistics (NCHS) defined a poisoning as the “event resulting from ingestion of or contact with harmful substances including overdoses or incorrect use of any drug or medication.”Citation11 NCHS reported that the age-adjusted death rate for poisoning doubled from 1985 through 2004 to 10.3 deaths per 100,000 population. The rise was most evident from 1998 to 2000 when the poison fatalities increased an average of 8.2% per year.Citation11
The CDC National Center for Injury Prevention and Control latest poison data available are for the year 2006.Citation12 Their system reports 37,286 deaths from a US population of 298,362,973 and produces an age adjusted rate of 12.37 per 100,000 population [ICD-10 codes: X40-X49,X60-X69,X85-X90,Y10-Y19, Y35.2, *U01(0.6, 0.7)].Citation12 This is in contrast to the 1,229 fatalities captured by NPDS during this time period.Citation1
In 2005 (latest CDC report for this comparative data), 23,618 (72%) of the 32,691 poisoning deaths in the United States were unintentional, and 3,240 (10%) were of undetermined intent.Citation13 Unintentional poisoning death rates have been rising steadily since 1992. NPDS reported 2,005,307 unintentional exposures out of 2,403,539 total exposures reported. Unintentional poisoning was second only to motor vehicle crashes as a cause of unintentional injury death in 2005.Citation13 Among people 35–54 years old, unintentional poisoning caused more deaths than motor vehicle crashes. In the United States in 2005, 5,833 (18%) of the 32,691 poisoning deaths were intentional; 5,744 were suicides and 89 were homicides.Citation13 NPDS reported 308,483 intentional exposures for 2005.
Supplementary Material
Download MS Word (5.2 MB)Supplementary Material
Download MS Word (3.1 MB)References
- Previous year’s reports of the American Association of Poison Control Centers are available on the AAPCC website at http://aapcc.org/annual.htm.
- Position statement: ipecac syrup. American Academy of Clinical Toxicology; European Association of Poisons Centres and Clinical Toxicologists. J Toxicol Clin Toxicol. 1997;35:699–709.
- American Academy of Clinical Toxicology European Association of Poisons Centres and Clinical Toxicologists. Position Paper: Ipecac Syrup. J Toxicol Clin Toxicol 2004; 42: 133–143.
- American Academy of Pediatrics Policy Statement. Poison treatment in the home. Pediatrics 2003;112:1182–1185.
- Sosin DM, DeThomasis J. Evaluation challenges for syndromic surveillance–making incremental progress. MMWR Morb Mortal Wkly Rep. 2004;53 Suppl:125–129.
- Ginsberg J, Mohebbi MH, Patel RS, Brammer L, Smolinske MS, Brilliant L. Detecting influenza epidemics using search engine query data. Nature. 2009;457:1012–1014.
- Polgreen PM, Chen Y, Pennock DM, Nelson FD. Using internet searches for influenza surveillance. Clin Infect Dis. 2008;47:1443–1448.
- FDA. (2008, June 7). FDA Warns Consumers Nationwide Not to Eat Certain Types of Raw Red Tomatoes. Retrieved from http://www. fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116908. htm.
- Ginsberg1 J, Mohebbi1 MH, Patel1 RS, Brammer L, Smolinske MS, Brilliant L Detecting influenza epidemics using search engine query data. Nature 2009; 457: http://dx.doi.org/10.1038/nature07634.
- IOM. Forging a Poison Prevention and Control System/Committee on Poison Prevention and Control, B Geyer, JA Alexander, P Blanc, D Emerson, JR Hedges, MS Kamlet, A Mickalide, BH Rumack, DP Schor, DA Spyker, A Stergachis, DJ Tollerud, DK Walker; Board on Health Promotion and Disease Prevention, Institute of Medicine of the National Academies, 2004 http://www.nap.edu/catalog/10971. html.
- Bergen G, Chen LH, Warner M, Fingerhut LA. Injury in the United States: 2007 Chartbook. Hyattsville, MD: National Center for Health Statistics. 2008.
- WISQARS (Web-based Injury Statistics Query and Reporting System) accessed October 10, 2009 http://webappa.cdc.gov/sasweb/ncipc/mortrate10_sy.html.
- CDC: Poisoning in the United States: Fact Sheet http://www.cdc.gov/ncipc/factsheets/poisoning.htm. Accessed 10 October, 2009.
- McGraw-Hill’s AccessMedicine, Laboratory Values of Clinical Importance (Appendix), Harrison’s Principles of Internal Medicine. McGraw-Hill Professional, 2004, http://www.accessmedicine.com/. Accessed 24 June, 2008.
- Goldfrank’s Toxicologic Emergencies, Eighth Edition, McGraw-Hill Companies, 2006.
- Dart RC, editor. Medical Toxicology, Third Edition. Philadelphia, Lippincott, Williams & Wilkins, 2004.
Disclaimer
The American Association of Poison Control Centers (AAPCC; http://www.aapcc.org) maintains the national database of information logged by the country's 61 Poison Centers (PCs) serving all 50 United States, Puerto Rico, and the District of Columbia. Case records in this database are from self-reported calls: they reflect only information provided when the public or healthcare professionals report an actual or potential exposure to a substance (e.g., an ingestion, inhalation, or topical exposure, etc.), or request information/educational materials. Exposures do not necessarily represent a poisoning or overdose. The AAPCC is not able to completely verify the accuracy of every report made to member centers. Additional exposures may go unreported to PCs and data referenced from the AAPCC should not be construed to represent the complete incidence of national exposures to any substance(s).
Appendix A – Acknowledgments
The compilation of the data presented in this report was supported in part through the U.S. Centers for Disease Control AAPCC Contract 200-2007-19121.
The authors express their sincere gratitude to Jim Hirt, AAPCC Executive Director and the staff at the AAPCC Central Office for their support during the preparation of the manuscript.
The authors wish to express their appreciation to the following individuals who assisted in the preparation of the manuscript: Lily H. Gong; Kathy Worthen; Laura Rivers; Carol Hesse, Mary Anne Stigall.
Poison Centers
We gratefully acknowledge the extensive contributions of each participating poison center and the assistance of the many health care providers who provided comprehensive data to the poison centers for inclusion in this database. We especially acknowledge the dedicated efforts of the Specialists in Poison Information (SPIs) who meticulously coded 4,333,012 calls made to U.S. Poison Centers in 2008.
The initial review of reported fatalities and development of the abstracts was the responsibility of the staff of the participating poison centers. These poison centers and individuals are listed at the beginning of this report.
Many individuals at each center participate in the review of their centers fatality cases. The following toxicology professionals summarized and prepared their center's fatality data for NPDS:
Alabama Poison Center
Perry Lovely, MD, ACMT
John Fisher, PharmD, DABAT, FAACT
Lois Dorough BSN, RN, CSPI
Arizona Poison and Drug Information Center
Jude McNally RPh, DABAT
Leslie Boyer MD, FACMT
Arkansas Poison & Drug Information Center
Henry F. Simmons, Jr., MD
Pamala R. Rossi, PharmD
Howell Foster, PharmD
Banner Good Samaritan Poison and Drug Information Center
Frank LoVecchio, DO, MPH
Steven C. Curry, MD
Kathleen Waszolek, RN, CSPI
Blue Ridge Poison Center
Christopher Holstege, MD
Nathan Charlton, MD
David Lawrence, MD
Brigid Wonderly, RN
Stephen Dobmeier, RN
California Poison Control System – Fresno/Madera Division
Richard J. Geller, MD, MPH
California Poison Control System – Sacramento Division
Timothy Albertson, MD, PhD
Judith Alsop, PharmD
California Poison Control System – San Diego Division
Richard F. Clark, MD
Lee Cantrell, PharmD
Shaun Carstairs, MD
Sean Nordt, MD, PharmD
Allyson Kreshak, MD
Alicia Minns, MD
Alex Miller, MD
California Poison Control System – San Francisco
Kent R. Olson, MD
Thanjira Jiranantakan, MD
Thomas E. Kearney, PharmD
Tanya Mamantov, MD
Craig Smollin, MD
Carolinas Poison Center
Michael C. Beuhler, MD
Jennifer Englund, MD
Marsha Ford, MD
William Kerns II, MD
Anna Rouse Dulaney, PharmD
Ashley Webb, MSc, PharmD
Central Ohio Poison Center
Marcel J. Casavant, MD, FACEP, FACMT
Heath Jolliff, DO, FACEP, FAAEM
S. David Baker, PharmD, DABAT
Julee Fuller-Pyle
Julie Lecky, BA
Central Texas Poison Center
Ron Kirschner, MD
Douglas J. Borys, PharmD
Children's Hospital of MI Regional Poison Center
Cynthia Aaron, MD
Lydia Baltarowich, MD
Keenan Bora, MD
Bram Dolcourt, MD
Matthew Hedge, MD, ACMT
Susan C. Smolinske, PharmD
Suzanne R. White, MD
Cincinnati Drug and Poison Information Center
G. Randall Bond, MD
Rachel Sweeney, RN
Connecticut Poison Center
Charles McKay, MD
Kevin O'Toole, MD
Jarret Lefberg, DO
Bernard C. Sangalli, MS
DeVos Children's Hospital Regional Poison Center
Bernard Eisenga PhD, MD
Bryan Judge, MD
Brad Riley, MD
Florida/USVI Poison Information Center – Jacksonville
Thomas Kunisaki, MD, FACEP, ACMT
Florida Poison Information Center – Miami
Jeffrey N. Bernstein, MD
Richard S. Weisman, PharmD
Florida Poison Information Center – Tampa
Cynthia R. Lewis-Younger, MD, MPH
Pam Eubank, RN, CSPI
Judy Gussett, RN, CSPI
Judy Turner, RN, CSPI
Georgia Poison Center
Robert Geller, MD
Brent W. Morgan, MD
Arthur Chang, MD
Ziad Kazzi, MD
Gaylord P. Lopez, PharmD
Richard Kleiman, MD
Carl Skinner, MD
Andre Matthews, MD
Hennepin Regional Poison Center
David J. Roberts MD, ABMT, ABMS
Elisabeth F. Bilden MD
Deborah L. Anderson PharmD
Samuel J. Stellpflug, MD
Illinois Poison Center
Michael Wahl, MD
Sean Bryant, MD
Indiana Poison Center
James B. Mowry, PharmD
R. Brent Furbee, MD
Iowa Statewide Poison Control Center
Edward Bottei, MD
Kentucky Regional Poison Center
George M. Bosse, MD
Henry A. Spiller, MS, RN
Long Island Regional Poison and Drug Information Center
Thomas R. Caraccio, PharmD, DABAT
Michael McGuigan, MDCM, MBA
Louisiana Poison Center
Thomas Arnold, MD
Mark Ryan, RPh
Maryland Poison Center
Suzanne Doyon, MD, FACMT
Patrick Dougherty, PharmD
Mississippi Regional Poison Control Center
Robert Cox MD, PhD, DABT, FACMT
Tanya Calcott, RN, CSPI
Missouri Regional Poison Center at SSM Cardinal Glennon Children's Medical Center
Anthony Scalzo, MD
Shelly Enders, PharmD, CSPI
National Capital Poison Center
Cathleen Clancy, MD, FACMT
Nicole Whitaker, RN, BA, BSN, MEd, CSPI
Nebraska Regional Poison Center
Claudia Barthold, MD
New Jersey Poison Information and Education System
John Kashani, DO
Steven M. Marcus, MD
New Mexico Poison and Drug Information Center
Steven A. Seifert, MD, FAACT, FACMT
New York City Poison Control Center
Maria Mercurio-Zappala, MS, RPh
Robert S. Hoffman, MD
Lewis Nelson, MD
Alex Manini, MD
Silas Smith, MD
Oladapo Odujebe, MD
Jen Prosser, MD
Brenna Farmer, MD
Samara Soghoian, MD
North Texas Poison Center
Brett Roth MD, ACMT, FACMT
Northern Ohio Poison Center
Susan Scruton, RN, CSPI
Lawrence S. Quang, MD
Alfred Aleguas, PharmD
Northern New England Poison Center
Theresa Maher, MFA
Tamas Peredy, MD
Oklahoma Poison Control Center
William Banner, Jr., MD, PhD, ABMT
Lee McGoodwin, PharmD, MS, DABAT
Oregon Poison Center
Zane Horowitz, MD
Sandra L. Giffin, RN, MS
Palmetto Poison Center
William H. Richardson, MD
Jill E. Michels, PharmD
Pittsburgh Poison Center
Kenneth D. Katz, MD
Rita Mrvos, BSN
Edward P. Krenzelok, PharmD
Puerto Rico Poison Center
José Eric Díaz-Alcalá, MD
Regional Center for Poison Control and Prevention Serving Massachusetts and Rhode Island
Nadeem Al-Duaij, MD, MPH
Michele Burns Ewald, MD
Mathew George, MD
Katherine O'Donnell, MD
Regional Poison Control Center – Children's Hospital of Alabama
Diane Smith, RN, CSPI
Valoree Stanley, RN, CSPI
Erica Liebelt, MD, FACMT
Michele Nichols, MD
Rocky Mountain Poison & Drug Center
Carol L. Hesse, RN, CSPI
Alvin C. Bronstein, MD, FACEP, FACMT
Jennie A. Buchanan, MD
Ryan Chuang, MD
Jason Hoppe, DO
Sean H. Rhyee, MD, MPH
Shawn M. Varney, MD, FACEP
Shan Yin, MD, MPH
Amy Zosel, MD
Mary Anne Stigall
South Texas Poison Center
Douglas Cobb, RPh
Cynthia Abbott-Teter, PharmD
Miguel C. Fernandez, MD
George Layton, MD
C. Lizette Villarreal, MA
Southeast Texas Poison Center
Wayne R. Snodgrass, MD, PhD, ABMT
Jon D. Thompson, MS, DABAT
Jean L. Cleary, PharmD, CSPI
Tennessee Poison Center
John G. Benitez, MD, MPH
Saralyn Williams, MD
Donna Seger, MD
Texas Panhandle Poison Center
Shu Shum, MD
Jeanie E. Jaramillo, PharmD
The Poison Control Center at the Children's Hospital of Philadelphia
Allison A. Muller, PharmD
Kevin Osterhoudt, MD
The Ruth A. Lawrence Poison and Drug Information Center
Ruth A. Lawrence, MD
Norma Barton, BS Pharm, CSPI
University of Kansas Hospital Poison Control Center
Jennifer Lowry, MD
Tama Sawyer, PharmD
Upstate NY Poison Center
Jeanna M. Marraffa, PharmD
Jamie Nelsen, PharmD
Christine M. Stork, PharmD
Utah Poison Control Center
Martin Caravati, MD, MPH
Virginia Poison Center
Rutherfoord Rose, PharmD
Kirk Cumpston, DO
Washington Poison Center
William T. Hurley, MD, FACEP
David Serafin, CPIP
West Texas Regional Poison Center
John F. Haynes, Jr., MD
Leo Artalejo III, PharmD
Hector L. Rivera, RPh
West Virginia Poison Center
Lynn F. Durback-Morris RN, BSN, MBA, DABAT
Anthony F. Pizon, MD, ABMT
Western New York Poison Center
Prashant Joshi, MD
Wisconsin Poison Center
David D. Gummin, MD
Mark A. Kostic, MD
Cathy Smith, CSPI
Fatality review team
The Lead and Peer review of the 2008 fatalities was carried out by the 29 individuals listed here. The authors and the AAPCC wish to express our appreciation for their volunteerism, dedication, hard work, and good will in completing this task in a very limited time.
Ann-Jeannette Geib*, MD, Clinical Assistant Professor of Emergency Medicine UMDNJ-Robert Wood Johnson Medical School New Brunswick NJ
Bernard C. Sangalli*, MS, DABAT, Director, Connecticut Poison Center
Charles McKay, MD, Associate Medical Director, Connecticut Poison Control Center, University of Connecticut School of Medicine
David Gummin, MD, Wisconsin Poison Center, Milwaukee, WI
Dean Olsen, DO, New York City Poison Center Staff, New York, NY
Edward P. Krenzelok, PharmD, FAACT, DABAT, Director, Pittsburgh Poison Center
Elizabeth J. Scharman, PharmD, DABAT, BCPS, FAACT, Director West Virginia Poison Center
Frank LoVecchio, DO, Co-Medical Director, Banner Good Samaritan Poison and Drug Information Center, Phoenix, AZ
Heath Jolliff, DO, Central Ohio Poison Center, Columbus, Ohio
Henry Spiller*, MS, DABAT, FAACT Kentucky Regional Poison Center, Louisville, KY
Howell Foster, PharmD, DABAT, Arkansas Poison & Drug Information Center
Jen Hannum, MD, Department of Emergency Medicine, Wake Forest University Baptist Medical Center, Winston-Salem, NC
Jennifer Lowry**, MD, Medical Director, Kansas Poison Center at the University of Kansas Hospital, Kansas City, MO
Jill E. Michels, PharmD, DABAT, Managing Director, Palmetto Poison Center, SC
Judith A. Alsop, PharmD, DABAT, California Poison Control System – Sacramento Division
Karen E. Simone, PharmD, DABAT, Director, Northern New England Poison Center, Maine Medical Center
Kathryn Meier, PharmD, DABAT, Senior Toxicology Management Specialist, California Poison Control System, San Francisco Division
Lewis Nelson, MD, New York City Poison Center, New York University School of Medicine
Luke Yip*, MD, FACMT, FACEM, FACEP, Rocky Mountain Poison and Drug Center, Denver CO
Lynn Durback-Morris*, RN, MBA, DABAT, CSPI, Supervisor of Operations, West Virginia Poison Center
Maria Mercurio-Zappala, RPh, MS, DABAT, FAACT Managing Director, NYC Poison Control Center
Mark Su, MD, FACEP, FACMT, Assistant Professor of Emergency Medicine, Director, Fellowship in Medical Toxicology, Department of Emergency Medicine, North Shore University Hospital, Manhasset, NY
Richard J. Geller, MD, MPH, FACP, Medical and Managing Director, California Poison Control System, Fresno/Madera
Robert B. Palmer, PhD, DABAT, Toxicology Associates, Denver, CO; Rocky Mountain Poison and Drug Center, Denver, CO
S. David Baker, PharmD, DABAT, Managing Director, Central Ohio Poison Center
Steven M. Marcus**, MD, NJ Poison Information and Education System, Departments of Preventive Medicine and Community Health and Pediatrics, NJ Medical School, University of Medicine and Dentistry of NJ.
Timothy Wiegand*, MD. Clinical Assistant Professor of Medicine, Maine Medical Center, and Associate Medical Director, Northern New England Poison Center, Portland, Maine
William T. Hurley, MD, Medical Director, Washington Poison Center
*These reviewers further volunteered to read the top ranked 200 abstracts and judged to publish or omit.
**These individuals reviewed the pediatric fatalities.
Surveillance team
Surveillance was carried out by a team of 10 medical and clinical toxicologists working across the country in a distributed system who provided daily monitoring of surveillance anomalies throughout 2008:
Alfred Aleguas, PharmD, DABAT
Alvin C. Bronstein, MD, FACEP, FACMT
Blaine (Jess) Benson, PharmD, DABAT
Douglas Borys, PharmD, DABAT
Henry A. Spiller, MS, DABAT, FAACT
Jeanna M. Marraffa, PharmD, DABAT
John Fisher, PharmD, DABAT, FAACT
Maria Mercurio-Zappala, RPH, MS, DABAT, FAACT
Richard G. Thomas, PharmD, DABAT
S. David Baker, PharmD, DABAT
Regional Poison Center (RPC) Fatality Awards
This year the AAPCC and the Fatality Review team recognized several Regional Poison Centers (RPCs) for their extra effort in a variety of categories reflected by their preparation of fatality reports and prompt responses to reviewer queries during the review process. The awards were presented at the September 2009, North Amercian Congress of Clinical Toxicology meeting in San Antonio, Texas:
First Center to Complete all Cases – Pittsburgh Poison Center, closed the last of their cases September 1, 2008
Highest Percentage with Autopsy Reports – Oklahoma Poison Control Center, 97.4% of 153 cases
Highest Overall Quality of Reports – Carolinas Poison Center, 5.38 of possible 10 for their 56 fatalities
Most Abstracts Published in 2008 Annual report – a 3-way tie – Children's Hospital of Michigan Regional Poison Control Center (Detroit), Hennepin Regional Poison Center (Minneapolis), and Carolinas Poison Center (Charlotte)
Outstanding Case Preparation – Long Island Regional Poison & Drug Information Center. Honorable mention: National Capitol, Oklahoma, Florida Tampa, New Jersey, Carolinas
Most Helpful Regional Poison Center Staff – Georgia Poison Center, Honorable mention: Carolinas, and Long Island.
