Preface
This thesis is based on studies performed at the Clinical Orthopaedic Research Unit during my employment as Research Assistant at the Aarhus University Hospital during the period 2005–2009.
I have had the exceptional privilege of guidance from three excellent mentors: Kjeld Søballe, Ole Rahbek, and Søren Kold. I was introduced to orthopaedic research by Kjeld Søballe in 2002, and I have valued his support, patience, and positive guidance highly ever since. As a doctor as well as a researcher Kjeld has been a life-size inspiration because of his always positive attitude, belief in success, and solicitous patient care. I am grateful for Kjeld’s faith in my ability to carry out research, for always encouraging me to pursue the answer to new research questions, and for giving me excellent working conditions in terms of extensive freedom, responsibility, and guidance whenever needed. I am deeply indebted to Ole Rahbek, who has made invaluable contributions to the development of ideas, methods and interpretations in all studies in the thesis. Ole has remained loyal and supportive throughout the project providing practical assistance, skilful supervision, constructive criticism as well as motivation in my daily work. Søren Kold has likewise provided insightful and excellent scientific advice and critique as well as key suggestions in the undertaking of the two final studies.
During the final months of my education Kristian Larsen contributed outstanding scientific and statistical guidance with data analysis as well as invaluable support during the writing process of the three last studies and my thesis. I have gained much scientific and practical knowledge from daily educational discussions with Kristian, and I dearly appreciate his humour, passion for problem solving, patience, readiness to help, and unique ability to break complicated things down into simple little pieces.
My appreciation and thanks also goes to the patients who generously volunteered to participate in the studies.
I am profoundly grateful to Rikke Mørup, Lone Løvgren Andersen and Inger Krog-Mikkelsen, at the for the Clinical Orthopaedic Research Unit in Aarhus, for their friendship and support, but also for their brilliant skills of problem solving, practical assistance with patient examinations, meticulous assessment of clinical and radiographic data, and for always making things run smoothly no matter what difficult obstacles may have challenged the daily work. The secretaries Karen Fousing and Tina Stenumgaard are thanked for their positive spirits and always persistent readiness help.
I highly value the rewarding discussions at research meetings and journal clubs with my fellow PhD students over the past years. Especially, I have enjoyed the comradeship, collaboration, and enthusiasm of my closest research colleagues in Aarhus Inger Mechlenburg and Stig Storgaard Jakobsen.
Niels Trolle Andersen has kindly and patiently offered first class statistical guidance with all studies in this thesis, and Edwin Stanton Spencer has provided superb and timely linguistic corrections to the thesis and manuscripts III–V.
The collaborative suggestion made by Kjeld Anton Nielsen made the second study possible, and I thank Kjeld for being a patient believer in the completion of the study.
I am truly grateful to Torben Bæk Hansen for providing office facilities in Holstebro and helping me combine research and family life during the final chapters of my PhD studies. I look forward to our future collaboration.
Above all, I wish to thank Claus Stilling, my beloved husband and father of our dear son Sebastian, for his curious interest in my research, his technical assistance regarding wear analyses and graphics, and for his constant support and encouragement that made it possible for me to achieve my goals. I owe much to my husband, grand mom, parents, and parents-in-law for their tolerance and practical help during my periodically long working hours as well as their support of me anyhow, anytime and anywhere.
Financial support
The studies in this thesis were supported by grants from the Danish Rheumatism Association, the Helga and Peter Korning Foundation, Maggie and Svend Frietszches Legat, the Guildahl Foundation, and Biomet Danmark Aps.
Maiken Stilling Holstebro, April 2009
List of papers
The papers of this thesis will be referred to in the text by their Roman numerals (I–V).
Those currently in print have been reprinted by permission of the copyright holder.
I. Stilling M, Rahbek O, Søballe K. Inferior survival of hydroxyapatite-versus titanium-coated cups at 15 years. Clin Orthop Relat Res. 2009 Mar 28. [Epub ahead of print]
II. Stilling M, Nielsen KA, Søballe K, Rahbek
O. Clinical comparison of the wear of polyethylene with zirconia or cobalt chrome femoral heads. Clin Orthop Relat Res. 2009 Oct; 467(19): 2644-50. Epub 2009 Mar 27.
III. Stilling M, Søballe K, Andersen NT, Larsen K, Rahbek O. Polyethylene wear analysis in plain radiographs. The number of radiographs influences results. Manuscript accepted for publication in Acta Orthopaedica, 2009.
IV. Stilling M, Kold S, Andersen NT, Søballe K, Rahbek O, Larsen K. Superior accuracy and precision of model based RSA compared with PolyWare for measurement of polyethylene wear in total hip arthroplasty. A phantom study of validity and reliability. Manuscript preparation.
V. Stilling M, Larsen K, Andersen NT, Søballe K, Kold S, Rahbek O. The final follow-up plain radiograph is sufficient for clinical evaluation of polyethylene wear in total hip arthroplasty. A study of validity and reliability. Manuscript preparation.
Study I was presented in part at the 53rd Annual Meeting of the Orthopaedic Research Society, San Diego, USA, 2007, and at the Annual Autumn Meeting of the Danish Orthopaedic Society, 2006.
Study II was given the third price for best poster presentation at “The Day of Research” at Aarhus University Hospital, 2008, and was presented in part at the 53rd Combined Meeting of the Orthopaedic Research Society, Honolulu, Hawaii, 2007, and at the Nordic Orthopaedic Federation 54th Congress, Amsterdam, the Netherlands, 2008.
Study III was presented in part at the 53rd Combined Meeting of the Orthopaedic Research Society, Honolulu, Hawaii, 2007, at the Nordic Orthopaedic Federation 54th Congress, Amsterdam, the Netherlands, 2008, and at the Annual Spring Meeting of the Danish Orthopaedic Society, 2008.
Abbreviations
| µm | = | Micrometers |
| 2D | = | Two-dimensional |
| 3D | = | Three-dimensional |
| AP | = | Anteroposterior |
| CI | = | Confidence interval |
| CMM | = | Coordinate measuring machine |
| CoCr | = | Cobalt-chrome |
| CTL | = | Cross-table lateral |
| CTX-1 | = | C-terminal telopeptide of type I Collagen |
| DPD | = | Deoxypyridinoline, a crosslink of type I Collagen |
| GUR, | = | Granular UHMWPE Ruhrchemie. Designation for the grades of UHMWPE produced by Ticona (formerly Ruhrchemie AG) |
| HA | = | Hydroxyapatite |
| HHS | = | Harris hip score |
| HXLPE | = | Highly cross-linked polyethylene |
| LA | = | Lateral |
| LOA | = | Limits of agreement, the same as the PI |
| NTX-1 | = | Crosslinked N-telopeptide of type I Collagen |
| PE | = | Polyethylene |
| PI | = | Prediction interval (1.96 × SD) |
| PW | = | PolyWare; polyethylene wear analysis software |
| RCT | = | Randomized clinical trial |
| RLL | = | Radiolucent line |
| RSA | = | Radio stereometric analysis |
| SD | = | Standard deviation |
| THA | = | Total hip arthroplasty |
| Ti | = | Titanium |
| Ti-6Al-4V | = | Titanium-6aluminum-4vanadium |
| TRAcP | = | Tartrate-resistant acid phosphatase, an enzyme that is expressed in high amounts by bone resorbing osteoclasts (type 5b) |
| UHMWPE | = | Ultra high molecular weight polyethylene |
| Y-ZrO2 | = | Yttrium stabilised zirconium oxide |
| Zr | = | Zirconia |
Definitions
| Aseptic loosening – | = | Mechanical loosening of an endo-prosthesis without signs of infection. |
| Biomaterials – | = | Materials intended to interface with biological systems to evaluate, treat, augment or replace any tissue, organ or function of the body (Citation271;272). |
| Creep – | = | The plastic deformation of material without production of wear debris. |
| Delamination – | = | Separation of a coating into layers or separation of the entire coating. |
| Effective joint space – | = | The peri-implant area accessible to joint fluid and thus also to wear debris. |
| Implant – | = | A medical device made from one or more biomaterials that is intentionally placed within the body, either totally or partially buried beneath an epithelial surface (Citation271). |
| Osteolysis – | = | An active resorption or dissolution of bone as part of an ongoing disease process in relation to joint prostheses. Radiographically it is evident as periprosthetic bone loss not present in the initial radiographs. |
| Press-fit – | = | Insertion of an implant into an undersized cavity. |
| Radiolucent lines – | = | Linear osteolytic lesion with sclerotic margins at the implant-bone interface. |
| Rigid body – | = | In RSA the number of markers forming a segment corresponding to either part of the body or object of interest. |
| Sealing effect – | = | The ability of an implant to reduce the effective joint space, thus reducing the access of joint fluid and wear debris to the bone-implant interface. |
| Stress shielding – | = | The non-anatomical reduction in bone density (osteopenia) as a result of removal of normal stress from the bone by an implant. See Wolff’s law. |
| Wear – | = | Wear is defined by the removal of material from prostheses. |
| Wolff’s – | = | Wolff’s law states that bone in a healthy person or animal will remodel in response to the loads it is placed under. Therefore, if the loading on a bone decreases, the bone will become less dense and weaker because there is no stimulus for continued remodeling, which is required to maintain bone mass. |
Contents
Summary 6
Introduction 8
Commencement of hip arthroplasty 8
Contemporary hip arthroplasty 8
Biomaterials 10
Ceramics 10
Metals 13
Polymers 14
Aseptic loosening 17
Wear 19
Wear measurement 20
Aim of the thesis 23
Materials and methods 24
Patients 24
Design 25
Ethics and permissions 26
Sample size 26
Implants 27
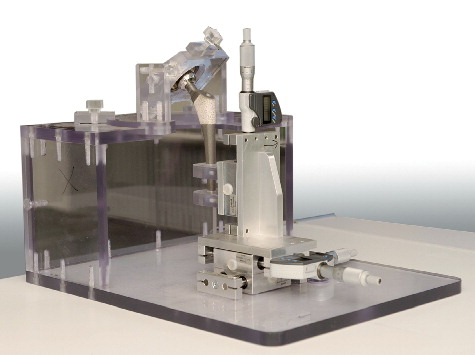
The phantom fixture 29
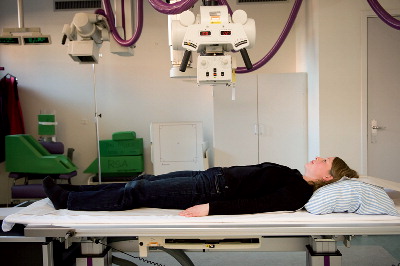
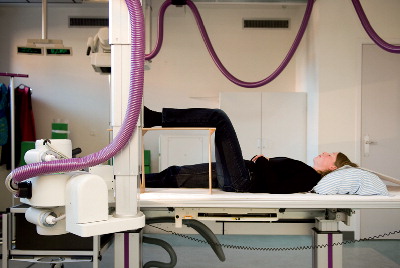
Radiographs 30
Timing of radiographic follow-up 31
Polyethylene wear measurement softwares 31
Evaluation of osteolysis 34
Clinical evaluation 34
Measurement agreements 34
Statistics 36
Results 38
Discussion 52
Key findings 52
Interpretation of results and comparison with the literature 52
Methodological considerations 59
Generalizability 61
Conclusions 63
Future research 64
References 65
Summary
Joint replacement is one of the greatest surgical successes in history, and countless attempts have been made in the past to improve the longevity of total hip arthroplasty (THA), including enhancement of implant designs, application of new surface coatings, and development of alternate bearing surfaces. New products have been enthusiastically embraced by surgeons despite lack of clinical support, but not unusually, further experience revealed unexpected drawbacks. Polyethylene (PE) wear has long been recognized as playing a central role in the aetiology of osteolysis and acetabular component failure. However, PE is still the gold standard counter-bearing surface of the femoral head in THA, and despite promising low-wear results of new PE products, there is a continued need for clinical studies to evaluate limitations not exposed by experimental studies. Assessment of low-wear bearing surfaces increases the demand for high precision and high accuracy methods of PE evaluation. Radiostereometric analysis (RSA) is considered gold standard of such measurements, but the method is limited by expense and equipment. Consequently, plain radiographs are still used in many descriptions of clinical wear. New software solutions for plain and stereo radiographs are frequently developed and present a persistent need for method validation and comparison.
The first aim of the thesis was to objectivise the clinical importance of hydroxyapatite (HA) coating as a contributor in third-body PE wear, osteolysis, and cup failure, and to focus on the potential of zirconia (Zr) femoral heads as a PE-wear reducing material. The second aim was to explore pit falls and conduct a comparison of three wear measurement methods (EGS-RSA, MB-RSA, and PolyWare), with two-dimensional (2D) and three-dimensional (3D) wear estimates.
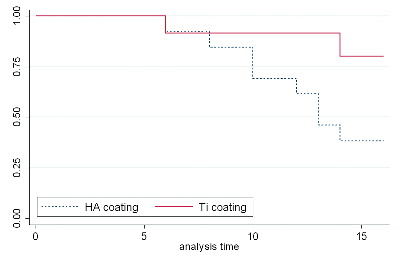
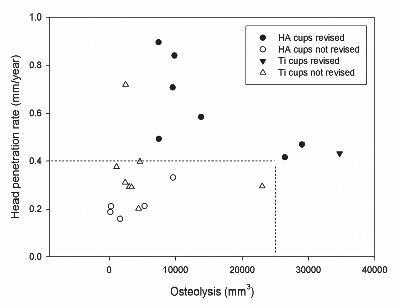
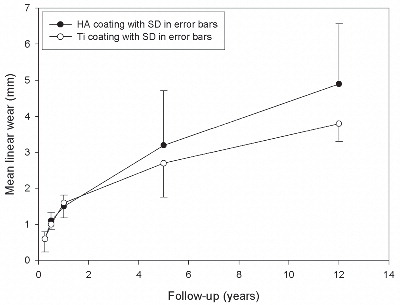
Study I: Twenty-six cementless THA components (HA vs. titanium coating) were evaluated in a randomized patient group with regard for need of cup revision after 15 years’ follow-up, and radiographic PE wear and osteolysis after a 12-year follow-up or at end-point revision. HA-coated cups displayed a higher revision rate. There was a positive association between high wear rate and revision, as well as between high osteolysis and revision.
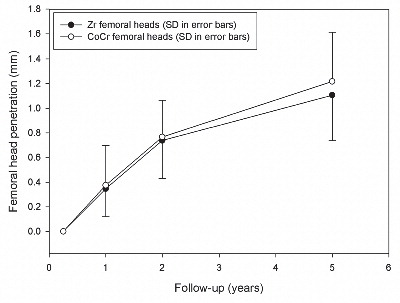
Study II: A clinical comparison was performed of PE wear with Zr or cobalt-chrome (CoCr) femoral heads in a young patient group of 69 hips with cementless acetabular shells. At a mean of 5 years, the wear rates were similar and there were no revisions.
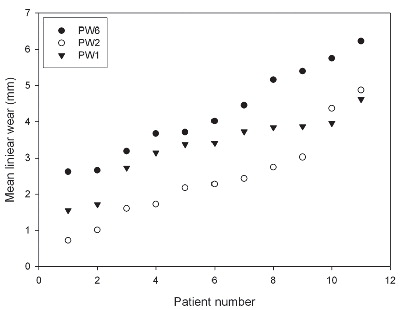
Study III: Linear PE wear in a long-term follow-up clinical series of 11 patients was evaluated by use of one, two, or six plain AP radiographs with the same wear measurement method (PolyWare). The number of radiographs used significantly influenced the magnitude of measured linear wear, and wear results with the PolyWare method based on different numbers of radiographs are not comparable.
Study IV: Two, new, model-based RSA techniques and a method (PolyWare) for plain radio-graphs were validated and compared in a phantom hip setup. Methods for 2D wear measurement were more precise (repeatable) and accurate than those for 3D wear measurement. The best concurrent validity was obtained between the MB-RSA and EGS-RSA techniques. PolyWare was the least accurate and precise method, and it demands a twofold larger sample size compared with RSA. Measurement of wear close to liner wear-through severely affects the accuracy of all methods.
Study V: As an extension of study III, the Poly-Ware method for plain radiographs using one or two radiographs was compared with EGS-RSA in a clinical series of 12 cementless hips with a minimum 5-year follow-up. Repeatability (precision) for 2D PE wear was similar for PolyWare using only one (the final) radiographs and EGS-RSA (“the gold standard”). The PolyWare method using only the final radiographs is applicable when the expected total 2D wear is above a total of 0.5 mm.
From the present clinical studies, it can be concluded that wear in older type non-crosslinked polyethylene liners exceed the defined tolerance of 0.2 mm/year for the development of osteolysis and failure. First-generation hydroxyapatite coating applied to first-generation modular cups resulted in high and early risk of revision, and the clinical performance of recently electrochemically deposited HA coatings should be followed closely. Although no negative effects of Zr femoral heads were observed, an expected clinical wear advantage of Zr femoral heads on PE compared with CoCr fem-oral heads on PE could not be demonstrated, and long-term follow-ups are needed. Close attention should be paid to the clinical performance of new ceramic products.
The methodological studies showed that measurement of PE wear on plain THA radiographs with the PolyWare method should be based on an equal number of radiographs per patient. A good agreement was established between EGS-RSA and PolyWare with use of only the final follow-up plain radiograph for 2D PE wear analysis. The Poly-Ware final follow-up radiograph method is ideal for clinical retrospective research with medium-to long-term follow-ups. It is easy and inexpensive to use, applicable in any hospital, and further alleviates the need for baseline images that are often lost, stored in hard copy, and of varying quality. For assessment of low-wear or short-term clinical follow-up, RSA should be used. Model-based RSA using scanned-surface cup models or computer-generated sphere models are highly accurate and on the level of marker-less RSA for PE wear analysis. Assessment of PE wear near wear-through of the liner is problematic for model-based RSA methods as well as for PolyWare, and it should not be attempted.
Introduction
Commencement of hip arthroplasty
Joint disease frequently results in serious functional deficits and general impairment. The first radical advances regarding surgical procedures of joint replacement in diseased and painful joints dates back to the last decades of the 19th century. The first attempts with arthroplasties were in the hip joint with substitution of only the femoral head. In 1891, Themistocles Gluck, Germany, experimented with ivory implants, but was troubled by infections and early revisions. Later methods of material interposition (muscle fascia, pig bladder, or even glass) were tried, and in 1939, Smith-Pedersen was partially successful with a hand-reamed molded cup for interposition between the femoral head and the acetabulum. In 1940, Dr. Austin T. Moore, John Hopkins Hospital, USA, performed one of the first metallic hip replacement surgeries as a hemi-arthroplasty bolted to the resected end of the femoral shaft. In spite of the inventive attempts mechanical failures, fractures, and infections were frequent complications.
The era of total joint replacement of the hip began in 1960 when Sir John Charnley, Wrightington Hospital, England (), introduced the revolutionary “Low Friction Arthroplasty,” which consisted of a metal femoral component, to be inserted and fixed by bone cement in the medullar femoral canal, in combination with a cemented Teflon ace-tabular cup (Citation44). The articulation was lubricated by synovial fluid. The Teflon cup yielded disastrous results because of accelerated in vivo wear and the resultant debris-induced foreign-body reaction (Citation48). Charnley replaced the Teflon cup by an ultra high molecular weight polyethylene (UHMWPE) cup in 1962. Charley also contributed to the improvement of infection prophylaxis with the proposal of a sterile-air operating theatre enclosure (Citation45), and after the risk of infection had decreased, the main challenges with total hip replacement were component fixation and wear. For over two decades, the Charnley Low Friction Arthroplasty design was the most used system in the world, and the basic principles in the Charnley design are still used today.
Contemporary hip arthroplasty ()
Hip arthroplasty is one of today’s most successful surgical procedures, effectively reliving pain and restoring physical function, and on a yearly basis, approximately 1.4 million UHMWPE components are implanted worldwide. Currently, more than 8000 primary total hip arthroplasties (THA) and more than 1400 revision THAs are performed annually in Denmark (Citation62), but the number is steadily increasing due to a longer lifespan and a general expectation of an active lifestyle, even at old age. The most common indications for THA are primary osteoarthritis (78%), fracture complications (13%), non-traumatic caput necrosis (2%), complications of childhood hip disorders (3.5%), and rheumatoid arthritis (1%) (Citation62).
As of standards, older people are treated with cemented arthroplasty due to their generally poor bone quality and the expectancy of a single arthroplasty lasting for life. Younger people are commonly treated with cementless components because they are expected to have good bone quality, but also because they sustain the risk of later revision surgery and cementless components provide more host bone for ease of revision and lasting fixation of the revision implant (Citation95). Cementless fixation relies on primary mechanical fixation at surgery followed by biological fixation within weeks due to bony ingrowth into a textured or porous implant surface. The overall risk of loosening and subsequent revision surgery is 8% after 10 years according the Danish Hip Arthroplasty Registry (DHR) (Citation62), and slightly better (5% after 10 years) in the Norwegian Registry (Citation96). However, younger patients (<50 years of age) sustain a higher risk of implant failure with a 13% 10-year revision rate according the DHR (Citation144). In comparison, the Finnish Hip Arthroplasty Registry report a 10% femoral stem revision after 10 years, and a 6 to 32% risk of acetabular component revision after 13 years, dependent on the component brand (Citation79). In general, the major causes for revision surgery are aseptic loosening (48%) and dislocation (22%) (Citation62). Several studies have pointed to an association between wear debris and aseptic loosening (Citation73;Citation75;Citation178;Citation183;Citation263;Citation269). The prevalence of pelvic osteolysis increases significantly with increasing wear of the acetabular component (Citation240), and the number of polyethylene (PE) particles in peri-implant tissue is significantly higher in areas with osteolysis (Citation132).
Hemi-spherical cups with a porous surface for press-fit (2 mm under-reaming) fixation is possibly the most promising cementless cup design because the tight rim-fit has been shown to seal off wear particles from the joint space, possibly aided by the porous coating (Citation196). Some surgeons favour augmented primary cup fixation by screws or pegs; however, cadaver studies have shown that this may not be necessary (Citation138;Citation273), and clinical mid-term results are in support of this (Citation217). The major disadvantage of screw holes in the cup is believed to be migration of wear particles via the screw holes or along the pegs, predisposing to osteolysis (Citation220). Furthermore, the gaps between the cup apex and the bottom of the acetabulum, that are often seen with press-fit procedure, extend the effective joint space, and this may facilitate particulate joint fluid to flush the acetabular bone through the screw-holes of the non-solid cup designs due to changes in hydrostatic pressures within the hip joint during gait (Citation216). This mechanism is considered to play a major role in the development of bone resorption (focal osteolysis) around the implant (Citation217).
Focus and improvement in fixation of implants during the 1980s and 1990s resulted in the gradual expansion of the indication for THA to younger and more active patients. This brought about an increased need for research and development of low-wear bearing surfaces. Many advances have been made over the past decades, but quite often steps forward later revealed unnoticed limitations, as was the case with Zr head fractures and in vivo grain transformation that resulted in high wear rates (Citation100;Citation107), Boneloc® bone cement that caused premature loosening and failure (Citation253), and Hylamer® PE that showed much higher clinical rates of wear than expected (Citation264). The lessons of the past are that in vitro results cannot be applied uncritically to the in vivo performance of implants. Any new implant should be evaluated in small-scale, randomized in vivo studies, after the completion of laboratory tests, and before recommendation for general use (Citation145).
While THA remains the single most effective method to treat advanced osteoarthritis of the hip, there is a general agreement that wear at the bearing surface remains one of the most important factors limiting long-term survival. Despite major advances in the production and sterilization of PE since the days of Sir John Charnley, PE wear and wear-related complications are still principal reasons for revision of THA. Wear-through of the PE liner, failure in the fixation of the acetabular metal shell, and wear-debris-mediated osteolysis are among the most common indications for revision of THA in patients with long-term clinical follow-up. Consequently, although numerous researchers have explored the field of PE debris, there is a continued need for research regarding PE wear, and analysis of head penetration into the PE liner remains paramount to the study of THA.
Biomaterials
A biomaterial is a nonviable material used in a medical device and intended to interact with biological systems (Citation271). Biomaterials may be described by their acceptance within a biological system as bio-tolerant (encapsulated in fibrous tissue implants), bioinert (in direct contact with the surrounding bone), and bioactive (distinguished by a direct chemical bond to the surrounding bone). According to the chemical composition, biomaterials may be classified as ceramics, metals, polymers, and composites. The following description will focus on the biomaterials of relevance to this thesis.
Ceramics
Ceramics in material science include all non-metallic and inorganic materials. Ceramics used for medical implants are of three types: oxide ceramics, glass ceramics, and calcium phosphate ceramics (Citation99).
Oxide ceramics are stabilized at the surface by an oxidized layer and have excellent tribological properties providing low-friction and low-wear. The oxide ceramics mostly used in joint replacement are zirconia (ZrO2), alumina (Al2O3), and oxinium (Zr2.5Nb). Marketed for hip arthroplasty, they usually make up the femoral head combined with a PE liner, but ceramic-on-ceramic systems are securing a foothold.
Glass ceramics are based on silica (SiO2) with high Na2O and CaO contents. The high CaO/P2O5 ratio (soluble calcium phosphate ions in a “bio-glass” ceramic structure) (FDA 45S5) makes bio-glass highly reactive to an aqueous medium and bioactive. Glass ceramics have no pores between crystals and are mechanically strong materials that can sustain repeated and quick temperature changes up to 800–1000 °C. Thus they are ideal for sealing to a variety of different metals, ranging from low expansion molybdenum to high expansion stainless steels and nickel-based super alloys. Bioglass has a Young modulus of 30–35 GPa, which is very close to that of cortical bone, and is used for non-loaded medical implants such as cochlear implants. Hydroxyapatite is formed on the surface of bioglass after implantation.
Calcium phosphate ceramics resemble the mineral phase of bone tissue and are bioactive. They can be coated on top of the porous surface of metal prostheses, i.e. in the form of hydroxyapatite, where they facilitate bonding or ingrowth of bone to the implant surface (Citation116).
Hydroxyapatite
The most prevalent mineral in bone tissue is hydroxyapatite (HA). HA is produced by precipitation of calcium phosphate into tricalcium phosphate at physiological pH and temperature, followed by an autocatalytic transformation into a crystalline form after contact with water (Citation26). HA is available as a powder with the chemical formula Ca10(PO4)6(PH)2 and a typical Ca/P molar ratio of 10:6. Classically, HA is applied to porous-coated or grit-blasted metal surfaces of orthopaedic implants by plasma-spraying, that is melting accelerated HA particles by injecting them into a high-temperature (15,000°C) plasma tail flame of ionized gas under a vacuum where the HA particles solidify on the metal substrate and build up a layer (Citation232). Many variables are determinative for the quality of the HA coating. To give an example, too high a temperature will make the hydroxyapatite powder vaporize or convert into other types of apatite, while a temperature too low may result in insufficient melting of the HA powder and result in unbonded particles in a lamellar structure with an insufficient adhesive strength (Citation232). Other factors of importance for the behaviour of the HA coating are the chemical composition (purity), the Ca/P ratio, the crystallinity, the microstructure (density), adhesive strength relative to the implant, the coating thickness, and the trace component analysis.
