Acknowledgements
This research was supported by grants from Sophies Mindes Research Foundation, Orthopaedic department “Kjøp smart fond” Rikshospitalet, The Aase Bye and Trygve J. B. Hoffs Fond for Medical Scientific Research, Norway and Adlerbert Research Foundation, Wilhelm, Martina Research Foundation and Hjalmar Svensson Research Foundation, The Swedish Research Foundation, Sweden.
| List of abbreviations | ||
| 4CF | = | Four corner fusion |
| ASA | = | American Society of Anesthesiologists |
| ASTM | = | American Society for Testing and Materials |
| c.p | = | Commercially pure |
| CaP | = | Calcium phosphate |
| CMC | = | Carpometacarpal |
| Co-Cr-Mo | = | Cobalt-chrome-molybdenum |
| CPP | = | Calcium phosphate phases |
| DASH | = | Disability of arm, shoulder and hand |
| DIP | = | Distal interphalangeal joint |
| DRUJ | = | Distal radioulnar joint |
| UD | = | Ulnar deviation |
| ECRB | = | Extensor carpi radialis brevis |
| ECU | = | Extensor carpi ulnaris |
| ERCL | = | Extensor carpi radialis longus |
| FDP | = | Flexor digitorum profundus |
| FDS | = | Flexor digitorum superficialis |
| FPL | = | Flexor pollicis longus |
| HA | = | Hydroxyapatite |
| ISO | = | International Organization for Standardization |
| MCP | = | Metacarpophalangeal joint |
| MOM | = | Metal-on-metal |
| MOP | = | Metal-on-polyethylene |
| NZW | = | New Zealand White |
| PIP | = | Proximal interphalangeal joint |
| PRC | = | Proximal row carpectomy |
| PRUJ | = | Proximal radioulnar joint |
| RD | = | Radial deviation |
| RTQ | = | Removal torque |
| SLAC | = | Scapho-lunate advanced collapse |
| SNAC | = | Scaphoid non-union advanced collapse |
| TFCC | = | Triangular fibrocartilage complex |
| THR | = | Total hip replacement |
| Ti6Al4V | = | Titanium-6Alumina-4Vanadium |
| TKR | = | Total knee replacement |
| TWA | = | Total wrist arthroplasty |
| UHMWPE | = | Ultra high molecular weight polyethylene |
| WA | = | Wrist arthroplasty |
Introduction
Motion
The radiocarpal and midcarpal motion comprises flexion (volar flexion), extension (dorsal flexion), radial- and ulnar deviation, and slight rotation (Palmer et al. Citation1985). The dorso-volar movement primarily occurs in the radiocarpal joint during which the scaphoid flexes/extends and rotates, while the lunate mainly flexes/extends. The remaining flexion and extension occurs in the midcarpal joint. Radio-ulnar deviation is mainly accomplished by a minor radiocarpal translation and angulation (with flexion of the scaphoid/extension of the capitate in RD and the opposite in UD) and a larger midcarpal angulation mainly by the capitate, making the midcarpal joint the major contributor to this particular motion (Craigen and Stanley Citation1995, Kaufmann et al. Citation2005). The mechanical center of rotation of the wrist was described by Youm and associates (1978), as being located in the proximal part of the capitate in the anterior and lateral planes. More recently, much attention has been focused on the so-called dart throwers motion (DTM), the movement of the hand from dorsoradially to ulnavolarly, a mobility exclusive for humans (Rohde et al. Citation2010). So-called dart-throwing is the maximum unrestricted motion the wrist can perform, and it is possible due to the lack of constraining ligaments between the lunate and the capitate. The DTM utilizes the midcarpal joint to the largest extent, which has its greatest freedom of motion in this oblique plane, and not in the coronal plane (Moritomo et al. Citation2007). Crucial to a wide range of wrist motions, it is important to preserve some radiodorsal and ulnovolar movement (Kijima and Viegas Citation2009). A recent report found the mechanical axis of the wrist to be oriented in the same plane as the DTM, obliquely to the direction of the flexion-extension (Crisco et al. Citation2011).
Biomechanics
The axis of forearm rotation passes near the centre of the radial head proximally and that of the ulnar head distally (though varying with load). Due to the difference in diameter, the maximum articular contact area over the DRUJ reaches 60% in the neutral position compared to less than 10% at the rims of the notch. The stability of the joint is enhanced by a bone rim on the dorsal side, and a cartilaginous lip on the palmar side, as well as the primary stabilizing ligaments, the TFCC, the ECU tendon sheath and secondary stabilizing structures mentioned above.
The carpal load has been stipulated to 10 times the applied force at the tip of the fingers, and can reach more than 500 kg in an adult man (Rikli et al. Citation2007). The midcarpal load is mainly transmitted through the scapho-lunate-capitate joint, less via the ulnar side of the wrist (Viegas et al. Citation1993). The load is transmitted from the carpus to the forearm in the neutral position mainly through the radiocarpal joint (80%), less through the ulnocarpal joint (20%). This changes with the position of the wrist. The load is increasingly transmitted through the ulnar side when the hand turns ulnawards and rotates into pronation (up to 50%) (Teurlings et al. Citation2000). The opposite occurs when the wrist moves towards radial deviation and supination.
Wrist degeneration
Inflammatory arthritis
Rheumatoid- and other inflammatory arthritis has previously been the major cause of generalized wrist joint destruction. The majority of patients with rheumatoid arthritis (RA) experience arthritis of the wrist joint, the third most frequent joint afflicted after the MCP and PIP joints. 50% of patients develop wrist symptoms within the first two years after the onset of the disease, and more than 90% within 10 years. 95% of the patients have bilateral wrist affection (Trieb Citation2008). Improved medical treatment has diminished the need for surgery during the past 10–15 years after peaking in the nineties. However, the incidence of the disease is the same and the majority of patients are in need of treatment (Louie and Ward Citation2010). The new biological therapeutic options (TNFα inhibitors) are very efficient, but due to their side effects the first choice for all patients is still the more traditional drugs (disease modifying anti-rheumatic drugs (DMARD’s), NSAIDS and cortisone) (Scott et al. Citation2010). More effective medication can to a larger extent preserve the patients’ joints but the need for surgery will probably arise later in the patient’s life, thus delaying, but not obliviating the requirement for wrist surgery in the future. RA and inflammatory wrist degeneration are characterized by panarthritis, influencing all surfaces and seldom leaving any part of the joint uninjured. Synovectomies are performed for painful inflammation, and to prevent extensor tendon rupture, but do not reduce the cartilage damage or concomitant degenerative changes. Limited fusions have been used, particularly radio-lunate or radio-scapho-lunate fusion, giving a stable and less painful wrist, at the expense of mobility. Resections are seldom an alternative for RA patients because the increased instability encountered can be especially problematic for these patients.
Osteoarthritis
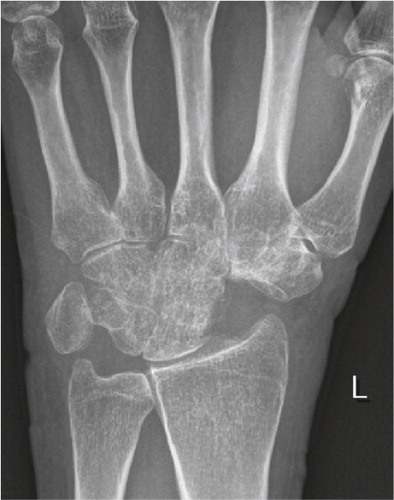
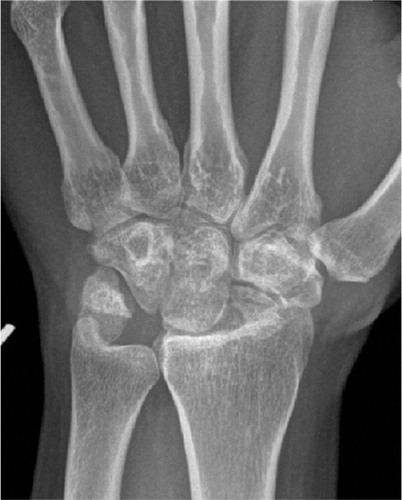
The non-inflammatory causes of wrist degeneration include post-traumatic conditions (sequelae after distal radius fracture, scaphoid fracture, and scapho-lunate and intercarpal ligament injuries), primary osteoarthritis and miscellaneous other disorders (septic arthritis, Kienböcks disease, Preisers disease and iatrogenic joint injury). Distal radius fracture and scaphoid fracture are very common injuries. Together they have an annual incidence of about 42/10,000 (Hove et al. Citation1995, Hove Citation1999), giving more than 20,000 fractures in Norway (of which 1,500–2,000 are scaphoid fractures) per year. Radiocarpal wrist degeneration after distal radius fracture is relatively rare. It is seen after complex intraarticular fractures where traumatic damage to the cartilage or residual untreated joint incongruence leads to degenerative arthritis (Catalano et al. Citation1997). The majority of fractures are ekstraarticular and advances in operative fracture treatment have reduced the incidence of secondary degeneration of the cartilage ().
SNAC and SLAC osteoarthritis
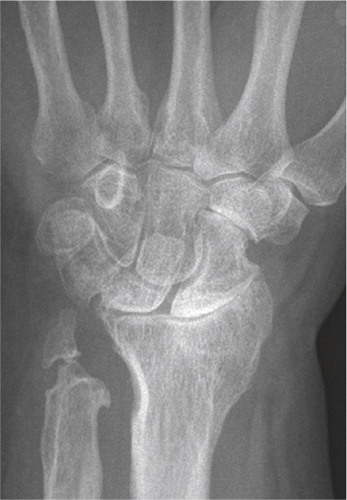
SNAC and SLAC changes describe the characteristic degeneration observed with time in untreated scaphoid non-union or scapholunate ligament injury. Whether it is due to a non-union or ligament rupture the scaphoid is unable to resist the collapsing tendency of the proximal row of carpal bones and this is the common aetiology behind the degenerative changes observed. The forces acting over the wrist press the scaphoid into flexion and the remaining proximal row into extension, with a concomitant shortening and collapse of the carpus. Degenerative changes are first seen between the radial styloid tip and the distal scaphoid. The arthrosis progresses between the radius and scaphoid distal to the fracture/non-union (in scaphoid non-union patients) or the whole radio-scaphoid joint (in scapho-lunate injuries). Further progression occurs in the midcarpal joint, between the scaphoid and lunate proximally and the capitate distally. The characteristic pattern that occurs is divided into three stages: SNAC/SLAC 1 (osteophytes at the radial styloid), SNAC/SLAC 2 (radio-scaphoid degeneration) and SNAC/SLAC 3 (midcarpal/scapho-luno-capitate degeneration) (Cooney et al. Citation1984, Watson and Ballet Citation1984). Up to 75–100% of patients with longstanding scaphoid non-union (> 5–10 years) demonstrate degenerative changes (Inoue and Sakuma Citation1996). In patients successfully treated for scaphoid fracture or non-union (without degenerative changes at the time of treatment), the degenerative process seems to halt. In patients with degenerative changes at surgery (i.e. non-unions) the degenerative process slows, but progression can be expected (Reigstad et al. Citation2009, Citation2012). For S-L ligament injuries there are no epidemiological or incidence surveys, hence an estimation of the need for surgery due to degeneration is difficult ().
Lunate malacia
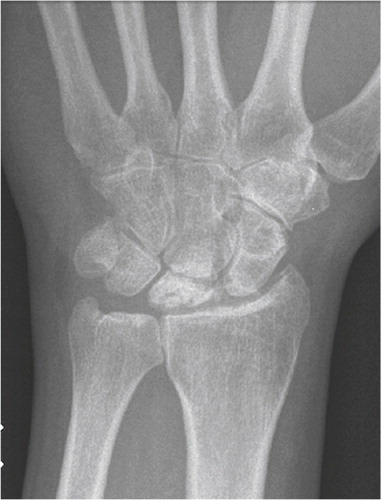
Lunate malacia (Kienböcks disease) is a multifactorial disease promoting softness and collapse of the lunate bone as a result of avascularity (Schuind et al. Citation2008). Numerous etiological or morphological factors have been postulated including the shape of the lunate bone, the length of the ulna (Gelberman et al. Citation1980), the shape of the radius (Tsuge and Nakamura Citation1993) and vascular vulnerability due to high intraosseus pressure (Schiltenwolf et al. Citation1996). The disease is staged according to Lichtman, from I–IV (Lichtman et al. Citation1977) focusing on the MR and radiological changes seen. The final stage IV includes carpal collapse and secondary wrist degenerative changes. The incidence of the disease is unknown, but young men are most often affected. Lunate malacia can ultimately lead to irreversible changes of the wrist joint, and the final salvage procedures are wrist arthroplasty or arthrodesis ().
Other causes of radiocarpal degeneration
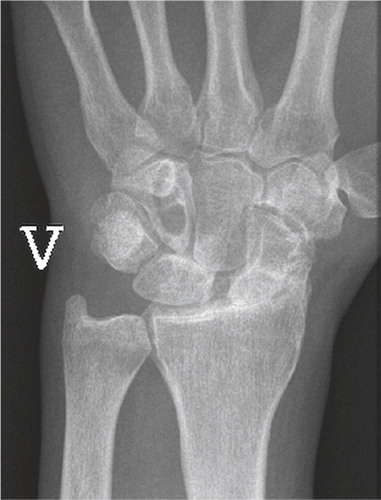
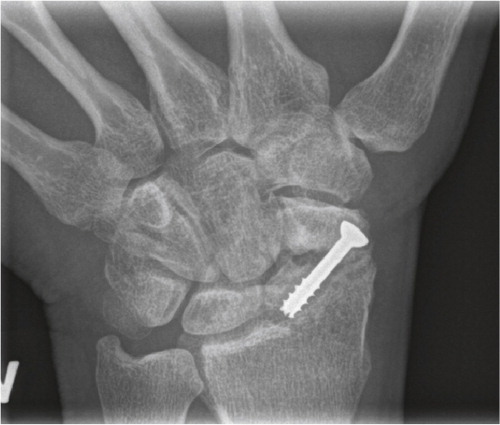
Iatrogenic injuries of the wrist joint are reported more frequently due to the great number of distal radius and scaphoid fractures operated. Incorrect placement of screws penetrating into the articulation can be devastating for the joint (Sahu Citation2011), especially when the patients are encouraged to start early motion (). The incidence is unknown.
Figure 4. Scaphoid fracture operated with a screw. Non-union and misplaced screw causing destruction the radio-scaphoid joint.
Primary wrist joint infection is rare, whereas postoperative joint infections are reported more often due to the same reasons as iatrogenic injuries.
Primary osteoarthritis: although being a very common joint disorder, it rarely affects the radiocarpal joint.
Preisers disease (Preiser Citation1910) is an extremely rare disorder with avascular necrosis of the scaphoid leading to radiocarpal degenerative changes. Definite aetiology and incidence is unknown and there is no standardized treatment algorithm (Imam Citation2009). The degenerative changes follow the SNAC pattern and the salvage treatment is similar.
Treatment options for radiocarpal degeneration
So far no non-surgical treatment can heal or delay cartilage degeneration in the wrist. Non-operative treatment includes analgesics like NSAIDs or paracetamol, change and adaption of the activity level and the use of different kinds of splinting devices, from slight to significant restriction of wrist motion.
Non-inflammatory degeneration may halt or stop if articular incongruence is corrected and the joint surface restored. Healing of scaphoid fractures and early non-unions has been demonstrated to halt the degenerative process (Duppe et al. Citation1994, Reigstad et al. Citation2012). Accurate reduction and fixation of complex intraarticular distal radius fractures can prevent degenerative changes (Raju and Kini Citation2011). The same may apply to successful suturing of scapho-lunate ligament injuries (Pomerance Citation2006) although the results are somewhat less encouraging compared to those seen after scaphoid and distal radius fractures. The restriction of activities and/or splinting the wrist in the early stages of lunate malacia might prevent carpal collapse and postpone degenerative changes. However, the natural history of the disease and the effect of the different treatments are uncertain as are the conclusions to be drawn (Schuind et al. Citation2008).
If symptomatic treatment is inadequate wrist pain can be treated by more limited surgical procedures hoping to postpone the need for total wrist arthrodesis or arthroplasty. The procedures include four-corner fusion, proximal row carpectomy and intercarpal fusions. The latter have rather narrow indications but they include radio-lunate arthrodesis, radio-scapho-lunate arthrodesis, triscaphe arthrodesis and scapho-capitate arthrodesis. These procedures are based on the concept of removing or fusing the damaged surfaces and depending the weight transmission and motion on the remaining uninjured surfaces. They almost invariably involve some loss of motion, but render the patient (ideally) with less or no pain. A brief presentation of the indications and procedures follows.
Four corner fusion
Four-corner fusion (4CF) can be indicated if there is intact cartilage on the lunate and on the lunate facet of the radius. Typical indications include SNAC, SLAC 2 and 3 wrists and in some instances after intraarticular distal radius fractures. The procedure involves removing the scaphoid, performing a radial styloidectomy and fusing the lunate, triquetrum, capitate and the hamate ().
The procedure may provide good pain relief, but only 50–60% of motion and 60–80% of the grip strength compared to the contralateral side. Complications include hardware problems, painful non-unions and progressive degenerative changes of the remaining joints (Mulford et al. Citation2009). Few prospective studies have been performed. Chung et al. (Citation2006)performed a prospective study on 11 patients with SNAC 2 wrists using a spider plate. From preoperatively to 1 year they found decreased motion (138°–112°) and strength (27–17 kg). A minor decrease in pain and an increase in overall satisfaction were observed. Three patients experienced hardware failure or persistent non-union. Similar results with decreased motion, persistent non-union and pain have been reported with K-wires, screws and staples, and at longer term follow-up conversion of an increasing number of wrists to total arthrodesis has been reported (Krakauer et al. Citation1994).
Proximal row carpectomy
Proximal row carpectomy (PRC) can be performed when the capitate and the lunate facets on the radius have intact cartilage. The indications include SNAC or SLAC 2 wrists, Kienböcks disease and sequelae after distal radius fracture. The scaphoid, lunate and triquetrum are removed and the capitate is positioned in the lunate fossa of the radius ().
The results seem to be comparable to 4CF concerning pain relief, grip strength and ROM (Mulford et al. Citation2009). Fewer complications have been reported, but progressive degenerative changes are seen in the majority of patients after more than 10 years follow-up. Conversion to arthrodesis was performed in almost 20% of the cases (DiDonna et al. Citation2004).
Overall the results are variable and the studies characterized by a low number of patients, retrospective designs and different effect parameters reported. So far, none of these procedures have shown overall convincing results or obtained more widespread use than the other.
Triscaphe arthrodesis, radiolunate, radioscapholunate and scaphocapitate arthrodesis have been used in selected patients and can in some instances be applied when the degenerative changes are minor and limited with the majority of joint surfaces intact. The procedures have not gained extensive use, probably due to technical problems, high non-union rates and relatively significant reduction of motion (Wolfe et al. Citation2011).
Total wrist arthrodesis
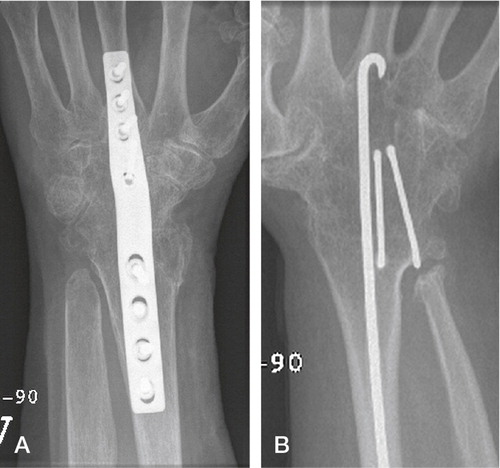
Total wrist arthrodesis has been the treatment of choice for destroyed wrist joints since the turn of the nineteenth century. When given no alternative treatment, the patients were satisfied with the pain relief and accepted the loss of motion and function, a situation similar to hip arthrodesis prior to efficient hip arthroplasties. Wrist arthrodesis was initially achieved using autograft from the tibia, the distal radius or the iliac crest without further fixation. During the 1960’s K-wires and large Steinmann-pins where used to achieve additional fixation, but rotational instability in the fixation and non-unions were problematic (Clayton Citation1965, Haddad and Riordan Citation1967). Mannerfeldt and Malmsten (Citation1971) introduced Rush rods and staples, achieving a high union rate (“union achieved in most cases”). During the 1970s, plate fixation became popular especially advocated by the AO group (Wright and McMurtry Citation1983). These two methods have been dominant although screw fixation, tension band wiring and bioabsorbable devices have been described (Hayden and Jebson Citation2005). The Mannerfeldt method is reserved for patients with inflammatory wrist degeneration while plate fixation is used in both inflammatory and non-inflammatory arthritis ().
