Abstract
Ceria nanoparticles (NPs) are widely used as fuel catalysts and consequently are likely to enter the environment. Their potential impacts on. biota at environmentally relevant concentrations, including uptake and toxicity, remain to be elucidated and quantitative data on which to assess risk are sparse. Therefore, a definitive assessment of the molecular and phenotypic effects of ceria NPs was undertaken, using well-characterised mono-dispersed NPs as their toxicity is likely to be higher, enabling a conservative hazard assessment. Unbiased transcriptomics and metabolomics approaches were used to investigate the potential toxicity of tightly constrained 4–5 nm ceria NPs to the unicellular green alga, Chlamydomonas reinhardtii, a sentinel freshwater species. A wide range of exposure concentrations were investigated from predicted environmental levels, to support hazard assessment, to supra-environmental levels to provide insight into molecular toxicity pathways. Ceria NPs were internalised into intracellular vesicles within C. reinhardtii, yet caused no significant effect on algal growth at any exposure concentration. Molecular perturbations were only detected at supra-environmental ceria NP-concentrations, primarily down-regulation of photosynthesis and carbon fixation with associated effects on energy metabolism. For acute exposures to small mono-dispersed particles, it can be concluded there should be little concern regarding their dispersal into the environment for this trophic level.
Introduction
Ceria nanoparticles (NPs) are widely used as catalysts, exploiting their oxygen storing capacity, in particular, as a diesel fuel additive with annual production estimated at up to 700 tonnes (Hendren et al., Citation2011; Park et al., Citation2008a; Xia et al., Citation2008). Other applications include in polishing and coatings and in the biomedical industry for treating inflammation and cancer as well as radiation protection of cells, again exploiting their strong antioxidant properties (Boxall et al., Citation2007; Celardo et al., Citation2011; Hoshino et al., Citation2001; Shah et al., Citation2012). Consequently these NPs are likely to enter the environment, yet their environmental fate and potential impacts on biota are relatively unknown (Boxall et al., Citation2007; Van Hoecke et al., Citation2009). Several uses of ceria NPs lead to non-point source discharge, potentially resulting in low-level and widespread exposure to multiple plant and animal species (Cassee et al., Citation2011). Recently the level of ceria NPs in the aquatic environment has been estimated, focusing on diesel as the primary source for environmental discharge (Boxall et al., Citation2007; Johnson & Park, Citation2012). Based on actual usage data of the number of vehicles, the consumption of fuel containing ceria NPs, and the fraction that will escape vehicle exhausts, Johnson & Park (Citation2012) predicted that direct deposition into UK waters is as low as 0.02 ng/L, rising to ca. 300 ng/L when considering rainfall data and run-off into water courses that are within 20 m of the roads. This prediction is yet to be confirmed by actual measurements and does little to estimate the levels of ceria NPs that may enter the environment from other industries (Boxall et al., Citation2007; Hoshino et al., Citation2001).
While information on the biological effects of ceria NP exposure is available, the findings are somewhat contradictory. Several studies have concluded that there are unlikely to be any adverse health effects of ceria NP exposure, even considering complete release of all ceria NPs (4.4 × 106 particles/cm3) when added to diesel fuel at a level of 5000 µg/L (Fall et al., Citation2007; Park et al., Citation2008a). Xia et al. (Citation2008) showed that ceria NPs were cytoprotective at 25 µg/L due to their antioxidant properties. Conversely, Park et al. (Citation2008b) observed a cytotoxic effect of ceria NPs on a human lung cell line; however, the concentration range tested (5–40 µg/L) was noticeably higher than expected environmental levels. Thill et al. (Citation2006) and Pelletier et al. (Citation2010) found some evidence of bacterial toxicity at extremely high concentrations (1.2–730 mg/L). Moreover, Zhang et al. (Citation2011) discovered that environmentally realistic exposure concentrations (ca. 140–14 000 ng/L) significantly decreased the mean lifespan of nematodes and could induce accumulation of reactive oxygen species (ROS) and oxidative damage in Caenorhabditis elegans. In the aquatic environment the situation is similarly conflicting. Van Hoecke et al. (Citation2009) reported no acute toxicity of ceria NPs to two crustaceans (Daphnia magna and Thamnocephalus platyurus) at concentrations as high as 1 and 5 mg/L, respectively, nor in Danio rerio (zebrafish) embryos up to 200 µg/L. At a molecular level, Lee et al. (Citation2009) determined a genotoxic effect of ceria NPs with increased DNA strand breaks in both D. magna and Cophilaxus riaparius at a concentration of 1 mg/L.
Studies investigating the exposure of aquatic plants and algae to ceria NPs are more in agreement. Rogers et al. (Citation2010) determined a 50% effect concentration (EC50) for growth inhibition of 10.3 mg/L for Pseudokirchneriella subcapitata (a freshwater green alga), while Van Hoecke et al. (Citation2009) determined an EC10 of 2.6–5.4 mg/L and Rodea-Palomares et al. (Citation2011) an EC50 of 2.4–29.6 mg/L for the same species. A study using the cyanobacterial strain Anabaena CPB4337 found an EC50 of 0.27–6.6 mg/L and several further responses in both P. subcapitata and Anabaena, including the generation of hydroxyl radicals, decreased chlorophyll concentrations, inhibition of photosynthesis and increased membrane disruption and cell damage, at 10 µg/L (Rodea-Palomares et al., Citation2012). Interestingly, none of these aquatic toxicity studies utilised predicted environmentally relevant concentrations and no adverse effects on aquatic organisms have been detected below 10 µg/L, which is ca. 30-fold higher than the maximum predicted levels in the aquatic environment (see above). There is a vast array of properties and levels of characterisation that need to be considered when investigating ceria NPs and the majority of these studies are limited in this context, making interpretation difficult (Baalousha et al., Citation2012a,Citationb). Additionally, nominal exposure concentrations have typically been reported, which do not allow for aggregation, absorptive losses or other dynamic changes during the exposure period.