The mechanical properties of the HA coating increase with decreasing coating thickness as coating defects are reduced, and generally a coating thickness of 50–75 µm is recommended (Citation238). Other general agreements regarding HA coatings include as high a purity as possible (95–97%), crystallinity of 70–90%, Ca/P ratio of 1.67, and adhesive strength between 5 MPa and 65 MPa, depending on the condition of the metal substrate. Furthermore, the strain between the HA coating and the metal substrate is minimized when the elastic modulus of both components is as close as possible (Citation232). The lower the crystallinity, the quicker the HA coating is resorbed into the bone tissue (Citation185). This is because a low crystalline coating releases more calcium and phosphate ions due to dissolution. This again enhances bone formation, and thus provides the HA coating a higher bioactivity (Citation90;Citation156). It has been shown that a 50% crystalline HA coating provided a 3-fold increase in fixation strength compared with a 75% crystalline HA coating after 16 weeks of implantation in the bones of dogs, but after 32 weeks, the fixation strength of the different crystalline HA coatings was the same (Citation185). HA disintegrates from the prostheses and this can happen in four ways: by osteoclastic resorption due to bone remodeling, by chemical dissolution at a natural pH, by delamination due to bond failure, and by mechanical abrasion due to lack of primary stability (Citation166). With older and thicker HA coatings, traces of HA are still evident after several years in situ (Citation166). Newer electrochemical HA coating principles with application of very thin (5 µm) and quickly resorbable (3 months) hydroxyapatite layers to implant surfaces are currently at their novice (Citation63). In addition, different molar ratios than used in the original are gaining favour.
Numerous experimental studies have proved the superior osteoconductive properties of HA during both stable and unstable conditions (Citation232;Citation233;Citation236), and HA has been demonstrated to posses the ability of bridging a peri-implant gap by bi-directional bone growth both with and without the presence of bone allograft in the gap (Citation234;Citation235). Furthermore, it has been shown that HA coating on grit-blasted implants had pronounced delamination of the HA coating in contrast to porous-coated implants, indicating a greater bonding strength of HA to porous coatings (Citation186;Citation188). On the other hand, grit-blasted implants had greater bone-ingrowth compared with porous-coated implants, indicating different surface activities. In the experimental setting the HA coating has been shown to seal off wear-particle migration into the bone-implant interface, and HA is thus believed to reduce macrophage-induced osteolysis, and prolong the lifespan of cementless implants (Citation195;Citation196).
Short-and mid-term studies have supported the notion that HA has a stabilizing effect on cementless implants in the clinical setting (Citation39;Citation164;Citation176;Citation239;Citation252), and longer-term revision rates of less than one percent for HA-coated femoral components are reported in the literature (Citation42;Citation43;Citation140;Citation191;Citation199). Two studies of HA-coated versus non HA-coated acetabular components demonstrated equal or improved fixation and reduced periprosthetic radiolucencies (Citation55;Citation204), but reports on PE wear rates reveal values higher than expected (range of 0.15 to 0.32 mm/ year) with HA-coated cups and medium-term revision rates between 13% and 40% (Citation22;Citation43;Citation74;Citation127;Citation200). In some situations, such as those in which the HA coating was applied directly to a smooth implant surface and subsequently flaked off, high rates of early failure might be readily explained (Citation43;Citation127). The major overall concern is disintegration of the HA coating in vivo, resulting in loss of fixation, formation of particulate HA debris, and abrasive third-body wear between the articulating surfaces of the prosthetic components (Citation15;Citation207;Citation243). Supporting this apprehension, HA particles have been detected on the PE surface of retrieved components (Citation14;Citation25), and loose HA particles may increase production of PE particles, leading to accelerated PE wear and liner revision, premature periprosthetic osteolysis, and aseptic implant loosening (Citation14;Citation166). Overall, HA coatings may not always be advantageous, and the release of HA particles from the implant surface could generate a clinical problem with few, if any, early warning signs (Citation20;Citation166) ().
Table 1. Uncemented HA-coated hemispherical acetabular cups, reports of revision and wear
Surgeons of today are divided into supporters or rejecters of HA coatings based on the proven superior osteoconductive effects and the signs of third-body wear. Indications that HA is responsible for severe clinical periprosthetic osteolysis is another concern (Citation82;Citation205;Citation208) that is contradicted by experimental studies of the sealing effect of HA (Citation195). More long-term reports are needed to clarify these issues.
Zirconia
Ceramic femoral heads have been developed to reduce wear of conventional metal-on-UMHWPE bearing surfaces in THA. Zirconia (Zr) femoral heads were introduced in 1985 to solve the problems of alumina head fracture and lessen concerns about PE wear debris. Several laboratory studies suggest an advantage of ceramic over metal heads (Citation36;Citation61;Citation136;Citation213), predicting 22% to 77% reduction of PE wear with Zr femoral heads, dependent on the head size (Citation66). One survey of 19 articles suggests that clinical wear studies have not demonstrated a similar advantageous wear profile for Zr heads but rather are contradictory and report wide variations (e.g., from less than 0.1 mm/year to more than 0.5 mm/year) in magnitude of wear (Citation193). Hernigou and Bahrami (Citation100), and von Schewelov et al. (Citation264) showed a higher wear rate of PE with Zr than with metal heads. Kim (Citation128), on the other hand, reported wear rates in favour of Zr heads. Furthermore, the studies report PE wear in relation to varying head sizes, types of PE, and methods used to measure PE wear, which makes comparisons difficult ().
Table 2. Summary of clinical publications regarding wear of 28-mm yttria-stabilized zirconia and metal femoral heads in articulation with UHMWPE
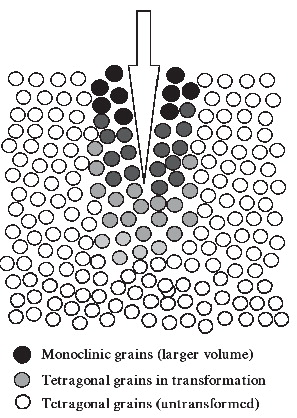
Zr is a three-phase crystalline material (mono-clinic, cubic, and tetragonal) that adapts to changes in temperature by volumetric compensations. It has long been suspected that Zr partially transforms in vivo from the tetragonal phase to the monoclinic phase, due to the physiological mechanical and hydrothermal stresses related to gait and exercise. Zr heads are commonly implanted in young patients with high-activity lifestyles, increasing the risk of frictional heating and mechanical stress (Citation52). In vitro ageing studies have predicted a 5% M-T phase transformation in a 20-year simulation model, and this has been accepted as a tolerable limit for human implants (Citation36). However, recent case reports demonstrate 30–80% monoclinic surface content in early revised heads (Citation51;Citation92), and recently it has been clarified that yttria-stabilized Zr shows an increased monoclinic content with age (Citation210).
The transformations corresponded to the contact areas with the PE, which indicates that tribological conditions acted as triggers. It still unclear why some Zr heads phase transform whereas others do not (Citation129), and the performance of individual Zr heads is impossible to predict.
The strong crystalline structure of Zr also accounts for its brittleness and low fracture toughness. The heat produced by activity in a lubricated joint induces surface metastability (see FootnoteFootnote on page 22) and volume expansion of the crystal grains of Zr, which leads to decreased hardness and increased roughness of the femoral head with potential surface micro-cracks that may propagate in an advancing crack-front and result in abrupt failure (Citation36;Citation51;Citation92;Citation136;Citation215). The sudden fractures of Zr happen because of the tensile notch effect of the accumulated stresses that continue toward the centre of the head (Citation9). This has been described both clinically (Citation153;Citation161) and experimentally (Citation163) ().
Metals, on the other hand, are ductile, and the energy of a micro-crack will be dissipated into the metal without catastrophic failure (). Thus fracture of metal femoral heads is not a problem. Stabilizing materials, such as yttrium, are added to Zr during manufacturing to better control phase transformation and subsequently limit volume expansion and crack initiation (Citation101), and this has improved the performance of ceramic orthopaedic components.
Figure 4. A micro-crack at the surface of ceramics (left) propagate due to build up tension, while tension in a micro-crack at the surface of metal (right) dissipate in the metal.
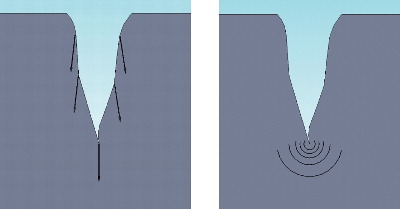
In 2001, all orthopaedic components made of yttria-stabilized Zr (Y-ZrO2) were withdrawn from the marked worldwide due to head fractures (Citation52;Citation153;Citation225). Later theories and experimental studies confirming the instability of Zr heads, (e.g., uncontrolled phase transformation, cracking, and time-dependent degradation, even at physiological temperatures) has caused concern for the patients of the more than 400,000 Zr heads already inserted (Citation52). More than 343 cases of failure with Zr femoral heads have been documented since 2000. Although these patients were revised at the time of implant failure, they were left with a future risk of potential severe adverse effects due third-body wear from the small fracture particles undoubtedly left behind (Citation150;Citation174).
The described fracture failures are particularly coherent with two batches of femoral heads sintered in “a tunnel furnace” after a change in the manufacturing process at St. Gobain-Desmarquest (Evreux, France). Furthermore, a “long neck” bore (8 mm) has been suspected to increase the fracture risk of Zr heads due to elevated stresses at the taper-bore interface (Citation134). Still it is not clearly understood which Zr heads are at greatest risk of fracturing or why. Prophylactic revision of yttria-stabilized Zr heads has not been advised, but a more cautious control of the already inserted implants has been encouraged. The study with Zr heads presented in this thesis did not use femoral heads from those two batches.
Metals
The most used metal implants in orthopaedic applications are stainless steel, chrome-cobalt, and titanium.
Stainless steel
Stainless steel is an iron-based alloy containing about 20% chromium, 17% nickel, and molybdenum. The type most used is 316L (ASTM), which has adequate mechanical properties for medical implants (Citation232). Stainless steel is usually annealed, cold-worked, or cold-forged to improve alloy strength. A potential problem for orthopaedic implants made of stainless steel is the relatively high modulus of elasticity, about 200 GPa, which is 10 times higher than that of bone.
Chromium-cobalt
Chromium-cobalt (CrCo) alloys have been used in medical appliances since the 1930s and are widely used in orthopaedic implants today. These alloys usually contain 30–60% cobalt, 20–30% chromium, 7–10% molybdenum, and various amounts of nickel. CoCr alloy have high corrosion and fatigue resistance and are ideally suited for articulating surface applications (Citation56). The wear of CrCo alloys is less than that of titanium and stainless steel. Although CrCo alloys are hard and tough, there is a constant metal release from prosthetic articulations; this, however, has been shown to be negligible for the articulation of PE on CoCr (Citation214).
Titanium alloy
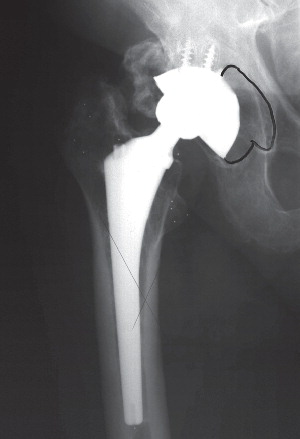
Commercially pure titanium (Ti) is characterized by a high corrosive resistance and is very biocompatible (Citation3). In addition, the elastic modulus of Ti is closer, but larger (5 times) than that of cortical bone, compared to other implant metals. The high elasticity of titanium may reduce stress shielding. However, pure Ti has poor mechanical properties, and therefore Ti alloys (Ti-6Al-4V) with similar elasticity and corrosive resistance but superior mechanical properties were developed (Citation97). Ti is oxidized with a stable oxide surface when exposed to air. Therefore tissues surrounding Ti implants are exposed to a ceramic surface rather than directly to the Ti metal. Good clinical results have been obtained using Ti for hip stems (Citation31;Citation98). illustrates proximally HA coated Ti stem with a magnum CoCr femoral head in a metal-on-metal articulation with a HA coated cup.
Polymers
A polymer (Greek: many parts) is a large molecule (macromolecule) composed of repeating structural units typically connected by covalent chemical bonds.
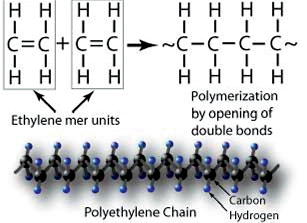
Polyethylene ()
PE is an outstanding material for orthopaedic joint replacement, providing excellent abrasion resistance, low friction, high impact resistance, a self-lubricating surface, insignificant water absorption, good chemical resistance, high energy absorption, and no temperature sensitivity in the human biological environment. PE is the most common bearing surface in THA, and the articulation of PE with a metal head (a hard-on-soft bearing couple) is still the gold standard.
Material properties of polyethylene
PE is a polymer consisting of only carbon and hydrogen in long CH2-chains. The polymer consists of crystalline lamellae embedded in non-crystalline amorphous regions. The PE used for orthopaedic implants today is ultra-high molecular weight PE (UHMWPE), defined as a linear PE with an average molecular weight higher than 3.1 million g/mol. GUR 1020 (molecular weight above 3.5 million g/mol) and GUR 1050 (molecular weight of 5.5–6 million g/mol) are the most commonly used resins in orthopaedic implants today. Calcium stearate, formerly used as catalyst in the production of PE, is no longer added.
The molecular chain of UHMWPE is more than one kilometre long and tangled like a string of spaghetti. This chain folding enables the molecule to form locally ordered, sheet-like regions known as crystalline lamellae. The crystalline lamellae are microscopic and invisible to the naked eye. The lamellae diffract visible light, giving UHMWPE a white, opaque appearance at room temperature. At temperatures above the melt temperature of the lamellae, around 137°C, UHMWPE becomes translucent. The lamellae are on the order of 10–50 nm in thickness, and 10–50 µm in length. The average spacing between lamellae is on the order of 50 nm. The relative amount of crystalline material for standard GUR 1050 PE is approximately 55%, with a crystal size of 39–75 nm. Particle size averages 140 µm.
The basic mechanical properties include stiffness, ductility, strength, and elongation to break. These measures relate to the PE material and not the shape of the implant. The modulus of elasticity is the ratio between stress per unit area and the resulting deformation. For PE, the elastic modulus reduces with strain (strain-softening). PE is a visco-elastic material, which has the potential for plastic deformation in addition to wear. Creep is the effect of long polymer chains in PE sliding over each other, resulting in slow material deformation. Creep does not result in the production of particulate debris. In a wear simulator, wear accounted for <30% of the change in PE thickness – the rest was plastic deformation or creep (Citation209). Femoral head penetration measurements in vivo cannot distinguish between creep, settling of the liner in the metal shell, back-side wear, and true wear (Citation246). The pattern of PE wear is typically high in the first period after surgery and then decreases with time.
The reason is that the femoral head penetrates into the acetabular polyethylene due to a combination of creep and wear. Creep decreases over time and is considered to be important within the first 6 to 12 months, after which PE wear is described as linear (the true wear) or in a “steady state” (Citation246). Bedding-in describes the backside of the PE liner wearing into a higher conformity with the metal shell, while running-in describes the initial fitting of the femoral head into the polyethylene liner, resulting in a larger contact surface with lower contact stresses and lower rates of wear (Citation229;Citation245). Ultimate strength is the stress maximum before component failure with a single stress load. Yield strength is the amount of stress that makes a plastic deformation in a component measurable. Fatigue strength is the stress below which no failure occurs regardless of the number of loading cycles. Elongation to break is the load over the elongated length of the polyethylene until it breaks.
Production of polyethylene
PE is polymerized into a powder (resin) by ethylene gas. The resin is consolidated prior to machining of implant components. Optimal consolidation is crucial for clinical performance of the PE, and three fabrication traditions exist: compression molding, ram extrusion, and hot isostatic pressing (HIPing) (Citation19). Compression molding comprises pressing the powder under temperatures over the melting point directly into the final shape. Ram extrusion is a process of compression and heating the polymer powder into cylindrical bars, which may later be machined into implants. HIPing is a multi-step conversion process beginning with the manufacture of a cylindrical compact through cold isostatic pressing which expels most of the air. Subsequently, the compacted “green” rods are sintered in a HIP (hot isostatic pressure) furnace in a low oxygen pouch to prevent degradation of the UHMWPE. The resulting rod stock is essentially isotropic due to the hydrostatic sintering process and may be considered a compression molded form of the resin. Finished implants are then made by either turning or milling operations (ArCom Processed Polyethylene). Important parameters in all three production methods are time, temperature, and pressure, which influence the density, crystallinity, and degree of consolidation of the PE. A study has shown a two-fold increase in wear of extruded bar cups compared to compression molded cups (Citation10).
Sterilization of polyethylene
Until 1995, UHMWPE was typically sterilized in an oxygen environment by gamma radiation (25–45 kGy) (11;58). A consequence of this procedure is accelerated oxidation of the UHMWPE due to breakage of chemical bonds (oxidative chain scission) and the creation of free radicals within the polymer (Citation247). Although free radicals can enhance the wear properties of PE through subsequent cross-linking, they leave PE vulnerable to oxidation (Citation160). Thus, if the PE is packaged in an air environment, oxygen present in the air during radiation sterilization can react with the free radicals and can adversely affect the mechanical properties of the polymer (Citation244). When this became evident, the sterilization of PE in air was abandoned by manufacturers and alternative sterilization strategies developed. Two fundamentally different methods were developed. The first approach is sterilization without radiation by surface treatment (ethylene oxide or gas plasma), eliminating the formation of free radicals and potential for oxidative damage to the PE during shelf and in vivo life. However, improvement in wear properties from radiation-induced cross-linking is also eliminated. The second approach is sterilization with radiation in a low-oxygen or oxygen-free environment and vacuum-barrier packaging in an inert gas such as nitrogen or argon, before or after radiation sterilization (Citation58). This method reduces the potential for shelf oxidation; however; free radicals generated during the radiation sterilization remain within the polymer, and the subsequent potential for in vivo oxidation is unknown (Citation247). The clinical performance of UHMWPE is superior when sterilized by gamma radiation compared to gas plasma sterilization (Citation247).
Cross-linking of polyethylene
Cross-linking of PE (HXLPE) can be achieved with high loads of irradiation, chemical agents, or peroxides – all of which result in a higher resistance to wear of the PE (Citation137;Citation177). Next, the material is thermally remelted or annealed to do away with all the free radicals, and finally it is sterilized with or without irradiation. Annealing is heating to a temperature lower than the melting point. In this way the mechanical properties are maintained; however, free radicals and the potential for post-treatment oxidation are still present. Remelting involves heating of the cross-linked PE to temperatures higher than the melting point. This makes free radicals in the crystalline regions accessible for elimination, but also the microstructure of the PE is changed (reduced crystallinity) and mechanical properties are reduced.
The first attempts of cross-linking (to produce cross-linked PE) were made in the early 1980s (Citation8;Citation88;Citation177), and at the end of the 1990s encouraging long-term clinical results were reported (Citation89). Laboratory studies showed a 90% decrease in wear rate with increasing cross-linkage (Citation137;Citation159). Further resources were then used to find the best way to benefit from the decrease in wear rate attributed to the cross-linking and simultaneously avoiding the negative consequences of oxidation (reduced toughness and resistance to crack fatigue). Clinical studies of short and mid-term follow-up reveal a 50–80% decrease in wear (Citation71;Citation203).
The size of the highly cross-linked wear particles is smaller, but the total number of particles similar to conventional PE (Citation202). However, the biological reaction of macrophages to cross-linked PE particles is higher compared with the reaction to non-crosslinked PE particles, which raises concern regarding a cell-mediated osteolytic response (Citation77).
Vitamin E polyethylene
Recently PE with the addition of vitamin E, a natural antioxidant, has been developed. Vitamin E hinders cascade oxidation reactions in the UHMWPE without remelting the irradiated cross-linked polymers, and thus a reduction in mechanical properties due to a decrease in crystallinity by the remelting process is avoided (Citation179;Citation181;Citation182). E-Poly highly cross-linked PE (HXLPE) is thus believed to surpass the limitations of first generation remelted and annealed highly cross-linked UHMWPE and provide both high mechanical strength and true oxidative stability (Citation180). Oxidative stability testing has shown that vitamin E prevents oxidative degradation of the PE without remelting, allowing the material to maintain mechanical properties and wear resistance over time (Citation179;Citation180;Citation182). In large (femoral) heads, as much as an 89% reduction in wear in comparison with traditional UHMWPEs is expected.
Aseptic loosening
Although joint replacements are highly successful, especially during their first decade of use, they do not last forever, and revision THA surgery remains a significant burden to the healthcare economies of Western countries. The main reason for revision surgery is aseptic loosening, defined as the mechanical loosening of a joint prosthesis without signs of infection. Particulate debris from joint replacements, especially PE particles, is believed to play a major role in the development of aseptic loosening. For each day of patient activity, around one hundred million microscopic UHMWPE wear particles are released into the tissues surrounding the hip joint (Citation137), where they activate the cellular systems in the local tissues controlling foreign-body immune reactions and bone turnover. Loosening does however not occur until the local bone loss is extensive, and the patient may remain clinically without symptoms until the components loosen.
In 1977, Willert described synovial thickening and scar tissue around artificial joints (Citation270), and he found huge amounts of wear debris in the articular capsule within granulation tissue that included macrophages and giant cells. He suggested that in cases where wear products were not sufficiently removed by the lymphatic system, the synovial membrane could extend to the bone-implant interface and contribute to implant loosening. More specifically, it is probably the total mass of biologically active cells (macrophages, lymphocytes, and fibroblast-like cells) in the synovial membrane (joint capsule) and the inter-facial membrane (Citation86) (fibrous membrane around loose implants) that result in the production of osteolytic mediators in the joint fluid. These mediators penetrate to the bone-implant interface and contribute to increased local bone resorption (Citation118;Citation274).
The particulate debris is mainly phagocytised by macrophages. Particles between 0.2 to 10 µm can be phagocytised, but it is primarily submicron particles that are found within macrophages (Citation21). The cellular response to wear particles includes a large variety of cytokines, chemokines, arachedonic acid metabolites, and degradative enzymes that interact in a complex network (Citation2;Citation49;Citation81;Citation84;Citation87;Citation112;Citation133;Citation168-170;Citation201). Monocytes and macrophages are recruited and some are differentiated into osteoclasts by activation of the RANK receptor RANKL (Citation50), but activated macrophages may even participate directly in bone lysis around the implant (Citation118). Among the most important cytokines in osteolysis are TNFα, IL-1, and IL-6, and these directly affect osteoclasts and osteoblasts and result in bone resorption. Furthermore, both cytokines induce secondary effects on neighbouring cells, resulting in bone-matrix degrading enzymes (16;Citation111;Citation190).
There is probably an inter-individual variation in the reaction to wear debris (Citation41;Citation115;Citation154;Citation155;Citation274), and further it has been suggested that the PE debris produced initially in vivo is smaller and more bio-active than particles produced at a later stage (Citation6). Third-body wear can generate particles with yet another biological significance, and the relationship between the implant time in situ and the biological response to particles leading to aseptic loosening is probably complex and difficult to determine.
Osteolysis
Wear of UHMWPE is generally recognized as the primary mediator for osteolysis (Citation12;Citation75) and the main problem limiting survival of cementless ace-tabular components (Citation94). The occurrence of peri-prosthetic osteolysis is multifactorial and indeed particle-related (Citation132), but a theory combining fluctuation of joint fluid under pressure and particle-mediated cell responses is the most likely. The space between a cementless total hip implant and the bone constitutes the path of least resistance and allows for joint fluid containing particles to access the endosteal surface of the femur and acetabulum (Citation148;Citation149;Citation266). Thus fluctuating pressure waves in the joint fluid during gait can promote bone resorption (Citation7;Citation258). The peri-implant area accessible to joint fluid and thus to wear debris is termed the effective joint space (Citation222).
There is a strong relationship between long-term true wear rates and the occurrence of osteolysis, and a PE wear threshold above 0.2 mm/year leads to long-term, large lytic bone destruction in most cases (80%) and in all cases with wear rates greater than 0.3 mm/year, regardless of the type of implant, fixation (cemented or cementless), and head size (Citation73;Citation240;Citation263). Wear rates above this critical wear threshold were shown to be associated with a substantially greater risk of loosening and revision. Several authors have shown that that true wear rates (steady-state wear rates), after the period of creep, seem to be constant (Citation73;Citation131;Citation246), and thus measurement of early true wear rates may enable the prediction of patients at risk of later development and osteolysis. A practical wear-rate threshold below 0.05 mm/year, below which osteolysis would be very rare, has been suggested (Citation75) and seems obtainable with the newer cross-linked PEs (Citation70;Citation203). The early detection of such small wear rates increases the demands placed on wear-measurement methods and underlines the need for investigation of the practical detection limit for the methods used for wear measurement now in use.
Radiographic osteolysis of cementless implants may appear according to two patterns. The first pattern results in expansile (cystic) lesions with indistinct margins. The expansile lesions begin at the bone-implant interface and expand into the cancellous bone, with a considerable loss of bone () (Citation278). High particle loads circulating in the effective joint space may facilitate this type of lesion, but the role of unsealed screw holes is still debated (Citation217). It has been recommended that cases of progressive osteolysis and impending wear-through be revised (Citation54). The second pattern is similar to what is seen in cemented implants, with a slower growing linear osteolytic lesion with sclerotic margins at the implant-bone interface. Linear osteolysis probably results from a soft-tissue membrane, formed by the biologic response to wear particles, dissecting along the implant-bone interface. These lesions begin at the implant periphery and progress to the central region of the interface (Citation223). Usually radiolucencies wider than 1 mm are considered significant, but it has been suggested that attention should be given to even thinner radiolucencies (0.3 mm) (Citation106). It is currently not clear when to revise only the worn liner and when to revise both the cup and liner (54;Citation143;Citation171;Citation251). There is a common consensus, however, that a complete radiolucency surrounding the entire implant should be considered a radiographic failure.
Biological markers of PE wear and osteolysis
Ion forms of polymers used in arthroplasty are not specific, and wear particles are often retained in the local. Thus it is difficult to measure PE wear directly, but mediators of the inflammatory reaction induced by PE wear products may be useful as surrogate markers. The challenge is to identify markers specifically associated with PE wear and osteoclastogenesis that are not elevated with other coexisting systemic conditions (i.e. osteoarthrosis) (Citation17).
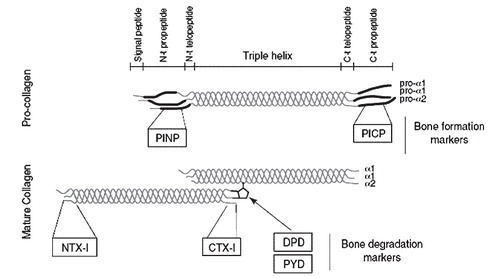
Indices of bone turnover (i.e. collagen fragments) can be evaluated biochemically by blood and urine samples, and may be associated with osteolysis (). Collagen type 1 is mainly present in the bones, and DPD, NTX-1 and CTX-1 are examples of collagen type 1 degradation markers. Activated macrophages and osteoclasts produce TRAcP and a resorption-index (CTX-1/TRAcP) can be calculated to provide information of osteoclast activity. Bone Specific Alkali Phosphatase (BSAP) and osteocalcin measured in serum are specific markers of bone construction or osteoblast activity. Measurements of bone resorption markers (osteoclast activity) in blood and urine (NTX-1) have recently been shown to be predictive of periprosthetic osteolysis, however a baseline value is needed for assessment of individual cases (Citation260;Citation268). Analysis of blood and urine samples forms an interesting and harmless potential for the monitoring of implant failure, and bisphosphonates make up the potential of a medical solution to decrease osteoclastic activity and lessen osteolytic periprosthetic damage (Citation250).
Wear
Wear is defined as the progressive removal of material from the prosthesis, resulting in particulate debris. The most important wear location in a normal THA would be between the acetabular component and the femoral head. PE wear has a multifactorial nature () and the complexity concerns many factors, i.e. material properties such as hardness, surface finish, and conformity of the femoral head and the socket. In addition, the femoral head size (Citation37;Citation103), liner thickness (Citation117;Citation139), implant design, bone cements, implant surface coatings, operative procedure and component placement, component fixation, the quality and manufacturing of PE, as well as the sterilization technique have been shown to play a role. The larger the femoral head, the greater the PE wear (Citation66;Citation142). Patient-related variables, such as young age and male gender associated with the activity level of the patient and the use of the implant, also influence the success of THA (Citation221). Patients below the age of 60 have been shown to walk 30% more than patients who were 60 years or older. Wear is traditionally described in terms of “use over time” but suggestions of redefinition to “function of use” or number of movement cycles have been proposed (Citation219). The average patient has a walking activity averaging 0.9 million cycles per year, and the most active patients have walking activity averaging 3.2 million cycles per year (Citation219). A 45-fold difference in the number of gait cycles from the least active patient to the most active patient has been described (Citation219), and can probably explain some of the large differences in wear seen within a group. In all series of THAs, there are some cases with wear several times greater than the average for the study, and this cannot simply be explained by difference in the wear resistance of the PE.
