| Abbreviations | ||
| BMD | = | bone mineral density |
| BMP | = | bone morphogenic protein |
| cox | = | cyclooxygenase |
| CDMP | = | cartilage-derived morphogenic protein |
| DEXA | = | dual energy x-ray absorptiometry |
| HO | = | heterotopic ossification |
| ip | = | intraperitoneal |
| mcl | = | medial collateral ligament |
| NSAID | = | non steroid anti inflammatory drug |
| PG | = | prostaglandin |
| PGHS | = | prostaglandin endoperoxide synthase |
Introduction
Cox inhibitors, both conventional NSAIDs and the specific cox-2 inhibitors, have commonly been used in musculoskeletal trauma and orthopedic surgery to reduce inflammatory responses and pain. These drugs are demonstrated to be efficient in the treatment of postoperative pain related to orthopedic surgery Citation1-7.
However, concerns in using cox inhibitors in orthopedic fracture treatment have been raised due to their possible negative effect on fracture healing Citation8-11, and similar concerns have also been expressed regarding soft tissue healing Citation12,13.
The present thesis was prepared on the basis of the continuing discussion in the literature to elucidate the effects of cox inhibitors on fracture healing, tendon healing and tendon-to-bone healing.
Tissue healing
Bone
Traditionally, the healing of fractures has been divided into primary (direct) and secondary (indirect) fracture healing. Primary healing occurs in presence of anatomic reduction and rigid fixation. The majority of fractures heal by secondary healing, and this process has been divided into five stages; hematoma formation, inflammation, formation of soft callus, formation of hard callus and remodeling Citation14. During recent years, researchers have increasingly focused on the cell and molecular biology of fracture healing. Distinct responses of fracture healing take place in all four components involved at the injury site; the bone marrow, cortex, periosteum and external soft tissue Citation15.
Overall, fracture healing is a complex physiological process, and remains to a great extent an unknown cascade of complex biological events. Intracellular and extracellular molecular signaling are involved, and systemic and local factors like growth factors, hormones and cytokines interact at the fracture site Citation16. The process involves thousands of activated genes; the transcriptional steps are required for the regulation of the inflammatory response, chondrogenesis, osteogenesis and remodeling Citation17:5. The initial days after fracture represent the fundamental stage of the healing process, and the role of the inflammation is important in initiating the repair process Citation18.
Tendon
Normal tendon healing occurs in three overlapping phases. In the initial inflammatory phase, erythrocytes and inflammatory cells invade the site of injury. This phase lasts for 4 to 7 days, and is followed by the proliferative phase. Within the first week, fibroblasts fill the gap between the two tendon stumps, and capillaries grow into the gap. Collagen synthesis may begin as early as on the third day and continues over the next 5 to 6 weeks. In the following remodeling phase, consolidation of the tissue from cellular to fibrous develops and at last maturation of the fibrous tissue to a more scar-like tendon tissue occurs. Collagen fibers near the tendon ends begin to align along the long axis of the tendon, and collagen organization and cross-linking continue over the course of a year. However, after 6 months, there are usually only minimal histological differences between repair tissue and normal tissue Citation19,20.
Tendon-to-bone
Tendons are attached to bone through either fibrocartilaginous or fibrous insertions. In fibrocartilaginous insertions, typically seen where tendons attach to epiphyses, a characteristic 4-layered architecture is seen, consisting of tendon, non-mineralized fibrocartilage, mineralized fibrocartilage and bone Citation21,22. Fibrous insertions characteristically occur where tendons and ligaments are attached to diaphyseal bone either as a periosteal insertion where the collagen fibers penetrate the bone indirectly via the periosteum, or as a bony insertion where they attach directly to the bone without showing fibrocartilage layers Citation23. When healing occurs between tendon and bone inside a bone tunnel, a fibrous insertion develops. Initially, a fibrous layer of scar tissue separates tendon and bone Citation24 and inflammatory cells are also present Citation25. Later, collagen fibers from the cancellous bone protrude through this fibrous tissue directly into the tendon in the presence of predominantly fibroblastic cells and less inflammatory cells Citation24,25. In a dog model there is continuity between the collagen fibers of the tendon and the surrounding trabecular bone after 26 weeks Citation24, indicating that a complete healing has occurred.
In contrast, in another study in rats, a tendon was mechanically healed in a bone tunnel after 6 weeks Citation26. When testing, the specimens ruptured at the muscle-tendon junction rather than the healing tendon-to-bone junction, maybe due to an intact suture supporting the reattachment during testing and not the healing alone. Most likely more time is needed before solid healing is also achieved in rats, but the time aspect remains uncertain.
Prostaglandin and cyclooxygenase
Prostaglandin (PG) was first identified by von Eular in 1936 Citation27. Piper and Vane asserted in 1971 that PGs was released from different tissues after stimulation of a PG biosynthesis Citation28.
In the acute inflammation cascade the synthesis of PGs begins with release of arachidonate from membrane phospholipids. Cyclooxygenase (cox), previously named prostaglandin endoperoxide synthase (PGHS), is the key enzyme required for the conversion of arachidonic acid to prostaglandin Citation29. Other eicosanoids, like thromboxane, are also synthesized by cox, and a total number of more than 100 related substances derived from arachidonic acid are recognized Citation30. Three cox isoforms have been identified, cox-1 Citation31, cox-2 Citation32 and cox-3 Citation33. The cox-3 isoform is detected in the central nervous system and is sensitive to inhibition by acetaminophen/paracetamol; the role of the cox-3 is far from elucidated Citation30. The cox-1 enzyme is produced constitutively in nearly all tissues and is mainly responsible for the biosynthesis of PGs involved in homeostatic regulation. Cox-2 is inducible and primarily involved in producing PGs in response to a wide spectrum of environmental insults and internal stimuli; i.e. in an acute inflammation Citation34.
After a fracture, the release of prostaglandins increase Citation35. Dead cells in the area results in an aseptic inflammatory response, and without this inflammation the bone resorption and formation necessary for healing cannot occur adequately Citation36. PGs have a direct effect on bone resorption through increased osteoclastic activity Citation37-41. Furthermore, PGs increase the replication and differentiation of the osteoblasts, resulting in enhanced bone formation Citation38,41. Thus, PGs are partly responsible for ensuring the balance between bone resorption and bone formation Citation34,42,43.
It is demonstrated that cox-2 plays a critical role in bone resorption Citation44, and induction of cox-2 in osteoblasts is reported to be essential to the acute stress response in a bone remodeling system Citation45. Furthermore, cox-2 is demonstrated to be specifically essential for fracture healing Citation46 and required for both intramembranous and endochondral bone formation Citation47. The inflammation is present in the early phase after fracture, and the cox-2 mRNA level shows peak expression during the first 2 weeks and a return to basal levels by 3 weeks.
Similarly, it is demonstrated that cox-2 regulates early stages of myofiber growth during muscle regeneration Citation48, and that cox-2 is important in the muscle healing process Citation49.
Cox inhibitors
Cox inhibitors are used in the treatment of inflammation and pain. Conventional cox inhibitors, the non steroid anti inflammatory drugs (NSAIDs), inhibit both the cox-1 and cox-2 enzymes. The newer, selective cox-2 inhibitors mainly target the cox-2 enzyme.
Vane demonstrated in 1971 that the therapeutic effects of aspirin and indomethacin were due to inhibition of the synthesis of prostaglandins Citation50. Other NSAIDs were developed, including naproxen, ibuprofen, piroxicam, diclofenac, ketorolac and others. After the discovery of the cox-2 enzyme Citation32, and the fact that cox-2 was inducible Citation51, the individual variations of activity against cox-1 and cox-2 between the different NSAIDs were elucidated Citation52,53. Two selective cox-2 inhibitors were introduced in 1999, celecoxib and rofecoxib, they are potent inhibitors of cox-2 and weaker inhibitors of cox-1 Citation30. Other cox-2 inhibitors have later been developed, such as valdecoxib, parecoxib, etoricoxib and others.
Cox inhibitors show a wide range of side effects, with variations between conventional cox inhibitors and cox-2 inhibitors, and also between the different conventional cox inhibitors and the different cox-2 inhibitors. This variation is probably due to the cox inhibitors variable potency against cox-1 and cox-2. It has been demonstrated that piroxicam and indomethacin show high gastrointestinal toxicity Citation54, and these drugs have a much higher potency against cox-1 than cox-2 Citation55.
Fewer serious gastrointestinal adverse events with rofecoxib compared to naproxen were reported Citation56. Furthermore, the same study demonstrated that the incidence of myocardial infarctions was higher in patients who were administered rofecoxib, leading to the withdrawal of rofecoxib from the market.
Cannon et al. demonstrated, however, that the rate of thrombotic cardiovascular events in patients with arthritis and treated with etoricoxib was similar to those in patients on diclofenac, when considering patients after long-term use of these drugs Citation57. Warner and Mitchell also guard against the increasingly prevalent idea that conventional cox inhibitors have inherently lower cardiovascular risks than the specific cox-2 inhibitors Citation58.
Use of cox inhibitors in orthopedic surgery and trauma
Cox inhibitors have been demonstrated to be efficient in the treatment of postoperative pain related to orthopedic surgery Citation1-7, and are widely in use to reduce opioid analgesia. In a review article the authors conclude that opioid consumption in all included trials was reduced on average by 35 % with cox-2 inhibitors Citation59. However, it was not possible to demonstrate any definite clinically beneficial effect with reduced opioid related adverse events like nausea.
Cox-2 inhibitors are advantageous in relation to surgery as they do not impair the platelet activation and aggregation which is mediated by cox-1 through the generation of thromboxane A2 Citation60. Therefore they are safer concerning perioperative bleedings Citation61-65. Cox-2 inhibitors are at least as effective against postoperative pain as the conventional cox inhibitors Citation66-68, and should probably be the drug of choice when cox inhibitors are indicated.
Recently 21 peer-reviewed articles and abstracts concerning the perioperative use of NSAIDs and cox-2 inhibitors were retracted after having been identified as fraudulent Citation69-72. These retractions also compromise every systematic review, meta-analysis and editorial that have included these fabricated data, and among other conclusions following these retractions, it was concluded that there is no longer evidence supporting the preemptive effect of NSAIDs and cox-2 inhibitors when administered before surgery Citation73.
Due to this, the entire area of perioperative pain management should be reassessed.
Effects of cox inhibitors on tissue healing
Cox inhibitors effects on fracture healing
It is well documented in experimental studies that cox inhibitors have a negative effect on bone metabolism and impair fracture healing, both for conventional cox inhibitors like indomethacin Citation74-80, ibuprofen Citation81-83, naproxen Citation84, ketorolac Citation85-87 and diclofenac Citation88, but also for newer specific cox-2 inhibitors like celecoxib Citation46,89,90, rofecoxib Citation46,84, etodolac Citation91, valdecoxib Citation92 and parecoxib Citation85. The cox-2 enzyme is critically involved in bone repair and required for both intramembranous and endochondral bone formation Citation47, the cox-2 function is specifically essential for fracture healing Citation46. This indicates that inhibition of the cox-2 enzyme is responsible for the impaired healing.
Even short-term administration of cox inhibitors have a negative effect on the healing process Citation88,89,93 indicating that the initial period after a fracture is important. However, the negative effect seems to be reversible after short-term administration Citation92,93
Clinical evidence of the effects on bone healing is sparse. In a retrospective study on femoral shaft fractures a marked association between nonunion and the use of NSAIDs after injury was noted Citation94. Delayed healing was also observed in patients administered NSAIDs and whose fractures had united.