While interactions of nanomaterials with the components of the environment are inevitable, the general consequences of such interactions remain unclear and sparse quantitative data are available on which to assess risk (Lowry et al., Citation2012; Pelletier et al., Citation2010; Seaton et al., Citation2010; Tejamaya et al., Citation2012). Thus, there still remains a critical need for interdisciplinary approaches to understand the degree of exposures to NPs, their uptake and potential toxic effects on human and environmental health (Cassee et al., Citation2011; Madl & Pinkerton, Citation2009; Maynard et al., Citation2006). Adverse outcome pathways (AOPs) represent a new concept in risk assessment that can bring a wide range of emerging tools and knowledge together to create a link between so-called molecular initiating events and adverse outcomes at higher levels of biological organisation, and have considerable potential to advance regulatory ecotoxicology (Ankley et al., Citation2010; Volz et al., Citation2011). Ecotoxicogenomic approaches, including transcriptomics and metabolomics, can provide an unbiased assessment of an organism’s response to NPs and hence help to identify these adaptive and toxic molecular response pathways (Ankley et al., Citation2006; Snape et al., Citation2004; Sturla et al., Citation2014). Such ‘omics approaches are increasingly being used in aquatic toxicology, including to elucidate the molecular effects of NP exposure (Poma & Di Giorgio, Citation2008; Poynton et al., Citation2012; Simon et al., Citation2013; Tsyusko et al., Citation2012).
Here we focused on the freshwater environment as an anticipated sink for NP discharges. The behaviour of metal oxide NPs in freshwater is complex, including adsorption onto and intake by biota, dissolution, aggregation and sedimentation. We focused on dispersed NPs as their toxicity is likely to be higher, enabling a conservative assessment of their maximum possible hazard to the freshwater environment (Klaine et al., Citation2008). An integrated ‘omics approach incorporating both transcriptomics and metabolomics was used to investigate the potential toxicity of tightly constrained and characterised ceria NPs to the unicellular green alga, Chlamydomonas reinhardtii, a sentinel species in the freshwater environment. Our aim was to achieve a more comprehensive and definitive assessment of the responses of this sentinel species to ceria NPs than reported previously, using well-defined nanoparticles and unbiased molecular analyses. We investigated a wide range of exposure concentrations from predicted environmental levels, to inform on hazard assessment, to supra-environmental levels to provide insight into the molecular toxicity pathways activated by ceria NP exposure. Consistent with the AOP concept and a weight of evidence approach to environmental risk assessment, these molecular measurements were made alongside standard phenotypic measures of algal growth.
Experimental section
Ceria NP synthesis and characterisation in exposure media
The PVP-coated ceria NPs tested in this study were synthesised and characterised as described previously (Merrifield et al., Citation2013). Larger, PVP-coated “bulk” particles of ca. 500–600 nm were prepared in a similar manner, with identical reagents, but produced by heating an unstirred suspension at 140°C for 4 h. Dissolution rates of Ce from the NPs were determined as described in Supplementary Information (SI).
Culturing of Chlamydomonas reinhardtii and ceria NP uptake and exposure studies
Chlamydomonas reinhardtii cultures (CCAP strain 11/32c) were grown and exposed under standard conditions, as described in SI. Specifically, growth inhibition (EC50) studies were of 72-h duration, under continuous light as detailed in standard test guidelines (OECD, Citation2002). Studies were conducted over a wide range of nominal ceria NP concentrations (0.5–80 000 µg/L), also for ionic Ce (CeNO3; 0.5–32 mg/L) and free PVP (0.56–56.44 mg/L). ‘Omics studies were performed at two dosing regimens: supra-environmental levels (80, 400, 2000 and 10 000 µg/L; n = 8) and predicted environmental concentrations (0.029, 0.144 and 0.72 µg/L; n = 4), including relevant controls. Measurements of ceria NP uptake by C. reinhardtii were conducted using ICP-MS, as described in SI.
Scanning electron microscopy of C. reinhardtii
Following the 72-h exposures, algal cells were collected, fixed, dehydrated, embedded in resin and sectioned on an ultra-microtome, as described in SI. These were imaged on a Jeol 7000 scanning electron microscope using backscatter and STEM modes (see SI). Elemental analysis was performed using an Oxford Instruments INCA EDS system with Si-Li x-sight detector.
Mass spectrometry-based metabolomics
Following exposure to the ceria NPs, metabolites were extracted from C. reinhardtii using a modified version of published methods (see SI). Polar metabolite extracts were dried in a centrifugal concentrator and stored at −80°C. Following resuspension, metabolites were measured using a hybrid 7-T Fourier transform ion cyclotron resonance mass spectrometer (LTQ FT Ultra, Thermo Scientific; negative ion mode using the SIM (selected ion monitoring)-stitching method from m/z 70–590), and data were processed as reported previously (see SI). Statistical analyses using MATLAB (version 7, The Mathworks, Natick, MA), PLS-Toolbox (Eigenvector Research, Wenatchee, WA) and R were used to discover any metabolic perturbations in C. reinhardtii in response to treatment as described in SI. MI-Pack software was used to putatively annotate the detected peaks, which were then investigated using MetaboAnalyst 2.0 for biochemical pathway enrichment (See SI).
Microarray-based transcriptomics
Transcriptomics methods are described in full in SI. Briefly, RNA was prepared from unexposed controls (n = 4), ionic Ce control (CeNO3; 0.008 µg/L; n = 7) free PVP control (1.94 mg/L; n = 4), and two ceria NP concentrations (0.144 µg/L; n = 4, and 10 000 µg/L; n = 7). A novel oligonucleotide microarray (Agilent Technologies, Santa Clara, CA) was designed using JGI sequence data and annotation, supplemented by Phytozome and Blast2GO. The 8 × 15 k array design is available from ArrayExpress, accession A-MEXP-2384. Cy3-labeling, hybridisations and scanning were performed (Agilent). Raw microarray data are available at ArrayExpress, accession E-MTAB-2454. Processed signal data were analysed using GeneSpring vGX7.3 (Agilent Technologies), MultiExperimental Viewer (MeV) v4.7 and GE workbench, while SAM analysis was carried out within MeV. Annotation enrichment (Algal Functional Annotation Tool and Blast2GO) compared transcripts statistically significantly altered in expression, as determined by SAM, with the list of all detected transcripts.
