Abstract
Objective: Many psychological and psychosocial interventions have been developed to treat regular users of cannabis, but it is unclear which intervention(s) are the most effective. This article aims to assess the effectiveness of psychological and psychosocial interventions for cannabis cessation, and to outline priorities for future research. Methods: A systematic review of the scientific literature. Eleven databases were searched in February 2014. Results: Twenty-six RCTs were identified; the majority were considered to be at a high risk of bias. Cognitive behavioural therapy (CBT) significantly improved outcomes compared with wait-list in five studies post-treatment, maintained at 9 months in the one study with later follow-up. Studies of motivational interviewing (MI) or motivational enhancement therapy (MET) gave mixed results, with some improvements over wait-list while some comparisons were not significant. Four studies comparing CBT against MI/MET gave mixed results; longer courses of CBT provided some improvements over shorter MI. Courses of other types of therapy (social support groups and case management) gave similar improvements to CBT. Vouchers for abstinence (contingency management) gave promising results in the short-term and at follow-up. Conclusion: Studies were heterogeneous, covering a range of interventions, comparators, populations and outcomes. CBT improved short-term outcomes in a clinically dependent self-selected population of cannabis users. Brief MI improved short-term outcomes at post-treatment in a younger non-clinically dependent population. There is some evidence that CBT may be more effective than briefer MI interventions although results were mixed. Contingency management may enhance long-term outcomes in combination with CBT in clinically dependent individuals.
Introduction
Cannabis is the most commonly used illicit drug worldwide (UNODC, Citation2013). The European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) annual report notes that cannabis is the illicit drug most likely to be used across Europe, with 11.2% of 15–34 year olds reporting use in the last year (European Monitoring Centre for Drugs and Drug Addiction, Citation2014). Routine data collected by the US government shows that there were a total of 300,000 admissions for cannabis use in 2012, making up 17.5% of admissions for substance abuse in the US for that year (U.S Department of Health and Human Services, Citation2014).
Not all cannabis use is problematic, but harm is most likely in weekly and daily users (Davis, Thomas, Jesseman, & Mazan, Citation2009). Cannabis dependence, defined by the International Classification of Disorders (ICD-10) as “a cluster of physiological, behavioural, and cognitive phenomena in which the use of cannabis takes on a much higher priority for a given individual than other behaviours that once had greater value” can develop from frequent use (World Health Organization, Citation2000). Not all individuals who use cannabis frequently develop dependence; it is not clear why some individuals develop dependence while others do not (van der Pol et al., Citation2013). The quantity of cannabis consumed per day and the level of harm sustained should be taken into account when ascertaining if an individual is dependent, and not just frequency of use (Asbridge, Duff, Marsh, & Erickson, Citation2014). Different interventions may be applicable depending on whether or not an individual is at risk of dependence; screening tools, such as The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST), recommend that those at moderate risk receive a “brief intervention”, while those at high risk receive an “intensive intervention” (WHO ASSIST Working Group, Citation2002).
Psychosocial and psychological interventions are used in clinical practice to treat individuals who regularly use cannabis (National Institute for Health and Care Excellence (NICE), Citation2007). Interventions, such as Cognitive Behavioural Therapy (CBT), motivational interviewing (MI) and contingency management, aim to change an individual’s behaviour through either changing the way the individual thinks or behaves (CBT), improving motivation to change and resolving ambivalence to change (MI) or providing individuals with tangible rewards (such as monetary vouchers) to reinforce behaviour change (contingency management).
A number of systematic reviews have been undertaken to assess the benefits of psychological and psychosocial interventions for regular cannabis users (Davis et al., Citation2015; Denis, Lavie, Fatseas, & Auriacombe, Citation2006; Dutra et al., Citation2008). All have found some positive effects of such interventions on cannabis users, but have only included a subset of the available evidence, for example, only including RCTs with non-active control conditions (Davis et al., Citation2015), non-intensive treatments (Dutra et al., Citation2008) or only individuals meeting the diagnostic criteria for cannabis dependence (Denis et al., Citation2006; Dutra et al., Citation2008).
The aim of this review is to assess the evidence for the effectiveness of a broad range of psychosocial and psychological interventions for cannabis cessation in adults.
Methods
Literature search and inclusion criteria
In February 2014, searches of electronic databases (listed in ) were undertaken. Search terms are available on request. Additional search methods included checking references within relevant reviews and studies (reference tracking) and contact with experts. Inclusion and exclusion criteria are listed in .
Box 1. Electronic databases
Box 2. Inclusion exclusion criteria
Data extraction and quality assessment
Titles and abstracts were screened by one reviewer and a 10% sample checked by a second reviewer. Full texts were screened by two reviewers. Data extraction was undertaken by one reviewer and checked by a second reviewer; any disagreements were resolved through discussion. Where studies comprised duplicate reports, the most recent and relevant report was used as the main source.
Two reviewers independently assessed the methodological quality of each study and discrepancies were resolved through discussion. Quality was assessed using an adapted version of the Cochrane Collaboration risk of bias assessment criteria. Two adaptations were made to these criteria. Firstly, the “5-and-20 rule” was utilised for incomplete outcome data – a level of risk was allocated to participant attrition, either being low risk (<5% attrition), intermediate risk (5–20%) or high risk (>20%) (Schulz & Grimes, Citation2002). Attrition at the final follow-up was used to assign a level of risk in studies with multiple follow-up points. The second adaptation was to add an “overall risk” criterion, where studies were categorised using the following criteria: “Low-risk” was allocated to studies where randomisation, allocation concealment, blinded outcome assessment and incomplete data were all determined to be “low risk”. “High-risk” was allocated to studies deemed to have undertaken inadequate randomisation (e.g. self-selection, sequential patients), and/or where allocation was not concealed, and/or where incomplete data was deemed to be “high risk”. “Unclear risk” was allocated to all other studies. These criteria were added to reduce the subjectivity of the Cochrane tool, in order to increase the reliability of the tool when used by two researchers, and to provide an overall picture of the risk of bias for each study.