Appendix B – data definitions
NPDS classifies all calls as either EXPOSURE (concern about an exposure to a substance) or INFORMATION (no exposed human or animal). A call may provide information about one or more exposed person or animal (receptors).
Reason for exposure
Specialists in Poison Information (SPIs) coded the reasons for exposure reported by callers to PCs according to the following definitions:
Unintentional general: All unintentional exposures not otherwise defined below.
Environmental: Any passive, non-occupational exposure that results from contamination of air, water, or soil. Environmental exposures are usually caused by manmade contaminants.
Occupational: An exposure that occurs as a direct result of the person being on the job or in the workplace.
Therapeutic error: An unintentional deviation from a proper therapeutic regimen that results in the wrong dose, incorrect route of administration, administration to the wrong person, or administration of the wrong substance. Only exposures to medications or products used as medications are included. Drug interactions resulting from unintentional administration of drugs or foods which are known to interact are also included.
Unintentional misuse: Unintentional improper or incorrect use of a nonpharmaceutical substance. Unintentional misuse differs from intentional misuse in that the exposure was unplanned or not foreseen by the patient.
Bite/sting: All animal bites and stings, with or without envenomation, are included.
Food poisoning: Suspected or confirmed food poisoning; ingestion of food contaminated with microorganisms is included.
Unintentional unknown: An exposure determined to be unintentional, but the exact reason is unknown.
Suspected suicidal: An exposure resulting from the inappropriate use of a substance for reasons that are suspected to be self-destructive or manipulative.
Intentional misuse: An exposure resulting from the intentional improper or incorrect use.
Medical outcome
No effect: The patient did not develop any signs or symptoms as a result of the exposure.
Minor effect: The patient developed some signs or symptoms as a result of the exposure, but they were minimally bothersome and generally resolved rapidly with no residual disability or disfigurement. A minor effect is often limited to the skin or mucus membranes (e.g., self-limited gastrointestinal symptoms, drowsiness, skin irritation, first-degree dermal burn, sinus tachycardia without hypotension, and transient cough).
Moderate effect: The patient exhibited signs or symptoms as a result of the exposure that were more pronounced, more prolonged, or more systemic in nature than minor symptoms. Usually, some form of treatment is indicated. Symptoms were not life-threatening, and the patient had no residual disability or disfigurement (e.g., corneal abrasion, acid-base disturbance, high fever, disorientation, hypotension that is rapidly responsive to treatment, and isolated brief seizures that respond readily to treatment).
Major effect: The patient exhibited signs or symptoms as a result of the exposure that were life-threatening or resulted in significant residual disability or disfigurement (e.g., repeated seizures or status epilepticus, respiratory compromise requiring intubation, ventricular tachycardia with hypotension, cardiac or respiratory arrest, esophageal stricture, and disseminated intravascular coagulation).
Death: The patient died as a result of the exposure or as a direct complication of the exposure.
Not followed, judged as nontoxic exposure: No follow-up calls were made to determine the outcome of the exposure because the substance implicated was nontoxic, the amount implicated was insignificant, or the route of exposure was unlikely to result in a clinical effect.
Not followed, minimal clinical effects possible: No follow-up calls were made to determine the patient's outcome because the exposure was likely to result in only minimal toxicity of a trivial nature. (The patient was expected to experience no more than a minor effect.)
Unable to follow, judged as a potentially toxic exposure: The patient was lost to follow-up, refused follow-up, or was not followed, but the exposure was significant and may have resulted in a moderate, major, or fatal outcome. Unrelated effect: The exposure was probably not responsible for the effect.
Confirmed nonexposure: This outcome option was coded to designate cases where there was reliable and objective evidence that an exposure initially believed to have occurred actually never occurred (e.g., all missing pills are later located). All cases coded as confirmed nonexposure are excluded from this report.
Death, indirect report
A reported death is coded as “indirect” if no inquiry was placed to the poison center. For example, if the case was obtained from a medical examiner who queries the PC about interpretation of post mortem reports.
Appendix C – abstracts of select cases
Abstracts of the 89 cases selected (see Selection of Abstracts for Publication) and 1 addendum from 1,315 human fatalities judged related to a poisoning exposure as reported to U.S. Poison Centers in 2008. A structured format for abstracts was optional in the preparation of the abstracts and was used in the abstracts presented. Abbreviations, units and normal ranges omitted from the abstracts are given at the end of this appendix
Abstracts
Case 6. Acute ethanol ingestion: undoubtedly responsible.
Scenario/Substances: 23 y/o male ingested an unknown amount of alcohol 10 hours prior, found comatose by EMS, given D50% en route to the ED without effect.
Physical Exam: Unresponsive male, BP 130–140/96, HR 120, without spontaneous respirations. Pupils fixed and dilated, gag reflex absent.
Laboratory Data:
Ca 7.8, anion gap 23, serum osmolality 357, pH 6.91. Ethanol 402 mg/dL. Toxicology screen, acetaminophen and salicylate not detected.
Clinical Course: In ED, patient was intubated, ventilated and sedated with IV propofol. Sodium bicarbonate was given. At 14 hours post-ingestion, 2 sequential cardiac arrests occurred, pupils remained fixed and dilated; the patient expired at 25 hours post-ingestion.
Autopsy Findings: Ante mortem ethanol in blood (specimen not dated or timed) 0.37 gm/100 ml. No other drugs detected. Death due to acute ethanol intoxication.
Case 44. Acute hydrofluoric acid Ingestion, Dermal: undoubtedly responsible.
Scenario/Substances: A 12 month old female got hydrofluoric acid on her hands and put her hands in mouth after the bottle of the product fell onto the child's walker. The child vomited 1–2 times at home and became lethargic per father and EMS who transported directly to tertiary care pediatric ED by helicopter.
Physical Exam: In ED awake, alert, no apparent distress. BP 92/38, HR 166, RR 38, T 37°C, O2 sat 100%. No oral burns noted and the remainder of exam unremarkable.
Laboratory Data: (2.5 hr post exposure):
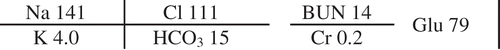
Calcium 6.8, ECG sinus tachycardia, ventricular rate of 175, QRS 54 ms, QT/QTc 256/436 ms, PR 94 ms, and nonspecific ST abnormality.
Clinical Course: In ED IV access, cardiac monitoring established with pulse oximetry and reverse trendellenburg. Irrigation of the child's hand occurred 2–3 h post exposure. The child was resting comfortably. While sleeping 4 hr post exposure, posturing with apnea and Torsade de pointes occurred. Positive pressure ventilation, CPR was started. Resuscitation included 6 g calcium, 6 g magnesium sulfate, 0.7 mg epinephrine, and defibrillation. Resuscitation was unsuccessful and she expired 4.5–5 h post exposure.
Autopsy Findings: No anatomic pathology was identified. The cause of death was ruled cardiac arryhthmia due to hypocalcemia due to hydrogen fluoride poisoning.
Case 57. Acute antifreeze (ethylene glycol) ingestion: undoubtedly responsible.
Scenario/Substances: 65 y/o male was brought to the ED due to loss of consciousness of unknown cause.
Laboratory Data: HCO3 4 (from ABG), Ca 8.9, Cr 2.0, BUN 13, osmolar gap 150.
Clinical Course: Upon arrival to the ED he was obtunded. Day 2 ethylene glycol was reported >3000 mg/L, glycolic acid >3000 mg/L, methanol and ethanol were negative. The patient was started on hemodialysis. Day 3 fomepizole was given prior to a second session of hemodialysis, post-dialysis ethylene glycol was 295 mg/L. On Day 4 he developed a fever of 41.1° C seizures, treated with sodium bicarbonate, IV lorazepam, and a cooling blanket. He did not respond and died on Day 4.
Autopsy Findings: Cause of death was ethylene glycol overdose.
Case 58. Acute ethylene glycol ingestion: undoubtedly responsible.
Scenario/Substances: 69 y/o male ingested 1 “cup” of antifreeze and presented an unknown time later to the ED with altered mental status.
Past Medical History: Hypertension, sleep apnea, osteomyelitis, hypercholesterolemia.
Physical Exam: Confused male with constricted pupils, BP 210/70, HR 103, RR 40.
Laboratory Data: ABG-pH 6.96, pCO2 11, PO2 131 (on O2), HCO3 2.3; K 6.8, Cr 1.8, BUN 11, anion gap 27, Serum ethylene glycol 156 mg/dL; serum methanol not detected; toxicology serum screen for ethanol, salicylate and acetaminophen not detected. Head CT: diffuse cerebral edema.
Clinical Course: Patient was intubated, sedated, received IV fluids, sodium bicarbonate, fomepizole, and mannitol. Hemodialysis performed. Multiple tonic clonic seizures treated with levetiracetam and valproic acid. Cr increased to 6.6. The patient remained unresponsive with anoxic brain injury. Comfort measures were instituted and he expired on Day 12.
Autopsy Findings: Pulmonary edema, cardiomegaly, acute tubular necrosis and oxalate crystal deposition in renal tubules. Cause of death was acute ethylene glycol poisoning.
Case 61. Acute crotalid envenomation: undoubtedly responsible.
Scenario/Substances: A 37 y/o male was bitten on his right thumb by a rattlesnake at home. He called his wife, who arrived 30 min later and found him blue and unresponsive with vomitus. EMS intubated, defibrillated and began CPR.
Past Medical History: Asthma; 2 prior rattlesnake bites with reported “allergy” to rattlesnake venom.
Physical Exam: Unresponsive; small (<1 cm) laceration on right thumb; pupils fixed and dilated. Respiratory and metabolic acidosis. Asystole converted to VF, then VT. Respiratory arrest; T 35.6°C.
Clinical Course: In the ED, the patient was given sodium bicarbonate, epinephrine, atropine, methylprednisolone, diphenhydramine, 6 vials of antivenom, norepinephrine, lidocaine, clindamycin, levoquinalone, IV fluids, midazolam, epinephrine, vecuronium, naloxone and vitamin K. At ∼4 hours post exposure, PT 19.6 seconds; PTT 40.5 seconds; fibrinogen 155 mg/dl; platelets 232; WBC 19.7; Hgb 19.1, Hct 56.8%. After stabilizing the patient, he was flown to a tertiary hospital. Six more vials of antivenom were given. No ecchymosis or oozing noted. Day 2, he was not on norepinephrine or sedation, still on ventilator; shaking; swelling to hand; thumb was black; on insulin infusion; on antibiotics; diphenhydramine and 4 units of fresh frozen plasma given; given. On Day 2 his platelets were 142; PTT 29.6 seconds; INR 1.5; fibrinogen 223 mg/dl; D-Dimer 11.09 mcg/mL; fibrin degradation product >40 mcg/mL. On Day 3 he had GCS 5T; on lantus, albuterol nebulizer, morphine, hydrocortisone, antibiotic; vitamin K; poor urine output; on NG feeding; tip of thumb necrotic. His platelets were 84, fibrinogen 330 mg/dl; AST 44; ALT 451; WBC 21.2; Hgb 11.5; Hct 33.2%. On Day 4, he had no cough or gag reflex; GCS 3T. On Day 5, the patient's family requested comfort measures only and he expired on Day 6
Autopsy Findings: Not done
Case 64. Acute jellyfish stings: probably responsible.
Scenario/Substances: A 70 y/o male was swimming in the ocean off a Florida beach when he was stung by a Portuguese Man O' War. Local wound care was applied by the lifeguard including application of vinegar and removal of nematocysts. The patient collapsed ∼25 min after the sting as he was walking away from the lifeguard stand. EMS found him in cardiopulmonary arrest and transported him to the ED.
Past Medical History: cardiac disease with a pacemaker/automatic cardioverter defibrillator.
Clinical Course: He arrived in the ED in cardiopulmonary arrest. Pacer spikes were noted on the electrocardiogram, but there were no spontaneous respirations or pulse, resuscitation was stopped, and he was pronounced dead.
Autopsy Findings: Not available
Case 66. Acute sulfuric acid ocular and dermal: undoubtedly responsible.
Scenario/Substances: 18 y/o male suffered burns oropharynx, neck, face, and chest, when acid was thrown in his face during an altercation. He was intubated by EMS during transport to the ED.
He presented in the ED with 2nd and 3rd degree burns to his face, neck, and upper chest. Stabilized and evacuated by air to an out of state burn center.
Physical Exam: 2nd and 3rd degree burns and edema of the oropharynx, neck, face, and chest.
Clinical Course: He was placed on a ventilator and received IV fluids and sedation while his skin and eyes were irrigated. He was stabilized and transferred to the burn center ICU. Esophagogastroduodenoscopy showed injury to his oropharynx. Bronchoscopy revealed inhalation injury at the level of the carina. He had debridement of the anterior neck and chest with placement of a graft. Subsequently he developed bilateral bronchopneumonia, abdominal compartment syndrome, and acute renal failure. A decompression laparotomy was performed but his respiratory function continued to decline and he expired on Day 8.
Autopsy Findings: Cause of death: complications of acid burns to the oropharynx, face, neck, and chest. Incidental findings were cardiomegaly and bilateral ventricular hypertrophy
Case 72. Acute nitrate/nitrite inhalation: undoubtedly responsible.
Scenario/Substances: A 33 y/o male was found unresponsive with“Jungle juice” (an ethanol beverage) and a head cleaner containing ethyl chloride found nearby.
Physical Exam: Unresponsive with agonal respirations, O2 sat in the 80s%. SBP in the 80s; cyanotic with mottled skin.
Laboratory Data: The methemoglobin level was reported after the patient expired at > 70%.
Clinical Course: The patient was brought to the ED by EMS. He was given 1 dose of methylene blue and naloxone with no apparent response. He became pulseless, was intubated and CPR was administered per ACLS protocols. He could not be resuscitated and expired shortly after arriving at the hospital.
Autopsy Findings: The cause of death was chemical asphyxiation due to methemoglobin due to “huffing” of volatile substances. Postmortem methemoglobin saturation was 41.2%.
Case 84. Acute antifreeze (ethylene glycol) ingestion: undoubtedly responsible.
Scenario/Substances: 50 y/o male became be very unsteady and lethargic. His wife assumed that he had taken too much of his methadone, but when she later attempted to wake him and he had a seizure, she called EMS.
Laboratory Data:
Day 1: ABG-pH 6.9/pCO2 32/pO2 111, total Ca 5.8, Na 155, K 5.4, CO2 8, Cr 3.5, CK 366, ethanol and acetaminophen not detected, urine drug screen negative; TProt in CSF 148 mg/L; glucose in CSF 146 mg/dL; PT 16.5, WBC 44.2, platelets 449. Urinanalysis: protein 75, glucose 50, blood 4+.
Day 2: K 2.8, HCO3 17, Cr 6.2, CK 812, CKMB 10.3, troponin 0.097, WBC 19.8, PT 16.7, d-dimer 3.79, serum osmolality 341, lactic acid 5.3, pH 7.18, ionized Ca 0.88, methanol not detected; ethylene glycol 24.8 mg/dL.
Day 3: AST 71, Total Ca corrected to 8.1 mg/dL. Day 4: WBC 17.8, Cr 3. Day 5: AST 109. Day 6: K 5.5, BUN 81, Cr 6.5. Day 7: BUN 81, Cr 6.7, Alk Phos 142, AST 71.
Clinical Course: Upon arrival to the ED, the patient was intubated, IV fluids, sodium bicarbonate, and antibiotics were initiated for hypotension, profound acidosis and suspected sepsis and he was admitted to the ICU. Fomepizole was started when his ethylene glycol 24.8 mg/dL was reported. He was in renal failure (Cr 4.7) with poor urine output. On Day 2, the patient was hemodialyzed and fomepizole was continued. Day 3: dialysis was performed and sodium bicarbonate and norepinephrine were continued. CxR revealed severe lung consolidation. IV calcium replacement was administered. An EEG was consistent with encephalopathy. Insulin and insulin glargine were given for hyperglycemia. On day 5, the patient showed minimal response including gag reflex, corneal reflex and pupillary reflex. Repeat EEG showed moderate abnormality with increased generalized slowing, poor organization of background activity suggesting diffuse encephalopathic process. The family elected for comfort measures only and the patient expired on Day 7.
Autopsy Findings: Not available.
Case 85. Acute antifreeze (ethylene glycol) ingestion: undoubtedly responsible.
Scenario/substance: 50 y/o male found unresponsive in the trunk of a car was suspected of drinking ethylene glycol after finding that a friend had died from ethylene glycol ingestion. He was reportedly taking his wife's alprazolam as well. He was transported to the ED.
Physical Exam: In the ED he was unresponsive to pain and had a gag reflex and responsive pupils. Vital signs were “stable” and he was not intubated.
Laboratory Data: pH 7.06, HCO3 8, K 5.8, Cr 1.36. Urine: no crystals, no ketones. Calculated osmolality 299, anion gap 24. Ethanol, salicylate and acetaminophen not detected.
Clinical Course: No response to naloxone; started on fomepizole, folate, pyridoxine, and thiamine based on acidosis and recent history of a friend with ethylene glycol poisoning. He was retransferred to a tertiary care hospital, intubated and begun on hemodialysis. Labs were BUN 27, Cr 2.7, K 7.9, HCO3 < 10, AST 36, lactate 2.4, INR 1.2. He received sodium bicarbonate and calcium chloride IV. Ethylene glycol 26,089 mg/L. Despite dialysis and fomepizole he developed ARDS and/or aspiration pneumonia and expired on Day 2.
Autopsy Findings: No autopsy performed.
Case 87. Acute antifreeze (ethylene glycol) ingestion: probably responsible.
Scenario/Substances: A 51 y/o female was found unresponsive at home in the early morning with a suicide note after having been seen the night before. Her parents managed her medications because of concerns about overdose. EMS found her breathing spontaneously but unresponsive; unresponsive to naloxone 2 mg IM en route to the hospital.
Past Medical History: depression, bipolar disorder, prior suicide attempts. Medications included lithium, duloxetine, zolpidem, simvastatin, lorazepam, trazodone, rabeprazole.
Physical Exam: ED: GCS 3, BP 155/72, HR 98, RR 21 (intubated, on ventilator), T (rectal) 35.4°C, pupils 4–5 mm. Exam otherwise unremarkable.
Laboratory Data: ABG-pH 6.89/pCO2 27/pO2 524/HCO3 5.2 on FiO2 100%. Na 147, K 5.1, HCO3 7; BUN 12, Cr 1.36, glucose 295. osmolality 332, osmol gap 18 mmol. Ethanol, acetaminophen, salicylate all not detected. Lithium 0.03 mmol/L. ECG showed sinus rhythm, QRS 78 ms, QTc 472 ms, CxR mild pulmonary congestion. UA showed trace protein and blood, questionable crystals.
Clinical Course: Naloxone IV and flumazenil IV were given in the ED without response. Based on the severe acidosis and the osmol gap, the home was searched for antifreeze – the patient's daughter called the hospital to report she had found a ¾ empty 1-gallon container of antifreeze in the bathroom. In addition, the family found an empty bottle of ziprasidone that had been filled a week before. Ethylene glycol level from admission was 17 mg/dL, reported 2 days after arrival. Fomepizole was started and IV sodium bicarbonate was given. The family declined permission for dialysis. The next day the patent remained completely unresponsive and the Cr had increased to 3.81. The patient's family requested comfort measures only and the patient expired on Day 2.
Autopsy Findings: The ME determined the cause of death to be ethylene glycol toxicity.
Case 90. Acute ethylene glycol ingestion: undoubtedly responsible.
Scenario/Substances: A 55 y/o female ingested an unknown amount of ethylene glycol and was found at home unresponsive with posturing.
Past Medical History: Prior CVA; medications included estrogen, buspirone, and sertraline.
Physical Exam: Unresponsive, BP 146/90, HR 72.
Laboratory Data: Initial ED values: ABG-pH 6.69/pCO2 24/pO2 229/HCO3 2.8, lactate 53 mg/dL, anion gap 27, BUN 9, Cr 1.1, glucose 140 mg/dL, CK and troponin: normal. Day 1: WBC 23.6, glucose 308, Cr 2.0, BUN 11, pCO2 7, Na 150, K 6.1, ethanol 14 mg/dL, serum osmolality 466. Labs 10 hr after admission; lactic acid 30 mg/dL, pH, 7.1.
Day 2, ethylene glycol level 4570 mg/L.
Day 3: ethylene glycol level 211 mg/dL, BUN, 11; creatinine 1.9, CT head: small subdural hematoma.