Supporting information
Supporting information is available from the Wiley Online Library and includes energy dispersive spectra of the ceria NPs, additional multivariate analyses of the metabolomics data, full KEGG pathway enrichment analysis of the transcriptomic and metabolomic changes, and lists of significantly changing metabolites and genes in C. reinhardtii following exposure to ceria NPs.
Results
Ceria NP synthesis and physicochemical characterisation
The thorough characterisation of NPs in complex matrices is challenging but essential for any assessments of exposure and effect (Hassellöv et al., Citation2008; Tiede et al., Citation2009). There is a lack of standard methods for assessing such interactions in ecotoxicological studies (Merrifield et al., Citation2013; Pelletier et al., Citation2010). However, information on size, morphology, surface properties, dispersion/aggregation and dissolution and their dynamic changes on exposure must be available to guide the interpretation of any toxicity (Thill et al., Citation2006; Tiede et al., Citation2009). The ceria NPs used here were synthesised and fully characterised, as previously described in Merrifield et al. (Citation2013). Briefly, the NPs were polyvinylpyrrolidone (PVP)-coated, made from 99.999% purity grade CeNO3 and characterised as 4–5 nm (by transmission electron microscopy; TEM) and 6–7 nm (by field flow fractionation; FFF, and dynamic light scattering; DLS) both as prepared and in the algal exposure media (). The difference between the TEM and DLS/FFF is explained by the thin coating of the polymer PVP, consistent with our previous work (Baalousha & Lead, Citation2012). NP properties were largely, though not entirely, conserved in the C. reinhardtii exposure media. In addition to size, aggregation remained minimal, no significant changes in electrophoretic mobility were observed and the NPs remained largely insoluble. However, while the prepared particles were essentially 100% Ce(III), they became ca. 70% Ce (III) and 30% Ce (IV) oxide in the media (measured from the ratio of the white lines in the electron energy loss spectrum; EELs), likely due to sorption of redox active species from the media. The NPs were sterically stabilised by the polymer coating and hence were dispersed under all conditions, despite the near-zero electrophoretic mobility. It was observed that at NP concentrations of 80 mg/L and above the exposure media became opaque and NP aggregates collected at the base of the exposure vessel, indicating their limit of dispersibility. The high stability and minimal dissolution of these NPs made them ideal for ecotoxicological studies (Merrifield et al., Citation2013). Ceria particles were also made at a larger size (500–600 nm, termed “bulk”), identically synthesised and PVP coated, for comparison to the NPs, to evaluate the dependency of any biological responses on nano-structure.
Figure 1. (A) Diameter of ceria NPs in exposure media after 0 and 72 h as measured by FFF (mean ± SD) and DLS (Z-average and polydispersity index (PDI)); (B) a representative STEM image of ceria NP taken after 72 h under exposure conditions (scale bar 5 nm); and (C) a typical EELs spectrum taken from centre of the nanoparticle.
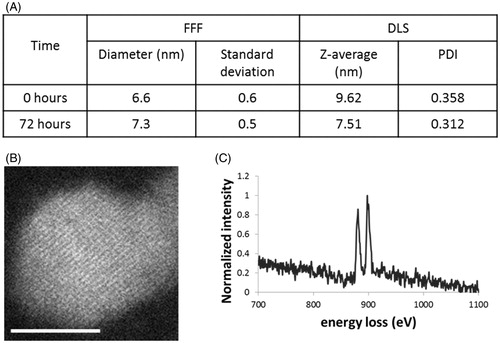
The essentially complete conservation of the tightly constrained particle properties within the exposure media, along with quantitative determination of this conservation, allows for greater understanding of any specific nanoscale effects on toxicity without uncertainties due to particle polydispersity, heterogeneity and dynamic changes caused by the media. Hypothetically, as the coating changes, no doubt the response changes, either due directly to the different coating or indirectly via dynamic changes of the NPs over the exposure period. In addition, the polymer coating and crystalline form suggests these NPs are relevant to ceria NPs used in the polishing, rather than other uses such as diesel combustion, but our primary concern is not representation of specific sectoral uses of NPs but to attempt to understand nanoscale effects in a more quantitative manner.
Ceria NPs do not affect C. reinhardtii growth rate under conditions tested
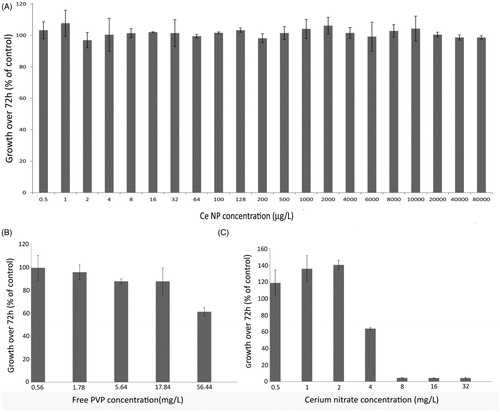
Microalgae, including C. reinhardtii, are keystone species in aquatic ecosystems and are often used in aquatic toxicity testing (Jamers et al., Citation2013). In accordance with international standards, we used a measure of growth inhibition over 72 h to indicate toxicity of ceria NPs to C. reinhardtii (OECD, Citation2002). An exceptionally wide range of exposure concentrations (0.5–80 000 µg/L) of ceria NPs were investigated to include both predicted environmental levels and those concentrations at which adverse effects have been reported previously (). No inhibition of algal growth was detected at any of the ceria NP concentrations tested (). For ionic cerium (CeNO3; positive control), a 50% effect concentration (EC50) for growth inhibition of 4.5 mg/L and a “no observed adverse effect level” (NOAEL) on growth of 2 mg/L were determined. These values lie within the range of previously reported concentrations for CeNO3 toxicity to freshwater green algae of 0.63–15.5 mg/L (Evseeva et al., Citation2010; Rogers et al., Citation2010). Direct toxicity of the free PVP, in the absence of NPs, was also investigated to account for any free PVP introduced from the ceria NP exposures. The lowest concentration of PVP that reduced the algal growth rate was determined to be 56.4 mg/L. Studies were repeated independently, confirming the reproducibility of these growth measures.