A good clinical trial on this subject is a study published by Burd Citation95, which observed a patient population randomly allocated into groups of indomethacin treatment, radiation or no treatment to evaluate heterotopic ossification after surgical treatment of acetabular fracture Citation96. These patients had concomitant long bone fractures distributed equally into the three groups, and there was a significant increase of nonunion in patients who received indomethacin compared to those who did not receive indomethacin. There were no nonunions in the acetabular fractures, however, indicating that fractures known to be vulnerable to nonunion or delayed union are exposed when administered cox inhibitors, but not fractures with fewer healing problems in mainly trabecular bone. This is further illustrated by two studies published showing no effect of cox inhibitors on fractures of the distal radius (Colles' fracture) Citation97,98. Nonunions following distal fractures of the radius are extremely rare, and only a few cases have been reported in the literature Citation99.
Cox inhibitors effects on tendon healing
The effects of cox inhibitors on tendon healing are less studied than fracture healing. It has been demonstrated that ibuprofen produced a deleterious effect on tendon rupture strength and impaired the healing process Citation100. In the healing process, tendon cells migrate to the injury site, proliferate and synthesize collagen. In an experimental study, celecoxib inhibited both the tendon cell migration and proliferation Citation101. It has also been demonstrated that indomethacin might have a negative effect on tendon healing in the early proliferative phase, but might be beneficial in the remodeling phase Citation102. This is in accordance with the findings in Virchenko's study, where parecoxib had a negative effect in the early phase of tendon healing, but during remodeling inflammation appeared to have a negative influence Citation103. This is probably the reason why indomethacin and celecoxib was reported to increase the failure stress in a healing tendon Citation104. In that study the cox inhibitors were administered until termination after 14 days. This is probably far into the remodeling phase in rats, when cox inhibitors may have a positive influence on healing. It is still unclear, however, how long these healing processes take in human compared to rat, but Virchenko suggests that a week in rats corresponds to a month in humans Citation103.
Cox inhibitors effects on tendon-to-bone healing
To achieve normal tendon-to-bone healing, bone ingrowth and mineralization are required Citation24. To our knowledge, only 2 experimental studies on cox inhibitors' effects on tendon-to-bone healing have been previously published. Cohen's study demonstrated that indomethacin and celecoxib impaired rotator cuff tendon-to-bone healing Citation105, confirmed with both mechanical testing and histological analysis. The cox inhibitors appeared to inhibit the formation and maturation of collagen at the tendon attachment site. In Ferry's study they also demonstrated a detrimental effect on the healing strength at the patellar tendon insertion, when celecoxib, valdecoxib and piroxicam were administered; no effect was seen with ibuprofen and naproxen. A detrimental effect on collagen production was seen with celecoxib, valdecoxib, piroxicam and naproxen, but not with ibuprofen Citation106. They suggested that the different absorption and metabolism between the drugs and the uncertainties of oral drug administration could explain the different inhibition effects between the conventional cox inhibitors.
Cox inhibitors effects on heterotopic ossification
Heterotopic ossification (HO), also named ectopic bone formation, is a common complication following implant surgery, i.e. hip replacement surgery Citation107. The pathogenesis of HO is unclear, but trauma to the soft tissue appears to induce the process. The process is similar to that observed during fracture healing with an initial acute inflammation Citation108.
Cox inhibitors have been widely used to prevent HO. Several review articles have been published during recent years confirming preventive effects Citation109-111, including a Cochrane review Citation112. The effect is best documented in total hip replacements Citation4,112-115, but cox inhibitors are also effective in preventing HO in acetabular fractures Citation96 and after spinal injuries Citation116. Indomethacin has been the drug of choice, but other conventional cox inhibitors have been shown to be effective as well Citation114,115,117,118.
During the last years, the specific cox-2 inhibitors have also proved to be as effective as conventional cox inhibitors Citation119-121. And indeed, findings do indicate that the formation of HO is mainly mediated by cox-2 Citation122.
Traditionally, cox inhibitors have been administered for a period of 4–6 weeks postoperatively in prevention of HO, but short-term administration for 2–10 days also seems to be effective Citation118,123,124.
Cox inhibitors effects on spinal fusion
Indomethacin has been shown to inhibit posterior spinal fusions in the rat Citation125 and the rabbit Citation87. Celecoxib delayed allograft healing and incorporation in femoral shaft fractures in mice Citation126. In a retrospective clinical study published on spinal fusion, a higher incidence of nonunion was found in patients receiving ketorolac, and an even higher incidence if they also smoked cigarettes Citation127. Corresponding findings were observed in another study, but here the patients used cox inhibitors over a longer period Citation128. In contrast, ketorolac did not increase the incidence of nonunions in adolescents Citation129. However, the incidence of nonunions in adolescents was as low as 2.5%, indicating a substantial healing potential in young patients.
Cox inhibitors effects on implant incorporation
Several studies have demonstrated impaired bone ingrowth into porous implants Citation130-132, and it is also demonstrated that cox-2 activity is essential for the osseointegration in a titanium implant Citation133. The detrimental effect of perioperatively administered indomethacin on bone ingrowth was transient in yet another study Citation134, but the model was unloaded. In a clinical setting, restricted weight-bearing might be prudent.
It is suggested that the most important cause of implant loosening is osteolysis due to an inflammatory reaction to particulate wear debris, in which particles of polyethylene, metal, and bone cement debris are phagocytosed by macrophages. This process is shown to be cox-2 dependent, and cox-2 contributes to accelerated bone loss in wear debris-induced osteolysis. It is also demonstrated that a cox-2 inhibitor inhibited titanium-induced PGE2 production and inflammation, and due to these effects, cox-2 inhibitors might reduce bone loss in inflammation-induced bone disease Citation135. Accordingly, cox inhibitors may have a positive effect on the prevention of implant loosening. However, titanium wear debris will probably appear some time after the implantation of an implant. Due to this, administration of cox inhibitors should perhaps be avoided during the first days postoperatively to secure bone ingrowth, but administered after some time to prevent wear debris osteolysis.
In a clinical study, there were no radiographical differences after 6 years in patients administered indomethacin 100 mg daily in 6 weeks to prevent HO after cementless total hip prosthesis Citation136. Equivalently, the migration of cementless acetabular implants were not different in patients administered with indomethacin 100 mg daily in 2 weeks to prevent HO compared with patients receiving irradiation or the control group Citation137. However, in yet another clinical study on HO prevention after cemented hip arthroplasty, there were significantly more revisions due to aseptic loosening or periprosthetic fractures in patients administered ibuprofen 400 mg 3 times daily in 7 or 21 days compared to the placebo Citation138.
Recently it was demonstrated that celecoxib did not affect fixation in total knee replacement after 2 years Citation139. However, the prostheses were fixed with bone cement. Due to this, fixation of the implants did not depend on bone ingrowth, and the cox-2 inhibitor could safely be administered.
Cox inhibitors effects on ligament healing
The literature supports neither a positive nor negative influence of cox inhibitors on ligament healing. In one study it was demonstrated that piroxicam increased the early healing strength of the medial collateral ligament (mcl) in rats after short-term administration, but the final strength was not affected Citation140. In another study, the short-term administration of celecoxib impaired the early healing of mcl in rats Citation141. Short-term administration of a specific cox-1 inhibitor (SC-560) did not have any influence on healing of the mcl in rats Citation142. When comparing short-term administration of different cox inhibitors, other analgesics and a placebo, piroxicam was found to increase the healing strength of mcl in rats, but no difference in healing strength was found in rats administered with naproxen, rofecoxib, butorphanol (opiate) or acetaminophen Citation143. In rabbits, no difference in strength was demonstrated in the healing of mcl after 2 weeks of ibuprofen administration Citation144.
It is demonstrated in several studies that early motion improves the healing of the mcl Citation145-147. Treatment with cox inhibitor after mcl injury could, due to the analgesic effect, improve early motion, and may thereby influence healing. However, it is demonstrated that analgesics in general do not appear to affect ligament healing in rats Citation143. Uncertainties related to oral administration, absorption of the drugs and degree of cox inhibition might explain some of the diversity in experimental findings.
Cox inhibitors effects on muscle healing
Piroxicam might delay muscle regeneration in the early period Citation148. It has been demonstrated, using both pharmacological and genetic approaches that the cox-2 pathway regulated early stages of myofiber growth during muscle regeneration, and that a cox inhibitor reduced the number of myoblasts Citation48. Also, the cox-2 pathway plays an important role in muscle healing, and prostaglandins are key mediators of the cox-2 pathway Citation149.
Other studies suggest a negative effect on myofiber growth with both decreased myonuclear addition and decreased inflammation, and a direct regulation of muscle cell activity by cox-2. Cox-2 activity is important for myofiber growth in multiple physiological conditions Citation49.
Aims of the studies
Conventional NSAIDs and specific cox-2 inhibitors are widely administered in relation to orthopedic trauma and surgery. Controversy exists as to whether these drugs have a negative effect on healing in bone and tendons. It has also been questioned whether the newer cox-2 inhibitors are safer than conventional NSAIDs, which mainly operate as cox-1 inhibitors. Furthermore, a common opinion has been that short-term administration of these drugs related to trauma or surgery is safe regarding tissue healing. The aims of the present studies have been to elucidate these effects of cox inhibitors. The following questions were particularly emphasized:
Is there a negative effect of short-term administration of the cox-2 inhibitor parecoxib and the conventional NSAID indomethacin on fracture healing?
Is there a negative effect of short-term administration of the cox-2 inhibitor parecoxib and the conventional NSAID indomethacin on tendon healing?
Is there a negative effect of short-term administration of the cox-2 inhibitor parecoxib and the conventional NSAID indomethacin on tendon-to-bone healing?
Summary of papers
Paper I – Negative effect of parecoxib on bone mineral during fracture healing in rats
Dimmen S, Nordsletten L, Engebretsen L, Steen H, Madsen JE. Acta Orthop 2008; 79(3): 438-44.
BMD at the fracture site up to 6 weeks and mechanical properties 6 weeks after fracture were studied in rats administered parecoxib for 1 week perioperatively. The aim of the study was to investigate the effect of short-term administration of parecoxib on BMD and fracture healing. The rats were administered parecoxib intraperitoneally (ip) for 7 days after a closed standardized tibial shaft fracture stabilized with an intramedullary nail. Placebo animals were administered saline. BMD at the fracture site was measured 2, 3 and 6 weeks after surgery. The rats were killed after 6 weeks, and the fractures were mechanically tested. We found that BMD was lower in the parecoxib group compared to the placebo group, but there were no statistically significant differences in mechanical properties between the parecoxib group and the placebo group.
Paper II – Parecoxib and indomethacin delay early fracture healing: a study in rats
Dimmen S, Nordsletten L, Madsen JE. Clin Orthop Relat Res 2009; 467(8): 1992-9.
BMD at the fracture site up to 3 weeks and mechanical properties 3 weeks after fracture were studied in rats administered parecoxib or indomethacin for 1 week perioperatively. The same fracture model as in Paper I was used. The aim of the study was to further investigate the early effect of short-term administration of parecoxib on BMD and early fracture healing. The rats were administered parecoxib or indomethacin ip for 7 days after a closed standardized tibial shaft fracture that was stabilized with an intramedullary nail, and placebo animals were administered saline. BMD at the fracture site was measured 2 and 3 weeks after surgery. The rats were killed after 3 weeks, and the fractures were mechanically tested. Parecoxib decreased BMD for 3 weeks after fracture, indomethacin for 2 weeks. Both parecoxib and indomethacin reduced the ultimate bending moment and the bending stiffness of the healing fractures after 3 weeks compared to the placebo group.
Paper III – Negative effects of parecoxib and indomethacin on tendon healing: an experimental study in rats
Dimmen S, Engebretsen L, Nordsletten L, Madsen JE. Knee Surg Sports Traumatol Arthrosc 2009; 17(7): 835-9.