Figure 7. A) Wrist arthrodesis using plate fixation. B) Wrist arthrodesis with the Mannerfelt method.
The results after total wrist arthrodesis are variable. The loss of function is substantial but it can to some degree be compensated by shoulder, elbow and forearm motion. In rheumatoid patients relatively high satisfaction has been reported. Solem et al. (Citation2006) found an excellent result with regards to pain relief in 28/40 patients in a long term follow-up study of mainly rheumatoid patients. In their series five patients had plate or rod removal, and in two radiocarpal union was not achieved on the first attempt. The DASH (Hudak et al. Citation1996) score was 38 and a 20% reduction in grip strength (compared to the other side) was observed. A comparison with the preoperative status was not performed, additional wrist surgery in the follow-up period and functional or occupational results were not reported (Solem et al. Citation2006). Adey et al. reported on the health status after arthrodesis in patients with post-traumatic joint degeneration after an average of six years. The grip strength was about 80% of the uninjured side, 14/22 reported persistent pain (of which four had severe pain). Although 15/22 were satisfied with the surgery, 20/22 were interested in a procedure that could restore some wrist motion (Adey et al. Citation2005). De Smet examined 36 non-rheumatoid patients with wrist arthrodesis after minimum 4 (mean 7) years. Pain resolved completely (mean VAS (0–10) = 2.5) in 20 patients at rest, but only 6 at activity (VAS = 5.4). The patients reported a relatively high DASH score = 44, only 11/35 could be reemployed at their previous job and the grip strength was 63% of the opposite side (up 10% from preoperative). 31 additional surgical procedures were necessary in 21 patients in the follow-up period, in two due to radiocarpal non-union (De Smet and Truyen Citation2003). Studies have shown that if given the choice, patients favour a procedure that could preserve some motion (Gaisne et al. Citation1991, Sauerbier et al. Citation2000, De Smet and Truyen Citation2003, Adey et al. Citation2005) and the patients with arthroplasty on one side and arthrodesis on the other are more satisfied with the arthroplasty. Palmer et al. (Citation1985) evaluated functional range of motion in healthy individuals and found that the vast majority of activities of daily living could be accomplished by 5° of flexion, 30° of extension, 10° of radial deviation and 15° of ulnar deviation. Ryu and Cooney (Citation1991) found that 40° of flexion and extension and 40° of radioulnar deviation (a total of 120°) gave a near normal wrist function, and as little as 25° of wrist motion gives a much better function compared to arthrodesis (Nelson Citation1997). However these reports used healthy volunteers with normal forearm rotation and upper extremity function as study objects, and the results are not applicable if other parts of the upper extremity are compromised. A study by Franko et al. (Citation2008) in healthy volunteers above 45 years of age compared unrestricted wrist motion with partially and highly restricted motion (ROM = 201° vs. 99° vs. 41°). Patients completed the DASH score and the PRWE (patient related wrist evaluation). They also developed a subjective scoring system (MASS, modern activity subjective survey) and an objective test battery (MATT, modern activity timed test) which evaluated activities of a contemporary lifestyle using a cell phone, a computer, a digital camera etc. They found a direct correlation between reduced range of motion and functional impairment, also demonstrated for modern activities.
In 2007, Synthes (the largest supplier worldwide of plate arthrodesis for the wrist) sold approximately 60 plates, the same year 9 arthroplasties were performed according to the Norwegian Arthroplasty Register (NAR Citation2010). The number of arthrodesis performed using other methods (ordinary plates, cramps, intramedullary pins) is not known, and many patients are reluctant to undergo surgery when arthrodesis is the only option.
Implants/biomaterials
Implants for bone fixation
Cobalt-chrome-molybdenum (Co-Cr-Mo) and titanium alloys (mainly Titanium-6Alumina-4Vanadium, Ti6Al4V) are the bulk metals mainly used for orthopaedic arthroplasties. The two metals differ in a variety of properties. Co-Cr-Mo is stronger, it can withstand great forces without deformation and it is bio-inert with a moderate ability to achieve bone-implant contact (Palmquist et al. Citation2009). In a highly polished form the alloy demonstrates very good wear performance, and has been the metal of choice in articulations (UHMWPE-metal and metal-metal) in orthopaedic arthroplasties. Using two different metals for fixation and articulation is a more complicated manufacturing process, and there is a tendency to choose the same metal for both fixation and articulation. There are some concerns with the use of Co-Cr-Mo as the material of choice in arthroplasties. The Youngs modulus/modulus of elasticity (i. e. the amount of deformation/strain with applied force/tension/stress in Pascal = N/m2) is high for Co-Cr-Mo (≈ 230 GPa), and much higher than that of cortical bone (≈ 10–30 GPa). The transmission of force from the implant to the bone will be asymmetric and can give stress risers in the proximal and distal junction between implant and bone. Where there is no loading of the bone Wolf’s law will apply and bone resorption can occur (stress shielding) (Sumner and Galante Citation1992). Some authors have expressed concern for this phenomenon and believe that it eventually can led to loosening of implants(Dujovne Citation1993), while others believe that bone resorption will stop and a new steady state will occur (Karachalios et al. Citation2004, Merle et al. Citation2011).
More attention has been focused on the lower bone tolerance and bone ingrowth capacity (giving less fixation and shorter component survival) compared to titanium alloy (Jinno Citation1998), the ion leakage in Co-Cr-Mo implants (especially from gritblasted or extensively porous coated implants) and the wear particles in the articulation. A weaker bond between the metal and applied bioactive coatings like HA has also been suggested (Filiaggi Citation1991, CitationSun 2001). There are two main concerns with the release and production of ions and aggregates of ions; i) they can stimulate a low-grade inflammation via cytokines (interleukin 1 and 6, tumour necrosis factor α and other) which have been implicated in osteolysis and aseptic loosening and ii) they can exert a cytotoxic effect and thereby indirectly be carcinogenic (Catelas and Wimmer Citation2011). The latter has been demonstrated in in vitro experiments, but in large cohorts (hip arthroplasties) which examine metal wear and occurrence of cancer, no increased risk has been seen (Visuri et al. Citation2010), see articulation below. The amount of wear in an articulation is dependent on the size of the articulation and the diametral clearance (difference in diameter on the ball and socket). The production of particles is greatest in the “setting in” phase and stabilizes with time. Differences in the activity level of the patients do not seem to affect the level of ions (Cobb and Schmalzreid Citation2006). On the other hand, modern metal-on-metal articulations with Co-Cr-Mo have demonstrated very low wear rates in simulators, excellent long term clinical performance and low wear rates in large diameter resurfacing arthroplasties.
The modulus of elasticity for titanium alloys (≈ 110 GPa) is closer to bone (≈ 10–30 GPa) than Co-Cr-Mo (≈ 230 GPa). Titanium alloy is light (50% of the density of Co-Cr-Mo), it is strong and it is versatile for loadbearing. Due to the lower elasticity it is not suited as a material in the articulation. The bone conducting ability has been well appreciated for titanium and its alloys for years, both in clinical and experimental orthopaedic and dental surgery (Lintner et al. Citation1986, Goldberg et al. Citation1995, Williams Citation2001, Reigstad et al. Citation2008). Extensive literature is available on the use of titanium in bone.
Surface modifications
The interface between the host bone and bearing metal has been subject to increasing attention and interest over the last decades. Increasing the surface area of the implants increases the area of implant adjacent to bone with increased bone present at the surface for bone fixation (Carlsson et al. Citation1988, Goldberg et al. Citation1995). The surface structure can improve the cell attachment to the implant and increase the biochemical interaction between implant and bone. A minimum roughness is proposed necessary to allow space for vascularisation and ingrowth of new bone (Predecki et al. Citation1972). The surface topography can be altered to promote bone ingrowth by subtractive/abrasive processes (particles removed from the surface creating pits or pores, giving a concave profile) or additive process (adding materials thereby creating bumps giving a convex surface). The superiority of a rough surface as compared to a smooth surface is well established, and all implants intended for bone ingrowth have some surface modification giving a rougher/ more irregular surface, which stimulates cell proliferation and osteoblast differentiation for stable bone anchorage and implant fixation. The pore size should mimic that of cancellous bone, the macropores (diameter > 100 μm) provide a scaffold for bone-cell colonization, while micropores (< 10 μm) allow body fluid circulation (LeGeros et al. Citation2003). The most common abrasive methods include (sand- or grit-) blasting and acid etching.
Abrasive methods
Silica (sand-blasting), TiO2 or alumina (Al2O3, corundum) are used to create the roughness. The particle size ranges from small to medium to large grit (25–250μm) and the final implant roughness depends upon particle size, time of blasting, pressure and distance from the source of particle to the implant surface (Wennerberg et al. Citation1996). A moderate roughness (sa = 1.0–2.0 μm) has been postulated as optimal (Wennerberg and Albrektsson Citation2009). The blasting process leaves remnants of the blasting material on the surface, but so far the bone response has been similar when comparing different blasting materials (TiO2 vs. Al2O3) of similar surface roughness (Wennerberg et al. Citation1996, Mueller et al. Citation2003). Rough surface implants have demonstrated excellent long term results in clinical studies and histological retrieval studies (Lintner et al. Citation1988, Reigstad et al. Citation2008).
Acid etching removes impurities as well as the oxide layer on the implants and creates pits and craters, giving a more homogenous rough surface compared to gritblasting. HNO3, HF, HCl or H2SO4 are the most commonly used solutions and the concentration and treatment time determines the amount of material removed. The method has been extensively used in the dental field as an additional implant treatment after gritblasting. Bone ingrowth and stable implant fixation has been demonstrated in experimental and clinical studies (Buser et al. Citation2004, Bornstein et al. Citation2005). Acid etching is uncommonly used in orthopaedic implants.
Additative processes/applying implant coating
Calcium phosphates have been used for decades in the bone-implant field of medicine due to its similarity with the mineral phase of bone. The calcium phosphates belong to a family of biocompatible substrates, where hydroxyapatite (HA) Ca10(PO4)6(OH)2 and tricalcium phosphate Ca3(PO4)2 have been the most common. They exist in different forms, both crystalline and amorphous, with variable calcium to phosphate ratio. At physiological pH HA is the most stable of the calcium phosphates. Bulk HA is not suitable for load-bearing due to brittleness and low fatigue resistance (Jarcho Citation1986), but a thin layer of HA on a metal substrate had the theoretical advantage of combining the load-bearing capacity of the metal and the biocompatibility of HA. Therefore HA has been widely used as coating on implants for bone fixation, usually applied by plasmaspraying technique. In its crystalline (cage-structure) form it is stable and not resorbable whilst in the amorphous (hydrated) form it is more soluble, and may be resorbed in a biological environment. The synthetic HA used in orthopaedic coatings usually have a crystallinity of > 70% and a calcium to phosphate ratio of 1.67. Although called HA, the applied coating usually comprises elements of other ions as well as different calcium phosphate phases (CPP). These elements have other biological and physiological properties (due to impurities and the thermal influence of the process (Locardi et al. Citation1993)) than pure HA. The plasmaspray process and final coating product is regulated by ISO and ASTM standards in Europe and USA. The technique of plasmaspraying coatings was developed during the eighties. The coating in powder form is sprayed through an arc with a temperature of over 5,000° C, creating the plasma form of the powder, hitting the implant substrate outside of the arc. The implant is relatively cold (< 300° C) and the coating is immediately created on the metal surface. The irregular surface is called porous coating. The mechanical properties of the metal is not affected (Ducheyne et al. Citation1986). For many years plasmaspraying was the only commercially available method of coating application, giving a coating thickness ranging from 40–200 μm. It has been extensively used on orthopaedic and odontologic implants. Thinner coatings are difficult to achieve with this technique if a complete cover is desired. Both experimental and clinical studies (including autopsies) have demonstrated encouraging long-term bone-implant fixation properties (Bloebaum et al. Citation1993, Soballe and Overgaard Citation1996, Vidalain Citation2011). The ion release from the underlying metal is also reduced in coated implants, although the effect seems to be limited to titanium implants (Ducheyne and Healy Citation1988). The bonding strength is stronger between titanium alloy and coating (mechanical and chemical bonding) compared to Co-Cr implants and coating (only mechanical bonding) (Sun et al. Citation2001). The mechanical strength of the plasmasprayed coating increases with decreasing thickness due to the brittleness and weaker resistance to tensile and shear forces of thicker coatings (Wang et al. Citation1993). Due to reports raising concern about thick plasmasprayed HA (Rokkum et al. Citation1999), thinner coatings have been applied, and also combinations of crystalline HA and the more soluble tricalcium phosphate (tricalcium phosphate alone has shown less bone ingrowth as compared to HA (Lind et al. Citation1999)). Theoretically the more soluble part of the coating could serve as a local reservoir of calcium and phosphate, thereby increasing the bone development in the immediate vicinity of the implant (Lee Citation2001). The optimal configuration of a plasmasprayed HA coating with regards to thickness, solubility and resilience has not been established and the potential benefits or side effects of coating resorption, reservoir effect for bone production, bone cell affinity, exposure of the underlying metal substrate, long term bonding capability and surface structure is still debated.
Plasmaspraying titanium particles (TiO2, titanium plasma spray, TPS) applied on implants creates a porous coating and has been used for a long period. The benefits postulated are i) creating a homogenous surface and covering the rough implant surface (reducing ion leakage), ii) utilizing the biocompatibility and bone conductive properties of titanium (if the coated metal substrate is made of a less bone compatible alloy), and iii) creating a porous surface suitable for direct bone ingrowth. Whether the observed effect in experimental studies comparing TPS implants with other implants is due to surface roughness differences or the coating itself has been difficult to prove (Wennerberg and Albrektsson Citation2009), and findings from cadaver (Chanlalit et al. Citation2011) and clinical studies (Becker et al. Citation2000) have not been encouraging. Although extensively, used especially in hip arthroplasties, (with good results (Klaassen et al. Citation2009, Lombardi et al. Citation2009)), the coating has not been compared with other surface modifications in clinical studies.
The drawbacks of plasmaspray techniques is the inability to coat internal surfaces, that is surfaces leeward to the spray direction (inside pores/gaps) and the bonding strength between the metal substrate and the plasmasprayed coating, rendering the coating susceptible to loosening/deflaking. To overcome the shortcomings of plasmaspray, many experimental methods have been developed for the application of bioactive calcium phosphate coatings. These include ion beam sputtering, sol gel deposition, electrophoretic deposition and electrochemical deposition. A thorough review on the different methods has been done by Narayanan and co-workers (Citation2008). So far the main commercial application has been the electrochemical deposition of calcium phosphate. A uniform coating is applied on any substrate that can conduct an electric current (which includes all metals used for bone fixation), and the coating will form on the exposed surfaces. The process is usually carried out in room temperature, where the metal is attached to an electrical current (as the cathode) in an aqueous solution of calcium and phosphorous ions. The different solubilities of different calcium phosphate phases (CPP) with varying pH is controlled at the cathode/electrolyte interface. The chemical composition and the thickness of the coating are dependent on the CPP concentration, the pH, the current and the processing time, and are unique for the different manufactures. The coating thickness can vary from nanoscale to 20 μm, and have different calcium-phosphate composition, including HA, tricalcium phosphate and brushite (a hydrated 1:1 calcium-phosphate) (Schmidmaier et al. Citation2002, Rossler et al. Citation2003, Becker et al. Citation2004). Clinical experiences with the electrochemical coatings compared to their plasmasprayed counterparts have so far been satisfactory in short term follow-up series (Boe et al. Citation2011).
Surface characterization
Surface topography: An important factor related to implant fixation is the surface topography. Qualitative measurements of surface irregularities on the micrometre level have been performed for decades, initially developed after the First World War for the aircraft industry. The profile of an object is examined by a stylus with a load applied moving mechanically over the surface, and the motion up and down creates an electrical signal which is converted to digital information, a profilometer similar to a traditional audio record player. The 2D (R) information can describe many different parameters, the most common is the average roughness, Ra (Dagnall Citation1986). The inability to measure small implants, measurement errors due to damage to the mechanical stylus and the geometry of the stylus influencing the result are some of the disadvantages of the mechanical 2D profilometer. Surfaces with pits or sharp spikes will yield the same roughness, and the profilometer cannot distinguish between valleys or peaks. Estimation of the optimal surface roughness using 2D methods has not been established, mainly due to different measurement methods used and a lack of a standardized method. Some surfaces are still described using 2D measurements by their manufacturers, but the introduction of 3D evaluation has become the method of choice. The dominant tool for micrometre evaluation of surfaces utilises an optical profilometer (measuring reflected light) and provides a 3D characterisation. Standardization of the measurement of surface irregularities of oral implants has been suggested (Wennerberg et al. Citation1996, Wennerberg and Albrektsson Citation2000). The 3D characterization of implants usually includes Sa (average height deviation), Sds (density of summits) and Sdr (developed surface area, comparing the surface with a flat reference area of the same size). The importance of measuring more than 2 implants (due to individual implant differences) as well as different parts of the implant (top, valleys, flanks), where emphasised by the authors.
For qualitative descriptive evaluation of implants electron microscopy is used. The SEM (scanning electron microscopy) method is used for visualisation of implant surfaces. Electrons are accelerated towards the surface, and the electrons emitted back from the surface are collected giving a picture for evaluation (Goldstein Citation1988). SEM cannot be used for quantitative measurements.
Surface chemical characterization: On hard surfaces the chemical composition is evaluated in the same manner as when studying the composition of stars and planets. Light reflected from the surface has wavelengths (spectres) characteristic for different chemical substances and elements. Spectroscopic analysis then enables us to identify the different substances and quantify the amount present on the surface.
Articulation
If stable implant fixation occurs, the main long term concern in arthroplasty surgery is wear in the articulation. The tribology (science and engineering of interacting surfaces in relative motion) of arthroplasties has had a huge development from the early designs with rough metal, soft polyester or polyethylene and brittle ceramics to the contemporary low wear articulations. The articulations in modern arthroplasties comprise metal on metal (MOM), ceramics–ceramics (“hard bearings”), metal–UHMWPE (MOP) or ceramics–polyethylene. Co-Cr-Mo in a highly polished form is the main metal alloy used for articulation. It has demonstrated a very high wear resistance in both MOP (60–150 μm (CitationNikolaou 2012) per year) and MOM (5 μm (Sieber et al. Citation1999) per year) articulations.
Metal-on-polyethylene
The MOP articulation is the most extensively used in THR and TKR (NAR Citation2010). The soft on hard articulation in modern arthroplasties provides long-term survival of the components. Still, the relatively high wear rate, especially in situations when cement, bone, HA or other (“third bodies”) products gain access to the articulation is of concern (Rokkum and Reigstad et al. Citation1998). The relatively small wear-particles (0.1–1μm) are phagocytised by macrophages leading to inflammation and bone resorption (Green et al. Citation1998). The production of polyethylene wear products, and the subsequent periprosthetic osteolysis and eventual loosening of the implants have led to increasing interest in refining the UHMWPE bearings (especially increasing the wear resistance by crosslinking the polyethylene) or the development of other types of bearing. For highly cross-linked UHMWPE the laboratory wear (Dumbleton et al. Citation2006) results and measured wear in clinical trials (Kuzyk et al. Citation2011) have been very encouraging, but the revision rate has so far not been affected (Nikolaou et al. Citation2012).