Ceria NPs are internalised by C. reinhardtii
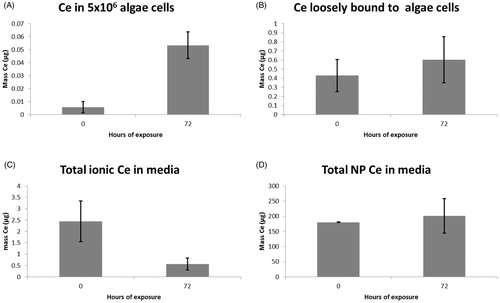
Given the lack of effect of ceria NPs on algal growth, we next sought to determine whether the NPs were taken up by the cells, and if so, to quantify this uptake. C. reinhardtii were exposed to 10 mg/L ceria NPs to assess uptake for the standard 72-h growth period used in toxicity assays (OECD, Citation2002). Ce was separated operationally into internalised/strongly bound NP mass (), loosely bound (), dissolved in media (), and particulate within the media (). Clearly, the majority of the ceria NPs remained within the media as individual nanoparticles, with ca. 1% or less present in the media as dissolved species. This concentration decreased over time, presumably due to absorptive losses rather than uptake, given that the mass of cerium associated with the algal cells was <1% of the dissolved cerium mass. Low masses of cerium were loosely associated with the cells and even lower masses were taken up or strongly associated with the algae. Of the ceria in suspension, <0.1% was internalised within or strongly bound to the algal cells.
Figure 3. Bar charts showing the increase of cerium (A) within or strongly bound to 5 × 106 algae cells, and (B) loosely bound to these cells, together with the masses of (C) dissolved and (D) particulate cerium in the media, all over a standard 72-h exposure period.
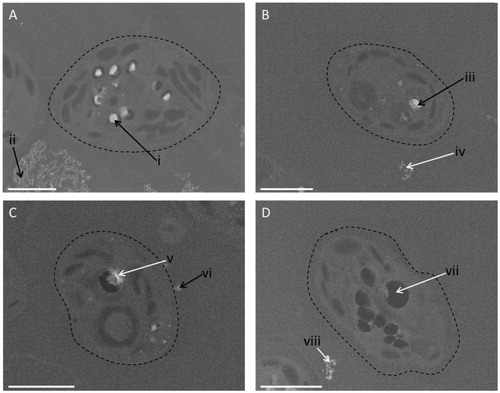
To assess whether this uptake of Ce was indeed as a result of cellular internationalisation or simply due to adsorption of the NPs to the outside of the C. reinhardtii, scanning electron microscopy (SEM) imaging coupled with energy dispersive spectroscopy (EDS) elemental analysis was conducted. Two widely differing ceria NP concentrations were used (80 and 10 000 µg/L), a single bulk ceria concentration (10 000 µg/L), and an unexposed control sample of C. reinhardtii; exposure conditions were the same for all the treatments. The measurements showed that the algal cells internalise the ceria NPs at both concentrations, with more Ce detected at the higher exposure concentration (). Furthermore, Ce was clearly compartmentalised within intracellular vesicles, an effect not previously reported for ceria in algal cells. Cerium was also detected surrounding the cells in both the highest ceria NP exposed () and the bulk ceria exposed samples (), although it is not possible to elucidate if the particles were actually bound to the cell surface. No internalised Ce could be detected following exposure to the bulk material and no Ce was detected in the unexposed control samples (). Furthermore, no settling of the particles was observed in either the stock suspension of bulk ceria or at the concentration of bulk particles used in the exposure study.
Figure 4. SEM images of algal cells exposed to a high (10 000 µg/L) concentration of ceria NPs (A), a low concentration (80 µg/L) of ceria NPs (B), bulk ceria (10 000 µg/L) (C), and unexposed control cells (D). The black arrows point to the areas where cerium was found using energy dispersive spectroscopy (EDS), while the white arrows are areas where no cerium was found (see Figure S1 for EDS spectra). The dashed line highlights the cell surface. Scale bar in each case is 2 µm.
Effects of ceria NPs on gene transcription and metabolism are restricted to supra-environmental exposure concentrations
Several recent studies predict that the environmental levels of ceria NPs are in the ng/L range (ca. 300 ng/L; Boxall et al., Citation2007; Johnson & Park, Citation2012) while the adverse effects of this class of nanomaterial on algal growth occur at supra-environmental levels, at ca. four orders of magnitude higher concentration (2.4–10.3 mg/L; Rodea-Palomares et al., Citation2011, Citation2012; Rogers et al., Citation2010; Van Hoecke et al., Citation2009). Given this, and our own observations above, we investigated the molecular responses of C. reinhardtii at several concentrations of ceria NPs within two widely differing concentration regimens; first, at high supra-environmental levels (80, 2000, 4000 and 10 000 µg/L), reflecting the concentrations that are reported to induce adverse growth effects in algae, and secondly, at lower environmentally realistic values (0.029, 0.144 and 0.72 µg/L) which encompass the estimated environmental levels of ceria NPs in aquatic systems. Additional exposures of C. reinhardtii to ionic Ce at 1 µg/L (0.01% of the highest NP concentration to match the dissolution rate of these NPs and 2000 times below the NOAEL; Merrifield et al., Citation2013), to bulk ceria at 10 000 µg/L (same concentration as the highest ceria NP treatment) and to free PVP at 1.94 mg/L (highest level of free PVP that could result from the highest ceria NP concentration) were included. The algal responses were probed using transcriptomics, to investigate gene regulatory mechanisms, alongside metabolomics to detect any downstream functional molecular responses.