Mechanical properties of healing tendons 2 weeks after injury were studied in rats administered parecoxib and indomethacin 1 week perioperatively. The aim of the study was to investigate the effect of short-term administration of parecoxib and indomethacin on tendon healing, utilizing an Achilles tendon transection model. The rats were killed after 2 weeks, and the tendon callus was measured and mechanical testing of the healing tendon was performed. We found that the transverse and sagittal diameters of the tendons were reduced in both the parecoxib group and the indomethacin group compared to placebo. Tensile strength in the healing tendons was reduced in rats administered both parecoxib and indomethacin, and stiffness was reduced in the parecoxib group.
Paper IV – The effect of parecoxib and indometacin on tendon-to-bone healing in a bone tunnel: an experimental study in rats
Dimmen S, Nordsletten L, Engebretsen L, Steen H, Madsen JE. J Bone Joint Surg Br 2009; 91(2): 259-63.
Mechanical properties of the healing tendon-to-bone interface in a bone tunnel 2 weeks after surgery were studied in rats administered parecoxib and indomethacin 1 week perioperatively. The aim of the study was to investigate the effect of short-term administration of parecoxib and indomethacin on tendon-to-bone healing in a bone tunnel. The rats were killed after 2 weeks, and mechanical testing of the healing tendon-to-bone interface was performed. We found that pullout strength, energy absorption and stiffness in the healing tendons-to-bone interface were reduced in rats administered parecoxib, and pullout strength and energy absorption were reduced in animals administered indomethacin compared to the placebo group.
General discussion
Ethical considerations
All four studies in this thesis are experimental studies in rats. Unfortunately, neither in vitro models to study the healing processes, nor human methods to test mechanical properties exist. Thus, animal studies were chosen. All studies were performed using animal models previously published from our group Citation150-154, and the number of animals was kept to a minimum in accordance with the statistical power analysis. Specially educated personnel supervised the animals on a daily basis. All experiments conformed to and were consented by the Norwegian Council of Animal Research Code for the Care and Use of Animals for Experimental Purposes.
In pharmaceutical studies one should be aware of the ethical considerations related to connections between scientists and the pharmaceutical industry. There may be a great divergence between the companies' aims and those of science Citation155. Large amounts of money are involved; in the year 2000 nearly 45 million prescriptions were written for celecoxib and rofecoxib in the United States alone Citation156. Scientists behind many studies received large grants or funds from the pharmaceutical companies, or they may even be employees of those companies. Recently it was revealed that at least 21 of anesthesiologist Scott Reuben's published papers were pure fiction (Scientific American, March, 2009 (http://www.sciam.com/article.cfm?id=a-medical-madoff-anesthestesiologist-faked-data), and these studies have been retracted Citation69-72.
The work of this thesis was supported by grants from Smith & Nephew Research Fund, Nordic Medical Supply Arthroscopy Fund and the Norwegian Olympic Center.
Methodological considerations
Experimental animals
Rats of the Wistar strain were used. In male rats, there is a quick metabolism of the two cox-2 inhibitors rofecoxib and celecoxib Citation157,158. This might also apply to parecoxib, the cox-2 inhibitor used in our studies. To ensure a half-life comparable to that in humans, we only used female rats in the studies. Rats have been widely used in orthopedic experimental research, and their anatomy and physiology are well known. The bone remodeling in rats is comparable to that in humans Citation159, and tendon healing and tendon-to-bone healing are also assumed to resemble human healing. However, the healing processes seem to be much faster in rats Citation159, and 2 weeks is therefore a considerable healing time in rat models Citation103. The onset of puberty in female rats is at 5 weeks of age Citation160. In rat adulthood, each month is roughly equivalent to 2.5 human years Citation161. However, these time perspectives do not necessarily represent corresponding times in rats and humans regarding the tissue healing processes.
Our animal laboratories are modern with excellent facilities. In all studies the animals were kept in pairs in wire-topped plastic cages with free access to tap water and standard laboratory rodent food (with 1.1% calcium, 0.8 % phosphorus and 1500 IU/kg vitamin D3) in a 12-hour light and 12-hour dark cycle. The animals were taken care of by educated and professional personnel. In all studies the animals gained weight during the course of the experiments.
Drug administration
For surgery and bone density measurements the animals were anesthetized with a combination of Hypnorm (fluanisone 5 mg/mL, fentanyl citrate 0.1575 mg/mL) and Dormicum (midazolam 2.5 mg/mL). Temgesic (buprenorphin 0.3mg/mL) 0.01ml/100 g body weight was given subcutaneously as analgesic. The animals were killed with a phenobarbital overdose.
Parecoxib (Dynastat, Pfizer, Pharmacia Europe EEIG, Kent, Great Britain) is a potent and selective inhibitor of cox-2 Citation162,163 for parenteral use and therefore ideal for administration in animal studies. Parecoxib is also claimed to become the anti-inflammatory drug of choice for parenteral treatment of postoperative pain in the future Citation164-166. Indomethacin (Confortid, Dumex-Alpharma A/S, Copenhagen, Denmark) is primarily a cox-1 inhibitor Citation55 and the best documented conventional cox inhibitor to delay fracture healing Citation46,75,76. This drug is also available for parenteral use, and is thus suitable for animal studies.
The cox inhibitors were administered ip twice daily to ensure the best absorption and sufficient blood concentrations. An even better method to ensure constant plasma concentration of the drugs, would be to use implantable minipumps Citation93. Parecoxib was administered in a dose of 0.05 mg/100 g body weight in the morning and the evening for 7 days, the first injection immediately before surgery. Indomethacin was administered in a dose of 0.0625 mg/100 g body weight at the same time points. These doses are equivalent to human doses. The half-life of cox-2 inhibitors is shown to be 3–5 times higher in male rats compared to humans Citation157,158,167,168, whereas the half-life in female rats and humans are comparable Citation157,168. Thus we chose to administer human doses, corrected for weight, to female rats.
Comparing the effects of indomethacin with parecoxib, however, is associated with some uncertainties due to the rats' different metabolism of different cox inhibitors. Any demonstrated difference could be dose related, and not drug related. Plasma concentration measurements may have increased the value of the drug comparisons.
Tibial fracture model
In study I and II the right hindlimb of the rats was fractured using a specially designed fracture forceps Citation169. Prior to this, a reaming of the medullary canal was performed using an 18 G cannula (BD venflon Pro 18 GA 1.26 IN (1.3 × 32 mm)). Post fracture, this cannula stabilized the tibia, together with a smaller cannula (Braun Sterican 19 G/1,5 IN (1.20 × 40 mm)) passing through the largest one and advancing down towards the ankle joint. On the innermost level, the stylet of a 22 G spinal cannula (Braun Spinocan 22 G x 3 ½ IN (0.73 × 88 mm)) was inserted. This stylet was kept inside the medullary canal when the tibia was fractured to facilitate the reduction of the fracture during fixation Citation152. The rotation was only secured by the press fit effect of the cannulas. Rotational instability was not a problem in this model, neither in these studies nor in previous studies from our group.
Tendon rupture model
In Paper III the Achilles tendon of the right hindlimb was cut, and a 3 mm long segment was removed. The tendon was left unsutured Citation104. The plantaris tendon was left intact, but carefully dissected free from the healing Achilles tendon before mechanical testing. Whether maintaining the plantaris tendon leads to more or less weight bearing may be questioned. The plantaris tendon is strong in rats, and can take over some function after the Achilles tendon is injured. However, even if the animals are allowed full weight bearing, they generally protect their operated hind limb. Due to this, we believe that keeping the plantaris tendon intact may result in increased loading of the operated limb, and tendon loading has been shown to increase PG release, DNA and protein synthesis Citation102. This factor might have significance for the healing after treatment with cox inhibitors. In cox inhibitor treated animals this would probably not have the same importance due to the impaired PG production, but in placebo animals increased PG could possibly increase healing, resulting in larger differences between the groups. The animals in our study were allowed full weight bearing with no immobilization. All tendons healed. No time study was performed to elucidate if the healing tendons would have regained full strength during the later course of healing.
Tendon-to-bone model
In study IV, no previous model in the rat was available, to our knowledge. We therefore developed our own model in a pilot study to confirm the surgical technique and decide the time point of termination. In the pilot study we killed the animals and did the dissections and mechanical testing at three time points; 10, 14 and 18 days. After 14 days we observed satisfactory healing and the best conditions for soft tissue dissection. This was in accordance with a tendon-to-bone healing study demonstrating that the tendon was attached securely within the bone tunnel after 14 days Citation25. Time studies would of course have been of great interest to elucidate if the impaired healing was transient and the healing would be restored in the long term. Our main aim was to elucidate if the healing was impaired at an early time point as this might have a direct impact on patient mobilization after reconstructions using tendon grafts in bone tunnels. The Achilles tendon was dissected free from the calf muscle proximally and all muscle fibers were removed from the tendon. The tendon was then pulled through a 2 mm drill hole in distal tibia and secured anteriorly to the periosteum with a suture with the ankle joint held in 90 degrees. The animals were allowed full weight on their injured legs, with no immobilization. Therefore, the tendon was put under tension during mobilization. After 14 days the Achilles tendons were meticulously dissected free from the bone. The only remaining contact between tendon and bone was inside the drill hole. This was probably the most crucial point in the surgical procedure. 2 tendons from each group slipped out during the dissection, probably due to improper healing of the tendon in the bone tunnel. These specimens were excluded.
BMD measurements
Lunar PIXIMUS (Lunar, Madison, Wisconsin, USA) is specially designed for BMD measurements on small animals using Dual Energy X-ray Absorptiometry (DEXA). By using this method, we were able to measure BMD at the fracture site on anesthetized animals at different time points during healing. The intramedullary nail had to be included in the measurements, but its constant value was subtracted from the calculated BMD values to estimate the real BMD at the fracture site. As the intramedullary nail was included in the measurements, the real difference in BMD in the fracture callus was probably larger than observed, since the nail represented an equal constant in all groups. To validate this method, we also measured BMD after killing and removal of the nail, and this showed equal results indicating that the DEXA measurement with the nail in place was a reliable method. In Paper I the BMD after treatment with parecoxib at 0.05 mg/100 g body weight did not differ significantly from placebo after 3 weeks (p < 0.06) in contrast with the results from Paper II where animals treated with the same dose of parecoxib, for the same period of time did show difference in BMD values when compared with the placebo group. However, in both studies, the values were borderline significant values, one just over and the other one just below a p-value of 0.05. Our first study was possibly underpowered, explaining the difference between the results in the two Papers. Furthermore, we used animals from two different producers and strains in these two studies, this may also have contributed to the small differences in BMD.
Mechanical bone testing
In study I and II a three-point cantilever bending test was used. The test was originally developed for the rat femoral shaft Citation170, but has been modified for tibial shaft testing Citation152. This test has recently been modified again by incorporating the three-point cantilever bending test apparatus in an MTS machine (Model 858 Minin Bionix with Test Star II controller, MTS Systems, Corporation Eden Prairie, Minnesota, USA) Citation154. Four-point bending tests or torsional tests are often used for mechanical testing of the soft callus due to concerns related to possible deformation of the soft callus from the fulcrum in three-point bending. However, in our three-point bending test, the distal tibia is fixed, and the proximal fragment is bent in an anterior direction, so that the fulcrum is not moving or sagging into the callus. The three-point bending test gains standardized fractures and reliable results, and have been used by our group in several previous studies published in peer-reviewed journals Citation150-153,170. Both the fractured right tibia and the intact left tibia were fractured and the bending moment, total energy, deflection and bending stiffness were registered and calculated. The ratios between the right and the left tibia for the four mechanical properties were calculated.
Figure 1. A typical load-displacement curve from the mechanical testing of (A) an intact tibia (gray line), and (B) a healing fractured tibia (gray line).