Data synthesis
Data were analysed via a narrative synthesis, based around grouping and tabulating the data in meaningful clusters, allowing results to be summarised to provide an overview of the direction of effect for each relevant subgroup (Popay et al., Citation2006). Studies were categorised according to their intervention and comparison groups (e.g. CBT versus wait-list, CBT versus MI, etc.). Results were tabulated for two key time points (post-treatment and latest follow-up). The latest follow-up time point was selected as the majority of trials did not utilise the same follow-up time points; selecting the latest follow-up provided information regarding the long-term effect of the interventions. Effect sizes and corresponding confidence intervals were included in the synthesis when reported by the studies. Where reported, Cohen’s D effect sizes were classified as small (threshold of d = 0.20), medium (d = 0.50), large (d = 0.80), or very large (d = 1.30) using the thresholds as described by Rosnow and Rosenthal (Citation1996). In order to synthesise the data, key outcomes were selected which were both clinically relevant and reported by the majority of studies.
Results
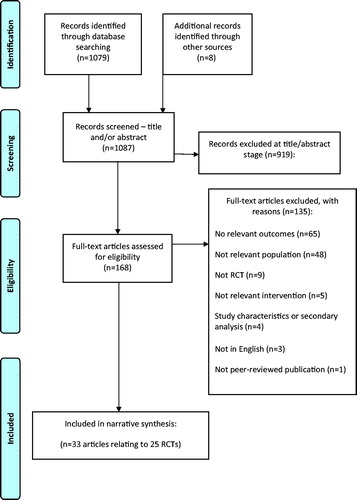
The searches identified 1087 citations. In total, 33 articles relating to 25 RCTs were included in this review. The full PRISMA flow chart is shown in .
Description of studies contributing to data synthesis
gives an overview of the characteristics of the included studies. Studies were undertaken in a range of countries and recruited a total of 7938 participants.
Table 1. Characteristics of included studies
Interventions varied considerably; single interventions consisted of multiple and overlapping components. Ten studies assessed CBT (versus wait-list, MI, or another intervention), five studies assessed contingency management (versus CBT or another intervention), nine studies mainly assessed MI (versus wait-list or another intervention) and one assessed web-based counselling.
All the included RCTs measured the effect of the intervention(s) on participant’s cannabis usage, although, the way in which this was measured varied greatly by study. Eleven studies measured participants’ severity of cannabis dependence (measured via self-report using various instruments, most frequently using the Severity of Dependence Scale (SDS) or Addiction Severity Index (ASI) (McLellan, Luborsky, Woody, & O'Brien, Citation1980; Swift, Copeland, & Hall, Citation1998). Fourteen studies measured participants’ number of cannabis related problems (measured using various instruments, including the Cannabis Problems Questionnaire (CPQ) (Copeland, Gilmour, Gates, & Swift, Citation2005). Nineteen studies measured participant’s usage of the intervention or session attendance. For the narrative synthesis, outcomes were grouped into four groups: cannabis usage, severity of dependence, number of dependence symptoms and number of cannabis problems.
Quality assessment
Most studies used an appropriately generated randomisation sequence, with 17 studies being deemed “low risk”, seven “unclear risk”, and one “high risk” for this measure. No studies blinded study participants to group allocation; we deemed this form of blinding to be impossible for the interventions under review. As many of the outcome measures were self-reported, outcomes were deemed to have been blinded if the outcome assessors were blinded to group allocation. This form of blinding was poorly reported – in 14 studies, blinding of outcome assessment was unclear or unreported. Participant attrition was well reported but high, ranging from 6% to 79% (mean 31%, median 25%); 17 studies were rated as high risk for this attribute (with attrition of more than 20% at the final follow-up time point). Regarding overall risk, 18 studies were deemed to be “high risk”, in seven studies the risk was unclear and no studies were deemed to be “low risk”.
Effectiveness of psychological and psychosocial treatments
and summarise the studies included in the seven comparison groups, including interventions and between group differences at post-treatment () and follow-up ().
Table 2. Results at post-treatment
Table 3. Results at follow-up
CBT versus wait-list control
Six studies (Babor et al., Citation2004; Copeland, Swift, Roffman, & Stephens, Citation2001; Hoch et al., Citation2012, Citation2014; Jungerman, Andreoni, & Laranjeira, Citation2007; Stephens, Roffman, & Curtin, Citation2000) compared CBT (4–14 sessions) versus wait-list control. Five (Babor et al., Citation2004; Copeland et al., Citation2001; Hoch et al., Citation2012, Citation2014; Jungerman et al., Citation2007) provided individual CBT sessions and one (Stephens et al., Citation2000) group sessions. CBT interventions also incorporated other components including case management (Babor et al., Citation2004) and a social support group (Stephens et al., Citation2000). The majority of studies only included participants who met the Diagnostic and Statistical Manual of Mental Disorders (DSM) criteria for cannabis dependence (Babor et al., Citation2004; Copeland et al., Citation2001; Hoch et al., Citation2012; Stephens et al., Citation2000). The remaining studies included participants who used cannabis a certain number of times a month (Hoch et al., Citation2014; Jungerman et al., Citation2007).