Multivariate analyses using principal components analysis (PCA) of the mass spectrometry-based metabolomics data revealed that there was a significant perturbation of metabolic function within the algal cells when exposed to ceria NPs at supra-environmental concentrations (80, 400, 2000 and 10 000 µg/L; and S2). Consistent with this observation, univariate analyses of individual peak intensities determined that 347 peaks (false discovery rate corrected, q < 0.05; of a total of 3379 peaks measured) changed significantly at an exposure concentration of 10 000 µg/L ceria NPs relative to unexposed controls. Of these, 68 were putatively annotated with a known metabolite name (Table S1). No metabolic effects were detected at any of the exposure concentrations near the predicted environmental levels of ceria NPs (0.029, 0.144 and 0.72 µg/L; and S3) and no individual peaks changed intensity significantly under this exposure regime. Furthermore, no metabolic effects were detected in the algal cells exposed to ionic Ce, bulk ceria or to free PVP, relative to unexposed controls (Figures S2 and S3), nor did any peaks change intensity significantly relative to controls.
Figure 5. PCA scores plots from analyses of (A) mass spectrometry-based metabolomics dataset and (B) microarray-based transcriptomics dataset measured from C. reinhardtii exposed to supra-environmental concentrations of ceria NPs: control (○), 10 000 µg/L ceria NPs (![]()
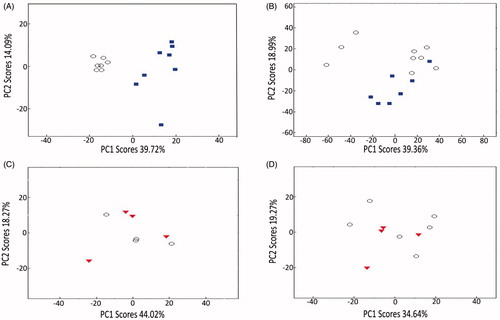
Microarray-based transcriptomics, necessarily conducted on a reduced experimental design but still encompassing environmentally realistic and supra-environmental levels, revealed the same dependency on relatively high concentrations of ceria NPs to induce any effects. Specifically, at a concentration of 10 000 µg/L, ceria NPs exerted significant perturbations on gene transcription, with Statistical Analysis of Microarrays (SAM) revealing that 357 gene transcripts were induced and 707 transcripts were repressed (q < 0.05; of a total of 12 211 detected), relative to a combined ionic Ce and untreated control group (Table S2). Similarly, multivariate PCA demonstrated a separation between the transcriptional profiles of 10 000 µg/L ceria NP-treated algal cells compared to control samples (). Consistent with the metabolomics data, in this experiment no significant differences in gene expression (q < 0.05) were detected between algal cells exposed to the environmentally realistic 0.144 µg/L ceria NPs and either untreated controls or a combined ionic Ce and untreated control group, and no separation between these treatment groups was apparent in the PCA scores plot (). Potentially, increasing the power of the analysis by examining further replicates could discover gene expression responses to 0.144 μg/L ceria NPs.
Focusing on the exposure of C. reinhardtii to the highest concentration of 10 000 µg/L ceria NPs, annotation enrichment analyses were performed for both the transcriptomics and metabolomics datasets. For the transcriptomics, gene ontology (GO) terms significantly enriched among repressed transcripts related predominantly to chloroplast, photosynthesis and response to stress (); no GO terms were significantly enriched among induced genes. KEGG pathway enrichment analysis showed that multiple molecular pathways were perturbed after exposure to supra-environmental ceria NP concentrations, the majority of which were represented by both transcripts and metabolites, indicating a consistency between gene expression and subsequent metabolic change ().
Figure 6. GO terms significantly enriched among gene transcripts repressed in algal cells following a 72-h exposure to 10 000 µg/L ceria NPs (SAM, q < 0.05; Test set), as identified by Blast2GO with Fisher’s Exact Test at FDR < 0.05 in comparison with all transcripts detected (Reference Set).
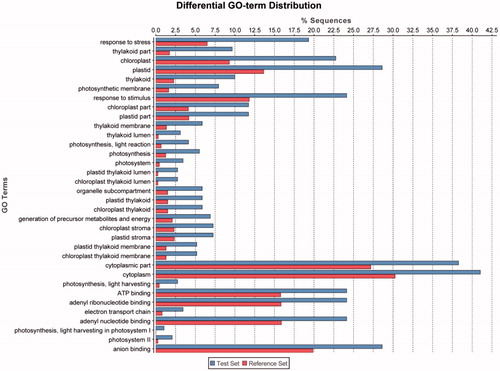
Table 1. Top 15 perturbed molecular pathways following KEGG pathway enrichment analysis of the transcriptomic and metabolomic changes elicited in C. reinhardtii in response to 10 000 µg/L ceria NPs.
Considering the perturbed pathways, most notable was the statistically significant transcriptional repression of genes encoding chloroplast photosynthetic proteins. For example, transcripts encoding light-harvesting proteins of photosystems I and II LHCA3,5 and 8, LHCB4 and 5, and LHCBM2,3,5,6 and 7 were significantly reduced in expression after 10 000 µg/L ceria NP exposure, as were chloroplast-encoded photosystem I transcripts psa d, e, f, g, h, I, j, k, and photosystem II transcripts psb 28, o, p3, s1, w and x. Oxidative stress-responsive transcripts tended to be repressed after NP exposure, with the GO term “response to stress” and the KEGG pathway “glutathione metabolism” significantly enriched amongst down-regulated transcripts. One transcript similar to glutathione peroxidase was induced, but superoxide dismutase FSD1, l-ascorbate peroxidase-like, glutathione s-transferases, glutathione reductase GSHR1 and two peroxiredoxin family transcripts were all repressed. KEGG pathway enrichment analyses identified substantial perturbations to metabolic pathways ( and S1), including a consistent repression of transcripts and metabolites in the carbon-fixation pathway (Tables S2 and S3; phosphoribulokinase PRK1, sedoheptulose-1,7-bisphosphatase SEBP1, ribose-5-phosphate isomerase RPI1 and putatively identified glyceraldehyde-3-phosphate, 3-phosphoglycerate, xylulose-5-phosphate, erythrose-4-phosphate, ribulose-5-phosphate/ribose-5-phosphate) and alterations to CoA and fatty acid biosynthesis, amino acid, nucleotide and carbohydrate metabolism.