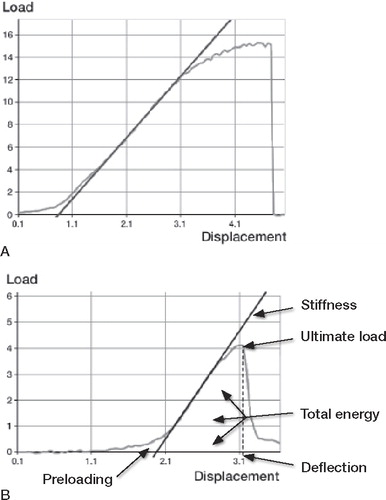
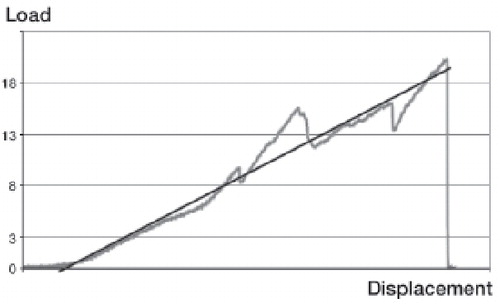
Figure 2. A typical load-displacement curve from the mechanical testing of a healing tendon (gray line).
The bending moment is the ultimate load the bone can withstand before fracture (top point of the gray load-displacement curve) multiplied by the moment arm by which the load is applied in the MTS machine. The total energy is the energy absorbed by the bone until fracture (the area under the curve until failure excluded the preloading). Deflection is the ultimate deformation in the bending of the bone before fracture (the point on the displacement axis corresponding to the fracture point). Stiffness is the ratio of the applied bending moment on the corresponding deflection in the straight, elastic part of the load-displacement curve (the black line).
Mechanical tendon testing
In Paper III a stretching test aligned parallel with the tendon was performed. The proximal tendon was spread like a fan and clamped between fine grained sand paper, and the calcaneus was held in a claw Citation104. No healing tendons slipped in the clamp during testing, but when testing intact tendons, they slipped out of the clamp before rupturing. Thus, ratios could not be compared like in studies I and II. The tensile strength (stretching force), total energy, deflection and stretching stiffness were registered.
Tensile strength was the ultimate load the tendon could withstand before rupture. Total energy was the energy absorbed by the tendon until rupture. Deflection was the ultimate stretching of the tendon before rupture. Stiffness was the ratio of the applied force on the corresponding deflection in the straight, elastic part of the load-displacement curve (black line).
Mechanical tendon-to-bone testing
In Paper IV a pull out test was performed. The calcaneus was held in the same claw as in Paper III, while the tibia was fixed in a custom made metal clamp. This clamp ensured that the drill hole was aligned with the direction of the force applied, and there was no contact between the tendon and the metal clamp. The ultimate pull out strength, total energy, deflection pullout and stiffness were registered.
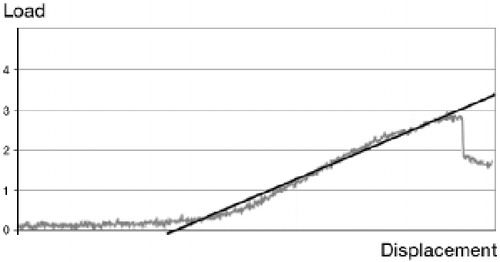
Pullout strength was the ultimate load that the tendon-to-bone junction could withstand before failure. Total energy was the energy absorbed by the tendon-to-bone interface until failure. Deflection was the ultimate stretching of the tendon-to-bone interface before failure. Stretching stiffness was the ratio of the applied force on the corresponding deflection in the straight, elastic part of the load-displacement curve (black line).
Statistical analysis
All the mechanical data were registered in TestStar software (MTS Systems, Corporation Eden Prarie, Minnesota, USA) and calculations were made in SPSS for Macintosh (SPSS Inc., Chicago, Illinois, USA). Statistical significance was set at p < 0.05.
Figure 3. A typical load-displacement curve from the mechanical pull out testing of a healing tendon-to-bone junction (gray line).
In Paper I, the two groups were compared by an unpaired Students t-test. For the DEXA measurements Bonferroni adjustment for multiple time-point tests were applied.
In Paper II–IV the three groups were compared using Analysis of Variance (ANOVA). Scheffe's post hoc test Citation171 was chosen to demonstrate if there was a negative effect of parecoxib and indomethacin on healing to give maximum protection against type I errors.
Results
In these studies we demonstrated that cox inhibitors reduced the BMD and impaired the mechanical strength in healing fractures. Tendon healing and tendon-to-bone healing were also impaired.
From our studies, and previous studies published on this topic, there should be no doubt about the cox inhibitors negative effect on the healing process. However, there are numerous uncertainties connected to the degree of significance for tissue healing in humans.
One of the important questions concerning cox inhibitors' effect on tissue healing is whether short-term administration of these drugs in relation to surgery or injury only temporarily delay the healing or definitely influence the final outcome.
In study I we demonstrated that parecoxib exerted an inhibitory effect on the mineralization of fracture callus, but the study suggested that the effect was temporary. The difference between BMD in the two groups was largest after 2 weeks, and after 6 weeks there were no significant differences. In study II we demonstrated similar results; the difference between BMD in animals administered indomethacin and the placebo group was largest after 2 weeks, but there were no significant differences after 3 weeks. Comparable results have been demonstrated in other studies using radiological analysis Citation89 or radiological bone density calculations Citation88, even though the duration of drug administration differed in the groups they compared. Fractures in animals administered cox inhibitors for a short time period had better radiological signs of healing compared to animals administered cox inhibitors for a longer period.
Concerning mechanical testing we did not find differences when testing after 6 weeks in study I. However, when testing earlier in the healing period (3 weeks, study II), there was a significantly decreased strength in animals administered both parecoxib and indomethacin compared to the placebo group. This also indicates that when administering cox inhibitors short-term only, the negative effects are reversible. Corresponding findings are seen in other studies Citation89,92. Histologically, significantly more fibrous tissue and less woven bone formation were found after 4 and 8 weeks, but not after 12 weeks Citation172. Initiating cox inhibitor treatment in the later course of fracture healing may also have a negative effect on the healing process, but not to the same extent as when treatment is administered around the time of fracture Citation89.
Thus, short-term administration of cox inhibitors delays fracture healing, but the healing process is probably restored when inhibition is removed.
Tendon healing is different from bone healing. In Paper III we demonstrated impaired tendon healing after short-term administration of parecoxib or indomethacin. It has previously been suggested that cox inhibitors might have a negative effect on the proliferative phase of tendon healing, but might have a positive effect in the remodeling phase Citation102. Accordingly it is demonstrated that inflammation appears to have a negative influence during remodeling Citation103. Long term application of cox inhibitors are reported to increase the failure stress in a healing tendon when administered until trial termination, i.e. during both the proliferative phase and the remodeling phase Citation104.
In Paper III the cox inhibitors were administered in the early phase only. Due to this, the proliferation phase was inhibited, and healing was impaired.
Consequently, short-term cox inhibitor administration delay tendon healing. However, administered in the later phase of healing cox inhibitors might be beneficial.
Tendon-to-bone healing is also different from both fracture healing and tendon healing. However, like in fractures and tendon injuries, the healing process is initiated with an inflammatory phase. In Paper IV we demonstrated that short-term administration of parecoxib and indomethacin impaired tendon-to-bone healing.
In Ferry's study Citation106 they administered the drugs until termination after 2 weeks, and patellar tendon-to-bone healing strength was decreased by the cox-2 inhibitors celecoxib and valdecoxib, and also by the conventional NSAID piroxicam, but not by naproxen and ibuprofen. Collagen content at the repair site was decreased for all but ibuprofen. The differences between the cox inhibitors were probably due to inadequate dosages or different metabolism of the drugs Citation106. In Cohen's study Citation105 they administered the drugs for 2 weeks, and demonstrated significantly lower failure loads in rotator cuff sutures after 2, 4 and 8 weeks in rats administered celecoxib or indomethacin, when compared to the placebo. The differences persisted at 8 weeks, and in contrast to findings in fracture healing, there was no indication that the impairment of tendon-to-bone healing was temporary. Thus, regarding tendon-to-bone healing, short-term administration impairs healing. It remains unclear whether the negative effects of cox inhibitors on tendon-to-bone healing are transient or persistent.
Another important question is whether inhibition of cox-1 or cox-2 is responsible for the negative effects on the healing process.
The cox-2 enzyme is inducible and involved in producing PGs related to acute inflammations, while the cox-1 enzyme is produced constitutively and involved in the homeostatic regulation Citation34. Therefore, one would expect that the inducible cox-2 was the enzyme involved in the healing process. In a study using mice genetically deficient for cox-1 or cox-2 enzyme, it was demonstrated that cox-2 is critically involved in bone repair and required for both intramembranous and endochondral bone formation Citation47. In another study using these mice, and also rats, administered both conventional cox inhibitors and cox-2 inhibitors, they also demonstrated that cox-2 function was specifically essential for fracture healing Citation46. Several other studies have also demonstrated that cox-2 inhibitors impair fracture healing Citation89-92. Furthermore, cox-2 plays an essential role in osseointegration of implants Citation133, and inhibits rotator cuff tendon-to-bone healing in rats Citation105. Cox-2 activity is also crucial during the early stages of muscle regeneration; muscle regeneration was attenuated by a cox-2 inhibitor but was unaffected by cox-1 inhibition, and cox-2 inhibition resulted in fewer myoblasts Citation48. Cox-2 also regulates growth of atrophied muscle Citation49.
In fracture calluses in rats, cox-2 mRNA levels showed peak expression during the first 2 weeks of healing in rats and returned to basal levels by 3 weeks Citation85. Accordingly, PGE2 reached the highest levels 24 hours after fracture and declined to baseline by 35 days Citation92. The PGE2 levels in the callus were reduced when cox inhibitors were administered to the animals Citation89,92. PGE2 was the first PG shown to influence bone metabolism Citation39, and a PGE2 agonist specifically promoted fracture healing Citation173. PGE2 has been assumed to be the major product of cox-2 activity and being the primary mediator of the anabolic activities in skeletal cells Citation174. Furthermore, cox-2 is induced early in response to muscle injury, and mRNA levels peak 4 days after injury Citation48.
In the present thesis, the specific cox-2 inhibitor parecoxib showed a more pronounced negative effect on fracture healing, tendon healing and tendon-to-bone healing compared to the conventional NSAID indomethacin. This is in accordance with findings reported in the literature in general. Considering findings in earlier published studies, we expected to find impaired healing in rats administered indomethacin in human doses adjusted for weight in rats. We hypothesized that even more reduced healing in rats administered parecoxib in human doses would argue for inhibition of cox-2 to be the responsible mechanism. However, due to uncertainties related to the metabolism of different NSAIDs and cox-2 inhibitors in rats, we cannot conclude that cox-2 inhibition is responsible for the impaired healing. Altogether, however, findings indicate that cox-2 is the enzyme responsible for initiating the healing process and that inhibition of cox-2 is responsible for the impaired healing. Due to this, inhibition of cox-2 during the early period after injury or surgery seems to have the most detrimental effects on tissue healing.
Impaired healing on the basis of cox inhibition is probably not due to inhibition of the inflammation alone. Several mechanisms are most likely involved.
PGs are synthesized in the inflammatory process, and are important to initiate bone healing Citation36. PGs also play an important role in regulating bone metabolism and preventing an imbalance between bone resorption and bone formation Citation34,42,43. When a cox-2 inhibitor was administered 2 weeks after fracture, a negative effect on healing was still observed Citation89. This suggests that cox-2 might have additional functions during fracture healing besides inhibiting PGs.
Angionesis is essential for successful fracture healing and endochondral ossification Citation175,176. Angionesis is also shown to be inhibited by cox-2 inhibitors Citation177, and vascularization of fracture callus in mice genetically deficient for cox-2 is reported to be reduced Citation46. This might lead to improper delivery of osteclasts and osteoblasts to the fracture area.
