Abstract
Background and purpose The methods of reconstruction for proximal femur bone tumors that are used most often include modular prosthetic replacement and allograft-prosthesis composite reconstruction. In modular prostheses, the abductors are detached from the insertion and then reinserted into the implant, and the iliopsoas is detached and left free. In the allograft-prosthesis composite, the detached tendons are fixated to the graft. We assessed whether the latter procedure provides functional advantages regarding gait.
Patients and methods We studied 2 groups of 10 patients, each with prosthetic reconstruction of the proximal femur either with modular prosthetic replacement or with allograft-prosthesis composite. Functional performance was analyzed by gait analysis 2.5–10 years after surgery. At that time, all the patients had good function according to the Musculoskeletal Society score.
Results Walking speed was reduced in all patients, and especially in patients with modular prosthetic replacement. Different hip extension patterns during late stance were found in the 2 groups. Surface EMG showed a typical prolonged muscle co-contraction pattern during gait, which was more evident in modular prosthetic patients.
Interpretation Although both procedures provided good functional outcome in the long-term follow-up, gait analysis revealed mechanical changes during gait that were probably related to the muscle reinsertion procedure. Direct fixation of the muscles to the bone graft appeared to result in a more efficient muscular recovery.
The 2 most widely used techniques for reconstruction after resection of a tumor in the proximal femur are modular prosthetic replacement (MP) and allograft-prosthetic composite reconstruction (APC) (Unwin et al. Citation1996, Giurea et al. Citation1998, Bickels et al. Citation2000, Fox et al. Citation2002). The most commonly used MP prostheses are designed with a trochanter muscle insertion device that allows direct fixation of the gluteus medius to the prosthesis (Kotz et al. Citation1986, Bickels et al. Citation2000). This kind of fixation may be insufficient, with lack of strength of the gluteal muscles and possible joint instability and impaired function (Schreiber et al. 1991, Rechl et al. Citation1999). Alternatively, the abductor muscles can be reinserted into the fascia lata—but also with impaired function (Giurea et al. Citation1998, Gottsauner-Wolf et. 1999, Anderson et al. Citation2002). The iliopsoas muscle is usually not re-attached, but is left free to heal without any fixation or is rotated anteriorly to close and reinforce the hip capsular repair. Apart from poor function, several authors have reported aseptic loosening and instability (Zwart et al. Citation1994, Sanjay and Moreau Citation1999, Mittermayer et al. Citation2001, Menedez et al. 2006, Chandrasekar et al. Citation2009).
The allograft-prosthesis composite (APC) implant was recently designed to reduce these complications. This implant is composed of a revision-type prosthesis inserted inside a bone allograft to which the residual abductors and the iliopsoas muscle tendons are biologically reinserted, which should reduce the risk of postoperative dislocation and give better function (Gitelis et al. Citation1988, Zehr et al. Citation1996, Giurea et al. Citation1998, Anract et al. Citation2000, Langlais et al. Citation2003, Farid et al. Citation2006, Biau et al. Citation2008, Donati et al. Citation2008, 2011). In a comparative study on MP and APC, however, Zehr et al. (Citation1996) found no differences in function and survival. In our own experience (Donati et al. Citation2001, 2002), function in APC patients—when assessed by the MSTS score—compared favorably with that in MP patients in whom a Trendelenburg gait was present in most cases. In almost all of these studies, however, the functional outcome was assessed by scoring systems that have recently been questioned for not providing objective and quantitative information about functional recovery (Rompen et al. Citation2002, Rosenbaum et al. Citation2008). Functional outcome has seldom been evaluated with laboratory-based computer-assisted gait analysis.
In the present study, using gait analysis we objectively assessed walking ability in patients treated with the APC implant or with the MP system with long-term follow-up. Our hypothesis was that the APC implant would provide better control of the hip during gait both in the sagittal plane and the coronal plane, due to the “biological” reconstruction of muscles.