Discussion
Importance of fully characterising ceria NPs to interpret their uptake and potential toxicity
We report that no inhibition of algal growth was detected at any of the ceria NP concentrations tested (0.5–80 000 µg/L), while an EC50 for growth inhibition of 4.5 mg/L and NOAEL of 2 mg/L were determined for ionic cerium (CeNO3); the lowest concentration of free PVP that reduced growth was 56.4 mg/L (). Considering the composition of the highest ceria NP exposure solution, these findings are self-consistent: first, the highest ceria NP exposure was estimated to contain PVP at a maximum concentration of 1.94 mg/L, which is ca. 30 times lower than the concentration of PVP required to reduce growth. Secondly, with a dissolution rate of 0.01% (Merrifield et al., Citation2013), the highest expected ionic cerium levels in the highest concentration ceria NP exposure is only 8 µg/L, which is over 500 times lower in concentration that our measured EC50 for growth inhibition of C. reinhardtii by ionic cerium. Therefore, neither PVP nor dissolved cerium would be expected to affect growth in the highest ceria NP exposure, and the absence of any effect of these NPs suggests that there is no nano-specific effect on algal growth up to 80 mg/L.
Our finding is perhaps surprising when compared to published EC50 values for algae exposed to ceria NPs, which range from 2.4–10.3 mg/L (Rodea-Palomares et al., Citation2011, Citation2012; Rogers et al., Citation2010; Van Hoecke et al., Citation2009). However, these earlier studies used commercial ceria NPs that were uncoated, resulting in large aggregates (100–500 nm) with potentially higher dissolution rates. It is conceivable that the adverse effects detected in these studies were caused by direct NP surface (specifically, ceria) interactions with algal cell surfaces, an effect that would not be expected to occur for PVP-coated NPs. In addition, the higher level of toxicity may also have resulted from the much greater rate of dissolution from the uncoated NP core, which was reported as high as 21% by Rogers et al. (Citation2010). The techniques used by Van Hoecke et al. (Citation2009) and Rodea-Palomares et al. (Citation2011, Citation2012) were unable to detect dissolved ceria and hence dissolution rates could not be determined. However, trace metal analysis of the commercial particles used by Rodea-Palomares et al. (Citation2011, Citation2012) determined notable levels of other metals in the NP stocks, including dissolved Zn (10–25 µg/L), the toxic effects of which are well documented. This emphasises the necessity to fully characterise NPs in environmentally relevant media to interpret their potential toxicity accurately. The PVP coating used in our study prevents NP aggregation and dissolution and hence allows for a true determination of the adverse effects of ceria NPs on C. reinhardtii. Furthermore, the extensive characterisation of the ceria NPs used here allows us to rationalise our algal uptake results. Previously it was speculated that NP aggregates that are larger than the pores in algal cell walls (5–20 nm diameter) cannot be taken up (Navarro et al., Citation2008). This could explain why we have definitively observed the internalisation of the ca. 5 nm mono-dispersed ceria NPs within our algal samples ( and ), unlike in earlier studies in which large ceria aggregates were formed and no internalisation was reported. This again highlights the important role that NP size can have on determining routes of uptake.
Molecular pathways affected by supra-environmental concentrations of ceria NPs
While neither metabolomics nor transcriptomics detected any significant molecular alterations in response to environmentally relevant concentrations of ceria NPs in this experiment, both approaches detected molecular responses to the supra-environmental level of 10 000 µg/L ceria NPs. These findings add further weight to the argument that the risks to C. reinhardtii posed by environmentally realistic concentrations of ceria NPs, for the coated and mono-dispersed particles studied here, are low (Johnson & Park, Citation2012). At supra-environmental concentrations, however, the PVP-coated nanoparticles induced molecular alterations that were nano-specific, i.e. the changes were not observed as a result of exposure to the isolated PVP coating or dissolved (ionic) Ce. Therefore an understanding of these responses is still valuable within a hazard assessment paradigm.
The observed repression of photosynthesis-related transcription is consistent with the decreased chlorophyll concentration and inhibition of photosynthetic oxygen production found when P. subcapitata were exposed to ceria NPs at similar concentrations (Rodea-Palomares et al., Citation2011). The earlier study also reported an increase in ROS after ceria NP exposure, but there was no evidence of oxidative stress in our study with C. reinhardtii implying little effect of ceria NP-induced ROS, rather a decrease in ROS with down-regulation of photosynthesis. Differences between the algal species, the physicochemical properties of the nanoparticles, or their subcellular locations, between this study and the findings reported by Rodea-Palomares et al. (Citation2012) may explain this apparent contradiction. Moreover and importantly, there was a lack of detection of a DNA-damage response despite the inclusion of probes on the microarray representing this biological response. The observed repression of transcripts and metabolites of the carbon fixation pathway and perturbation of CoA and fatty acid biosynthesis, amino acid, nucleotide and carbohydrate metabolism pathways is consistent with repression of photosynthesis. In summary, a 72-h exposure to the supra-environmental concentration of 10 000 µg/L ceria NPs primarily mediated a repression of both photosynthesis and carbon fixation that resulted in wide-ranging changes to cellular metabolism in C. reinhardtii.