Five studies (Babor et al., Citation2004; Hoch et al., Citation2012, Citation2014; Jungerman et al., Citation2007; Stephens et al., Citation2000) reported post treatment (5–18 weeks) outcomes. All five reported significantly better results for CBT (4–14 sessions) than for wait-list on all key outcomes (cannabis usage, severity of dependence, dependence symptoms, and cannabis problems). Effect sizes were small to very large where reported. Full intervention session attendance ranged from 65% (Hoch et al., Citation2014) to 86% (Hoch et al., Citation2012).
Only one study (Copeland et al., Citation2001) reported between-group data at a later follow-up (9 months) point than post-treatment. This study reported significantly better results for CBT (six sessions) than wait-list on all key outcomes at 9 months post-baseline except days used (p NR).
CBT or psychotherapy versus brief MI
Four studies (Babor et al., Citation2004; Budney, Higgins, Radonovich, & Novy, Citation2000; Copeland et al., Citation2001; Stephens et al., Citation2000) compared CBT (6–14 sessions) versus brief MI/MET (1–4 sessions). Three studies (Babor et al., Citation2004; Budney et al., Citation2000; Copeland et al., Citation2001) provided individual CBT sessions while one (Stephens et al., Citation2000) compared group CBT against individual MET. CBT interventions also included case management (Babor et al., Citation2004) and a social support group (Stephens et al., Citation2000). All studies included only participants who met the DSM criteria for cannabis dependence.
Three CBT studies reported between group data post-treatment (at 12–18 weeks). One study (Babor et al., Citation2004) reported significant between group differences, finding that 9-session CBT was significantly better than 2-session MET on most key outcomes (all except joints per day), with small to medium effect sizes (d = 0.4–0.5, for days used and dependence symptoms). Full intervention session attendance ranged from 69% (Copeland et al., Citation2001) to 86% (Stephens et al., Citation2000).
Three studies reported between group data at later follow-ups. Results were mixed, with significant differences identified in cannabis usage outcomes in one study (Babor et al., Citation2004) at 9 and 15 months, but not at 9 months (Copeland et al., Citation2001) or 16 months (Stephens et al., Citation2000) in the other two studies. A significant difference was found between groups in the one study that reported severity of dependence at 9 months (p = 0.04) (Copeland et al., Citation2001) and dependence symptoms at 9 months (p < 0.01, d = 0.31) (Babor et al., Citation2004), but no significant differences were found when measured at 16 months in another study (Stephens et al., Citation2000). All three studies that reported cannabis problems found no significant between group differences at 9 months (Babor et al., Citation2004; Copeland et al., Citation2001), 15 months (Babor et al., Citation2004) or 16 months (Stephens et al., Citation2000).
CBT versus other interventions (or different CBT format or duration)
Four studies (Jungerman et al., Citation2007; Kadden, Litt, Kabela-Cormier, & Petry, Citation2007; Sobell, Sobell, & Agrawal, Citation2009; Stephens, Roffman, & Simpson, Citation1994) compared CBT (4–10 sessions) against another intervention, comprising a social support group (Stephens et al., Citation1994), case management sessions (Kadden et al., Citation2007) or compared individual versus group CBT (Sobell et al., Citation2009) or CBT over different durations (Jungerman et al., Citation2007). One study only included participants who met the DSM criteria (Kadden et al., Citation2007), while two included participants who consumed cannabis at a certain frequency (Jungerman et al., Citation2007; Stephens et al., Citation1994) and one study included individuals without “severe dependence” (Sobell et al., Citation2009). All four studies showed no significant between group differences at post-treatment for cannabis usage outcomes, but showed mixed results for cannabis problems and severity of dependence outcomes, with the same study (Jungerman et al., Citation2007) showing significant results for each. The same study also identified significant differences for dependence symptoms outcomes. Two studies reported average number of sessions attended across both study groups, reporting 76% (Stephens et al., Citation1994) and 58% (Kadden et al., Citation2007) attendance, with no significant differences between groups. At later follow-up points, no significant differences were identified for any key outcomes at 14 or 15 months.
Telephone or web-based CBT or counselling versus wait-list or other interventions
Three studies (Gates, Norberg, Copeland, & Digiusto, Citation2012; Rooke, Copeland, Norberg, Hine, & McCambridge, Citation2013; Tossmann, Jonas, Tensil, Lang, & Struber, Citation2011) compared telephone or web-based interventions versus wait-list or education controls. Interventions included telephone-CBT (Gates et al., Citation2012), web-CBT (Rooke et al., Citation2013) and web-counselling (Tossmann et al., Citation2011). One study included participants only if they met the DSM criteria for cannabis dependence (Tossmann et al., Citation2011), while two included participants based on their cannabis intake (Gates et al., Citation2012; Rooke et al., Citation2013).