Patients and methods
2 groups of patients were retrospectively recruited from subjects treated at the Rizzoli Institute with proximal femur bone tumor resection, either with modular prosthetic replacement (MP) or allograft-prosthesis composite (APC). The inclusion criteria were: (1) presence of gluteus medius tendon to be re-attached onto the trochanter of the implant; (2) absence of implant complications; (3) no local or distant tumor recurrence; (4) no neurological damage due to surgery or chemotherapy; (5) good overall function (80% of the maximum score) assessed with the Musculoskeletal Tumor Society (MSTS) scoring system (Enneking et al. Citation1993). The study was approved by the local scientific committee and all the patients signed an informed consent form.
The MP group consisted of 10 men with a mean age of 41 (25–47) years. In all cases, the implant used was the KMFTR (Stryker-Howmedica, Kiel, Germany). The mean length of resection was 16 (12–20) cm; 6 patients received neoadjuvant chemotherapy. The mean follow-up time after surgery at the time of gait analysis was 118 (45–179) months.
The APC group consisted of 7 men and 3 women with a mean age of 31(19–59) years. The mean length of resection was 14 (6–20) cm; 4 patients received neoadjuvant chemotherapy. The mean follow-up time was 60 (31–124) months ().
Table 1. General data on patients
In both groups, the surgical technique consisted of intraarticular resection of the proximal femur and excision of part of the vastus. The rectus was always spared; the gluteus medius muscle and iliopsoas were always detached, but leaving enough muscle length to be reinserted onto the implant. The 2 muscles were always reinserted onto the allograft tendon in the APC group, whereas in the MP group only the gluteus medius was re-attached using the trochanter device of the prosthesis and the iliopsoas was left free. After surgery, all patients were mobilized with 2 crutches and no weight bearing was allowed for 1 month. Then partial weight bearing was allowed along with muscle strengthening, joint mobilization, and walking recovery. In the APC group, full weight bearing was allowed after the osteotomy line had healed (3 months), whereas in the MP group free walking was permitted during the second postoperative month. In both groups, gait rehabilitation lasted not less than 1 year after the surgical procedure.
Clinical assessment
The manual muscle test (Medical Research Council scale) was used to score the strength in the following groups of hip muscles: extensors and flexors, abductors, adductors (minimum score 1, maximum 5). Functional evaluation was based on the Musculoskeletal Tumor Society (MSTS) (Enneking et al. Citation1993) scoring system, with numerical values from 0 to 5 points assigned for each of the following 6 categories: pain, function, emotional acceptance, use of supports, walking ability, and gait. The patient’s score for the various parameters was expressed as a percentage of the total possible score of 30 points.
Instrumental assessment
Functional evaluation of gait consisted of the acquisition of 3 walking trials along a 10-meter walkway. Gait was assessed using the 3D ELITE system (BTS, Milan), which estimates the body segment movements in space and measures kinematic and kinetic parameters. Marker positioning and kinematics analysis were performed according to the CAST protocol (Cappozzo et al. Citation1995). An electromyographic telemetry system (Telemg; BTS) was used to record the surface EMG signal from 8 muscles: bilateral erector spinae muscle (HES homolateral, CES contralateral) and in the treated limb, gluteus medius (GM), rectus femoris (RF), medial hamstrings (MH), lateral hamstrings (LH), gastrocnemius (GAS), and tibialis anterior (TA). The EMG signals were acquired at the same time as the kinematic and kinetic data, and were then processed offline by means of a statistical detection algorithm to obtain muscle on-off timing (Bonato et al. Citation1998), normalized to the duration of the stride. The data from both groups were compared with those from 10 healthy volunteers (mean age 28 (20–33) years, 7 men) (Benedetti et al. Citation1998).
Statistics
Relevant parameters were extracted from the gait analysis curves in order to allow a comparative statistical analysis. The ANOVA test was used for parameters with homogeneity of variance and the Sheffe test was used for paired analysis. For parameters in which the Levene test for homogeneity of variances was significant, the non-parametric Kruskal-Wallis test was used, followed by the paired analysis according to the Mann-Whitney test. All statistical analyses were performed using SPSS version 11.0.
Results
Clinical assessment
The manual muscle test showed reduced strength (mean 4) in all the hip muscles tested. Although muscular strength was always less in the MP group, particularly for hip abductors, these differences were not statistically significant (Figure 1, see supplementary data).