Conclusion
We have reported an extensive investigation into the molecular and phenotypic effects of ceria NPs to C. reinhardtii, a sentinel algal species in freshwater ecosystems, including all essential components of a nanotoxicology study: synthesis of tightly constrained NPs, physico-chemical characterisation of the nanomaterials in the exposure media; quantification of the uptake of this material into the cells and imaging to determine its location; an assessment of the effects of exposure on the algal phenotype, i.e. its growth rate using standardised OECD protocols; and extensive molecular measurements spanning both regulatory (gene expression) and functional (metabolic) responses. We report clear evidence for the internalisation of the mono-dispersed, PVP-coated, ceria NPs into intracellular vesicles within the C. reinhardtii. Despite this, no significant effect on algal growth rate was measured at any of the wide-range of ceria NP exposure concentrations which included predicted environmental levels. Furthermore, molecular perturbations were only detected when C. reinhardtii was exposed to very high concentrations of the ceria NPs, ca. 3000 times above the predicted environmental levels of this nanomaterial. The principal molecular changes associated with ceria NP exposure include a down-regulation of photosynthesis and carbon fixation with associated effects on energy metabolism. We recommend that these molecular changes can direct the construction of adverse outcome pathways in regard to on-going hazard assessment of ceria NPs. Overall we conclude that for acute exposures to small (4–5 nm) mono-dispersed particles, there should be little concern regarding their dispersal into the environment for this specific trophic level. However, our discovery of the internalisation of the ceria by the algal cells raises further questions. First, what are the molecular and phenotypic effects of the ceria NPs in a longer term exposure scenario, and second, what are the repercussions of this internalisation within the aquatic food chain with regards to the bioavailability and potential toxicity of ceria to primary consumers? Such questions must be addressed as part of a rigorous hazard assessment of this important and widely used class of nanomaterial.
Supplementary material available online
Supplemental Material.pdf
Download PDF (817.4 KB)Acknowledgements
We acknowledge Paul Stanley and Theresa Morris for their assistance with the electron microscopy and the Birmingham Functional Genomics laboratory staff for helping with the transcriptomics.
Declaration of interest
We thank the UK Natural Environment Research Council (NE/H008764/1 and NE/H013148/1) and the Center for Environmental Nanoscience and Risk for financial support. The FT-ICR mass spectrometer used in this research was obtained through the Birmingham Science City Translational Medicine: Experimental Medicine Network of Excellence project, with support from Advantage West Midlands (AWM).
References
- Ankley GT, Daston GP, Degitz SJ, Denslow ND, Hoke RA, Kennedy SW, et al. 2006. Toxicogenomics in regulatory ecotoxicology. Environ Sci Technol 40:4055–64
- Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, et al. 2010. Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment. Environ Toxicol Chem 29:730–41
- Baalousha M, Lead JL. 2012. Rationalizing nanomaterial sizes measured by atomic force microscopy, flow field-flow fractionation and dynamic light scattering: sample preparation, polydispersity and particle structure. Environ Sci Technol 46:6134–42
- Baalousha M, Ju-Nam Y, Cole PA, Gaiser B, Fernandes TF, Hriljac JA, et al. 2012a. Characterization of cerium oxide nanoparticles – part 1: size measurements. Environ Toxicol Chem 31:983–93
- Baalousha M, Ju-Nam Y, Cole PA, Gaiser B, Fernandes TF, Hriljac JA. 2012b. Characterization of cerium oxide nanoparticles – part 2: nonsize measurements. Environ Toxicol Chem 31:994–1003
- Boxall ABA, Chaudhry Q, Sinclair C, Jones A, Aitken R, Jefferson B, et al. 2007. Current and Future predicted environmental exposure to engineered nanoparticles. Central Science Laboratory Report for Department of Environment Food and Rural Affairs, Central Science Laboratory, York, UK
- Cassee FR, Van Balen EC, Singh C, Green D, Muijser H, Weinstein J, et al. 2011. Exposure, health and ecological effects review of engineered nanoscale cerium and cerium oxide associated with its use as a fuel additive. Crit Rev Toxicol 41:213–29
- Celardo I, Pedersen JZ, Traversa E, Ghibelli L. 2011. Pharmacological potential of ceium oxide nanoparticles. Nanoscale 3:1411–20
- Evseeva T, Geras’kin S, Majstrenko T, Brown J, Belykh E. 2010. Comparative estimation of 232Th and stable Ce(III) toxicity and detoxification pathways in freshwater alga Chlorella vulgaris. Chemosphere 81:1320–7
- Fall M, Guerbet M, Park B, Gouriou F, Dionnet F, Morin J. 2007. Evaluation of cerium oxide and cerium oxide based fuel additive safety on organotypic cultures of lung slices. Nanotoxicology 1:227–34
- Hassellöv M, Readman JW, Ranville JF, Tiede K. 2008. Nanoparticle analysis and characterization in environmental risk assessment of engineered nanoparticles. Ecotoxicology 17:344–61
- Hendren CO, Mesnard X, Dröge J, Wiesner MR. 2011. Estimating production data for five engineered nanomaterials as a basis for exposure assessment. Environ Sci Technol 45:2562–9
- Hoshino T, Kurata Y, Terasaki Y, Susa K. 2001. Mechanism of polishing of SiO2 films by CeO2 particles. J Non-Cryst Solids 283:129–36
- Jamers A, Blust R, De Coen W, Griffin JL, Jones OAH. 2013. Copper toxicity in the microalga Chlaydomonas reinhardtii: an integrated approach. Biometals 26:731–40