Two studies measured outcomes at post-treatment time points (Gates et al., Citation2012; Rooke et al., Citation2013). Mixed results were identified for cannabis use outcomes, with significant reductions in usage for a 6 week CBT/MI intervention, with a small effect size (d = 0.30) (Rooke et al., Citation2013), and significant reductions in joints per day for a 3 week telephone CBT/MI intervention (Gates et al., Citation2012). Other cannabis use outcomes in the same two studies reported insignificant results (Gates et al., Citation2012; Rooke et al., Citation2013). The effect of web or telephone interventions on severity of dependence was mixed, with one study showing a significant improvement (Gates et al., Citation2012) and another showing insignificant results and small effect (d = 0.30) (Rooke et al., Citation2013). One study reported the effect on cannabis problems and reported a significant decrease (Gates et al., Citation2012). All three studies reported telephone or web session completion, reporting 51% (Tossmann et al., Citation2011), 58% (Rooke et al., Citation2013) and 81% (Gates et al., Citation2012) attendance.
All three studies reported follow-up outcomes at 3 months. Cannabis usage outcomes were mixed, with some significant improvements in two studies providing 6–7 week interventions (Rooke et al., Citation2013; Tossmann et al., Citation2011), but no significant improvements in a study providing a 3 week intervention (Gates et al., Citation2012). Severity of dependence outcomes followed a similar pattern to that at post-treatment. Improvements in cannabis problems were significant in the one study reporting such outcomes (Gates et al., Citation2012).
Brief MI versus wait-list or assessment only
Ten studies (Babor et al., Citation2004; Copeland et al., Citation2001; de Dios et al., Citation2012; Gmel, Gaume, Bertholet, Fluckiger, & Daeppen, Citation2013; Humeniuk et al., Citation2012; Lee, Neighbors, Kilmer, & Larimer, Citation2010; Lee et al., Citation2013; Stein, Hagerty, Herman, Phipps, & Anderson, Citation2011; Stephens, Roffman, Fearer, Williams, & Burke, Citation2007; Stephens et al., Citation2000) compared a brief intervention (1–2 sessions of MET, MI or personalised feedback) versus wait-list or assessment only. One study assessed a web-based intervention (personalised feedback) (Lee et al., Citation2010). One study provided a group MI session (Gmel et al., Citation2013) while the other nine provided individual sessions. Three studies included participants only if they met the DSM criteria for cannabis dependence (Babor et al., Citation2004; Copeland et al., Citation2001; Stephens et al., Citation2000) while four studies included participants who consumed a certain quantity of cannabis per month (1–15 d used per month) (de Dios et al., Citation2012; Lee et al., Citation2013; Stein et al., Citation2011; Stephens et al., Citation2000), while this was not reported in two studies (Gmel et al., Citation2013; Humeniuk et al., Citation2012). Five studies implemented an upper age limit for study inclusion, ranging from 19 to 29 years (de Dios et al., Citation2012; Gmel et al., Citation2013; Lee et al., Citation2010, Citation2013; Stein et al., Citation2011).
Five studies reported between-group data post-treatment, showing a mixed picture with some significant effects. Cannabis use outcomes largely showed significant improvements, with all studies reporting at least one significant outcome. Effect sizes (reported in one study) were small to medium (d = 0.29–0.59) (Babor et al., Citation2004). Three studies reported dependence symptoms, identifying a significant improvement (Babor et al., Citation2004; Stephens et al., Citation2000, Citation2007) but a small to medium effect size in the one study that reported effect sizes (Babor et al., Citation2004). Three studies reported the effect on cannabis problems, with one reporting a significant effect (Stephens et al., Citation2000). Eight studies (Babor et al., Citation2004; Copeland et al., Citation2001; de Dios et al., Citation2012; Humeniuk et al., Citation2012; Lee et al., Citation2013; Stein et al., Citation2011; Stephens et al., Citation2000, Citation2007) reported session attendance, ranging from 80% to 100%.
At later follow-ups, seven studies reported mixed between-group results, with some significant effects. Cannabis use outcomes were mixed; studies reporting at shorter follow-up time-points (3 months) were more likely to report significant between group differences that those reporting long-term outcomes (6–9 months). One study reported RRs (risk ratios) at 3 and 6 months, showing reduced RR of cannabis usage at 3 months (RR = 0.96 and 0.76) but increased risk at 6 months (RR = 1.11) (Lee et al., Citation2013). One study reported significant improvements in severity of dependence at 9 months (Copeland et al., Citation2001). Three studies reported participant’s cannabis problems between 3 and 9 months, with significant results reported in one study (Copeland et al., Citation2001).
Brief MI versus other interventions
Three studies (Fernandes et al., Citation2010; Fischer, Jones, Shuper, & Rehm, Citation2012; Stephens et al., Citation2007) compared a brief intervention (one session of MI or telephone MI) versus education controls (regarding cannabis or general health). All MI sessions were individual (not group). Two studies selected participants who used cannabis a certain number of days/uses per week (Fischer et al., Citation2012; Stephens et al., Citation2007); this was not reported in one study (Fernandes et al., Citation2010).
One study (Stephens et al., Citation2007) of MI (one session) versus education control reported post treatment outcomes, reporting significant improvements in cannabis usage, dependence and cannabis problems outcomes. The same study reported session attendance, where 89% of participants attended a MI session and 94% attended a “cannabis education” session.
At later follow-ups, all three studies reported cannabis use outcomes at time points ranging from 3 to 12 months, with one finding a significant result at 6 months (OR = 1.6) (Fernandes et al., Citation2010). One study reported both dependence symptoms and cannabis problems at 6 and 12 months, finding a significant decrease in dependence symptoms at both time points, but no significant differences in cannabis problems (Stephens et al., Citation2007).