Wear is a complex mechanism, and although many contributing factors have been described, many elements probably remain unknown.
Wear mechanisms can be separated into four wear modes. Mode 1 wear, also termed adhesive wear, exists between the two articulating bearing surfaces and involves pulling away particles from the surfaces. Mode 2 wear refers to the condition of primary bearing surfaces rubbing against each other in a manner not intended by the designer and describes metal rubbing against metal such as seen in PE-wear-through or a dislocated hip. Mode 3 wear, also named abrasive wear, involves third bodies. This type of wear occurs when particulate material (cement particles, bone pieces, hydroxyapatite, and metal) is interposed between the bearing surfaces and the surface becomes abraded. Outward scratching of metal or grain pull-out from phase-transformed Zr may also result in abrasive wear, which could be classified as two-body wear. Mode 4 wear, or fretting wear, refers to the rubbing between two materials that are not intended for motion, such as fretting between the metal shell and a PE insert (back-side wear).
UHMWPE particulate debris from peri-implant tissue of failed cementless total hip implants has been analyzed by scanning electron microscopy, and a mean size of 0.5 µm (range 0.2–2.0 µm) was determined. Most particles were found to be spheroids; however, fibrils, typically 0.2–0.3 µm in width and up to 10 µm long, were also seen (Citation218). Many particles were aggregated as a carpet-like mesh of 50 to 80 µm (Citation228). It is the submicron particles (0.3–1.0 µm) that have the major effect on macrophages and bone remodelling (Citation194;Citation212). Wear debris is present in lymph nodes and distant organs as well as in the peri-prosthetic tissues (Citation198). The size of the PE particles keeps them mainly in the local environment, whereas metallic debris has been found in the bone marrow, liver, and spleen (Citation40;Citation255). Lately two case-reports of severe and rare complications of PE wear has been reported; a case of penetration of a metallic femoral head through the acetabular shell (Citation230), and a case of recurrent femoral deep vein thrombosis from a pelvic mass induced by polyethylene wear debris following total hip arthroplasty (Citation198).
Wear measurement
The earliest motivations for wear measurements were the determination of PE wear-through, while today’s interest is more directed towards the role of PE wear debris in periprosthetic osteolysis. Radio-graphic techniques for wear measurement are commonly used to determine whether a new PE-bearing material has better wear properties than a previous material or to monitor wear performance against some historically determined baseline.
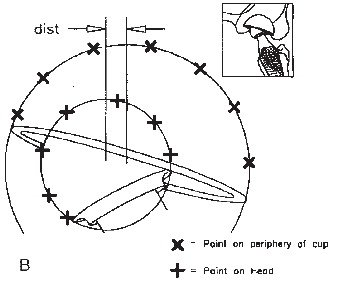
Radiographic images are essentially single-point-perspective geometric projections of radiopaque objects, and methods used to measure femoral head penetration can be distinguished from one another based on the strategy used to determine the relative positions of the femoral head and cup. Shadow-comparing methods determine the relative positions of the head and acetabular component by direct comparison of the radiographic shadows of the two objects (Citation46;Citation47;Citation93;Citation135;Citation142;Citation151;Citation229). Shadow-comparing methods require only knowledge of the femoral head diameter to assess femoral head penetration. Shadow-casting methods cast the radiographic shadows of one or both components back to towards the point source of the beam to determine the relative positions of each object (Citation67;Citation126;Citation176). Shadow-casting methods require either detailed knowledge of the geometry of the acetabular component or digitization of reproducibly distinguishable features of its shadow. With the various shadow-comparing and the shadow-casting methods currently available, it is assumed that the head has worn a straight cylindrical path through the acetabular bearing, and a linear vector of wear is reported or calculated.
The measure of interest is a change in penetration rates with time and the determination of steady-state wear rates. A number of methods can be valuable in clinical studies of THA, provided appropriate quality control of the radiographs and digital resolution is assured. Radiostereometric analysis (RSA) methods, because of their higher precision, can give important early information on device performance from a small number of patients, whereas methods for plain radiographs, such as the PolyWare and Martell methods, are indicated for the assessment of PE wear in high-wear bearings or studies of long-term follow-up. In vivo PE wear occurs in multiple directions, which readily explains why several clinical methods that assume a single direction of wear underestimate the true amount of wear (Citation275-277). Innovations in PE processing over the past decade have sparked clinical and industrial interest in utilizing early wear measurements as predictors of long-term wear (Citation192).
Between the second and the tenth postoperative year, head penetration is approximately constant – that is linear over time. Therefore, radiographically determined head penetration patterns can be used to estimate when complete liner wear-through will likely happen. This may be useful for determining how frequently a patient should return for follow-up examinations – or when to schedule the patient for revision surgery. However, specific phenomena may change the head penetration patterns and obscure the linearity of radiographic wear, i.e. third-body wear debris and changes in the surface smoothness of bearing surfaces. Small values of femoral head penetration are most susceptible to wear measurement error, and each specific wear measurement method accounts for variability and limitations.
Manual methods
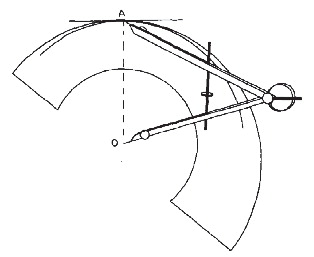
The first attempt to measure PE wear was the uniradiographic method described by Charnley and Cubic (Citation46), which was soon modified to the duoradiographic method (Citation91). These techniques were developed for cemented PE cups. Livermore et al. (Citation142) later described a method that used a transparent overlay with concentric circles (). This method relies on the visual determination of the edge of the femoral head that is not obscured by the metal cup. Although manual techniques have been used successfully in series of long-term follow-up of patients with a relatively large total femoral head penetration, these techniques can result in a high variability among different users, and they lack precision to determine useful information in short-term in vivo follow-up or in low-wear bearing (Citation53;Citation72). However, these methods are the simplest and cheapest to apply to clinical radio-graphs.
Computer-assisted methods for plain radiographs
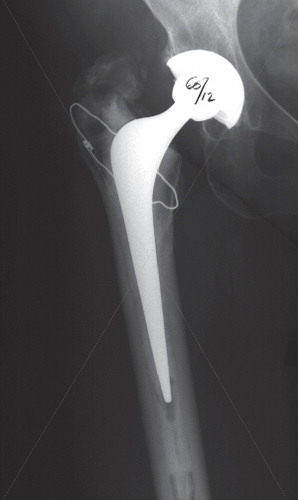
In an attempt to improve the precision of manual wear measurement, computerized techniques were developed. These techniques use either one plain radiograph (anteroposterior) to determine two-dimensional linear PE wear, or two plain radio-graphs (anteroposterior and cross-table lateral), to determine three-dimensional linear and volumetric PE wear (Citation67;Citation68;Citation93;Citation135;Citation151;Citation229). These programmes combine the use of image analysis techniques with the determination of bone landmarks and edge-detection algorithms to determine the change in the position of the femoral head centre with respect to the acetabular component centre. The computerized techniques function by modelling the margins of the femoral head and acetabular shell, each with a fitted ellipse. The precision of these techniques therefore depends on the level of contrast at the implant borders and the amount of margin of the femoral head that is obscured. These techniques are sensitive to the quality and projection of clinical radiographs, the congruency of patient positioning during the examination, and the variability of head penetration in a clinical patient group (Citation76;Citation76;Citation184;Citation184;Citation249;Citation249). These techniques are widely used and applicable in retrospective and large series because they use conventional radio-graphs and do not require in vivo bead marking of the bones and the use of a calibration cage during roentgen examinations as is the case with RSA. The Martell method (Hip Analysis Suite) has been shown to overestimate true wear, while the Devane method (PolyWare) () has been shown to underestimate the true wear as assessed by a coordinate measuring machine on retrieved cups (Citation105). Laboratory studies infer that computerized methods are superior to manual methods (Citation13;Citation172); however, this has not been confirmed in the clinical setting (Citation13;Citation76). Experience with computerized software is necessary prior to engagement in the evaluation of clinical patient series. And in addition computer, scanner, and personnel to operate the equipment as well as the cost of the software’s should be considered.
Computerized methods for stereo radiographs
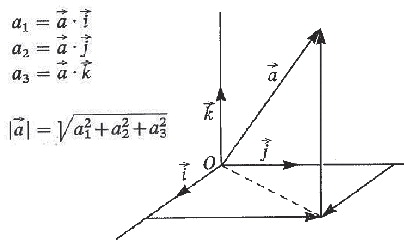
Radiostereometric analysis (RSA) was developed by Selvik et al. (Citation226) and is considered the most accurate method of determining the magnitude of relative displacements from radiographs for multiple applications, including the evaluation of growth plate integrity, joint kinematics, fracture healing, implant stability, and femoral head penetration (Citation32 ;Citation102;Citation125;Citation146;Citation211;Citation256). Several software packages for RSA have been developed () (29;Citation108;Citation120;Citation256;Citation257;Citation265). Formerly, prestudy bead marking of the implants or PE was required to confine a rigid body segment, and in assessment of implant migration, fracture healing, and joint kinematics, intra-operative bead marking of the bones (reference rigid body segments) is still essential. Tantalum beads size 0.8 mm and 1.0 mm are commonly used because of their high radiographic density and biocompatibility. A pair of stereo radiographs is obtained with the patient in relation to a uni-planar or bi-planer calibration box, which allows for the reconstruction of a three-dimensional coordinate system. By use of automated computerized analysis software, the relative displacement of two rigid bodies can be calculated from sequential stereo radiographs (Citation32;Citation126). The placement of tantalum beads in vivo along with the costly and specialized roentgen setup limit this method to small groups of patients and selected research organisations. Digital RSA methods have been shown to yield results close to the true value (accuracy) and with high precision (Citation33;Citation262).
Aim of the thesis
The problem limiting longevity of total hip arthroplasty during the past two decades was, and still is, osteolysis and aseptic loosening, which most commonly occurs in association with polyethylene wear particles. The overall aim of this thesis was to evaluate radiographic polyethylene wear in two clinical patient series to identify implant materials that might lead to increased wear, osteolysis, and revision. During these investigations we became inquisitive regarding the limitations of the wear measurement software that we used, and therefore in three succeeding methodological studies we further investigated possible inaccuracies and measurement problems with this software and in addition its agreement with RSA, the gold standard of radiographic wear measurement.
The individual studies that make up this PhD thesis had the following specific aims:
Study I To investigate whether there was a difference in cup survival rates with or without HA coating, and whether any difference in survival rates was associated with the amount and rate of wear and the amount of osteolysis.
Study II To assess the mid-term polyethylene wear characteristics and clinical performance in a patient group with CoCr femoral heads compared to a group with Zr femoral heads.
Study III To investigate whether polyethylene wear analysis with the PolyWare software of a single, two, or multiple plain radiographs in the same clinical series of patients could result in different estimates of wear.
Study IV To compare three different wear measurement methods in a hip phantom by intra-method repeatability, criterion concurrent validity between methods, and criterion concurrent validity between the methods and the true wear. Study V To establish the intra-method repeatability and criterion concurrent validity between methods for measurements of polyethylene wear in a clinical patient series with total hip arthroplasty.
Materials and methods
Patients
Studies I and III
The patient material comprise a long-term (12 year radiographic, and 15 years survival) follow-up of 27 eligible patients that were operated in 1990-91 and prospectively enrolled to random allocation of either a Ti- (n = 13) or a HA-coated (n = 15) THA for a femoral stem migration study (Citation239). The patients were all operated by one surgeon (CB) by the posterolateral surgical approach at Aarhus University Hospital. One patient who entered the study with bilateral surgery, one Ti hip and one HA hip, died 1 year after surgery of causes unrelated to THA. He was unrevised on both THA’s according to the patient record and was excluded from investigation in the entire study (survival, wear, and osteolysis).
We included patients with osteoarthritis of the hip and age older than 18 and younger than 67 years and excluded those with congenital hip disorders, osteoporosis (ie, those under medical treatment), bone metabolic disorders, rheumatoid arthritis, malignant disease, and femoral neck fractures. All patients meeting the inclusion and exclusion criteria were offered participation in the migration study until allocation of 28 hips had been reached.
26 patients remained for determination of survival based on revision at 15 years. Radiographs of 22 of the included total 26 patients were available for measuring linear wear (accessible postoperative and follow-up radiographs), and 25 patients had radiographs (last follow-up) available for quantification of osteolysis. The further censuring of patients for the different investigations in this study is described in .
Figure 13. Diagram of the censuring of patients in study I. A = aseptic loosening, O = osteolysis, T = trauma.
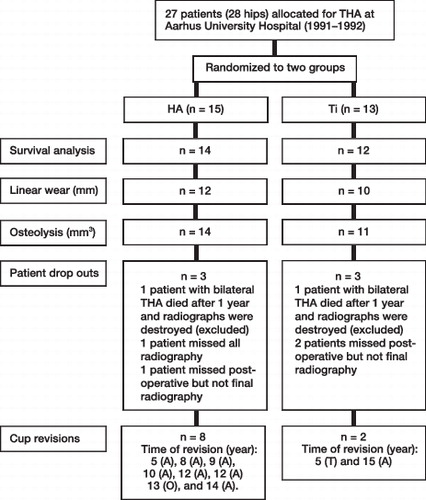
We assessed radiographic polyethylene wear and osteolysis to the 12-year follow-up or end point revision at a minimum of 5 years (mean, 10.9 years; range, 5–12.6 years).
Distribution of gender, age, weight, cup size, liner thickness, average follow-up time, and hip side was similar between the patient groups ().
Table 3. Patient demographics (mean, range) of study I
Study II
The patient material include a medium-term (5 year) retrospective follow-up of 68 patients (70 hips), younger than 65 years, having THA for primary or secondary osteoarthritis. From 1996 to 1997, CoCr femoral heads were used in all eligible 33 patients (33 hips), and from 1998 to 1999, Zr femoral heads were used in all eligible 35 patients (37 hips). The patients were all operated by one surgeon (KAN) at Randers Regional Hospital by the posterolateral approach. One patient with a Zr femoral head lacked all radiographs after 3 months in the radiographic folder, and was excluded from the entire study, and the total number of patients included in the study was therefore 33 patients (33 hips) with CoCr femoral heads, and 34 patients (36 hips) with Zr femoral heads. The minimum clinical follow-up was 56 months (mean, 65 months; range, 56–77 months). Five-year radiographs were missing for six of the 67 patients (9%) and we used the latest radiographs (24 to 37 months) to measure wear in these cases.
Study IV
There were no patients involved in this study, which was a phantom study.
Study V
The patient material was a selected group of twelve patients who had primary total hip arthroplasty (THA) between December 2001 and October 2003. These twelve patients were a subgroup out of 44 patients enrolled in an ongoing multicenter randomized clinical trial (RCT) evaluating two surgical techniques of the femoral component by radiostereometric analysis.
We invited all available patients from the RCT, operated at one center (Aarhus University Hospital), who had a minimum 5 year clinical follow-up (average 6.1 year; range 5.3–7.1 years) for an additional clinical and radiographic double examination follow-up. Of the 18 invited patients, twelve responded and accepted participation. They were all seen and radiographed in January and February of 2009.
Criteria of inclusion were osteoarthritis of the hip, and age older than 18 and younger than 70 years. Criteria of exclusion were osteoporosis (i.e. those under medical treatment), neuromuscular or vascular leg disease, bone metabolic disorders, insufficient bone stock for total cementless THA, rheumatoid arthritis, malignant disease, planned pregnancy, and femoral neck fracture. Four surgeons performed all THAs using a posterolateral approach.
Design
Studies I and II
In the clinical studies the outcomes of implant survival, linear polyethylene wear, and osteolysis were assessed retrospectively by querying the Danish Hip Arthroplasty Registry (DHR) and all local patient records, and by evaluation of radiographic images. All patients included in these two studies were registered in DHR, a nationwide clinical database on primary THAs, revisions, and postoperative complications in Denmark since the beginning of 1995, and thus no patients were lost to final clinical follow-up of revisions and complications. According to levels of evidence for the primary research question as adopted by the American Academy of Orthopaedic Surgeons both studies were classified as Level III therapeutic studies: retrospective comparative studies. In study I we retrospectively assessed parameters in a prospective randomized patient group. In study II two consecutive patient series were compared in retrospect.
Study III
This was a methodogical study assessing the importance of the number of plain radiographs used for polyethylene wear analysis with the Digital Poly-Ware software. We used the radiographic material of patients with 12 year follow-up from study I.
Study IV
In a hip phantom we compared true simulated wear with the measured wear by two model based RSA methods applicable for wear analysis and by Poly-Ware. We assessed the entire scale of clinically relevant wear from 0.01 mm to 8.7 mm, where the highest value was close to wear-through of the liner. The study was prepared in accordance with the Standards for Reporting of Diagnostic Accuracy (STARD) initiative (Citation30).
Study V
In a small prospective patient series of cementless THA we retrospectively compared the polyethylene wear measured by one model based RSA method and the Digital PolyWare method and compared the results. The study was prepared in accordance with the Standards for Reporting of Diagnostic Accuracy (STARD) initiative (Citation30).
Ethics and permissions
Studies I, II and III
In study I the RCT of the femoral stem was initially (in 1990) approved by the local ethical committee and informed consent from all participants was obtained at the time of surgery. The procedures followed the ethical standards of the local ethical committee on human experimentation, and were in accordance with the Helsinki Declaration of 1995, revised in 2000. The protocol of the retrospective radiographic follow-up studies I and II were reviewed by the Central Denmark Region Committees on Biomedical Research Ethics, and were classified as register research projects as they involved only radiographs kept on file and no human biological material. Therefore, and according to the Act on Biomedical Research Ethics Committee System and the Processing of Biomedical Research Projects § 8.3, no ethical approval was needed. Study I and II were registered with the Central Denmark Region (RM) and the Danish Data Protection Agency (record number RM 1-16-02-1-08/049; 2007-58-0010 and record number RM 1-16-02-1-08/07; 2007-58-0010, respectively) and permission was granted to employ a database for the project. Study III involved the radiographs of study I, and no further approval for this study was obtained.
Study IV
There were no ethical considerations for this project, which was a phantom study.
Study V
The original RCT and the retrospective polyethylene sub-study were approved by the local ethical committee (record number 20000065 and 20080196, respectively) and informed consent from all participants was obtained. The procedures followed the ethical standards of the local ethical committee on human experimentation, and were in accordance with the Helsinki Declaration of 1995, revised in 2000. Study V was registered with the Danish Data Protection Agency (record number RM 1-16-02-31-09; 2007-58-0010) and permission was granted to employ a database for the project.
Sample size
Study I
The first project at commencement of my PhD education was study I. The sample size was fixed from the start, as the patient material was collected from the first RSA study performed at Aarhus University Hospital (Citation239). It was easily spotted in the patient records that the HA group sustained more and earlier failures related to excessive wear of the acetabular component compared with the Ti group. A calculation of risk difference at 40.48% (95% CI: 7.06–73.89) was performed. With the number of patients available (n=26) a calculation of power, based on an expected clinically relevant failure difference of 40% between the groups (alpha = 0.05), revealed a power of 50 or less. The difference in proportion was based on the existing literature of HA coated hemispheric cups failures (cup revision rate of 39.5% at 8 years, and 43% at 6 years follow-up) (Citation127;Citation263). In spite of the small sample size available our curiosity for a correlation of HA cup failure to wear-problems and osteolysis made us proceed with these evaluations.
Study II
With the second study, again, the sample size was fixed from the start to the available cohorts, but to test the chance of significant results we performed a preliminary calculation of sample size based on long term (12 years) PE wear data of a similar study (mean 0.134 mm/year; standard deviation 0.14 mm/year) (Citation100). Early (mean 3.7 years) true wear rates (eliminating creep) above 0.1 mm/year has been strongly and significantly associated with later osteolysis (Citation73) and therefore we targeted a mean difference in PE wear rate of 0.1 mm/year which suggested 31 patients per group with a power of 80% (alpha = 0.05).
Study IV
In the phantom study, we were interested in the agreement of the measured wear along the entire scale of clinically relevant wear with the true simulated wear, and we did not perform a calculation of sample size.
Studies III and V
With study III and V we used the available 12- and 5-years of follow up radiographs, respectively, to compare differences in wear measured by different wear measurement methods, and we did not perform a pre-study calculation of power.
Implants
Studies I and III
All components (femoral stems and acetabular cups) were similar except for the coating (which for a given patient was the same for the stem and the cup). One group had implants with porous Ti coating with HA and the other group had implants with a similar porous Ti coating without HA. The cups were fixed with two to three titanium screws. The femoral component was a solid Ti6A14V alloy core Bi-Metric® design (Biomet Inc, Warsaw, IN) with a collarless straight stem and a circumferential Ti plasma spray porous coating to the proximal ¼ ().
The acetabular metal shell was a hemispheric Ti plasma-spray porous Universal® design (Biomet Inc, Warsaw, IN) with rim flair, holes for optional bone screw supplemental fixation, and a Hexloc® locking mechanism (Biomet Inc) for the liner (). The femoral heads (Biomet Inc, Warsaw, IN) were all 28-mm chrome-cobalt alloys. The Ti-coating applied to all components had a pore size of 300 µm. The HA-coated components had an additional 50- to 75-µm layer of spray-dried synthetic HA deposited by plasma spraying. The HA crystallinity was 90%. The surface roughness (Ra) was 41 µm for the HA components and 47 µm for the Ti components. The Universal® shell and Hexloc® locking mechanism were discontinued in 1994.
The liner was a 10° face GUR 415 bar extruded UHMWPE in all cases (). A postextrusion thermal cycle (annealing), known to slightly increase crystallinity, density, and rigidity, was conducted before machining to maintain the PE bar in stable shape. To reduce oxidation, the PE was packaged in an inert argon environment. We used six different PE thicknesses (range, 3.39–11.46 mm). The overall mechanical properties for this PE were reportedly superior () than the recommended standard of the American Society of Testing and Materials (Citation1).
Table 4. Properties of ultrahigh-molecular-weight polyethylenea
Study II
The THA was a so-called hybrid with a cemented femoral component and an uncemented acetabular component. The acetabular components were all uncemented, titanium plasma-sprayed, and hydroxyapatite-coated and of the same design (Mallory-Head, Solid Finned Ringloc® metal shells; Biomet Inc, Warsaw, IN) (). They were inserted using the same technique (approximately 2-mm press-fit by coating thickness, line-to-line reaming). In all cases, the PE liners were a compression-molded, ultrahigh-molecular-weight PE (UHMWPE) resin, consolidated, packed, and sterilized by gamma irradiation in argon gas in the range of 2.5 to 4 Mrad (ArCom®; Biomet Inc).
Figure 17. Bi-Metric® solid cup with Ti plasma-spray porous coating and Ringloc® locking mechanism (Biomet Inc).

All femoral components were of the same design (Exeter®; Stryker, Kalamazoo, MI) () and inserted using the same technique, including distal plugging, pulsatile lavage of the medullary canal, and pressurized injection of vacuum-mixed Simplex® P polymethylmethacrylate bone cement (Stryker Howmedica, Rutherford, NJ).
All femoral heads were 28 mm. The femoral heads inserted during 1996 and 1997 were CoCr heads (Howmedica Osteonics Corp, Allendale, NJ), and the femoral heads inserted during 1998 and 1999 were of yttria-stabilized zirconia (Y-ZrO2) ceramic (Prozyr®) and produced by St GobainDesmarquest Céramiques (Evreux, France) by sintering and hot isostatical pressing ().
Study V
All components (femoral stems and acetabular cups) were cementless. The femoral component was a solid Ti6A14V alloy core Bi-Metric® design (Biomet Inc, Warsaw, IN) with a collarless straight stem and a circumferential titanium plasma-spray and hydroxyapatite porous coating to the proximal ¼ ().
The acetabular component was a titanium plasma-spray and hydroxyapatite coated Mallory-Head Solid or Dome Holed Finned Ringloc® metal shell (Biomet Inc, Warsaw, IN) (). The cups were inserted using the same technique (approximately 2-mm press-fit by coating thickness, line-to-line reaming). The femoral stems were inserted by randomization to two surgical techniques of preparation of the medullar canal (bone rasping versus bone compaction). The femoral heads (Biomet Inc, Warsaw, IN) were all chrome-cobalt alloys (Biomet Inc, Warsaw, IN), and in eleven cases 28 mm and in one case 22 mm in diameter. In all cases, the PE liners were of the Hi-Wall type, and consisted of compression-molded, ultrahigh-molecular-weight PE (UHMWPE) resin, consolidated, packed, and sterilized by gamma irradiation in argon gas in the range of 2.5 to 4 Mrad (ArCom®; Biomet Inc).
The phantom fixture
A custom-made phantom fixture for the femoral head and acetabular shell was constructed (Eurocon CNC & Process ApS, Denmark) of radiolu-cent materials, allowing for radiographic exposure of the components in all directions (). The femoral head could be moved independently in craniocaudal, mediolateral and anteroposterior directions by use of three digital dial micrometers, each with a resolution of 0.001 mm (Hofmann GmbH, Achim, Germany). If desired, the cup could be rotated and fixed in any preferred combination of abduction (tilt) and anteversion. A wire marked the horizontal plane. A 56-mm Mallory-Head Solid Finned Ringloc® metal shell (Biomet Inc, Warsaw, IN) and a 28-mm cobalt-chromium femoral head (Biomet Inc, Warsaw, IN) fixed on a Bi-Metric® femoral stem (Biomet Inc, Warsaw, IN) were mounted in the phantom fixture with the ball concentrically within the cup (zero wear position).
For radiographic imaging the phantom was placed on the side imitating a right hip. After obtaining the radiographs the metal shell was reverse engineered by laser scanning to 5000 triangular elements (TNO Industrie en Techniek, Eindhoven, The Netherlands).
Displacement protocol
The cup tilt and anteversion was kept constant at 45° and 25°, respectively. A displacement protocol of 5 predefined 3D wear vectors was defined for each of 3 wear categories; low wear (10µm, 20µm, 30µm, 40µm, and 50µm), medium wear (100µm, 200µm, 300µm, 400µm, and 500µm), and high wear (1000µm, 2000µm, 3000µm, 4000µm, and 5000µm) in order to assess the total scale of clinical wear. Thus 15 wear advancements were obtained as well as a baseline value (vector of 0µm) with the femoral head centered in the acetabular shell (simulating a postoperative radiograph). For each wear simulation all three micrometers were advanced according to the displacement protocol in the superior, medial, and posterior directions correspondingly between new radiographs. In the position of the 5000µm vector the femoral head almost touched the dome of the acetabular shell (simulated wear through of 8.7 mm). For comparison with the measured wear, we calculated the true vector length by use of Pythagoras Theorem.
When “a” defined the advancement of the micrometer, and “r” defined the radius of the fem-oral head the calculations of true wear followed these formulas:
– 2D linear wear = √ (a2 + a2)
– 3D linear wear = √ (a2 + a2 + a2)
– Volumetric wear = (√ (a2 + a2 + a2)) × π × r2.
The calculated “true wear” ranged between 0.014 and 7.071, 0.017 and 8.660, and 11 and 5333 for the two-dimensional (2D) linear vectors (mm), the tree-dimensional (3D) linear vectors (mm), and the volumetric wear (mm3), respectively.