Clinical Course: After transfer to the second hospital, IV sodium bicarbonate and fomepizole were given. Hemodialysis was started when the ethylene glycol level returned; fomepizole was continued. Seizures occurred and a lorazepam infusion was started; norepinephrine was required to support BP. On Day 3 hemodialysis was repeated Comfort measures were instituted on Day 4 and he expired on Day 5.
Autopsy Findings: A hospital blood sample contained ethylene glycol 5300 mg/L. No other substances were detected on toxicology screens. Blunt head trauma with an acute, small subdural hemorrhage was also found.
Case 98. Acute cyanide Unknown: undoubtedly responsible.
Scenario/Substances: A 72 y/o male intentionally ingested cyanide. A the suicide note at the scene warned responders of the presence of cyanide. The medical examiner's office requested guidance about the handling of the body.
Autopsy Findings: Pulmonary congestion and edema,
cerebral edema, lacerations, abrasions, and contusions throughout the body. Postmortem toxicology: cyanide 18 mg/L in femoral blood; carboxyhemoglobin saturation of heart blood 6.7%, and ethanol 0.04 g/100 mL in femoral blood. Cause of death was cyanide poisoning.
Case 107. Acute ethylene glycol monobutyl ether Inhalation/nasal: probably responsible.
Scenario/Substances: 25-y/o male inhaled the fumes from an aluminum cleaner while at work cleaning a truck 3 weeks prior to clinical evaluation. He became acutely ill, but recuperated in a couple of days and returned to work. Two days prior to presenting he returned to work, repeated the exposure and the symptoms returned but were more severe. The patient presented to his primary care physician's office with complaints of acute shortness of breath, cough, hemoptysis, dizziness, severe chest pains with deep breathing, and a sore throat. The patient was started on steroids and a budesonide/formoterol inhaler. He stated he felt better after 2 days of treatment, but his symptoms suddenly worsened during the night and his physician instructed him to go to the ED.
Past Medical History: He had history of depression, alcohol abuse, hypertension and 1-pack per day smoking for 8 years.
Physical Exam: On arrival in the ED, with shortness of breath, coughing, hemoptysis, dizziness, chest pain with cough, and a sore throat. bilateral lung crackles, tachycardia, and depressed affect. BP 118/69, HR 109, RR 25 and T 37.6°C.
Laboratory Data: Electrolytes, BUN, Cr, glucose, ALT, AST, CK, CK-MB, and, troponin were WNL. WBC 21.5, Hgb 8.9, Hct 26, ABG-pH 7.46/pCO2 33.9/pO2 67.3
Clinical Course: The patient was admitted with acute dyspnea secondary to chemical pneumonitis. His CxR showed diffuse bilateral alveolar infiltrates. He was started on methylprednisolone, ciprofloxacin, urosemide. He had hemolytic anemia. Day 4 he received 2 units of packed red blood cells early in the day and later became hypertensive and tachycardic with respiratory distress requiring intubation and ventilation and a propofol infusion. Day 6 a bumetanide infusion and dialysis for oliguric renal failure with metabolic acidosis, hyperkalemia and anemia. He was started on vancomycin and piperacillin with tazobactam infusions after blood cultures for fever. The patient had a cardiac arrest on Day 6, was resuscitated, but died on Day 7.
Autopsy Findings: Severe pulmonary congestion and edema related to pneumonia, fibrosis, and diffuse aveolar damage, hepatic inflammation with cirrhosis, severe renal edema and congestion. The cause of death was complications of cleaning chemical inhalation.
Case 115. Acute cleaner (alkali) Ingestion (with aspiration): undoubtedly responsible.
Scenario/substance: A 54 y/o male accidentally drank an alkaline degreaser solution that had been stored in a diet green tea plastic bottle. He presented to the local ED ∼30 min post-ingestion.
Past Medical History: obesity
Physical Exam: Respiratory distress with posterior oropharyngeal edema.
Clinical Course: The patient's airway continued to swell. Attempts to intubate were unsuccessful, as were attempts at cricothyrotomy; the anesthesia team performed an emergency tracheotomy. The patient suffered a cardiorespiratory arrest, and attempts at resuscitation were unsuccessful. The patient expired ∼2 hr post-ingestion.
Autopsy Findings: The patient had massive upper airway edema. The base of the tongue and epiglottis were erythematous and markedly edematous. The upper airway was occluded by the supraglottic tissues which had marked erythema and edema. The esophagus showed subtle mucosal erythema. The proximal fundus of the stomach had mucosal erythema and focal mucosal erosion. Aortic blood was negative for ethanol. The cause of death was determined to be asphyxia secondary to massive upper airway edema and laryngospasm secondary to sodium hydroxide ingestion and aspiration.
Case 119. Acute cleaner (acid) ingestion: undoubtedly responsible.
Scenario/Substances: A 70 y/o female was taken to the ED after a suspected ingestion of a toilet bowl cleaner containing 15–25 % hydrochloric acid, and resembled a smoothie drink often consumed by the patient.
Past Medical History: Alzheimer dementia and hypertension.
Clinical Course: The patient was awake and not complaining of any pain on examination in the ED, HR 120, RR 28. Initial K 5.9. The patient developed rapidly progressive abdominal pain and suffered perforation of her bowel. She was not taken to surgery and died within 24 hours after presentation.
Autopsy Findings: Extensive necrosis of stomach, duodenum and small bowel.
Case 130. Acute food poisoning, bacterial ingestion: probably responsible.
Scenario/Substances: 26 y/o male noticed gradual onset of cramping abdominal pain and development of watery diarrhea during the night and a temp of 38.7°C. The patient had eaten at a restaurant 3 days earlier which had been associated with an atypical E Coli food poisoning outbreak.
Past Medical History: Healthy; the patient denied any history of peptic ulcer disease or similar abdominal problems.
Physical Exam: The patient presented to the ED after awaking with bloody diarrhea. T 36.6°C, BP 147/85, HR 81, RR 20, O2 sat 97% on room air. Abdomen, peri umbilicular tenderness.
Laboratory Data: Bilirubin 1.7, AST 43, WBC 14.8, 79 segs and 8 bands, INR 1.01, PTT 26, Hgb 17.1, Hct 49%; calculated osmolality 278 mOsm/L. Day 2: WBC 48,000 with 22 % bands and 58 % segs; CO2 18;; Later bilirubin 1.5; Glucose 156; K 5.1; Hgb 18.4; Platelets 118, Hct 57.6 %; Urinalysis: nitrites positive, blood trace, white cells trace, ketones trace, glucose trace, protein 3+, casts 5–10 /hpf fine granular.
Clinical Course: Admitted to noncritical care unit to have stools checked for C. difficile, shigella, and salmonella as well as amylase, lipase, SED rate and a urinalysis. He received normal saline IV for 4 hours and then D5 1/2 normal saline with K. Started on metronidazole, levofloxacin and vancomycin. WBC increased to 29.0. Abdominal cramping with tenderness in the left and right lower quadrants. Day 2 transferred larger hospital where his condition deteriorated. A flexible sigmoidoscopy examination demonstrated normal appearing rectal mucosa and a large amount of blood in the colon. Ciprofloxacin therapy was initiated. Developed DIC, thrombocytopenia, ARDS, and cardiac arrest. Resuscitated successfully and hemodialysis initiated. The patient had a second cardiopulmonary arrest and expired on the Day 4.
Autopsy Findings: Hemorrhagic necrotizing colitis with relative rectosigmoid sparing, stomach and small bowel ischemic changes. Hemorrhagic mesenteric lymph nodes and serosanguinous ascites (1000 ml). Acute pneumonia with bilateral serosanguinous effusions (400 ml). Hemorrhagic infarction of the liver and the spleen was congested with hemorrhage and hilar vein thrombosis. Adrenal hemorrhagic necrosis. Heart demonstrated edema and ischemic changes. Cerebral edema with ischemic changes. Renal cortical necrosis and extreme edema. Ethanol was not detected. Multiple postmortem blood, colon and stool cultures were negative. A nasopharyngeal swab for virus culture was negative. Multiple tissue sections submitted to the CDC for immunohistochemical testing and serologic studies showed no evidence of infection with E. coli O111. Several hundred individuals became sick with an acute intestinal illness and ∼70 were hospitalized for severe bloody diarrhea and abdominal pain. Many patients subsequently tested positive for E. coli O111. A single restaurant where the patient dined was reported to be a common source.
Case 131. Acute foreign body ingestion: undoubtedly responsible.
Scenario/Substances: He tried to swallow cocaine packets while in police custody. Cardiac arrest before arrival to the ED and EMS resuscitated.
Physical Exam: In the ED: BP 98/67, HR 101,, O2 sat 97%. ET tube in place, not responsive, fixed pupils equal at 5–6 mm, muscle spasms.
Laboratory Data: Na 167, Cr 1.8, CK 3085. Urine drug screen positive for cocaine metabolite.
Clinical Course: ICU bronchoscopy showed small bags of cocaine which were removed. He became hypertensive and was treated with labetalol but never became responsive. Developed diabetes insipidus and renal insufficiency. Declared brain dead, was given, comfort care was instated and expired Day 4.
Autopsy Findings: Complications of foreign body obstruction, cocaine use, atherosclerotic heart disease, pulmonary emphysema, pleural effusions. Serum cocaine 0.16 mg/L, benzoylecgonine 2.84 mg/L, cocaethylene not detected.
Case 164. Acute carbon monoxide inhalation: undoubtedly responsible.
Scenario/Substances: 43 y/o male attempted suicide by redirecting the car exhaust into the passenger compartment. He was found pulseless, after being missing for 15 min. CPR was initiated and he was transferred to an ED where he was resuscitated. He was then transferred to a tertiary care facility for hyperbaric oxygen.
Past Medical History: Depression
Clinical Course: Vital signs at the tertiary care facility 200/120, HR 147, RR 14. The patient is intubated and unresponsive with a GCS 3. He had non specific movements of his head occasionally. Pupils were equal and reactive 3 mm. Otherwise exam was unremarkable. On admission the ABG-pH 7.39/pCO2 32/pO2 543, lactic acid 4.7 mMol/L
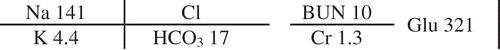
anion gap 18, troponin 0.22, WBC 24.2, Hgb 16.5, Hct 50.5, platelets 407, carboxyhemoglobin 8%. Day 2 troponin 3.51 and later to 6.52. AST 71, ALT 32, CK 702. He received hyperbaric oxygen therapy for 3 hours. He declined neurologically while in the ICU; his pupils became fixed and constricted and the corneal reflex was lost. His blood pressure became unstable despite fluid resuscitation and vasopressors. He experienced an episode of SVT treated with amiodarone. He failed an apnea test and, after discussion with the family, comfort measures were instituted and he expired.
Autopsy Findings: ME documented an initial Carboxyhemoglobin 50.5%.
Case 202. Acute mercury parenteral: undoubtedly responsible.
Scenario/Substances: A 36 y/o male apparently injected himself with elemental mercury an unknown time before arriving at ED. He had complaints of fever, diarrhea, headache, cough and pruritus.
Past Medical History: The patient had been to the ED about 10 days prior with complaints of fever, sore throat, generalized itching and a macular rash. He was given diphenhydramine, but symptoms did not improve.
Clinical Course: In the ED complaining of fever, diarrhea and headache on the left side. CxR and CT scan showed mercury deposits in the lungs and within the right heart cavities. Succimer and IV antibiotics were started on Day 3. On Day 5, the patient had nausea, emesis, diarrhea, numbness and tingling of the feet, weakness in the extremities, blurred vision and was producing blood-stained sputum. On Day 6, the patient's urine mercury was 552 mcg/dl, serum mercury was 224 mcg/dl, urine lead was 24 mcg/dl and cadmium was 6.1 mcg/dl. On Day 7, he was transferred to the ICU and oral n-acetylcysteine was begun. On Day 8, he was febrile, had diffuse bone pain, was agitated, tachycardic and had urticarial rash on his chest and abdomen. On Day 9, succimer was held due to leukopenia. His WBC was 1.9. On Day 11, his AST peaked at 465, his ALT peaked at 344 and his CK peaked at 43002. He was intubated on Day 10 and vasopressors were begun on Day 11. DMPS (2,3-dimercaptopropane-1-sulfonate) was started on Day 12, as well as dialysis. DMPS was discontinued on Day 16 because of hypotension. The patient was anuric by Day 17. He expired on Day 18.
Autopsy Findings: Focal myocardial necrosis, pulmonary edema and bilateral pleural effusion and ascites. Blood mercury level was 39 mcg/dl. Cause of death was acute elemental mercury poisoning. Manner of death was undetermined.
Case 203. Acute mercury inhalation/nasal: undoubtedly responsible.
Scenario/substance: A 55 y/o male was boiling off elemental mercury to isolate gold from electronic components. Over the next 2 days he developed flu like symptoms, progressive chest pain, shortness of breath and swollen gums. Sheriff's Department reported that methamphetamine laboratory chemicals and paraphernalia were also discovered at the site.
Physical Exam: In the ED, 2 days after exposure, HR 80s, T 38.1°C
Laboratory Data: ABG-pH 7.4/pCO2 33/pO2 51; WBC was 20.9, urine positive for THC and methamphetamine; urinalysis positive for trace protein, urobilinogen, blood +1; Cr 0.9, BUN 15. Mercury level on admission 1,108 mcg/L. All other laboratory values WNL.
Clinical Course: Patient admitted to ICU and placed on oxygen. ECG normal sinus rhythm with HR in the 80s. IV fluids were initiated prior to transfer to a larger hospital. Day 2 he was intubated and ventilated. IV fluids, oxygen and BAL were administered. CxR indicated ARDS. Day 5 penicillamine administration was added. Day 9 he was hypotensive and required epinephrine and norepinephrine. Chelation with succimer was initiated. He continued to deteriorate and penicillamine was discontinued. Comfort measures were instituted and he expired on Day 10.
Autopsy Findings: No autopsy was performed. Final cause of death listed as ARDS due to toxic effects of mercury vapor inhalation.
Case 204. Acute arsenic ingestion: undoubtedly responsible.
Scenario/Substances: 57 y/o male ingested 3 grams of arsenic pentoxide 5 hours prior to arrival at ED with hematemesis.
Physical Exam: BP 92/62, HR 102, RR 20, O2 sat 98%. Agitated, diaphoretic, anuric; HEENT: no burns; Lungs: clear, tachycardic; generalized abdominal tenderness and bloating, NEURO: nonfocal, SKIN: no rash
Laboratory Data: Initial local hospital:
WBC 16.6, Hgb 19.9, platelets 395, glucose 2598 mg/dL, Cr 2.0, K 2.6, HCO3 19, ALT 17. Tertiary care hospital: WBC 25.1, Hgb 16.7, glucose 39, Cr 1.8, K 2.6, lactate 8.2 mg/dL, pH 7.1, INR 1.3, troponin 17.5 ng/mL, urinary arsenic 2,800 μg/L, inorganic arsenic 264 μg/L, serum arsenic 252 μg/L.
Clinical Course: Upon transfer to the tertiary care hospital, NG aspiration of the metallic stomach contents seen on abdominal xray yielded a white residue. He was intubated and received BAL IM, IV fluids, potassium, norepinephrine and antibiotics. Endoscopy and CVVHD were performed. Day 2: Maximum rates of norepinephrine, dopamine and vasodopressin were required to support BP. The patient's family elected the institution of comfort measures and he expired on Day 2.
Autopsy Findings: Not available
Case 206. Acute on chronic chloroflurocarbon Inhalation/nasal: undoubtedly responsible.
Scenario/Substances: 19 y/o male witnessed at home inhaling fluorinated hydrocarbons multiple times on the day of presentation.
Past Medical History: Inhalant abuse
Physical Exam: Mechanically ventilated, BP120/72, HR 80, RR 16, T 36.7°C, O2 sat 88%. CxR showed interstitital edema which progressed to infiltrate.
Clinical Course: In ED had shortness of breath and chest pain; transferred to tertiary care hospital. Pulmonary embolism notfound, developed severe respiratory distress and hypoxemia which required intubation and ventilation with PEEP. He required high pressure ventilation with 100% oxygen for 9 days with failed attempts to wean. Fever prompted empiric antibiotics. Steroids were administered. Day 13: hypercarbia, hypoxemia, acidosis continued followed by a fatal cardiac arrest.
Autopsy Findings: not performed
Case 214. Acute charcoal lighter fluid injection: undoubtedly responsible.
Scenario/Substances: 40 y/o female came to the ED after intentionally injecting 40 ml of charcoal lighter fluid into her vein.
Past Medical History: History of prior suicide attempts, alcohol abuse, marijuana use, back surgery, knee surgery, s/p hysterectomy.
Physical Exam: Altered mental status, diaphoretic, systolic BP 99, HR 94.
Laboratory Data: ABG-pH 7.46, pCO2 31, pO2 70, serum bicarbonate 19, O2 sat 97% (3 L O2 NC). Salicylate, APAP, ethanol, urine pregnancy and urine tox negative. ECG: ST segment elevation.
Clinical Course: Admitted to ICU, tachypneic (RR 30–40), with abdominal pain. Respiratory status declined requiring intubation. Hypotension developed and IV vasopressin and phenylephrine were given which increased systolic BP to 94–103. Body temperature increased to 104.7 F and hyperglycemia developed. The patient expired 18 hours after presentation.
Autopsy Findings: No autopsy was performed. Cause of death was multiple organ failure and self injection IV with charcoal lighter fluid. Toxicology reported blood alcohol level of 0.10%.
Case 218. Acute mineral spirits ingestion (with aspiration): undoubtedly responsible.
Scenario/Substances: An 11 month old male became ill after ingesting an unknown amount of paint thinner. The product was in its original container, but the cap had been left off. A sibling had used the product to clean a floor and had misplaced the safety cap. The child experienced rapid onset of cough, altered mental status and an extended generalized tonic-clonic seizure of 40 min duration. Responding EMS reported finding an actively seizing child with HR 132 and RR 22.
Physical Exam: In the ED the child was unresponsive, cyanotic, actively seizing, HR 130, BP 85/61 and T 37.8°C. He exhibited a “strong paint thinner odor”.
Laboratory Data: A chest X-ray taken on arrival was consistent with ARDS.
Clinical Course: The child was intubated, ventilated and given bronchodilators in the ED. A pneumothorax required chest tubes. Adequate oxygenation could not be accomplished. An NG tube on back-aspiration revealed a paint thinner-like odor. Rectal diazepam was given for the seizures, which remained refractory to treatment. The child was anuric with “multi-organ system failure”. Death occurred 12 hours after presentation.
Autopsy Findings: Marked pulmonary edema and congested lungs. Hemorrhagic gastrointestinal fluids. Cerebral edema. A comprehensive postmortem blood toxicology screen was negative. Analysis of small intestines revealed the presence of petroleum distillates. Cause of death: multiple organ failure secondary to petroleum hydrocarbon toxicity.
Case 223. Acute mushrooms, cyclopeptides ingestion: undoubtedly responsible.
Scenario/Substances: 53 y/o female developed nausea and vomiting 1 day after picking, cooking and eating wild mushrooms. Symptoms began 10–12 hours after ingestion. She was brought to ED where they were concerned about possible Amanita mushroom poisoning.
Laboratory Data: AST 68, ALT 54, Cr 1.2, INR 1.1. Day 2: AST 304, ALT 223; Day 3 AST 2,060, ALT 1,723, pH 6.8, lactate 10 mmol/L, INR 5.5.
Clinical Course: Initial treatment was multiple dose activated charcoal (q 2 – 4 hr); Penicillin G 1,000,000/Kg/day IV divided every 8 hours for 24 hours, and cimetidine 300mg IV q 6 hr. Day 2: Increased LFTs and PCC recommended and she received N-acetylcysteine IV. Mental status was alert and oriented with resolution of GI complaints.
Day 3: Patient became lethargic, pH 7.1, intubation required, refractory hypotension requiring vasopressors, with HR 143. Dialysis was considered, but the patient was too hypotensive. Day 4: Multiple cardiac arrests occurred before the fatal arrest. A sample of the meal was examined by a mycologist who confirmed Amanita bisporigera.
Autopsy Findings: Severe hepatic necrosis.
Case 224. Acute mushrooms, cyclopeptides ingestion: probably responsible.
Scenario/Substances: 55 y/o female picked mushrooms while hiking in upstate New York; developed weakness, dizziness, nausea and vomiting 24 hr after ingestion; presented to the ED 2 days later.
Physical Exam: Abdominal tenderness.