Contingency management (vouchers for abstinence) versus other interventions
Five studies (Budney, Moore, Rocha, & Higgins, Citation2006; Budney et al., Citation2000, Citation2011; Kadden et al., Citation2007; Litt, Kadden, & Petry, Citation2013) compared contingency management (vouchers for abstinence assessed via urine tests), alone or in combination with CBT, versus other interventions. One study also assessed computer-based CBT plus contingency management (Budney et al., Citation2011). Comparators included CBT (Budney et al., Citation2000, Citation2006; Kadden et al., Citation2007) (9–14 sessions), MET (Budney et al., Citation2000, Citation2011) (2–4 sessions), case management (Kadden et al., Citation2007; Litt et al., Citation2013) (nine sessions) and CBT + vouchers (CBT plus vouchers for abstinence) for completed CBT homework (Litt et al., Citation2013). All studies included cannabis users only if they met the DSM criteria for cannabis dependence.
Four studies reported between-group data post-treatment; results favoured either CBT + vouchers or vouchers alone over CBT alone. Cannabis use outcomes showed a majority of significant differences between groups, with the results favouring either CBT + vouchers or vouchers alone over CBT alone. One study reported effect sizes for 6 weeks or more of continuous abstinence, which favoured vouchers over CBT and CBT plus vouchers over CBT alone (Budney et al., Citation2006). One study reported cannabis problems, and another reported severity of dependence, both reporting non-significant differences between contingency management and other interventions (Budney et al., Citation2006, Citation2011). Session attendance was reported by two studies and was similar between interventions, ranging from 61% to 69% (Budney et al., Citation2006; Litt et al., Citation2013).
Later follow-ups indicated that positive results were maintained for combined treatment with CBT + vouchers. However, the beneficial short-term results for vouchers alone were less likely to be maintained long-term. Four studies reported between-group data at 14–15 months, with all four reporting cannabis use outcomes, the majority of which were not significantly different between groups (Budney et al., Citation2006, Citation2011; Kadden et al., Citation2007; Litt et al., Citation2013). Severity of dependence and dependence symptoms were reported at 14 months in one study comparing case management, contingency management and CBT, finding no significant differences between groups (Kadden et al., Citation2007). Two studies found no significant differences between groups for cannabis problems at 12 and 14 months (Budney et al., Citation2006; Kadden et al., Citation2007).
Discussion
Across six studies of CBT (4–14 sessions) versus wait-list, CBT was significantly better on most outcomes (cannabis use, severity of dependence, cannabis problems), post-treatment (in all five studies with data) and at 9 months (in the one study with later follow-up). Four studies comparing CBT (6–14 sessions) against briefer MI/MET (1–4 sessions) gave mixed results, with two studies showing better results for CBT post-treatment and at 9–16 months, while two further studies showed few between-group differences; both CBT and MI gave significant improvements from baseline. One study of CBT versus social support group (10 sessions each) and another of CBT versus case management (nine sessions each) showed no significant differences between groups but all groups significantly improved from baseline with changes maintained at 14–15 months. One study each of telephone-CBT, web-CBT and web-counselling all showed significant improvements over wait-list or education control post-treatment and at 3 months. Ten studies assessing brief MI/MET (1–2 sessions) versus wait-list or assessment only (AO) gave mixed results, with brief MI appearing significantly better on some outcomes but not others, post-treatment and at 3–9 months. Results were similar for three studies comparing brief MI against education controls. Compared with studies assessing other interventions, those assessing brief MI tended to include younger participants due to six of the 13 studies (de Dios et al., Citation2012; Fischer et al., Citation2012; Gmel et al., Citation2013; Lee et al., Citation2010, Citation2013; Stein et al., Citation2011) implementing an upper age limit for study inclusion, ranging from 19 to 29 years. Five studies assessed contingency management (monetary vouchers for abstinence) in a clinically dependent population. Vouchers alone and CBT plus vouchers gave better results than CBT or MET alone post-treatment (three studies), while at 14–15 months positive results were maintained for CBT plus vouchers but less so for vouchers alone (two studies). A minority of the studies (n = 9) reported effect sizes for between group differences at post-treatment, and five studies reported such figures at later outcome assessments.
This review is inclusive in scope, including a wide range of studies, interventions and outcomes. Only RCTs were included, resulting in only the highest level evidence being analysed. This inclusivity resulted in a number of limitations. First, the inclusivity of studies resulted in the reporting of a diverse and heterogeneous group of outcomes. Peters, Nich and Carroll (Citation2011) suggest a framework for evaluating outcomes in RCTs of cannabis cessation therapies, suggesting that frequency of cannabis use, severity of cannabis use, and psychosocial functioning provide the best model of end of treatment outcomes (Peters et al., Citation2011). In this review, we presented all key outcomes reported by the included studies, grouped into four categories (cannabis use, severity of dependence, dependence symptoms, and cannabis problems). Second, many studies did not report between group differences at post-treatment or follow-up, instead reporting change from baseline in each group, thus limiting the comparison that can be made between treatments. Those that did report between group differences did not always report effect sizes. The studies that did report effect sizes only report confidence intervals in the minority of cases. Finally, the inclusion criteria used to recruit participants into the studies were diverse. The majority of studies that assessed CBT recruited participants who met the DSM criteria for cannabis dependence, while other studies, assessing other interventions, recruited those who utilised cannabis a certain frequency per month. Therefore, currently we can only assess the effectiveness of CBT on “clinically dependent” individuals, and other interventions on individuals who may not be dependent on cannabis and have a wide range of cannabis usage.