The mean MSTS score in the MP group was 87% (80–93) and in the APC group it was 90% (81–99) (). In the APC group 2 patients had no restrictions, 4 had restrictions in recreational activities, and 4 had restrictions in occupational activities. In the MP group, 1 patient had no restrictions and 9 patients had restrictions in recreational activities. In the MP group, 2 patients reported no pain, 3 reported mild pain, and 5 had an intermediate score between the first 2. In the APC group 6 patients reported no pain, 1 reported mild pain, and 3 had an intermediate score between the first 2. Limping was clinically present in 4 patients in the MP group (1 mild and 3 moderate) and in 3 ACP patients (mild).
Instrumental assessment
Time-distance parameters. In both groups there was reduced cadence, which was lower in the MP group (p < 0.001) (Table 2, see supplementary data). Stride length (normalized to height) and speed of progression were reduced in both groups, the latter substantially in the MP group (p = 0.006).
Kinematic parameters. No statistically significant difference was found in movement of the pelvis in the coronal and transverse planes relative to the controls, whereas in the sagittal plane the anterior tilt during stance was reduced in both groups.
At heel contact, the hip flexion angle was significantly reduced in both groups (Table 2, see supplementary data). The APC group showed a more flexed hip in stance phase (p = 0.004) and at toe-off. Conversely, at toe-off, the MP group showed greater hip extension.
The range of hip motion in the sagittal and coronal planes was significantly reduced in both groups, as was hip flexion in the swing phase. Compared to the control patients, however, the MP group showed reduced hip adduction in stance and increased abduction in the swing. In the transverse plane, both groups showed greater internal rotation in stance phase than the controls (p = 0.001).
Kinetic parameters. Consistently with kinematics, the extensor moment was reduced in the MP group (p = 0.003). Both groups had reduced hip flexion, adduction moment, and external rotation moments (p < 0.001) (Table 2, see supplementary data).
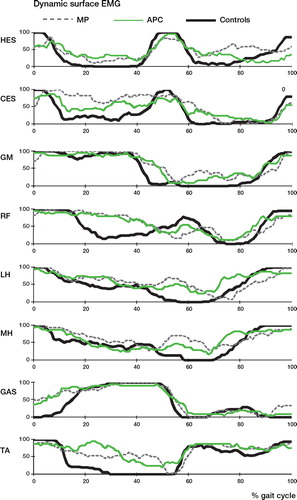
Surface dynamic EMG. In all the MP patients and in 50% of the APC patients, EMG showed a prolonged activation of the erector spinae muscles contralateral to the operated limb during the stance phase. Some patients, particularly in the MP group, also presented activation of the homolateral spinal erectors in the swing phase. Another recurrent abnormality was the presence of a peak of hamstring activity (both medial and lateral) in the transition between the stance and the swing phase, which was more frequent in the MP group (). Furthermore, all patients in both groups presented a prolonged activation of the rectus femoris in co-contraction with hamstrings in about 70% of cases, and prolonged activity of tibialis anterior in co-contraction with the gastrocnemius, which was also prematurely activated during stance.
Figure 2. Patterns of muscular activity presented as a percentage of the number of patients with on/off muscle activity during the gait cycle (compared to control data). HES: homolateral erector spinae; CES: contralateral erector spinae; GM: gluteus medius; RF: rectus femoris; MH: medial hamstrings; LH: lateral hamstrings; GAS: gastrocnemius; TA: tibialis anterior.
Discussion
Reconstruction of a large part of the proximal femur after bone tumor resection can lead to severe gait impairment. Both the prosthetic device implant and muscle detachment and reinsertion can account for gait abnormalities.
Although good functional recovery (MSTS higher than 80%) was an inclusion criterion in this study, generally MP patients more often had pain problems or restriction in activities than APC patients. Also, evaluation of hip muscles in the MP group showed a greater reduction in the strength of hip flexors and hip abductors than in the APC group, but without there being any statistically significant differences. The reduction in muscular strength, already described in literature (Schreiber et al. 1991, Rechl et al. Citation1999, Farid et al. Citation2006, Lee et al. Citation2009), is an expected consequence because—as already stated—the operation requires the detachment and reinsertion of hip muscles, which can lead to change of tension and suboptimal lever arm.