Metal-on-metal
The most common MOM articulation is the Co-Cr-Mo ball and socket and it has been extensively used since its introduction in the early sixties after promising hip arthroplasty results presented by McKee and Watson-Ferrar (Citation1966). The advantage of the Co-Cr-Mo articulation is the very low wear rate (20–180 times lower than conventional metal–UHMWPE) and its self-polishing function (postulated to remove irregularities caused by third body wear) (McKellop et al. Citation1996, Zywiel et al. Citation2011). A larger head (usually avoided in metal–UHMWPE articulations due to a larger volumetric wear) would be more stable, thereby minimizing the rate of dislocations (Bystrom et al. Citation2003). The clinical performance of modern THA with MOM articulation in young patients has been promising (Delaunay et al. Citation2008). The main local concerns from wear particles in MOM articulations include periprosthetic soft tissue reactions, periprosthetic osteolysis as well as metal-induced immune responses. In vitro experiments have demonstrated reduced cellular function including osteoblasts and fibroblasts (Germain et al. Citation2003, Fleury et al. Citation2006). Local tissue reactions in hip resurfacing arthroplasties have been reported and histologic changes have been characterised by extensive necrosis and the presence of B and T lymphocytes as well as plasmacells. Whether the reaction is due to an allergic response to normal amounts of metal ions or a toxic reaction to high amounts of ions is not known (Pandit et al. Citation2008). The systemic concerns of metal ions include renal failure, the accumulation of ions in the liver and a possible carcinogenic- and teratogenic effect (Heath et al. Citation1971, Zywiel et al. Citation2011). The most extensive local problems have been seen after use of poorly engineered implants, in cases of implant malposition or cases of impingement between implant components and in large diameter resurfacing hip arthroplasties (Cobb and Schmalzreid Citation2006, Mabilleau et al. Citation2008, Langton et al. Citation2011, Seppanen et al. Citation2012). Systemic effects have been difficult to demonstrate and serious systemic side effects like cancer have not been confirmed (Makela et al. Citation2012, Smith et al. Citation2012). These studies have a short observation period (3.6 and 7 years), and might be too short to detect an increased risk. Increased levels of systemic metal-ions are also detected in conventional MOP arthroplasties (MacDonald et al. Citation2003, Luetzner et al. Citation2007), but so far no increased cancer risk has been observed in long term (13 years) follow-up studies (Visuri et al. Citation2010).
Ceramic–ceramic
Ceramic–ceramic has the theoretical advantage of the hard bearings (very low wear) without the production of potentially harmful wear products. The development of brittle and wear resistant ceramics with incremental improvements in the manufacturing and control processes paved the way for more extensive use of ceramics in articulations. Aluminium-oxide (alumina) and zirconium have been the main ceramics used, but zirconium was abandoned due to poor clinical performance (Norton et al. Citation2002). The wear characteristics are excellent (100 and > 2000 fold decrease in linear wear compared to MOM and MOP respectively) (Prudhommeaux et al. Citation2000) and the wear particles do not appear to affect cellular function in vitro (Germain et al. Citation2003). The combination with UHMWPE sockets has demonstrated excellent long term results (Urban et al. Citation2001, Reigstad et al. Citation2008). Early all-ceramic articulations performed unsatisfactory, mainly due to loosening of the ceramic cups due to low bone conducting properties (O’Leary et al. Citation1988). Using a metal backing with better bone conducting properties for the ceramic acetabular component has improved the performance substantially, demonstrating better wear characteristics compared to ceramic–UHMWPE (Lewis Citation2010). The earlier zirconium problems, the brittleness/fracture risk and ceramic squeaking from the articulation (Hamilton et al. Citation2010, Mai et al. Citation2010) are the main reason why we thus far have not seen a shift towards all-ceramic articulations despite their promising results.
The history of total wrist arthroplasty
The replacement of the arthritic hip joint has been referred to as “the operation of the century” due its predictable functional outcome and pain relieving effect, as well as its cost effectiveness in terms of quality of life adjusted favourable results (Learmonth et al. Citation2007). Knee replacements have similar results, while replacements in other joints (shoulder, elbow, wrist, cmc, mcp, pip, ankle and mtp) have been less predictable. Initial promising results with wrist arthroplasties have been less encouraging in the longer term and the procedure has been reserved for low demand patients.
The first artificial wrist arthroplasty has been attributed to Themistocles Glück (1853–1942), a Rumanian-German surgeon. In May 1890 he implanted his first knee arthroplasty made out of ivory, and in June 1890 the first wrist arthroplasty of the same material was implanted, both performed at the Kaiser-und-Kaiserin-Friedrich-Kinderkrankenhaus in Berlin. The indication for the latter was tuberculosis in the wrist in a 21-year old male. The implant was described as a ball and socket articulation with forks on each side for stable fixation in both the ulna and radius proximally and in the metacarpals distally. Having septic arthritis as the indication this prosthesis of course was bound to fail. Gluck claimed that the patient after one year had a good range of motion but he developed a chronic fistula. Further follow-up was not reported. A skeleton exhibiting his implants in the hip, knee, ankle, shoulder, elbow and wrist was displayed in his hospital, but disappeared at the end of World War II. There are no pictures or drawings available of his wrist implant (Ritt et al. Citation1994).
Swansons silicone implant
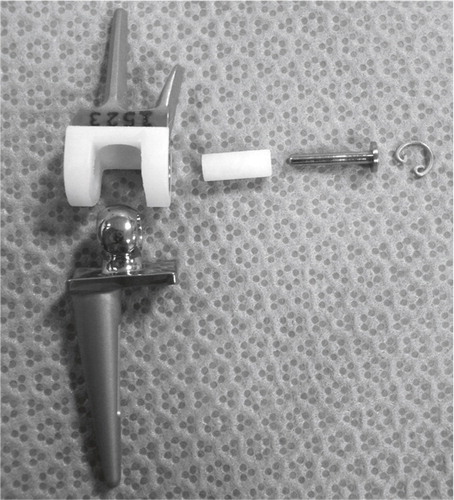
Albert Swanson introduced his silicone implants in the 1960s. Silicone was thought to be totally inert; it was durable and elastic and could sustain a substantial load without deformation or breakage. A wide range of silastic implants was developed including mcp, pip and dip joint as well as cmc 1, os lunatum substitution and ulnar and radial head replacements. His wrist arthroplasty was introduced in 1967 comprising a double stemmed, flexible hinge implant. A barrel-shaped midsection was selected, and the core of the implant was reinforced with Dacron to provide axial stability and resistance to rotational force. In 1974 the original silicone rubber was changed to a high performance silicone elastomer. This had been mechanically tested with more than 200,000,000 flexion repetitions to 90° without evidence of material fatigue or fracture (Swanson et al. Citation1984). The barrel-shaped midsection was developed to create a wider and better joint space, and to prevent subsidence into the bone. The implant was not intended for bone fixation, and motion between the implant and bone was assumed to be an advantage, whereby the implant could adjust to the patient’s rotational requirements with little resistance. For wrists with severe instability of the joint, stiffness or deviation in a non-functional position this constrained replacement was considered a good choice, with its tendency to normalize the wrist position. The operation included a proximal row carpectomy (with inclusion of the proximal part of the head of the capitate) distally, removal of the distal radius and the ulnar head, and the creation of two square surfaces with smoothened edges. After reaming of the medullary canal the implant was inserted. From 1982 the mid-section of the implant was secured with metal liners (titanium grommets) to protect the silicone from sharp bone edges creating wear debris and fractures of the implant (). The palmar and dorsal ligaments were reefed allowing 30° of flexion/extension and 10° ulnar and radial deviation on passive manipulation.
Figure 8. The Swanson wrist replacement with metal grommets. (Courtesy of Wright Medical Technology.)

Excessive motion after surgery was avoided because it was thought to increase the chance of implant failure. Tendon transfer for wrist balancing was performed in the same operation. Swanson reported his results achieving good pain relief, acceptable range of motion (average 88° flexion, extension, radial and ulnar deviation), grip strength and radiological results with a relatively low complication rate (25 reoperations in 181 wrists including 9 implant fractures) (Swanson Citation1984). His results have been difficult to reproduce by others, and an increasing amount of surgeons reported high complication rates, implant failure/fracture (up to 50%, mainly between the barrel and the distal stem) and destructive synovitis attributed to silicone wear debris (Jolly et al. Citation1992, Stanley and Tolat Citation1993, Schill et al. Citation2001, Kistler et al. Citation2005). The documentation concerning this implant is mainly retrospective, many of the patients have been lost to follow-up, and the deteriorating health of these predominantly rheumatoid patients is demonstrated by a mortality rate within few years (up to 25%) in the reports. Although promising at first, the use of Swanson’s silicone arthroplasty has since diminished. It never became an alternative for patients with higher wrist demands. The arthroplasty is sold by Wright Medical Technology (http://www.wmt.com/physicians/). I have contacted them to get the annual figures of wrist arthroplasty sales in the US and worldwide, but this could not be provided. In Norway the implant has not been in use for the last 15–20 years according to the Wright Medicals three latest agents and the Norwegian Arthroplasty register.
Volz wrist arthroplasty
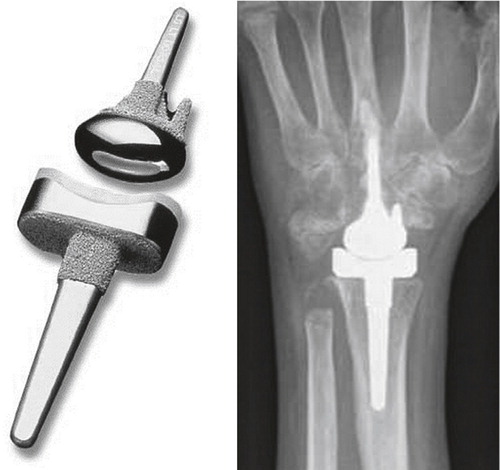
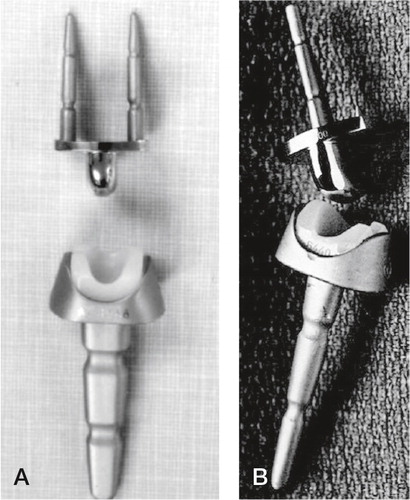
Parallel with the development and introduction of Swanson’s silicone replacement came the huge advancements in hip replacement, especially the success of the Charnley low friction arthroplasty using metal articulating with polyethylene and methylmetacrylate bone cement. This inspired Robert Volz to design a wrist implant employing the same concepts, applied for clinical use in 1973. The proximal component for cemented fixation in the medullary canal of the radius was connected to a polyethylene insert. The distal component had initially two prongs for cemented fixation in the second and third metacarpals (through the trapezoid and the capitate), but this was later reduced to one prong and two pegs in the modified version ().
Figure 9. A) The first Volz arthroplasty with two distal prongs, and B) the modified Volz wrist arthroplasty with one central distal prong. (Courtesy of Dr Hamas.)
The surface of the components was profiled for better implant-cement fixation to prevent loosening between the bone and implant when distractive forces were applied to the wrist. The articulation consisted of a hemispherical design with two different dimensions available for the concave polyethylene radius component and the distal chrome-cobalt convex surface. This allowed a maximum of 90° flexion-extension and 50° radial-ulnar deviation before impingement occurred. The resection of bone included the distal ulna and radius (from the level of Listers tubercle), the proximal carpal row, and the head of the capitate to the level of the trapezoid. Volz reported his initial experience in 17 wrists after a maximum observation period of 13 months with the distal double-prong version in 1976. He achieved good pain relief, range of motion and stability (Volz Citation1976). A tendency for ulnar drift was observed, and the results in 25 wrists with the modified distal component were published in 1984. In the latter series both cemented and uncemented fixation of the distal component was used. His clinical results after a mean follow-up of 3.2 years were even better, having a total ROM of approximately 100° with no radioulnar imbalance. No revisions due to dislocation, infection, implant fracture or loosening had been performed (Volz Citation1984). Bosco et al. (Citation1994) reported satisfactory results in 17 patients after an average of 8.4 years, despite having complications. There were four loose distal components and one loose proximal component, where loosening was defined as the presence of a radiolucent line of more than 2 mm or cement fracture. Four metacarpal components had perforated the bone, and an increasing collapse of the carpus was observed (a reduction of 24% using the index from Youm (Youm and Flatt Citation1980)). Three out of four posttraumatic patients experienced loosening, and the author recommended the arthroplasty for low-demand RA patients. Gellmann et al. (1997) reported on 14 wrists (13 patients) after an average of 6.5 years, finding good pain relief and a mean total wrist ROM = 57°. He observed a high degree of complications; two articular dislocations (one chronic), seven wrists showed radiolucency about one or both components, migration was observed in two radial and five metacarpal components, in two wrists both components had subluxed and in one patient the components were dislocated. Only one revision had been performed. The patient satisfaction was high. Similar results were reported by Menon (Citation1987) in 18 wrists (16 patients), but function evaluated with grip strength or ADL was not reported. A final change of the design was introduced in 1988, the CFV (Clayton, Ferlic and Volz) prosthesis. The results did not improve, they reported failures and revisions in 6/15 and problems with the remainder including tenosynovitis, carpal tunnel syndrome and soft tissue balancing (Menon Citation1987). The use of the prosthesis was discontinued in 1993.
Meuli wrist arthroplasty system
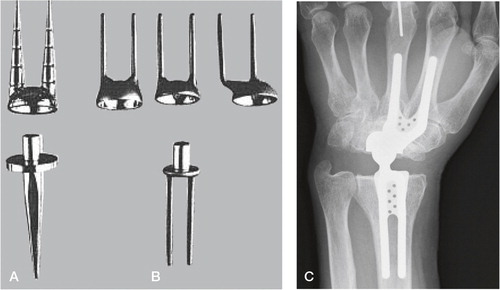
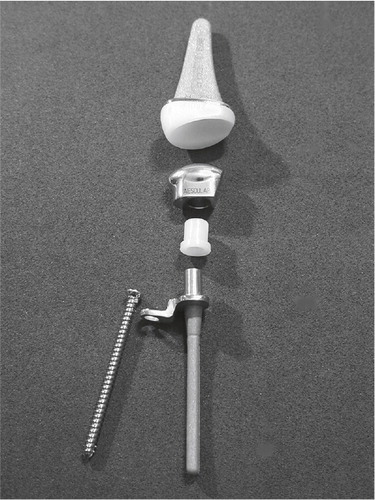
Hans Christoph Meuli from Berne, Switzerland developed and introduced the arthroplasty bearing his name in 1972. Altogether three versions have been in clinical use. The first version was intended for cemented fixation, having a ball-and-socket articulation, with the ball (made out of polyester at first, then changed to UHMWPE) on the proximal side. The proximal component had one prong and distal component had two prongs, and were made out of Protasul 10 (Ti6AlNb) and could be bent for individual adjustments prior to fixation in the radius and the 2. and 3. metacarpal. The bone resection needed included the distal ulna, the radius distal to the ulnar joint line, the scaphoid, lunate and the capitate to the level of the trapezoid. Initial problems in his series of 41 patients included polyester synovitis, dislocations, technical errors, stem breakage, infection and ulnar deviation. The latter was due to the centre of rotation being placed too far radially. The ball was changed to a UHMWPE, and the socket was positioned eccentrically ulnarward in the Meuli II ().
Figure 10. A) The Meuli I (polyester head), B) Meuli II (polyethylene head) and C) The Meuli III, metal-on-UHMWPE articulation. (Courtesy of Dr Mraz.)
Technical errors occurred with the first patients, and these were attributed to the necessary learning curve (Meuli Citation1980, Citation1984). Cooney et al. (Citation1984) published their reoperation rate in 140 Meuli I and II arthroplasties. Soft tissue balancing was a major problem. They report 9% revisions due to dislocation, 3% revision due to loosening and 3% revision due to deep infection. No subjective clinical outcomes were given. The number of follow-ups, drop-outs and deceased were not mentioned, and no radiographic evaluation was performed. They recommended the Meuli prosthesis to be abandoned. In 1986 the Meuli III was introduced, where the concept was thoroughly changed. The components were made out of corundum rough blasted protasul-100 (Ti6Al7Nb), including the ball, which was coated with titanium nitride (presumed hard and wear resistant). The UHMW polyethylene was inserted in the cup in the distal component, and it was intended for cementless fixation (having the opportunity to be cemented if the bone stock was inadequate). His own results with the Meuli III (38 wrists) were satisfactory, with adequate pain reduction, mean total ROM 90° and unchanged grip strength. No dislocations were seen, but eight (six distal and two proximal) components loosened and were revised. The distal loosenings were attributed to incorrect positioning during the initial period (Meuli Citation1997). In a more recent retrospective report from Strunk and Bracker (Citation2009), comparing Meuli, Biax and Universal II, 15 Meuli III had been operated (two were dead), and were contacted after a mean of 9 years. They were subjectively satisfied (RA patients), having a mean approximate total wrist ROM = 70°. Three patients had experienced dislocations, two of these were stable after closed reduction, and one was converted to an arthrodesis. Two revisions were performed due to deep infection, both converted to arthrodesis. Radiologically only one of the remaining arthroplasties was firmly attached to bone, the other exhibited signs of loosening (mainly around the carpal/distal component). The arthroplasty is no longer available.
Trispherical wrist arthroplasty
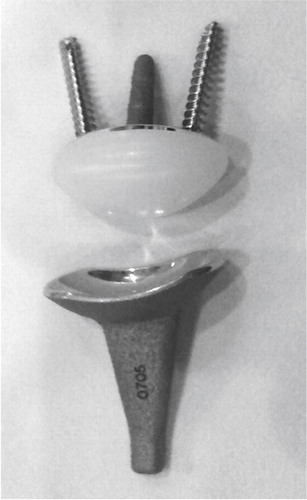
The Trispherical wrist arthroplasty was developed at the Hospital for Special Surgery using vitallium (a chrome-cobalt alloy) and UHMW polyethylene in a hinge articulation. The mechanism allowed flexion-extension (maximum 170°) in the hinge, radial-ulnar deviation (maximum 30°) and some rotation along the sloppy barrel. The proximal component was cemented in the radius after resection of the distal ulna. A proximal row carpectomy was performed, and the most proximal part of the capitate and triquetrum were resected. The distal component had one longer and larger (cemented in the third metacarpal) prong and one shorter and offset (cemented in the second metacarpal and the remaining scaphoid) prong ().
In 1990 the inventors published results after an average of 9 years follow-up. 34 patients (44 implants) all suffering from RA were operated between 1977 and 1983, 26 patients with 35 arthroplasties were available for review. Only two wrists had been revised, one due to implant loosening, the other due to persistent pain. Radiolucent lines were present in 7 wrist, (6 distally and in one case around both components), and three wrists presented with migration of the distal component, two of them perforating dorsally in the third metacarpal. Average total ROM at follow-up was 70°, and the majority of patients reported little or no pain (Figgie et al. Citation1990). In Citation1997, Lorei et al. published results after revised Trispherical arthroplasties, identifying 8 revisions out of 87 primary procedures, giving a 9% revision rate after average follow-up of 8.7 years (Lorei et al. Citation1997). This represented only the registered failures, and no follow-up details of the primary procedure were given. The 8 failures were due to loss of extension (2), metacarpal perforation (4), radius erosion (1) and infection (1). The only available publications concerning this arthroplasty have been reported by the group behind the development of this device. In 1999, CitationO’Flynn et al. reported a patient who had bilateral arthroplasties, one being converted to an arthrodesis after 8 years (due to distal implant loosening). After 9 years the remaining arthroplasty became painful, and she experienced an acute loss of motion. The hinge mechanism had failed, and the revision revealed a disengagement of the hinge and metallosis, and wear of the polyethylene insert (O’Flynn et al. Citation1999). This is the latest report concerning this implant, and the production of this arthroplasty was stopped.
Guepar wrist arthroplasty
The Guepar wrist arthroplasty was developed by Alnot in Paris. He introduced a proximal all-polyethylene component for cemented fixation. The articulation was egg-shaped with an ulnar translation to centralize the rotation. The distal component was made out of cobalt-chrome. Two long screws were fixed to a small plate and screwed through the capitate and trapezoid into the medulla of the 2. and 3. metacarpals respectively. A relatively generous resection was performed, including the distal radius and ulna, and a straight cutting plane at the level of the proximal capitaten ().
Figure 12. A) The Guepar wrist arthroplasty with microscrew. B) Radiologically loose distal and proximal components. (Courtesy of Dr Hubach.)