Radiographs
All radiographs were obtained at Aarhus University Hospital (study I, III, IV, and V) or Randers Regional Hospital (study II). All stereo radio-graphs were digital, but the initial plain radio-graphs (obtained prior to 2006 for study I, II, III and V) were stored in hard-copy and were digitized to tagged image files at a resolution of 300 dots per inch at 100% scale in a high-resolution optical scanner (Epson Expression 10000xl Pro A3).
Antero-posterior radiographs ()
For plain AP radiographic images the patients (study I, II, III, and V) were positioned supine, and the phantom was placed on the side imitating a right hip (study IV). The distance between the beam source and the femoral head was 100 cm, with the plain films 15 cm below the femoral head. The digital grayscale tiff-format AP radiographs of the phantom (study IV) had a size of 2364 × 2964 pixels while the digital pelvic radiographs (study V) had a size of 2080 × 1711 pixels. The final radiographs of study V were collected as double exams, with complete reposition of the radiographic equipment and the leg of the patient between exams, and it was the same radiographer to perform all double examination radiographs.
Cross-table lateral radiographs ()
For plain cross-table lateral (CTL) radiographic images the patients (study I, II, III, and V) were positioned supine and the, and uninvestigated hip was elevated on a leg support. The phantom was placed on the side imitating a right hip (study IV). The distance between the beam source and the femoral head was 100 cm, and the plain films were kept vertical next to the hip being investigated. The cross-table lateral radiographs had a size of 2364 × 2964 pixels (grayscale tiff-format). The final radio-graphs of study V were collected as double exams, with complete reposition of the radiographic equipment and the leg of the patient between exams, and it was the same radiographer to perform all double examination radiographs.
Stereo radiographs ()
A standard digital RSA-setup of two synchronized ceiling-fixed roentgen tubes (Arco-Ceil/Medira; Santax Medico, Aarhus, Denmark), angled towards each other at 40°, and a uniplaner carbon calibration box (Box 24, Medis Specials, Leiden, The Netherlands) was used. The films were placed in the drawer below the calibration box. The exposure was adjusted according to the body mass of the patient, but the standard was 85 kV and 15 mAs. The patient was placed supine (image to the right). All stereo-radiographs were fully digital (FCR Profect CS; Fujifilm, Tvedbæk, Denmark). The stereo radiographs, when converted from the original 30MB single-image color DICOM file-format to a feasible image-size for the RSA software, had a size of 2080 × 2529 pixels (grayscale BMP file-format).
Timing of radiographic follow-up
Studies I and III
Plain radiographs for study I were obtained, according to the prospective RSA protocol, within a week following surgery and after bearing weight, at 3 months, 6 months, 1 year, 2 years, 5 years, and 12 years.
Study II
Plain radiographs for study II were obtained on a routine basis within a week following surgery and after bearing weight, at 3 months, 1 year, 2 years, and 5 years.
Study IV
Plain anteroposterior (AP), cross table lateral as well as stereo radiographs of the hip phantom were obtained consecutively for each new simulated wear position. A total of 16 AP radiographs, 16 LA radigraphs, and 16 stereo-radiographs were made. The radiographs were obtained in one stretch during one day in January 2009, at the University Hospital of Aarhus, Denmark. Thus the phantom was not moved from the examining table.
Study V
Plain radiographs for study V were obtained, according to the prospective RSA protocol, within a week following surgery and after bearing weight, and at 5 years.
The patients of study V were further seen consecutively within one month of primo 2009 for a 5 to 7 year follow-up radiographic series of double examination AP, LA and stereo-radiographs. It was the same radiographer to obtain all the follow-up images.
Polyethylene wear measurement softwares
PolyWare ()
Figure 27. Graphic output of wear analysis using PolyWare Pro 3D Digital. To the left digital edge-detection by fitting circles to the borders of the cup and head is shown, and to the right a solid model is applied at the end of analysis.
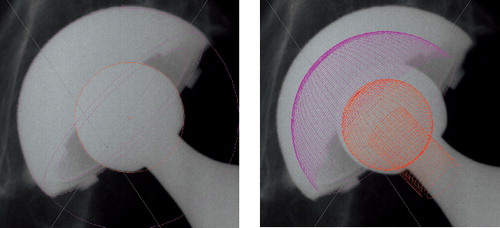
In all five studies we utilized the polyethylene wear measurement software PolyWare Pro 3D Digital vs. 5.10 (Draftware Developers, Conway, SC). Devane et al. developed this 3D measurement technique for the measurement of polyethylene wear in metal-backed acetabular cups (Citation67;Citation68). The technique relies on computer-assisted technology to create a three-dimensional solid model of the acetabular component and femoral head on the basis of back projection of the radiographs (so-called shadow-casting) and model knowledge of the implant provided in a software library (). With this technique, 2D wear (in the frontal plane) can be estimated on the basis of serial anteroposterior plain radiographs, and 3D wear can be estimated by incorporating penetration on lateral radiographs. Volume calculations are not based on the solid model capabilities, but rather on a formula based on the wear tract being a cylinder and the angle of femoral head displacement within the acetabular cup.
The initial step in analysis is to flip all right hip AP images to simulate the projection of a left hip. Then the beam-centre in the radiograph is marked and a small area around the cup and head is cropped for improved visual effect. Three points on the outline contour of the acetabular dome and opening, as well as on the femoral head, is then marked and border-circles are applied to the image followed by a solid 3D model (image above). The software allows for the analysis of serial radiographs with respect to a reference radiograph, as well as for using only the final follow-up radiograph and assume zero wear (femoral head displacement) at the time of surgery.
Devane reported a three-dimensional accuracy of approximately 0.15 mm (on the basis of the mean absolute difference between the measured and true displacements) and a volume calculation that was within 8% of the true amount of the polyethylene removed with this digitizer tablet computerized method. In 1999, precision (0.089 mm) for 3D wear measurement was improved with a more automated software version (PolyWare Pro/3D Digital Version Rev 4 and 5) including custom built filters and an edge-detector, as well as contemporary image processing software. We used this software version, as it allow for manual overrule of the automated edge-detection when this is visually not correct, as can be the case with some clinical radiographs (Citation59). In 2005 a fully automated software version (PolyWare Auto Rev 6.0) was marketed, and reported to have a precision of 0.028 mm. That was a threefold improvement in accuracy compared to the earlier software version. The accuracy was defined as mean plus standard deviation of the absolute difference between measured and true separations, and was tested in a phantom jig (6 positions of separating two steel balls from 0.05 mm to 0.3 mm at 0.050 mm increment).
Model-Based RSA ()
Figure 28. Graphic output of wear analysis using Model-Based RSA. Elementary geometric sphere models are matched to the defined ROI’s (red areas) of the borders of the femoral head and cup.
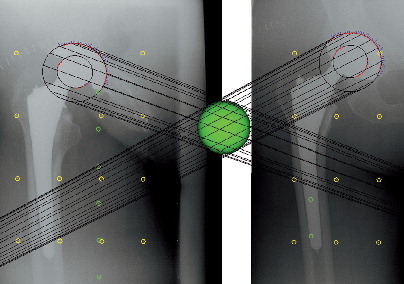
In study IV and V we used the Model-Based RSA vs. 3.2 software (Medis Specials, Leiden, The Netherlands). RSA is an accurate technique for the measurement of the position of an object in space using roentgen rays (Citation227).
RSA was originally described by Göran Selvik in 1972 as an accurate manual method for measurement of skeletal and implant movements (Citation226). Since then several advances in the technical accuracy by new mathematical algorithms have been proposed and software programmes have been refined to a user friendly and less time-consuming appliance. The advantage of the RSA software is that it allows for early evaluation of e.g. implant fixation and the degree of wear in relatively small study populations (Citation124). We used a fully digitized marker-free RSA method with 3D implant models in substitution of the tantalum beads. This technique is based on minimizing the differences between the virtual projections of a 3D surface model of an implant and the actual projection of the implant as it appears in the radiographic image. If the implant is non-symmetrical, its projection will appear unique. Thus the orientation of the implant in the stereo-radiograph can be estimated from it’s projection by repeat micro adjustments of the model until a minimal difference in the outline remains (Citation121). Algorithms to minimize the difference between the actually projected contour of an implant and the virtually projected contour of a model of that same implant have been evaluated (Citation122), and currently the Model-Based RSA software (MB-RSA) uses the iterative inverse perspective matching (IIPM) algorithm, and the choice of two algorithm based on minimization of the difference (DIF) between the actual contour and the virtual contour (DIF DoNLP and DIF DHSAnn). The implant models must be to be added to the personal software license as reverse engineered models, created by laser scanning of actual implants, or computer assisted drawings, provided by the implant manufacturers. For scanned models 5000 triangular elements has been recommended (Citation121). In 2008 a new attribute of the model based RSA software, featuring computer generated geometrical shape implants (EGS-RSA), was released. For this software an EGS algorithm is used to match the EGS model with the radiographic implant projection. The EGS-RSA system has been validated for use with femoral stems (Citation120) but it has never been validated for measurement of polyethylene wear prior to this thesis. With model based RSA (EGS-RSA or MB-RSA) the cost and trouble of implant bead-marking and re-sterilization, along with the risk of compromised or altered implant fixation strength, is avoided (Citation124). Further RSA using implant models provides the potential for retrospective analysis of implant components obtained in former radio-graphic series.
The initial step in analysis is to calibrate the stereo-radiograph according to the known 3D position of the top (control) and bottom layer of markers (fiducial) in the calibration box that was used at the time of the radiographic recording. The control markers transform the projection lines from the roentgen foci. The fiducial markers are used to calculate the position of the implant models (or implant markers) to the point with the shortest distance (smallest crossing line error) between the projection lines. For model based wear analysis no bone markers are used, and the next step is to match the outer contour of the implant cup and femoral head model to the projections of the implant in the stereo radiograph by mathematical algorithms. This is a fully automated function. Thereafter the relative penetration of the geometrical centre of the femoral head with respect to the geometrical centre of the cup can be calculated in successive examinations, because the outline of the cup (the rigid body or fix point) is matched in the consecutive stereo radiographs. The results are given as translation vectors along the X, Y and Z coordinate axes () and 2D or 3D wear vectors may be calculated according to Pythagoras theorem. As the models for hemispherical cups and heads are symmetrical, it is not possible to calculate rotations. With MB-RSA (Study IV) we had to reverse the femoral head penetration analysis with the femoral head as the fixed reference, and subsequently change the signs of the measured migrations to obtain the correct wear directions, to make the wear analysis function.
Figure 29. Translation vectors (X, Y, and Z) for the femoral head with respect to the cup in RSA wear analysis.
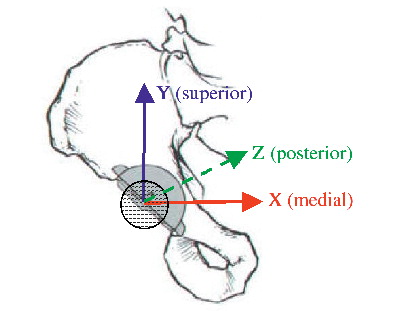
The accuracy of RSA has not improved substantially during the past 35 years, since Selvik measured migration in analogue radiographs on an analogue measuring table (Citation124). The general reported accuracy of RSA ranges between 0.05 mm and 0.5 mm for translations, and between 0.15° and 1.15° for rotations (95% confidence intervals) (Citation123). Kaptein et al. validated EGS-RSA in a phantom study of a femoral stem, and reported X-translation (SD 0.069 mm) and Y-translation (SD 0.082 mm) to be more precise than Z-translation (SD 0.136 mm). The precision of Model-based RSA using surface models (MB-RSA) was shown to be better than EGS-RSA when using the optimal model (the scanned model of the implant in the roentgen image) but poorer than EGS-RSA when using a model obtained from scanning of a different implant than the radiographed. Both model based RSA methods had translational standard deviations slightly larger (Z-direction SD of 0.14 mm and 0.21 mm for EGS-RSA and non-optimal model MB-RSA, respectively) than marker-based (Z-direction SD of 0.12 mm) in this femoral stem component phantom experiment (Citation120). Due to the high accuracy of RSA adequate statistical power can be reached with relatively small sample sizes.
Evaluation of osteolysis
Study I
All patients had some degree of expansile pelvic osteolysis on the last (minimum five years) available radiographs. The osteolytic area was marked in Indian-ink directly on the AP radiographs by an experienced orthopaedic consultant with subspecialty in hip surgery. The images were then digitized with a transmission-light scanner (Mustek P3600 A3 pro, Irvine, CA) and the osteolytic area quantified by the PolyWare Pro 3D Digital 5.10 software. The measure was given in mm3 as the software assumed the lesion to be of equal size in the third dimension (z-axis).
Study II
The five year radiographs were evaluated for aseptic loosening (progressive radiolucent lines along the implant edges) and osteolysis (periprosthetic bone resorption with radiographic evidence of progressive bone loss not present in the initial radio-graphs) (Citation267). The location of osteolytic lesions was described according to the three zones defined by DeLee and Charnley (Citation65), and its area was measured digitally and expressed in mm2.
Clinical evaluation
In study I and II clinical data on complications and implant revision was assessed by querying the DHR. We further read through all patient records of these patients as they had all been seen for a minimum five-year (study II) or minimum five-year, end point revision or twelve-year follow-up (study I). In study II a preoperative and a 3 month Harris hip score (HHS) was obtained, to ensure and evaluate that all patients regained walking function after surgery and was relieved of pain. In study V we obtained HHS at the consecutive 5 to 7 year follow-up to certify the activity level of the patients.
Measurement agreements
Bias
It is not likely, that different methods will agree exactly, by giving identical results for all investigated individuals. The variation of wear measurements within the same method consist of a systematic variation (bias) and a random variation. The systematic variation can be corrected for if known, whereas this is not possible for the random variation. In investigation of different wear measurement methods we were interested in knowing the systematic difference between methods as well as the random variation to be able to judge the clinical effect. Bland and Altman suggested plotting the average and differences between methods (Citation23), and when there was no obvious relation, to summarize the lack of agreement by calculating the mean differences (bias) and the standard deviations of the differences.
The differences are expected to follow a normal (Gaussian) distribution, because a lot of variation between subjects is removed when calculating the differences. Provided that the differences within the limits of agreement (LOA), defined as the mean difference ± 1.96 x standard deviations (SD) of the differences, are not clinically important, the methods may be used interchangeably (Citation23). The LOA are only estimates of values that apply to the whole population, and a second sample would result in different LOAs. The estimated LOA between methods can be compared by a paired t test.
Repeatability
Precision has been defined as the closeness of agreement between independent test-results obtained under stipulated conditions according to definitions in ISO 1998 (Citation197). Under repeatability conditions (independent test results are obtained with the same method on identical test items in the same laboratory by the same operator using the same equipment, within a short interval of time) repeatability and precision are synonymous. Reproducibility refers to the action of performing something more than once. Reproducibility has been defined as precision under reproducibility conditions (test results are obtained by the same method on identical test items in different laboratories with different operators, using different equipment).
Repeatability (random variation) of two measurement methods limits the amount of agreement which is possible. Thus, if one method has poor repeatability the agreement between the two methods being compared is bound to be poor. Repeatability may be assessed by repeated measurements on a series of subjects, and plotted as the average versus the difference. The mean difference (bias) should not be statistically significantly different from zero (Citation23). 95% of the differences are expected to fall within the LOA. The repeatability coefficient (SDdif-intra) can then be calculated as the square root of the sum of squared differences divided by n = √(∑dif2/n) (Citation23). The measures of repeatability (SDdif-intra or equivalent the width of LOAintra) can be compared pair wise by looking at the ratios, and tested by an F-test.
The agreement of repeated measurements (bias) by each of two methods on the same subjects can be compared by a corrected standard deviation of differences (SDdif-inter) (Citation23). SDdif-inter consist of the random variation with-in each method (SDdif-intra) plus some between method random variations. Bland and Altman described this method in 1986 (Citation23), which may be used to analyze the repeatability of a single measurement method or to compare measurements by two observers (inter-observer variability).
Accuracy
Reports of accuracy measurements and precision are often confusing as the terms have been used synonymously, however, they are not synonyms (Citation197). Accuracy is the closeness of agreement between a test result and the accepted reference (the “true”) value (Citation197). The coherence of bias, precision and accuracy is illustrated in . Accuracy can be assessed in the clinic only in retrieval studies, but in phantom studies accuracy may be assessed as the agreement with the known (simulated) true value. Accuracy is related to measurement error, as the systematic component (bias) of a measurement is related to the trueness of the instrument (Citation197).
Figure 30. Graphic illustration of the relationship between accuracy, precision (repeatability), and bias. A) demonstrates low accuracy and low precision; the method is useless. B) demonstrates high accuracy but low precision (scatter); a large sample size is needed. C) demonstrates high precision (little scatter) with poor accuracy and a large bias; the systematic variation can be corrected for if known. D) shows an accurate and precise method. (Reprint from McCalden et al. J Bone Joint Surg Am 87: 2323–2334, 2005).
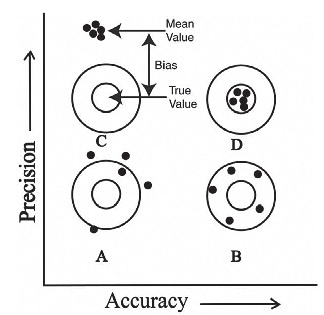
Validity
According to the OED standard definition validity describes a test that is sound and sufficient, that is, a test measures what it purports to measure. There are several varieties of validity, including construct validity, content validity, and criterion validity. Criterion validity defines the correlation of a measurement and an external criterion of the studied phenomenon and two aspects can be distinguished; concurrent validity and predictive validity. The sub-aspect concurrent validity define the time-chronological correlation of two methods (Citation113). In studies III, IV, and V we used concurrent validity for comparison of time-chronological wear measurements by different wear measurement methods, while criterion validity (study IV) was reserved for the comparison of wear measurements by different methods with the true wear.
Statistics
In general, statistical significance was assumed at p < 0.05, and the Intercooled Stata® 9.0 and 10.0 (StataCorp LP, College Station, TX) software packages besides Microsoft Office Excel 2003 was used for statistical computations.
Study I
We compared survival rates of the cups by a log-rank test at 15 years’ follow-up. According to a Shapiro-Wilk test (Citation5), head penetration and wear rates were normally distributed when converted to log scale. Similarity or differences in variances of the log scale wear data for the two groups was tested by an F test. The log mean values of the two groups were compared by a two-sample t test with unequal variances (head penetration) and a two-sample t test with equal variance (wear rates). Head penetration (mm) of the longest follow-up per patient (minimum 5 years, maximum 12.6 years) and head penetration rates (mm/year) are presented on a normal scale for interpretational reasons. Mean osteolysis between the groups were compared by the Mann-Whitney U-test. The associations between wear rate and revision and between osteolysis and revision were assessed by the Mann-Whitney U-test.
Study II
According to a Shapiro-Wilk test (Citation5), the mean annual femoral head penetration rates followed a Normal distribution when converted to log scale. Similarity or differences in variances of the log scale penetration rates for the two groups was tested by an F test. The log mean values of the two groups were compared by a two-sample t test with equal variances (wear rates). With linear wear data normality could not be achieved by transforming the data (log scale and cubic transformation) and thus they were tested by a non-parametric test (Mann-Whitney U-test). Linear wear (mm) are presented on a normal scale for interpretational reasons. Continuous demographic input variables between the groups were compared by a two-sample t test with equal variances, and categorical variables were tested by a chi-squared test though cells with observations of 5 and below were tested by a Fisher’s Exact test. Difference in HHS (pre-operative to 3 month postoperative) between the groups was tested non-parametrically (Mann-Whitney U-test).
Studies III, IV and V
Repeatability (random variation) was assessed as the standard deviation of the difference (SDdif-intra) between the first and the second repeat measurement (Study III and IV) or between the double examinations (Study V) within the methods with LOA (LOAintra) defined as (SDdif-intra × ± 1.96). The bias between the first and the second measurements was estimated as the mean difference between the two measurements of the simulated wear in the phantom. The differences between the two measurements followed a normal distribution (Shapiro-Wilk test (Citation5)) and were tested by a paired t-test. The data was further presented as Bland-Altman plots and scatter plots with lines of equality (Citation23). The measures of repeatability (SDdif-intra or equivalent the width of LOAintra) of the three methods were compared pair wise by looking at the ratios, and tested by an F-test.
Concurrent validity for each strategy/method was established by calculating the average value and the difference between two repeat measurements (Study III and IV) or double examinations (Study V). Further the standard deviation of the difference (SDdif-inter) between these methods was estimated, and from the SDdif-inter we calculated according to Altman (Citation5) LOAinter as (SDdif-inter × ± 1.96), and further calculated the bias with a 95% confidence interval. The bias was investigated as the difference in means between the two wear measurement methods. The differences followed a normal distribution (Shapiro-Wilk test (Citation5)) and were tested by a paired t-test. Data was further presented in Bland-Altman plots and scatter plots with lines of equality (Citation23).
In study V, the phantom study, criterion validity was further assessed in a regression model. Under perfect conditions (measured wear = calculated true wear) the slope would be 1 and the intercept zero. We calculated the slopes (regression coefficient) with 95% confidence intervals, and the intercept on the y-axis with the p-value for testing the intercept equal to 0. We further calculated the standard variation around the lines (SDline), equivalent to root means square (RMS), and the coefficient of determination (r2). The SDline for the different methods were compared pair wise by Pitman’s test. Data was further presented in Bland-Altman plots and scatter plots with lines of equality.
Results
Study I
Revisions
At final follow-up more (p = 0.045) HA cups had been revised than Ti cups: eight of 14 HA cups (57%) and two of 12 Ti cups (17%) (). At revision surgery, aseptic loosening, massive ace-tabular osteolysis, and metallosis were clinically evident for all revised cups, except for one Ti cup that was revised after a traumatic fall at 5.6 years.
All of the cups (Ti and HA) revised within the 12-year follow-up period had wear rates of more than 0.4 mm/year or osteolysis of more than 25,000 mm3 (). The wear rate was higher (p = 0.0001) in revised patients than in non-revised patients. The volume of osteolysis was higher (p = 0.003) in revised patients than in non-revised patients.
PE wear
The head penetration rate for the HA group at a mean of 10.6 years (0.46 mm/year; SD, 0.26; range, 0.16–0.90 mm/year) was similar to (p = 0.33) that of the Ti group (0.38 mm/year; SD, 0.14; range, 0.20–0.72 mm/year) at a mean of 11.1 years. Mean head penetration in the HA group at a mean of 10.6 years (4.8 mm; SD, 2.6; range, 1.97–10.56 mm) was also similar to (p = 0.25) that of the Ti group (3.8 mm; SD, 0.9; range, 2.51–5.36 mm) at a mean of 11.1 years. Wear of the PE continued in both study groups throughout the period of follow-up (). During the first 6 months, the PE wear was quite large in both groups, illustrating a combination of wear and bedding-in. After 1 year, linear head penetration continued in both study groups but at a curve of less steepness. Reflected by variance, the distribution of wear was wider (p = 0.017) in the HA group (SD, 2.6; range, 1.97–10.56 mm) than in the Ti group (SD, 0.9; range, 2.51–5.36 mm).
Osteolysis
Osteolytic lesions () were visible on the plain radiographs in all 25 patients at the latest available follow-up. The mean measure of osteolysis was 9320 mm3 (SD, 8838; range, 178–29,028 mm3) in the HA group and 7531 mm3 (SD, 10,915; range, 1091–34,698 mm3) in the Ti group (p = 0.30).
Study II
PE wear
There was no difference (p = 0.73) between the mean annual femoral head penetration rate for the CoCr group (n = 33; 0.25 mm/year; SD, 0.16 mm/ year; range, 0.05–0.81 mm/year) and the Zr group (n = 36; 0.23 mm/year; SD, 0.12 mm/year; range, 0.07–0.66 mm/year). Reflected by variance, the distribution of wear was similar (p = 0.46) between the groups. Fifty-eight percent of the Zr heads had a wear rate of more than 0.2 mm/year compared with 51% of the CoCr heads. Mean linear wear at 5 years was similar (p = 0.80) in the Zr group (1.11 mm; SD, 0.53 mm; range, 0.15–2.05 mm) compared with the CoCr group (1.22 mm; SD, 0.74 mm; range, 0.28–3.78 mm) ().
Clinical results
There was no difference in HHS comparing the score obtained prior to surgery with the 3 month post-operative score (p = 0.25).
Complications in the CoCr group consisted of two femur fractures at 7 months and 3 years post surgery, but no component revisions were performed and the patients were included for wear measurements after the fractures because they returned to their habitual functional level after recovery. Two patients had one episode of early posterior hip dislocation and were treated with closed reduction. There were no postoperative infections in either group. There were no revisions in any of the patients within the 5-year follow-up period. Radiographic evaluation of all last-examination AP radiographs revealed no sign of aseptic loosening (progressive expansile osteolysis or RLL with cup migration) of any acetabular or femoral components. Only one patient in the CoCr group had an evident DeLee and Charnley type 3 acetabular osteolysis of 22.4 mm2 () at 5-year follow-up on the AP conventional radiograph.
Study III
Measured wear by three strategies
Observed median wear and range for the eleven patients was 3.4 mm (1.6–4.6), 2.3 mm (0.7–4.9), and 4.0 mm (2.6–6.2) for the PW1 (final radio-graph), PW2 (first and last radiograph), and PW6 (serial radiographs incl. the first and the last), respectively ().
Repeatability
No bias (p > 0.42) was observed. LOA around the bias were ±0.56, ±0.37, and ±1.22 mm for PW1, PW2, and PW6, respectively. SDdif-intra, bias, LOA around the bias, 95% confidence interval (CI) around the bias, and p-value for paired t-test are presented in and . The relative repeatability were significantly different between both PW1 and PW6 (p < 0.001), and PW2 and PW6 (p = 0.02) ().
Figure 38. Bland-Altman plots and scatter plots with lines of equality for repeatability measures for each of the three methods (Study III). In the Bland-Altman plots; the x-axis: average of two measurements, y-axis: difference between two measurements (y = measurement 1 – measurement 2), red lines: 95% limits of agreement, dashed line: bias from 0, long solid green line: y = 0 line, dots: individual double measures. In the scatter plots; x-axis: first measurement; y-axis: second measurement; maroon lines: lines of equality. PW, PW2 and PW16: See legend in below.
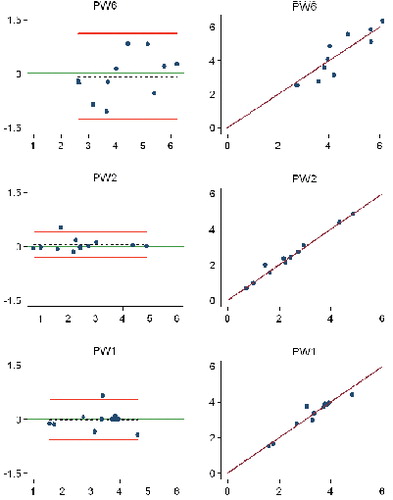
Table 5. Repeatability of radiographic double wear measurements within the methods (study III)
Concurrent validity
A significant bias between all pair wise comparisons were observed (p < 0.02) with 0.81 mm between PW1 and PW2, 1.00 mm between PW1 and PW6, and 1.81 mm between PW2 and PW6. Limits around the bias were 2.52 mm between PW1 and PW2, 2.24 mm between PW1 and PW6, and 1.19 mm between PW2 and PW6. SDdif-inter, bias, LOA around the bias, 95% confidence interval (CI) around the bias, and p-value for paired t-test are presented in and .