In the last few years, gait analysis has emerged as an assessment tool for the functional outcome of limb salvage procedures, but there have been very few studies on patients with hip replacement for bone tumors (De Visser et al. Citation2000, 2003, Kawai et al. Citation2000, Rompen et al. Citation2002). To our knowledge, this is the first time that gait function has been quantitatively investigated in APC patients and compared to that in MP subjects with different fixation of residual muscles.
In agreement with the literature (De Visser et al. Citation2000, Kawai et al. Citation2000, Rompen et al. Citation2002), MP patients had reduced walking speed due both to a reduction in cadence and to a reduction in stride length, not only compared to the controls but also compared to the APC patients. From a kinematic point of view, in the sagittal plane, MP patients maintained the hip more extended than the control group at toe-off, with a consistent reduction in the extensor moment and increased activity of the hamstrings in this gait phase. This hip extension pattern was also found by Rompen et al. (Citation2002) in most of their patients. Although the low speed of progression could account for this, it is reasonable to believe that this pattern may be related to loss of function of the iliopsoas muscle (detached and left free in these patients), which in this phase of gait contributes to the flexion of the hip joint to trigger the swing phase. The limb advancement would be compensated for by the increased hip abduction and by the homolateral erector spinae muscle activation in the swing phase, evident in the MP patients.
In the coronal plane, no true Trendelemburg sign was evident from gait analysis data (the pelvis does not show any upper displacement on the operated side). However, the hip patients tended to have reduced adduction in stance phase on the operated side. Since the gluteus medius is reinserted onto the prosthesis in MP patients, according to Kaway et al. (2000) it can be inferred that its performance in stabilizing the pelvis under loading is not optimal.
APC patients walked with higher speed of progression than did MP patients, because of higher cadence. From a kinematic point of view, in contrast to MP patients, these patients showed a less extended hip both during stance and at toe-off. In most of the APC patients also, increased hamstring activity was evident in this phase. Since APC reconstruction implies iliopsoas reinsertion at the allograft tendon, it is possible that in some cases a suboptimal tensioning was achieved or a protective pattern of the allograft was adopted by the patients, resulting in a functionally flexed hip. Unfortunately, we do not have data on passive extension of hip range of motion in patients as the MSTS score only assesses the active hip flexion. On the coronal plane, APC patients tend to have a slightly greater adduction in the stance phase than MP patients. Although this difference was not statistically significant, it supports the hypothesis that the “biological” reinsertion of muscle to the graft results in more efficient recovery of the abductor mechanism (Giurea et al. Citation1998).
The above-mentioned gait abnormalities are reflected in the change in EMG pattern in the affected leg. In all the MP patients, erector spinae muscles controlateral to the operated limb are extremely active during stance, and their contraction could be considered to be compensation aimed at controlling the pelvis moving the trunk toward the support limb, thus reducing the adduction moment and therefore reducing the need for abductors use. Furthermore, in 6 of 10 MP patients the abnormal activation of the ipsilateral erector spinae muscles during the mid-swing could be interpreted as an auxiliary mechanism to advance the affected limb through elevation of the pelvis, as found in the kinematic data.
Finally, EMG records showed a typical prolonged co-contraction pattern during stance phase, both for the hamstrings-quadriceps and for tibialis anterior-gastrocnemius muscle couples, which was more often evident in MP patients. Prolonged quadriceps activation, in most patients in co-contraction with hamstrings, was already reported in the literature in patients with femoral prosthesis (Bach Citation1996). In a series of 10 patients with proximal femur prosthesis or saddle prostheses, De Visser et al. (Citation2000) described a prominent burst at the stance-swing transition for the biceps femoris, which was interpreted as a task in active flexion of the knee. De Visser described similar findings for tibialis anterior-gastrocnemius, and these were explained as a need for joint stabilization in patients—where the resection of muscles, ligaments, and joint structures can cause a loss of proprioceptive feedback, which is normally important for the control of locomotion.
Our study had some limitations. The number of patients was small; many of them encountered problems during the postoperative period which meant that they were not eligible for the study, or they did not survive. The difference in follow-up after surgery was the second limitation: the mean follow-up time in the MP group was 10 years, as opposed to 5 years in the APC group. The reason for this difference was mainly due to the later introduction of the APC procedure at our institute. However, we believe that additional follow-up time would not have changed the functional performance substantially.