Corresponding mechanisms are seen after muscle injury where the intramuscular number of inflammatory cells as macrophages are decreased Citation48. Muscle regeneration is impaired when inflammatory cells are depleted and stimulated when they are increased Citation178. Macrophages are necessary in the repair process to phagocytose cellular debris and secrete growth factors, cytokines and PGs that are implicated in myogenesis Citation179,180. The majority of macrophages in injured muscle are claimed to invade from circulation and are not a result of the proliferation of resident macrophages Citation181. Therefore, impaired release of vasodilatory PGs by cox-2 inhibition might also lead to improper delivery of inflammatory cells to the injury site, not only in muscle, but in tendon and bone as well.
Bone morphogenetic proteins (BMPs), which are important regulators of bone formation, might also be affected by cox inhibition. In a study using bone marrow stromal cell cultures obtained from mice genetically deficient for cox-2, findings suggested that reduced PGE2 level due to cox-2 inhibition interfere with BMP-2 signaling events leading to reduced levels of 2 genes necessary for bone formation Citation47. Another study demonstrated similar findings where the expression of BMP-7 was increased upon PGE2 treatment Citation182. Also concerning tendon healing, and probably tendon-to-bone healing, these mechanisms might be present as BMPs, and may be necessary for normal repair. Other BMPs, like the cartilage-derived morphogenic proteins (CDMPs) is demonstrated to improve early tendon healing Citation183-185, and reduced PGE2 due to cox inhibition, might interfere with CDMP-2 signaling.
It is also shown that mice, genetically deficient for cox-2, have elevated plasma levels of both Ca2+ and PTH consistent with primary hyperparathyroidism, suggesting that the cox enzymes are regulators of BMD and bone strength Citation186. The same study demonstrates lower BMD, decreased bone strength and decreased trabecular volume in mice genetically deficient for cox-2.
In summary, findings from different studies indicates that fracture healing, tendon healing and tendon-to-bone healing, regulated through the cox-2 pathway, involve multiple mechanisms, not only the inhibition of inflammation.
Altogether, short-term administration of cox inhibitors impairs the healing process in bone and tendons. Administered in doses equivalent to human doses, the inhibition is more pronounced with cox-2 inhibition than with cox-1 inhibition.
Clinical considerations
An important question concerning cox inhibitors and tissue healing in relation to this thesis is the clinical relevance of the findings; results of experimental animal studies cannot be directly extrapolated to the human clinical setting. However, as long as experimental studies demonstrate that cox inhibitors are potentially deleterious to healing of bone, tendon and tendon-to-bone, these studies should be our guidance and make clinicians aware of the possible deleterious effects on musculoskeletal tissue healing of these drugs.
Aspenberg asserted in an Editorial in 2002 that cox inhibitors should be avoided after skeletal surgery Citation8. Few studies have been published since that would cause one to change this view. However, cox inhibitors could probably be considered for administration after fractures that usually incur few or no healing problems, like distal radial fractures and acetabular fractures. This is in accordance with findings in Burd's study were 29% of patients administered indomethacin had a nonunion of a long bone fracture, but all acetabular fractures in the same patients healed Citation95. Concerning arthroplasties, it has recently been demonstrated that administration of cox inhibitors to patients after implantation of cemented knee prostheses did not affect the outcome after 2 years Citation139. It is likely that cox inhibitors can be administered when implanting cemented prostheses, where bone ingrowth into the implant is not required. One should be aware, however, of a possibly elevated risk of infections. In a prospective randomized study on patients undergoing coronary artery bypass surgery, there was an increased incidence of sternal wound infections in patients administered cox-2 inhibitors compared to the placebo Citation187. Considered the severity infected arthroplasties in particular, and deep infections in relation to orthopedic surgery in general, it is important to elucidate if cox inhibitors have an effect on wound healing and wound infections.
No prospective randomized clinical trial on long bone fractures with or without cox inhibitor treatment have been performed. However, an association between the use of NSAIDs after injury and nonunion in human has been observed for femoral shaft fractures in retrospective studies Citation94,95. This supports the findings from a large number of experimental studies. It is not unlikely that these findings also apply to all human fractures known to be vulnerable to nonunion and delayed union and other similar bone healing situations. Although the negative effect of short-term administration of cox inhibitors on fracture healing seems to be transient in the experimental studies, this might not be the case in humans, as the fracture healing potential is much better in rats compared to humans. So, cox inhibitors should be avoided in situations were unimpaired healing is essential for the outcome, i.e. fractures with known healing problems, spinal fusions, arthrodesis, osteotomies and after bone grafting procedures. Moreover, cox inhibitors should probably be avoided in implant surgery where bone ingrowth is essential.
Concerning soft tissue healing in a clinical setting, cox inhibitors are widely used for sports related injuries and after surgery. Lately, however, concerns also have been expressed regarding the treatment of muscle, ligament and tendon injuries as long as they might be potentially deleterious to tissue healing Citation12,13. Prospective randomized clinical trials have not been performed in terms of tendon or tendon-to-bone healing,. Based on experimental studies, however, cox inhibitors should probably be avoided in the proliferation phase, but might be considered for administration in the later remodeling phase during soft tissue healing in tendons and ligaments. Furthermore, after procedures requiring tendon-to-bone healing like rotator cuff repairs, tendon reinsertions and cruciate ligament reconstructions cox inhibitors should probably be avoided altogether.
There are still numerous unknown aspects of the use of cox inhibitors. The negative effects on healing of musculoskeletal tissue demonstrated in experimental studies should be thoroughly elucidated in clinical studies before cox inhibitors are administered to these patients.
Conclusions
There is a negative effect of short-term administration of the cox-2 inhibitor parecoxib on fracture healing.
There is a negative effect of short-term administration of the cox-2 inhibitor parecoxib and the conventional NSAID indomethacin on the earlier fracture healing process.
There is a negative effect of short-term administration of the cox-2 inhibitor parecoxib and the conventional NSAID indomethacin on tendon healing.
There is a negative effect of short-term administration of the cox-2 inhibitor parecoxib and the conventional NSAID indomethacin on tendon-to-bone healing.
References
- Dahl V, Raeder JC, Drosdal S, Wathne O, Brynildsrud J. Prophylactic oral ibuprofen or ibuprofen-codeine versus placebo for postoperative pain after primary hip arthroplasty. Acta Anaesthesiol Scand 1995; 39-3: 323-6.
- Dahl V, Dybvik T, Steen T, Aune AK, Rosenlund EK, Raeder JC. Ibuprofen vs. acetaminophen vs. ibuprofen and acetaminophen after arthroscopically assisted anterior cruciate ligament reconstruction. Eur J Anaesthesiol 2004; 21-6: 471-5.
- Fogarty DJ, O'Hanlon JJ, Milligan KR. Intramuscular ketorolac following total hip replacement with spinal anaesthesia and intrathecal morphine. Acta Anaesthesiol Scand 1995; 39-2: 191-4.
- Fransen M, Anderson C, Douglas J, MacMahon S, Neal B, Norton R, Woodward M, Cameron ID, Crawford R, Lo SK, Tregonning G, Windolf M. Safety and efficacy of routine postoperative ibuprofen for pain and disability related to ectopic bone formation after hip replacement surgery (HIPAID): randomised controlled trial. Bmj 2006; 333-7567: 519.
- Hubbard RC, Naumann TM, Traylor L, Dhadda S. Parecoxib sodium has opioid-sparing effects in patients undergoing total knee arthroplasty under spinal anaesthesia. Br J Anaesth 2003; 90-2: 166-72.
- Malan TP, Jr., Marsh G, Hakki SI, Grossman E, Traylor L, Hubbard RC. Parecoxib sodium, a parenteral cyclooxygenase 2 selective inhibitor, improves morphine analgesia and is opioid-sparing following total hip arthroplasty. Anesthesiology 2003; 98-4: 950-6.
- McLoughlin C, McKinney MS, Fee JP, Boules Z. Diclofenac for day-care arthroscopy surgery: comparison with a standard opioid therapy. Br J Anaesth 1990; 65-5: 620-3.
- Aspenberg P. Avoid cox inhibitors after skeletal surgery! Acta Orthop Scand 2002; 73-5: 489-90.
- Dahners LE, Mullis BH. Effects of nonsteroidal anti-inflammatory drugs on bone formation and soft-tissue healing. J Am Acad Orthop Surg 2004; 12-3: 139-43.
- Einhorn TA. Do inhibitors of cyclooxygenase-2 impair bone healing? J Bone Miner Res 2002; 17-6: 977-8.
- Goodman SB, Ma T, Genovese M, Lane Smith R. COX-2 selective inhibitors and bone. Int J Immunopathol Pharmacol 2003; 16-3: 201-5.
- Paoloni JA, Orchard JW. The use of therapeutic medications for soft-tissue injuries in sports medicine. Med J Aust 2005; 183-7: 384-8.
- Warden SJ. Cyclo-oxygenase-2 inhibitors : beneficial or detrimental for athletes with acute musculoskeletal injuries? Sports Med 2005; 35-4: 271-83.
- McKibbin B. The biology of fracture healing in long bones. J Bone Joint Surg Br 1978; 60-B-2: 150-62.
- Einhorn TA. The cell and molecular biology of fracture healing. Clin Orthop Relat Res 1998-355 Suppl: S7-21.
- Dimitriou R, Tsiridis E, Giannoudis PV. Current concepts of molecular aspects of bone healing. Injury 2005; 36-12: 1392-404.
- Hadjiargyrou M, Lombardo F, Zhao S, Ahrens W, Joo J, Ahn H, Jurman M, White DW, Rubin CT. Transcriptional profiling of bone regeneration. Insight into the molecular complexity of wound repair. J Biol Chem 2002; 277-33: 30177-82.
- Tsiridis E, Giannoudis PV. Transcriptomics and proteomics: advancing the understanding of genetic basis of fracture healing. Injury 2006; 37 Suppl 1: S13-9.
- Sharma P, Maffulli N. Tendon injury and tendinopathy: healing and repair. J Bone Joint Surg Am 2005; 87-1: 187-202.
- Platt MA. Tendon repair and healing. Clin Podiatr Med Surg 2005; 22-4: 553-60, vi.
- Cooper RR, Misol S. Tendon and ligament insertion. A light and electron microscopic study. J Bone Joint Surg Am 1970; 52-1: 1-20.
- Benjamin M, Evans EJ, Copp L. The histology of tendon attachments to bone in man. J Anat 1986; 149:89-100.
- Benjamin M, Kumai T, Milz S, Boszczyk BM, Boszczyk AA, Ralphs JR. The skeletal attachment of tendons–tendon ”entheses”. Comp Biochem Physiol A Mol Integr Physiol 2002; 133-4: 931-45.
- Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am 1993; 75-12: 1795-803.
- Liu SH, Panossian V, al-Shaikh R, Tomin E, Shepherd E, Finerman GA, Lane JM. Morphology and matrix composition during early tendon to bone healing. Clin Orthop Relat Res 1997-339: 253-60.
- Hibino N, Hamada Y, Sairyo K, Yukata K, Sano T, Yasui N. Callus formation during healing of the repaired tendon-bone junction. A rat experimental model. J Bone Joint Surg Br 2007; 89-11: 1539-44.
- von Eular US. On specific vasodilating and plain muscle stimulating substances from accessory genital glands in man and certain animals (prostaglandin and vesiglandin). J Physiol 1936; 88-2: 213-34.
- Piper P, Vane J. The release of prostaglandins from lung and other tissues. Ann N Y Acad Sci 1971; 180:363-85.
- Needleman P, Turk J, Jakschik BA, Morrison AR, Lefkowith JB. Arachidonic acid metabolism. Annu Rev Biochem 1986; 55:69-102.
- Botting RM. Cyclooxygenase: Past, present and future. A tribute to John R. Vane (1927-2004). Journal of thermal biology 2006; 31:208-19.