Figure 39. Bland-Altman plots and scatter plots with lines of equality for concurrent validity between the three methods (Study III). In the Bland-Altman plots; the x-axis: average of the measurements of two methods, y-axis: difference between measurements of two methods, red lines: 95% limits of agreement, dashed line: bias from 0, long solid green line: y = 0 line, dots: individual double measures. In the scatter plots; maroon lines: lines of equality. PW, PW and PW : See legend in below.1 2 6
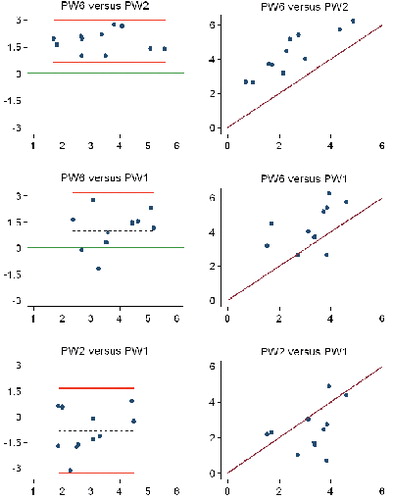
Table 6. Comparison of repeatability and concurrent validity between methods (Study III)
Study IV
Repeatability
The systematic variation (bias) within the methods (mean difference of two wear measurements in the same radiographs) along with the intra-method random variation of repeatability (LOAintra, mm) and a 95% confidence interval (p value) around the bias is displayed in for 2D and 3D methods of RSA and PolyWare. A graphic overview is given in . Comparison of repeatability between methods is presented in .
Figure 40. Bland-Altman plots and scatter plots with lines of equality for repeatability measures within each of the three methods (study IV). In the Bland-Altman plots: X-axis: average of two measurements. Y-axis: difference between two measurements (y = measurement1 – measurement2). Red lines: 95% limits of agreement. Dashed black line: bias from 0. Long solid green line: y = 0, line of perfect average agreement. Navy dots: individual double measures. In the scatter plots: X-axis: first measurement. Y-axis: second measurement. Maroon lines: lines of equality. EGS-RSA = radiostereometric analysis using sphere models. MB-RSA = radiostereometric analysis using scanned cup models. PW = PolyWare.
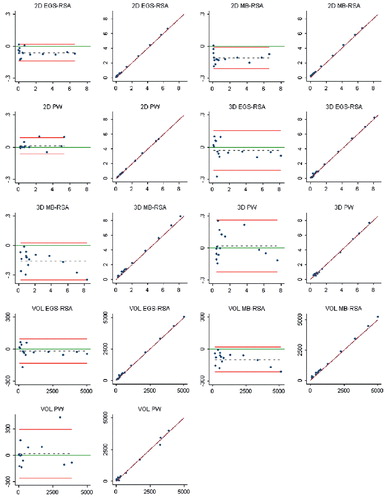
Table 7. Repeatability of two measurements with the 3 wear measurement methods (study IV)
Concurrent validity
The systematic variation (a mean value of two measurements with a method) between methods along with the random variation between methods (LOAinter, mm) and a 95% confidence interval (p value) around the bias is displayed in for 2D and 3D methods of RSA and PolyWare. A graphic overview is given in .
Figure 41. Bland-Altman plots and scatter plots with lines of equality for concurrent validity between the three methods (study IV). In the Bland-Altman plots: X-axis: average of the measurements of two methods. Y-axis: difference between measurements of two methods. Red lines: 95% limits of agreement. Dashed black line: bias from 0. Long solid green line: y = 0 line, line of perfect average agreement. Navy dots: individual double measures. In the scatter plots: Maroon lines: lines of equality (45 degree line, slope of 1). EGS-RSA = radiostereometric analysis using sphere models. MB-RSA = radiostereometric analysis using scanned cup models. PW = PolyWare.
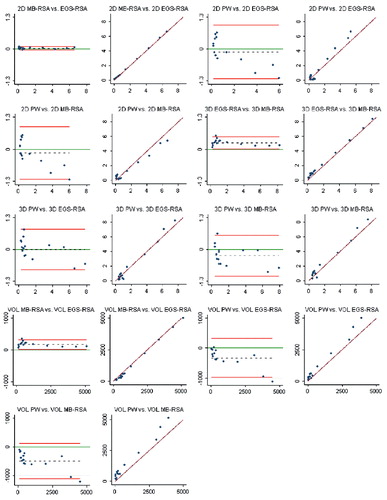
Table 8. Concurrent validity between methods (study IV)
Criterion validity
Only 2D EGS-RSA had a slope not significantly different from 1 ( and , ), and was the only method with an intercept not significantly different from 0 (p=0.21). With all methods, measuring the highest wear measure (close to wear-through of the liner) was difficult, as shown in . Therefore a sensitivity analysis was performed, in which we compared measured wear by the three methods to the true wear, using the same regression model, but excluded the highest observation ().
Figure 42. Regression lines (best fit lines) of criterion validity for 2D and 3D wear by method (study IV). X-axis: micrometer vector (mm). Y-axis: measured wear by method (mm). 2D = two-dimensional. 3D = three-dimensional. RSA EGS = radiostereometric analysis using sphere models. MB-RSA = radiostereometric analysis using a cup model. PW = PolyWare . Individual regression lines (best fit lines) of criterion validity for the three wear measures by methods and the true wear (study IV). In the scatter plots: Red dashed lines: 95% prediction interval of lines. Solid green lines: lines of equality (45 degree line, slope of 1). Black dashed line: best fit line of point measures. Navy dots: individual measures. EGS-RSA = radiostereometric analysis using sphere models. MB-RSA = radiostereometric analysis using a cup model. PW = PolyWare.
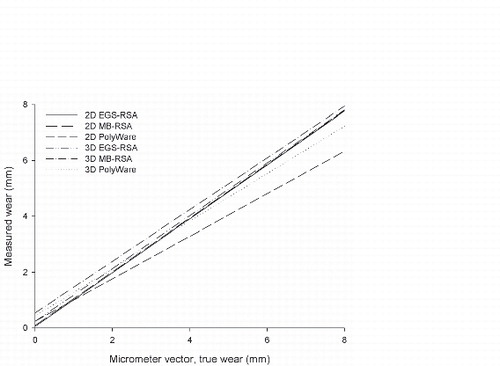
Figure 43. Individual regression lines (best fit lines) of criterion validity for the three wear measures by methods and the true wear (study IV). In the scatter plots: Red dashed lines: 95% prediction interval of lines. Solid green lines: lines of equality (45 degree line, slope of 1). Black dashed line: best fit line of point measures. Navy dots: individual measures. EGS-RSA = radiostereometric analysis using sphere models. MB-RSA = radiostereometric analysis using a cup model. PW = PolyWare.
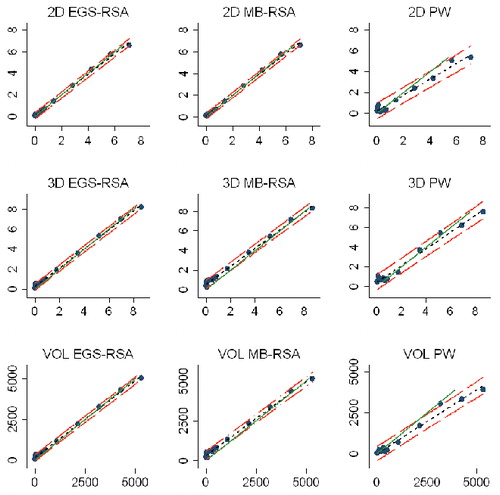
Figure 44. Bland-Altman plots illustrating measures of criterion validity between measured wear with the three wear measurement methods and the true wear (study IV). In the Bland-Altman plots: X-axis: average of measured wear and true wear. Y-axis: difference between measured wear and true wear. Red lines: 95% limits of agreement. Dashed black line: bias from 0. Long solid green line: 0 line (true wear), line of perfect average agreement. Navy dots: individual measures. EGS-RSA = radiostereometric analysis using sphere models. MB-RSA = radiostereometric analysis using a cup model. PW = PolyWare.
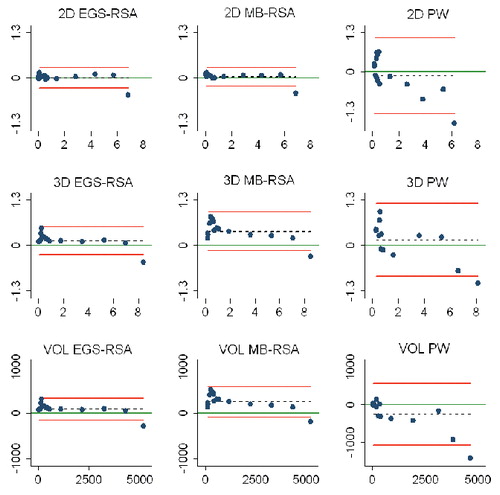
Table 9a. Criterion validity (measured wear against true wear) of the three methods presented in a regression model. Results are ordered by the most accurate method first and the least accurate method last (study IV)
Table 9b. Criterion validity (measured wear against true wear) of the three methods presented in a regression model without the highest value (near wear through). Results are ordered by the most accurate method first and the least accurate method last (study IV)
Volumetric wear
All measures of volumetric wear were based on calculations of the linear wear. In PolyWare, these calculations were given directly in the software output and a detailed description can be encountered in Devane’s first publication (Citation67). For the RSA methods, volumetric wear measures were calculated on the basis of the 3D linear wear. The volumetric results are presented for repeatability in , and , for concurrent validity in and , and for criterion validity in , , and , , .
Table 10. Comparing methods by repeatability and criterion validity (study IV)
Pose estimation ()
Figure 45. Models are fitted to the ROI marked on the periphery of the cup in the stereo radiograph and the projection lines to the roentgen foci are visible. A) A scanned implant model. B) A computer generated sphere.
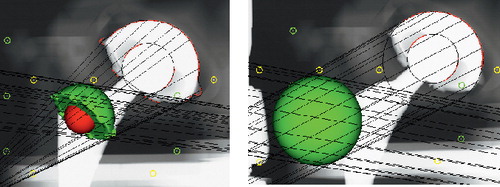
The mean differences of the pose estimation with the spheres (EGS-RSA) were 0.08 mm (SD 0.03) for the femoral head sphere, and 0.11 mm (SD 0.03) for the cup sphere. The mean differences of the pose estimation with the sphere model used for the femoral head in MB-RSA were 0.08 mm (SD 0.03), and 0.19 mm (SD 0.03) for the scanned cup model.
Study V
Demographic data
Patient demographics are summarized in .
Table 11. Patient demographics (study V)
Repeatability
Repeatability was evaluated within methods and revealed no clinically relevant or statistically significant bias between any two pairs of radiographic double examinations of PE wear. The relative repeatability between 2D PW1 and 2D EGS-RSA (“gold standard”) was 1.02 (p=0.95). The data are presented with median (range), SDdif-intra, bias (±LOA), 95% CI for bias, and p values for bias in and in Bland-Altman plots and scatter plots with lines of equality (Citation23) in . Concurrent validity of repeatability between methods is presented in .
Figure 46. Bland-Altman plots and scatter plots with lines of equality for repeatability measures for each of the three methods (study V). In the Bland-Altman plots; the x-axis: average of two measurements, y-axis: difference between two measurements (y = measurement 1 – measurement 2), red lines: 95% limits of agreement, dashed line: bias from 0, long solid green line: y = 0 line, dots: individual double measures. In the scatter plots; x-axis: first measurement; y-axis: second measurement; maroon lines: lines of equality. EGS-RSA = radiostereometric analysis using sphere models, PW = PolyWare using only the final follow-up radiographs, PW2 = PolyWare using the postoperative and the final follow-up 1 radiographs.
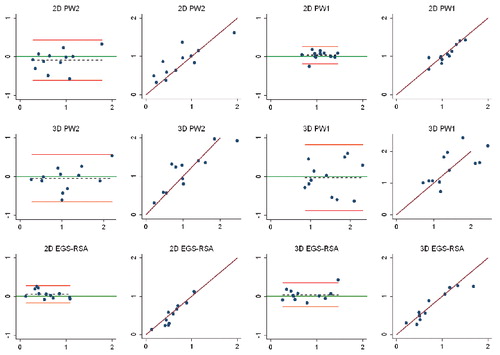
Table 12. Repeatability of radiographic double examination wear measurements within the methods (study V)
Concurrent validity
Concurrent validity exposed a statistically signifi-cant (p<0.04) bias between all pairwise comparisons of methods, except between 2D PW2 and 2D EGS-RSA. The data are presented with SDdif-inter, bias (±LOA), 95% CI for bias, and p values for bias in and in Bland-Altman plots and scatter plots with lines of equality (Citation23) in .
Figure 47. Bland-Altman plots and scatter plots with lines of equality for concurrent validity between the three methods (study V). In the Bland-Altman plots; the x-axis: average of the measurements of two methods, y-axis: difference between measurements of two methods, red lines: 95% limits of agreement, dashed line: bias from 0, long solid green line: y = 0 line, dots: individual double measures. In the scatter plots; maroon lines: lines of equality. EGS-RSA = radiostereometric analysis using sphere models, PW1 = PolyWare using only the final follow-up radiographs, PW2 = PolyWare using the postoperative and the final follow-up radiographs.
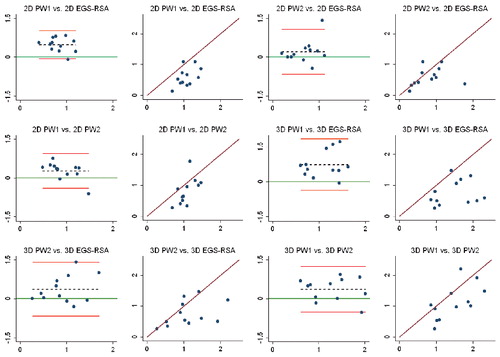
Table 13. Comparison of repeatability and concurrent validity between methods (study V)
Discussion
Key findings
To our knowledge, this is the first thesis to validate the Model-Based RSA software (Medis Specials and Leiden University Medical Center, The Netherlands) for PE wear measurement and to compare PE wear measured with the PolyWare software with that measured with RSA software. Moreover this thesis reveals the accuracy and precision of Model-Based RSA for PE wear measurement compared with PolyWare. RSA using scanned implant models and RSA using computer-generated sphere models (EGS) performed similarly. The accuracy of Model-Based RSA was shown to be below the expected clinical PE wear rate per year of most prostheses, even crosslinked PE liners, and Model-Based RSA may allow measurement of PE wear in individual patients so as to be a predictor of osteolysis and prosthesis survival.
In addition, this thesis presents the longest available, randomized follow-up of HA-versus Ti-coated implants, and it confirms the inferior survival of first-generation HA-coated implants with individual cases of excessive wear and osteolysis that were possibly related to HA third-body wear.
Interpretation of results and comparison with the literature
First-generation HA-coated implants (study I)
We reported a high 15-year cup revision rate of 57% and 17% with HA-and Ti-coated cups, respectively. The cases of high PE wear in this study could be related to HA particles separating from the coating, which led to increased backside wear and articulate wear on the PE, a mechanism described by Bauer, Rokkum and Morscher (Citation14;Citation166;Citation207). We have however, no certainty of this because, unfortunately, the liners were not saved for histology. Disintegration of the HA coating is influenced by a number of factors, including the purity, crystallinity, and porosity of the HA; manufacturing procedures; coating technique used; the thickness of the HA coating; the surface on which HA is deposited (smooth or rough); the adhesive strength of HA to the substrate (Citation237); and the speed of HA resorption (Citation24;Citation187;Citation189). Plasma-sprayed calcium phosphate coatings have been applied in different qualities and with various coating thicknesses ranging from 40 µm (Citation204) to 150 µm (Citation206) to 300 µm (Citation166;Citation207). Thinner plasma-sprayed HA coatings (50 µm) have been recommended, because they give a stronger fixation and reduce the risk of HA fracture (Citation232;Citation237). The implants used in this study had a coating thickness of 70 µm and were first-generation HA coatings. The manufacturing of HA coatings have changed since then. Today, plasma-sprayed HA deposition has been replaced by much thinner (approximately 5 µm) and electrochemically deposited HA coatings that ensure an even distribution and a quick resorption (Citation63). Medium-and long-term clinical results with these new coating techniques have not yet appeared.
The high PE wear rates found, also for non-HA coated cups, might be explained by the inferior quality of the UMHWPE liner in combination with a poor PE locking mechanism (Hexloc®) (Citation27). Also, the screw-hole design of the acetabular cups allow exit of PE wear debris directly to the bone and offer an entrance for HA particles, which accelerate backside wear (Citation141;Citation204). We found that a femoral-head penetration rate of more than 0.4 mm/year was directly associated with cup failure and revision. We believe the reason to be that the PE particles liberated in the wear process induce a cell-mediated reaction, which results in osteolysis (Citation60). A PE wear rate threshold of 0.1–0.2 mm/ year has been described to induce osteolysis in the long term (Citation73;Citation75;Citation240;Citation263), and many reports of wear with HA-coated cups () indicate annual wear rates much higher than that. We also saw a tendency for cups with high quantities of osteolysis to become loose and necessitate revision, and we reason that the cause is the PE particle load. A recent study from the Finnish Arthroplasty Registry suggests the most common reason for revision of the Universal® cup (combined data for the Ti-and HA-coated cups) is wear leading to liner exchange, with a 13-year revision rate of 26% (Citation79). Other studies of the same acetabular component reveal revision rates between 13% and 26% with a mean of 7 and 10 years’ follow-up (Citation114;Citation162). Several authors describe a large main group of well-performing cups and a smaller group of cup failures due to osteolysis, wear, aseptic loosening, and in rare cases PE fracture (). The higher variation of femoral head penetration within the HA group as opposed to the Ti group (SD 0.26 mm/year and 0.14 mm/year, respectively) which we uncovered supports the concerns expressed in other studies with individual cases of high PE wear with HA-coated cups that have lead to implant failure (Citation22;Citation24;Citation38;Citation74;Citation127;Citation206;Citation263). Apparently, there is no clear association between implants prone to wear failure and clinical performance (Citation127;Citation167) and thus expansile (cystic) osteolysis and loosening may progress without warning signs (Citation18;Citation166;Citation206). For some cups, there is a plausible explanation of failure, e.g., HA coating applied to smooth surfaces (Citation43;Citation127;Citation205;Citation263), very thick coatings (Citation166;Citation207), the presence of screw holes (Citation22;Citation82), a poor locking mechanism (Citation162), and older types of UHMWPE. However, for other HA-coated cup designs, the reasons for the wear rate, osteolysis, and revision are not entirely transparent (Citation74;Citation173) and this raises concern about the continued use of HA coating for acetabular cups.
Zirconia as an articulate bearing material (study II)
Zirconia has good biocompatibility, as well as mechanical and sliding characteristics, when evaluated in the experimental setting (Citation36;Citation136;Citation213). Despite this, several clinical studies have reported a higher annual wear rate of Zr-on-UHMWPE than was expected on the basis of laboratory studies (Citation100;Citation134;Citation264). Clinical publications with components similar to those investigated in our study are few and their results are conflicting (). Only one author (Citation128) reports a superior clinical wear performance with Zr in comparison with metal heads.
The high wear rates with Zr heads reported in this study could be related to the high-activity lifestyle of young patients (Citation221). Vigorous use of a THA may increase the risk of frictional heating and mechanical stress in the articulation (Citation136) and lead to increased wear. For the Zr-on-PE bearing, this mechanism is now well established as a partial tetragonal to monoclinic phase transformation resulting from in vivo physiologic, mechanical, and hydrothermal stresses (Citation107;Citation129), which increase Zr surface roughening by grain pullout and provide the potential for accelerated PE wear. This theory explains why Zr-on-PE in our study (Citation241) and other medium-term studies has a wear performance similar to CoCr-on-PE at mid-term follow-up (Citation57;Citation100;Citation134). Furthermore, the continuous deterioration of Zr in vivo (Citation129) explains the report of progressive wear with Zr-on-PE bearing couples from mid-term to a minimum of 10 years’ follow-up, as described by Hernigou and Bahrami (Citation100), as well as the reduced survival of Zr-on-PE bearing couples (63%) at mid-term (5.8 years), described by Allain et al. (Citation4).
Zirconia femoral heads were withdrawn from the commercial market in 2001 due to incidents of head fracture after a change in the manufacturing procedure. We did not experience Zr head fractures at mid-term follow-up. However, reported cases of catastrophic results after revision of fractured ceramic heads (Citation107;Citation153;Citation174) and complete wear-through of the metal shell by a ceramic head (Citation147) encourage continued sharing of knowledge of Zron-PE bearing couples in the literature.
Considerations on wear measurement methods (studies III, IV, and V)
Contemporary image processing techniques have been developed for application of PE wear measurement in scanned or digital images of hip pros-theses. The ability to measure very small amounts of wear has been improved by use of image processing technology to minimize the observer as a source of error.
Optimally, evaluation of new implants, including improved crosslinked PEs, should be performed after a short follow-up period, which requires tools of a high accuracy and precision. Although RSA is considered to be the most accurate and precise PE wear analysis method (the gold standard) (Citation110;Citation262), many radiographic in vivo studies, especially retrospective studies, are restricted to wear measurements on plain radiographs. Several computer-assisted methods for PE wear assessment on plain radiographs are available (Citation83;Citation157;Citation262), but the agreement between PE wear measured in stereo and plain radiographs is considered in only a few published clinical studies (Citation34;Citation109;Citation110;Citation262). Several factors may complicate the direct comparison of wear results obtained by different wear analysis techniques, as they each have specific limitations. The algorithms for determination of wear in software packages for stereo radiographs differ from the algorithms in software packages for plain radiographs. Thus exact agreement between PE wear measurements based on different type/angle radiographs evaluated with different software packages cannot be anticipated, but some similarity is to be expected. Results obtained by different wear measurement methods should thus be validated and judged against other methods, and the reproducibility between research institutions should be assured.
Wear estimates may also be dependent on a number of factors not attributed to the analysis methods, such as the inclusion of the radiographs selected for baseline analysis (inclusion or exclusion of the creep period) (Citation246), mobilization of the patient before the baseline radiograph, and possibly the number of radiographs analysed.
Does the number of radiographs influence results? (study III)
We observed a large difference in measured median PE wear in the same patients using a different number of radiographs for analysis (one, two, or six). Using a six radiograph strategy, the estimated PE wear was almost twice the PE wear observed using a two radiograph strategy. This bias was consistent for the individual measurements () except for patients with wear close to wear-through of the liner. One explanation for the observed rather high random variation in repeatability using six radiographs could be the inherent problem that each of the five PE wear estimations contributes with positive values and sums up variances from examination to examination. It therefore seems that a multiple radiograph strategy is most favourable for monitoring the development of wear over time, and less favourable for the precise estimate of wear at a given time point. Comparing concurrent validity between the use of one, two, and six radiograph strategies, we observed large systematic variations of 0.8 to 1.8 mm, and none of the strategies tested similar for concurrent validity. The systematic variation can be corrected for if known whereas this is not possible for the random variation. The random variation was lowest (± 1.2 mm) when comparing two or serial (six) radio-graphs, and thus these numbers of radiographs seem most comparable.
In theory, the two strategies using the fewest radiographs (one and two radiographs) should have had the lowest random variation and been in closest agreement. The final follow-up radiograph is the same in both strategies, and thus the difference must arise from the software handling of the starting point. Using only the final radiograph for analysis (PW1), the software decides the position of zero PE wear (baseline) from CAD knowledge of the cup component and size along with information about the femoral head size. With the two radiographs strategy, the actual baseline position of the cup and head, as estimated from the baseline radiograph, is used for the PE wear measurement to the final follow-up radiograph. More research is needed to clarify what causes the differences between PW1 and PW2 and to clarify whether these differences are problematic only for the Universal component implant brand.
Our results confirm the limitations of comparing mean PE wear results based on analysis of a varying number of plain AP radiographs. Inter-study results of PE wear with PolyWare using two or multiple serial radiographs correlate well and seem comparable. However, care should be taken when mixing strategies, and we do not advice comparing PE wear by assessing an unequal number of available radiographs per patient.
Which number of radiographs better reflect the true wear? (study V)
Our experience with PolyWare in study III lead us to question whether it was more accurate to use only the final radiographic follow-up or both the postoperative and the final radiograph follow-ups. We assessed this question in a group of unrevised patients with medium-term follow-up, in whom both stereo radiographs and plain radiographs were available. PE wear measurements obtained by EGS-RSA (gold standard) were considered in close agreement with the true wear (study IV). We found that PolyWare overestimated PE wear by a mean of 0.2 to 0.5 mm in comparison with EGSRSA, depending on the number of radiographs used for analysis. Ebramzadeh et al. reported a similar clinical overestimate of 0.18 mm. We established concurrent validity with 2D wear estimates of EGS-RSA and PolyWare using two radio-graphs (baseline and final) based on statistical testing. However, the random variation was smallest (LOA ± 0.55 mm) between EGS-RSA and Poly-Ware using only the final radiographic follow-up for measurement of 2D PE wear, and because the systematic variation (bias) can be corrected for when known, we recommend using the PolyWare wear measurement method with only of the final radiographic follow-up (PW1).
The difference between RSA and PolyWare increased in the 3D wear measurements, and we do not think PolyWare is applicable in the clinical setting with 3D PE wear measurements. Concerns with clinical 3D PE wear measurement using plain radiographs have been reported by Bragdon et al. who compared marker-based digital RSA and the Martell wear measurement method (Hip Analysis Suite) on plain radiographs (Citation34). Bragdon et al. suggested calculation and comparison of the steady-state wear between methods to level out the differences. In our patient series, unfortunately, no 1- or 2-year plain radiographic follow-ups were available, and thus this was not possible.
Using only the final plain radiographic follow-up for wear estimates with the PolyWare method (PW1) comes within ± 0.55 mm (LOA) of the true value, while using two radiographs (PW2) comes within ± 0.9 mm. Random variation of one half of a millimeter is sufficient for comparative studies assessing differences between two groups, and furthermore the 0.5 mm (bias) overestimation should apply systematically for both groups and can thus be corrected for if desirable. Furthermore, limiting the radiographic assessment to the final follow-up will improve the possibility of being able to use good quality radiographs as digital radiographic equipment has become standard in the most radio-graphic departments. In retrospective clinical studies, the need for only the final radiograph, should make it possible to define a prestudy protocol aimed at obtaining the last follow-up radiographs. This should limit problems of projection variation in radiographs in future retrospective studies, potentially decrease the number of patients needed for evaluation, and make analyses less time consuming.
Accuracy of radiographic PE wear measurements (study IV)
In vivo accuracy can only be assessed in combination with revision surgery and coordinate machine measures (CMMs) of the true wear values. CMMs determine only the articulate wear, while radio-graphic methods estimate both the articulate and the back-side wear. With a phantom, a zero-wear situation as well as the difference between a measured and calculated true wear situation may be assessed. The accuracy or detection limit of the software programs has become progressively more important with the continuous development of improved low-wear bearing couples. Multiple studies have established that PE wear contributes to osteolysis and implant failure at rates higher than 0.2 mm/year (Citation73;Citation75;Citation178;Citation240;Citation263), and therefore it is important to utilize wear measurement techniques with an accuracy at or superior to this threshold.
The definition of accuracy differs between authors (Citation197), and it has been suggested that accuracy should be presented with measures of the systematic differences (e.g., Bland-Altman Plots (Citation23)) (Citation262). The ISO standards for presentation of accuracy as described by Ranstam et al. (Citation197) were suggested as a better solution than merely presenting the means and standard deviations. These standards report the accuracy as the sum of precision and bias; however, these measures may appear to be more valuable when presented separately.
RSA is considered the gold standard of wear measurements, and digital RSA has been shown to reveal highly accurate measurements of PE wear (Citation28;Citation33;Citation262). RSA accuracy data are reported in the range of 0.092–0.35 mm for RSA (Citation130;Citation226). Von Schewelov et al. report a regression coefficient of 0.94 with a phantom wear assessment using 3D marker-based digital RSA (Umeå system) compared with the true value (Citation262). Our results with 3D wear measurements of newer and marker-less RSA methods using models and fully digital films had a similar accuracy, with slopes between 0.93 and 0.95.