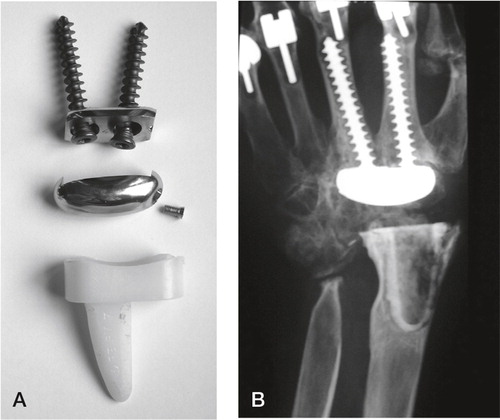
The egg-shaped ball was fixed to the plate in the distal component via a microscrew. In a retrospective follow-up of 72 wrists after mean four years 11 wrist were revised, 5 due to unscrewing of the microscrew and loosening of the distal articulation, 4 proximal loosenings (attributed to wear products from the articulation) and 2 distal loosenings. In the remainder 56% osteolysis and bone resorption under the carpal plate was observed to increase with time. The authors believed the cause was micromotion between the plate and screws (Fourastier et al. Citation1996). The arthroplasty gave good pain relief, but reduced motion compared to preoperative (47° vs. 39° flexion–extension). The published material concerning this arthroplasty has been given by Alnot and his group in Paris, but no results have been published for the last 15 years. Hubach referred to his experience in 17 revision arthroplasties with the TMW arthroplasty at the arthroplasty Instructional Course during the FESSH meeting in Oslo 2011. 9 of the prior failures were Guepar wrist arthroplasties.
Biax wrist arthroplasty
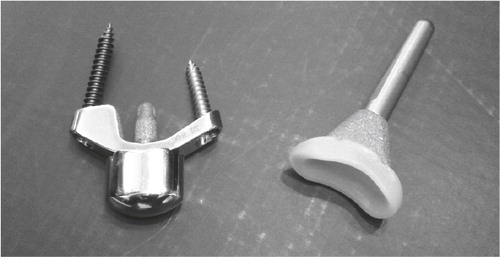
Biaxial (motion in two axes, i.e. flex/ext and rad/ulnar dev) prosthesis was developed at the Mayo clinic between 1978 and 1982 by Cooney, Beckenbaugh and Linscheid. The components were made of chrome-cobalt, with an ellipsoidal shaped head (distally) articulating with a UHMW polyethylene bearing (proximal). Both components had porous coating on the proximal part (made of the same material as the bulk implant), and were intended for cemented fixation, though press fit fixation was possible. Proximally fixation is limited to the radius, and ulnar head excision is part of the procedure described by the inventors, but the DRUJ can be spared. A PRC is performed, along with a resection of the most proximal part of the capitate and trapezoid, leaving approximately 2.5 cm for the articulation. Distally the long stem is cemented in the capitate and the third metacarpal, while the radial stud is fitted in the trapezoid ().
5 year follow-up results from the Mayo Clinic were published in 1996 (Cobb and Beckenbaugh Citation1996). 64 wrists in 52 patients had been operated solely on the basis of wrist degeneration due to RA (63) or JRA (1), both cemented and cementless fixation was used. In 11 wrists the implant had been removed (8 distal loosenings, 1 dislocation, 1 infection and 1 soft tissue imbalance), 6 patients were dead and one lost to follow-up. The remaining 46 patients rated their symptoms as better or much better (91%) and the pain as none or mild (97%) at the final follow-up. Forearm rotation was unaffected by the procedure, and total ROM increased from 78° preoperatively to 90°. Three patients experienced dislocation and three other had subluxations, all were stable after closed reduction. Radiographic loosening was seen in 14 patients (8 revised at follow-up). Takwale et al. (Citation2002) retrospectively reviewed 66 out of 76 (5 dead, 5 lost to follow-up) Biaxial implants in RA wrists at a mean follow-up of 52 months. Both cemented and uncemented techniques were used, and the distal ulna was resected in all patients. They experienced 6 early dislocations, stable after closed (5) or open (1) reduction. The subjective outcome was excellent or good in 41/66, fair or poor in 25/66 (using the Hospital for special surgery HSS scoring system). 50 patients had mild (9) or no pain (41) pain, total ROM was 66°. However 11 patients had soft tissue imbalance leading to abnormal resting position and reduced function. 14/66 distal components were loose (one of these also had a loose proximal component), 5 had been revised. Series with short follow-up and better results have been published, but the authors have not repeated their examinations (Courtman et al. Citation1999, Stegeman et al. Citation2005). Kretschmer and Wannske (Citation2003) used the Biax on predominantly post-traumatic or degenerative wrist arthritis patients, though avoiding patients with heavy manual work. The initial experiences in 21 patients were promising, but in his series of 42 patients followed for 2.6 years the results were less encouraging. Polyethylene wear on the dorsal rim had led to loosening in 7, and permanent dislocation in 2 patients, a complication not earlier described for the Biax. 9 of the remaining 31 patients had radiographic osteolysis (2 around both components, 4 around the distal component with metacarpal perforation of the tip of the stem, and 3 without). Four patients had early radiocarpal dislocation, treated by closed reduction and temporary k-wire fixation, being stable thereafter. Cementless fixation was used for all components (Kretschmer and Fansa Citation2007). A serious polyethylene-metal debris wear in a patient was described by Groot et al. (Citation2006), confirming the problem experienced by Kretschmer. Similar results in a rheumatoid population were reported by Harlingen et al. (Citation2011). After 6 years, 7/40 wrist had required revision due to infection (2), loosening (3), malposition (1) or distal component breakout (1). 22 out of the remaining 32 (one patient died during the follow-up period) were radiographically loose. Altogether 4 intraoperative and 27 postoperative complications were seen. The patients reported satisfaction with the procedure, reduced DASH score and functional wrist motion. The long stemmed revised distal Biaxial component was developed in the 1990s. It was intended for revision purposes, but was also used for primary arthroplasties. In a series by Rizzo and Beckenbaugh (Citation2003) 14 patients with 17 implants were retrospectively examined after mean 74 months. Cement was used on the distal component, and uncemented fixation proximally. There were no dislocations, no revisions, no radiological loosenings, they found high patient satisfaction, good range of motion and strength. Despite these encouraging results and being one of the most widely used wrist arthroplasties worldwide, the DePuy Company withdrew the implant in 2004 without any explanation.
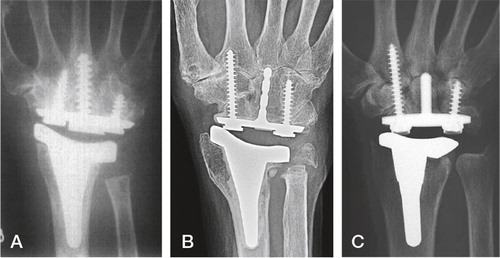
Destot wrist arthroplasty
The only arthroplasty intended for use in posttraumatic wrist arthritis was the Destot prosthesis developed by a group of French and Belgian surgeons (The Destot group) during the nineties ().
Figure 14. Sandblasted and porous coated steel proximal and distal components, UHMWPE radial cup, proximal steel carpal ball, condylar UHMWPE cylinder and distal steel component. (Courtesy of Dr Levadoux.)
Levadoux and Legrè published their experience in 28 out of 35 SLAC and SNAC wrists after 47 (12–72 months), 80% were men with a mean age of 62.5 years (Levadoux and Legre Citation2003). Having a double articulation allowing rotation distally and flexion/extension and ulnar-radial deviation the developers hoped for more motion than other contemporary arthroplasties. The resection preserves the DRUJ, removing the lunate, scaphoid and the proximal capitates. The capitate and 3. metacarpal are reamed and additional distal fixation achieved with a 4.5 mm cancellous screw in the trapezoid and 2. metacarpal. Proximally a medullary gouge is used to prepare the bone bed. The size of the intermediate mobile condylar-carpal component is chosen to re-establish the carpal height. Although the clinical results were good, a high degree of revisions and distal loosenings were observed. Six distal loosenings with twisting out of the second metacarpal screw and two metacarpal stem fractures were seen (the patients with metacarpal failures where asymptomatic according to the authors). Revisions included three wrists revised due to pain (fused or revised with new arthroplasty) one early infection (treated with surgical cleaning, healing uneventfully but later experienced metacarpal stem fracture) and one late infection (revised to arthrodesis). The 4-year survival rate was 85% (five revisions, survival given for all 35 wrists). The degree of patient satisfaction is reported for all patients, including the fused wrists (7 having moderate or severe pain, 23 satisfied or very satisfied with the end result). For the 21 patients with an arthroplasty the total ROM increased from 78° to 123°, forearm rotation from 105° to 167° and grip strength from 20 to 32 kg. Although good results were achieved in a high demand patient group, further follow-up has not been undertaken (personal communication, M Levadoux), and the company distributing the arthroplasty (3S Ortho) has withdrawn the product.
Anatomic Physiologic wrist
Anatomic Physiologic wrist (APH) was designed and introduced by Radmer, Reimer Andresen and Martin Sparmann in Berlin, Germany. The arthroplasty consisted of cobalt-chrome components coated with HA for press-fit fixation in the radius and the distal carpal row/proximal 2.–4. metacarpals. The articulation was egg/ellipsoid, with a radial inclination of 10° on both sides (). The articulating surfaces were covered with titanium-niobium for optimal wear properties. The resection comprised the distal radius and ulna and a proximal row carpectomy with a resection at the level of the proximal capitate bone. The distal component introduced a new fixation principle, comprising a coronal plate interrupted by pegs for fixation in the distal carpal row/proximal 2.–4. metacarpals. The bearing surface was mobile. The initial results in 30 RA patients after 18 months were very encouraging with a satisfactory pain reduction, increasing ROM (from average 58° to 101°) and grip strength (from average 18 kPa to 29 kPa). There were three early complications, one progressive soft tissue imbalance with subsequent dislocation (arthrodesis), one dislocation of the carpal component (revised with a new one) and one deep infection (arthrodesis) (Radmer et al. Citation1999).
Figure 15. The APH wrist. (From Radmer et al., Journal of Hand Surgery, 1999, Reprint permission from Rightslink.)

4 years later the results from 40 patients with an average after 52 months were catastrophic. Radiolucent lines greater than 2 mm were present in 30 out of 36 remaining patients (30 on the distal side, and 5 also on the proximal side), 33 had migration of the carpal component, where 9 also perforated the third metacarpal. Subsidence was also noted in 14 of the radial components. 36 out of 37 remaining arthroplasties were revised due to frank loosening in 33 patients (12 of both components) and material failures in three. Metal debris was found extensively in all patients (in the capsule, around the flexor tendons and extensor tendons) during revision surgery, attributed to titanium (Radmer et al. Citation2003). The APH was withdrawn from the market.
Contemporary arthroplasties
Universal wrist arthroplasty system
Universal/UTW 2 was initially developed by Jay Menon. This primarily uncemented (cementing is optional), unconstrained arthroplasty has a press fit radial component with a tie mesh for bone ingrowth in the metaphyseal region. The articulation plate (made of titanium) has a 20° inclination similar to the articulating surface on the radius. The distal carpal plate is ovoid, and has a titanium tie mesh covering the back side. Fixation is achieved with three titanium screws (one 6.5 mm in the capitate, and two 4.5 mm in the scaphoid/trapezoid and the hamate bones). A UHMW polyethylene egg-shaped component articulates with the radius component. The surgical technique includes an intercarpal arthrodesis at the level of the distal carpal resection (through the proximal pole of the capitate, including the distal scaphoid), resection of the ulnar head and an inclined resection of the distal radius. In 1998 Menon published the results in his first 31 (37 wrists) patients after mean 6.7 years. He achieved good pain relief (88% did not have pain) and motion increasing the total ROM (active or passive is not given) from 73° (preoperatively) to 96° (postoperatively). 5 patients experienced dislocations, 3 were openly reduced, one closed and one converted to wrist fusion. Two patients had radial component loosening and were revised with new cemented components. Two others had deep infections (one salvaged by wrist fusion, and one by antifungal therapy), and one final patient was converted to arthrodesis due to muscular imbalance. A radiographic analysis or report was not given (Menon Citation1998). Divelbiss et al. (Citation2002) reported their short term results from 19 patients (22 wrists) with the revised UTW arthroplasty. The distal component had been altered, having a centrally placed peg with indentations, and screws on each side. The radial-sided distal screw is longer for purchase in the second metacarpal. Cement was used on the proximal side and for the distal peg. The clinical results were similar to Menon’s original series including a DASH score reduction from 46 to 32 after one year, but only 8 wrists had passed the 2 year follow-up. Three patients experienced dislocations, one was finally converted to an arthrodesis, one was stable when the UHMW polyethylene was changed to a larger size and the third was stable after treatment with open reduction and temporary external fixation. Osteolytic lines were seen around six screws, no implants were determined to be loose. The majority of patients where later followed up after 7.3 years, where 9/19 wrists had been revised due to distal loosening, 1/19 due to chronic instability. Two others were loose but not revised. The second edition was also reported by Murphy et al. (Citation2003) where the treatment was compared to arthrodesis after mean 26 months of follow-up. The patients returned questionnaires (DASH and PRWF). No radiological or objective functional outcomes were reported (such as range of motion, grip strength, loosening, bone unions etc.), and complications compared after a retrospective chart study. Out of 27 arthroplasties four dislocated, one was converted to arthrodesis and one needed capsular reconstruction. A finite element and laboratory experiment was performed comparing the original toroidal/teardrop articulation with a strict ellipsoidal articulation. The original toroidal articulation was extremely sensitive to rotation, creating incongruence and instability (which was the major concern with the early models) and substantial peak stress on the volar and dorsal rims of the radial component. The ellipsoidal articulation demonstrated a more stable situation, i.e. a greater rotational resistance (which was desired by the developing team postulating that low rotational stability was the initiating step in dislocation), and a more stable contact area throughout rotation (although the toroidal shape had a greater contact area up to 5° of rotation) (Grosland et al. Citation2004). The study was supported by the developing company and these findings had been included in final version of the implant, where the distal carpal plate also was changed to a press fit, titanium coated stem without indentations ().
Figure 16. The development of the Universal arthroplasty system. A) Menon’s first edition. (From J Menon, Journal of Arthroplasty 1998, reprint permission from Rightslink). B) The second edition (Universal) with the central peg with indentations. C) The contemporary Universal 2. B) and C) Courtesy of Dr van Winterswijk.
The arthroplasty and the technique is described and published in three different places by Adams where he refers his results presented at different annual meetings (but never published in peer reviewed journals) or to results from the first implant version (Adams Citation2004a, Citation2004b, Anderson and Adams Citation2005). He has tight connections to the company, from which his research and publications concerning this arthroplasty has been supported economically. Recently van Winterswijk and Bakx (Citation2010) published their experience after a follow-up of mean 46 months in 15 patients (17 wrists, 8 Universal 1 and 9 Universal 2) of whom all but one were RA patients. Four were men. Clinical results were good, pain and DASH score were reduced and an increase in ROM (from 68° to 91°) was seen. They found two complications, one early dislocation (stable after closed reduction) and one distal loosening. The patient with osteoarthritis was less satisfied than the rheumatoid patient.
Total modular wrist arthroplasty (TMW)
The total modular wrist system (TMW) was developed by Peter Hubach and introduced in 1999. This wrist arthroplasty has an optional DRUJ prosthesis. The system is primarily intended for uncemented fixation, the components are made out of titanium alloy distally and cobalt-chrome proximally, and plasmasprayed with hydroxyapatite or Bonit®. An uncoated radius component is available for cemented fixation. A straight proximal resection including the distal ulna and a distal straight resection at the level of the proximal capitate are performed, leaving a gap of 19–21 mm. The distal carpal plate is HA/ Bonit®-coated, and fixation is achieved with three HA/Bonit®-coated titanium alloy metacarpal screws (2.–4. metacarpals). The radius component is made of chrome-cobalt, and intended for press-fit fixation. The articulation has a reverse egg shape with the convex part on the proximal side and the UHMW polyethylene socket on the distal side. A constrained articulation is also available ().
Figure 17. The total modular wrist system (TMW). A) The constrained version (upper), the unconstrained version (lower). B) Implant in situ. (Courtesy of Dr Hubach and OrthoCube.)
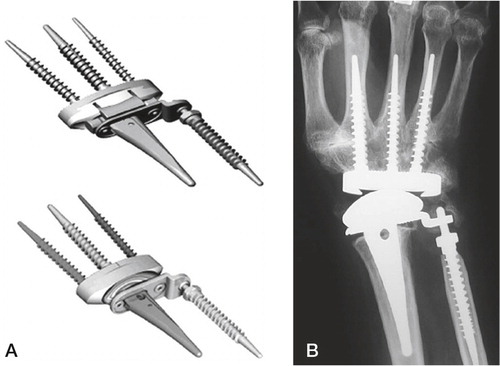
Two different radius plates are offered, one with the DRUJ arthroplasty connection. The ulna component is screwshaped and a peg for articulation with a circular UHMWPE insert. Rahimtoola and Bucher published their preliminary results in 2004 (Rahimtoola and Hubach Citation2004). 32 patients were followed mean 20 months postoperatively. The patient satisfaction was good, with a mean increase in ROM of 25° (from 63° to 88°). 5 wrists demonstrated osteolysis and signs of loosening, one patient had a traumatic dislocation, and five others experienced progressive subluxation and required tendon release (3) or change of the polyethylene insert (1). These short term results have not been confirmed by others. Hubach’s results from 60 primary and 18 revision arthroplasties after a mean follow-up of 4.2 years were reported at the Annual Finnish Rheumatoid Hand Surgeon meeting in 2008 in Tampere. Of the 60 primary joint replacements, 14 were revised (7 loosenings, 5 instabilities and 2 radius fractures). Total ROM averaged 99° and the patients were satisfied with the achieved pain reduction. These results have not been published elsewhere. The arthroplasty is sold by Orthocube, Switzerland, and they have provided their total sale figures (116 worldwide, 2008 Frank Claus, Orthocube, personal communication).
Maestro wrist arthroplasty
The Maestro™ Wrist Reconstructive System (WRS), is developed in cooperation with James Strickland, Andrew Palmer and Thomas Graham, and has been available since January 2005. The arthroplasty has a press fit proximal radius component. Stability is achieved by the ti-alloy stem fixed to a Co-Cr-Mo radial body (coated with Macrobond, a thick titanium plasmaspray), intended for uncemented fixation. An UHMWPE insert is fixed to the proximal component. The distal component consists of a monoblock structure, with an ellipsoid Co-Cr-Mo articulation. The carpal plate has a central peg for fixation in the capitate, and two screws for additional radial (trapezoid/2. metacarpal) and ulnar (hamate) fixation. Both screws are made of titanium ().
The undersurface of the distal component is coated and plasmasprayed with Macrobond. Resection is performed using a resection guide (the proximal cut can optionally be DRUJ saving), and the distal cut has a convex fashion, approximately at the level of the head of the capitate. The articulation is centred, without inclination or volar angulation. By courtesy of Biomet Norway, I have been provided with the instructional lecture by dr Strickland. More than 300 procedures have been performed between 2005 and 2008. From the 25 performed by the developers no major complications have occurred. 14 patients have more than two years of follow-up, achieving pain reduction and good ROM (average 122°). No loosenings or dislocations were observed. Dellacqua reported on the technique and his clinical results in 19 patients after 27 months. He found a minor increase in motion (to 115°), and 57% of patients reported good or excellent according to the Lamberta wrist score. Radiological results were not reported (Dellacqua Citation2009). Nydick et al. (Citation2012) published their retrospective results in 22 patients after 2.3 (0.4–4.6) years in a mixed patient population (rheumatoid and non-rheumatoid). One wrist was converted to arthrodesis due to infection, one patient had a volar dislocation, treated by closed reduction and two patients had wrist contracture. The wrist motion increased from 120 to 128° and the pain reduction was satisfactory. Preoperative DASH was not performed.