- Hemler M, Lands WE. Purification of the cyclooxygenase that forms prostaglandins. Demonstration of two forms of iron in the holoenzyme. J Biol Chem 1976; 251-18: 5575-9.
- Simmons DL, Levy DB, Yannoni Y, Erikson RL. Identification of a phorbol ester-repressible v-src-inducible gene. Proc Natl Acad Sci U S A 1989; 86-4: 1178-82.
- Chandrasekharan NV, Dai H, Roos KL, Evanson NK, Tomsik J, Elton TS, Simmons DL. COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci U S A 2002; 99-21: 13926-31.
- Dubois RN, Abramson SB, Crofford L, Gupta RA, Simon LS, Van De Putte LB, Lipsky PE. Cyclooxygenase in biology and disease. Faseb J 1998; 12-12: 1063-73.
- Dekel S, Lenthall G, Francis MJ. Release of prostaglandins from bone and muscle after tibial fracture. An experimental study in rabbits. J Bone Joint Surg Br 1981; 63-B-2: 185-9.
- Simmons DJ. Fracture healing perspectives. Clin Orthop Relat Res 1985-200: 100-13.
- Schelling SH, Wolfe HJ, Tashjian AH, Jr. Role of the osteoclast in prostaglandin E2-stimulated bone resorption: a correlative morphometric and biochemical analysis. Lab Invest 1980; 42-3: 290-5.
- Lin CH, Jee WS, Ma YF, Setterberg RB. Early effects of prostaglandin E2 on bone formation and resorption in different bone sites of rats. Bone 1995; 17-4 Suppl: 255S-9S.
- Klein DC, Raisz LG. Prostaglandins: stimulation of bone resorption in tissue culture. Endocrinology 1970; 86-6: 1436-40.
- Dietrich JW, Goodson JM, Raisz LG. Stimulation of bone resorption by various prostaglandins in organ culture. Prostaglandins 1975; 10-2: 231-40.
- Nefussi JR, Baron R. PGE2 stimulates both resorption and formation of bone in vitro: differential responses of the periosteum and the endosteum in fetal rat long bone cultures. Anat Rec 1985; 211-1: 9-16.
- Kawaguchi H, Pilbeam CC, Harrison JR, Raisz LG. The role of prostaglandins in the regulation of bone metabolism. Clin Orthop Relat Res 1995-313: 36-46.
- Norrdin RW, Jee WS, High WB. The role of prostaglandins in bone in vivo. Prostaglandins Leukot Essent Fatty Acids 1990; 41-3: 139-49.
- Okada Y, Lorenzo JA, Freeman AM, Tomita M, Morham SG, Raisz LG, Pilbeam CC. Prostaglandin G/H synthase-2 is required for maximal formation of osteoclast-like cells in culture. J Clin Invest 2000; 105-6: 823-32.
- Pilbeam CC, Fall PM, Alander CB, Raisz LG. Differential effects of nonsteroidal anti-inflammatory drugs on constitutive and inducible prostaglandin G/H synthase in cultured bone cells. J Bone Miner Res 1997; 12-8: 1198-203.
- Simon AM, Manigrasso MB, O'Connor JP. Cyclo-oxygenase 2 function is essential for bone fracture healing. J Bone Miner Res 2002; 17-6: 963-76.
- Zhang X, Schwarz EM, Young DA, Puzas JE, Rosier RN, O'Keefe RJ. Cyclooxygenase-2 regulates mesenchymal cell differentiation into the osteoblast lineage and is critically involved in bone repair. J Clin Invest 2002; 109-11: 1405-15.
- Bondesen BA, Mills ST, Kegley KM, Pavlath GK. The COX-2 pathway is essential during early stages of skeletal muscle regeneration. Am J Physiol Cell Physiol 2004; 287-2: C475-83.
- Bondesen BA, Mills ST, Pavlath GK. The COX-2 pathway regulates growth of atrophied muscle via multiple mechanisms. Am J Physiol Cell Physiol 2006; 290-6: C1651-9.
- Vane JR. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nature - New Biology 1971; 231-25: 232-5.
- Xie WL, Chipman JG, Robertson DL, Erikson RL, Simmons DL. Expression of a mitogen-responsive gene encoding prostaglandin synthase is regulated by mRNA splicing. Proc Natl Acad Sci U S A 1991; 88-7: 2692-6.
- Mitchell JA, Akarasereenont P, Thiemermann C, Flower RJ, Vane JR. Selectivity of nonsteroidal antiinflammatory drugs as inhibitors of constitutive and inducible cyclooxygenase. Proc Natl Acad Sci U S A 1993; 90-24: 11693-7.
- Meade EA, Smith WL, DeWitt DL. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem 1993; 268-9: 6610-4.
- Garcia Rodriguez LA, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet 1994; 343-8900: 769-72.
- Vane JR, Botting RM. New insights into the mode of action of anti-inflammatory drugs. Inflamm Res 1995; 44-1: 1-10.
- Bombardier C, Laine L, Reicin A, Shapiro D, Burgos-Vargas R, Davis B, Day R, Ferraz MB, Hawkey CJ, Hochberg MC, Kvien TK, Schnitzer TJ. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group. N Engl J Med 2000; 343-21: 1520-8, 2 p following 8.
- Cannon CP, Curtis SP, FitzGerald GA, Krum H, Kaur A, Bolognese JA, Reicin AS, Bombardier C, Weinblatt ME, van der Heijde D, Erdmann E, Laine L. Cardiovascular outcomes with etoricoxib and diclofenac in patients with osteoarthritis and rheumatoid arthritis in the Multinational Etoricoxib and Diclofenac Arthritis Long-term (MEDAL) programme: a randomised comparison. Lancet 2006; 368-9549: 1771-81.
- Warner TD, Mitchell JA. COX-2 selectivity alone does not define the cardiovascular risks associated with non-steroidal anti-inflammatory drugs. Lancet 2008; 371-9608: 270-3.
- Romsing J, Moiniche S, Mathiesen O, Dahl JB. Reduction of opioid-related adverse events using opioid-sparing analgesia with COX-2 inhibitors lacks documentation: a systematic review. Acta Anaesthesiol Scand 2005; 49-2: 133-42.
- Funk CD, Funk LB, Kennedy ME, Pong AS, Fitzgerald GA. Human platelet/erythroleukemia cell prostaglandin G/H synthase: cDNA cloning, expression, and gene chromosomal assignment. Faseb J 1991; 5-9: 2304-12.
- Hegi TR, Bombeli T, Seifert B, Baumann PC, Haller U, Zalunardo MP, Pasch T, Spahn DR. Effect of rofecoxib on platelet aggregation and blood loss in gynaecological and breast surgery compared with diclofenac. Br J Anaesth 2004; 92-4: 523-31.
- Leese PT, Hubbard RC, Karim A, Isakson PC, Yu SS, Geis GS. Effects of celecoxib, a novel cyclooxygenase-2 inhibitor, on platelet function in healthy adults: a randomized, controlled trial. J Clin Pharmacol 2000; 40-2: 124-32.
- Leese PT, Talwalker S, Kent JD, Recker DP. Valdecoxib does not impair platelet function. Am J Emerg Med 2002; 20-4: 275-81.
- Noveck RJ, Hubbard RC. Parecoxib sodium, an injectable COX-2-specific inhibitor, does not affect unfractionated heparin-regulated blood coagulation parameters. J Clin Pharmacol 2004; 44-5: 474-80.
- Weber EW, Slappendel R, Durieux ME, Dirksen R, van der Heide H, Spruit M. COX 2 selectivity of non-steroidal anti-inflammatory drugs and perioperative blood loss in hip surgery. A randomized comparison of indomethacin and meloxicam. Eur J Anaesthesiol 2003; 20-12: 963-6.
- Daniels SE, Grossman EH, Kuss ME, Talwalker S, Hubbard RC. A double-blind, randomized comparison of intramuscularly and intravenously administered parecoxib sodium versus ketorolac and placebo in a post-oral surgery pain model. Clin Ther 2001; 23-7: 1018-31.
- Mehlisch DR, Desjardins PJ, Daniels S, Hubbard RC. Single doses of parecoxib sodium intravenously are as effective as ketorolac in reducing pain after oral surgery. J Oral Maxillofac Surg 2003; 61-9: 1030-7.
- Rasmussen GL, Steckner K, Hogue C, Torri S, Hubbard RC. Intravenous parecoxib sodium foracute pain after orthopedic knee surgery. Am J Orthop 2002; 31-6: 336-43.
- Heckman JD. Retractions. J Bone Joint Surg Am 2009; 91-4: 965.
- Retraction. Anesthesiology 2009; 110-3: 689.
- Neal JM. The effect of cyclooxygenase-2 inhibition on acute and chronic donor-site pain after spinal-fusion surgery: erratum retraction. Reg Anesth Pain Med 2009; 34-2: 184.
- Shafer SL. Notice of retraction. Anesth Analg 2009; 108-4: 1350.
- White PF, Kehlet H, Liu S. Perioperative analgesia: what do we still know? Anesth Analg 2009; 108-5: 1364-7.
- Hogevold HE, Grogaard B, Reikeras O. Effects of short-term treatment with corticosteroids and indomethacin on bone healing. A mechanical study of osteotomies in rats. Acta Orthop Scand 1992; 63-6: 607-11.
- Sudmann E, Dregelid E, Bessesen A, Morland J. Inhibition of fracture healing by indomethacin in rats. Eur J Clin Invest 1979; 9-5: 333-9.
- Rø J, Sudmann E, Marton PF. Effect of indomethacin on fracture healing in rats. Acta Orthop Scand 1976; 47-6: 588-99.
- Keller J, Bunger C, Andreassen TT, Bak B, Lucht U. Bone repair inhibited by indomethacin. Effects on bone metabolism and strength of rabbit osteotomies. Acta Orthop Scand 1987; 58-4: 379-83.
- Engesaeter LB, Sudmann B, Sudmann E. Fracture healing in rats inhibited by locally administered indomethacin. Acta Orthop Scand 1992; 63-3: 330-3.
- Elves MW, Bayley I, Roylance PJ. The effect of indomethacin upon experimental fractures in the rat. Acta Orthop Scand 1982; 53-1: 35-41.
- Allen HL, Wase A, Bear WT. Indomethacin and aspirin: effect of nonsteroidal anti-inflammatory agents on the rate of fracture repair in the rat. Acta Orthop Scand 1980; 51-4: 595-600.
- Tornkvist H, Lindholm TS, Netz P, Stromberg L, Lindholm TC. Effect of ibuprofen and indomethacin on bone metabolism reflected in bone strength. Clin Orthop Relat Res 1984-187: 255-9.
- Altman RD, Latta LL, Keer R, Renfree K, Hornicek FJ, Banovac K. Effect of nonsteroidal antiinflammatory drugs on fracture healing: a laboratory study in rats. J Orthop Trauma 1995; 9-5: 392-400.
- Lindholm TS, Tornkvist H. Inhibitory effect on bone formation and calcification exerted by the anti-inflammatory drug ibuprofen. An experimental study on adult rat with fracture. Scand J Rheumatol 1981; 10-1: 38-42.
- Goodman S, Ma T, Trindade M, Ikenoue T, Matsuura I, Wong N, Fox N, Genovese M, Regula D, Smith RL. COX-2 selective NSAID decreases bone ingrowth in vivo. J Orthop Res 2002; 20-6: 1164-9.
- Gerstenfeld LC, Thiede M, Seibert K, Mielke C, Phippard D, Svagr B, Cullinane D, Einhorn TA. Differential inhibition of fracture healing by non-selective and cyclooxygenase-2 selective non-steroidal anti-inflammatory drugs. J Orthop Res 2003; 21-4: 670-5.
- Ho ML, Chang JK, Wang GJ. Effects of ketorolac on bone repair: A radiographic study in modeled demineralized bone matrix grafted rabbits. Pharmacology 1998; 57-3: 148-59.