Devane et al. developed and tested the first semi-automatic PolyWare version and reported a 3D accuracy of ± 0.15 mm for 3D linear wear, and 411 mm3 for volumetric wear (or values within 8% of the true volume) (Citation67). Kang et al. estimated the 2D measurement error of the software to 0.15 mm and the 3D measurement error to 0.21 mm in a phantom setting (Citation119). Next, Ebramzadeh et al. concluded that the 2D measurement error (mean difference from the micrometer true value) with PolyWare was 0.14 mm (mean and median) with laboratory radio-graphs of a phantom (Citation76). In a retrieval study, Poly-Ware has been shown to underestimate 2D PE wear by 20% and 3D PE wear by 18% (Citation105), and we saw similar values of underestimates in our study IV.
Based on our observations, the PolyWare software is generally less accurate for measurement of wear compared with RSA; however, it is seemingly suitable for measurements in the range of 1 to 5 mm. This is sufficient for clinical use because liner thicknesses in the smallest and biggest diameter metal-backed cups articulating with 28 mm heads range from approximately 4 to 11 mm. For assessment of low wear and medium wear (range 0.01–0.5 mm), we found that RSA methods were superior to PolyWare. Accuracy with the volumetric wear measurements were poor and based on calculations and further assumptions from the clinical wear estimates. In general, we do not advise using volumetric wear measures of PE wear.
Precision of radiographic PE wear measurements (study IV)
In the clinical setting, repeatability is often assessed as double radiographic examinations at one follow-up in order to reveal the total error due to patient pose, the radiographic equipment, and the PE wear measurement software (Citation126;Citation175). Precision is typically stated as the absolute mean value of all recorded differences between two double examinations with a standard deviation of 1.96 (95% confidence interval) to represent the total error. When double examination radiographs are unavailable, as typical in a retrospective situation, repeatability of measurements of the same radiographs to assess only the error of the observer and the instrument may be feasible. When comparing repeatability of PE wear measurements, one should therefore be aware whether double examination (radiographic and software precision) or single examination (software precision) images were used.
The precision of marker-based radiostereometric and plain radiographic computer-assisted methods for PE wear measurements in THA have been investigated in other research institutions with use of phantom experiments (Citation28;Citation33;Citation59;Citation105;Citation262), but due to differences in study designs, a direct comparison of results is not easy. Von Schewelov et al. showed the precision of marker-less digital RSA wear measurements (Umeå system) to be between 0.17 mm and 0.22 mm in a phantom study, depending on the amount of measured wear (Citation262). These numbers were calculated on the basis of formulas presented by Ranstam et al. (Citation197) and correspond to our report of SDintras which were approximately 0.05 mm for 2D and 0.10 mm for 3D model-based RSA wear measurements. The 95% prediction interval around the line ( and ) is probably a more reliable estimate of precision in our study and revealed a 3D precision of 0.27 mm and 0.37 mm for EGS-RSA and MBRSA, respectively. However, excluding the near wear-through measure, which is not included in the study by von Schwelow et al., improved precision to 0.10 mm and 0.08 mm for EGS-RSA and MB-RSA, respectively. Von Schwelov et al. performed 57 repeat wear measurements (19 radiographs in each of three wear positions compared with 0 wear, 3D vector range 0.2–1.5 mm), whereas we performed 15 repeat wear measurements (15 radiographs in 15 wear positions compared with 0 wear, 3D vector range 0.017–8.660 mm). Repeatability in our study thus reflects the entire repertoire of wear seen in the clinic, and each radiograph is different with respect to the visibility of the femoral head within the cup. Furthermore, we included repeat wear measurements of substantially lower wear (0.01–0.01 mm) than did von Schewelov et al., which probably tested the lower detection limit of the RSA software, and, in addition, our category of high wear exceeded by far the higher wear limit in their study. In another phantom hip wear study of marker-based RSA with the Umeå system, Brag-don et al. obtained five roentgen datasets of 17 pairs of stereo radiographs, as they advanced the femoral head in one direction at a time in a total of four wear positions (single vector range 0.05–0.2 mm) (Citation33). The precision that they (Citation33) reported for the resultant 3D vector (60.43 µm ∼ 0.06 mm) is comparable with our data regarding the SDline (0.08–0.10 mm). Borlin et al. (Citation28) used the phantom stereo radiographs obtained by Bragdon et al. (Citation33) but grouped the examinations pairwise to generate independent measurements for investigation of a marker-less femoral head penetration method (Umeå system). They reported precision in a similar fashion as we did (95% prediction interval of the RMS), with precision measures in the individual three x, y, and z axes of 0.08 mm, 0.1 mm, and 0.13 mm, respectively.
The precision of PolyWare was shown to be 0.02 mm (max 0.28 mm) for 2D wear measures, 0.07 mm (max 0.41 mm) for 3D wear measures, and 24 mm3 (max 178 mm3) for volumetric wear measures of repeat analysis of the same zero wear laboratory radiographs (Citation59). These values, we believe, reflect the bias of our reported intra-method repeatability (); however, our bias was lower, which could be explained by the fact that we did not change the cup angulations (Citation59). We believe the random variation of repeatability should be reported, and on the basis of our LOAintras we observed a good repeatability for all 2D methods, with a LOAintra of approximately ± 0.1 mm around the bias but slightly worse for 3D methods, with an approximate LOAintra of ± 0.2 mm. Thus repeatability (LOAinter) was poorer by two-fold with 3D compared with 2D wear measurements by Poly-Ware.
Can PE wear close to wear-through of the liner be measured? (study IV)
For the assessment of high wear close to wear-through of the liner, accuracy was poor with both the RSA and the PolyWare methods and consequently resulted in values lower than the true value (). The negative effect of the near wear-through value is also seen clearly in and with inclusion and exclusion of the near wear-through value, respectively. Thus inclusion of the highest value resulted in a twofold worse accuracy for 2D RSA measures, and a slightly worse accuracy for 3D RSA measurements. The PolyWare accuracy was not improved by removal of the highest value, although as seen in , it was a problematic value for the software. According to the same plot, the explanation may be that PolyWare also had problems with the small wear measures.
We speculate that the reason for the problem in measurement of values near wear-through is the decrease in visible head contour because of overlap with the cup, resulting in a decreased area for adjustment of circles with edge-detection and regions of interest. The problem with obscured free borders of the femoral head with increasing wear is present with both the RSA and the PolyWare methods, and further studies should focus on the upper limit, as well as the lower limit, of reliable wear measurements.
Sample size (study IV)
Many variables that are difficult to control for, and are independent of the method used, have been shown to influence precision of PE wear measurements such as patient factors (male gender, tall patients, active patients, and young patients) (Citation221), radiographic quality (Citation249), multidirectional wear patterns (Citation277), hip angulations (Citation59;Citation80), intra-observer variance (Citation78), and the manufacturing tolerances of acetabular components (Citation105). For a given patient series, these variables could be termed the “biological variation”. In addition to the biological variation, there is also the variation of the applied PE wear measurement method, which may be investigated under ideal conditions in a phantom study. When the biological variation and the method variation are known, the sample size for a clinical study may be estimated, and additionally, the difference in sample size when choosing between different wear measurement methods.
The differences in the sample size (n) of PE wear measurements by PolyWare and EGS-RSA can be calculated as: n /n 2 2 2 pw egs = (sdbio + sd ) / (sdbio +pw sd 2 egs ). This formula can be used when the biological variation in PE wear among patients (sd 2 bio ) and the measurement variation of the two methods sdegs and sdpw are known, and are based on the assumption that the same conditions of power and difference in PE wear exist between the investigated patient groups. Assuming that sdbio = 0.25, and from our study IV sdpw = 0.34, and sdegs = 0.13, the difference in sample size, independent of the mean PE wear or wear rate, between PolyWare and EGS-RSA is then 0.178/ 0.079 = 2.2. It is up to the researcher to decide between an expensive (high establishment and running costs) and highly accurate RSA setup with few patients or a low-cost but less accurate PolyWare setup that requires at least twice as many patients to obtain a sufficient sample size.
2 D or 3D wear measurements? (studies III, IV, and V)
It has been suggested that AP plain radiographs used for assessment of 2D wear with PolyWare and similar plain methods should suffice for a determination of the major vector (Citation73;Citation105;Citation248). Clinically, PolyWare has been shown to underestimate 2D linear wear by 20%, 3D wear by 18%, and volumetric wear by 13% (Citation105). In the phantom experiment (study IV) we found PolyWare to underestimate the true simulated 2D wear by 24%, supporting the theory that the major wear vector is seen in the frontal plane. However, true wear in the clinical setting is multidirectional (Citation275;Citation277) on the individual patient level (Citation275), and furthermore, the PE wear tract is probably not a tight cylinder around the femoral head (Devane et al., 1995a).
We found accuracy and repeatability to be better with 2D than with 3D PolyWare and RSA techniques. On the other hand, von Schewelov et al. showed better accuracy and precision for RSA with 3D digital measures (Citation262). For RSA it can be questioned whether 2D wear constitutes a useful estimate for research purposes. As the multidirectional wear measurements better described the clinical situation, maybe a slight loss of precision and accuracy could be accepted in order to gain a more relevant measure.
In the phantom (study IV), PolyWare had a better accuracy with 3D than with the 2D measurements of simulated wear, and thus the addition of the third wear axis (LA) was important for this software. These observations were made on the basis of high quality digital radiographs obtained on the same day, by the same equipment and radiographer. However, in the clinical setting, poor quality plain lateral radiographs have been shown and discussed to severely affect the accuracy and precision (Citation105;Citation248;Citation249). On the basis of these discussions and our own experience lateral radiographs, we do not advise to use 3D estimates of PE wear measurements in clinical plain radiographs.
Radiographic projection differences are difficult to control and offer some explanation for the differences in magnitude of measured PE wear with use of a few versus multiple radiographs, which we observed (studies III and V). This observation further stresses the use for a strict protocol for the patient pose with standard hip radiographs and for double examination radiographs with complete repositioning of the patient between exposures in research projects. Recently, a mathematical correction algorithm has been suggested that would make 2D wear measurements in plain radiographs less sensitive to radiographic projection differences and approximate 3D “true” linear wear values obtained by RSA (Citation254).
What about the patient pose during radio-graphic examination? (studies III and IV)
Plain AP radiographs used for wear analysis are not calibrated (position coordinates), and in retrospective studies, radiographs are often not obtained according to a standardized protocol. The clinical positioning of patients with the risk of slight changes in hip angulations between radiographic follow-ups has been shown experimentally to influence wear results (Citation59;Citation80). A plausible theoretical explanation for this is that the radiographic shadows of the components vary with angular displacements, making the basis for automatic edge-detection different between follow-ups. Two authors describe PolyWare to be sensitive to different cup positions/ hip angulations, which results in 3D measurement errors of <0.25 mm (median) (Citation76) and 2D measurement errors of mean 0.4 mm (max 0.86 mm) (Citation59) with low abduction angles (20°–35°) and modest anteversion (0°–10°). We kept the anteversion and tilt constant in our phantom study in order to make fair comparisons of PolyWare and RSA, and thus we did not encounter the problems of variation in pelvic angulations. Wear analysis with PolyWare is based on non-calibrated radiographs, and, because it is difficult to strictly control the pelvic angulations in clinical anteroposterior radiographs (Citation80), we have reservation in believing that the clinical accuracy of PolyWare will match the accuracy of our phantom study (study IV). Pelvic angulations in study V may be some of the explanation for the differences observed between PolyWare and RSA.
There are no guarantees that the head will be located in the deepest point of the wear tract at the time of the radiograph (Citation152). Several PE wear studies have addressed the potential of weight-bearing supine radiographs for PE wear analysis as a solution to this problem. The overall conclusion is that the measured differences are without clinical relevance (35;Citation152;Citation165;Citation231;Citation261).
Reference examination for wear measurements (studies I, II, III and V)
Charnley was the first to measure radiographic PE wear in two dimensions. His measurements were based on radiographic examinations with the patient supine and with the postoperative radio-graph as the baseline (Citation47). Thus his measurements included creep. Since then, much attention has been given to defining, calculating, and excluding the initial and delimited period in clinical follow-up based on theories of creep (bedding-in) of the PE liner (Citation85;Citation158;Citation246), but no consensus has been reached. Creep may be dependent on various factors, including acetabular component design, patient activity (friction heating), the type/quality of PE, and perhaps also the sterilization method. “True in vivo wear” can be described in retrieval studies by CMM, and while this offers an accurate estimate of the articulate wear including creep, backside wear cannot be quantified (Citation105). It is thus problematic to correlate the defined “true in vivo wear” obtained by CMM with radiographic measures of wear that include both articulate and backside wear, and it gets even more complicated when the first post-operative period is excluded because of theories of creep (Citation105). In addition, the exclusion of a variable period of “bedding-in” (6 weeks to 24 months), done in some, but not all studies, inevitably results in different magnitudes of reported wear and wear rates, even though efforts are made to calculate intercepts and the steady-state wear. Thus inter-study PE wear comparison is difficult, and there is a need for a standardization guide for the presentation of polyethylene wear results and precisions.
Using only the final plain radiograph for analysis (study V) will include the period of creep, but improve the chance of collecting good quality and digital radiographs. Also, it allows for the definition of a prestudy protocol to obtain last follow-up radiographs in a retrospective clinical study targeting PE wear. This should limit problems of projection variation in radiographs in future retrospective studies, potentially decrease the number of patients needed for evaluation, and make analyses less time consuming. Keeping in mind the limitations regarding PE wear measurement with medium-to long-term follow-up with older type PWs and larger groups of patients, the PolyWare method is optimal, simple, and in relatively close agreement with the gold standard of RSA.
Methodological considerations
Study I
We attempted full follow-up with the original randomized patient group; however, hard-copy radio-graphs of four patients were lost, and these patients could not be assessed for radiographic PE wear. As these patients were distributed evenly among the groups (two with Ti cups, two with HA cups) we do not think this had a severe influence on results.
All available radiographs were collected, and the observer measuring wear was masked to the groups.
We were interested in describing both the pattern of wear and the time-dependent wear, and thus we chose to measure wear in serial radiographs. Given the variation in wear among patients, an approach with fewer radiographs or addressing a steady-state wear might have been more applicable.
Revised cups were not collected for retrieval analysis due to the retrospective design, and thus the true amount of wear and the involvement of HA particles in third-body wear remains uncertain.
Apparently the small sample size likely explains the lack of statistical significance with comparison of wear and osteolysis (Type 2 error).
Case-mix should have been optimal because the groups were randomized, and although there were an equal number of both genders in the HA cup group, there were more females than males in the Ti cup group, which potentially could have contributed to a higher wear rate in the HA cup group. The level of activity was, however, not recorded and could have influenced PE wear with one or both of the groups.
We compared mean wear in the two groups from the postoperative baseline until the last radiograph available (revision, death, or to the 12-year follow-up) for a minimum of 5 years. Ideally, all patients would have been followed up for the same length of time, preferably long term, but excluding revisions from a wear analysis potentially removes the worst cases of PE wear and distorts conclusions. Thus we chose to include all available patients and used the last available radiographs. In some patients six radiographs were included in the analysis, whereas in others only five, which we later learned could be problematic. There were more revisions in the HA group, thus fewer images were available, and consequently mean duration of follow-up was shorter for this group. The slightly unequal number of radiographs per patient in the groups studies could, in the worst case, have evened out the wear measured between groups rather than increase the existing difference.
Study II
Although no patients were lost to follow-up, missing radiographs deprived us of the option of medium-term wear analysis in six patients (follow-up 24 to 37 months), which could have introduced selection bias. None of these patients were revised and according to their patient record at the 5-year clinical follow-up, they had all returned to work shortly after surgery and were still working and doing well. Therefore we do not think that the lack of the final image in these particular six patients severely affected our conclusions.
All available data were collected. It was diffi-cult to mask the observation of wear measurement between groups, although attempts were made, due to the lower density of zirconia, which is evident on the radiographs.
We were interested in describing both the pattern of wear and the time-dependent wear, and thus we chose to measure wear in serial radiographs. An approach with fewer radiographs or addressing a steady-state wear might have been more relevant.
We were unable to show the expected difference between the groups, and a mid-term endpoint is probably not sufficient with the given sample size and the chosen method of wear analysis. Although we performed a relevant calculation of sample size and included a sufficient number of patients accordingly, there was a 20% chance of a type two error.
The patients were not randomized, but the potentially confounding variables we assessed were similar, and the comparator (CoCr femoral heads) was relevant. Even when controlled for other factors, PE wear is greater in males than in females (Citation224). Thus the smaller proportion of females in both groups might have biased the wear rates accordingly. The activity level of this young patient group was not determined and may have influenced the measured femoral-head penetration.
The density of Zr is less than that of CoCr and this could potentially cause difficulties in outlining the contour of the femoral head (Citation262). The automatic detection of the femoral head was successful in 98% of the images. Thus we conclude, that the radiographs were of good quality and the wear measurement method applied in this study effectively counteracted this problem.
Study IV
We did not replicate all in vivo variable penetration patterns (non-cylindrical and different directions) because all motion imparted to the phantom followed the three coordinate axes equally (cylindrical).
The position of the phantom remained unchanged between examinations, and thus we did not explore the errors arising from differences in patient pose between examinations.
Radiographic quality was better than is the standard for clinical plain radiographs, and thus repeatability and validity could be expected to be lower in the clinical situation.
We only evaluated the accuracy and precision of a 28-mm femoral head and a 56-mm acetabular component (liner thickness 8.8 mm). However, we do not think the latter severely affected the generalizability of our results because it has been shown that RSA can accurately detect femoral head penetration, irrespective of the direction of penetration in the cup, the cup size, and the cup brand (Citation262).
Cup anteversion and tilt were kept constant because, with PolyWare, these changes may affect both repeatability and validity.
We did not test differences between 2D and 3D methods in any analysis because this would inevitably result in an advantage for 2D methods as they do not have the extra variance of a third dimension.
In general
No distinction between creep, articulate wear, and backside wear could be made with any of the wear measurement methods used in this thesis.
The PolyWare method was validated for clinical evaluation of longer-term follow-ups and series of high wear (Citation69;Citation105), and we used the method according to these recommendations. We only used AP images, as the CTL radiographs were of varying projection and quality, and we thus believe to have used the software within the described limits.
Cups that had migrated according to visual judgement were not included in the analyses. Cup migration was, however, not assessed by accurate methods such as RSA, because we either did not have the stereo radiographs needed (studies I and III) or no bone markers were inserted in the pelvis (study V).
The post-operative clinical plain radiographs were all obtained according to the standards of the departments of radiology, although not according to a specified study protocol. Thus the leg was not placed in a soft foam positioner, or rotation-stabilized by a fixture, and most likely this affected the projection between radiographs obtained over a long follow-up period.
The plain radiographs, except for the final radio-graphs of study V, were all hardcopy printed films that we digitized for computed wear analysis. Physical degradation and varying resolution could have influenced our wear analyses, since the first radiographs (study I) were obtained in 1990.
Addressing wear measures close to wear though of the liner (study I and III) was attempted in a few cases (studies I and III) prior to new knowledge (study IV) and this could have affected the results (presumably lower wear than the measured)
Many biological variables are impossible to control for, such as small changes in the radiographic setup and calibration from follow-up to follow-up, under or over exposure of radiographs that affect the sharpness of component borders, patient position (pelvis) and leg rotation, body size and soft tissue mass of the patients, and angulations and size of component. Wear measurements based on uncalibrated plain radiographs would naturally be more sensitive to some of these changes than calibrated stereo radiographs, thus it is important to assure that samples are of a sufficient size. Despite all these potential problems with plain radiographs, we did not exclude any patients /AP radiographs due to poor quality. An indicator, that this is correct, is that the border of the femoral head was sufficiently visible for the software automatic edge-detection to work well in more than 95% of the radiographs. On the contrary, the majority of the CTL radiographs were useless and consequently not used other than to judge anteversion or retro-version of the cups.
In studies I, II and III we only used AP hip radio-graphs and in study V we only used pelvic antero-posterior radiographs. Thus the ray centre could not have been very different between follow-ups.
A disadvantage of both PolyWare and Model-Based RSA is that these methods are only applicable for uncemented cups. This is because the lack of metal-contour in all polyethylene cups make it impossible to measure wear in cemented cups. For cemented cups wear can be assessed by marker-based RSA when the PE cups are marked with tantalum beads at the time of surgery. For non-hemispheric cups wear assessment can be performed with Model-Based RSA using scanned surface models, but not with EGS-RSA.
We followed the recommendations of the instruction manuals with the used software programs, also with regard to the resolution of the scanned primary hardcopy plain radiographs.
Generalizability
Study I
Our results of poor survival of HA-coated cups are based on discontinued components, and it is unclear whether they apply to newer HA coating technologies, such as thinner HA coatings and electrochemical HA coatings, crosslinked PEs, and newer implant designs, i.e., with better liner locking mechanisms. We suggest further investigations of newer components with regard to survival, wear, and osteolysis.
Study II
The published mid-term clinical results for Zr are contradictory and our study does not support the use of Zr rather than CoCr heads. There is a need for more evaluation of the long-term wear performance and potential side effects of Zr femoral heads.
Study IV
The practical experience at the Clinical Orthopaedic Research Unit in Aarhus with both PE wear measurement methods investigated in this PhD study is extensive, which probably demonstrated the accuracy and precision of the software programmes at their best. Nevertheless the software packages are computerized, and the manual influence of results is limited. Thus, after overcoming an initial and expected learning curve with the software, the results of our phantom study should be highly reproducible in any research department in which digital RSA is available.
The accuracy and precision shown with the three methods in our study are a result of perfect conditions and cannot be expected to improve in the clinical situation. However, with good quality radiographs and a careful protocol for obtaining these, the clinical accuracy and repeatability of the wear measurement softwares might come close to the results presented in the current study. Ideally, these software methods should be compared in a clinical series as well.
Studies III and V
The results may partially be related to the populations, the physical position of implants in the patients, and prosthetic brands investigated. Thus results in these studies may not be completely reproducible in other institutions but the general conclusions are expected to apply externally as a benchmark for various brands of hemispheric metal shells with UHMWPE liners and metal fem-oral heads when using good quality radiographs.
Conclusions
Study I
Survival of first-generation medium-thickness HA-coated cups is inferior compared with similar Ti-coated cups. Individual cases of excessive PE wear and premature cup failure might be related to third-body wear from delaminated HA. These findings apply to first-generation modular cups with first-generation medium-thickness HA-coatings, and may not apply to other cup designs and new HA coating technologies. We advise close follow-up of patients with HA-coated cups similar to those used in our study.
Study II
Mid-term clinical results for Zr are contradictory and our study does not support the use of Zr rather than CoCr femoral heads. There is a need for more evaluation of the long-term wear performance and potential side effects of Zr femoral heads, not least, because of the recently reported catastrophic cases of fractured ceramic heads and wear-through of the metal shell (Citation147;Citation150;Citation174).
Study III
There are limitations of comparing mean PE wear results based on analysis of a varying number of plain AP radiographs. Inter-study results of PE wear with PolyWare using two or multiple serial radiographs correlate well and seem comparable. We do not advise comparing PE wear in groups by assessing an unequal number of available radio-graphs per patient.
Study IV
Marker-free (model based) RSA is applicable for measurement of small amounts of wear at the threshold of osteolysis prediction (0.2 mm/year) and for small sample sizes. RSA using scanned implant-models and RSA using EGS-models may be used interchanging when measuring PE wear in hemispheric cups. PolyWare was the least precise and least accurate method; however, it is a good and low-cost alternative to RSA but demands a twofold larger sample size. Neither model-based RSA nor PolyWare is applicable for measurement of PE wear close to wear-through of the liner. Repeatability and validity of these three methods should be further tested in a clinical setting.
Study V
The PolyWare method using only final radio-graphic AP images is easy and inexpensive to use, applicable for 2D wear measurements above 0.5 mm total, and offers a simple and fast setup applicable in most hospitals for the assessment of PE wear. The PolyWare method using only the final radiographic AP images has a clinical repeatability similar to EGS-RSA (“the gold standard”) and is ideal for retrospect research because it alleviates the need for baseline images that are often lost, stored in hard copy, and of varying quality. For low PE wear assessments, the PolyWare software does not comprise the needed accuracy, and for such situations RSA is recommended. For assessment of medium-term or long-term wear measurements in larger groups of patients, the PolyWare method is optimal, simple, and in relatively close agreement with the gold standard of RSA.
Future research
To clarify the severe and negative long-term side-effects of HA, which were seen in study I, a randomized study of modern acetabular components with a time-fashionable HA coating should be initiated and compared with similar components without HA. Potentially, a comparative study of large sample size could offer some clarification, and we are currently performing such a study.
The last patients from study II have just been seen for a minimum 10-year radiographic follow-up that included clinical data obtained from the Danish Registries and questionnaires with respect to the hip (HHS pain and disability scores) and daily living. We are looking forward to processing and publishing these data.
To follow-up on the phantom data in study IV, we have just collected the final data for a clinical validity and reliability study of PolyWare, MBRSA, and EGS-RSA. Currently, data analysis is ongoing.
The variation of wear measurements in a clinical series constitutes a combination of biological variation and method variation. Biologic variation may not be changed, but future research should focus on determining the optimal number of wear measurement repetitions necessary to reduce wear measurement scatter to a minimum (Citation259).
Methodological studies should focus on the upper limit, as well as the lower limit, of reliable wear measurements with various wear measurement methods.
Future studies should address the described problems of pelvic angulations with PolyWare in comparison with RSA.
Newer types of PEs constantly hit the marked. The most recent suggestion for reduction of particulate PE wear is the addition of vitamin E to the HXLPE product. Investigations are currently ongoing in the US, and we await the initial results with suspense.
Notes
(Footnote)
A system is in a metastable state when it is in equilibrium (not changing with time) but is susceptible to fall into lower-energy states with only slight interaction. It is analogous to being at the bottom of a small valley (weakly stable state) when, passing a small hill (unstable transition state), there is a deeper valley close by (strongly stable state) – a local stability of a system at a local (but not global) minimum of a potential.
References
- ASTM Standard specification for ultra-high molecular-weight polyethylene powder and fabricated form for surgical implants. ASTM Standard F648. 1984. West Conshohocken, PA: American Society for Testing and Materials.
- al Saffar N, Revell PA. Interleukin-1 production by activated macrophages surrounding loosened orthopaedic implants: a potential role in osteolysis. Br J Rheumatol 1994; 33(4):309-16.
- Albrektsson T, Branemark PI, Hansson HA, Lindstrom J. Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand 1981; 52(2):155-70.
- Allain J, Le MS, Goutallier D, Voisin MC. Poor eight-year survival of cemented zirconia-polyethylene total hip replacements. J Bone Joint Surg Br 1999; 81(5):835-42.
- Altman DG Practical Statistics for Medical Research. 1995 ed. Chapman & Hall; 1995.
- Aspenberg P, Herbertsson P. Periprosthetic bone resorption. Particles versus movement. J Bone Joint Surg Br 1996; 78(4):641-6.
- Aspenberg P, Van der Vis H. Fluid pressure may cause periprosthetic osteolysis. Particles are not the only thing. Acta Orthop Scand 1998; 69(1):1-4.
- Atkinson JR, Cicek RZ. Silane crosslinked polyethylene for prosthetic applications. II. Creep and wear behaviour and a preliminary moulding test. Biomaterials 1984; 5(6):326-35.
- Bal BS, Garino J, Ries M, Rahaman MN. A review of ceramic bearing materials in total joint arthroplasty. HIP Int 2007; 17(1):21-30.
- Bankston AB, Keating EM, Ranawat C, Faris PM, Ritter MA Comparison of polyethylene wear in machined versus molded polyethylene. Clin Orthop 1995; (317):37-43.
- Bargmann LS, Bargmann BC, Collier JP, Currier BH, Mayor MB Current sterilization and packaging methods for polyethylene. Clin Orthop 1999; (369):49-58.