Remotion wrist arthroplasty
The Remotion wrist arthroplasty system has been developed by Cooney (Mayo) and Gupta (Louisville). The arthroplasty consist of a proximal Co-Cr-Mo monoblock with a volar (10°) and ulnar (10°) inclination, mimicking the distal radius surface. The proximal part of the radial component is coated with plasmasprayed titanium and the articulating surface is highly polished. Recently a minor change on the dorsoradial part of the proximal component was done to provide a better fit. The distal/carpal component is made of the same material with a peg for press fit fixation in the capitate, where the undersurface and peg are plasmasprayed with titanium. Two 4.5 mm cancellous screws (Co-Cr-Mo) placed through the carpal plate in the direction of the second and fifth metacarpal provide additional fixation. An egg-shaped UHMPE insert is fixed to the distal component by a snap fit, and allows some rotation of the polyethylene on the peg. Resection includes the distal radius (where sparing of the DRUJ is optional) and a straight resection is performed at the level of the proximal capitate. The remaining carpal bones are fused. The distal fixation is based on the carpal bones, mainly the capitate. The arthroplasty allows 40° of flexion and extension and 40° of radioulnar deviation. It is available in 4 sizes, with a standard or a plus version of the polyethylene insert (). The features of the design and the technique have been published by one of the inventors, but their clinical results have not been reported (Gupta Citation2008).
Herzberg recently published the first results in a series of 20 wrists in 19 patients (average 56 years of age) followed for more than one year (average 2.7 years) (Herzberg Citation2011). 13 wrists had been destroyed by inflammatory arthritis, seven had degenerative arthritis of other etiology. The clinical results were quite good, no revisions had been performed although 2 patients experienced loosening of the proximal or distal component (both in rheumatoid patients, but with minimal clinical symptoms). Average ROM of 71° was achieved in the rheumatoid group (a minor decrease compared to preoperatively), 49° in the degenerative group (a minor increase compared to preoperatively). Radial deviation was the lowest, averaging 4°. The majority of patients were satisfied with the procedure, all patients were better than prior to surgery, and 14/19 had a good or excellent result according to the Meuli score (Meuli and Fernandez Citation1995).
Summary
Mallory’s statement in 1988 addressing hip arthroplasties ‘‘all prostheses will fail sometime. It is a race between the life of the patient and the life of the prosthesis’’ is definitely applicable to the arthroplasty of the wrist. So far none of the arthroplasty designs introduced have managed to withstand the long term strain in a normally demanding wrist. All designs have demonstrated inherent weaknesses, some apparent early on whilst others have surfaced after some time. The arthroplasties employed have been limited to older patients with low functional demands, and only minor wrist load has been permitted by manufacturers.
Some of the lessons learned are: Cemented fixation can be used in the radius, but stable cemented fixation is difficult to achieve distally due to the small size of bones, the limited amount of cancellous bone and the inability to apply modern cementing techniques. The wrist is subject to great forces of tension, and cement has little tensional resistance. The multiplanar wrist motion will wear out any constrained devices in all but the weakest patients. The articulation must allow flexion-extension, radial-ulnar deviation and some pro-supination (rotation).
The specifications for the development of a durable wrist arthroplasty must include: a strong durable bone conducting metal implant providing maximal fixation to the minimal bone available, and a low wear bearing with a non-constrained articulation allowing three degrees of freedom (flexion-extension, radio-ulnar deviation and rotation).
Aims of study
To compare a new resorbable calcium-phosphate coating with plasmasprayed HA-coated and uncoated titanium-alloy implants for bone fixation.
To analyse and modify a new wrist arthroplasty based on clinical and radiological performance in a pilot study.
To analyse the clinical and radiological results of a new wrist arthroplasty in patients with post-traumatic osteoarthritis.
Synopsis
Paper 1
Improved bone ingrowth and fixation with a thin calcium phosphate coating intended for complete resorption
The study involved a comparison of gritblasted Ti-6Al-4V screw-shaped implants with and without a 15 ± 5 μm Bonit® coating. Bonit® is claimed to be a resorbable calcium phosphate coating (consisting mainly of brushite, a hydroxyapatite precursor) which is electrochemically deposited on the implant substrate. 14 NZW rabbits were operated with one screw in the distal femur and two screws in the proximal tibia, coated screws were utilized on one side, uncoated on the other. After 6 (n=6) and 12 (n=7) weeks of insertion in an unloaded situation the rabbits were euthanized. Biomechanical removal torque, and histomorphometric qualitative and quantitative measurements were performed. The biomechanical removal torque test showed significantly increased values for the coated implants after 12 weeks (p = 0.0018), but not after 6 weeks (p = 0.093) of integration. Higher bone-implant contact was found for the coated implants in the tibia after 6 weeks and for both tibial and femoral screws after 12 weeks (p < 0.05). There was no difference in the inflammatory reaction around the implants, and possible grains of the coating could be detected after 6 weeks, but not after 12 weeks of follow-up. The unloaded short term study showed promising results for the easily applicable and resorbable coating (Bonit®) compared to uncoated titanium-alloy implants.
Paper 2
Different patterns of bone fixation with hydroxyapatite and resorbable CaP coatings in the rabbit tibia at 6, 12, and 52 weeks
In the second study we compared the resorbable electrochemically deposited calcium phosphate coating (Bonit®) with a (40 μm) plasma-sprayed hydroxyapatite (HA) coating, applied on the same type of gritblasted screw-shaped Ti-6Al-4V implants. The screws were implanted in the proximal cortical region of rabbit tibias for 6 (n=10), 12 (n=9), and 52 (n=9) weeks. The removal torque results demonstrated stronger bone-to-implant fixation for the HA than Bonit®-coated screws at 6 (p = 0.005) and 12 (p = 0.028) weeks. After 52 weeks, the fixation was in favour of the Bonit®-coated screws, but the difference was statistically insignificant (p = 0.086). Qualitative histological examination demonstrated coat flaking and delamination of the HA with multinucleated giant cell activity and bone resorption. This seemed to preclude any significant increase in fixation comparing the HA implants at 6 versus 12 weeks and 12 versus 52 weeks. The Bonit®-coated implants exhibited increasing fixation from 6 to 12 weeks and from 12 to 52 weeks. The coating was resorbed within 6 weeks, with minimal activity of multinucleated giant cells or bone resorption. A different fixation pattern was observed for the two coatings with a sharper but time limited increase in fixation for the HA-coated screws and a slower but more steadily increasing fixation pattern for the Bonit®-coated screws. The coat flaking and giant cell activity with subsequent bone resorption had potential deleterious consequences for the HA screws and seemed to preclude an expected increase in fixation with time.
Paper 3
New concept for total wrist replacement
A novel, uncemented modular wrist prosthesis with screw fixation, metal-on-metal coupling, and ball-and-socket articulation was developed. The system was based upon experiences with long-term, durable and uncemented arthroplasties in other joints. Wrist prototypes were tested in cadavers and modified. The screws for bone fixation were made out of Bonit® coated gritblasted titanium-alloy (Ti6Al4V), the modular ball and socket from highly polished Co-Cr-Mo. The prosthesis was constructed to minimize the bone removal in the wrist and to spare the distal radioulnar joint. The instruments required for the systems implantation were also designed. Ethical approval was received from the Regional Ethical Committee and after informed consent was obtained, eight non-rheumatoid patients (scheduled for wrist arthrodesis) were operated on. The patients were followed yearly thereafter. After 7 to 9 years, the fixed center of the ball-and-socket articulation was shown to provide good stability and mobility. Pain and grip strength was satisfactory. No dislocations, metacarpal fractures or cut-outs, or mechanical failures of the implants occurred. Two distal screws loosened (both revised with new distal screws) most likely due to motion between the capitate and the third metacarpal. One early inflammation and one late haematogenous infection occurred (both revised to arthrodesis). Although the four non-revised and the two patients with revised distal implants had a good result at follow-up (increasing the ROM from 64° to 125°, with unaffected forearm rotation and satisfactory pain reduction), the revision rate was high. None of the patients regretted choosing arthroplasty over arthrodesis. We propose modifications to the implant with reduction in the diameter of the screws and the height of the threads, and rounding of the distal tip. The surgical technique should include: release of the third CMC joint; alignment of the capitate and the third metacarpal and arthrodesis of this joint with bone chips to increase implant fixation and longevity.
Paper 4
Promising 1-6 years results with the Motec wrist arthroplasty in SNAC and SLAC patients
The Motec cementless wrist arthroplasty was introduced in 2006. The implant is made of Bonit® or plasmasprayed HA-coated gritblasted titanium-alloy (Ti6Al4V) screws for bone fixation with a modular ball-and-socket Co-Cr-Mo articulation. The arthroplasty is based on the principle of bone implant fixation, low-wear articulation, minimal bone removal, simple technique and sparing of the distal radioulnar joint. 30 patients (20 men) with destroyed SNAC (16) or SLAC (14) wrists were operated upon and followed prospectively. The mean age at surgery was 52 (31–71) years. Nineteen out of 30 were working (seven in heavy manual labour) preoperatively. All prostheses integrated well radiologically. At a mean follow-up of 3.2 (1.1–6.1) years, no dislocation or implant breakage occurred. Two painful wrists, one of them with infection/inflammation (negative bacterial cultures), were fused. One arthroplasty loosened after 5 years, with infection suspected. The remainder demonstrated intimate bone-implant contact. The clinical results were excellent with markedly decreased DASH (> 20 points) and pain scores, along with increased motion and grip strength compared to preoperatively. None of the patients used analgesic medications at follow-up, and the majority were working (18/30, 6 in heavy manual labour). Knowing the outcome, 27/30 would have chosen arthroplasty again. The new wrist arthroplasty has functioned well after short-term follow-up in a posttraumatic, high demand patient group.
Materials and methods
Paper 1 and 2
A similar set up was used in the first two papers.
Implants
In the first study 84 screw shaped implants (3.75 mm in outer diameter, 8 mm long; 6 mm treaded, 2 mm square headed, pitch height 0.6 mm) were prepared by turning from rods of Ti6Al4V (Mechanical shop, University of Gothenburg, Sweden). After ultrasonically degreasing in trichloroethylene and rinsing (twice) in absolute ethanol the screws were blasted with Al2O3 particles of a size of 50–75 μm in a custom-mode motorised rotation chamber (ELOS Medical AB, Timmersdala, Sweden). Post-blasting they were treated in trichloroethylene and absolute ethanol as before. Half of the batch was set aside as control samples, while the other half of the batch received a 15 ± 5 μm thick coating of Bonit® (Patent WO 02/05862, DOT Medical Solutions Laboratories Gmbh, Rostock, Germany) (test implants). The coating was electrochemically applied at room temperature in an aqueous solution of calcium and dihydrogenphosphate salts. The calcium: phosphate ratio of the coating was 1.1 ± 0.1 according to the manufacturer. After application of the coating the implants were heated to a maximum of 130°C for 20 minutes to instantly remove moisture and prevent the slow transformation to HA. All implants were finally heat sterilised in an oven at 150°C for 1 hour.
In the second study 180 screwshaped (the initial 60 are reported) implants were prepared by turning from rods, according to the same specifications as in paper 1, (from Edstraco AB, Stockholm, Sweden) with similar gritblasting. Half the batch received a 15 ± 5 μm coating of Bonit® (DOT Medical-Solutions Laboratories GmbH, Rostock, Germany) as in paper 1. The other half of the batch received a standardised plasmasprayed coating of HA (MedicalGroup, Lyon, France) with a mean thickness of 40 μm, a crystallinity > 60% and a Ca/P ratio of 1.67–1.76 according to the manufacturer (). The coating of HA covered only one side of the screw thread, see below. All screws were finally sterilised by gamma radiation at the respective coating company.
Surface characterisation
Surface chemical analysis was performed on one unused gritblasted, one Bonit® and one HA screw using X-ray Photoelectron Spectroscopy (Physical Electronics (PHI5500) Chanhassen, Minnesota, USA).
Surface roughness was characterised by an interferometer (MicroXam TM®, Phase-Shift, Arizona, USA). Three unused gritblasted, three Bonit® and three HA screw implants were measured on nine sites each (three tops, three valleys and three flank areas). Each measured area was 264 × 200 μm. Three surface parameters were described (Thomas Citation1999): Sa, which is a pure height descriptive parameter measuring the average height deviation in micrometers. Sds = Density of summits/μm2. This parameter describes how close the individual irregularities are, thus this is a pure spatial descriptive parameter. Sdr is a parameter describing, in percent, the developed surface area compared with an equally sized absolute flat reference area. This parameter is influenced by variations in height and in spatial direction, and is therefore called a hybrid parameter.
Scanning electron microscope (SEM, LEO 1550 Geminy, Germany) was used to qualitatively investigate the sample surfaces of one uncoated, one Bonit®-coated and one HA-coated screw. In addition light microscopical examination of the screw surfaces was done on cut and ground sections of one Bonit®-coated, one HA-coated and one uncoated screw.
Animals and anaesthesia
The first study was approved by the local animal ethics committee at the University of Gothenburg, Gothenburg, Sweden and performed in Gothenburg. The second study was approved by the animal ethics committee at the University of Oslo (UiO), Oslo University Hospital, Rikshospitalet, Oslo and performed at UiO, Oslo.
The first and second studies involved 14 and 30 adult female New Zealand White rabbits respectively. The rabbits were given standard food and water, and they were kept in separate cages prior to the experiment as well as after. Before surgery the rabbits were anaesthetised by an intramuscular injection of phentanyl/fluanizon (Hypnorm®, Vet. Janssen, Saunderton, England) at a dose of 0.5 ml/kg, and 2.5 mg diazepam (Stesolid®, Dumex, Copenhagen, Denmark) intraperitoneally. Further injections with Hypnorm® were administered as needed. The hind legs were shaved and disinfected prior to injection of local anaesthesia with 1 ml 0.5% lidocain (Xylocain®, Astra-Zeneca AB, Södertälje Sweden) in the operating field. The skin and facial layers were opened under sterile conditions and closed in separate layers. The periosteum on the insertion sites, i.e. the tibial tuberosity and the medial femoral condylar region was gently pulled away (and not resutured), followed by low rotary drilling with a graded series of drills and tapping with tailor made instruments during profuse saline cooling. Three screws were inserted in each leg (two implants in the tibia and one in the femur), i.e. uncoated vs. Bonit®-coated in the first study, and Bonit®-coated vs. HA-coated in the second ().
Figure 21. Peroperative view. A) The femur screw is placed. B) Prior to closure. Three screws in situ placed in the knee region, one in the distal condylar femur region and two in the proximal tibia.
Postoperative buprenorfin 0.25 mg/kg (Temgesic®, Reckitt and Coleman, USA) was administrated. All animals were given antibiotic prophylaxis (trimetoprim 40 mg/ml and sulfamethoxazol 200 mg/ml (Borgal®, Intervet International B.V Boxmeer, Netherland) 0.5 ml/kg subcutaneously, which was continued for 5 days with 0.2 ml/kg × 2 in the drinking water). The follow-up time was scheduled to be 6- and 12 weeks (seven rabbits in each group) in the first study, 6, 12 and 52 weeks (10 rabbits in each group) in the second. One rabbit died after three weeks in the first study leaving 6 rabbits for the 6 week evaluation and 7 for the 12 week. In the second study one rabbit in the 12 weeks group did not recover after surgery and died after 10 days, and one rabbit in the 52 weeks group was found dead after 40 weeks. The remaining 10 rabbits in the 6 weeks group and nine in the 12 and 52 week groups fared well. All rabbits were euthanized with an overdose of pentobarbital (Apoteksbolaget, Uppsala, Sweden) intravenously.
The distal tibial implants were used for biomechanical removal torque tests, while the proximal tibial screws and the femoral implants with surrounding bone tissue were prepared for histomorphometrical evaluation. All screws are reported from the first experiment, while the removal torque results were reported from the second study.
Biomechanical test
Removal torque tests: The torque needed to loosen the test and control implants from the bone bed in vivo is performed using custom-made electronic equipment with the animal in full anaesthesia allowing for similar conditions of sample testing. The square headed implant is connected to a pin fitting the removal torque jig. The device had a strain gauge transducer enabling direct readings of the peak loosening torque in Newton centimetres (Ncm) (Johansson Citation1998, Johansson Citation2001). This equipment is routinely used for biomechanical tests in our laboratories and has been involved in several PhD theses and thus various in vivo studies (Wennerberg Citation1996, Ivanoff Citation1999, Franke-Stenport Citation2002, Sul Citation2002, Sundfeldt Citation2002). In the second study a baseline test for the removal torque was done on a recently euthanized rabbit (refrigerated and thawed), which was operated as the other rabbits, but the removal torque test was done immediately after surgery to investigate the persistence of the two coatings after the removal torque test.
Sample preparation and evaluation of sections
In the first study the proximal tibial and femoral screws were resected en bloc with surrounding bone tissue and immersed in 4% neutral buffered formaldehyde for one week. Further treatment followed the internal guidelines of the laboratories. In brief this involved dehydration in graded series of ethanol, pre-infiltration in diluted resin, infiltration and embedding in pure resin (TechnovitVLC 7200, Kültzer, Germany). Undecalcified cut and ground sections were prepared using the Exakt cutting and grinding equipment (Exakt Apparatebau, Norderstedt, Germany) and prepared according to the procedure described by Donath (Donath Citation1988) and Johansson(Johansson and Morberg Citation1995). All specimens were divided in the same anatomical manner, i.e. along the long axis of the implants. Sections with an initial thickness of 200 μm were prepared and further ground to approximately 10 μm. All sections were histologically stained in 1% toludine-blue in 1% Borax solution mixed in proportions of 4:1 with 1% pyronin-G solution followed by cover slipping. Qualitative histological descriptions and quantitative histomorphometrical evaluations were performed in a Leitz Aristoplan light microscope (Leitz Gmbh, & Co. KG, Oberkochen, Germany). The microscope is equipped with a Leitz Microvid unit connected to a PC (Johanssen Citation1991) and a mouse enabling “direct measurements to be performed through the eye piece of the microscope” and semi-automatic computer-based evaluations. Cut and ground sections of one unused gritblasted and one Bonit® screw were also performed.
In the second study the samples were prepared by a similar manner. Bulk staining with basic fuchsine added to the dehydration steps in ethanol was carried out on the 6 week samples. This technique has been referred to as a staining suitable for the observation of micro-cracks in the light microscope (Burr Citation1985). However since we found it difficult to observe cracks, the two other groups received the routine histological staining involving 1% toludine blue in 1% Borax solution mixed in proportions of 4:1 with 1% pyronin-G solution followed by cover slipping. A Leitz Aristoplan light microscope (Leitz Gmbh, & Co. KG, Oberkochen, Germany) was used for qualitative histology and for bone length measurements on the removal torque loosened implants. Cut and ground sections of one unused Bonit® and HA screw as well as one Bonit® and HA implant from a dead rabbit (which was operated like the others and removal torque tested immediately afterwards) were also examined in the microscope for evaluation of the coating and for its stability/endurance after the surgical procedure with screwing and removal torque testing. The proximal tibial and femur samples were prepared for histomorphometric analysis, but this is not part of the thesis.
The histomorphometrical quantifications in study one involved ( and ):
Figure 22. Illustration of the histomorphometric evaluation. A) Screw in situ in the cortical region of the rabbit tibia. B) Bone-implant contact. C) Bone area within the threads. D) Outfolded mirror image.
Figure 23. Histological section at 400 x magnification of a test sample demonstrating histomorphometric measurements at higher magnification within one thread. Implant in black. Direct bone-implant contact in red (% of length between the threads), yellow line demarcates bone-area within the thread minus green areas (% of area).
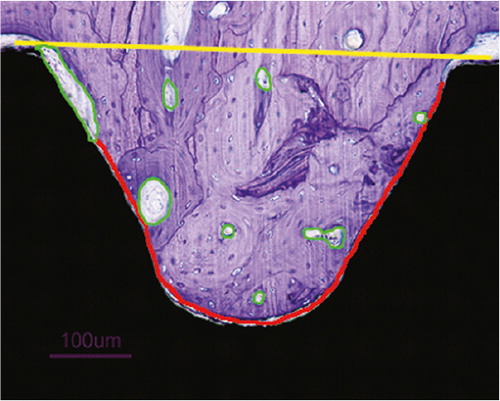
bone to implant contact in all threads around the implants
bone contact in the 3 best consecutive threads in the cortical region of the tibial implants
bone area in all threads around the implants
bone area in the 3 best consecutive threads in the cortical region of the tibial implants
bone area in the out-folded mirror images to the 3 best inner threads. This investigation may reflect time dependent remodelling effects (Johanssen Citation1991).