Laboratory Data: Initial AST 4200, ALT 5000, INR >20 HCO3 10.
Clinical Course:
Admitted with fulminant hepatic failure 3 days after ingestion of wild mushrooms. Treatment with N-acetylcysteine started and transfer to a liver center. 6 hr after admission: BP declined, vasopressors and intubation required. Bleeding from venipuncture sites noted, patient was considered too unstable for transfer. Patient expired 14 hours after presentation from cardiac arrest.
Autopsy Findings: Cause of death was acute fulminant hepatic necrosis due to ingestion of poisonous mushroom.
Case 228. Acute paraquat ingestion: undoubtedly responsible.
Scenario/Substances: An 8 y/o male ingested an unknown blue herbicide that had been stored in a soda bottle.
Clinical Course: At initial ED presentation, he had normal vital signs and had 1 episode of vomiting, but was otherwise asymptomatic during evaluation. CxR 4 hours post ingestion was normal and he was sent home. The child developed increasing vomiting and returned to the ED 10 days later, ataxic, lethargic, and confused with continued emesis, HR, 110; RR 50–57, O2 sat 96% with O2 supplementation. Laboratory results at that time were:
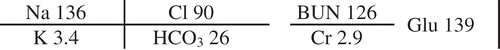
Salicylate was not detected, CK 58, AST 35, ALT 11, lactate 2.6 mg/dL, venous pH 7.44 and pCO2 40, UA showed 1+ Hgb. The herbicide was unavailable, but the parents believed the product was a chlorphenoxy compound. He was admitted for new onset renal failure, anion gap metabolic acidosis, altered mental status, hypoxia and tachypnea. A CxR revealed mediastinitis with a large right pneumothorax. During Day 1 vitals remained stable with improvement in clinical status. Paraquat was considered as a possible etiology, but no specific therapy was recommended as they were considered too risky given the unclear etiology and improving renal function. On Day 2, a chest tube was placed; IV fluids, electrolyte replacement, and supplemental oxygen were continued. Renal function continued to improve. His BP increased and nicardipine was initiated. On Day 3, chest tube removal was not tolerated and his oxygen requirement increased. A repeat CxR revealed increased consolidation and furosemide was administered for fluid overload. Alkalosis persisted; ABG pH was 7.53. Later that day, his respiratory status further declined (O2 sat 80%), and he was intubated. CxR revealed bilateral atelectasis, pulmonary edema, and questionable pulmonary fibrosis. Anemia was treated with packed red blood cells. On Day 4, paraquat was reconsidered as a potential cause of injury; unconventional therapies were discussed including cyclophosphamide in combination with high dose steroids. The pneumothorax recurred; antibiotics were started and high flow oscillator ventilation for poor oxygenation instituted; his lungs developed hemorrhage. Except for a low serum magnesium, his electrolytes, liver function, and renal function were normal. An ABG demonstrated pH, 7.43; pCO2, 48; and pO2 about 50.On Day 5, the CxR improved although ventilation remained difficult. There was no improvement in ABG; pO2 was only 27. Pulmonary surfactant was administered but not tolerated. Free air was discovered in his abdomen and epinephrine was added for a drop in BP and HR. Continued anemia was treated with additional packed red blood cells. On Day 6, the child's status further declined with continued decreases in O2, BP, HR, and intermittent changes in heart rhythm. On day 7, dopamine was started to maintain BP but he became hypertensive and died after an unsuccessful resuscitation.
Autopsy Findings: Pleural and peritoneal effusions. Lung parenchyma was moderately congested without obvious consolidation or focal lesions. Lung sections showed diffuse alveolar damage with fibrosis and intraalveolar hemorrhage. The small bronchi were plugged with mucus and acute inflammatory cells. Analysis of urine sample obtained at the final healthcare facility on day 14 from ingestion, revealed paraquat, 0.74 mcg/mL. Cause of death was diffuse alveolar damage and fibrosis with intraalveolar hemorrhage consistent with paraquat poisoning. The ME ruled the death as accidental secondary to respiratory failure due to paraquat ingestion.
Case 242. Acute copper sulfate ingestion: probably responsible.
Scenario/Substances: 59 y/o female may have ingested a copper sulfate containing root killer. The caller was not sure if this was a suicide attempt or if the product was diluted.
Past Medical History: Bipolar disorder.
Physical Exam: Patient was mumbling her words and difficult to understand. BP 220/110; Coffee ground-like emesis.
Laboratory Data: K 7.8. Post intubation ABG-pH 7.07/pCO2 76/pO2 396, Methemoglobin 2.5%.
Clinical Course: Treated for hypertension and hyperkalemia. Within a few hours, she became hypotensive and required hemodynamic support. Sodium bicarbonate given for acidosis. Within ∼8 hours from she became bradycardic, had a cardiac arrest and resuscitation was unsuccessful.
Autopsy Findings: Not available
Case 243. Acute strychnine ingestion: undoubtedly responsible.
Scenario/substance: A 61 y/o male ingested trazodone, propoxyphene-acetaminophen, hydrocodone, acetaminophen-diphenhydramine, beer, and arsenic powder. Initial symptoms included drowsiness, diaphoresis and multiple episodes of emesis. Emesis eventually became bloody.
Past Medical History: Depression
Physical Exam: Drowsy and vomiting, BP 141/74; HR 85, RR 18. Muscle contractions were present.
Laboratory Data: Initial electrolytes, liver function tests, and coagulation studies were normal. Ethanol was 30 mg/dL, acetaminophen 148 mg/L at 5 hr, salicylate was not detected.
Clinical Course: The patient received IV fluids, antiemetics, IV N-acetylcysteine and IM BAL. Within 5 hour of presentation, vomiting had subsided and mental status was normal. However, nurses reported an odd tremor. He went on to develop severe muscle spasms, hematuria, and delirium. About 8 hours later, the patient went into sudden cardiac arrest and resuscitation was unsuccessful.
Autopsy Findings: Postmortem venous blood: caffeine, dihydrocodeine 0.031 mg/L, hydrocodone 0.19 mg/L, propoxyphene 0.96 mg/L, norpropoxyphene 1.9 mg/L, oxycodone 0.060 mg/L, and strychnine 1.2 mg/L. No arsenic was detected. The death was attributed primarily to strychnine.
Case 250. Acute oxycodone ingestion: undoubtedly responsible.
Scenario/Substances: A 4 y/o female was found unresponsive and after a suspected carbon monoxide exposure. EMS found the child to be in full arrest, initiated ACLS resuscitation, gave naloxone, and transported her to the ED.
Clinical Course: Resuscitation was unsuccessful and the child was pronounced dead upon arrival to the ED.
Autopsy Findings: Subclavian blood: oxycodone > 2 mg/L (lethal per ME: 0.10 - 8.0 mg/L), oxymorphone 0.20 mg/L (oxycodone metabolite) and midazolam 0.54 mg/L (toxic per ME: 1.0 - 1.5 mg/L). Further investigation revealed the child had been playing with and is presumed to have ingested some of a grandparent's medication.
Case 253. Acute methadone ingestion, inhalation/nasal: undoubtedly responsible.
Scenario/substance: 12 y/o male found to be behaving abnormally and his 16 y/o brother suspected drug use. The next morning he was found unresponsive. The family found him pulseless and apneic and performed CPR and EMS.
Past Medical History: Attention-deficit hyperactivity disorder. Medications included amphetamine/ dextroamphetamine and atomoxetine.
Physical Exam: On initial presentation, the patient was in cardiac arrest. He was resuscitated to a pulse, 90’s; blood pressure, 60/30 to 110/60; T, 96° F.
Laboratory Data: During resuscitation, ABGs & electrolytes were ABG-pH 6.73/pCO2 47/pO2 574/HCO3 6.2,
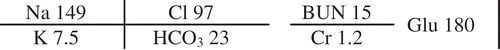
troponin was 1.9 ng/mL; CK, 393, MB fraction 23.2, myoglobin, 1957 mcg/L. His urine drug screen was positive for methadone, benzodiazepines, and THC.
Clinical Course: In the ED the patient received standard PALS care with a loading dose of phenobarbital. Naloxone and flumazenil were given with no response. Return of spontaneous circulation occurred 90 min after his arrest, but return of spontaneous respiration did not occur. After intubation and resuscitation, the patient was transferred to a tertiary care facility's ICU. On arrival, BP 78/56; HR 103, and he had significant cardiac dysfunction. He was on an induced hypothermia protocol, which was discontinued after 6 hours due to refractory hypotension. Treatment with dopamine, epinephrine, insulin, phenytoin, and steroids was provided. On Day 2 HR 102; BP 94/58; RR 24 (ventilator), T, 98.5° F with a warming blanket, Na 150, K 3.2; ionized Ca 5.4; BUN 33; Cr, 3; ALT, 2088; AST, 4729; bilirubin, 1.1; INR 2.94; lactate, 10.7 mmol/L. ABG-pH 7.2/pCO2 37/pO2 64/HCO3 14.7 /BE -13, phenytoin 11 mcg/mL. On Day 3 an EEG showed no cortical brain activity. A cerebral perfusion study on Day 4 supported the diagnosis of brain death. Comfort measures were instituted and he expired on Day 3.
Autopsy Findings: Bloody fluid (antemortem) methadone 0.24 mg/L, nicotine (trace amounts). The cause of death was methadone and benzodiazepine toxicity.
Case 266. Acute acetaminophen ingestion: undoubtedly responsible.
Scenario/Substances: A 17 y/o female arrived 28 hours post acetaminophen ingestion.
Past Medical History: Schizophrenia, bi-polar disorder, anxiety, prior suicide attempts. She was paraplegic from a previous motor vehicle collision. Medications included quetiapine, venlafaxine, escitalopram, and lorazepam.
Physical Exam: She presented afebrile with nausea and vomiting.
Laboratory Data: The initial acetaminophen level was 138 mcg/mL ∼28 hours post ingestion, AST 1,946, ALT 1,699, bilirubin 3.6 and PT 55.7. Day 2 AST 10,050, ALT 7,940, INR 12.6, bilirubin 4.5.
Clinical Course: IV N-acetylsteine was started and transferred for possible transplantation. Liver function and enzymes worsened. On Day 2 intracranial pressure began to rise and was treated with pentobarbital and mannitol. CT scan the following day showed herniation. Thefamily elected comfort measures only and the patient expired on Day 6.
Autopsy Findings: Cause of death was acute liver failure due to hepatic necrosis due to acute acetaminophen intoxication. Manner of death was suicide.
Case 281. Acute methadone ingestion: undoubtedly responsible.
Scenario/Substances: 19 y/o male found unresponsive at home without spontaneous pulse or respirations after suspected abuse of methadone. He received 20 min of CPR by EMS with return of pulses and was transported to the ED.
Past Medical History: No prior hospitalizations or surgeries.
Physical Exam: BP 120/70, HR 100, RR 16 on mechanical ventilation, pupils fixed at 5 to 6 mm.
Laboratory Data: Na 145, K 3.7, Cl 110, bicarbonate 21 (was 16), BUN 27, Cr1.5, PT 15.4, INR 1.42, CK 5,059, WBC 21.0, platelets 310, APAP & ASA not detected, head CT negative for acute intracranial event.
Clinical Course: The patient arrested again in the ED treated successfully with an additional 40 min of resuscitation. He was then transferred to a tertiary hospital. On the second Day he was given naloxone 8 mg resulting in tachycardia (from 50 to 90), hypertension (SBP 200 ) and piloerection. Methadone 92 ng/mL on admission and 54 ng/mL 48 hrs post ingestion. However his level of consciousness did not change (Glasgow coma score) and he remained ventilator dependent. His initial EEG was found to have burst suppression and subsequent EEGs were isoelectric. Based on the isoelectric EEG, negative cold calorics, brainstem reflexes and no other signs of brain function he was pronounced dead on Day 4.
Autopsy Findings: ME gave cause of death as methadone intoxication.
Case 288. Acute salicylate ingestion: undoubtedly responsible.
Scenario/Substances: 20 y/o female ingested salicylate 325 mg tablets. From a bottle of 500 tablets, 379 were missing.
Physical Exam: In the ED ∼14 hours after the ingestion she complained of nausea and vomiting and of being weak and tired. She denied tinnitus or abdominal pain. HR 67 to 110 (highest when she was actively vomiting), BP 124/85, RR 28, afebrile, O2 sat 100% on room air.
Laboratory Data: salicylate 101 mcg/mL, ABG-pH 7.49/pCO2 16/pO2 130, Na 143, K 4.3, Cl 105, HCO3 13, BUN 10, Cr 1.1, glucose 137, tox screen: negative for tricyclic antidepressants, positive for salicylate.
Clinical Course: Four hours after presentation to the ED (18 hours post ingestion) she had a seizure and arrested. While attempting to place a Quentin catheter for dialysis she developed a widened QRS, went into VT and then VF and was unable to be resusciated. Her organs were harvested for transplantation.
Autopsy Findings: External examination only due to tissue donation. Cause of death was severe metabolic acidosis due to acute salicylate intoxication. Antemortem blood salicylate was 700 mcg/mL.
Case 290. Acute methadone ingestion: undoubtedly responsible.
Scenario/Substances: A 20 y/o female presented in cardiac arrest after consuming unknown amounts of carisoprodol, alprazolam, and methadone. The medications were not believed to be hers. EMS found the patient cyanotic and cold to touch, pupils were fixed and dilated. During intubation gastric contents were noted in the pharynx. CPR was begun, she was found to be in VF, treated with defibrillation.
Past Medical History: chronic pain, anxiety, previous suicide attempts.
Clinical Course: ED: resuscitation that included naloxone (6 mg), epinephrine, and atropine was applied. The patient was declared dead before other suggested therapies could be attempted.
Autopsy Findings: External: several tattoos and piercings, multiple non-fatal contusions. Internal exam showed vascular congestion, patchy pneumonia, and cellular debris consistent with aspiration pneumonia. Myocardial ischemia on microscopy was attributed to hypotension. Aortic blood: methadone 0.27 mg/L, alprazolam 0.057 mg/L trace meprobamate and atropine. Ileac vein methadone 0.30 mg/L, Liver methadone 1.7 mg/kg. The cause of death was methadone toxicity.
Case 291. Acute fentanyl (transdermal) inhalation: undoubtedly responsible.
Scenario/Substances: A 20 y/o male was found unresponsive by his parents in his home after smoking a 25 mcg fentanyl patch in a tin foil pipe. He was found face down in his pillow in the bedroom. The father attempted CPR at home and in their car en route to the ED while the mother drove.
Past Medical History: drug abuse including alcohol and marijuana since 14 y/o, smoking heroin.
Physical Exam: On arrival in the ED, the patient was unresponsive, and asystolic. He received 4 mg of naloxone without response.
Clinical Course: Resuscitative efforts continued for 45 min in the ED without response. He was transported to a regional hospital. Lab: Na 138, Cl 108, lactic acid 4.6 mmol/L, Cr 2.0, AST 831, salicylate 4.8 mg/dL, acetaminophen not detected, urine toxicology screen negative. Absent brainstem reflexes. He was stabilized on pressor therapy with dopamine and norepinephrine. The patient was challenged with escalating doses of naloxone up to a total dose of 15 mg without response. He developed multi-organ failure with shock liver and acute renal failure. The parents requested comfort measures only and the patient expired on Day 2.
Autopsy Findings: Antemortem blood: negative for carisoprodol and meprobamate, fentanyl 9.6 ng/mL, norfentanyl 1.2 ng/mL. Cause of death: acute fentanyl intoxication.
Case 342. Acute salicylate ingestion: probably responsible.
Scenario/Substances: 25 y/o female found unresponsive. She was brought to the ED by EMS, who reported that all pill counts and medications were “accounted for.” The patient was unable to provide any history. It was mentioned that she had reported feeling hot earlier and had taken her clothing off.
Past Medical History: schizophrenia, bipolar disorder, and diabetes. Medications included risperidone, metformin, glipizide, atenolol, clonazepam and levothyroxine.
Physical Exam: Initial exam in the ED revealed BP 102/63, HR 164, RR 44, T 41.1°C. She was unconscious and in severe respiratory distress. Skin was warm and dry. Pupils were not recorded. Bowel sounds were present. There was no rigidity or abnormal muscle movements.
Laboratory Data: ABG-pH 7.47/pCO2 22/pO2 224 on 15 L nonrebreather mask. Na 143, K 3.5, CO2 17, BUN 27, Cr 1.9, CK 472, WBC 25.0.
Clinical Course: Serum salicylate was 96.5 mg/dL; ethanol nondetectable; urine tox screen negative. The patient was treated with IV fluids, sodium bicarbonate (boluses and infusion), and application of ice packs and a cooling blanket. The T fell to T 39.4°C. She remained deeply comatose. Before the planned hemodialysis could be carried out the patient developed complete heart block followed by asystole and could not be resuscitated. She expired ∼3 h after arrival in the ED.
Autopsy Findings: Not available.
Case 380. Acute on chronic acetaminophen/butalbital/ caffeine ingestion: undoubtedly responsible.
Scenario/Substances: 29 y/o male presented to hospital after an intentional ingestion of sertraline and acetaminophen/diphenhydramine combination tablet.
Past Medical History: Psychiatric disorder
Physical Exam: BP 175/99, HR 144 and afebrile. The patient was confused and agitated alternating with episodes of obtundation. Pupils were noted to be dilatated and the patient had positive bowel sounds. Patient was confused.
Laboratory Data: ALT 37, AST 20, troponin 0.015, ammonia 90 mM/L.
The maximal AST 447; ALT 95 on Day 3. EKG sinus tachycardia with a rate of 144, QRS 99, QTc 551.
Clinical Course: Intubated on presentation and given activated charcoal. He started on IV acetylcysteine initially 150 mg/kg over 1 h followed by 12.5 mg/kg for 4 h and 6.25 mg/kg for 16 h, the patient was converted to intermittent 70 mg/kg at ∼24 h post ingestion. When his acetaminophen levels started to rise he was started on whole bowel irrigation with polyethylene glycol solution. An attempt was made to extubate the patient on Day 2 but he vomited, aspirated and was subsequently reintubated. He had a myocardial infarction on Day 4, comfort measures were instituted and he expired.
Autopsy Findings: Cause of death: Complications of Acetaminophen intoxication. Antemortem blood acetaminophen 350 mg/mL, and sertraline 29 ng/mL.
Case 423. Acuity unknown methadone ingestion: probably responsible.
Scenario/Substances: A 32 y/o male found by family at home, not breathing, with no detectable pulse and evidence of vomiting and aspiration; was successfully resuscitated by EMS prior to ED arrival. Family reported the patient had been started on methadone less than a week earlier and increased dosage within the last few days. One day earlier, patient noted to have trouble breathing.
Physical Exam: Bradycardia.
Clinical Course: The patient received naloxone 6 mg which improved HR followed by naloxone infusion prior to transfer to another hospital. Upon arrival, hypotension required vasopressors; CT head: changes consistent with anoxic brain injury and herniation with compression of the brainstem. The patient expired 19 hr after presentation.
Autopsy Findings: Herniation of the cerebellar tonsils and symmetric flattening of gyri. Post-mortem blood: methadone 0.30 mcg/mL, methadone metabolite (EDDP) 0.19 mcg/mL.
Case 441. Acuity unknown fentanyl transdermal ingestion: Dermal: undoubtedly responsible.
Scenario/Substances: 34 y/o male was found dead in a car with family pictures and was taken to the medical examiner. Tablets of citalopram were found at the scene.
Autopsy Findings: Cerebral edema, pulmonary edema and early decompositional changes noted. Nine fentanyl patches (25 mcg/hr) found on left chest. Post-mortem blood: fentanyl 12 ng/mL, norfentanyl 6.2 ng/mL, alprazolam 0.25 mg/L. Cause of Death was mixed drug intoxication (fentanyl and alprazolam).
Case 515. Acute on chronic methadone ingestion: undoubtedly responsible.
Scenario/Substances: 41 y/o male was found cyanotic in coma, possibly down for 12 hours with a suicide note. He responded to naloxone and was placed on a continuous infusion. He said he took methadone 280 mg.
Past Medical History: seizures, COPD, heroin abuse, previous suicide attemps, prior gunshot to head, dilated cardiomyopathy. Medications included clonidine, albuterol, ibuprofen, and methadone
Physical Exam: T 33.9°C; after naloxone BP 116/49, HR 79, RR 25, and O2 sat 99% on 2 liters nasal O2.
Laboratory Data: ABG-pH 7.02/pCO2 46/pO2 135,
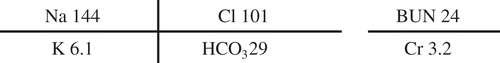
lactic acid 7.7, CK 20,000, QRS 142, QTc 449. Toxic alcohol panel was negative.