- Barrack RL, Folgueras A, Munn B, Tvetden D, Sharkey P Pelvic lysis and polyethylene wear at 5-8 years in an uncemented total hip. Clin Orthop 1997; (335):211-7.
- Barrack RL, Lavernia C, Szuszczewicz ES, Sawhney J. Radiographic wear measurements in a cementless metal-backed modular cobalt-chromium acetabular component. J Arthroplasty 2001; 16(7):820-8.
- Bauer TW. Severe osteolysis after third-body wear due to hydroxyapatite particles from acetabular cup coating. J Bone Joint Surg Br 1998; 80(4):745.
- Bauer TW. Hydroxyapatite: coating controversies. Orthopedics 1995; 18(9):885-8.
- Bauer TW Particles and periimplant bone resorption. Clin Orthop 2002; (405):138-43.
- Bauer TW, Shanbhag AS Are there biological markers of wear? J Am Acad Orthop Surg 2008; 16 Suppl 1:S68-S71.
- Bauer TW, Taylor SK, Jiang M, Medendorp SV An indirect comparison of third-body wear in retrieved hydroxyapatite-coated, porous, and cemented femoral components. Clin Orthop 1994; (298):11-8.
- Bellare A, Cohen RE. Morphology of rod stock and compression-moulded sheets of ultra-high-molecular-weight polyethylene used in orthopaedic implants. Biomaterials 1996; 17(24):2325-33.
- Benko TZ, Santiago-Martin A, Ruddlesdin C, Selzer G. Aseptic loosening of 2 rim-fix, hydroxyapatite-coated acetabular cups. J Arthroplasty 2002; 17(4):519-23.
- Benz EB, Federman M, Godleski JJ, Bierbaum BE, Thornhill TS, Spector M. Transmission electron microscopy of intracellular particles of polyethylene from joint replacement prostheses: size distribution and cellular response. Biomaterials 2001; 22(21):2835-42.
- Blacha J. High osteolysis and revision rate with the hydroxyapatite-coated ABG hip prostheses: 65 hips in 56 young patients followed for 5-9 years. Acta Orthop Scand 2004; 75(3):276-82.
- Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986; 1(8476):307-10.
- Bloebaum RD, Beeks D, Dorr LD, Savory CG, DuPont JA, Hofmann AA Complications with hydroxyapatite particulate separation in total hip arthroplasty. Clin Orthop 1994; (298):19-26.
- Bloebaum RD, Zou L, Bachus KN, Shea KG, Hof-mann AA, Dunn HK Analysis of particles in acetabular components from patients with osteolysis. Clin Orthop 1997; (338):109-18.
- Blumenthal NC, Posner AS. Hydroxyapatite: mechanism of formation and properties. Calcif Tissue Res 1973; 13(3):235-43.
- Bobyn JD, Tanzer M, Krygier JJ, Dujovne AR, Brooks CE Concerns with modularity in total hip arthroplasty. Clin Orthop 1994; (298):27-36.
- Borlin N, Rohrl SM, Bragdon CR RSA wear measurements with or without markers in total hip arthroplasty. J Biomech 2005 Jun 27.
- Borlin N, Thien T, Karrholm J. The precision of radiostereometric measurements. Manual vs. digital measurements. J Biomech 2002; 35(1):69-79.
- Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, . Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. Clin Biochem 2003; 36(1):2-7.
- Bourne RB, Rorabeck CH, Patterson JJ, Guerin J Tapered titanium cementless total hip replacements: a 10- to 13-year followup study. Clin Orthop 2001; (393):112-20.
- Bragdon CR, Estok DM, Malchau H, Karrholm J, Yuan X, Bourne R, . Comparison of two digital radiostereometric analysis methods in the determination of femoral head penetration in a total hip replacement phantom. J Orthop Res 2004; 22(3):659-64.
- Bragdon CR, Malchau H, Yuan X, Perinchief R, Karrholm J, Borlin N, . Experimental assessment of precision and accuracy of radiostereometric analysis for the determination of polyethylene wear in a total hip replacement model. J Orthop Res 2002; 20(4):688-95.
- Bragdon CR, Martell JM, Greene ME, Estok DM, Thanner J, Karrholm J, Comparison of femoral head penetration using RSA and the Martell method. Clin Orthop 2006; (448):52-7.
- Bragdon CR, Thanner J, Greene ME, Malchau H, Digas G, Harris WH, Standing versus supine radiographs in RSA evaluation of femoral head penetration. Clin Orthop 2006; (448)9:46-51.
- Cales B Zirconia as a sliding material: histologic, laboratory, and clinical data. Clin Orthop 2000; (379):94-112.
- Callaghan JJ, Brown TD, Pedersen DR, Johnston RC. Orthopaedic crossfire--Larger femoral heads: a triumph of hope over reason! In the affirmative. J Arthroplasty 2003; 18(3 Suppl 1):82-4.
- Canales Cortes V, Panisello Sebastia JJ, Herrera RA, Peguero BA, Martinez MA, Herrero BL, . Ten-year follow-up of an anatomical hydroxyapatite-coated total hip prosthesis. Int Orthop 2006; 30(2):84-90.
- Capello WN, D’Antonio JA, Manley MT, Feinberg JR Hydroxyapatite in total hip arthroplasty. Clinical results and critical issues. Clin Orthop 1998; (355):200-11.
- Case CP, Langkamer VG, James C, Palmer MR, Kemp AJ, Heap PF, . Widespread dissemination of metal debris from implants. J Bone Joint Surg Br 1994; 76(5):701-12.
- Case CP, Langkamer VG, Lock RJ, Perry MJ, Palmer MR, Kemp AJ. Changes in the proportions of peripheral blood lymphocytes in patients with worn implants. J Bone Joint Surg Br 2000; 82(5):748-54.
- Chambers B, St Clair SF, Froimson MI. Hydroxyapatite-coated tapered cementless femoral components in total hip arthroplasty. J Arthroplasty 2007; 22(4 Suppl 1):71-4.
- Chang JK, Chen CH, Huang KY, Wang GJ. Eight-year results of hydroxyapatite-coated hip arthroplasty. J Arthroplasty 2006; 21(4):541-6.
- Charnley J. Anchorage of the femoral head prosthesis to the shaft of the femur. J Bone Joint Surg Br 1960; 42-B:28-30.
- Charnley J. A sterile-air operating theatre enclosure. Br J Surg 1964; 51:195-202.
- Charnley J, Cupic Z The nine and ten year results of the low-friction arthroplasty of the hip. Clin Orthop 1973; (95):9-25.
- Charnley J, Halley DK Rate of wear in total hip replacement. Clin Orthop 1975; (112):170-9.
- Charnley J, Kamangar A, Longfield MD. The optimum size of prosthetic heads in relation to the wear of plastic sockets in total replacement of the hip. Med Biol Eng 1969; 7(1):31-9.
- Chiba J, Rubash HE, Kim KJ, Iwaki Y The characterization of cytokines in the interface tissue obtained from failed cementless total hip arthroplasty with and without femoral osteolysis. Clin Orthop 1994; (300):304-12.
- Childs LM, Paschalis EP, Xing L, Dougall WC, Anderson D, Boskey AL, . In vivo RANK signaling blockade using the receptor activator of NFkappaB: Fc effectively prevents and ameliorates wear debris-induced osteolysis via osteoclast depletion without inhibiting osteogenesis. J Bone Miner Res 2002; 17(2):192-9.
- Clarke IC, Brown S, Pezzotti G, Green DD, Donaldson TD Undesirable phase transformations in Zirconia hip balls - mechanical and hydrothermal trigger mechanics. Abstract, 53rd Annual Meeting of the Orthopaedic Research Society, San Diego, February 2007.
- Clarke IC, Manaka M, Green DD, Williams P, Pezzotti G, Kim YH, . Current status of zirconia used in total hip implants. J Bone Joint Surg Am 2003; 85-A473-84.
- Clarke JC, Black K, Rennie C, Amstutz HC Can wear in total hip arthroplasties be assessed from radiographs? Clin Orthop 1976; (121):126-42.
- Claus AM, Walde TA, Leung SB, Wolf RL, Engh CA, Sr Sr. Management of patients with acetabular socket wear and pelvic osteolysis. J Arthroplasty 2003; 18(3 Suppl 1):112-7.
- Coathup MJ, Blackburn J, Goodship AE, Cunningham JL, Smith T, Blunn GW. Role of hydroxyapatite coating in resisting wear particle migration and osteolysis around acetabular components. Biomaterials 2005; 26(19):4161-9.
- Cohen J, Wulff J. Clinical failure caused by corrosion of a vitallium plate. Case report, new testing methods for crevice corrosion, and new techniques for fashioning cobalt chromium alloys to be used in surgical implants. J Bone Joint Surg Am 1972; 54(3):617-28.
- Cohn RM, Gonzalez D, V, Peterson M, Cornell CN. Similar wear in total hip arthroplasties with metallic or zirconia femoral heads. HSS J 2008; 4(2):107-11.
- Collier JP, Sutula LC, Currier BH, Currier JH, Wooding RE, Williams IR, Overview of polyethylene as a bearing material: comparison of sterilization methods. Clin Orthop 1996; (333):76-86.
- Collier MB, Kraay MJ, Rimnac CM, Goldberg VM. Evaluation of contemporary software methods used to quantify polyethylene wear after total hip arthroplasty. J Bone Joint Surg Am 2003; 85-A(12):2410-8.
- Cooper RA, McAllister CM, Borden LS, Bauer TW. Polyethylene debris-induced osteolysis and loosening in uncemented total hip arthroplasty. A cause of late failure. J Arthroplasty 1992; 7(3):285-90.
- Cuckler JM, Bearcroft J, Asgian CM Femoral head technologies to reduce polyethylene wear in total hip arthroplasty. Clin Orthop 1995; (317):57-63.
- Danish Hip Arthroplasty Registry Yearly Report, 2008.
- Daugaard H, Elmengaard B, Bechtold JE, Jensen T, Soballe K The effect on bone growth enhancement of implant coatings with hydroxyapatite and collagen deposited electrochemically and by plasma spray. J Biomed Mater Res A 2009 Mar 16.
- Delank KS, Drees P, Menzel N, Hansen T, Duschner H, Eckardt A. Increased polyethylene wear after cementless ABG I total hip arthroplasty. Arch Orthop Trauma Surg 2006; 126(8):509-16.
- DeLee JG, Charnley J Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop 1976; (121):20-32.
- Derbyshire B, Fisher J, Dowson D, Hardaker C, Brummitt K. Comparative study of the wear of UHMWPE with zirconia ceramic and stainless steel femoral heads in artificial hip joints. Med Eng Phys 1994; 16(3):229-36.
- Devane PA, Bourne RB, Rorabeck CH, Hardie RM, Horne JG Measurement of polyethylene wear in metal-backed acetabular cups. I. Three-dimensional technique. Clin Orthop 1995; (319):303-16.
- Devane PA, Bourne RB, Rorabeck CH, MacDonald S, Robinson EJ Measurement of polyethylene wear in metal-backed acetabular cups. II. Clinical application. Clin Orthop 1995; (319):317-26.
- Devane PA, Horne JG Assessment of polyethylene wear in total hip replacement. Clin Orthop 1999; (369):59-72.
- Digas G, Karrholm J, Thanner J, Herberts P. 5-year experience of highly cross-linked polyethylene in cemented and uncemented sockets: two randomized studies using radiostereometric analysis. Acta Orthop 2007; 78(6):746-54.
- Digas G, Karrholm J, Thanner J, Malchau H, Herberts P Highly cross-linked polyethylene in cemented THA: randomized study of 61 hips. Clin Orthop 2003; (417):126-38.
- Dorr LD, Wan Z Ten years of experience with porous acetabular components for revision surgery. Clin Orthop Relat Res 1995; (319):191-200.
- Dowd JE, Sychterz CJ, Young AM, Engh CA. Characterization of long-term femoral-head-penetration rates. Association with and prediction of osteolysis. J Bone Joint Surg Am 2000; 82-A(8):1102-7.
- Duffy P, Sher JL, Partington PF. Premature wear and osteolysis in an HA-coated, uncemented total hip arthroplasty. J Bone Joint Surg Br 2004; 86(1):34-8.
- Dumbleton JH, Manley MT, Edidin AA. A literature review of the association between wear rate and osteolysis in total hip arthroplasty. J Arthroplasty 2002; 17(5):649-61.
- Ebramzadeh E, Sangiorgio SN, Lattuada F, Kang JS, Chiesa R, McKellop HA, . Accuracy of measurement of polyethylene wear with use of radiographs of total hip replacements. J Bone Joint Surg Am 2003; 85-A(12):2378-84.
- Endo M, Tipper JL, Barton DC, Stone MH, Ingham E, Fisher J. Comparison of wear, wear debris and functional biological activity of moderately crosslinked and non-crosslinked polyethylenes in hip prostheses. Proc Inst Mech Eng [H] 2002; 216(2):111-22.
- Engh CA, Jr. Jr., Sychterz CJ, Young AM, Pollock DC, Toomey SD, Engh CA, Sr Sr. Interobserver and intraob-server variability in radiographic assessment of osteolysis. J Arthroplasty 2002; 17(6):752-9.
- Eskelinen A, Remes V, Helenius I, Pulkkinen P, Nevalainen J, Paavolainen P. Uncemented total hip arthroplasty for primary osteoarthritis in young patients: a mid-to long-term follow-up study from the Finnish Arthroplasty Register. Acta Orthop 2006; 77(1):57-70.
- Foss OA, Klaksvik J, Benum P, Anda S. Polyethylene acetabular wear in hip prostheses: computer-simulated quantification of error caused by changes in pelvic orientation and direction of wear. Acta Orthop 2008; 79(5):618-23.
- Frokjaer J, Deleuran B, Lind M, Overgaard S, Soballe K, Bunger C. Polyethylene particles stimulate monocyte chemotactic and activating factor production in synovial mononuclear cells in vivo. An immunohistochemical study in rabbits. Acta Orthop Scand 1995; 66(4):303-7.
- Gallo J, Langova K, Havranek V, Cechova I. Poor survival of abg I hip prosthesis in younger patients. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub 2008; 152(1):163-8.
- Geerdink CH, Grimm B, Vencken W, Heyligers IC, Tonino AJ. The determination of linear and angular penetration of the femoral head into the acetabular component as an assessment of wear in total hip replacement: A comparison of four computer-assisted methods. J Bone Joint Surg Br 2008; 90(7):839-46.
- Gelb H, Schumacher HR, Cuckler J, Ducheyne P, Baker DG. In vivo inflammatory response to polymethylmethacrylate particulate debris: effect of size, morphology, and surface area. J Orthop Res 1994; 12(1):83-92.
- Glyn-Jones S, Lardy-Smith P, Gill HS, Murray DW. The creep and wear of highly cross-linked polyethylene: a three-year randomised, controlled trial using radiostereometric analysis. J Bone Joint Surg Br 2008; 90(5):556-61.
- Goldring SR, Jasty M, Roelke MS, Rourke CM, Bringhurst FR, Harris WH. Formation of a synovial-like membrane at the bone-cement interface. Its role in bone resorption and implant loosening after total hip replacement. Arthritis Rheum 1986; 29(7):836-42.
- Goldring SR, Schiller AL, Roelke M, Rourke CM, O’Neil DA, Harris WH. The synovial-like membrane at the bone-cement interface in loose total hip replacements and its proposed role in bone lysis. J Bone Joint Surg Am 1983; 65(5):575-84.
- Grobbelaar CJ, du Plessis TA, Marais F. The radiation improvement of polyethylene prostheses. A preliminary study. J Bone Joint Surg Br 1978; 60-B(3):370-4.
- Grobbelaar CJ, Weber FA, Spirakis A. Clinical experiences with gammairradiation cross-linked polyethylene. A 14 to 20 yeasrs follow-up report. S Afr Bone Joint Surgery 1999; 9:140-7.
- Gross KA, Berndt CC, Goldschlag DD, Iacono VJ. In vitro changes of hydroxyapatite coatings. Int J Oral Maxillofac Implants 1997; 12(5):589-97.
- Halley DK, Charnley J Results of low friction arthroplasty in patients thirty years of age or younger. Clin Orthop 1975; (112):180-91.
- Haraguchi K, Sugano N, Nishii T, Miki H, Oka K, Yoshikawa H. Phase transformation of a zirconia ceramic head after total hip arthroplasty. J Bone Joint Surgery Br 2001; 83-B(7):996-1000.
- Hardinge K, Porter ML, Jones PR, Hukins DW, Taylor CJ. Measurement of hip prostheses using image analysis. The maxima hip technique. J Bone Joint Surg Br 1991; 73(5):724-8.
- Harris WH Wear and periprosthetic osteolysis: the problem. Clin Orthop 2001; (393):66-70.
- Harris WH, Maloney WJ Hybrid total hip arthroplasty. Clin Orthop 1989; (249):21-9.
- Havelin LI, Engesaeter LB, Espehaug B, Furnes O, Lie SA, Vollset SE. The Norwegian arthroplasty register: 11 years and 73,000 arthroplasties. Acta Orthop Scand 2000; 71(4):337-53.
- Head WC, Bauk DJ, Emerson RH, Jr Jr Titanium as the material of choice for cementless femoral components in total hip arthroplasty. Clin Orthop 1995; (311):85-90.
- Head WC, Emerson RH, Jr. Jr., Higgins LL. A titanium cementless calcar replacement prosthesis in revision surgery of the femur: 13-year experience. J Arthroplasty 2001; 16(8 Suppl 1):183-7.
- Heimke G Ceramics. Handbook of biomaterials evaluation. Scientific, technical, and clinical testing of implant materials. In: von Recum AF, editor. Macmillian Publishing Company, New York.; 1986. p. 38-54.
- Hernigou P, Bahrami T. Zirconia and alumina ceramics in comparison with stainless-steel heads. Polyethylene wear after a minimum ten-year follow-up. J Bone Joint Surg Br 2003; 85(4):504-9.
- Heuer AH. Transformation Toughening in Zro2-Containing Ceramics. Journal of the American Ceramic Society 1987; 70(10):689-98.
- Hildebrand H, Aronson S, Kullendorff CM, Selvik G. Roentgen stereophotogrammetric short-term analysis of growth rate in children operated for Crohn’s disease. Acta Paediatr Scand 1991; 80(10):917-23.
- Hirakawa K, Bauer TW, Hashimoto Y, Stulberg BN, Wilde AH, Secic M. Effect of femoral head diameter on tissue concentration of wear debris. J Biomed Mater Res 1997; 36(4):529-35.
- Hopper RH, Jr. Jr., Young AM, Engh CA, Jr. Jr., McAuley JP. Assessing the pattern of femoral head penetration after total hip arthroplasty. J Arthroplasty 2004; 19(7 Suppl 2):22-9.
- Hui AJ, McCalden RW, Martell JM, MacDonald SJ, Bourne RB, Rorabeck CH. Validation of two and three-dimensional radiographic techniques for measuring polyethylene wear after total hip arthroplasty. J Bone Joint Surg Am 2003; 85-A(3):505-11.
- Hultmark P, Hostner J, Herberts P, Karrholm J. Radiographic evaluation of Charnley cups used in first-time revision: repeated observations for 7-15 years. J Arthroplasty 2003; 18(8):1005-15.
- Hummer CD, III III, Rothman RH, Hozack WJ. Catastrophic failure of modular zirconia-ceramic femoral head components after total hip arthroplasty. J Arthroplasty 1995; 10(6):848-50.
- Hurschler C, Seehaus F, Emmerich J, Kaptein BL, Windhagen H. Comparison of the model-based and marker-based Roentgen Stereophotogrammetry methods in a typical clinical setting. J Arthroplasty 2009; 24(4):594-606.
- Ilchmann T, Franzen H, Mjoberg B, Wingstrand H. Measurement accuracy in acetabular cup migration. A comparison of four radiologic methods versus roentgen stereophotogrammetric analysis. J Arthroplasty 1992; 7(2):121-7.
- Ilchmann T, Mjoberg B, Wingstrand H. Measurement accuracy in acetabular cup wear. Three retrospective methods compared with Roentgen stereophotogrammetry. J Arthroplasty 1995; 10(5):636-42.
- Imai S, Konttinen YT, Jumppanen M, Lindy O, Ceponis A, Kemppinen P, . High levels of expression of collagenase-3 (MMP-13) in pathological conditions associated with a foreign-body reaction. J Bone Joint Surg Br 1998; 80(4):701-10.
- Ingham E, Fisher J. The role of macrophages in osteolysis of total joint replacement. Biomaterials 2005; 26(11):1271-86.
- International Epidemiological Association Inc A Dictionary of Epidemiology. Third Edition ed. Oxford University Press, 1995.
- Isaac DL, Forder J, Skyrme AD, James SE. The Biomet Bi-Metric total hip arthroplasty and universal acetabular cup: high polyethylene failure rate in the medium term. J Arthroplasty 2007; 22(5):697-700.
- Ise K, Kawanabe K, Matsusaki T, Shimizu M, Onishi E, Nakamura T. Patient sensitivity to polyethylene particles with cemented total hip arthroplasty. J Arthroplasty 2007; 22(7):966-73.
- Jarcho M Calcium phosphate ceramics as hard tissue prosthetics. Clin Orthop 1981; (157):259-78.
- Jasty M, Goetz DD, Bragdon CR, Lee KR, Hanson AE, Elder JR, . Wear of polyethylene acetabular components in total hip arthroplasty. An analysis of one hundred and twenty-eight components retrieved at autopsy or revision operations. J Bone Joint Surg Am 1997; 79(3):349-58.
- Kadoya Y, Revell PA, Al-Saffar N, Kobayashi A, Scott G, Freeman MA. Bone formation and bone resorption in failed total joint arthroplasties: histomorphometric analysis with histochemical and immunohistochemical technique. J Orthop Res 1996; 14(3):473-82.
- Kang JS, Park SR, Ebramzadeh E, Dorr LD. Measurement of polyethylene wear in total hip arthroplasty--accuracy versus ease of use. Yonsei Med J 2003; 44(3):473-8.
- Kaptein BL, Valstar ER, Spoor CW, Stoel BC, Rozing PM. Model-based RSA of a femoral hip stem using surface and geometrical shape models. Clin Orthop 2006; 448:92-7.
- Kaptein BL, Valstar ER, Stoel BC, Rozing PM, Reiber JH. A new model-based RSA method validated using CAD models and models from reversed engineering. J Biomech 2003; 36(6):873-82.
- Kaptein BL, Valstar ER, Stoel BC, Rozing PM, Reiber JH. Evaluation of three pose estimation algorithms for model-based roentgen stereophotogrammetric analysis. Proc Inst Mech Eng [H ] 2004; 218(4):231-8.
- Karrholm J. Roentgen stereophotogrammetry. Review of orthopedic applications. Acta Orthop Scand 1989; 60(4):491-503.
- Karrholm J, Gill RH, Valstar ER. The history and future of radiostereometric analysis. Clin Orthop 2006; 448:10-21.
- Karrholm J, Hansson LI, Selvik G Longitudinal growth rate of the distal tibia and fibula in children. Clin Orthop 1984; (191):121-8.
- Karrholm J, Herberts P, Hultmark P, Malchau H, Nivbrant B, Thanner J Radiostereometry of hip prostheses: Review of methodology and clinical results. Clin Orthop 1997; (344):94-110.
- Kim SY, Kim DH, Kim YG, Oh CW, Ihn JC. Early failure of hemispheric hydroxyapatite-coated acetabular cups. Clin Orthop Relat Res 2006; 446:233-8.
- Kim YH. Comparison of polyethylene wear associated with cobalt-chromium and zirconia heads after total hip replacement. A prospective, randomized study. J Bone Joint Surg Am 2005; 87(8):1769-76.
- Kim YH, Kim JS. Tribological and material analyses of retrieved alumina and zirconia ceramic heads correlated with polyethylene wear after total hip replacement. J Bone Joint Surg Br 2008; 90(6):731-7.
- Kiss J, Murray DW, Turner-Smith AR, Bulstrode CJ. Roentgen stereophotogrammetric analysis for assessing migration of total hip replacement femoral components. Proc Inst Mech Eng [H ] 1995; 209(3):169-75.
- Kobayashi A, Donnelly WJ, Scott G, Freeman MA. Early radiological observations may predict the long-term survival of femoral hip prostheses. J Bone Joint Surg Br 1997; 79(4):583-9.
- Kobayashi A, Freeman MA, Bonfield W, Kadoya Y, Yamac T, Al-Saffar N, . Number of polyethylene particles and osteolysis in total joint replacements. A quantitative study using a tissue-digestion method. J Bone Joint Surg Br 1997; 79(5):844-8.
- Koreny T, Tunyogi-Csapo M, Gal I, Vermes C, Jacobs JJ, Glant TT. The role of fibroblasts and fibroblast-derived factors in periprosthetic osteolysis. Arthritis Rheum 2006; 54(10):3221-32.
- Kraay MJ, Thomas RD, Rimnac CM, Fitzgerald SJ, Goldberg VM. Zirconia versus Co-Cr femoral heads in total hip arthroplasty: early assessment of wear. Clin Orthop 2006; 453:86-90.
- Krismer M, Bauer R, Tschupik J, Mayrhofer P. EBRA: a method to measure migration of acetabular components. J Biomech 1995; 28(10):1225-36.
- Kumar P, Oka M, Ikeuchi K, Shimizu K, Yamamuro T, Okumura H, . Low wear rate of UHMWPE against zirconia ceramic (Y-PSZ) in comparison to alumina ceramic and SUS 316L alloy. J Biomed Mater Res 1991; 25(7):813-28.
- Kurtz SM, Muratoglu OK, Evans M, Edidin AA. Advances in the processing, sterilization, and crosslinking of ultra-high molecular weight polyethylene for total joint arthroplasty. Biomaterials 1999; 20(18):1659-88.
- Kwong LM, O’Connor DO, Sedlacek RC, Krushell RJ, Maloney WJ, Harris WH. A quantitative in vitro assessment of fit and screw fixation on the stability of a cementless hemispherical acetabular component. J Arthroplasty 1994; 9(2):163-70.
- Learmonth ID, Hussell JG, Smith EJ. Inadequate polyethylene thickness and osteolysis in cementless hip arthroplasty. J Arthroplasty 1997; 12(3):305-9.
- Lee JM, Lee CW. Comparison of hydroxyapatite-coated and non-hydroxyapatite-coated noncemented total hip arthroplasty in same patients. J Arthroplasty 2007; 22(7):1019-23.
- Lieberman JR, Kay RM, Hamlet WP, Park SH, Kabo JM. Wear of the polyethylene liner-metallic shell interface in modular acetabular components. An in vitro analysis. J Arthroplasty 1996; 11(5):602-8.
- Livermore J, Ilstrup D, Morrey B. Effect of femoral head size on wear of the polyethylene acetabular component. J Bone Joint Surg Am 1990; 72(4):518-28.
- Lombardi AV, Jr. Jr., Berend KR. Isolated acetabular liner exchange. J Am Acad Orthop Surg 2008; 16(5):243-8.
- Lucht U. The Danish Hip Arthroplasty Register. Acta Orthop Scand 2000; 71(5):433-9.
- Malchau H Introducing new technology: a stepwise algorithm. Spine 2000 1; 25(3):285.
- Malchau H, Karrholm J, Wang YX, Herberts P. Accuracy of migration analysis in hip arthroplasty. Digitized and conventional radiography, compared to radiostereometry in 51 patients. Acta Orthop Scand 1995; 66(5):418-24.
- Malizos K, Roidis NT, Poultsides L, Basdekis G, Moraitis T, Xenakis T Protrusio of a ceramic femoral head through the acetabular metallic shell, extensive metallosis and ’bubble sign’. Orthopedics 2009; 32(2).
- Mallory TH, Lombardi AVJr., Fada RA, Adams JB, Kefauver CA, Eberle RW Noncemented acetabular component removal in the presence of osteolysis: the affirmative. Clin Orthop 2000; (381):120-8.