- Johnson AC, Park B. 2012. Predicting contamination by the fuel additive cerium oxide engineered nanoparticles within the United Kingdom and the associated risks. Environ Toxicol Chem 31:2582–7
- Klaine SJ, Alvarez PJJ, Batley GE, Fernandes TF, Handy RH, Lyon DY, et al. 2008. Nanomaterials in the environment: behaviour, fate bioavailability and effects. Environ Toxicol Chem 27:1825–51
- Lee S, Kim S, Choi J. 2009. Genotoxicity and ecotoxicity assays using the freshwater crustacean Daphnia magna and the larva of the aquatic midge Chironomus riparius to screen the ecological risks of nanoparticle exposure. Environ Toxicol Pharmacol 28:86–91
- Lowry GV, Espinasse BP, Badireddy AR, Richardson CJ, Reinsch BC, Bryant LD, et al. 2012. Long-term transformation and fate of manufactured Ag nanoparticles in a simulated large scale freshwater emergent wetland. Environ Sci Technol 46:7027–36
- Madl AK, Pinkerton KE. 2009. Health effects of inhaled engineered and incidental nanoparticles. Crit Rev Toxicol 39:629–58
- Maynard AD, Aitken RJ, Butz T, Colvin V, Donaldson K, Oberdörster G, et al. 2006. Safe handling of nanotechnology. Nature 444:267–9
- Merrifield RC, Wang ZW, Palmer RE, Lead JR. 2013. Synthesis and characterisation of polyvinylpyrrolidone-coated cerium oxide nanoparticles. Environ Sci Technol 47:12426–33
- Navarro E, Baun A, Behra R, Hartmann NB, Filser J, Miao A-J, et al. 2008. Environmental behaviour and ecotoxicity of engineered nanoparticles to algae, plants and fungi. Ecotoxicology 17:372–86
- OECD. 2002. Guidelines for Testing of Chemicals, No 201, Freshwater Alga and Cyanobacteria, Growth Inhibition Test. Organisation for Economic Cooperation and Development, Paris, France
- Park B, Donaldson K, Duffin R, Tran L, Kelly F, Mudway I, et al. 2008a. Hazard and risk assessment of a nanoparticulate cerium-oxide based diesel fuel additive – A case study. Inhal Toxicol 20:547–66
- Park E, Choi J, Park Y, Park K. 2008b. Oxidative stress induced by cerium oxide nanoparticles in cultured BEAS-2B cells. Toxicology 245:90–100
- Pelletier DA, Suresh AK, Holton GA, McKeown CK, Wang W, Gu B, et al. 2010. Effects of engineered cerium oxide nanoparticles on bacterial growth and viability. Appl Environ Microbiol 76:7981–9
- Poma A, Di Giorgio ML. 2008. Toxicogenomics to improve comprehension of the mechanisms underlying responses of in vitro and in vivo systems to nanomaterials: a review. Curr Genomics 9:571–85
- Poynton HC, Lazorchak JM, Impellitteri CA, Blalock BJ, Rogers K, Allen HJ, et al. 2012. Toxicogenomic responses of nanotoxicity in Daphnia magna exposed to silver nitrate and coated silver nanoparticles. Environ Sci Technol 46:6288–96
- Rogers NJ, Franklin NM, Apte SA, Batley GE, Angel BM, Lead JR, et al. 2010. Physico-chemical behaviour and algal toxicity of nanoparticulate CeO2 in freshwater. Environ Chem 7:50–60
- Rodea-Palomares I, Boltes K, Fernández-Pinas F, Leganés F, García-Calvo E, Santiago J, et al. 2011. Physicochemical characterization and ecotoxicological assessment of CeO2 nanoparticles using two aquatic microorganisms. Toxicol Sci 119:135–45
- Rodea-Palomares I, Gonzalo S, Santiago-Morales J, Leganés F, García-Calvo E, Rosal R, et al. 2012. An insight into the mechanisms of nanoceria toxicity in aquatic photosynthetic organisms. Aquat Toxicol 122:133–43
- Seaton A, Tran L, Aitken R, Donaldson K. 2010. Nanoparticles, human health hazard and regualtion. J R Soc Interface 7:S119–29
- Shah V, Shas S, Shah H, Rispoli FJ, McDonnell KT, Workeneh S, et al. 2012. Antibacterial activity of polymer coated cerium oxide nanoparticles. Plos One 7:1–13
- Simon DF, Domingos RF, Hauser C, Hutchins CM, Zerges W, Wilkinson KJ. 2013. Transcriptome sequencing of the effects of metal nanoparticle exposure on the transcriptome of Chlaydomonas reinhardtii. Appl Environ Microbiol 79:4744–85
- Snape JR, Maund SJ, Pickford DB, Hutchinson TH. 2004. Ecotoxicogenomics: the challenge of integrating genomics into aquatic and terrestrial ecotoxicology. Aquat Toxicol 67:143–54
- Sturla SJ, Boobis AR, FitzGerald RE, Hoeng J, Kavlock RJ, Schirmer K, et al. 2014. Systems toxicology: from basic research to risk assessment. Chem Res Toxicol 27:314–29
- Tejamaya M, Römer I, Merrifield RC, Lead JL. 2012. Stability of citrate, PVP and PEG coated silver nanoparticles in ecotoxicology media. Environ Sci Technol 46:7011–17
- Thill A, Zeyons O, Spalla O, Chauvat F, Rose J, Auffan M, et al. 2006. Cytotoxicity of CeO2 nanoparticles for Escherichia coli. Physico-chemical insight of the cytotoxicity mechanism. Environ Sci Technol 40:6151–6
- Tiede K, Hassellöv M, Breitbarth E, Chaudry Q, Boxall ABA. 2009. Considerations for environmental fate and ecotoxicity testing to support environmental risk assessments for engineered nanoparticles. J Chromatogr A 1216:503–9
- Tsyusko OV, Unrine JM, Spurgeon D, Blalock BJ, Starnes D, Tseng M. 2012. Toxicogenomic responses of the model organism Caenorhabditis elegans to gold nanoparticles. Environ Sci Technol 46:4115–24
- Van Hoecke K, Quik JTK, Mankiewicz-Boczek J, De Schamphelaere KAC, Elsaesser A, Ven Der Meeren P, et al. 2009. Fate and effects of CeO2 nanoparticles in aquatic ecotoxicity tests. Environ. Sci Technol 43:4537–46
- Volz DC, Belanger S, Embry M, Padilla S, Sanderson H, Schimer K, et al. 2011. Adverse outcome pathways during early fish development: a conceptual framework for identification of chemical screening and prioritization strategies. Toxicol Sci 123:349–58
- Xia T, Kovochich M, Liong M, Mädler L, Gilbert B, Shi H, et al. 2008. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 10:2121–34
- Zhang H, He X, Zhang Z, Zhang P, Li Y, Ma Y, et al. 2011. Nano-CeO(2) exhibits adverse effects at environmental relevant concentrations. Environ Sci Technol 45:3725–30