On the removal torque tested implants a rough estimation of the shear strength could be calculated by using the removal torque and bone length measurements from one central cut and ground section of each loosened implant. The bone length was measured using the 4x ocular magnifier. The microscope is connected to a computer using the Leitz Microvid (Ernst Leitz Wetzlar GMBH, Germany) unit. The microscope oculars are calibrated using a known scale with the aid of the computer program, and the bone lengths are measured in mm and reported with two decimals. We examined the length of bone in close contact with the implant through the cortical region on the original ground specimen. The soft tissue regions in the cortical regions were excluded. The bone marrow was without cancellous bone in this part of the tibia. The formula: T / π x d x rl x l was applied where T = removal torque (Ncm), d = mean diameter of the implant (3.45 mm), rl = lever arm (= radius i.e. 1.725 mm) and l = the entire bone tissue length along the implant surface (mm) in the cortical region(Derezende and Johansson Citation1993). The measurement is a reflection of the shear strength between implant and bone (Johansson Citation2010, Stenport and Johansson Citation2008). The test was performed in both studies, but only reported from the second (see below).
Paper 3 and 4
Implants
In the third study two variations of the same implant were used. In the first two patients a cone shaped, short and thick screw (made out of gritblasted Ti6Al4V) with rounded ends and high and wide threads (cutting in the proximal and non-cutting in the distal implants) along the entire implant was used ().
In the last 6 patients, longer and thinner screws with lower cutting threads and without threads distally were used. The screws were cross cut distally for an exact fit. A metal-on-metal (highly polished Cr-Co-Mo) ball-and-socket articulating mechanism was employed. The modular articulating system was fixed with a Morse cone to the screw shaped implants in the capitate/third metacarpal and the radius. An articulation diameter of 18 mm provided almost 80° of motion in all directions before impingement occurred between the implant components. All implants were coated with Bonit® (DOT Medical, Rostock, Germany). The instruments required for the surgical procedure were also developed to make surgery as straight forward and easy to learn as possible ().
Figure 25. The instruments developed for implantation. From the top, cannulated radius reamer and distal reamer, implant screwdriver, countersinker for the socket, impactor for the ball and socket, pins for the cannulated system and trial components for final implant selection.

In the fourth study, the Motec total wrist arthroplasty (Swemac Orthopaedics AB, Linköping, Sweden) was used (). The implant had been thoroughly changed from the experience gained during the developmental period (study 3). The screw-shaped implants are made of gritblasted titanium alloy, with cutting threads except for the most distal part. The diameter and the height of the threads were reduced.
Figure 26. The Motec total wrist arthroplasty. Thicker and shorter proximal screw, thinner and longer distal screw. Courtesy of Swemac Orthopaedics AB, Linköping, Sweden.
The blasted outer surfaces of the screws were coated with Bonit® (DOT Medical, Rostock, Germany) (54 components: 26 metacarpal and 28 radial) or hydroxyapatite (HA) (MedicalGroup, Lyon, France) (6 components: 4 metacarpal and 2 radial), according to the surgeon’s preference. Bonit® is a 15 ± 5 μm thick, resorbable, electrochemically deposited calcium-phosphate coating. The plasmasprayed HA is approximately 40 μm thick with a crystallinity exceeding 60%, according to the manufacturer. Three lengths of radial (32, 38 and 44 mm) and 5 of capitate/third metacarpal (45, 50, 55, 60 and 65 mm) screws are available, the latter in 2 different diameters. The cobalt-chrome-molybdenum articulations are highly polished with a surface roughness of Ramax= 0.08 μm and a difference in diameter between the ball and socket of 0.09–0.17 mm. The last 16 articulations received an extra smooth (Ra = 0.04 μm) wear resistant chromium-nitride surface coat of 4-6 μm (Medthin-Silver, IonBond AG, Olten, Switzerland). The articulation is available in two diameters (15 and 18 mm); 18 mm was chosen for all our patients. The modular taper lock coupling includes balls with 3 neck lengths and a total length variation of 5 mm. The maximum motion in all directions permitted by the coupling approaches 80° with the long and standard necks and 65° with the short neck, limited by impingement between the cup and the neck or the cup and the distal screw. The instruments for the surgery were refined and kept to a minimum (). The prosthesis was CE approved according to the ISO standard 14360 in 2006.
Patients
None of the patients are included in both study three or four.
In the third study, The Regional Committee for Medical and Health Research Ethics in Norway (S-99082, 1999) allowed insertion of the prosthesis in patients who had given informed consent. The implants were offered to non-rheumatoid patients admitted for wrist arthrodesis and were ineligible for more limited wrist procedures. The characteristics of the eight patients (seven males) operated from February 2001 to June 2003 are shown in . The median age of the patients was 52 (range, 23–76) years. Two patients were retired; the remaining 6 were employed.
Table 1. Patient characteristics
Four of the patients had prior surgery in the affected wrist.
In the fourth study, all patients from January 2006 to January 2011 with scaphoid non-union advanced collapse (SNAC) and scapho-lunate advanced collapse (SLAC) who were scheduled for wrist arthrodesis were offered arthroplasty; 30/35 chose the latter. The indication was heavy wrist pain associated with destruction of the radiocarpal joint, thus contraindicating four-corner fusion. If in doubt, a CT scan was taken to visualize the joint surfaces. Degeneration in the radiolunate joint was considered a contraindication for limited carpal arthrodesis or resections. The prospective follow-up study was approved by the hospitals data authority (22 February 2006). 30 patients (20 men) were included and followed-up prospectively. 16 SNAC and 14 SLAC wrists were operated; 23 right and 23 dominant hands. The mean age at surgery was 52.4 (31.0–71.4) years. 16 patients were graded ASA 1, 12 ASA 2 and two ASA 3. 22 previous wrist surgeries (fracture treatment, ligament reconstruction, bone resection or limited arthrodesis) had been performed on 13 patients, with multiple surgeries in eight cases. Five patients had bilateral osteoarthritis of the wrist and eight of the ipsilateral distal radioulnar joint; one had previously undergone Darrach’s procedure.
Surgical technique
A longitudinal incision ulnar to the Lister’s tubercle commenced 2–3 cm proximal to the wrist joint and concluded just distal to the third carpometacarpal (CMC 3) joint. The extensor retinaculum was elevated subperiosteally to the radial styloid and close to the distal radioulnar joint. A longitudinal capsular incision was made from the radius to the CMC 3 joint. The lunate, the proximal 2/3 of the scaphoid and the radial styloid were removed. The distal radioulnar joint was avoided. The entry point of the distal guide wire was centrally on the head of the capitate, transversing the CMC 3 joint and entering the diaphyseal canal of the third metacarpal. The proximal guide wire entered the ridge between the lunate and the scaphoid fossa approximately in the midpoint. The principles of the operation are shown in .
Figure 28. A) The K-wire is drilled centrally through the capitate and into the third metacarpal. B) Reaming of the screw canal. C) Screwing home the distal component. D) Drilling the K-wire centrally into the radius. E) Reaming of the canal. F) The radius screw is in place and the socket has been punched into the screw. The ball is locked into the distal screw.
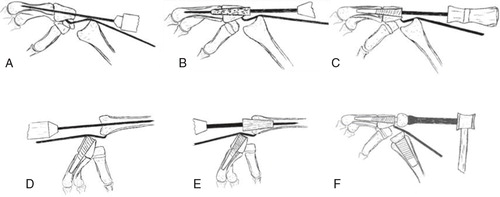
Prophylactic antibiotic treatment with cefalotin 2 g × 4 (Keflin®, EuroCept, Ankeveen, Netherlands) was used for one day postoperatively. A forearm cast was applied for median 30 (range, 24–38) days.
In the fourth study, the principle of the surgery was similar, although we emphasized some new parts of the surgery. The CMC 3 joint was opened. Cartilage and sclerotic bone was removed in a wedge-shaped fashion to establish an arthrodesis in slight extension giving a straight line from the entry point in the capitate through the CMC 3 and passing the isthmus of the metacarpal. Under fluoroscopic control, the guide wire was passed through the capitate, across the CMC 3 joint and into the third metacarpal, ending in the caput. The reamer was introduced over the guide wire and advanced until filling the proximal metacarpal diaphysis, optimally passing through the isthmus. The appropriate size of capitate/third metacarpal screw was inserted, and filling in resected spongious bone completed the CMC 3 arthrodesis. This aimed to provide the maximal continuous bone-implant contact. On the proximal side, the distal radius was opened with an awl in the middle of the ridge between the lunate and scaphoid fossae. The guide wire was passed, with the aid of the fluoroscope, centrally in the medullary canal up to the junction between the distal and middle one third of the radius (which secures centralization of the position in the distal radius). When the reamer engaged both cortices in the lateral view, the appropriate size of radius screw was found. After countersinking for the cup, the screw was driven in. Trial cup and balls optimised tension and range of motion. The triquetrum was excised if ulnar impingement was suspected. The patients received intravenous cefalotin/Keflin® (EuroCept Pharmaceuticals, Netherlands) 2 g × 4 or clindamycin/Dalacin® (Pfizer Inc. New York, USA) 600 mg × 4 (with the first dose preoperatively). A plaster slab secured slight wrist extension and radial deviation. The sutures were removed after two weeks and another cast applied for four weeks, after which a wrist exercise program was commenced. During the cast period, the patient was encouraged to use the shoulder, elbow, forearm and fingers on the operated side.
Follow-up
In the third study, standard wrist radiographs were taken postoperatively and at outpatient clinical follow up after 3, 6, and 12 months with yearly follow up thereafter. In the fourth study, follow-up was scheduled at 6 weeks, 6 months, 1 year and yearly thereafter. The patients graded radial and ulnar sided wrist pain at rest and in activity using a scale of 0–10 with 0 indicating no pain. Active range of motion (AROM) and passive ROM (PROM) of the wrist (flexion + extension + radial + ulnar deviation) as well as forearm rotation were measured using a handheld goniometer. At follow-up the grip strength was assessed with Biometrics G100 E-link Hand Dynamometer (Biometrics Ltd, Gwent, United Kingdom). The measurements were performed by physiotherapists unrelated to the treatment and unaffiliated with our department. The patients completed the DASH score(Hudak Citation1996), translated and validated into Norwegian (CitationFinsen 2001, Citation2008). The DASH questionnaire has been developed to assess global upper extremity function and is a valid instrument for the evaluation of single or multiple disorders in the upper extremity at one or many points in time(Beaton Citation2001). In the fourth study the DASH and grip strength measurement were also performed preoperatively and key pinch was measured with JAMAR® Key pinch dynamometer (J.A. 88 Preston Corp., Clifton, NJ, USA) at follow-up and compared with the contralateral side.
Radiology: AP and lateral radiographs pre- and postoperatively and at all follow-ups were compared and examined for CMC 3 arthrodesis, bone-implant contact and migration. Subsidence of the distal component was measured from the distal end of the metacarpal screw to the distal end of the metacarpal head. Periprosthetic radiolucent lines were registered. Loosening was defined either as a continuous radiolucent line surrounding, or measurable migration of the component. Focal osteolysis was defined as any round or scalloped area of bone loss. Revision was defined as any change of the arthroplasty components or conversion to arthrodesis, and considered a failure.
Statistical methods
In the first study the Wilcoxon signed rank test was used for comparison of related samples (test vs. control for the 6- and 12 week samples) and Mann-Whitney U test for independent samples (6 week results compared to 12 week results) due to a non-parametric distribution of the measurements. P values < 0.05 were considered statistically significant. In the second study the measured parameters exhibited a normal distribution demonstrated by the q-q plot. Student t-test was used for intra-individual comparisons. For inter-individual comparisons an independent t-test was used and adjusted for Bonferroni. An * was given for the Bonferroni-corrected p-values in the results section. Confidence intervals (CI) are given for the comparisons, and p values < 0.05 are considered statistically significant. In the third study the data is presented as median (range). No statistical comparisons were made due to the low number of patients. In the fourth study data is given as mean (SD). Student t-test was used to compare continuous variables. All p-values were 2-tailed, and the significance level was set to 0.05. A Kaplan Meyer survival plot was estimated using the time from the primary operation to revision of any cause or death. P < 0.05 was considered statistically significant. The data was analysed with SPSS for Windows version 16.01 (SPSS Inc., Chicago, IL, USA).
Results
Study 1 and 2
Surface characterization
The surface of all implants comprised calcium, phosphorous, oxygen and carbon and traces of zinc. The uncoated implant also had aluminium and traces of lead and copper on the surface, and the HA-coated implants also revealed nitrogen on the surface.
Qualitative differences between the three different screws were revealed by scanning electron microscopy (). The SEM surface of the HA screws also demonstrated a rough surface, but less spiky as compared to the Bonit® surface.
Figure 29. A) The uncoated SEM surface, demonstrating a more homogenous, less rough surface. B) The Bonit®-coated SEM surface demonstrating a spiky and relatively rough surface. C) The SEM surface on the HA-coated screw.

Light microscopical investigation of the sections taken from the non inserted implants revealed no coating on the gritblasted screws, while the Bonit® coating was seen as a continuous rim on the surface of the implant. The Bonit® sample showed black particles in the evenly distributed coating layer. The HA coating was unequally distributed with variations in thickness, the thickest being around 40–50 μm. The coating was consistently found on the same flank side of the thread, diminishing towards the other; the latter probably being “leeward” during the plasmaspraying process ().
Figure 30. Histological picture of unused A) Bonit® screw revealing a continuous thin coating layer with black particles embedded and B) HA screw with the coating mainly on two thirds of the threads.
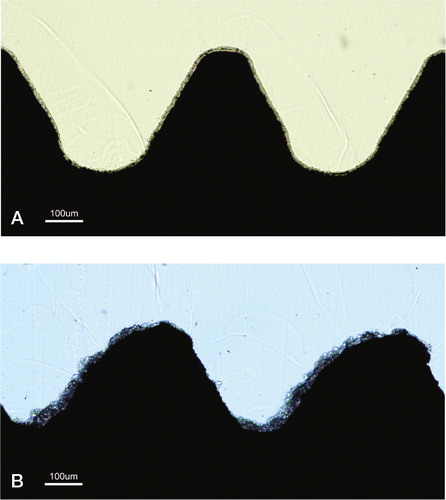
Microscopical evaluation of the baseline screws tested in the second study demonstrated persistent coatings on both samples after insertion and removal torque testing.
Surface roughness
In the first study the Bonit® implants had a considerably rougher surface than the uncoated screws. The average height deviation was much greater and the surface had a much denser structure demonstrated by the parameter Sds. As a result the surface enlargement was approximately 225 % compared with 33 % for the control ().
Table 2. Interferometric surface roughness analyses from study 1. Mean (SD)
In study 2 the Sa and Sdr parameters were significantly higher for the HA than the Bonit® screws. The density of summits, were significantly greater on the Bonit® surface due to the spiky appearance as demonstrated with scanning electron microscope ().
Table 3. Interferometric surface roughness analysis from study 2. Mean (SD)
Removal torque
In the first study there was as a difference in removal torque values in favour of the Bonit®-coated screws, although not significant at 6 weeks (). For both types of screws there were significant increases in removal-torque values from 6 to 12 weeks.
Table 4. The removal torque results in Ncm (SD) [range]
In the second study, the HA screws demonstrated the strongest fixation at 6 weeks, moderately increasing at 12 weeks, without further enhanced fixation at 52 weeks. The Bonit®-coated screws exhibited increasing fixation with time. Comparing the two screws, the fixation was in favour of HA after 6 and 12 weeks, while the opposite was the case after 52 weeks, however the latter was not statistically significant ().
Table 5. The removal torque results in Ncm (SD)
The increased fixation of Bonit® at 6 vs. 12 and 12 vs. 52 weeks was statistically significant (p = 0.005 and p = 0.004, respectively). The increased fixation of HA was insignificant between the 6 vs. 12, and 12 vs. 52 weeks (p = 0.5 and p = 1.0, respectively).
Bone length and shear strength
Bone length and shear strength were recorded in both studies, but not preferred as part of the manuscript by one of the reviewers evaluating the first study. In the second study the two coatings exhibited different patterns of bone length and shear strength values with increasing time. A uniform picture was found for the Bonit®-coated implants demonstrating a steady increase in both removal torque and bone length with time, giving correspondingly enhanced shear strength throughout the follow-up (due to a greater increase in removal torque as compared to bone length) ().
Table 6. Bone length in mm. Mean (SD)
The HA screws demonstrated its greatest bone length at 12 weeks, while the removal torque exhibited a minimal increase with time thus giving shear strength peak at 6 and 52 weeks. The bone length was 65% and 61% greater for the Bonit® compared to the HA screws at 6 and 52 weeks, respectively, at 12 weeks the bone length was approximately identical. The difference in bone length adjacent to the Bonit®-coated implants compared to HA resulted in 300% higher shear strength in favour of the HA screws at 6 weeks (p = 0.005) ().
Table 7. Shear strength values in N/mm2. Mean (SD)
Quantitative histomorphometric analysis (performed on the non-removal torque implants in the first study): Bone to implant contact: The bone contacts were greater for the Bonit®-coated samples compared to the controls both at 6 and 12 weeks but not statistically significant for all threads at 6 weeks. All other contact parameters were significantly greater for the Bonit®-coated samples.
Bone to implant contact increased for both the test Bonit® and control implants from 6 to 12 weeks, but this difference was not significant, as shown in .
Table 8. Bone–implant contact (%). Mean (SD) [range]
Bone area
Irrespective of time- and area parameters tested there were no significant differences observed between Bonit® and control samples. In general the quantitative data of areas in all threads showed no increase with time. The relation between the 3 best inner areas compared to the mirror images demonstrated about a 1 – 1 relation ().
Table 9. Bone area (%). Mean (SD) [range]
Qualitative histological description
Study 1
3 weeks samples
Femur: Survey inspection of test and control samples revealed extensive callus formation on both sides of the implants. In this area the cells appeared to be chondrocyte-like and had a “reddish” staining. One could observe dark purple/red areas inside the bone tissue as well. In the bone tissue under the callus formation there were clear demarcation lines observed between old and newly formed bone. Newly formed bone trabeculae were observed inside the threads and appeared like rims on the thread implant surfaces. An ongoing bone remodelling was present with osteoclasts resorbing old bone and osteoid rims seen on new formed bone areas. No differences could be observed between test and control samples.
Tibia: Survey inspection of test and control samples revealed greater callus-like formation on the inferior side compared to the superior. The periosteal thickening /formation was greater than the endosteal bone tissue formation. It seemed like the new bone formation in the marrow threads was greater on the test side. Multinucleated giant cells could be observed in close vicinity to the implant surfaces, especially in the marrow cavity.
6 weeks samples
Femur: Survey inspection revealed a greater callus-like formation on the superior side compared to the inferior, although some small thin bone trabeculae were seen on the latter forming a shell-like structure on top of the old cortical layer. Inside this structure the tissue had a more bone-marrow like appearance compared to the superior side. The area on the superior side was filled with thicker trabeculae and some areas of light purple stained cartilage-like material. There was also a large amount of cells and blood vessels. The cartilage-like areas were entrapped in the tissue surrounded by new bone trabeculae extending from this cartilage like tissue outwards in the callus. An ongoing bone remodelling (osteoid with osteoblasts and osteoclastic resorption of bone) could be observed on the trabeculae. Most of the bone tissue in the threads was newly formed and only a few areas demonstrated contact with old cortical un-remodelled bone. Osteoid like tissue could also be seen in close relation to the implants. Multinucleated giant cells, light stained macrophages and cells with seemingly phagocytised material could be observed close to the implant surfaces.
Tibia: The survey inspections of the tibia samples revealed a greater periosteal reaction (with a large amount of bone trabeculae incorporated in this area) on the superior side compared to the inferior. The superior side seemed to have a more pronounced thickening /corticalization than the inferior side. Most of the bone tissue in the thread region was newly formed bone. Clear cement-lines between old and newly formed bone were observed. Osteoid like tissue with osteoblast seams was located close to the interface. Higher magnification revealed cells with particles/debris presumably internalised. Multinucleated giant cells could be observed in close vicinity to the implant surfaces as well.
12 weeks sample
Femur: Survey inspections of both materials revealed remnants of a possible callus formation on both sides of the implants. It seemed as if the superior side had a greater callus formation than the inferior and it showed a more pronounced corticalization. The former also had a thin bone shell demarcating the outer area of pre-existing callus area compared to the opposite side. The tissue under this shell had a marrow like structure. Higher magnification demonstrated some cells in this region with internalised “material”. Bone remodelling units could be seen close to the interface region. Osteoid rims with osteoblasts were also located close to the implant surface. In soft tissue regions in the interface one could observe blood vessels, macrophages and multinucleated cells. The latter were either large and light stained, or smaller and darker stained in close vicinity to the implant surface.