To our knowledge, this is the first review that has attempted to synthesise all evidence regarding available psychological and psychosocial treatments for cannabis cessation. Other systematic reviews have included a similar set of studies, but have undertaken a meta-analysis. The most recent of which undertook a meta-analysis of 10 studies, two of which were not included in this review (due to participants being referred via the criminal justice setting in one study) (Sinha, Easton, Renee-Aubin, & Carroll, Citation2003), and the study not reporting outcomes for marijuana using population only in another (Davis et al., Citation2015; Roffman, Stephens, Simpson, & Whitaker, Citation1988). Behavioural therapies outperformed control conditions when all outcome and time variables were combined (Hedges’ g = 0.44). Dutra et al. reviewed psychosocial interventions for cannabis dependence, including five studies that included regular cannabis users, two of which were not included in this review (due to participants being referred via the criminal justice setting in one study (Carroll et al., Citation2006) and participants being, on average, less than 18 years of age in another (Martin & Copeland, Citation2008), finding a large effect on cannabis use (d = 0.81, 95% CI = 0.25–1.36)). The present review did not identify conclusive evidence to support these findings. Meta-analysis, although providing clinically relevant estimations of effect sizes, is not always relevant, especially when undertaken on a set of heterogeneous studies (Higgins & Green, Citation2011). Although studies in this review were grouped by intervention and comparator, there was still considerable variation within the groups. The most notable heterogeneity within each comparison group within this review was the length and frequency of the intervention, the outcomes measures and follow-up time points. The present review did not include a number of studies included in previous reviews. This possibly limited the scope for undertaking meta-analyses, but did prevent further heterogeneity from being present in the data.
Future studies should carefully consider trial methodology. Many studies in the present review either did not follow up participants at post-treatment or later follow-ups, or did not undertake between group inferences at these time points. Studies should follow up patients beyond treatment cessation, and may wish to include an inactive control arm. Wait-list controls with long-term follow-up are also valuable, although this needs to be balanced against ethical considerations and acceptability to trial participants.
Conclusions
This systematic review has identified a disparate evidence base that differed most notably in the nature and length of the interventions, the comparator groups, the populations studied and the outcomes measured (differing in metrics used, statistics reported, and follow-up periods). There was a distinct lack of between group comparisons at long-term follow-up time points. Individuals recruited to the trials differed considerably in their cannabis use at baseline, with a cohort of trials (involving mostly CBT and contingency management interventions) requiring individuals to meet the DSM criteria for cannabis dependence. Based on the available evidence, courses of CBT improved outcomes in a self-selected population of cannabis users who are clinically dependent, although there is a lack of evidence to support the long-term effect of such interventions. Brief MI improved outcomes at post-treatment in a younger non-clinically dependent population, but this was not sustained in the long term (3–12 months). There is some evidence that CBT (6–14 sessions) may be more effective than briefer MI interventions although results were mixed. Contingency management may also enhance long-term outcomes in combination with CBT in a clinically dependent population. Intervention retention varied within comparison groups, although brief MI sessions appeared to have the highest attendance.
Declaration of interest
This project was funded by the NIHR Health Technology Assessment Programme (project number 13/70/01). It will be published in full in Health Technology Assessment , Vol. 19, No. 56. See the HTA Programme website (www.hta.ac.uk ) for further project information. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Department of Health. The authors report that they have no conflicts of interest.
References
- Asbridge, M., Duff, C., Marsh, D.C., & Erickson, P.G. (2014). Problems with the identification of “problematic” cannabis use: Examining the issues of frequency, quantity, and drug use environment. European Addiction Research, 20, 254–267.
- Budney, A.J. (2013). Development and efficacy test of computerized treatment for marijuana dependence. Retrieved from https://www.clinicaltrials.gov/ct2/show/study/NCT00594659.
- Babor, T.F., Carroll, K., Christiansen, K., Donaldson, J., Herrell, J., & Kadden, Ret al. (2004). Brief treatments for cannabis dependence: Findings from a randomized multisite trial. Journal of Consulting and Clinical Psychology, 72, 455–466.
- Budney, A.J., Higgins, S.T., Radonovich, K.J., & Novy, P.L. (2000). Adding voucher-based incentives to coping skills and motivational enhancement improves outcomes during treatment for marijuana dependence. Journal of Consulting & Clinical Psychology, 68, 1051–1061.
- Budney, A.J., Moore, B.A., Rocha, H.L., & Higgins, S.T. (2006). Clinical trial of abstinence-based vouchers and cognitive-behavioral therapy for cannabis dependence. Journal of Consulting & Clinical Psychology, 74, 307–316.
- Budney, A.J., Stanger, C., Costello, P., Fearer, S., Walker, D.D., & Bickel, W.K. (2011). Computer-assisted delivery of behavioral treatment for cannabis use disorders: Preliminary results from a controlled trial and implications for dissemination. Proceedings of the 73rd Annual Scientific Meeting of the College on Problems of Drug Dependence, 2011 June 18–23, Hollywood, FL (Vol. 22, Abstract 85).
- Carroll, K.M., Easton, C.J., Nich, C., Hunkele, K.A., Neavins, T.M., Sinha, R., … Bruce JR. (2006). The use of contingency management and motivational/skills-building therapy to treat young adults with marijuana dependence. Journal of Consulting & Clinical Psychology, 74, 955–966.
- Copeland, J., Gilmour, S., Gates, P., & Swift, W. (2005). The Cannabis Problems Questionnaire: Factor structure, reliability, and validity. Drug and Alcohol Dependence, 80, 313–319.