- Martin GJ, Jr., Boden SD, Titus L. Recombinant human bone morphogenetic protein-2 overcomes the inhibitory effect of ketorolac, a nonsteroidal anti-inflammatory drug (NSAID), on posterolateral lumbar intertransverse process spine fusion. Spine 1999; 24-21: 2188-93; discussion 93-4.
- Beck A, Krischak G, Sorg T, Augat P, Farker K, Merkel U, Kinzl L, Claes L. Influence of diclofenac (group of nonsteroidal anti-inflammatory drugs) on fracture healing. Arch Orthop Trauma Surg 2003; 123-7: 327-32.
- Simon AM, O'Connor J P. Dose and time-dependent effects of cyclooxygenase-2 inhibition on fracture-healing. J Bone Joint Surg Am 2007; 89-3: 500-11.
- Bergenstock M, Min W, Simon AM, Sabatino C, O'Connor JP. A comparison between the effects of acetaminophen and celecoxib on bone fracture healing in rats. J Orthop Trauma 2005; 19-10: 717-23.
- Endo K, Sairyo K, Komatsubara S, Sasa T, Egawa H, Ogawa T, Yonekura D, Murakami R, Yasui N. Cyclooxygenase-2 inhibitor delays fracture healing in rats. Acta Orthop 2005; 76-4: 470-4.
- Gerstenfeld LC, Al-Ghawas M, Alkhiary YM, Cullinane DM, Krall EA, Fitch JL, Webb EG, Thiede MA, Einhorn TA. Selective and nonselective cyclooxygenase-2 inhibitors and experimental fracture-healing. Reversibility of effects after short-term treatment. J Bone Joint Surg Am 2007; 89-1: 114-25.
- Meunier A, Aspenberg P. Parecoxib impairs early metaphyseal bone healing in rats. Arch Orthop Trauma Surg 2006; 126-7: 433-6.
- Giannoudis PV, MacDonald DA, Matthews SJ, Smith RM, Furlong AJ, De Boer P. Nonunion of the femoral diaphysis. The influence of reaming and non-steroidal anti-inflammatory drugs. J Bone Joint Surg Br 2000; 82-5: 655-8.
- Burd TA, Hughes MS, Anglen JO. Heterotopic ossification prophylaxis with indomethacin increases the risk of long-bone nonunion. J Bone Joint Surg Br 2003; 85-5: 700-5.
- Burd TA, Lowry KJ, Anglen JO. Indomethacin compared with localized irradiation for the prevention of heterotopic ossification following surgical treatment of acetabular fractures. J Bone Joint Surg Am 2001; 83-A-12: 1783-8.
- Adolphson P, Abbaszadegan H, Jonsson U, Dalen N, Sjoberg HE, Kalen S. No effects of piroxicam on osteopenia and recovery after Colles' fracture. A randomized, double-blind, placebo-controlled, prospective trial. Arch Orthop Trauma Surg 1993; 112-3: 127-30.
- Davis TR, Ackroyd CE. Non-steroidal anti-inflammatory agents in the management of Colles' fractures. Br J Clin Pract 1988; 42-5: 184-9.
- Villamor A, Rios-Luna A, Villanueva-Martinez M, Fahandezh-Saddi H. Nonunion of distal radius fracture and distal radioulnar joint injury: a modified Sauve-Kapandji procedure with a cubitus proradius transposition as autograft. Arch Orthop Trauma Surg 2008.
- Kulick MI, Smith S, Hadler K. Oral ibuprofen: evaluation of its effect on peritendinous adhesions and the breaking strength of a tenorrhaphy. J Hand Surg [Am] 1986; 11-1: 110-20.
- Tsai WC, Hsu CC, Chou SW, Chung CY, Chen J, Pang JH. Effects of celecoxib on migration, proliferation and collagen expression of tendon cells. Connect Tissue Res 2007; 48-1: 46-51.
- Almekinders LC, Baynes AJ, Bracey LW. An in vitro investigation into the effects of repetitive motion and nonsteroidal antiinflammatory medication on human tendon fibroblasts. Am J Sports Med 1995; 23-1: 119-23.
- Virchenko O, Skoglund B, Aspenberg P. Parecoxib impairs early tendon repair but improves later remodeling. Am J Sports Med 2004; 32-7: 1743-7.
- Forslund C, Bylander B, Aspenberg P. Indomethacin and celecoxib improve tendon healing in rats. Acta Orthop Scand 2003; 74-4: 465-9.
- Cohen DB, Kawamura S, Ehteshami JR, Rodeo SA. Indomethacin and celecoxib impair rotator cuff tendon-to-bone healing. Am J Sports Med 2006; 34-3: 362-9.
- Ferry ST, Dahners LE, Afshari HM, Weinhold PS. The effects of common anti-inflammatory drugs on the healing rat patellar tendon. Am J Sports Med 2007; 35-8: 1326-33.
- Neal B, Gray H, MacMahon S, Dunn L. Incidence of heterotopic bone formation after major hip surgery. ANZ J Surg 2002; 72-11: 808-21.
- Puzas JE, Miller MD, Rosier RN. Pathologic bone formation. Clin Orthop Relat Res 1989-245: 269-81.
- Neal BC, Rodgers A, Clark T, Gray H, Reid IR, Dunn L, MacMahon SW. A systematic survey of 13 randomized trials of non-steroidal anti-inflammatory drugs for the prevention of heterotopic bone formation after major hip surgery. Acta Orthop Scand 2000; 71-2: 122-8.
- Fijn R, Koorevaar RT, Brouwers JR. Prevention of heterotopic ossification after total hip replacement with NSAIDs. Pharm World Sci 2003; 25-4: 138-45.
- Board TN, Karva A, Board RE, Gambhir AK, Porter ML. The prophylaxis and treatment of heterotopic ossification following lower limb arthroplasty. J Bone Joint Surg Br 2007; 89-4: 434-40.
- Fransen M, Neal B. Non-steroidal anti-inflammatory drugs for preventing heterotopic bone formation after hip arthroplasty. Cochrane Database Syst Rev 2004-3: CD001160.
- Kjaersgaard-Andersen P, Nafei A, Teichert G, Kristensen O, Schmidt SA, Keller J, Lucht U. Indomethacin for prevention of heterotopic ossification. A randomized controlled study in 41 hip arthroplasties. Acta Orthop Scand 1993; 64-6: 639-42.
- Persson PE, Sodemann B, Nilsson OS. Preventive effects of ibuprofen on periarticular heterotopic ossification after total hip arthroplasty. A randomized double-blind prospective study of treatment time. Acta Orthop Scand 1998; 69-2: 111-5.
- Vielpeau C, Joubert JM, Hulet C. Naproxen in the prevention of heterotopic ossification after total hip replacement. Clin Orthop Relat Res 1999-369: 279-88.
- Banovac K, Williams JM, Patrick LD, Levi A. Prevention of heterotopic ossification after spinal cord injury with COX-2 selective inhibitor (rofecoxib). Spinal Cord 2004; 42-12: 707-10.
- Reis HJ, Kusswetter W, Schellinger T. The suppression of heterotopic ossification after total hip arthroplasty. Int Orthop 1992; 16-2: 140-5.
- Pritchett JW. Ketorolac prophylaxis against heterotopic ossification after hip replacement. Clin Orthop Relat Res 1995-314: 162-5.
- Romano CL, Duci D, Romano D, Mazza M, Meani E. Celecoxib versus indomethacin in the prevention of heterotopic ossification after total hip arthroplasty. J Arthroplasty 2004; 19-1: 14-8.
- Saudan M, Saudan P, Perneger T, Riand N, Keller A, Hoffmeyer P. Celecoxib versus ibuprofen in the prevention of heterotopic ossification following total hip replacement: a prospective randomised trial. J Bone Joint Surg Br 2007; 89-2: 155-9.
- van der Heide HJ, Rijnberg WJ, Van Sorge A, Van Kampen A, Schreurs BW. Similar effects of rofecoxib and indomethacin on the incidence of heterotopic ossification after hip arthroplasty. Acta Orthop 2007; 78-1: 90-4.
- Rapuano BE, Boursiquot R, Tomin E, Macdonald DE, Maddula S, Raghavan D, Lane JM, Helfet DL. The effects of COX-1 and COX-2 inhibitors on prostaglandin synthesis and the formation of heterotopic bone in a rat model. Arch Orthop Trauma Surg 2008; 128-3: 333-44.
- Amstutz HC, Fowble VA, Schmalzried TP, Dorey FJ. Short-course indomethacin prevents heterotopic ossification in a high-risk population following total hip arthroplasty. J Arthroplasty 1997; 12-2: 126-32.
- van der Heide HJ, Koorevaar RC, Lemmens JA, van Kampen A, Schreurs BW. Rofecoxib inhibits heterotopic ossification after total hip arthroplasty. Arch Orthop Trauma Surg 2007; 127-7: 557-61.
- Dimar JR, 2nd, Ante WA, Zhang YP, Glassman SD. The effects of nonsteroidal anti-inflammatory drugs on posterior spinal fusions in the rat. Spine 1996; 21-16: 1870-6.
- O'Keefe RJ, Tiyapatanaputi P, Xie C, Li TF, Clark C, Zuscik MJ, Chen D, Drissi H, Schwarz E, Zhang X. COX-2 has a critical role during incorporation of structural bone allografts. Ann N Y Acad Sci 2006; 1068:532-42.
- Glassman SD, Rose SM, Dimar JR, Puno RM, Campbell MJ, Johnson JR. The effect of postoperative nonsteroidal anti-inflammatory drug administration on spinal fusion. Spine 1998; 23-7: 834-8.
- Deguchi M, Rapoff AJ, Zdeblick TA. Posterolateral fusion for isthmic spondylolisthesis in adults: analysis of fusion rate and clinical results. J Spinal Disord 1998; 11-6: 459-64.
- Sucato DJ, Lovejoy JF, Agrawal S, Elerson E, Nelson T, McClung A. Postoperative ketorolac does not predispose to pseudoarthrosis following posterior spinal fusion and instrumentation for adolescent idiopathic scoliosis. Spine 2008; 33-10: 1119-24.
- Trancik T, Mills W, Vinson N. The effect of indomethacin, aspirin, and ibuprofen on bone ingrowth into a porous-coated implant. Clin Orthop Relat Res 1989-249: 113-21.
- Keller JC, Trancik TM, Young FA, St Mary E. Effects of indomethacin on bone ingrowth. J Orthop Res 1989; 7-1: 28-34.
- Pablos AB, Ramalho SA, Konig B, Jr., Furuse C, de Araujo VC, Cury PR. Effect of meloxicam and diclofenac sodium on peri-implant bone healing in rats. J Periodontol 2008; 79-2: 300-6.
- Chikazu D, Tomizuka K, Ogasawara T, Saijo H, Koizumi T, Mori Y, Yonehara Y, Susami T, Takato T. Cyclooxygenase-2 activity is essential for the osseointegration of dental implants. Int J Oral Maxillofac Surg 2007; 36-5: 441-6.
- Cook SD, Barrack RL, Dalton JE, Thomas KA, Brown TD. Effects of indomethacin on biologic fixation of porous-coated titanium implants. J Arthroplasty 1995; 10-3: 351-8.
- Zhang X, Morham SG, Langenbach R, Young DA, Xing L, Boyce BF, Puzas EJ, Rosier RN, O'Keefe RJ, Schwarz EM. Evidence for a direct role of cyclo-oxygenase 2 in implant wear debris-induced osteolysis. J Bone Miner Res 2001; 16-4: 660-70.
- Wurnig C, Schwameis E, Bitzan P, Kainberger F. Six-year results of a cementless stem with prophylaxis against heterotopic bone. Clin Orthop Relat Res 1999-361: 150-8.
- Ince A, Sauer U, Wollmerstedt N, Hendrich C. No migration of acetabular cups after prophylaxis for heterotopic ossification. Clin Orthop Relat Res 2007; 461:125-9.