In conclusion, even in patients with a good functional score (MSTS > 80%), when function was assessed by gait analysis, subtle impairment of gait was found depending on the surgical procedure.
Supplementary data
Figure 1 and Table 2 are available at our website (www.actaorthop.org), identification number 5474
Supplementary Material
Download PDF (45.9 KB)MGB conceived and designed the study, analyzed and interpreted the data, and drafted the article. EB acquired data. CM analyzed the data and also drafted the article. DD was the surgeon who operated the patients; he also conceived and designed the study and revised the article.
No competing interests declared.
- Anderson ME, Hyodo A, Zehr RJ, Marks KE, Muschler GF. Abductor reattachment with a custom proximal femoral replacement prosthesis. Orthopedics 2002; 25 (7): 722-6.
- Anract P, Coste J, Vastel L, Jeanrot C, Mascard E, Tomeno B. Proximal femoral reconstruction with megaprosthesis versus allograft prosthesis composite: a comparative study of functional results, complications and longevity in 41 cases. Rev Chir Orthop Reparatrice Appar Mot 2000; 86 (3): 278-88.
- Bach CM. Gait analysis in patients with distal femoral resection replaced by a hinge knee design. Wien Klin Wochenschr 1996; 108 (6): 184-6.
- Benedetti MG, Catani F, Leardini A, Pignotti E, Giannini S. Data management in gait analysis for clinical applications. Clin Biomech (Bristol. Avon) 1998; 13 (3): 204-15.
- Biau DJ, Davis A, Vastel L, Tomeno B, Anract P. Function, disability, and health-related quality of life after allograft-prosthesis composite reconstructions of the proximal femur. J Surg Oncol 2008; 97: 210–5.
- Bickels J, Meller I, Henshaw RM, Malawer M. Reconstruction of hip joint stability after proximal and total femur resections. Clin Orthop 2000; (375): 218–30.
- Bonato P, D'Alessio T, Knaflitz M. A statistical method for the measurement of muscle activation intervals from surface myoelectric signal during gait. IEEE Trans Biomed Eng 1998; 45 (3): 287-99.
- Cappozzo A, Catani F, Della Croce U, Leardini A. Position and orientation of bones during movement: anatomical frame definition and determination. Clin Biomech 1995; 10: 171-8.
- Chandrasekar CR, Grimer RJ, Carter SR, Tillman RM, Abudu A, Buckley L. Modular endoprosthetic replacement for tumours of the proximal femur. J Bone Joint Surg (Br) 2009; 91 (1): 108-12.
- De Visser E, Mulder T, Schreuder HW, Veth RP, Duysens J. Gait and electromyographic analysis of patients recovering after limb-saving surgery. Clin Biomech (Bristol. Avon) 2000; 15 (8): 592–9.
- De Visser E, Veth RP, Schreuder HW, Duysens J, Mulder T. Reorganization of gaitafter limb-saving surgery of the lower limb. Am J Phys Med Rehabil 2003; 82 (11): 825-31.
- Donati D, Zavatta M, Gozzi E, Giacomini S, Campanacci L, Mercuri M. Modular prosthetic replacement of the proximal femur after resection of a bone tumour. J Bone Joint Surg (Br) 2001; 83 (8): 1156-60.
- Donati D, Giacomini S, Gozzi E, Mercuri M. Proximal femur reconstruction by an allograft prosthesis composite. Clin Orthop 2002; (394): 192-200.
- Donati D, Colangeli M, Colangeli S, Di Bella C, Mercuri M. Allograft-prosthetic composite in the proximal tibia after bone tumor resection. Clin Orthop 2008; (466) (2): 459-65.
- Donati D, Di Bella C, Frisoni T, Cevolani L, DeGroot H. Alloprosthetic composite is a suitable reconstruction after periacetabular tumor resection. Clin Orthop 2011; (469) (5): 1450-8.
- Enneking WF, Dunham W, Gebhardt MC, Malawer M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the Musculoskeletal System. Clin Orthop 1993; (286): 241-6.
- Farid Y, Lin PP, Lewis VO, Yasko AW. Endoprosthetic and allograft prosthetic composite for reconstruction of the proximal femur bone neoplasms.Clin Orthop. 2006; (442): 223–9.