- Copeland, J., Swift, W., Roffman, R., & Stephens, R. (2001). A randomized controlled trial of brief cognitive-behavioral interventions for cannabis use disorder. Journal of Substance Abuse Treatment, 21, 55–64.
- Davis, C.G., Thomas, G., Jesseman, R., & Mazan, R. (2009). Drawing the line on risky use of cannabis: Assessing problematic use with the ASSIST. Addiction Research & Theory, 17, 322–332.
- Davis, M.L., Powers, M.B., Handelsman, P., Medina, J.L., Zvolensky, M., & Smits, J.A. (2015). Behavioral therapies for treatment-seeking cannabis users: A meta-analysis of randomized controlled trials. Evaluation & the Health Professions, 38, 94–114.
- de Dios, M.A., Herman, D.S., Britton, W.B., Hagerty, C.E., Anderson, B.J., & Stein, M.D. (2012). Motivational and mindfulness intervention for young adult female marijuana users. Journal of Substance Abuse Treatment, 42, 56–64.
- DeMarce, J.M., Stephens, R.S., & Roffman, R.A. (2005). Psychological distress and marijuana use before and after treatment: Testing cognitive-behavioral matching hypotheses. Addictive Behaviors, 30, 1055–1059.
- Denis, C., Lavie, E., Fatseas, M., & Auriacombe, M. (2006). Psychotherapeutic interventions for cannabis abuse and/or dependence in outpatient settings. [Review] [58 refs][Update in Cochrane Database Syst Rev. 2013;6:CD005336; PMID: 23780682]. Cochrane Database of Systematic Reviews, CD005336.
- Dutra, L., Stathopoulou, G., Basden, S.L., Leyro, T.M., Powers, M.B., & Otto, M.W. (2008). A meta-analytic review of psychosocial interventions for substance use disorders. American Journal of Psychiatry, 165, 179–187.
- European Monitoring Centre for Drugs and Drug Addiction. (2014). European Drug Report. Trends and developments 2014. Lisbon: EMCDDA.
- Fischer, B., Dawe, M., McGuire, F., Shuper, P.A., Capler, R., Bilsker, D. … Rehm, J. (2013). Feasibility and impact of brief interventions for frequent cannabis users in Canada. Journal of Substance Abuse Treatment, 44, 132–138.
- Fernandes, S., Ferigolo, M., Benchaya, M.C., Moreira, T.C., Pierozan, P.S., Mazoni, C.G., … Barros, H.M. (2010). Brief motivational intervention and telemedicine: A new perspective of treatment to marijuana users. Addictive Behaviors, 35, 750–755.
- Fischer, B., Jones, W., Shuper, P., & Rehm, J. (2012). 12-Month follow-up of an exploratory “brief intervention” for high-frequency cannabis users among Canadian university students. Substance Abuse Treatment, Prevention, & Policy, 7, 15.
- Gates, P.J., Norberg, M.M., Copeland, J., & Digiusto, E. (2012). Randomized controlled trial of a novel cannabis use intervention delivered by telephone. Addiction, 107, 2149–2158.
- Gmel, G., Gaume, J., Bertholet, N., Fluckiger, J., & Daeppen, J.B. (2013). Effectiveness of a brief integrative multiple substance use intervention among young men with and without booster sessions. Journal of Substance Abuse Treatment, 44, 231–240.
- Higgins, J.P., & Green, S. (1 March 2011). Cochrane handbook for systematic reviews of interventions. Chichester (UK): The Cochrane Collaboration (2015).
- Hoch, E., Buhringer, G., Pixa, A., Dittmer, K., Henker, J., & Seifert, A. (2014). CANDIS treatment program for cannabis use disorders: Findings from a randomized multi-site translational trial. Drug & Alcohol Dependence, 134, 185–193.
- Hoch, E., Noack, R., Henker, J., Rohrbacher, H., Pixa, A., Buhringer, G. … Wittchen, H.U. (2008). Tailoring CBT to problem profiles of patients with cannabis use disorders. Sucht, 54, 306.
- Hoch, E., Noack, R., Henker, J., Pixa, A., Hofler, M., Behrendt, S., … Wittchen, H.U. (2012). Efficacy of a targeted cognitive-behavioral treatment program for cannabis use disorders (CANDIS). European Neuropsychopharmacology, 22, 267–280.
- Humeniuk, R., Ali, R., Babor, T., Souza-Formigoni, M.L., de Lacerda, R.B., Ling, W., … Vendetti, J. (2012). A randomized controlled trial of a brief intervention for illicit drugs linked to the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) in clients recruited from primary health-care settings in four countries. Addiction, 107, 957–966.
- Jungerman, F.S., Andreoni, S., & Laranjeira, R. (2007). Short term impact of same intensity but different duration interventions for cannabis users. Drug & Alcohol Dependence, 90, 120–127.
- Kadden, R.M., Litt, M.D., Kabela-Cormier, E., & Petry, N.M. (2007). Abstinence rates following behavioral treatments for marijuana dependence. Addictive Behaviors, 32, 1220–1236.
- Lee, C.M., Kilmer, J.R., Neighbors, C., Atkins, D.C., Zheng, C., Walker, D.D., & Larimer, M.E. (2013). Indicated prevention for college student marijuana use: A randomized controlled trial. Journal of Consulting and Clinical Psychology, 81, 702–709.
- Lee, C.M., Neighbors, C., Kilmer, J.R., & Larimer, M.E. (2010). A brief, web-based personalized feedback selective intervention for college student marijuana use: A randomized clinical trial. Psychology of Addictive Behaviors, 24, 265–273.