Tibia: The periosteal and endosteal thickening was greater on the 12-week samples compared with the 6 weeks samples. Internal comparisons revealed that the endosteal formation was possibly not as pronounced as the periosteal formation. The bone tissue in close contact with the implants had been remodelled. Haversian canals could be seen inside the thread areas. Irrespective of material, osteoid like tissue was observed on the implant surfaces. Some thread-bottom areas in the marrow cavity demonstrated soft tissue structures with a loose capsule like formation filled “bone forming islands” in the centre-region and large multinucleated giant cells in “direct” contact with the implants. The thread tops revealed a more dense capsule formation separating the implant from the bone marrow cavity. Irrespective of implant material, one could observe cells with black and yellow like particles that seemed to have been internalised/phagocytised. Macrophages and light stained elongated multinucleated giant cells in close connection to the surfaces were seen.
In the first study, no major qualitative differences were seen between test samples and controls when examined with ordinary light microscopical technique within the same time point.
Study 2
In the second study, the histological examination on the Bonit® screws on the removal torque samples demonstrated a uniform pattern of loosening between implant and bone after application of the removal torque. Coating remnants were not observed in the interface region when inspected in the light microscope at any observation time. Callus formation (i.e. periosteal bone tissue formation) and immature young bone was observed at 6 weeks, maturing through 12 weeks with remodelling and conversion to a lamellar structure after 52 weeks. The bone-implant length increased gradually. Multinucleated giant cells and macrophages were seen occasionally in the interface region. The overall histological picture was consistent with that of “calm” normal bone remodelling (and ).
Figure 31. Photomicrographs of sections. a) Six-week Bonit® screw, bulk staining with basic fuchsine. b) Overview of a 52-week sample demonstrating the shape and position of the screw and the macroscopic appearance in the tibial bone. c) Magnification of a 52-week Bonit® screw demonstrating the bone-implant contact. Toluidine blue routine histological staining in b) and c).
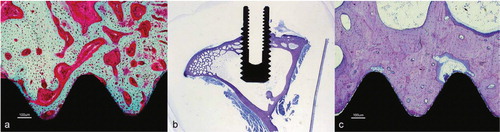
Figure 32. Close up of a Bonit®-coated screw at 12 weeks. The entire interface region reveals remodelled bone (RB) tissue. Except macrophages being observed in the soft tissue regions in the interface no inflammatory reactions were observed. Cement lines (CL) are clearly visible between new formed bone and old cortical bone (OB). Both bone forming and bone resorption cavities can be observed. Toluidine blue routine histological staining.
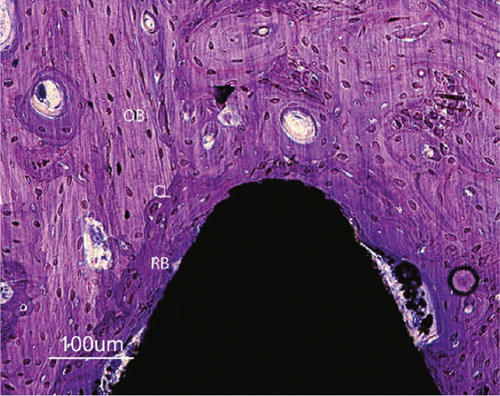
The HA-implant interface revealed detachment both between the bulk implant and coating (HA adherent to the bone) as well as between the HA coating and bone (HA adherent to the implant), both patterns being demonstrated along the very same implant at all times of follow-up. When blood was seen in the interface between the coating and bone/implant, the detachment probably occurred during the removal torque test. Detachment was not seen through the coating or through the bone outside the coating.
The bone in the vicinity of the HA-coated screws demonstrated a less uniform picture as compared to the Bonit® implants. At 6 weeks immature young bone was observed in the contact area, as well as resorption of bone and HA. At 12 weeks the most active histological picture was observed, with remodelling of bone and resorption of HA substituted by immature bone or soft tissue in the contact zone and with remnants of HA in the vicinity. Remodelling cavities were also seen further away from the implant often containing HA particles. A similar, but less active picture was found at 52 weeks, where more resorption and substitution of HA was seen. HA was also observed in remodelling cavities further away in the bone tissue. The larger particles where surrounded by cells, minor particles were internalised in cells. The most active and complex histological picture was seen at 12 weeks. This had diminished at 52 weeks. Multinucleated giant cells were frequently observed, often containing granules of HA. The bone length was shorter compared to the Bonit® implants at 6 and 52 weeks; at 12 weeks they were almost the same length ( and ).
Figure 33. Photomicrographs of HA-coated implants: a) 6-week bulk staining with basic fuchsine. b) Overview of the 52-week sample demonstrating the shape and position of the screw and the macroscopic appearance. c) Magnification of the 52-week HA screw. Toluidine blue routine histological staining in b) and c).
Figure 34. A 12-week close up of a HA-coated implant demonstrating loose HA particles (arrows) in the soft tissue outside thread peak as well as in bone remodelling cavities further away from the implant. Older cortical bone (OB) can be observed separated from younger bone by cement lines (CL). Note regions of more woven bone (WB) (immature) structure internalized in the remodelled bone. Toluidine blue routine histological staining.
Study 3
Since the third study can be considered a case series the individual results from each patient are given after which some general results are given and reflections made. A brief summary of each of the eight patients follows:
Patient 1 (first edition). A periprosthetic radiolucent line was seen in the distal metacarpal at 3 months, whereas intimate bone-implant contact was observed in the capitate and the radius. The clinical result was excellent, but deteriorated after two years when the metacarpal lines progressed to the capitate, and the distal screw loosened. The prosthesis was removed and arthrodesis of the wrist performed. Although pain-free, the patient missed wrist motion, especially at work, and he insisted on rearticulation, which was performed after three years. The revision prosthesis was well integrated radiologically, and wrist function was good three years following rearticulation ().
Figure 35. Patient 1 (first edition prosthesis). A) after 1 year, intimate bone-implant contact in the radius and capitate, radiolucent line surrounding the metacarpal part, B) after 2.5 years, a loose distal component with distal screw migration, C) arthrodesis, asymptomatic open CMC 3 joint, D) 3 years after rearticulation, intimate bone-implant contact and CMC 3 arthrodesis after bone transplantation. The DRUJ is unaffected.
Patient 2 (first edition) showed a line surrounding the metacarpal part of the screw at 1 year. Intimate bone-implant contact was seen in the capitate and radius. The wrist function was excellent.
Patient 3 (second edition). A perforation of the ulnar and dorsal cortex of the metacarpal occurred during the reaming. After six months intimate bone implant contact was seen in the radius and the capitate, while a radiolucent line was present in the metacarpal interface radially and distally along the implant. After two years, the distal screw loosened in the capitate as well. A revision screw was inserted, but septic loosening occurred (staphylococcus epidermidis). The whole prosthesis was removed 18 months after revision, and an iliac crest bone graft and plate arthrodesis was performed as a one stage procedure. Kloxacillin (Diclocil, Bristol-Myers Squibb Company, New York, USA) 1 g × 4 daily was given for 8 weeks and the arthrodesis healed uneventfully.
Patient 4 (second edition). Reaming caused a near complete perforation of both the ulnar cortex of the metacarpal and volarly in the radius. A surrounding radiolucent line was observed from 3 months along the metacarpal part of the implant, but the prosthesis demonstrated intimate bone-implant contact in the capitate and radius. DRUJ osteoarthritis was treated with the Darrach’s procedure after four years. The wrist function was good at follow-up.
Patient 5 (second edition). The distal screw was inserted uneventfully, but a perforation of the dorsal cortex occurred in the radius. Radiologically the prosthesis exhibited intimate bone-implant contact along both components. However, a dorsal tenosynovitis of the wrist developed and synovectomy revealed the joint to be full of whitish material. This raised suspicion of infection and the well-fixed prosthesis was removed. CRP/SR was not taken preoperatively. All cultures from the joint were sterile. Histology showed inflammation mainly with lymphocytes. The patient received kloxacillin (Diclocil, Bristol-Myers Squibb Company, New York, USA) 1 g × 4 daily for 3 months. After two years she was rearticulated with a new prosthesis. Darrach’s procedure was performed concomitantly. Cultures obtained during the reoperation were sterile. The new prosthesis shows intimate bone-implant contact, and the wrist function is good three years following revision.
Patient 6 (second edition). A near complete perforation of the radial metacarpal cortex was experienced at surgery. The radial screw was inserted close to the DRUJ but did not penetrate it. After 3 months the distal screw exhibited a surrounding radiolucent metacarpal line, though not in the capitate or radius. The patient developed DRUL osteoarthritis, and the Darrach’s procedure was performed after two years. The wrist functioned well at follow-up.
Patient 7 (second edition). The reaming nearly perforated the dorsal radius, but the bone defect healed well. Both components exhibited intimate bone-implant contact from 3 months on and the wrist function was excellent ().
Figure 36. Patient 7 (second edition). A) postoperative image showing near perforation of the dorsal cortex of the radius, B) 1 year postoperatively demonstrating intimate bone-implant contact along both components, traces of bone radially and ulnarly C) At 6 years, ectopic bone has rebuilt the radial styloid after resection, spots of mature bone are seen in the soft tissue ulnarly. The DRUJ is unaffected.
Patient 8 (second edition). The reamer perforated the ulnar diaphysis of the metacarpal. A radiolucent line surrounded the metacarpal part of the implant after 3 months, but no line was found in the capitate or radius. The wrist functioned well. After 5 years the distal component loosened, and a fistula developed at the level of the radial styloid while the patient was waiting for revision. At revision a focal osteolysis surrounded the distal 1/5 of the proximal implant adjacent to the joint. CRP and ESR were 76 and 25 respectively. Abiotrophia defective (a nutritional variant of streptococcus normally occurring as mouth flora) grew in all cultures. The infection was considered to be blood borne. The prosthesis was removed, gentamycin (Septopal®, Biomet Merck Norge AS, Oslo, Norway) was administered locally and clindamycin (Dalacin, Orifarm, Oslo, Norway) 300 mg × 4 orally for 3 months. A wrist arthrodesis was subsequently performed with an iliac bone graft and a plate, healing uneventfully.
Radiographic evaluation: CMC 3 fusion was not seen in any patient. All radius screws were integrated to bone without any radiolucent line. The two loosenings of the metacarpal screws started with a radiolucent line at 3-6 months, gradually surrounding the implant. With the exception of patient 6 in which the cup may have interfered with the DRUJ, the joint was unaffected by the prosthesis. A summary of the results is given in .
Table 10. Radiological results
Functional evaluation: The prosthesis provided excellent pain relief and patient satisfaction (). No patient required analgesics due to wrist pain. One patient retired during the follow-up period, the remaining 5 continued to work. All patients would have chosen the arthroplasty again if they had known the outcome. The median total active range of motion was 117 and 192°, and the grip strength 34 and 43 kg in the operated and non-operated wrist, respectively. Pronation and supination were unaffected ().
Table 11. Patient satisfaction at follow-up
Table 12. Range of motion (°) and grip strength (kg)
Study 4
In the fourth study, the mean operation time in the 30 patients was 103 (SD = 20, range 74–170) minutes. A concomitant Darrach’s procedure was performed in one patient with symptomatic distal radioulnar osteoarthritis. There were no peroperative complications. One postoperative superficial wound infection recovered after a week of oral antibiotics, and there have been no signs of deep infection after 3 years of follow-up. The mean cast period was 44 days (SD = 8, range 17–62). One patient declined follow-up after cast removal, however four years later the prosthesis has not been revised. All the remaining patients were followed-up according to the schedule for a mean of 3.2 years (SD = 1.5, range 1.1–6.1).
Radiographic analysis: All prostheses integrated well radiologically without subsidence, osteolysis or lines ().
Figure 37. A) Preoperative SLAC wrist, B) Frontal 3 years follow-up C) Lateral projection at 3 years follow-up. Intimate bone-implant contact without lines or osteolysis.
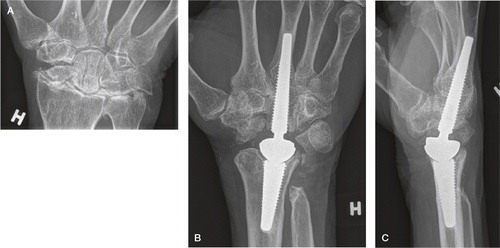
Two painful but well-fixed arthroplasties with stable articulations and no soft tissue metallosis were revised and converted to arthrodesis without affecting the pain: One initially satisfied patient experienced increasing tenderness after 6 months and the wrist was fused at 16 months. The fusion procedure using an iliac bone crest and an arthrodesis plate was uncomplicated. The other patient developed increasing pain after 5 months. Exploration at 7 months elicited inflammation with greenish pus. Multiple bacteria cultures of pus, bone, synovia, as well as scrapings and swabbings from the articulation and screws were all negative. The components were replaced with a gentamicin-impregnated cement spacer. The patient insisted on preserving motion, and, after 4 months of with antibiotic treatment, another arthroplasty was inserted. Similar pain recurred without signs of loosening and total fusion was performed 8 months later. Both fusions healed and the latter patient is working at follow-up. One prosthesis loosened before the 5-year follow-up (). The patient suffered from an anal fissure the preceding year and, therefore, raised suspicion of septic loosening. Although surgical intervention has been advised, minor complaints have made the patient reluctant up till now.
Figure 38. A) At 4 years, well integrated arthroplasty and well-functioning wrist. B) At 5.5 years, loosening of both components with enveloping double lines, subsidence and compensatory metacarpal diaphyseal widening.
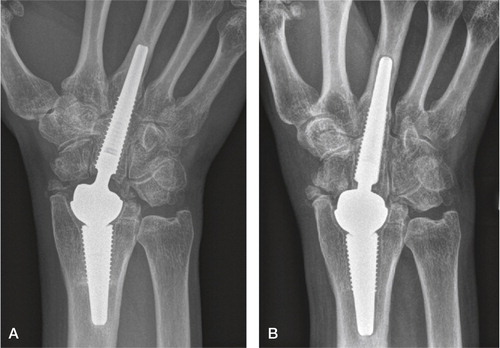
Five other patients underwent additional wrist surgery during the follow-up period due to DRUJ pain or pain with radiocarpal motion: one Darrach’s procedure was performed after 0.8 years, and four bone resections were undertaken due to impingement. The surgery gave better functional outcome in these patients, and these five, as well as the remaining 23 patients, all have their original arthroplasty in situ.
Three patients developed focal periarticular osteolysis in the radius without affecting the clinical outcome, in the most advanced case the majority of the radial styloid () was affected. They all stabilised after 1 year.
Figure 39. Focal periarticular radial osteolysis filling out the radial styloid at 3 years. Sclerotic line limiting the non-progressing lesion. Excellent clinical outcome.
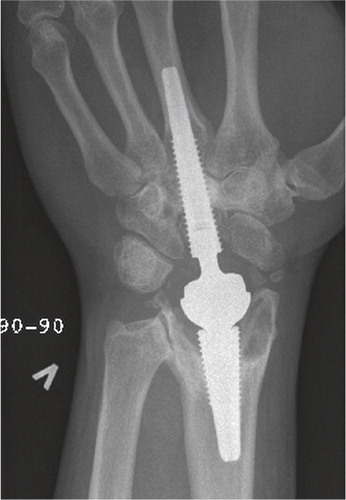
One arthroplasty demonstrated early periprosthetic lines in the capitate simultaneously with intimate third metacarpal bone-implant contact (). Another case showed intimate capitate bone-implant contact with metacarpal lines (). Both wrists were stable without signs of loosening and the wrist function was excellent. In these two patients the CMC 3 fusions had failed, (which also was the case in the revised wrist with inflammation); all other CMC 3 fused.
Figure 40. At 5 years, periprosthetic capitate lines without progression. Still intimate bone-implant contact in the metacarpal and radius.
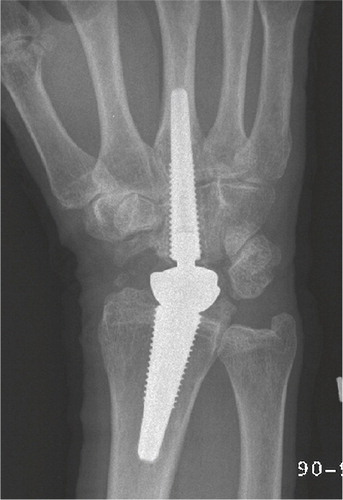
Figure 41. At 3 years, periprosthetic metacarpal lines without progression. Intimate bone-implant contact in the capitate and radius.
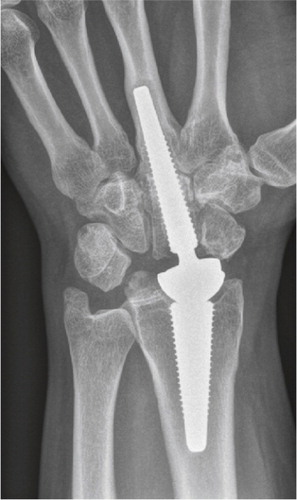
All the remaining prostheses demonstrated persistent bone integration without migration, osteolysis or lines. No dislocation or implant breakage occurred. With two revised arthroplasties, the six-year estimated survival was 93% (CI = 5.1–6.1) ().
The clinical performance increased rapidly during the first year, slowly up to 3 years and stabilizing ().
Table 13. The clinical result. Mean (SD)
Satisfactory pain relief, increased range of motion and grip strength was achieved. The mean key pinch at the most recent follow-up was 8.9 kg (SD = 2.7, range 5–14) compared with 9.5 kg (SD = 3.3, range 2–14) contralaterally (p = 0.44). No patient used analgesics for wrist pain. The majority of the employed patients had returned to work (). Three of five patients with long-term sick leave preoperatively where disabled at follow-up due to non-wrist related issues. With the exception of the two patients with revised prostheses, all patients would have chosen arthroplasty again knowing the outcome.
Table 14. Working status
Discussion
Study 1 and 2
There are some weaknesses of the two animal studies. The first study had a low number of animals, necessitating the use of large difference in measured values (rtq/histomorphometri) between the groups of implants in order to demonstrate statistical significance. A higher number of animals could have demonstrated significant differences between the implants where none was found in the study. Unfortunately there is no standardised available data for bone implant/shear strength in these animal experiments, making a power analysis difficult. A type II statistical error (not finding a significant difference where one exists due to a low number of n) could be the case for some of the non-significant findings. Another weakness in this animal setup is the non-weight bearing situation the screws were subjected to. The bone continuity will only expose the implants to shear forces, while tension and compression forces for the most part would surpass the implant. The ideal situation in our case would be a type of arthroplasty for the animals, giving information on a range of issues including wear and fixation. The drawback of a more complex experimental setup includes bias and confounders; problems or interesting findings may be camouflaged and therefore not reported. The relatively simple, well established and reliable model used in our studies has the advantage of reducing the potential technical, systematic and individual shortcomings and should therefore not be underestimated. Using skilled, trained personnel from the technician to the scientist is a major strength in this situation. Another weakness of the second study was the irregular HA coating. We ordered a complete, thin plasmasprayed coating on the implants which proved difficult to obtain on small, screwshaped implants.
Much attention has been given to the characterisation of implant surfaces. Albrektsson considered the optimal surface roughness to be Sa ≈ 1.5 μm in an oral implants review (Albrektsson and Wennerberg Citation2004). A rougher or a smoother surface than Sa ≈ 1.5 μm on titanium implants exhibited less bone ingrowth in one short and one long term study by Wennerberg et al. (Wennerberg et al. Citation1997, Citation1998). The optimal Sds and Sdr are presently not known. Both our studies demonstrated that the surface roughness (Sa) of Bonit® was favourable (≈ 1.5 μm) compared to both the uncoated gritblasted implants (≈ 0.7 μm) and to HA-coated implants (≈ 3.0 μm). After 3–6 weeks all Bonit® is resorbed (both theoretically and evaluated microscopically), exposing the alloy surface, which is similar to the original gritblasted roughness. Thus from 6 weeks on, none of the surfaces have an optimal roughness according to Albrektsson. The difference in surface roughness alone cannot explain the different fixation pattern.