- Fox EJ, Hau MA, Gebhardt MC, Hornicek FJ, Tomford WW, Mankin HJ. Long-term follow up of proximal femoral allografts. Clin Orthop 2002; (397): 106-13.
- Gitelis S, Heligman D, Quill G, Piasecki P. The use of large allografts tumor reconstruction and salvage of the failed total hip arthroplasty. Clin Orthop 1988; (231): 62-70.
- Giurea A, Paternostro T, Heinz-Peer G, Kaider A, Gottsauner-Wolf F. Function of reinserted abductor muscles after femoral replacement. J Bone Joint Surg (Br) 1998; 80 (2): 284-7.
- Gottsauner-Wolf F, Egger EL, Giurea A, Antosch M, Olsen D, Rock MG, Sim FH. Biologic attachment of an allograft bone and tendon transplant to a titanium prosthesis. Clin Orthop 1999; (358): 101-10.
- Kawai A, Backus SI, Otis JC, Inoue H, Healey JH. Gait characteristics of patients after proximal femoral replacement for malignant bone tumour. J Bone Joint Surg (Br) 2000; 82 (5): 666-9.
- Kotz R, Ritschl P, Trachtenbrodt J. A modular femur-tibia reconstruction system. Orthop 1986; 9 (12): 1639-52.
- Langlais F, Lambotte JC, Collin P, Thomazeau H. Long-term results of allograft composite total hip prostheses for tumors. Clin Orthop 2003; (414): 197-211.
- Lee SH, Ahn YJ, Chung SJ, Kim BK, Hwang JH. The use of allograft prosthesis composite for extensive proximal femoral bone deficiencies: a 2- to 9.8-year follow-up study. J Arthrop 2009; 24 (8): 1241-8.
- Menendez LR, Ahlmann ER, Kermani C, Gotha H. Endoprosthetic reconstruction for neoplasms of the proximal femur. Clin Orthop 2006; (450): 46-51.
- Mittermayer F, Windhager R, Dominkus M, Krepler P, Schwameis E, Sluga M, Kotz R, Strasser G. Long-term follow up of uncemented tumor endoprostheses for the lower extremity. Clin Orthop 2001; 388: 167-77.
- Rechl H, Reinisch M, Plotz W, Burgkart R, Gradinger R. Soft tissue reconstruction about the proximal femur. Oper Tech Orthop 1999; 9 (2): 115- 20.
- Rompen JC, Ham SJ, Halbertsma JP, van Horn JR. Gait and function in patients with a femoral endoprosthesis after tumor resection: 18 patients evaluated 12 years after surgery. Acta Orthop Scand 2002; 73 (4): 439–46.
- Rosenbaum D, Brandes M, Hardes J, Gosheger G, Rodl R. Physical activity levels after limb salvage surgery are not related to clinical scores-objective activity assessment in 22 patients after malignant bone tumor treatment with modular prostheses. J Surg Oncol 2008; 98: 97–100.
- Sanjay BKS, Moreau PG. Limb salvage surgery in bone tumour with modular endoprosthesis. Int Orthop 1999; 23: 41-6.
- Schreiber A, Exner GU, von Hochstetter AR. Muscle reattachment – especially the gluteal muscles – after proximal femoral resection and replacement by tumor prosthesis. In: Limb salvage: major reconstructions in oncologic and nontumoral conditions (ed Langlais F, Tomeno B. Berlin). Springer Verlag 1991; 395-8.
- Unwin PS, Cannon SR, Grimer RJ, Kemp HB, Sneath RS, Walker PS. Aseptic loosening in cemented custom-made prosthetic replacements for bone tumours of the lower limb. J Bone Joint Surg (Br) 1996; 78 (1): 5-13.
- Zwart HJ, Taminiau AH, Schimmel JW, van Horn JR. Kotz modular femur and tibia replacement. Acta Orthop Scand 1994; 65 (3): 315-8.
- Zehr RJ, Enneking WF, Scarborough MT. Allograft-prosthesis composite versus megaprosthesis in proximal femoral reconstruction. Clin Orthop 1996; (322): 207-23.