Clinical Course: Compartment syndrome in his upper extremeties treated with fasciotomy. He remained sedated, on the ventilator after surgery, with worsening oliguric renal failure and hypotension requiring norepinephrine. AST peaked at 3,281 and Cr at 6.6 mg/dl. He expired on Day 2.
Autopsy Findings: Severe hemorrhagic bilateral pulmonary edema. Pericardial blood methadone 0.63 mg/L and EDDP (methadone metabolite) 0.1 mg/L.
Case 518. Acute butalbital/ acetaminophen/caffeine ingestion: undoubtedly responsible.
Scenario/substance: A 41 y/o female was found unresponsive and cyanotic by her spouse. She had last been seen 24 hours prior. A bottle of acetaminophen with diphenhydramine was found near her bed.
Past Medical History: fibromyalgia depression. Medications included carisoprodol, acetaminophen with hydrocodone, and venlafaxine
Physical Exam: In the ED she was cyanotic, HR 90–100, T (rectal) 32.8°C; withdrew to pain and occasionally moved extremities.
Laboratory Data: ABG-pH 6.99/HCO3 10, CK 7488, INR 8.0; aPTT 78 seconds, acetaminophen 46 mcg/mL; salicylate 12.4 mg/dL; ethanol 57 mg/dL. Her urine drug screen was positive for cyclic antidepressants and benzodiazepines.
Clinical Course: In the ED, 4 mg naloxone and flumazenil were given with no response. She was intubated without any sedation and received sodium bicarbonate; norepinephrine, dopamine, and epinephrine. N-acetylcysteine and morphine infusions were begun. The patient's repeat HR 111; BP 84/37; RR 30. The initial EEG showed minimal brain activity. She remained hypotensive over the next 36 hours. She received fresh frozen plasma, whole blood, and insulin. She was begun on continuous veno-venous hemodiafiltration for refractory acidosis. Her CK rose to 38,480, troponin to 53.6, INR to 9, AST 7560, ALT 6876, glucose 760. She developed aspiration pneumonitis and pancreatitis. The family requested comfort measures only and the patient expired on Day 2.
Autopsy Findings: Massive centrilobular necrosis of the liver, moderate to severe bronchopneumonia, acute tubular necrosis of the kidney, full thickness necrosis and hemorrhage of the small intestine. Admission blood oxymorphone <0.05 mg/L. Femoral blood (antemortem): acetaminophen 53 mcg/mL, diphenhydramine 0.33 mcg/mL, hydroxyzine 0.076 mcg/mL, O-desmethylvenlafaxine 0.40 mcg/mL, venlafaxine 0.29 mcg/mL. Aortic blood (post-mortem): diphenhydramine 0.63 mcg/mL, O-desmethylvenlafaxine 0.75 mcg/mL, morphine 1.3 mg/L (likely from palliative care), venlafaxine 0.72 mcg/mL. Live: diphenhydramine 14 mg/kg, O-desmethylvenlafaxine 2.2 mg/kg, venlafaxine 3.5 mg/kg. The cause of death was multi-system organ failure due to oxymorphone, acetaminophen, and diphenhydramine toxicity.
Case 520. Acute fentanyl transdermal ingestion: undoubtedly responsible.
Scenario/Substances: 41-y/o white female presented to the ED with nausea and vomiting after an episode of coffee grounds emesis with epigastric and right upper quadrant “stabbing “pain. Admitted to the hospital a fentanyl patch applied. She ingested her fentanyl patch while outside on a smoking break. She returned to her room where she was later found unresponsive, bradycardic, hypotensive and apneic.
Past Medical History: Renal cell carcinoma, status post nephrectomy, ovarian cancer, right lower extremity deep venous thrombosis, gastroesophageal reflux, pancreatitis.
Physical Exam: HR 111, BP 136/97, RR 16, T 37.8°C, O2 sat 98% on 50% FiO2, PEEP 5 cm H2O. Intubated with coarse breath sounds, persistent left lateral gaze, pupils 3 mm, equal and reactive. GCS 5, responsive to deep tactile stimulation.
Laboratory Data: Hgb 11.1, WBC 22.4 INR 2.0, K 3.1, Ca 7.9, phosphate 2.2 mEq/L, albumin 2.9 gm/dL, AST 239, ALT 1,503, CK 4,610, CK-MB 64.6, troponin-I - 3.92, ABG-pH 7.35/pCO2 42/pO2 108/HCO3 23.1
Clinical Course: After resuscitation CPR, intubation, vasopressors, and naloxone infusion and transferred to the ICU. No response to naloxone and she was weaned from her vasopressors with maintenance of adequate blood pressure. Head CT showed no acute abnormality, echocardiogram showed diminished LV systolic function; acute renal failure and rhabdomyolysis developed which responded to bicarbonate and fluid resuscitation. She was subsequently found to have evidence of diffuse anoxic brain injury on head CT. Comfort measures were instituted and she in died in hospice.
Autopsy Findings: External examination only. Cause of death as listed by the coroner was complications of substance abuse.
Case 535. Chronic acetaminophen ingestion: undoubtedly responsible.
Scenario/substance: 42-y/o male alcoholic ingested acetaminophen hourly for 1 week to treat chronic pain.
Past Medical History: Alcoholism, pancreatitis, hypertension, hypothyroidism and antisocial personality disorder.
Physical Exam: In ED he presented with nausea, vomiting, anorexia, abdominal pain, dark urine. Alert and oriented, with jaundice, dehydration, tremor and ataxia.
Laboratory Data: Admission acetaminophen 76 μg/mL. AST peaked at 14,782 and then fell to 83 prior to death. Venous pH 7.18. Peak values Cr 5.2, INR 4.8, ammonia 131, bilirubin 26.5. K fell to 1.6.
Clinical Course: Treated with IV N-acetylcysteine, vitamin K, fresh frozen plasma and lactulose. Due to his alcoholism he was not a transplant candidate. On Day 2, the patient became somnolent and had encephalopathy (Grade IV) on Day 3 with oliguria elevated ammonia, unresponsive and required intubation. Developed renal failure, persistent acidosis, hypokalemia, and pancytopenia and was dialyzed. Insulin and multiple antibiotics administered. He deteriorated, mechanical ventilation was withdrawn and he was transferred to hospice for comfort care. He became bradycardic, hypotensive, arrested and expired on Day 10.
Autopsy Findings: No autopsy was performed.
Case 556. Acute hydrocodone, multiple drugs parenteral: undoubtedly responsible.
Scenario/Substances: 44 y/o female admitted 17 days earlier for COPD exacerbation and was to be discharged on Day 7, but had a witnessed grand mal seizure (no seizure history). The seizure was attributed to excessive meperedine administration, no further meperedine was administered, and no further seizure activity occurred. On Day 17, the patient was very anxious about her discharge and upcoming legal problems and complained of constipation and was noted to be diaphoretic and slightly tachycardic but her physical exam wasunremarkable. Later on Day 17she was cyanotic, dyspneic, diaphoretic and hypotensive with complaints of cramping and abdominal pain. A cup of white paste which appeared to be made from crushed tablets and water along with a syringe was found in her room. Bottles of hydrocodone with acetaminophen, tramadol, and promethazine, all of which had been filled the previous day, were found empty in her room. The patient denied injecting this mixture into her indwelling central line but a white powdery mixture was suctioned from her central line. She had apparently crushed 55 hydrocodone/ acetaminophen tablets, 33 tramadol tablets, and 20 promethazine tablets, mixed them with water and injected this mixture into her central line.
Past Medical History: Hypertension, asthma, COPD, HIV (14 + years), migraines with aura, chronic pain, head injury 4 years prior, anemia, bipolar depression, substance abuse history included opiates, cocaine, and tobacco. Her medications included efavirenz + emtricitabine +tenofovir combination product, paroxetine, salmeterol, and albuterol.
Physical Exam: Post injection, the patient was cyanotic and dyspneic, BP 91/57, HR 127, O2 sat 95% on nasal O2. Her lungs were clear without wheezes. She complained of cramping and abdominal pain, had diffuse, moderate tenderness in her lower abdomen with some bowel sounds in the LLQ. CT of chest, abdomen, and pelvis were negative for bowel infarction.
Laboratory Data: Urine drug screen was positive for opiates and benzodiazepines shortly after the injection. Glucose was 425 (repeat, 252), amylase 216, lipase 24, lactic acid 4.2 mmol/L, Na 128, K 7.6, Cl 92, HCO3 20, BUN 40, Cr 1.54, calcium 6.9, troponin and CK normal., albumin 2.5 g/dl, total bilirubin 2.6, AST 459, ALT 607, phosphorous 15 mg/dl. Initial
Clinical Course: The patient was transferred to the MICU. ECG after injection showed sinus tachycardia with mild ST depression which evolved to ST elevations and markedly peaked T waves 3.5 hrs later then signs of an acute anterior infarct 20min later. K was markedly elevated and calcium, insulin, and glucose were given. Sodium bicarbonate was administered for a worsening anion gap acidosis. Despite documentation of a “physiologic” acetaminophen level, n-acetylcysteine was started. The patient had a cardiac arrest, CPR including epinephrine, atropine, lidocaine, naloxone, amiodarone, was unsuccessful and the patient died.
Autopsy Findings: Aortic blood: hydrocodone 0.37 mg/L, morphine 0.076 mg/L, paroxetine 0.31 mg/L; atropine dihydrocodeine, lidocaine, N-desmethyltramadol, tramadol were present; and promethazine was trace. Iliac vein blood: hydrocodone 0.31 mg/L, morphine 0.063 mg/L, and tramadol 1.4 mg/L. Urine (bladder) hydrocodone 0.081 mg/L, morphine <0.050 mg/L. Lungs histopathology: massive small arterial vessel and capillary foreign bodies composed of refractile, polarizable, clear layered sheet like material and dark purple colored crystalline material surrounded by early thrombus formation. Scattered interstitial granulomata with similar material were noted. Other organ systems were unremarkable other than being consistent with preexisting disease states. The cause of death hydrocodone and tramadol toxicity in combination with acute massive micro-foreign body thromboemboli consistent with parenteral injection of medication.
Case 587. Acute on chronic salicylate ingestion: undoubtedly responsible.
Scenario/Substances: 47 y/o female with a 3 day history of headache, blurred vision and unsteady gait presented to the ED reporting having taken 4 baby aspirin tablets 3–4 times per day for an unknown period of time.
Past Medical History: Prior admission (6 mos earlier) for salicylate poisoning with cerebral edema requiring hemodialysis after intentional overdose.
Physical Exam: BP 133/82, HR 78, RR 16.
Laboratory Data: Venous blood gas: pH 7.47, PCO2 30, calculated HCO3 21.

WBC 7.8, Hgb 14.4, Hct 43, platelets 308, PT 10.4 sec, INR 1.0, APAP not detected, salicylate 46.6 mg/dL. ECG: normal sinus rhythm, QRS and QTc interval. CT head showed diffuse cerebral edema.
Clinical Course: IV sodium bicarbonate bolus then infusion was given to alkalinize the serum. Hemodialysis was initiated. 6 hr after admission, salicylate: 32 mg/dL; patient lethargic. Day 2: intubation, mechanical ventilation with sedation and paralysis performed. Salicylate: undetectable. ICP monitor showed increased pressure, therapeutic hypothermia and mannitol infusions. Day 6: CT head shows right-sided epidural hematoma which was surgically evacuted. Post operatively ICP was normal until Day 8 when ICP was again elevated and a myocardial infarction occurred. Pentobarbital coma was induced for ICP but the patient expired on Day 15.
Autopsy Findings: Cerebral swelling with bilateral tonsillar and left uncal herniation, evidence of hypoxic-ischemic encephalopathy, and small residual epidural hemorrhage; Severe coronary artery atherosclerosis, cardiac dilation and hypertrophy, left ventricular endocardial fibrosis, and severe aortic atherosclerosis: Acute bronchopneumonia, mild COPD, pulmonary congestion and edema, pleural effusions and ascites; Left renal artery stenosis with scarring of the left kidney. The cause of death was complications of acetylsalicylic acid overdose.
Case 588. Acute acetaminophen/hydrocodone ingestion: undoubtedly responsible.
Scenario/Substances: 47 y/o female was found down at home for an unknown time after taking an estimated 100 acetaminophen with hydrocodone and an unknown number of carisoprodol tablets.
Past Medical History: History of of chronic pain with increasing abdominal pain recently, fibromyalgia, obsessive/compulsive disorder and multiple prior suicide attempts.
Physical Exam: Obtunded and unresponsive; obvious respiratory effort; hypotensive with regular heart rate and rhythm.
Laboratory Data: Initial APAP level 396 mcg/mL, INR 1.7, liver transaminases in the 300’s. Day 2: INR 4.5 (after phytonadione). Later AST 14,012, ALT 2789, and ammonia 44 mcg/mL.
Clinical Course: The patient was intubated in the ED and given IV fluids for hypotension with no effect. The patient was transferred to a liver transplant center. Hepatic failure worsened, INR 12.1, bilirubin 5 mg/dL. Cerebreal edema was noted; the patient was denied transplant candidacy due to the history of drug dependence and non-compliance with medical directives.The patient remained unresponsive, experienced seizure activity and expired on Day 8.
Autopsy Findings: Blood: acetaminophen 434.4 mg/L, carisoprodol 12.2 mg/L, hydrocodone 0.86 mg/L, hydromorphone 26.4 mg/L. Cause of death: multi-organ failure due to acute intoxication with hydrocodone, acetaminophen and carisoprodol. Manner of death was suicide.
Case 607. Acute acetaminophen/caffeine/salicylate ingestion: undoubtedly responsible.
Scenario/Substances: A 48 y/o female ingested ∼30 tablets of Extra Strength Excedrin. She was taken to the ED ∼2 hours after the ingestion.
Physical Exam: On arrival in the ED awake and alert, and complaining of nausea and vomiting. T 36.4°C, BP 143/86, RR 20.
Laboratory Data: At 2.5 hours after ingestion, salicylate 36 mg/dL, acetaminophen 121 mg/L, AST 68, ALT 79, BUN 8, Cr 0.8, Na 143, K 3.6, HCO3 14.4, urine toxicology screen was negative.
Clinical Course: In the ED N-acetylcysteine was begun by NG tube and anti-emetics were given. Eight hours after presentation she became “restless and shaky and with cramps in the legs”. HR 146, RR 22–24. BP 149/100 and she was afebrile. A salicylate level 5.5 hours after presentation was 51.4 mg/dL and 81 mg/dL at 11 hours. Twelve hours after presentation she became agitated, HR 224. An ABG-pH 7.35, pCO2 12, HCO3 6.6. Intubation and ventilation were initiated. Hemodialysis was not performed on this patient. Following a dose of IV labetalol, given in an attempt to control heart rate, the patient suffered a cardiac arrest from which she could not be resuscitated.
Autopsy Findings: No autopsy was performed.
Case 612. Chronic acetaminophen ingestion: undoubtedly responsible.
Scenario/Substances: A 48 y/o female with chronic pain was taking acetaminophen (extended release) for headache 8 to 10 tablets or more on a daily basis. She was on a steadily decreasing dose of sustained release oxycodone 10 mg bid and fentanyl patch 25 mcg q 72 hours. Over 3 weeks, she developed very dark urine and became fatigued. PCP Dx dehydration and increased oral fluid intake. She became increasingly nauseated, and jaundiced. PCP referred her to the ED.
Past Medical History: History of hypertension, anxiety, chronic headaches, mechanical low back pain, degenerative knee arthritis, chronic narcotic use, ethanol, marijuana and crystal methamphetamine abuse. Medications included: fentanyl, sustained release oxycodone, acetaminophen, naproxen, alprazolam, clonazepam, quetiapine, olmesartan, gabapentin, fluoxetine, escitalopram, cyclobenzaprine and bismuth subsalicylate.
Physical Exam: In the ED the patient denied confusion, trouble walking or any motor weakness. No ascites noted. Admitted to the ICU, HR 108; mental status was intact, flapping asterixis, grossly jaundiced, icteric sclera and palmar erythema.
Laboratory Data: Acetaminophen not detected, INR 1.9 to 7.8; glucose 85 to 145; BUN 6 to 8; Cr 0.7 to 3.4; albumin 1.8 to 2.7; TProt 4.9 to 6.7; alk phos 200 to 300; bilirubin 14.1 to 17.7; AST 1246 to 4090; ALT 640 to 909; ammonia 79 to 90. Abdominal CT no portal hypertension small ascites related to inflammation.
Clinical Course: Hepatitis screens negative. She reported she had stopped taking acetaminophen ∼5 days prior to admission. Hepatic function and enzymes were abnormal. Admitted to ICU for IV N-acetylcysteine, later changing to oral dosing. Encephalopathic and was started on lactulose. On admission Cr 0.8 and BUN 8. Cr increased but not BUN, urine output was 90 cc. Decreasing mental status with hypotension resolved with IV fluids. Encephalopathy worsened and the lactulose was increased. Transferred to a liver transplant center, but denied due to social issues and BMI qualification. The patient's family elected the institution of comfort measures she expired on Day 14.
Autopsy Findings: Cause of death hepatic necrosis due to acetaminophen. Manner of death was accidental.
Case 633. Acute morphine ingestion: probably responsible.
Scenario/substance: A 51 y/o female was found to have altered level of consciousness by a friend at home. EMS found her apneic and asystolic, Initiated CPR, intubated, and gave epinephrine, atropine, and naloxone with return of sinus rhythm prior totransport to the ED.
Past Medical History: Fibromyalgia, chronic back pain, bipolar disorder, migraine headaches, meningitis. A friend reported that the patient was taking liquid morphine for chronic lower back pain and sometimes doubled up on her dose.
Medications included pregabalin, duloxetine, diazepam, acetaminophen/hydrocodone, and topiramate.
Physical Exam: ED: apneic with, fixed and dilated pupils, GCS 3, BP 71/41, HR 77, T (rectal) 34.4°C, O2 Sat 100%.
Laboratory Data: WBC 19.5, Hgb 9.9, K 5.9, HCO3 17, BUN 23, Cr 2.7, Glucose 322, AST 1330, ALT 1163, CK 2321, urine tox screen: positive for amphetamines, benzodiazepines and opiates;acetaminophen not detected.
Clinical Course: The patient received dopamine for hypotension, head CT suggested cerebral edema. She was admitted to the ICU where IV hydration and supportive care were continued. She had no change in her neurologic status over the course of several days, EEG was consistent with brain death. The patient's family requested comfort measures only and the patient expired.
Autopsy Findings: no significant abnormalities on gross examination. Microscopic examination: scattered aggregates of bile-like pigments consistent with aspiration. Premortem blood (afternoon of admission): diazepam 0.12 mg/l, nordiazepam 0.30 mg/l, d-methamphetamine 0.51 mg/l, d-amphetamine 0.13 mg/dl, morphine 0.84 mg/l, topiramate 3.6 mg/l, THC, fentanyl, and ethanol not detected, Cause of death: cardiopulmonary arrest due to acute multi-drug toxicity.
Case 689. Acute salicylate ingestion: probably responsible.
Scenario/substance: 55 year-old female was found by her husband and told him she had taken acetaminophen, aspirin and a cold medication.
Past Medical History: Depression
Laboratory Data: Initial acetaminophen 74 mcg/mL (unknown time after ingestion), salicylate 34 mg/dL. Later salicylate at unknown time 84 mg/dL. ABG-pH 7.43/PCO2 17/PO2 89/HCO3 11.
Clinical Course: Admitted to ICU. IV N- acetylcysteine given. Day 2: transferred to hospital floor, became febrile (T 40°C), with confusion, had a grand mal seizure followed by a cardiac arrest and death. The patient did not receive activated charcoal or sodium bicarbonate therapy.
Autopsy Findings: Post-mortem blood: salicylate 83 mg/dL, acetaminophen 39 mg/L. doxylamine 0.09 mg/L; urine drug screen negative. Cause of death was acute salicylate toxicity with pulmonary edema.
Case 703. Acute salicylate ingestion: undoubtedly responsible.
Scenario/Substances: 56 y/o male intentionally ingested 9 grams of aspirin, developed nausea and vomiting and was transported to the ED three hours post ingestion.
Physical Exam: Awake, alert and oriented. BP 202/78 HR 95 RR 30–40 (initial), then 20, 96% O2 sat. T 36.9°C.