- Persson PE, Nilsson OS, Berggren AM. Do non-steroidal anti-inflammatory drugs cause endoprosthetic loosening? A 10-year follow-up of a randomized trial on ibuprofen for prevention of heterotopic ossification after hip arthroplasty. Acta Orthop 2005; 76-6: 735-40.
- Meunier A, Aspenberg P, Good L. Celecoxib does not appear to affect prosthesis fixation in total knee replacement: A randomized study using radiostereometry in 50 patients. Acta Orthop 2009; 80-1: 46-50.
- Dahners LE, Gilbert JA, Lester GE, Taft TN, Payne LZ. The effect of a nonsteroidal antiinflammatory drug on the healing of ligaments. Am J Sports Med 1988; 16-6: 641-6.
- Elder CL, Dahners LE, Weinhold PS. A cyclooxygenase-2 inhibitor impairs ligament healing in the rat. Am J Sports Med 2001; 29-6: 801-5.
- Bogatov VB, Weinhold P, Dahners LE. The influence of a cyclooxygenase-1 inhibitor on injured and uninjured ligaments in the rat. Am J Sports Med 2003; 31-4: 574-6.
- Hanson CA, Weinhold PS, Afshari HM, Dahners LE. The effect of analgesic agents on the healing rat medial collateral ligament. Am J Sports Med 2005; 33-5: 674-9.
- Moorman CT, 3rd, Kukreti U, Fenton DC, Belkoff SM. The early effect of ibuprofen on the mechanical properties of healing medial collateral ligament. Am J Sports Med 1999; 27-6: 738-41.
- Hart DP, Dahners LE. Healing of the medial collateral ligament in rats. The effects of repair, motion, and secondary stabilizing ligaments. J Bone Joint Surg Am 1987; 69-8: 1194-9.
- Gomez MA, Woo SL, Amiel D, Harwood F, Kitabayashi L, Matyas JR. The effects of increased tension on healing medical collateral ligaments. Am J Sports Med 1991; 19-4: 347-54.
- Woo SL, Gomez MA, Sites TJ, Newton PO, Orlando CA, Akeson WH. The biomechanical and morphological changes in the medial collateral ligament of the rabbit after immobilization and remobilization. J Bone Joint Surg Am 1987; 69-8: 1200-11.
- Almekinders LC, Gilbert JA. Healing of experimental muscle strains and the effects of nonsteroidal antiinflammatory medication. Am J Sports Med 1986; 14-4: 303-8.
- Shen W, Prisk V, Li Y, Foster W, Huard J. Inhibited skeletal muscle healing in cyclooxygenase-2 gene-deficient mice: the role of PGE2 and PGF2alpha. J Appl Physiol 2006; 101-4: 1215-21.
- Kaastad TS, Reikeras O, Narum S, Madsen JE, Haug E, Obrant KJ, Nordsletten L. Effect of intensive training on lower leg structural strength: an in vivo study in ovariectomized rats. Scand J Med Sci Sports 1997; 7-4: 220-5.
- Madsen JE, Hukkanen M, Aune AK, Basran I, Moller JF, Polak JM, Nordsletten L. Fracture healing and callus innervation after peripheral nerve resection in rats. Clin Orthop Relat Res 1998-351: 230-40.
- Nordsletten L, Madsen JE, Almaas R, Rootwelt T, Halse J, Konttinen YT, Hukkanen M, Santavirta S. The neuronal regulation of fracture healing. Effects of sciatic nerve resection in rat tibia. Acta Orthop Scand 1994; 65-3: 299-304.
- Nordsletten L, Skjeldal S, Kirkeby OJ, Ekeland A. Muscle contraction increases the strength of healing tibial fracture in the rat. Acta Orthop Scand 1994; 65-2: 191-4.
- Melhus G, Solberg LB, Dimmen S, Madsen JE, Nordsletten L, Reinholt FP. Experimental osteoporosis induced by ovariectomy and vitamin D deficiency does not markedly affect fracture healing in rats. Acta Orthop 2007; 78-3: 393-403.
- Landefeld CS, Steinman MA. The Neurontin legacy–marketing through misinformation and manipulation. N Engl J Med 2009; 360-2: 103-6.
- Seidenberg AB, An YH. Is there an inhibitory effect of COX-2 inhibitors on bone healing? Pharmacol Res 2004; 50-2: 151-6.
- Paulson SK, Zhang JY, Breau AP, Hribar JD, Liu NW, Jessen SM, Lawal YM, Cogburn JN, Gresk CJ, Markos CS, Maziasz TJ, Schoenhard GL, Burton EG. Pharmacokinetics, tissue distribution, metabolism, and excretion of celecoxib in rats. Drug Metab Dispos 2000; 28-5: 514-21.
- Halpin RA, Geer LA, Zhang KE, Marks TM, Dean DC, Jones AN, Melillo D, Doss G, Vyas KP. The absorption, distribution, metabolism and excretion of rofecoxib, a potent and selective cyclooxygenase-2 inhibitor, in rats and dogs. Drug Metab Dispos 2000; 28-10: 1244-54.
- Vignery A, Baron R. Dynamic histomorphometry of alveolar bone remodeling in the adult rat. Anat Rec 1980; 196-2: 191-200.
- Engelbregt MJ, Houdijk ME, Popp-Snijders C, Delemarre-van de Waal HA. The effects of intra-uterine growth retardation and postnatal undernutrition on onset of puberty in male and female rats. Pediatr Res 2000; 48-6: 803-7.
- Ruth EB. Metamorphosis of the pubic symphysis. I. The white rat (Mus norvegicus albinus). Anat. Rec. 1935; 64-1: 1-7.
- Talley JJ, Bertenshaw SR, Brown DL, Carter JS, Graneto MJ, Kellogg MS, Koboldt CM, Yuan J, Zhang YY, Seibert K. N-[[(5-methyl-3-phenylisoxazol-4-yl)-phenyl]sulfonyl]propanamide, sodium salt, parecoxib sodium: A potent and selective inhibitor of COX-2 for parenteral administration. J Med Chem 2000; 43-9: 1661-3.
- Cheer SM, Goa KL. Parecoxib (parecoxib sodium). Drugs 2001; 61-8: 1133-41; discussion 42-3.
- Stichtenoth DO, Frolich JC. The second generation of COX-2 inhibitors: what advantages do the newest offer? Drugs 2003; 63-1: 33-45.
- Kranke P, Morin AM, Roewer N, Eberhart LH. Patients' global evaluation of analgesia and safety of injected parecoxib for postoperative pain: a quantitative systematic review. Anesth Analg 2004; 99-3: 797-806, table of contents.
- Romsing J, Moiniche S. A systematic review of COX-2 inhibitors compared with traditional NSAIDs, or different COX-2 inhibitors for post-operative pain. Acta Anaesthesiol Scand 2004; 48-5: 525-46.
- Halpin RA, Porras AG, Geer LA, Davis MR, Cui D, Doss GA, Woolf E, Musson D, Matthews C, Mazenko R, Schwartz JI, Lasseter KC, Vyas KP, Baillie TA. The disposition and metabolism of rofecoxib, a potent and selective cyclooxygenase-2 inhibitor, in human subjects. Drug Metab Dispos 2002; 30-6: 684-93.
- Paulson SK, Hribar JD, Liu NW, Hajdu E, Bible RH, Jr., Piergies A, Karim A. Metabolism and excretion of [(14)C]celecoxib in healthy male volunteers. Drug Metab Dispos 2000; 28-3: 308-14.
- Ekeland A, Engesaeter LB, Langeland N. Mechanical properties of fractured and intact rat femora evaluated by bending, torsional and tensile tests. Acta Orthop Scand 1981; 52-6: 605-13.
- Engesaeter LB, Ekeland A, Langeland N. Methods for testing the mechanical properties of the rat femur. Acta Orthop Scand 1978; 49-6: 512-8.
- Hilton A, Armstrong R. Stat Note 6, post hoc ANOVA tests. Microbiologist 2006; 7-3: 34-6.
- Brown KM, Saunders MM, Kirsch T, Donahue HJ, Reid JS. Effect of COX-2-specific inhibition on fracture-healing in the rat femur. J Bone Joint Surg Am 2004; 86-A-1: 116-23.
- Li M, Ke HZ, Qi H, Healy DR, Li Y, Crawford DT, Paralkar VM, Owen TA, Cameron KO, Lefker BA, Brown TA, Thompson DD. A novel, non-prostanoid EP2 receptor-selective prostaglandin E2 agonist stimulates local bone formation and enhances fracture healing. J Bone Miner Res 2003; 18-11: 2033-42.
- Huntjens DR, Danhof M, Della Pasqua OE. Pharmacokinetic-pharmacodynamic correlations and biomarkers in the development of COX-2 inhibitors. Rheumatology (Oxford) 2005; 44-7: 846-59.
- Hausman MR, Schaffler MB, Majeska RJ. Prevention of fracture healing in rats by an inhibitor of angiogenesis. Bone 2001; 29-6: 560-4.
- Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med 1999; 5-6: 623-8.
- Jones MK, Wang H, Peskar BM, Levin E, Itani RM, Sarfeh IJ, Tarnawski AS. Inhibition of angiogenesis by nonsteroidal anti-inflammatory drugs: insight into mechanisms and implications for cancer growth and ulcer healing. Nat Med 1999; 5-12: 1418-23.
- Lescaudron L, Peltekian E, Fontaine-Perus J, Paulin D, Zampieri M, Garcia L, Parrish E. Blood borne macrophages are essential for the triggering of muscle regeneration following muscle transplant. Neuromuscul Disord 1999; 9-2: 72-80.
- Robertson TA, Maley MA, Grounds MD, Papadimitriou JM. The role of macrophages in skeletal muscle regeneration with particular reference to chemotaxis. Exp Cell Res 1993; 207-2: 321-31.
- Cantini M, Massimino ML, Bruson A, Catani C, Dalla Libera L, Carraro U. Macrophages regulate proliferation and differentiation of satellite cells. Biochem Biophys Res Commun 1994; 202-3: 1688-96.
- Tidball JG. Inflammatory cell response to acute muscle injury. Med Sci Sports Exerc 1995; 27-7: 1022-32.
- Paralkar VM, Grasser WA, Mansolf AL, Baumann AP, Owen TA, Smock SL, Martinovic S, Borovecki F, Vukicevic S, Ke HZ, Thompson DD. Regulation of BMP-7 expression by retinoic acid and prostaglandin E(2). J Cell Physiol 2002; 190-2: 207-17.
- Virchenko O, Fahlgren A, Skoglund B, Aspenberg P. CDMP-2 injection improves early tendon healing in a rabbit model for surgical repair. Scand J Med Sci Sports 2005; 15-4: 260-4.
- Forslund C, Aspenberg P. Tendon healing stimulated by injected CDMP-2. Med Sci Sports Exerc 2001; 33-5: 685-7.
- Forslund C, Aspenberg P. Improved healing of transected rabbit Achilles tendon after a single injection of cartilage-derived morphogenetic protein-2. Am J Sports Med 2003; 31-4: 555-9.
- Myers LK, Bhattacharya SD, Herring PA, Xing Z, Goorha S, Smith RA, Bhattacharya SK, Carbone L, Faccio R, Kang AH, Ballou LR. The isozyme-specific effects of cyclooxygenase-deficiency on bone in mice. Bone 2006; 39-5: 1048-52.
- Ott E, Nussmeier NA, Duke PC, Feneck RO, Alston RP, Snabes MC, Hubbard RC, Hsu PH, Saidman LJ, Mangano DT. Efficacy and safety of the cyclooxygenase 2 inhibitors parecoxib and valdecoxib in patients undergoing coronary artery bypass surgery. J Thorac Cardiovasc Surg 2003; 125-6: 1481-92.