- Manley MT, D’Antonio JA, Capello WN, Edidin AA Osteolysis: a disease of access to fixation interfaces. Clin Orthop 2002; (405):129-37.
- Mariconda M, Silvestro A, Mansueto G, Marino D Complete polyethylene wear-through and secondary breakage of the expansion cup in a ceramic-polyethylene total hip arthroplasty. Arch Orthop Trauma Surg 2009 26.
- Martell JM, Berdia S. Determination of polyethylene wear in total hip replacements with use of digital radiographs. J Bone Joint Surg Am 1997; 79(11):1635-41.
- Martell JM, Leopold SS, Liu X. The effect of joint loading on acetabular wear measurement in total hip arthroplasty. J Arthroplasty 2000; 15(4):512-8.
- Masonis JL, Bourne RB, Ries MD, McCalden RW, Salehi A, Kelman DC. Zirconia femoral head fractures: a clinical and retrieval analysis. J Arthroplasty 2004; 19(7):898-905.
- Matthews JB, Besong AA, Green TR, Stone MH, Wroblewski BM, Fisher J, . Evaluation of the response of primary human peripheral blood mono-nuclear phagocytes to challenge with in vitro generated clinically relevant UHMWPE particles of known size and dose. J Biomed Mater Res 2000; 52(2):296-307.
- Matthews JB, Green TR, Stone MH, Wroblewski BM, Fisher J, Ingham E. Comparison of the response of primary human peripheral blood mononuclear phagocytes from different donors to challenge with model polyethylene particles of known size and dose. Biomaterials 2000; 21(20):2033-44.
- Maxian SH, Zawadsky JP, Dunn MG. In vitro evaluation of amorphous calcium phosphate and poorly crystallized hydroxyapatite coatings on titanium implants. J Biomed Mater Res 1993; 27(1):111-7.
- McCalden RW, Naudie DD, Yuan X, Bourne RB. Radiographic methods for the assessment of polyethylene wear after total hip arthroplasty. J Bone Joint Surg Am 2005; 87(10):2323-34.
- McDonald MD, Bloebaum RD. Distinguishing wear and creep in clinically retrieved polyethylene inserts. J Biomed Mater Res 1995; 29(1):1-7.
- McKellop H, Shen FW, Lu B, Campbell P, Salovey R. Development of an extremely wear-resistant ultra high molecular weight polyethylene for total hip replacements. J Orthop Res 1999; 17(2):157-67.
- McKellop H, Shen FW, Lu B, Campbell P, Salovey R. Effect of sterilization method and other modifications on the wear resistance of acetabular cups made of ultra-high molecular weight polyethylene. A hip-simulator study. J Bone Joint Surg Am 2000; 82-A(12):1708-25.
- Mclean CR, Dabis H, Mok D. Delayed fracture of the ceramic femoral head after trauma. J Arthroplasty 2002; 17(4):503-4.
- Meijerink HJ, Gardeniers JW, Buma P, Lemmens JA, Schreurs BW Hydroxyapatite does not improve the outcome of a bipolar hemiarthroplasty. Clin Orthop 2004; (421):143-50.
- Michaud RJ, Rashad SY. Spontaneous fracture of the ceramic ball in a ceramic-polyethylene total hip arthroplasty. J Arthroplasty 1995; 10(6):863-7.
- Moilanen T, Stocks GW, Freeman MA, Scott G, Goodier WD, Evans SJ. Hydroxyapatite coating of an acetabular prosthesis. Effect on stability. J Bone Joint Surg Br 1996; 78(2):200-5.
- Moore KD, Barrack RL, Sychterz CJ, Sawhney J, Yang AM, Engh CA. The effect of weight-bearing on the radiographic measurement of the position of the femoral head after total hip arthroplasty. J Bone Joint Surg Am 2000; 82(1):62-9.
- Morscher EW, Hefti A, Aebi U. Severe osteolysis after third-body wear due to hydroxyapatite particles from acetabular cup coating. J Bone Joint Surg Br 1998; 80(2):267-72.
- Morscher EW, Hefti A, Aebi U. Severe osteolysis after third-body wear due to hydroxyapatite particles from acetabular cup coating. J Bone Joint Surg Br 1998; 80(2):267-72.
- Murray DW, Rushton N. Macrophages stimulate bone resorption when they phagocytose particles. J Bone Joint Surg Br 1990; 72(6):988-92.
- Murray DW, Rushton N Mediators of bone resorption around implants. Clin Orthop 1992; (281):295-304.
- Nordsletten L, Hogasen AK, Konttinen YT, Santa-virta S, Aspenberg P, Aasen AO. Human monocytes stimulation by particles of hydroxyapatite, silicon carbide and diamond: in vitro studies of new prosthesis coatings. Biomaterials 1996; 17(15):1521-7.
- O’Brien JJ, Burnett RS, McCalden RW, MacDonald SJ, Bourne RB, Rorabeck CH. Isolated liner exchange in revision total hip arthroplasty: clinical results using the direct lateral surgical approach. J Arthroplasty 2004; 19(4):414-23.
- Ohlin A, Selvik G. Socket wear assessment. A comparison of three different radiographic methods. J Arthroplasty 1993; 8(4):427-31.
- Ohnsorge JA, Davis J, Maus U, Saklak M, Weisskopf M, Wirtz DC. Early polyethylene wear and excessive acetabular granuloma in an uncemented HA-coated total hip arthroplasty-midterm results of a prospective study. HSS J 2006; 2(2):114-20.
- Oldenburg M, Wegner R, Baur X. Severe Cobalt Intoxication Due to Prosthesis Wear in Repeated Total Hip Arthroplasty. J Arthroplasty 2009; 24(5):825.
- Onsten I, Carlsson AS, Besjakov J. Wear in uncemented porous and cemented polyethylene sockets: a randomised, radiostereometric study. J Bone Joint Surg Br 1998; 80(2):345-50.
- Onsten I, Carlsson AS, Sanzen L, Besjakov J. Migration and wear of a hydroxyapatite-coated hip pros-thesis. A controlled roentgen stereophotogrammetric study. J Bone Joint Surg Br 1996; 78(1):85-91.
- Oonishi H, Kadoya Y. Wear of high-dose gamma-irradiated polyethylene in total hip replacements. J Orthop Sci 2000; 5(3):223-8.
- Oparaugo PC, Clarke IC, Malchau H, Herberts P. Correlation of wear debris-induced osteolysis and revision with volumetric wear-rates of polyethylene: a survey of 8 reports in the literature. Acta Orthop Scand 2001; 72(1):22-8.
- Oral E, Christensen SD, Malhi AS, Wannomae KK, Muratoglu OK. Wear resistance and mechanical properties of highly cross-linked, ultrahigh-molecular weight polyethylene doped with vitamin E. J Arthroplasty 2006; 21(4):580-91.
- Oral E, Godleski Beckos CA, Lozynsky AJ, Malhi AS, Muratoglu OK. Improved resistance to wear and fatigue fracture in high pressure crystallized vitamin E-containing ultra-high molecular weight polyethylene. Biomaterials 2009; 30(10):1870-80.
- Oral E, Greenbaum ES, Malhi AS, Harris WH, Muratoglu OK. Characterization of irradiated blends of alpha-tocopherol and UHMWPE. Biomaterials 2005; 26(33):6657-63.
- Oral E, Wannomae KK, Hawkins N, Harris WH, Muratoglu OK. Alpha-tocopherol-doped irradiated UHMWPE for high fatigue resistance and low wear. Biomaterials 2004; 25(24):5515-22.
- Orishimo KF, Claus AM, Sychterz CJ, Engh CA. Relationship between polyethylene wear and osteolysis in hips with a second-generation porous-coated cementless cup after seven years of follow-up. J Bone Joint Surg Am 2003; 85-A(6):1095-9.
- Orishimo KF, Sychterz CJ, Hopper RH, Jr. Jr., Engh CA. Can component and patient factors account for the variance in wear rates among bilateral total hip arthroplasty patients? J Arthroplasty 2003; 18(2):154-60.
- Overgaard S, Bromose U, Lind M, Bunger C, Soballe K. The influence of crystallinity of the hydroxyapatite coating on the fixation of implants. Mechanical and histomorphometric results. J Bone Joint Surg Br 1999; 81(4):725-31.
- Overgaard S, Lind M, Glerup H, Bunger C, Soballe K. Porous-coated versus grit-blasted surface texture of hydroxyapatite-coated implants during controlled micromotion: mechanical and histomorphometric results. J Arthroplasty 1998; 13(4):449-58.
- Overgaard S, Soballe K. Polyethylene wear, osteolysis and acetabular loosening with an HA-coated hip prosthesis. J Bone Joint Surg Br 2000; 82(2):305-6.
- Overgaard S, Soballe K, Josephsen K, Hansen ES, Bunger C. Role of different loading conditions on resorption of hydroxyapatite coating evaluated by histomorphometric and stereological methods. J Orthop Res 1996; 14(6):888-94.
- Overgaard S, Soballe K, Lind M, Bunger C. Resorption of hydroxyapatite and fluorapatite coatings in man. An experimental study in trabecular bone. J Bone Joint Surg Br 1997; 79(4):654-9.
- Pap T, Pap G, Hummel KM, Franz JK, Jeisy E, Sains-bury I, . Membrane-type-1 matrix metalloproteinase is abundantly expressed in fibroblasts and osteoclasts at the bone-implant interface of aseptically loosened joint arthroplasties in situ. J Rheumatol 1999; 26(1):166-9.
- Paulsen A, Pedersen AB, Johnsen SP, Riis A, Lucht U, Overgaard S. Effect of hydroxyapatite coating on risk of revision after primary total hip arthroplasty in younger patients: findings from the Danish Hip Arthroplasty Registry. Acta Orthop 2007; 78(5):622-8.
- Pedersen DR, Brown TD, Hillis SL, Callaghan JJ. Prediction of long-term polyethylene wear in total hip arthroplasty, based on early wear measurements made using digital image analysis. J Orthop Res 1998; 16(5):557-63.
- Piconi C, Maccauro G, Angeloni M, Rossi B, Lear-month ID. Zirconia heads in perspective: a survey of zirconia outcomes in total hip replacement. HIP Int 2007; 17(3):119-30.
- Quinn JM, Sabokbar A, Athanasou NA. Cells of the mononuclear phagocyte series differentiate into osteoclastic lacunar bone resorbing cells. J Pathol 1996; 179(1):106-11.
- Rahbek O, Overgaard S, Jensen TB, Bendix K, Soballe K. Sealing effect of hydroxyapatite coating: a 12-month study in canines. Acta Orthop Scand 2000; 71(6):563-73.
- Rahbek O, Overgaard S, Lind M, Bendix K, Bunger C, Soballe K. Sealing effect of hydroxyapatite coating on peri-implant migration of particles. An experimental study in dogs. J Bone Joint Surg Br 2001; 83(3):441-7.
- Ranstam J, Ryd L, Onsten I. Accurate accuracy assessment: review of basic principles. Acta Orthop Scand 2000; 71(1):106-8.
- Regis D, Sandri A, Costa A, Bartolozzi P, Mazzilli G. Recurrent femoral deep vein thrombosis: rare complication of a pelvic mass induced by polyethylene wear debris following total hip arthroplasty. A case report. Thromb Res 2008; 121(4):593-5.
- Reikeras O, Gunderson RB. Excellent results of HA coating on a grit-blasted stem: 245 patients followed for 8-12 years. Acta Orthop Scand 2003; 74(2):140-5.
- Reikeras O, Gunderson RB. Long-term results of HA coated threaded versus HA coated hemispheric press fit cups: 287 hips followed for 11 to 16 years. Arch Orthop Trauma Surg 2006; 126(8):503-8.
- Revell PA, Al-Saffar N, Kobayashi A. Biological reaction to debris in relation to joint prostheses. Proc Inst Mech Eng [H] 1997; 211(2):187-97.
- Ries MD, Scott ML, i S Relationship between gravi-metric wear and particle generation in hip simulators: conventional compared with cross-linked polyethylene. J Bone Joint Surg Am 2001; 83-A Suppl 2 Pt 2:116-22.
- Rohrl SM, Li MG, Nilsson KG, Nivbrant B. Very low wear of non-remelted highly cross-linked polyethylene cups: an RSA study lasting up to 6 years. Acta Orthop 2007; 78(6):739-45.
- Rohrl SM, Nivbrant B, Strom H, Nilsson KG. Effect of augmented cup fixation on stability, wear, and osteolysis: a 5-year follow-up of total hip arthroplasty with RSA. J Arthroplasty 2004; 19(8):962-71.
- Rokkum M, Brandt M, Bye K, Hetland KR, Waage S, Reigstad A. Polyethylene wear, osteolysis and acetabular loosening with an HA-coated hip prosthesis. A follow-up of 94 consecutive arthroplasties. J Bone Joint Surg Br 1999; 81(4):582-9.
- Rokkum M, Reigstad A. Total hip replacement with an entirely hydroxyapatite-coated prosthesis: 5 years’ follow-up of 94 consecutive hips. J Arthroplasty 1999; 14(6):689-700.
- Rokkum M, Reigstad A, Johansson CB. HA particles can be released from well-fixed HA-coated stems: histopathology of biopsies from 20 hips 2-8 years after implantation. Acta Orthop Scand 2002; 73(3):298-306.
- Rokkum M, Reigstad A, Johansson CB, Albrektsson T. Tissue reactions adjacent to well-fixed hydroxyapatite-coated acetabular cups. Histopathology of ten specimens retrieved at reoperation after 0.3 to 5.8 years. J Bone Joint Surg Br 2003; 85(3):440-7.
- Rose RM, Radin EL Wear of polyethylene in the total hip prosthesis. Clin Orthop 1982; (170):107-15.
- Roy ME, Whiteside LA, Katerberg BJ, Steiger JA, Nayfeh T Not all zirconia femoral heads degrade in vivo. Clin Orthop 2007; (465):220-6.
- Ryd L. Micromotion in knee arthroplasty. A roentgen stereophotogrammetric analysis of tibial component fixation. Acta Orthop Scand Suppl 1986; 220:1-80.
- Sabokbar A, Pandey R, Quinn JM, Athanasou NA. Osteoclastic differentiation by mononuclear phagocytes containing biomaterial particles. Arch Orthop Trauma Surg 1998; 117(3):136-40.
- Saikko V. Wear of Polyethylene Acetabular Cups Against Zirconia Femoral Heads Studied with A Hip-Joint Simulator. Wear 1994; 176(2):207-12.
- Saikko V, Nevalainen J, Revitzer H, Ylinen P. Metal release from total hip articulations in vitro: substantial from CoCr/CoCr, negligible from CoCr/PE and alumina/PE. Acta Orthop Scand 1998; 69(5):449-54.
- Santos EM, Vohra S, Catledge SA, McClenny MD, Lemons J, Moore KD. Examination of surface and material properties of explanted zirconia femoral heads. J Arthroplasty 2004; 19(7 SUPPL.):30-4.
- Schmalzried TP, Akizuki KH, Fedenko AN, Mirra J. The role of access of joint fluid to bone in periarticular osteolysis. A report of four cases. J Bone Joint Surg Am 1997; 79(3):447-52.
- Schmalzried TP, Brown IC, Amstutz HC, Engh CA, Harris WH. The role of acetabular component screw holes and/or screws in the development of pelvic osteolysis. Proc Inst Mech Eng [H] 1999; 213(2):147-53.
- Schmalzried TP, Campbell P, Schmitt AK, Brown IC, Amstutz HC. Shapes and dimensional characteristics of polyethylene wear particles generated in vivo by total knee replacements compared to total hip replacements. J Biomed Mater Res 1997; 38(3):203-10.
- Schmalzried TP, Dorey FJ, McKellop H. The multi-factorial nature of polyethylene wear in vivo. J Bone Joint Surg Am 1998; 80(8):1234-42.
- Schmalzried TP, Guttmann D, Grecula M, Amstutz HC. The relationship between the design, position, and articular wear of acetabular components inserted without cement and the development of pelvic osteolysis. J Bone Joint Surg Am 1994; 76(5):677-88.
- Schmalzried TP, Huk OL Patient factors and wear in total hip arthroplasty. Clin Orthop 2004; (418):94-7.
- Schmalzried TP, Jasty M, Harris WH. Periprosthetic bone loss in total hip arthroplasty. Polyethylene wear debris and the concept of the effective joint space. J Bone Joint Surg Am 1992; 74(6):849-63.
- Schmalzried TP, Jasty M, Harris WH. Periprosthetic bone loss in total hip arthroplasty. Polyethylene wear debris and the concept of the effective joint space. J Bone Joint Surg Am 1992; 74(6):849-63.
- Schmalzried TP, Shepherd EF, Dorey FJ, Jackson WO, dela RM, Fa’vae F, The John Charnley Award. Wear is a function of use, not time. Clin Orthop 2000; (381):36-46.
- Schmauder S, Schubert H. Significance of Internal-Stresses for the Martensitic-Transformation in Yttria-Stabilized Tetragonal Zirconia Polycrystals During Degradation. Journal of the American Ceramic Society 1986; 69(7):534-40.
- Selvik G. Roentgen stereophotogrammetry. A method for the study of the kinematics of the skeletal system. Acta Orthop Scand 1989; Suppl 232:1-51.
- Selvik G, Alberius P, Aronson AS. A roentgen stereo-photogrammetric system. Construction, calibration and technical accuracy. Acta Radiol Diagn (Stockh) 1983; 24(4):343-52.
- Shanbhag AS, Jacobs JJ, Glant TT, Gilbert JL, Black J, Galante JO. Composition and morphology of wear debris in failed uncemented total hip replacement. J Bone Joint Surg Br 1994; 76(1):60-7.
- Shaver SM, Brown TD, Hillis SL, Callaghan JJ. Digital edge-detection measurement of polyethylene wear after total hip arthroplasty. J Bone Joint Surg Am 1997; 79(5):690-700.
- Sherman RA, Damron TA Penetration of a metallic femoral head through the acetabular shell. J Arthroplasty 2008 Oct 8.
- Smith PN, Ling RS, Taylor R. The influence of weight-bearing on the measurement of polyethylene wear in THA. J Bone Joint Surg Br 1999; 81(2):259-65.
- Soballe K. Hydroxyapatite ceramic coating for bone implant fixation. Mechanical and histological studies in dogs. Acta Orthop Scand 1993; Suppl 255:1-58.
- Soballe K, Hansen ES, Brockstedt-Rasmussen H, Bunger C. Hydroxyapatite coating converts fibrous tissue to bone around loaded implants. J Bone Joint Surg Br 1993; 75(2):270-8.
- Soballe K, Hansen ES, Brockstedt-Rasmussen H, Hjortdal VE, Juhl GI, Pedersen CM, Gap healing enhanced by hydroxyapatite coating in dogs. Clin Orthop 1991; (272):300-7.
- Soballe K, Hansen ES, Brockstedt-Rasmussen H, Pedersen CM, Bunger C Bone graft incorporation around titanium-alloy- and hydroxyapatite-coated implants in dogs. Clin Orthop 1992; (274):282-93.
- Soballe K, Hansen ES, Rasmussen H, Jorgensen PH, Bunger C. Tissue ingrowth into titanium and hydroxyapatite-coated implants during stable and unstable mechanical conditions. J Orthop Res 1992; 10(2):285-99.
- Soballe K, Overgaard S. The current status of hydroxyapatite coating of prostheses. J Bone Joint Surg Br 1996; 78(5):689-91.
- Soballe K, Overgaard S, Hansen ES, Brokstedt-Rasmussen H, Lind M, Bunger C. A review of ceramic coatings for implant fixation. J Long Term Eff Med Implants 1999; 9(1-2):131-51.
- Soballe K, Toksvig-Larsen S, Gelineck J, Fruensgaard S, Hansen ES, Ryd L, . Migration of hydroxyapatite coated femoral prostheses. A Roentgen Stereophotogrammetric study. J Bone Joint Surg Br 1993; 75(5):681-7.
- Sochart DH Relationship of acetabular wear to osteolysis and loosening in total hip arthroplasty. Clin Orthop 1999; (363):135-50.
- Stilling M, Nielsen KA, Soballe K, Rahbek O Clinical Comparison of Polyethylene Wear with Zirconia or Cobalt-Chromium Femoral Heads. Clin Orthop 2009; (467):2644-50. Epub 2009 Mar 27.
- Stilling M, Rahbek O, Soballe K Inferior survival of hydroxyapatite versus titanium-coated cups at 15 years. Clin Orthop 2009 Mar 28.
- Sun L, Berndt CC, Khor KA, Cheang HN, Gross KA. Surface characteristics and dissolution behavior of plasma-sprayed hydroxyapatite coating. J Biomed Mater Res 2002; 62(2):228-36.
- Sutula LC, Collier JP, Saum KA, Currier BH, Currier JH, Sanford WM, The Otto Aufranc Award. Impact of gamma sterilization on clinical performance of polyethylene in the hip. Clin Orthop 1995; (319):28-40.
- Sychterz CJ, Engh CA, Jr. Jr., Swope SW, McNulty DE, Engh CA Analysis of prosthetic femoral heads retrieved at autopsy. Clin Orthop 1999; (358):223-34.
- Sychterz CJ, Engh CA, Jr. Jr., Yang A, Engh CA. Analysis of temporal wear patterns of porous-coated acetabular components: distinguishing between true wear and so-called bedding-in. J Bone Joint Surg Am 1999; 81(6):821-30.
- Sychterz CJ, Orishimo KF, Engh CA. Sterilization and polyethylene wear: clinical studies to support laboratory data. J Bone Joint Surg Am 2004; 86-A(5):1017-22.
- Sychterz CJ, Yang AM, McAuley JP, Engh CA Two-dimensional versus three-dimensional radiographic measurements of polyethylene wear. Clin Orthop 1999; (365):117-23.
- Sychterz CJ, Young AM, Engh CA Effect of radio-graphic quality on computer-assisted head penetration measurements. Clin Orthop 2001; (386):150-8.
- Tanko LB, Mouritzen U, Lehmann HJ, Warming L, Moelgaard A, Christgau S, . Oral ibandronate: changes in markers of bone turnover during adequately dosed continuous and weekly therapy and during different suboptimally dosed treatment regimens. Bone 2003; 32(6):687-93.
- Terefenko KM, Sychterz CJ, Orishimo K, Engh CA, Sr Sr. Polyethylene liner exchange for excessive wear and osteolysis. J Arthroplasty 2002; 17(6):798-804.
- Thanner J. The acetabular component in total hip arthroplasty. Evaluation of different fixation principles. Acta Orthop Scand 1999; Suppl 286:1-41.
- Thanner J, Freij-Larsson C, Karrholm J, Malchau H, Wesslen B. Evaluation of Boneloc. Chemical and mechanical properties, and a randomized clinical study of 30 total hip arthroplasties. Acta Orthop Scand 1995; 66(3):207-14.
- The B, Flivik G, Diercks RL, Verdonschot N A new method to make 2-D wear measurements less sensitive to projection differences of cemented THAs. Clin Orthop 2008; (466):684-90.
- Urban RM, Jacobs JJ, Tomlinson MJ, Gavrilovic J, Black J, Peoc’h M. Dissemination of wear particles to the liver, spleen, and abdominal lymph nodes of patients with hip or knee replacement. J Bone Joint Surg Am 2000; 82(4):457-76.
- Valstar ER, de Jong FW, Vrooman HA, Rozing PM, Reiber JH. Model-based Roentgen stereophotogrammetry of orthopaedic implants. J Biomech 2001; 34(6):715-22.
- Valstar ER, Vrooman HA, Toksvig-Larsen S, Ryd L, Nelissen RG. Digital automated RSA compared to manually operated RSA. J Biomech 2000; 33(12):1593-9.
- Van der Vis H, Aspenberg P, Marti RK, Tigchelaar W, Van Noorden CJ Fluid pressure causes bone resorption in a rabbit model of prosthetic loosening. Clin Orthop 1998; (350):201-8.
- Vickers AJ How many repeated measures in repeated measures designs? Statistical issues for comparative trials. BMC Med Res Methodol 2003 Oct 27; 3:22.
- von Schewelov T, Carlsson A, Dahlberg L. Cross-linked N-telopeptide of type I collagen (NTx) in urine as a predictor of periprosthetic osteolysis. J Orthop Res 2006; 24(7):1342-8.
- von Schewelov T, Onsten I, Markusson P, Carlsson A. Weight bearing radiographs are not necessary for measurement of polyethylene penetration in total hip prostheses: a radiostereometric study of 111 patients examined in weight-bearing and supine position. Acta Orthop 2006; 77(1):104-8.
- von Schewelov T, Sanzen L, Borlin N, Markusson P, Onsten I Accuracy of radiographic and radiostereometric wear measurement of different hip prostheses - An experimental study. Acta Orthop Scand 2004; 75(6):691-700.
- von Schewelov T, Sanzen L, Onsten I, Carlsson A. Catastrophic failure of an uncemented acetabular component due to high wear and osteolysis: an analysis of 154 omnifit prostheses with mean 6-year follow-up. Acta Orthop Scand 2004; 75(3):283-94.
- von Schewelov T, Sanzen L, Onsten I, Carlsson A, Besjakov J. Total hip replacement with a zirconium oxide ceramic femoral head: a randomised roentgen stereophotogrammetric study. J Bone Joint Surg Br 2005; 87(12):1631-5.
- Vrooman HA, Valstar ER, Brand GJ, Admiraal DR, Rozing PM, Reiber JH. Fast and accurate automated measurements in digitized stereophotogrammetric radiographs. J Biomech 1998; 31(5):491-8.
- Walter WL, Walter WK, O’Sullivan M. The pumping of fluid in cementless cups with holes. J Arthroplasty 2004; 19(2):230-4.
- Wan Z, Dorr LD. Natural history of femoral focal osteolysis with proximal ingrowth smooth stem implant. J Arthroplasty 1996; 11(6):718-25.
- Wilkinson JM, Hamer AJ, Rogers A, Stockley I, Eas-tell R. Bone mineral density and biochemical markers of bone turnover in aseptic loosening after total hip arthroplasty. J Orthop Res 2003; 21(4):691-6.
- Wilkinson JM, Hamer AJ, Stockley I, Eastell R. Polyethylene wear rate and osteolysis: critical threshold versus continuous dose-response relationship. J Orthop Res 2005; 23(3):520-5.
- Willert HG. Reactions of the articular capsule to wear products of artificial joint prostheses. J Biomed Mater Res 1977; 11(2):157-64.
- Williams DF Difinitions in biomaterials. Progress in biomedical engineering. Elsevier Science Publishers, B.V., Amsterdam, New York; 1987.
- Williams DF, Black J, Doherty. P.J. Second consensus conference on definitions in biomaterials. Biomaterials-tissue interfaces, Advances in biomaterials, 525-533. 1992. London, Elsevier Publishers.
- Won CH, Hearn TC, Tile M. Micromotion of cementless hemispherical acetabular components Does press-fit need adjunctive screw fixation? . J Bone Joint Surg Br 1995; 77(3):484-9.
- Wooley PH, Nasser S, Fitzgerald RH, Jr Jr The immune response to implant materials in humans. Clin Orthop 1996; (326):63-70.
- Wroblewski BM. Direction and rate of socket wear in Charnley low-friction arthroplasty. J Bone Joint Surg Br 1985; 67(5):757-61.
- Yamaguchi M, Bauer TW, Hashimoto Y. Three-dimensional analysis of multiple wear vectors in retrieved acetabular cups. J Bone Joint Surg Am 1997; 79(10):1539-44.
- Yamaguchi M, Hashimoto Y, Akisue T, Bauer TW. Polyethylene wear vector in vivo: a three-dimensional analysis using retrieved acetabular components and radiographs. J Orthop Res 1999; 17(5):695-702.
- Zicat B, Engh CA, Gokcen E. Patterns of osteolysis around total hip components inserted with and without cement. J Bone Joint Surg Am 1995; 77(3):432-9.