Laboratory Data:
ABG-pH 7.51/pCO2 27.1/pO2 107 (2L, NC)

WBC 19.6, Hgb 18.0, platelets 233, PTT 32.7, INR 1.0. APAP not detected, ASA 85.6 mg/dL. Urine pH 6.5 prior to IV sodium bicarbonate.
Clinical Course: Serial oral activated charcoal and nephrology consultation for dialysis recommended on admission. Repeat salicylate 7 hours post ingestion: 116 mg/dL; ABG-pH 7.46/pCO2 21/pO2 56/HCO3 15; glucose 451 mg/dL. IV fluids and sodium bicarbonate given continuously from ED arrival; insulin infusion later added for hyperglycemia. 10 hr post ingestion: agitation required sedation, while being prepared for dialysis, the patient arrested and expired.
Autopsy Findings: No autopsy was performed. Toxicology listed salicylate 994 without units but a normal range of 20–300 was provided by the testing laboratory.
Case 766. Acute salicylate ingestion: probably responsible.
Scenario/Substances: A 73 y/o female was brought to an ED with altered mental status. Her husband reported to ED staff that she may have consumed unknown amount of salicylate over an unknown period of time.
Past Medical History: Nonspecific psychiatric history.
Physical Exam: Awake but non-verbal. BP 150/57, HR 98, RR 28 and O2 sat 99%.
Laboratory Data: Salicylate 113 mg/dL, ABG-pH 7.4/pCO2 27/HCO3 17.9, BUN 33, Cr 1.1, WBC 21.0.
Clinical Course: She developed irreversible cardiac arrest about 2 hours after presentation as she was being transported to have a dialysis catheter inserted.
Autopsy Findings: No structural cause of death. Femoral blood salicylate 98.5 mg/dL. Jugular blood salicylate 116 mg/dL, naproxen 78 mcg/mL, donepezil was 0.11 mcg/mL. Coroner cause of death was suicide caused by multiple drug ingestion.
Case 819. Acute bupropion ingestion: undoubtedly responsible.
Scenario/Substances: 17 y/o female ingested about 190 bupropion 150 mg tablets.
Past Medical History: Previous history of bupropion overdose with seizure activity.
Clinical Course: Admitted to the ICU and intubated, ventilated and IV fluids. Hypotension treated with vasopressors. Partial complex seizures were unsuccessfully treated with fosphenytoin and benzodiazpines. After successful cardioconversion, 20% Intralipid therapy was initiated. Despite aggressive supportive care the patient expired within 12 hours of hospitalization.
Autopsy Findings: Recent contusion at the tip of the tongue. Pulmonary congestion and edema with pleural effusions, ascites and pericardial effusion. Multiple scars on the front of the forearms/wrists. 144 tablets in the stomach and 43 tablets present in the small intestine. Cause of death was seizure disorder due to bupropion overdose. Manner of death was suicide.
Case 821. Acute on chronic bupropion ingestion: undoubtedly responsible.
Scenario/Substances: An 18 y/o male took bupropion and gabapentinin as a suicide gesture. He had called and texted several friends and his mother after the ingestion.
Past Medical History: Bipolar disease in the manic phase, suicidal ideation with several involuntary holds, first occurring in the 4th grade, marijuana use. Medications included bupropion, aripiprazole, gabapentin and lorazepam.
Clinical Course: When he arrived in the ED by ambulance which he had called himself he was awake and talking. HR 111 BP 138/78, RR 16, T 37.7°C, O2 sat 98% on room air. Electrolytes were normal, HCO3 was 17. Three hours post ingestion his speech was slurred and he had difficulty following directions. At 3.5 hr he had a grand-mal seizure. He received activated charcoal and was placed in 4-point soft restraints. Seizures increased in frequency until he was in status. He developed a wide complex bradycardia and arrested. Resuscitation attempts with atropine, epinephrine and sodium bicarbonate were unsuccessful. A pacemaker was placed but he could not be resuscitated and died about 5.5 hours after the ingestion.
Autopsy Findings: Severe pulmonary congestion and edema, gastric contents consistent with activated charcoal administration, Wellbutrin tablet fragments in the gastric contents. Blood concentrations : bupropion 2200 ng/mL, hydroxybupropion 2600 ng/mL, aripiprazole 330 ng/mL, lorazepam 22 ng/mL. No other drugs were detected. Cause of death acute bupropion toxicity.
Case 824. Acute on chronic antidepressant ingestion: contributory.
Scenario/substance: 19 y/o female found by her parents in her room with a decreased level of consciousness after taking an unknown amount of her own bupropion extended release tablets and clonazepam. A suicide note was found and her left wrist had a laceration. EMS transported her to the ED.
Past Medical History: morbid obesity, depression, bipolar disorder, and previous suicide attempts.
Physical Exam: The patient was drowsy with an abnormal affect upon arrival in the ED. Her pupils were equal and reactive to light. T (axillary) 39.3°C, BP 129/70, sinus tachycardia 115, RR 16.
Laboratory Data: APAP, ASA and EtOH not detected. Her urine drug screen was positive for benzodiazepines. Na 153, K 6.3, CK 1974, ABG-pH 6.3/pCO2 93.
Clinical Course: 125 grams of activated charcoal were given via NG tube 1 hour after admission. 1 hour after receiving naloxone and flumazenil she had 3 seizures. She was paralyzed, sedated and intubated. A norepinephrine infusion was started for hypotension and she was admitted to the ICU. She was given a loading dose of phenytoin, which was replaced with propofol and alorazepam infusion. She received multiple antibiotics for positive sputum cultures, chest tube insertions for bilateral pneumothoraces. By Day 9 she had develoed ARDS and extensive subcutaneous emphysema, hyperglycemia, and electrolyte imbalance. Comfort measures were instituted and she expired on Day 12.
Autopsy Findings: The lung parenchyma was firm, variegated red and exuded copious amounts of edema fluid with several thromboemboli in the small arteries, and organization. Microscopic analysis showed an organizing pneumonia, alveolar edema and hyalie membrane formation, with acute pulmonary thromboemboli. The liver showed shock liver with certilobular necrosis. The gross examination of the brain was normal. The cause of death was reported to be complications of drug overdose, with morbid obesity being a contributing factor. The manner of death was suicide.
Case 836. Acute olanzapine, carvedilol, bupropion ingestion: probably responsible.
Scenario/Substances: 29 y/o male found cyanotic and unresponsive at home with 3 empty medication bottles (olanzapine, carvedilol, bupropion) nearby. Patient was last seen awake and alert the evening prior to arrival. EMS gave 2 mg of naloxone IV, oxygen and 50% dextrose without effect.
Past Medical History: Substance abuse.
Physical Exam: Unresponsive, dyspneic, with agonal respirations, BP 117/74, HR 70, RR 16, T 33.9°C, O2 sat 93%, pupils 2–3 mm, minimally responsive. Neuro: rigidity, minimally responsive to noxious stimuli and sternal rub. Laboratory Data:

Ca 8.4, T. Tprot 7.3, albumin 4, bilirubin 0.2, AST 24, ALT 36, CPK 136, INR 0.4, PTT 30.6, ABG-pH 7.44/pO2 138/pCO2 37, O2 sat. 99.3%; urine pH 6; specific gravity 1.025, CPK MB 1.2, troponin I 0, WBC 4.1, Hgb 14.8, Hct 42.6, platelets 161, ethanol 104 mg/dL, APAP negative; ASA 3.3 mg/dL, urine tox negative for benzodiazepines, barbiturates, opioids, cocaine, amphetamine, PCP, methadone and THC. CT head and CxR were unremarkable.
Clinical Course: O2 and IV fluids were given in ED. Clinical deterioration ensured over the next few hours; the patient expired on Day 2 from cardiac arrest.
Autopsy Findings: Bupropion: femoral blood 8.49 mg/L; antemortem blood 0.63 mg/L; liver 6.05 mg/Kg; gastric contents 397 mg recovered. Olanzapine: femoral blood 0.17 mg/L; antemortem blood 1.03 mg/L; liver 4.73 mg/Kg
gastric contents 12.1 mg recovered. ethanol: antemortem blood 0.08 g%; blood, vitreous: not detected.
Phenytoin: femoral blood 14.7 mg/L. Lorazepam: blood: therapeutic concentration.
Case 844. Acute on chronic antidepressant ingestion: undoubtedly responsible.
Scenario/substance: A 36 y/o female was reported to intentionally ingest 21 bupropion (extended release) 300 mg, 21 sertraline 100 mg, and 21 cyclobenzaprine 10mg. She was transported by EMS to ED.
Past Medical History: Bipolar disorder, cocaine abuse, prior drug overdoses including 1 less than 1 month earlier. Medications included bupropion, sertraline, and cyclobenzaprine.
Physical Exam: Initially, the patient was slightly drowsy HR 128. She was also noted to have tremors, and possible seizure during transport
Laboratory Data: Low serum glucose, elevated CK, normal cardiac enzymes, urine drug screen positive for cocaine.
Clinical Course: Within 30 min of arrival, the patient became markedly agitated and combative; given lorazepam, haloperidol, and diphenhydramine. Cardiac arrest followed 5 min after onset of agitation. Resuscitation, including intubation, sodium bicarbonate, atropine, and epinephrine, was unsuccessful. She expired within 1 hour of initial presentation.
Autopsy Findings: Autopsy intact tablets of bupropion in her stomach (7 tablets). Aortic blood: for bupropion 3.0 mg/L, threobuproprion > 40 mg/L, sertraline 1.4 mg/L, norsertraline 2.0 mg/L, benzoylecgonine 0.23 mg/L, cocaethylene 0.024 mg/L, and diphenhydramine 0.28 mg/L. Atropine, erythro bupropion, and morpholinol bupropion were detected, but not quantified, cycyclobenzaprine of < 0.25 mg/L and cocaine and ethanol were not detected. Vena cava blood demonstrated bupropion 2.3 mg/L, norsertraline 7.5 mg/L, sertraline 4.7 mg/L and threobupropion 22 mg/L. Liver: norsertraline of 36 mg/kg, sertraline of 13 mg/kg and threobupropion of 140 mg/kg. Death was felt to be a due to a combination of bupropion, sertraline and cocaine.
Case 866. Acute sertraline ingestion: probably responsible.
Scenario/substance: A 31-y/o female was found unresponsive and cyanotic at home by her boyfriend. According to the boyfriend the patient had prescriptions for hydrocodone, carisoprodol and diazepam. EMS found her in asystole. She responded to epinephrine with return of spontaneous circulation, unresponsive to naloxone or to D50 given for a blood glucose of 28 mg/dL and she was transported to the ED.
Past Medical History: depression, anxiety
Physical Exam: Atraumatic, GCS was 3, pupils fixed and dilated, lungs clear tachycardia.
Laboratory Data: WBC 12, K 6, Cr 2.6, glucose 140, AST 800, ALT 500, troponin 0.33, CK 336. Urine toxicology screen positive for benzodiazepines and opiates; APAP, ASA and EtOH were not detected.
Clinical Course: She was intubated upon arrival in the ED. Head CT showed no acute intracranial injury. She became hypotensive, dopamine infusion was started and she was admitted to the ICU. BP 151/106, HR 110, T 37.1°C, O2 Sat 87% bagged. Norepinephrine was added for pressor support, but her condition continued to deteriorate and she was noted to be posturing on Day 3. EEG's showed no significant brain activity. The patient's family elected the institution of comfort measures she expired on Day 7.
Autopsy Findings: Ischemic infarction of basal ganglia, citalopram 1300 ng/mL, urine positive for oxazepam, temazepam, morphine and hydrocodone. Cause of death “narcotic and sedative drug intoxication.”
Case 914. Acute on chronic venlafaxine (extended release): ingestion: undoubtedly responsible.
Scenario/Substances: A 58 y/o female was found in her car with lacerations to her wrists. She reported taking a large number of tablets (later determined to be ∼100 tablets of extended release venlafaxine 150 mg). She seized in the ambulance en route to the ED.
Past Medical History: Depression
Physical Exam: Altered mental status (postictal), HR 150’s and hypotensive, volar wrist lacerations bilaterally.
Laboratory Data: Initial: K 3.1, lactate 2.0 mmol/L, ABG-pH 7.29/pCO2 49/pO2 67/HCO3 23. Several hours later ABG-pH 7.33/pCO2 44/pO2 125/HCO3 22; CK (maximum) 451, troponin 1.42. From day of admission, serum venlafaxine 20,000 ng/mL Carbon monoxide and urine drugs of abuse screen were negative.
Clinical Course: She was intubated and received adenosine for SVT without effect. Fluid and norepinephrine were given for hypotension. Whole bowel irrigation was started and then stopped due to problems with gastric/duodenal access. Further investigation revealed a large bezoar, most of which was removed endoscopically and found to be comprised, at least in part, of venlafaxine. The patient developed symptoms of cardiac failure ∼24 hours into the course, with an ejection fraction of < 15%. Insulin infusion was started and intraaortic balloon pump placed. She went to the cardiac catheterization lab to determine possible CAD and had a cardiac arrest in the lab. She was resuscitated and placed on dopamine. Comfort measures were instituted on Day 5 and she expired.
Autopsy Findings: Cause of death was listed as venlafaxine overdose.
Case 928. Acute diphenhydramine ingestion: undoubtedly responsible.
Scenario/Substances: A 25 y/o male was found unconscious on the floor of his apartment. Near his body were empty boxes originally containing 120 capsules of diphenhydramine 25 mg (3 g total)
Past Medical History: depression, psoriasis.
Physical Exam: On transport to the ED, the patient was unconscious. T 42.2°C, HR 80–110, BP 94/48.
Laboratory Data: ECG QRS prolonged, AST 2052, total bilirubin 1.4, BUN 29, Cr 2.5. Ethanol, APAP and salicylate were undetected. CK 208,000.
Clinical Course: IV hydration and sodium bicarbonate was given, with subsequent narrowing of the QRS width. 90 min after presentation, T was 43.3°C. Pancuronium was administered for hyperthermia, but was not effective.
Autopsy Findings: Postmortem toxicology revealed a diphenhydramine level of 7.2 mg/L. (based on a Vd of 3.5 L/kg this 86.4 kg body contained 2.18 g of diphenhydramine). No other toxic, traumatic or other cause of death was revealed by autopsy.
Case 943. Acute on chronic antineoplastic drug ingestion: undoubtedly responsible.
Scenario/Substances: 58 y/o female was prescribed methotrexate once weekly but took it before dialysis three times a week for unknown time period.
Past Medical History: Rheumatoid arthritis, end stage renal disease on hemodialysis
Laboratory Data: WBC 0.3, platelets 20, Hgb 11.5.
Clinical Course: Patient was hospitalized for 2 days before the dosing error was discovered and admission labs returned. Leucovorin 180 mg IV was given and dialysis performed. Hemorrhagic shock occurred with bleeding from all orifices and into soft tissues. The family decided to withdraw medical care and the patient expired
Autopsy Findings: Not available
Case 944. Acute methotrexate injection: probably responsible
Scenario/Substances: 67 y/o male admitted for scheduled chemotherapy with high-dose methotrexate (MTX) 20 g and bevacizumab for glioblastoma. Renal function was adequate and urine alkalinization was accomplished prior to MTX infusion. One day after the infusion an abnormally high serum level of MTX was found; the patient was confused and slightly agitated.
Physical Exam: Confused, GCS 14; BP 118/68, HR 78, RR 21 T 37.1 C.
Past Medical History: Glioblastoma multiforme, congestive heart failure, CAD, hypertension, gout.
Laboratory Data: Initial: BUN 3, Cr 0.7: Post-infusion: MTX 403 umol/L (toxic at >1.0 umol/L); 12–24 h post infusion: WBC 2.5, platelets 44, Hct 31.
Clinical Course: High-dose leucovorin, sodium bicarbonate infusion, and continuous hemodialysis were applied. Serum MTX declined over 16 hours to 1.43 umol/L. The MTX concentrations fluctuated between 0.33 and2.09 umol/L. The course was complicated by, mental status changes, anuria, renal failure, pancytopenia, sepsis, and atrial fibrillation. One dose of carboxypeptidase was administered. Despite resolution of toxic MTX levels, mental status declined, multisystem organ failure occurred. Comfort measures were instituted and she expired on Day 62.
Autopsy Findings: autopsy not performed.
Case 950. Acute metoprolol and flecainide ingestion: undoubtedly responsible.
Scenario/Substances: 14 y/o male ingested unknown amounts of his father's metoprolol and flecainide. Two empty bottles of flecainide and 1 empty bottle of metoprolol were found at the scene. The patient's citalopram medication bottle did not appear to have any missing medication. Prehospital the patient was awake and talking.
Past Medical History: Depression with treatment that included citalopram.
Physical Exam: In the ED: unresponsive, hypotensive (weak pulse), and bradycardic.
Laboratory Data: ECG showed a wide complex bradycardia and a long QTc.
Clinical Course: Upon arrival in ED he had a generalized seizure and remained unresponsive, intubated. He was never tachycardic. Resuscitation included epinephrine, atropine, sodium bicarbonate, high dose insulin, calcium chloride, and glucagon, but the patient remained pulseless and he was pronounced dead in the ED.
Autopsy Findings: Blood concentrations: flecainide 12 mcg/mL, citalopram 420 ng/mL, metoprolol 140 ng/mL. Cause of death was mixed drug overdose.
Case 959. Acute ingestion of a cardiac glycoside-containing aphrodisiac (Piedra): undoubtedly responsible
Scenario/Substances: 35 y/o male took one pill of “piedra”, believed to be an aphrodisiac. EMS found him with chest and epigastric pain of several hours duration and brought him to the ED.
Physical Exam: Bradycardia and hypotension in the field treated with atropine 1 mg. ED: BP 118/66, HR 110
Laboratory Data:
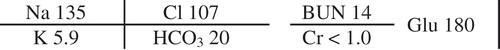
Digoxin 2.9 mcg/mL, initial K 7, WBC 20.5, Hct 48, platelets 484. ECG: narrow complexes with a variable AV block after atropine.
Clinical Course: The patient was treated with insulin, glucose, bicarbonate and albuterol. Bradycardia recurred, atropine 0.5 mg was given and improved BP and HR. Based on the history, bufotenin was suspected based on prior local reports of cardiac glycoside poisoning with ingestion of a resin-like aphrodisiac intended for topical use called “rock hard” or “love stone” or “piedra”. Digibind, 10 vials, was given with improvement in nausea, vomiting and abdominal pain, BP and HR. Over the next 20 hrs, bradycardia, VT, and varying degrees of AV block recurred. Digibind fragments were given by slow IV infusion plus bolus of 1–2 vials as needed for vital sign instability. 35 vials in total were given as well as 2–3 doses of activated charcoal q 4 hr to the 3rd dose which produced emesis, treated by metoclopramide and normal saline IV. On Day 2 after complaining of thirst, VT developed into VF which, despite ACLS protocols with the inclusion of digibind IV, was fatal.
Autopsy Findings: Not available
Case 979. Acute verapamil ingestion: undoubtedly responsible.
Scenario/Substances: 47 y/o female presented to the ED 15 hr after self-reported ingestion of verapamil with the intention of self harm. A history of several episodes of vomiting prior to ED arrival was obtained.
Past Medical History: Medications: sucralafate, escitalopram, atorvastatin, acetaminophen/ hydrocodone, omeprazole, polyethylene glycol, sennacot, montelukast, zolpidem, synthroid, and vitamin B12.
Physical Exam: Awake, lethargic; BP 91/68, HR 41.
Laboratory Data: ABG-pH 7.24/pCO2 44/pO2 70, salicylate 2.8 mg/dL; glucose 113; BUN 85; Cr 3.6.
Clinical Course: The patient quickly became hypotensive to systolic BP 56; ECG: sinus bradycardia with first degree heart block, QRS 154 ms, QTc 366 ms. 1mg of atropine and 1 gram of calcium gluconate were given without response. Vasopressor and high-dose insulin therapy was started as the patient remained awake and anxious. 12 hours after admission the patient became unresponsive, required intubation.
Day 2, echocardiogram revealed left ventricular ejection fraction of 77%. ECG: remained junctional rhythm, mean arterial pressure in the 60s on vasopressors. CxR revealed non-cardiogenic pulmonary edema. Renal function declined: 38 hr after admission, patient had a fatal asystolic cardiac arrest.
Autopsy Findings: The cause of death was determined to be secondary to complications of an acute verapamil overdose.
Post mortem whole blood revealed verapamil 2685 ng/mL, norverapamil 1733 ng/mL.
Case 1009. Acute on chronic beta blocker ingestion: undoubtedly responsible.
Scenario/Substances: 55 y/o male ingested a full bottle of his medication, refused to go to the hospital and family called EMS after he stood up and collapsed.