- Litt, M.D., Kadden, R.M., Kabela-Cormier, E., & Petry, N.M. (2008). Coping skills training and contingency management treatments for marijuana dependence: Exploring mechanisms of behavior change. Addiction, 103, 638–648.
- Litt, M.D., Kadden, R.M., & Petry, N.M. (2013). Behavioral treatment for marijuana dependence: Randomized trial of contingency management and self-efficacy enhancement. Addictive Behaviors, 38, 1764–1775.
- Litt, M.D., Kadden, R.M., Stephens, R.S., & Marijuana Treatment Project Research Group. (2005). Coping and self-efficacy in marijuana treatment: Results from the marijuana treatment project. Journal of Consulting & Clinical Psychology, 73, 1015–1025.
- Lozano, B.E., Stephens, R.S., & Roffman, R.A. (2006). Abstinence and moderate use goals in the treatment of marijuana dependence. Addiction, 101, 1589–1597.
- Martin, G., & Copeland, J. (2008). The adolescent cannabis check-up: Randomized trial of a brief intervention for young cannabis users. Journal of Substance Abuse Treatment, 34, 407–414.
- McLellan A.T., Luborsky L., Woody G.E., & O'Brien C.P. (1980). An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. The Journal of Nervous and Mental Disease, 168, 26–33.
- Moore, B.A., & Budney, A.J. (2013). Relapse in outpatient treatment for marijuana dependence. Journal of Substance Abuse Treatment, 25, 85–89.
- National Institute for Health and Care Excellence (NICE). (2007). NICE Clinical Guideline 51: Drug misuse: Psychosocial interventions. London: NICE.
- Peters, E.N., Nich, C., & Carroll, K.M. (2011). Primary outcomes in two randomized controlled trials of treatments for cannabis use disorders. Drug & Alcohol Dependence, 118, 408–416.
- Popay, J., Roberts, H., Sowden, A., Petticrew, M., Arai, L., Rodgers, M., …. Duffy, S. (2006). Guidance on the conduct of narrative synthesis in systematic reviews. ESRC Methods Programme.
- Roffman, R.A., Stephens, R.S., Simpson, E.E., & Whitaker, D.L. (1988). Treatment of marijuana dependence: Preliminary results. Journal of Psychoactive Drugs, 20, 129–137.
- Rooke, S., Copeland, J., Norberg, M., Hine, D., & McCambridge, J. (2013). Effectiveness of a self-guided web-based cannabis treatment program: Randomized controlled trial. Journal of Medical Internet Research, 15, e26.
- Rosnow, R., & Rosenthal, R. (1996). Computing contrasts, effect sizes, and counternulls on other people's published data: General procedures for research consumers. Psychological Methods, 1, 331–340.
- Schulz, K.F., & Grimes, D.A. (2002). Sample size slippages in randomised trials: Exclusions and the lost and wayward. Lancet, 359, 781–785.
- Sinha, R., Easton, C., Renee-Aubin, L., & Carroll, K.M. (2003). Engaging young probation-referred marijuana-abusing individuals in treatment: A pilot trial. American Journal on Addictions, 12, 314–323.
- Sobell, L.C., Sobell, M.B., & Agrawal, S. (2009). Randomized controlled trial of a cognitive-behavioral motivational intervention in a group versus individual format for substance use disorders. Psychology of Addictive Behaviors, 23, 672–683.
- Stein, M.D., Hagerty, C.E., Herman, D.S., Phipps, M.G., & Anderson, B.J. (2011). A brief marijuana intervention for non-treatment-seeking young adult women. Journal of Substance Abuse Treatment, 40, 189–198.
- Stephens, R.S., Roffman, R.A., & Curtin, L. (2000). Comparison of extended versus brief treatments for marijuana use. Journal of Consulting & Clinical Psychology, 68, 898–908.
- Stephens, R.S., Roffman, R.A., Fearer, S.A., Williams, C., & Burke, R.S. (2007). The Marijuana Check-up: Promoting change in ambivalent marijuana users. Addiction, 102, 947–957.
- Stephens, R.S., Roffman, R.A., & Simpson, E.E. (1994). Treating adult marijuana dependence: A test of the relapse prevention model. Journal of Consulting & Clinical Psychology, 62, 92–99.
- Swift, W., Copeland, J., & Hall, W. (1998). Choosing a diagnostic cut-off for cannabis dependence. Addiction, 93, 1681–1692.
- Tossmann, H.P., Jonas, B., Tensil, M.D., Lang, P., & Struber, E. (2011). A controlled trial of an internet-based intervention program for cannabis users. Cyberpsychology, Behavior and Social Networking, 14, 673–679.
- U.S Department of Health and Human Services. (2014). Substance abuse treatment admissions by primary substance of abuse, according to sex, age group, race, and ethnicity year = 2012, United States.
- UNODC. (2013). World Drug Report 2013. New York: United Nations.
- van der Pol, P., Liebregts, N., de, G.R., Korf, D.J., van den Brink, W., & van, L.M. (2013). Predicting the transition from frequent cannabis use to cannabis dependence: A three-year prospective study. Drug and Alcohol Dependence, 133, 352–359.
- WHO ASSIST Working Group. (2002). The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): Development, reliability and feasibility. Addiction, 97, 1183–1194.
- World Health Organization. (2000). ICD-10: International statistical classification of diseases and related health problems. Geneva: WHO.