Past Medical History: Hypertension
Physical Exam: ED initial: nonpalpable BP, HR 60, after glucagon BP 105, temp 36.2°C, pupils fixed and dilated.
Laboratory Data: Hgb 7.8, RBC 2.23, WBC 3.1, platelets 93. Labs repeated Hgb 13.9, RBC 3.98, WBC 5.6, platelets 139, ABG-pH 7.22/pCO2 38/pO2 110, blood ethanol 322.
Clinical Course: EMS observed agonal breathing, unconscious, unresponsive and sinus bradycardia of 53. IV naloxone, normal saline, glucagon, pacer pads, intubated. The family requested DNR and life support was removed.
Autopsy Findings: Cardiomegaly, fibrosis and fatty changes of liver with pulmonary and systemic visceral congestion. Mild myocyte hypertrophy and focal interstitial fibrosis of the myopericardium, portal tracts exhibited lymphocyte infiltrate with fibrosis and marked hepatosteatosis. Post mortem blood: y ethanol 187, metoprolol 95,000 ng/mL, delta-9 THC 5.8 ng/mL, and positive for nicotine and cotinine. Cause of death was suicide from overdose of metoprolol.
Case 1045. Acute amiodarone ingestion: undoubtedly responsible.
Scenario/Substances: 82 y/o female presented to the hospital after an intentional ingestion of 28 tablets of amiodarone 200 mg.
Past Medical History: Arthritis, arrhythmia, chronic renal insufficiency and insulin-dependant diabetes mellitus.
Physical Exam: The patient was obtunded, hypothermic BP 70/30; HR 30–40.
Laboratory Data: APAP & ASA not detected, tricyclic antidepressant screen was negative; urine drug screen was negative; troponin was negative; bicarbonate 15 mEq/L; pH 7.38; pCO2 34; pO2 157; BUN ; Cr 2.5 ; glucose 135, Glucose declined to 40. EKG bradycardia with a rate of 40, QRS 120.
Clinical Course: Intubated on presentation, given activated charcoal and started on sodium bicarbonate infusion for the prolonged QRS, given a bolus of dextrose, atropine and an infusion of glucagon and insulin, and dopamine. Whole bowel irrigation with polyethylene glycol. The patient initially responded, but hypotension and bradycardia recurred. A transvenous pacemaker was inserted but blood pressure did not improve. Insulin was titrated up to 3 u/kg/hr and dextrose 25 gm/hr and BP rose to 100 systolic on Day 2. The insulin and glucagon infusions were discontinued on Day 4, and the patient had recurrence of hypotension and bradycardia which again responded to high dose insulin and glucagon. Pulmonary edema developed and an EEG on Day 6 that showed poor electrical activity. Comfort measures were instituted and she expired.
Autopsy Findings: Not provided
Case 1048. Chronic digoxin ingestion: contributory.
Scenarios/Substances: 86 y/o female was brought to the ED with nausea, vomiting, diarrhea, and lower extremity edema. While ambulating from the car to the hospital she fell and struck her head. She arrived in the ED awake and alert, however she was bradycardic, hypotensive and hypothermic.
Past Medical History: Atrial fibrillation, CAD (s/p CABG), mitral and tricuspid stenosis, hypothyroid disease. Medications: digoxin, coumadin, furosemide, amlodipine and benazepril, spironolactone, and levothyroxine.
Physical Exam: Awake, alert and shivering; BP 60/48, P 35–40, T 31.1°C, RR 18, O2 sat 99% on room air. Pupils reactive and 3 mm. Skin, cool and dry, HEENT normal. Lungs bilateral basilar rales. Heart, grade 3/6 mid systolic possible diastolic murmur at the apex. abdomen normal, extremities pitting lower edema, weak pulses, moved all extremities, slightly confused.
Laboratory Data: WBC 4.6, Hgb 9.6, Hct 32, platelets 296,
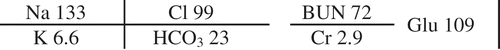
ionized Ca 1.13, Mg 2.9, phosphorous 6.9, CK 104, CK-MB 18.3 ng/ml, CK-MB Index 17.6%, troponin 0.06, acetaminophen 12.6 mcg/mL, ALT 391, AST 431, alk phos 103, bilirubin 0.7, direct bilirubin 0.3, INR 13.5, PTT 63.2 sec, digoxin 3.5 μg/L, B-type natriuretic peptide 125 pg/mL, urinalysis: specific gravity 1.03, glucose 100, large blood, protein 300 mg/dL; ECG: atrial fibrillation, ventricular rate 35–40, diffuse low voltage, QRS 130ms, QTc 490 ms, no ST changes; CxR: Enlarged cardiac silhouette; right pleural effusion; Head CT: No acute pathology; chronic atrophy and lacunar infarcts.
Clinical Course: Warming blanket, 3 mg atropine IV had no effect in HR; 3 vials digibind (120 mg) IV; sodium polystyrene 30 g PO; 25 g D50 and 10 U regular insulin IV; vitamin K 5 mg and 4 units of fresh frozen plasma. A urinary catheter was placed. Hypotension (70–80 systolic BP) despite 2 L normal saline; norepinephrine10 μg/min given. Hypothermia resolved over 5 hours; HR remained 40–50 with systolic BP 90 norepinephrine, mental status worsened; dialysis not feasible due to coagulapathy. 10 hr after admission, K 5.9, free digoxin level 2.7, serum Cr 3.1. Unsuccessful attempts to wean off the norepinephrine; patient became refractory to NE and dopamine was added. No significant clinical improvement was seen, urine output declined. The patient's family elected the institution of comfort measures and she expired on Day 4.
Autopsy Findings: No autopsy was performed.
Case 1057. Acute on chronic amlodipine ingestion (with aspiration): undoubtedly responsible.
Scenario/substance: A 91 y/o female mistakenly ingested 10.8 gm of recently prescribed diltiazem extended release formulation.
Past Medical History: Atrial fibrillation, dementia, anxiety, and osteoarthritis. Medications included: furosemide, levothyroxine, digoxin, esomeprazole, doxepin, temazepam, and warfarin.
Clinical Course: At 2 hours after ingestion, she exhibited depressed mental status, BP 80/50, PR 50. Skin was cool and clammy with peripheral cyanosis. ECG demonstrated complete AV block with a functional escape. Initial blood studies were normal, but the patient developed progressive hyperglycemia (450 mg/dl) and metabolic acidosis (pH 7.1, serum bicarbonate 17, lactate 3.7 mmol/L) during resuscitation procedures. K 3.5 mEq/L, digoxin 1.7 ng/mL, acetaminophen and salicylate were not detected. A CxR showed pulmonary edema. A bedside cardiac ultrasound demonstrated global hypokinesis that progressively worsened. Cardiac enzymes did not suggest ischemia. INR 2.1, Cr 1.55. Other treatments given included intubation, and administration of norepinephrine, glucagon, Ca gluconate, and high dose insulin. CxR was consistent with aspiration or pulmonary edema. External pacing was added without consistent capture. Asystole occurred 5 hour after ingestion and the patient was pronounced dead.
Autopsy Findings: Peripheral blood (antemortem) diltiazem 3.8 mg/L. Cause of death was accidental diltiazem overdose.
Case 1060. Acute on chronic flecainide ingestion: undoubtedly responsible.
Scenario/Substances: A 23 month old 11 kg male on oral flecainide for SVT, ingested ∼100 mL of 10 mg/mL (total 1 gram) flecainide. The child had a seizure, became somnolent, and was transported to the ED via private vehicle.
Past Medical History: SVT diagnosed in utero; preterm birth at 32 weeks; febrile seizures; ear infection.
Physical Exam: In the ED the patient was lethargic, appropriate but after a grand mal tonic-clonic seizure became bradycardic at HR 50 with widened QRS, QT and PR intervals and was unresponsive.
Laboratory Data: Initial flecainide level was 3.19 mcg/mL (reference range 0.2 to 1 mcg/mL), K 2.6; Ca 7.5; TProt 4.9, AST 106; ALT 58. WBC 62.8 with left shift; Hgb 10.2, Hct 29.9, ABG-pH 7.35/pCO2 25/pO2/HCO314.
Clinical Course: Intubated, CPR performed; VT resembling Torsades de Pointes, MgSO4 given. Epinephrine, atropine, and sodium bicarbonate administered. Two intraosseous lines were placed due to difficulty with IV access. HR increased to 80’s - 90’s with continued widened QRS and widened intervals. BP was 62/50. Cardioversion was attempted at 5 joules, then synchronized 10 joules with HCO3 along with an amiodarone bolus. Wide complex tachycardia with palpable pulses continued, but BP was not obtainable. A 20% lipid bolus was given at 2 mL/kg and subsequent infusion of 8 mL/kg over 1 hour was planned, but interrupted and took several hours. The patient was transffred to a tertiary care center and given an isoproterenol infusion for bradycardia and hypotension; systolic BP in the 70’s with continued wide ventricular complexes. External pacing pads increased the HR to 80; internal jugular catheter for transvenous pacing with good capture took place. Another lipid bolus at 12 mL/kg and an infusion of 8 mL/kg/hour was given. Severe neurological abnormality with lack of cortical or brain stem responses and no carotid arterial supply to the supratentorial or infratentorial brain was noted. The patient was pronounced brain dead on Day 2.
Autopsy Findings: Hypoxic encephalopathy due to aberrant cardiac rhythms due to flecainide intoxication. Blood positive for flecainide. Manner of death was accidental.
Case 1063. Acute antihistamine ingestion: undoubtedly responsible.
Scenario/substance: 21y/o male found on the floor with a suicide note and multiple empty bottles of diphenhydramine. Maximum possible ingestion was estimated at 8700 milligrams.
Past Medical History: depression and previous suicide attempts.
Physical Exam: The patient was confused upon arrival to the emergency room. T 36.9°C, HR 115, BP 170/101, RR 42.
Laboratory Data: APAP & ASA not detected, urine drug screen was negative.
Clinical Course: The patient was intubated and ventilated. He experienced 3 consecutive seizures and was treated with lorazepam 3mg. Whole bowel irrigation was initiated and IV fluids given. He was sent to the ICU and expired within several hours of his arrival to the ED.
Autopsy Findings: Stomach contained ∼100 ml of white, pasty, granular pill type material with a slight blue tinge Antemortem blood diphenhydramine 3.26 mg/L. The death was determined to be the result of intentional overdose with diphenhydramine and the manner of death was suicide.
Case 1076. Acute on chronic loperamide ingestion: undoubtedly responsible.
Scenario/substance: 21 y/o male ingested up to 800 loperamide tablets (1600 mg) and was found apneic and pulseless at home. EMS initiated CPR including epinephrine, naloxone, and intubation and restored rhythm.
Past Medical History: Chronic diarrhea and abdominal pain; previous loperamide overdose.
Physical Exam: In ED hypotensive (64/28) and agitation required propofol.
Laboratory Data: pH 7.26, anion gap, glucose 283, Cr 1.7.
Clinical Course: Developed myoclonus, opisthotonous, rigidity, severe anoxic encephalopathy and aspiration pneumonia. Transferred to hospice and expired 11 days after admission.
Autopsy Findings: Cause of death was anoxic encephalopathy and bronchopneumonia due to resuscitated cardiac arrest and loperamide intoxication.
Case 1095. Acute drotrecogin alpha: parenteral: contributory.
Scenario/Substances: A 22 y/o female presented to a HCF acutely ill, was diagnosed with meningitis and received infusion of 8900 mcg of drotrecogin alpha over 1 hour instead of over the intended 12 hours.
Past Medical History: Present diagnosis of meningitis; otherwise normal healthy adult
Laboratory Data: Before medication error: INR 1.42, PTT 37; ∼9 hours after error: INR 4.18; PTT > 300, fibrinogen 105.
Clinical Course: Patient was admitted to ICU in critical condition with full supportive measures and was noted to have active bleeding. Her condition continued to deteriorate. She was also in acute renal failure and continuous renal replacement therapy was initiated. She arrested and was resuscitated twice. She received fresh frozen plasma x 12 units, cryoprecipitate x 2 units, and platelet phoresis x 10 units. She developed a pneumothorax and required placement of chest tube. She expired on the day after medication error.
Autopsy Findings: Not available.
Case 1133. Acute alprazolam ingestion and parenteral administration: undoubtedly responsible.
Scenario/substance: 26 y/o female hospitalized for asthma. Found in cardiopulmonary arrest on the day after the self-administered alprazolam overdose. Six empty 10 ml syringes were found under her pillow, as well as a bottle of alprazolam with 64 missing tablets and two empty 1 mL vials of promethazine injectable, 25 mg/mL. A nearly empty bottle of fabric softener was also found.
Past Medical History: Major depression, anxiety disorder, borderline personality disorder, bipolar disorder, Munchhausen Syndrome, Munchhausen Syndrome by Proxy, hypertension, asthma.
Laboratory Data: Na 149, K 9.5, Hgb 10, platelets 5 (254 on admission), glucose 551, WBC 17.5. Urine and blood screens positive for benzodiazepine, caffeine. Blood alprazolam 120 ng/mL (range in 7 fatal suicides: 122–2100 ng/mL).
Clinical Course: Found apneic, pulseless. Resuscitation attempted with intubation, epinephrine, naloxone, flumazenil, sodium bicarbonate, calcium, insulin/glucose and dopamine. Resuscitation unsuccessful and the patient expired.
Autopsy: Acute alprazolam toxicity and foreign body granulomas of both lungs, extensive. Lung sections showed birefringent foreign body material.
Case 1136. Acute olanzapine ingestion: undoubtedly responsible.
Scenario/Substances: A 28 y/o female called her boyfriend and told him that she had taken an overdose of her medications. EMS found her in a room with pills scattered on the floor and bottles of atenolol, pregabalin, quetiapine and alprazolam. She was in cardiac arrest with PEA. CPR initiated with intubation, an intraosseous line was started and atropine and epinephrine were given prior to transport to the ED.
Past Medical History: depression and prior suicide attempts.
Physical Exam: On arrival in the ED she remained pulseless and apneic, with a slow, wide complex rhythm.
Clinical Course: Despite aggressive resuscitation efforts including epinephrine, atropine, sodium bicarbonate glucagon for 30 min in the ED the patient expired.
Autopsy Findings: There was ∼40 mL of beige-colored particulate fluid possibly consisting of multiple pill fragments. Blood was positive for benzodiazepines, ethanol, caffeine, nicotine, quetiapine and bupropion metabolite. Ethanol 102 mg/dL, alprazolam 390 ng/mL (therapeutic range 10–100 ng/mL), quetiapine 4500 ng/mL (therapeutic range 286–828 ng/mL). The cause of death was acute mixed drug ingestion.
Case 1140. Acute doxylamine ingestion: undoubtedly responsible.
Scenario/Substances: A 30 y/o male ingested 200 tablets of Unisom and then called 911. There were multiple empty packages of Unisom and antiretroviral medications at his bedside. When EMS arrived, he was initially conversant with garbled speech then subsequently had a grand mal seizure lasting about 45 seconds. He was given D50W for glucose on the mid 60’s. Activated charcoal was withheld in the field to minimize aspiration hazard and transported him to the ED.
Past Medical History: He was on antiretroviral medication. He had been admitted previously to the ED with altered mental status. He used alcohol, marijuana and diazepam to manage environmental stress and depression. Medical record indicated he had access to tricyclic antidepressants, ziprasidone, escitalopram and lorazepam.
Physical Exam: On arrival in the ED, the patient was unresponsive; the BP was 212/133, HR 117, O2 Sat 96% on nonrebreathing bag. After intubation, pupils were dilated and non-reactive, the oropharynx, lungs and abdomen were unremarkable. Extremities were flaccid after the seizure; there as no evidence of injury.
Laboratory Data: Initial glucose 153, Na 148, K 2.1, HCO3 6, lactic acid 35 mmol/L, INR 1.6, Hct 47.1%, WBC 12.4, platelets 381, salicylate 3.2 mg/dL, APAP 6.8 mg/L, valproic acid, phenytoin and digoxin were not detected. Carbamazepine 0.5mg/L, phenobarbital 1.5 mg/L
Clinical Course: The patient had a grand mal seizure lasting 40 seconds in the ED treated by lorazepam. The ECG showed a wide complex tachycardia with a rate of about 120. He developed VT which deteriorated to PEA after DC countershock. CPR was performed including epinephrine, atropine, and naloxone but he remained pulseless with a bradycardia. He was given 3 boluses of sodium bicarbonate followed by bicarbonate IV infusion, which resulted in a transient palpable pulse for only 5–10 seconds. He remained bradycardic and in PEA and did not respond to continued CPR and ACLS and he was pronounced dead
Autopsy Findings: The stomach contained 400 mL of green turbid fluid without identifiable food material and a white-green pill residue within the pylorus. Both lungs were congested with moderately edematous parenchyma. There was atherosclerotic coronary artery disease, but no evidence of occlusive thrombus or regional wall infarction. Blood levels: citalopram <0.1 mg/l, diphenhydramine 14.1 mg/l, doxylamine 17.5 mg/l. Gastric contents: diphenhydramine:1,320 mg, doxylamine 124 mg. Cause of death: acute Unisom (diphenhydramine and doxylamine) intoxication
Case 1199. Acute cocaine ingestion: undoubtedly responsible.
Scenario/Substances: 14 y/o male in ingested an unknown amount of either heroin or crack cocaine during an police arrest. In custody vomited up a baggie and then promptly re-ingested the contents.
Clinical Course: Arrived at the ED in full arrest and resuscitation was not successful and he was pronounced dead shortly after arrival, within an hour of ingesting the contents of the baggie.
Autopsy Findings: Stomach contents were positive for cocaine. Blood: cocaine 10.5 mg/L, benzoylecgonine 3.14 mg/L. The cause of death was acute cocaine intoxication.
Case 1204. Acute MDMA ingestion: undoubtedly responsible.
Scenario/Substances: 18 y/o female was driving with a friend when she suddenly lost consciousness and began vomiting. She reportedly had ingested “home-made, double-strength” MDMA. She was brought to the ED by private vehicle. A pill with an unrecognized imprint was brought with the patient that appeared to have been an illicit manufacture.
Physical Exam: Unresponsive to stimuli, with twitching noted
Laboratory Data: Urine drug screen showed positive for methamphetamine and cocaine. Na 160, K 4.9, BUN 12, Cr 1.1 Ca 9.6, CK 239, CK-MB 17.8, Troponin 2.3.
Clinical Course: The patient experienced a short episode of VT that resolved spontaneously. She was intubated and placed on mechanical ventilation. HR 119. CT showed evidence of cerebral edema and she was transferred to a tertiary medical facility. The patient continued to deteriorate with intermittent ventricular arrhythmias and increasing hypotension requiring pressors. Repeat CT showed increasing cerebral edema with closures of ventricles. Pupils were 6 mm and fixed. There was no response to stimuli. After 48 hours the patient was declared dead and became an organ donor.
Autopsy Findings: Blood from the initial ED arrival revealed MDMA 274 ng/mL and was negative for methamphetamine and cocaine
Case 1210. Acute heroin Parenteral: undoubtedly responsible.
Scenario/Substances: 21 y/o female was found unresponsive in a bathroom stall by a co-worker. A syringe was found in the stall near the patient. EMS found her comatose, administered naloxone 4 mg IV without response, intubated her and brought to ED.
Past Medical History: No known medical problems or family history of disease.
Physical Examination: Hypotensive, on respirator; GCS 3. Pupils fixed, with dolls eye movements, corneal reflexes absent. No trauma, rashes or track marks.
Laboratory Data:

Acetaminophen and salicylate not detected, urine pregnancy test negative; ethanol <10; urine tox positive for opiates; CxR: pulmonary edema; head CT: no noted bleed or injury.
Clinical Course: Hypotension requiring dopamine, norepinephrine, and phenylephrine infusions. Upon ICU transfer, the patient had PEA, was resuscitated before having an asystolic rhythm 1 hr later. The patient expired 7 hours after initial presentation.
Autopsy Findings: Pulmonary congestion and edema. Post mortem blood concentrations: codeine <0.1 mg/L, morphine 0.10 mg/L; vitreous: codeine <0.01 mg/L, morphine 0.05 mg/L, and 6-monoacetyl morphine 0.01 mg/L. Cause of death was accidental heroin intoxication.
Case 1212. Acute on chronic heroin parenteral: probably responsible.
Scenario/Substances: 22 y/o female, known drug abuser arrested and released and was sent to a crisis center and then home. Found by her parents unconscious in respiratory arrest with a syringe still in her arm and six empty and 2 full bags of heroin. EMS found her in cardiac arrest, CPR with intubation, epinephrine, atropine and naloxone with return of vital signs.
Past Medical History: Prior admissions for drug overdose including one 2 weeks prior from which she had been discharged to rehabilitation.
Physical Exam: On arrival to the ED her BP was 139/86, HR 124, T was 33.3°C.
Laboratory Data: ABG-pH 7.16/pCO2 40, QRS 100 ms, QT/QTc 372/499 ms, Na 137, K 3.6, Cl 96, BUN 11, Cr 1.8, glucose 146. AST 419, ALT 278, INR 1.3, salicylate, acetaminophen, ethanol were not detected. Head CT showed cerebral edema and diffuse subarachnoid hemorrhage, intraventricular hemorrhage and new infarctions of the basal ganglia.
Clinical Course: Patient was treated to correct her acidosis, but she remained moribund. She developed polyuria consistent with diabetes insipidus treated with vasopressin. Brain perfusion study showed no cerebral flow. Repeat head CT shortly before death showed protrusion of the cerebellar hemispheres into the foramen magnum. Declared dead on Day 2 and organs were harvested for transplantation.
Autopsy Findings: Cerebral edema and congestion, atelectasis of both lower lobes of lung. Premortem blood: morphine or fentanyl were not detected. Post mortem urine revealed acetaminophen level of 2.35 mg/L and free morphine 0.09 mg/L.
Case 1217. Acute caffeine ingestion: undoubtedly responsible.
Scenario/Substances: A 23 y/o female ingested 100 fat-burner weight loss capsules (cola nut, cactus extract, white willow bark, grapefruit extract, chitosan, caffeine 250 mg, green tea, guarana and garcinia).
Past Medical History: Venlafaxine for depression, cocaine abuse, prior suicide attempts and psychiatric hospitalizations. Smoked 1 pack per day.
Clinical Course: She suddenly became diaphoretic and tachycardic en route to the ED. When she arrived at the ED 1 hour after the ingestion she was anxious, but otherwise asymptomatic. BP 135/109, HR 139, RR 28. On physical exam she was diaphoretic. Following the gastric lavage (90 min post-ingestion), which returned a large quantity of reddish-purple soup and pill fragments that were consistent with the ingestion, the patient had a tonic seizure and was given lorazepam. Seven min following the seizure, the patient went into a VF and was resuscitated. CK exceeded 500,000 on Day 1. Activated charcoal was given and ∼36 hours after presentation, she was stable enough to undergo a session of hemodialysis. At ∼43.5 hours after initial presentation, she went into an unspecified heart block and then VF from which she could not be resuscitated. A caffeine level drawn upon presentation returned following the patient's death: 189 mg/L.
Autopsy Findings: Not available
Case 1243. Acute cocaine ingestion: undoubtedly responsible.
Scenario/Substances: 32 y/o male swallowed cocaine during a law enforcement traffic stop and while in police custody, started vomiting. An estimated 50 - 60 rocks of cocaine were seen in his emesis. EMS were called and found the patient unresponsive in cardiac arrest. He was revived at the scene with naloxone and a dopamine infusion was started prior to the onset of seizure activity.
Laboratory Data: Admission: urine drug screen positive for cocaine and PCP, APAP & ASA not detected, AST 91, ALT 139. Post intubation on Fi02 50%. ABG-pH 7.35/pCO2 31.2/pO2 231/HCO3 21/BE -5.
Clinical Course: In the ED the patient had fixed, dilated pupils, was intubated and ventilated. An infusion of naloxone was started prior to transfer to the ICU with a BP 90/77, HR 134. The dopamine infusion was stopped and a phenylephrine infusion started. The naloxone infusion continued. Day 2
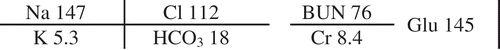
measured serum osmolarity 330 mOsm/kg. Day 5: BUN 103, Cr>10. He had episodes of hyperthermia (to 38.4°C) which also appeared to affect his BP (up to 185/105). His HR stayed in the 140 to 150 range. He received labetalol, mannitol, furosemide, and antibiotics. All medications, with the exception of antibiotics, pantoprazole, IV fluids, and total parenteral nutrition were stopped on Day 4. He was dialyzed on Days 3 - 6 for acute renal failure. His pupils remained fixed and dilated and he started posturing. He remained unresponsive on a ventilator throughout hospitalization. An EEG performed on Day 8 showed acute anoxic encephalopathy. A CT scan showed bilaterally thrombosed internal carotids without evidence of cerebral blood flow. Comfort measures were instituted and he expired on Day 8.
Autopsy Findings: Massive pulmonary edema. The brain was edematous and soft, with a small subarachnoid hemorrhage over the brainstem. Both carotid arteries were thrombosed. Antemortem blood was positive for cocaine metabolites. A brain sample showed ecgonine methyl ester at 10.3 mg/kg, cocaine at 2.71 mg/kg and benzoylecgonine at 4.66 mg/kg. Cause of death: cocaine intoxication.
Case 1246. Acute on chronic cocaine ingestion: undoubtedly responsible.
Scenario/Substances: A 32 y/o male swallowed several baggies of cocaine when he was stopped by law enforcement. He was transported to the ED.
Physical Exam: BP 200/120, HR 142, highest, T 36.7°C, diaphoretic and shivering.
Laboratory Data: Urine tox screen was positive for cocaine, THC, opiates. acetaminophen not detected.
Clinical Course: Admitted to the ICU where whole bowel irrigation was started. Developed diarrhea and a distended abdomen. His T increased to 37.7°C. A nicardipine infusion was started for hypertension resulting in a BP of 138/68; Plantar reflex was present; pupils were 2+ and sluggish, on propofol; intubated and placed on a ventilator; seizures every 3 minutes lasting about 60 seconds and he was started on benzodiazepines and phenytoin. The ambient T of the room was cooled and patient T to 36.4°C. His abdomen was no longer distended; the patient was placed on a labetalol infusion for persistent hypertension. Tonic-clonic activity and EEG showed epileptiform activity in the frontal lobe which stopped, but started in other areas of the brain;Glasgow coma scale 4. Significant anoxic brain injury was suspected. Day 9: he arrested and could not be resuscitated.
Autopsy Findings: Healing abrasions on the face, torso and extremities; empty cellophane packet found in gastric contents, pulmonary edema, cardiac hypertrophy. Antemortem blood: cocaine 95 ng/mL, benzoylecgonine 2500 ng/mL, no other drugs detected. Cause of death was anoxic/ischemic encephalopathy secondary to cocaine intoxication.
Case 1251. Acute cocaine ingestion: undoubtedly responsible.
Scenario/Substances: A 38 y/o male ingested “a large amount of crack cocaine” During a police stop. In the ensuing altercation the patient was “tasered” several times before being handcuffed. EMS arrived, but he refused to answer questions, refused an IV line, and become anxious and sweaty. He was transported to the hospital and admitted to EMS that he had ingested a couple of large packages of cocaine.
Clinical Course: He continued to be uncooperative, HR 160, BP 180/systolic. Shortly after arrival in the ED he abruptly seized and became bradycardic and hypotensive. He was intubated and resuscitative measures were unsuccessful and the patient expired less than 1 hour after arrival.
Autopsy Findings: Stomach contained ∼16 white-beige objects measuring ∼1 cm in diameter consistent with ingested crack cocaine. The heart was enlarged (480 gm) with concentric 2 cm left ventricular hypertrophy with evidence of atherosclerotic cardiovascular disease and scattered epicardial petechiae. Antemortem blood: cocaine 3200 ng/mL, benzoylecgonine 3100 ng/mL, delta-9-thc 2.9 ng/mL, delta-9-carboxy-THC 31 ng/mL, lorazepam 300 ng/mL (therapeutic range 50–240 ng/mL) [he had been given lorazepam in the hospital after the seizure.]. The cause of death was acute cocaine intoxication.
Case 1253. Acute cocaine ingestion: probably responsible.
Scenario/Substances: 39 y/o male brought to ED by police 2 days following an arrest when police suspected ingestion of cocaine.
Physical Exam: Hypertension, tachycardia, and agitation.
Laboratory Data: Initial: Cr 0.9, troponin 0.02, urine toxicology positive for cocaine and benzodiazepines; Day 5: Cr 3.5; Day 6: Cr 6.0, troponin 9.2.
Clinical Course: CT scan abdomen showed a large number of foreign bodies. On the day of admission surgery was performed to remove 15 “packets” believed to contain cocaine from the stomach. Post-operatively he became agitated; CT abdomen showed two foreign bodies in the colon.
Day 2: Colonoscopy removed two more “packets”. The packets were 2–3 inch thin balloons. One packet removed during colonoscopy was ruptured.
Day 3: He continued hypertensive and tachycardic (BP 180/110 HR 120) on a mechanical ventilator. A third CT scan of the abdomen did not show any foreign bodies. IV lorazepam drip and phentolamine were given for hypertension without resolution. Whole bowel irrigation was initiated, but was discontinued due to abdominal distention. IV infusion of nicardipine was used to control BP, but HR remained elevated. The patient expired during cardiac arrest on Day 6.
Autopsy Findings: The medical examiner stated that the brain, myocardium, bowels and kidneys appeared grossly normal, but in the distal small bowel 2 small burst “packets” were found.
Case 1260. Acute cocaine exposure: contributory.
Scenario/Substances: 42 y/o male found unresponsive and profoundly hypertensive in a known drug house and brought to the ED.
Past Medical History: Significant for cocaine use and smoking and history of head trauma 1 week prior to admission.
Physical Exam: Unresponsive, BP 240/140 to 300/200, HR 53–190. Pupils fixed and dilated (6 mm), absent corneal reflexes bilaterally.
Laboratory Data: ECG: QRS 120 ms, QTc 464 ms; Head CT: actively bleeding large subarachnoid hemorrhage. Chemistries were WNL, except glucose: 178; salicylate, acetaminophen, ethanol; negative; urine drug screen positive for cocaine.
Clinical Course: The patient was intubated, given sublingual nitroglycerin and started on nitroprusside infusion. No respiratory effort over the ventilator was noted; life support was withdrawn four hours after ED arrival and the patient expired.
Autopsy Findings: The cause of death was subarachnoid and intraventricular hemorrhage due to ruptured cerebral artery aneurysm. Cocaine was found to be a contributory cause. Iliac blood: benzoylecgonine 0.23 mg/L, ecgonine methyl ester was positive.
Case 1265. Acuity unknown cocaine Unknown: probably responsible.
Scenario/substance: 45 y/o male became agitated after a drug stop and police restraint. He was tasered once, got up and tried to run again, and was tasered a second time and became unresponsive. EMS was called, noted PEA, and administered naloxone, atropine and epinephrine. He was intubated and ventilated during transport to the ED.
Past Medical History: left ventricular hypertrophy, psychiatric disorder, and cocaine abuse.
Physical Exam: In the ED: T 39.9°C, BP 148/108, HR 140. His pupils were 3 mm and reactive to light. He was intubated and mechanically ventilated.
Laboratory Data: glucose 300, ethanol 14 mg/dl, APAP & ASA not detected. ABG-pH 6.90/pCO2 47/pO2 527/HCO3 9.3, urine drug screen was positive for cocaine, ammonia 334 umm/L, AST 123, ALT 106.
Clinical Course: Metabolic acidosis was treated with multiple doses of sodium bicarbonate. Despite aggressive care in the ICU, he expired about 8.5 hours after admission to the ED,
Autopsy Findings: Generalized cerebral edema early degenerative neuronal changes consistent with hypoxia. Bronchilitis. Focal hemorrhages at the attachments of the chordae tendinae of the heart, and microscopic revealed acute infarction with hemorrhage. Cause of death: 1) excited delirium, with cardiac dysrhythmia, myocardial infarction and hypoxic brain injury, metabolic acidosis and hyperthermia. 2) Cocaine intoxication with benzoyl ecgonine 1.56 mg/L, and ecgonine methyl ester at 0.371 mg/L in antemortem blood. Cocaine and cocaethylene were not detected. 3) Complications of cardiac arrest following violent suggle with restraint and deployment of electronic control device. The manner of death was deemed accidental.
Addendum
Case. Acute N-acetylcysteine parenteral.
Scenario/substance: An 18 y/o female took an unknown amount of acetaminophen 2 hours before arriving in the ED.
Past Medical History: The patient had recently found out she was pregnant.
Laboratory Data: Initial acetaminophen was 200 mcg/mL at 4 hours (all times refer to hours post ingestion), AST 20, ALT 14.
Clinical Course: In the ED, she was awake and alert, equal reactive pupils HR 123, BP 116/69, RR 18–20; weight 50 kg. She received activated charcoal and IV N-acetylcysteine (NAC) was started at 3.5 hours: Loading Dose 150 mg/kg over 1 hr, Second Dose 50 mg/kg/hr x 4 hr (instead of over 4 hr), Third Dose and 100 mg/kg/hr x 16 hr (instead of over 16 hr). Seizures began approximately 17 hours and continued until 23 hours. The patient aspirated charcoal and gastric contents and was intubated. Normal saline, phenytoin, lorazepam, and propofol were given. Day 2 vital signs were: HR 113, BP 133/63, RR 20. Approximately 26 hours: acetaminophen < 10 mcg/mL, AST 30, ALT 16, INR 2.1. NAC was re-started ∼29 hours (dosage according to package insert instructions). At 32 hours her BP was 67/34, pupils 9 mm and non-reactive, no cough, gag, or corneal reflexes were found leading to the Dx of brain death. During evaluation for organ donation, the patient developed a bradydysrhythmia, leading to asystole and expired at 41 hours.
Autopsy findings: Cerebral edema, herniation, acute pneumonia due to aspiration of gastric contents, acute myocardial ischemia. The liver was not grossly or microscopically necrotic. The cause of death was “complications from acetaminophen toxicity” but did not exclude the possibility of a NAC overdose.
NB: The above abstract is included as an addendum to this report because of the potential importance of the case and the initial lack of agreement between the regional poison center (RPC) and the fatality managers as to RCF based on the information then available. Details of the initial physical exam and dose administered were not received until the time of page proofs. The RPC authors believed the appropriate RCF = 1, undoubtedly responsible and opined “Given the initially reported NAC dose, the elevated INR consistent with an extremely high serum NAC level, the cerebral injury and lack of hepatic injury, death appears to be much more likely due to NAC overdose”.
Abbreviations & Normal ranges for Abstracts
Disclaimer – all laboratories are different, units and normal ranges are provided for general guidance only. These values were taken from Harrison's.Citation14, Goldfrank.Citation15 or Dart .Citation16
Serum electrolyte summary table
![]()
ABG = arterial blood gases
ABG-pCO2 = partial pressure of carbon dioxide[38–42] mmHg
ABG-pH = hydrogen ion concentration [7.38–7.42] mmHg
ABG-pO2 = partial pressure of oxygen [90–100] mmHg
ACLS = advanced cardiac life support, protocol for the provision of cardiac resuscitation
Alk phos = alkaline phosphatase [13–100] U/L
ALT = Alanine aminotransferase [7–41] U/L = (SGPT)
AMA = against medical advice
Ammonia = [250–80] mcg/dL = [15–47] mcmol/L
ARDS = acute respiratory distress syndrome
AST = Aspartate aminotransferase [12–38] U/L = (SGOT)
AVblock = atrio-ventricular block
BAL – British anti-Lewisite
BE = base excess, mmol/L
Bicarbonate = [22–26] mEq/L
Bilirubin = total [0.3–1.3] mg/dL, direct [0.1, 0.4] mg/dL, indirect [0.2, 0.9] mg/dL
BLQ = below the limit of quantitation
BMI = body mass index
BP = Blood Pressure, systolic/diastolic, mmHg (Torr)
BUN - see Urea nitrogen
C = degrees Centigrade
Ca = calcium, [8.7–10.2] mg/dL
CAD = coronary artery disease
CABG = coronary artery bypass graft
CK = creatinine kinase (CPK), total: [39–238] U/L females, [51–294] U/L males
Cl = chloride [102–109] mEq/L
COHb = COHb
COPD = chronic obstructive pulmoary disease
CPR = cardio pulmonary resuscitation
Cr = creatinine [0.5–0.9] mg/dL females, [0.6–1.2] males,
CT = computed tomography (CAT scan)
CVA = cerebrovascular accident
CVVHD = continuous venovenus hemodialysis
CxR = chest radiograph, chest xray
Day = when capitalized, Day = hospital day, i.e., days since admission
Dx = diagnosis
ECG = electrocardiogramECG, leads = I, II, III, aVR, aVL, aVF, V1, V2, V3, V4, V5, V6
ED = emergency department, in these abstracts refers to the initial health care facility
EDDP = principal methadone metabolite
EEG = electroencephalogram
ELISA = enzyme-linked immunosorbent assay
EMS = emergency medical services, paramedics, the first responders
ER = extended release (sustained release)
FiO2 = fraction of inspired oxygen
g/dL = grams per deciliter
GCS = Glasgow Coma Score, ranges from 3 to 15
Glu = glucose, fasting [75–110] mg/dL
HCF = health care facility
HCG = human chorionic gonadotropin test for pregnancy
HCO3 = bicarbonate
HCP = health care provider
Hct = hematocrit [35.4–44.4] females, [38.8–46.4]% males
Hgb = hemoglobin [12.0–15.8] g/dL females, [13.3–16.2] g/dL males
HIV = human immunodeficiency virus
HR = heart rate, beats per min
hr = hour(s)
ICU = intensive care unit
IgE = immunoglobulin E
IM = intramuscular
INR = international normalized ratio (PT to control) [0.8-1-2]
IU/L = international units per Liter
IV = intravenous
K = potassium, [3.5–5] mEq/L
kg = kilogram
L = Liter
Lactate = lactic acid [4.5–14.4] mg/dL arterial, [4.5–19.8] mg/dL venous
Leukocyte count = white blood count [3.54–9.06] 103/mm3
mcg/dL = micrograms per deciliter
mcg/L = micrograms per Liter
mcg/min = micrograms per minute
mcg/mL = micrograms per milliliter
mcmol/L = micromoles per liter
MDA = 3,4-methylenedioxyamphetamine
MDMA = methylenedioxymethamphetamine (ecstasy)
ME = medical examiner
mEq = milliequivalents
mEq/L = milliequivalents per Liter
Mg = magnesium [1.5–2.3] mg/dL
mg = milligrams
mg/dL = milligrams per deciliter
mg/kg = milligrams per kilogram
mg/L = milligrams per Liter
min = minutes
mmol/L = millmoles per Liter
mosm/kg = milliosmoles per kilogram
mosm/L = milliosmoles per Liter
MRI = Magnetic Resonance Imaging
ms = milliseconds
NG = nasogastric
ng/mL = nanograms per milliliter
NS = normal saline
O2 sat = oxygen percent saturation [94–100]% at sea level
OR = operating room
Osm = osmole
PALS = pediatric advanced life support
PC = poison center ( = PCC, or Poison Control Center)
PCP – primary care provider
PEA = pulseless electrical activity
PEEP = positive end expiratory pressure
Platelets = platelet count [150–400] × 109/L
PO = per os (“by mouth” in Latin)
Potassium = [3.5–5] mEq/L
PR = P-R interval [120–200] msec on the ECG
PT = prothrombin time, INR is preferred, but PT may be used if INR is not available
PTA Prior to admission
PTT = partial thromboplastin time [26.3–39.4] sec
QRS = ECG QRS complex duration [60–100] msec
QT = Q to T interval on the ECG waveform, varies with heart rate
QTc = QT interval corrected for heart rate, usually QTcB = QT/RR½ (Bazett correction) 1–15 y-o [<440] msec, adult male [<430] msec, adult female [<450] msec
RBC = red blood cell(s)
RR = respiratory rate, breaths per minute
sec = seconds
SVT = supraventricular tachycardiaF
s/p = status post
T (oral) = Temperature (oral) [36.4, 37.2]°C or
T (rectal) = Temperature (rectal) [36.4, 37.2]°C or
T (tympanic) = Temperature (tympanic) [36.4, 37.2]°C
THC = tetrahydrocannabinol
Tprot = total protein
Troponin I = normal range [0–0.08] ng/mL, Cut-off for MI > 0.04 ng/mL
U/dL = units per deciliter
U/L = units per liter
U/mL = units per milliliter
UA = urinalysis
Urea nitrogen (BUN) = [6–17] mg/dL
VF = Ventricular fibrillation
VT = Ventricular tachycardia
WBC = white blood count, see leukocyte count
WNL – within normal limits
y/o = years old