Research on thin resorbable calcium phosphate coatings has so far been mainly experimental (Schmidmaier et al. Citation2002, Rossler et al. Citation2003). In vitro studies with Bonit® have showed osteoblast activation and coating resorption in an MG-63 osteoblast-cell culture (Becker Citation2004). Preliminary reports of in vivo animal and human dental investigations have suggested faster bone in-growth with Bonit® coated implants as well as resorption of the coating without inflammatory reaction (Szmukler-Moncler et al. Citation2000, Massei et al. Citation2001). A study in goats compared titanium-alloy implants with two different calcium-phosphate coatings (electrochemical HA and brushite), Ti-plasmaspray and uncoated implants. At 3 and 15 weeks the mechanical strength was tested and histomorphometric measurements performed (Biemond et al. Citation2011). They confirmed the brushite to be resorbable at 3 weeks, while the thin HA layer was not completely resolved. The mechanical push out test was in favour of brushite (but not significant), HA (significant) and uncoated (significant) at 15 weeks, but not at 3 weeks. The bone implant contact was similar between the four different implants, while the bone-ingrowth depth was in favour of brushite and HA at both 3 and 15 weeks. The authors concluded that the “biomimetic calcium phosphate coating appeared to work as an accelerator of bone ingrowth”. Recently a larger human dental study with the Bonit® coating was published (Palarie et al. Citation2012). 311 implants (all implants similar) in 124 patients were evaluated, and after one year the survival of the implants were comparable to the literature on other types of well-functioning dental implants without coating. Unfortunately, this prospective series using the same type of implant in a large, but inhomogeneous group of patients makes any conclusion on the coating difficult. Their conclusion was “neither special disadvantages nor benefits were found, and long-term results are further needed”. The positive effect of thin calcium phosphate coatings might be relatively limited.
The first study demonstrated that the Bonit®-coated implants resulted in a stronger biomechanical implant fixation compared to the un-coated Ti6Al4V gritblasted controls. This observation may be related to the rougher surfaces of the Bonit®-coated screws or a stimulating effect of the coating itself. Some of the effect is related to the surface roughness, and this is in agreement with other studies performed in similar animal models (Wennerberg et al. Citation1995, Wennerberg Citation1996, Wennerberg and Albrektsson Citation2009). Initial osteoblast activation followed by coating degradation and release of calcium and phosphorous ions into the interface as a reservoir for new synthesis of hydroxyapatite has been proposed as a mechanism for the in vitro osteoblast experiments performed by Becker et al. (Citation2004). The primary surface roughness-effect of the coating may have diminished quickly. Our experiments demonstrated a continuing activity in the interface between implant and bone resulting in increased bone-implant contact, bone length and shear strength on the Bonit®-coated screws as compared to uncoated and with longer in- situ time. The Bonit® coating effect is limited to the immediate vicinity of the implants, demonstrated by the similarity in bone response further away from the interface. In the first study the bone area measurements together with the mirror images (both of which measure the secondary changes in bone stock and quality) did not show any differences between the Bonit® and uncoated implants. The histological picture was also similar between the two groups and no cytotoxic effect orinflammatory response was seen. The long term follow-up in our second study revealed the same “calm” histological picture for the Bonit® screws confirming the biofriendly effect. In the first study the Bonit® covered implants showed a better bone to implant fixation both biomechanically and histomorphometrically. The findings in the condylar spongious region of the rabbit femur confirms earlier findings in a pig model and in human biopsies (Szmukler-Moncler et al. Citation2000, Massei et al. Citation2001). The findings in the cortical region of the tibia are however new observations and interesting with regard to orthopaedic implants. The cortical region is the anchorage region for many orthopaedic prostheses and implants, and increased primary fixation here may prolong the implant survival and prevent aseptic loosening (Willert and Buchhorn Citation1999).
Experimental and clinical non-resorbable plasmasprayed HA coatings have demonstrated bone-HA contact (Gottlander and Albrektsson Citation1992, Hayashi et al. Citation1999). Plasmasprayed HA coatings have a strong and unquestionable osteoconductive effect, and have the ability of gap filling (Soballe and Overgaard Citation1996), but the coatings are also prone to delamination and degradation with subsequent increase in low grade inflammation by multinucleated giant cells and macrophages (Gottlander et al. Citation1997, Rokkum et al. Citation2002). Although bone implant contact remained strong, the bone amount in the area and mirror area diminished with time in the experimental study by Gottlander et al. (Citation1997). The same picture including coating degradation or delamination has been observed with thicker plasmasprayed HA coated implants (Morscher et al. Citation1998, Rokkum et al. Citation1999, Citation2002).
The coating covered impurities from the production and sandblasting, as demonstrated by the surface chemical investigation on both the Bonit® and HA samples giving them a more homogenous surface as compared to the uncoated screws. In the HA case, the covering was only partial when viewed by the light microscopy, and for the Bonit® coating it is almost totally resorbed after three weeks. Although an initial bone response could be impeded by differences in surface heavy metals such as alumina, lead or copper (Hallab et al. Citation2002), but this effect is difficult to measure in vivo and would be similar. Ion release from different surface roughnesses was equal in a similar animal model, and the effect is probably neglectable (Wennerberg et al. Citation2004).
A steady increase in the bone length and the removal torque of the Bonit®-coated screws was seen during the observation period. The resorption of Bonit® after 3–6 weeks resulted in direct bone-metal contact and probably constituted a stable situation during the degradation process as seen in our first study. Our findings are confirmed by others (Biemond et al. Citation2011). The histological picture with bone remodelling into a lamellar structure and minimal inflammatory response probably increased implant stability. We did not experience any negative effect of the Bonit® at short, medium or longer term follow-up, and fear of side effects can be cancelled. The bone remodelling matched the resorption phase to the repair rate. The ability of blasted titanium and titanium-alloy implants to form strong bone-implant fixation and the biocompatibility of the metals have been demonstrated in the same animal model as well as in clinical and retrieval studies of humans (Lintner et al. Citation1988, Johansson et al. Citation1998, Reigstad et al. Citation2008).
Generally, the removal torque, bone-implant length and shear strength are expected to increase with time and loading (Johansson et al. Citation1998, Cui et al. Citation2000). Also an increase in shear strength in the bone implant interface with time is expected due to maturation of the bone with resorption of callus and woven bone and conversion to a stronger lamellar bone structure (Cui et al. Citation2000).The rapid and strong bone response to HA is important as to achieve direct bone-implant contact, and is especially desired in the clinical situation where joint replacements requires early loading. In the second study the bone-implant length was in favour of Bonit® at 6 weeks compared to HA, but the interface bonding was weaker (lower removal torque and shear strength).
For the HA-coated screws the process regarding removal torque and bone length subsided after 12 weeks although the interfacial fixation (shear strength) of the intact bone-implant areas still remained strong (demonstrated by the high removal torque but short bone-length). This is consistent with the histological picture we found for the HA screws with flaking of the coating, delamination and multinucleated giant cell activation. At 52 weeks the HA bone length decreased, but more mature lamellar bone structure gave a stronger point fixation preventing a reduced removal torque. The new thinner plasma sprayed HA coating used in our study exhibited a bone reaction similar to the more widely used thicker coatings, but less volume of HA on our implants may have limited the inflammatory soft tissue response. The resorption of much HA at 52 weeks can explain the less active histological picture as compared to the 12 weeks samples. These mechanisms explain the undulating bone length and shear strength as well as the disordered histological picture adjacent to the HA-coated screws. At the intact interface between bone and HA the point fixation was stronger for the HA at all observation periods but statistically significant only at 6 weeks. We attribute this difference to the stronger bioactivity and bone stimulating effect of plasmasprayed HA. Potential side effects of HA coatings should be considered when choosing surface modifications on clinical orthopaedic and dental implants. In the second study the higher removal torque values (almost the twice of Bonit® after 6 weeks) at 6 and 12 weeks in favour of HA may be attributed to stronger bioactive properties. The ability to induce early intimate contact between bone and HA coated compared to Ti-alloy implants has been demonstrated by Soballe (Citation1993), observing higher push-out values, more bone ingrowth and improved gap healing up to 16 weeks in a dog model. Chang et al. found superior attachment and bone-implant contact with different plasma sprayed HA coating compared with sandblasted commercially pure (c.p.) titanium up to 26 weeks (Chang et al. Citation1999). These two studies did not however perform any qualitative histological evaluation of the tissue response in the vicinity of the implants.
The significance of the lack of HA on one side of the thread is difficult to estimate. The discontinuity of the HA might weaken the bond between the implant and coating and increase the deflaking tendency. We found persistent coating on the baseline test implants after screwing in and removal torque testing, at least exhibiting shear resistance of the coating. Whether a continuous coating would have increased the fixation or increased the side effects remains unanswered, but it demonstrates the difficulties in the application of thin plasmasprayed coatings. Long term negative effects of HA have been reported in experimental and clinical studies (Gottlander and Albrektsson Citation1991, Rokkum et al. Citation1999) with delamination and flaking of the coating, foreign body inflammation and implant loosening. Morscher et al. (Citation1998) experienced excellent short term results with a thick HA coated hip arthroplasty, but these results deteriorated after longer observation. Ten out of 25 revisions were due to severe osteolysis in the acetabulum and the proximal femur. HA was found in the articulating surface (embedded in the polyethylene and scratching the chrome-cobalt head) as well as in the granulomatous tissue in the osteolytic areas, demonstrated with SEM and energy-dispersive X-ray microanalysis. Histological examinations were not performed. An in vitro study by Velar et al. (Citation2009) found polymorph nuclear response as well as pro-inflammatory mediators to HA-particles, postulated to contribute to implant associated inflammation which we also found.
Study 3 and 4
A weakness of the third study includes a lack of a patient rated subjective scoring like the DASH score prior to surgery. The grip strength (in the third study) and key pinch (in the fourth study) not being recorded preoperatively is also a weakness.
Arthrodesis has been considered a permanent painless solution for destroyed wrists and is less disabling than in other joints due to the compensatory motion of the forearm, elbow and shoulder. Long-term follow-up studies have demonstrated that some fused wrists are tender, the functional disability is troublesome, and the majority of patients miss a minimum of movement (Adey et al. Citation2005). With a stiff wrist, any decreased range of motion in more proximal joints will lead to a relatively greater reduction of hand function than a stiff wrist alone. A painless wrist with a range of motion exceeding 110° provides almost normal wrist function (Ryu et al. Citation1991), and as little as 25° of motion permits significantly more activities of daily living than arthrodesis (Nelson Citation1997). Wrist arthrodesis is currently the gold standard for treatment of a destroyed wrist; a randomised prospective comparison of arthrodesis to wrist arthroplasty would be interesting. A low-quality retrospective comparison of wrist arthrodesis and the Universal wrist arthroplasty in rheumatoid patients was performed by Murphy et al. (Citation2003). A chart review on complications was done and questionnaires sent to the patients. There was a trend toward a better functional outcome in the arthroplasty patients, the remaining outcomes were similar. No conclusion was attempted. A systematic review on wrist arthrodesis vs. wrist arthroplasty in rheumatoid patients found comparable or better results for wrist arthrodesis (Cavaliere and Chung Citation2008). The majority of studies included old and abandoned arthroplasties, and no non-English papers were included. Performing a prospective, randomised study between arthrodesis and arthroplasty renders some inherent problems. It could be criticised of being a comparison of apples and oranges, the definition of primary end points would be debatable (motion vs. non-motion, hardware failure vs. revision, wrist scoring schemes etc.). Furthermore, the recruitment of patients could be difficult when informing about the treatment options (motion vs. non-motion). In our department, many of the patients were referred to us for wrist arthroplasty surgery after having been offered and declined an arthrodesis at the local hospital. Persuading them to be included in a study where arthrodesis was an option would be difficult.
Wrist arthroplasty has traditionally been limited to elderly patients and patients with general disabilities such as rheumatoid arthritis with bilaterally destroyed wrists and with a low demand lifestyle (Figgie Citation1990, Adams Citation2004). Well-functioning and long lasting wrist prostheses for non-inflammatory active patients have not yet been documented.
In study 3 and 4, we were able to, for non-rheumatoid patients, establish stability of the wrist, reduce the amount of bone removed and spare the distal radioulnar joint. Unlike other reports on wrist prostheses (Bosco et al. Citation1994, Menon Citation1998, Kretschmer and Fansa Citation2007) we saw no instability or dislocations, and there were no problems with preoperatively normal distal radioulnar joint. The implant developed differs from most of the reported total wrist prostheses by transforming the wrist to a ball and socket joint with a metal-on-metal bearing and a fixed centre of articulation. Conversion of the complex wrist joint into a single ball-and-socket articulation proved to be stable and mobile. Instability, dislocation and wear have been seen in egg-shaped arthroplasties like the Biax and the Universal Total Wrist (Divelbiss et al. Citation2002, Stegeman et al. Citation2005, Kretschmer and Fansa Citation2007). The Meuli III arthroplasty with reverse ball and socket articulation demonstrated distal implant perforation and loosening. Despite this, good stability was reported by Meuli and Fernandez on mixed rheumatoid and non-rheumatoid patients (Meuli and Fernandez Citation1995, Meuli Citation1997), although dislocations have been reported in rheumatoid patients by others (Strunk and Bracker Citation2009).
In the normal wrist the centre of rotation for flexion and extension and radial/ulnar deviation is assumed to be located in the head of the capitate (Youm et al. Citation1978), which is slightly distal to the centre of our prosthesis. We have not found this transfer of the centre to be of any disadvantage for articulation. The ball-and-socket concept has been stable and resulted in a good range of motion.
According to Youm et al. (Citation1978) some rotation of the wrist occurs in the radiocarpal joint. In our opinion an ellipsoidal articulation gives stress risers in rotation as well as in radial and ulnar deviation. A distal convex polyethylene component may increase this problem. A ball and socket articulation eliminates the difficulty, transforming the shear forces to compression, which may reduce wear.
Periprosthetic reactions to MOM articulations are likely rare in non-weight bearing joints. In patients with large resurfacing arthroplasties of weight bearing joints, pseudotumour formation and elevated blood ion levels have been reported (Mahendra et al. Citation2009). The problems have been attributed to mechanical factors of specific designs (Delaunay et al. Citation2010, Underwood et al. Citation2011, Seppanen et al. Citation2012), where impingement has given high wear. So far no conclusions have been drawn concerning the MOM joint replacements (Haddad et al. Citation2011) but caution is encouraged for large diameter MOM in weight bearing joints. The diametral clearance in MOM articulations is an important factor to diminish wear. A clearance that is too tight or a too wide may cause wear problems. The optimal difference has been estimated to be around 100 μm (Dowson et al. Citation2004) in a hip simulator study. The diametral clearance of Motec wrist is 0.09–0.17 mm according to the manufacturer, which is within the optimal range. A small diameter, non-weight bearing articulation like the wrist has a much lower wear than that of the hips and knees. So far we have not had indications of wear or particle related problems with the developmental arthroplasty or the Motec wrist. The new chromium-nitride surface coating used in approximately half the patients in the latter study has demonstrated a low wear rate experimentally (Williams et al. Citation2003). This may improve the wear properties and longevity of the implant.
In study 3 and 4 in non-rheumatoid patients we were able to establish stability of the wrist, reduce the amount of bone removed and spare the distal radioulnar joint. Unlike other reports on wrist prostheses (Bosco et al. Citation1994, Menon Citation1998, Kretschmer and Fansa Citation2007) we saw no instability or dislocations, and there were no problems with preoperatively normal distal radioulnar joint. The cementless fixation of the radial component proved effective especially on the radial side, but as with other wrist prostheses (Bosco Citation1994, Cobb and Beckenbaugh Citation1996, Strunk and Bracker Citation2009) we encountered problems with the fixation of the distal component. The critical step of the procedure was the reaming and the insertion of the distal screw. We encountered difficulties with the first edition of the distal component which had a very short engagement in the metacarpal. The stability was solely dependent on the fixation to the capitate, and the motion of the CMC 3 joint seemed to prevent bone-implant integration in the metacarpal. With the longer distal screw of the second edition we experienced two other main problems. The angulation of the CMC 3 joint in the frontal and lateral planes (flexion and ulnar deviation) caused a risk of the reamer perforating the metacarpal in the proximal cortical region (complete in two cases, incomplete in two others). The cutting threads of the reamers were too sharp, especially at the tip, making it easy to engage and ream the cortex on one side instead of being forced distally along the medullary canal. The squared distal end of the screw engaged the bone before the end of the reamed cut-out, preventing the implant from being “screwed home” or necessitating overreaming precluding exact maximal primary bone-implant contact and support. During reaming for the proximal screw we experienced the same difficulties and near-perforation of the cortex. The problems with reaming and perforation of the cortex decreased integration of the distal components, whereas all radius screws integrated well. In the third study no effort was done to fuse the third carpometacarpal joint. In the first two patients we predicted erroneously that a relatively thick screw secured both in the capitate and the third metacarpal would fuse the third carpometacarpal joint, improving the fixation of the component. In our two cases of loosening of the distal screw, the process started around the tip and progressed proximally. Movement between the metacarpal and the screw may eventually also loosen the screw from the capitate, although this did not happen in all patients with open carpometacarpal joints. The extensive reaming needed for the relatively large threads of the distal screws created the problem of little primary contact between the implant and the bone. To minimize these issues, particularly with fixation of the distal screw, the prosthesis, instruments, and surgical technique needed to be addressed before the final concept was introduced. A reduction in the diameter of the screws along with a reduction in the height of the threads aimed to prevent overreaming in the small and/or thin metacarpal bones. The rounded tip from the edition used in the first two patients was reintroduced to minimise risk of perforation of the sides and promoted a central positioning of the screws. The instruments and the technique were also changed; the angle of the cutting threads on the reamer was reduced to force the reamer centrally in the medullary canal, avoiding engagement of the cortex on either side. Distally the wedgeshaped opening and arthrodesis of the CMC 3 joint created a “one bone” for distal fixation. In the radius a blunt guidewire was introduced up to the proximal one third of the radius to secure the centralization of the screw in the distal part of the radius.
The results from the final, fully developed version used in study 4 suggest that these difficulties have been solved in the short term. The rounded tips on the reamers and screws contributed to keeping the implants within the diaphysis. To be capable of withstanding the physical demands of a normal lifestyle, osseointegration is a prerequisite for lasting cementless fixation of wrist prostheses. Fixation of the distal component has been problematic with previous wrist arthroplasties. Most designs have limited the attachment to the carpal bones and the most proximal part of the metacarpals 2-4, and report osteolytic lines and loosening despite fixation with two (Meuli) (Cooney et al. Citation1984) or three (Biax, Universal and Universal 1, Guepar) prongs (Kretschmer and Fansa Citation2007, Harlingen et al. Citation2011, Ward et al. Citation2011). Primary screw fixation proved sufficiently stable for achieving secondary fixation by bone ingrowth. The osteoconductive coating has probably promoted bone apposition and osseointegration consistent with the development of radiologically intimate bone-implant contact in all patients. Achieving “one bone” of the capitate and the metacarpal 3, provided a long and solid bone block with distal cortical attachment similar to the fixation of cementless femoral stems (Reigstad et al. Citation2008).
Infected wrist arthroplasties appear to be more frequent than for hips and knees (Levadoux and Legre Citation2003, Strunk and Bracker Citation2009, Rizzo et al. Citation2011). We suspected one infection without loosening and one late septic loosening in the last study. In the first arthroplasty study one of the patients experienced implant loosening after a haematogenous infection. Thin soft tissue covering and multiple prior operations may contribute to an increased infection risk. Broad-spectrum antibiotic prophylaxis and meticulous aseptic and atraumatic technique is mandatory during arthroplasty as well as in subsequent surgery. The liberal use of antibiotics is also advised should infections arise in other parts of the body and for surgical procedures known to have a high risk of infection such as extensive dental work.
The scalloped juxta-articular radius osteolysis appears to be a parallel to periarticular femoral osteolysis of hip arthroplasties and is not associated with loosening (Zicat et al. Citation1995). The two patients with either periprosthetic lines in the capitate and intimate bone contact in the metacarpal or the lines in the metacarpal with bone contact in the capitate, were probably caused by motion due to unsuccessful CMC 3 fusion. So far, screw fixation in the metacarpal or capitate only, has secured a painless function of the wrist, but these two patients have less distal fixation than the other patients, and we monitor them carefully. In the patients were minor additional surgery was performed in the follow-up period, the functional result increased and the surgery was worthwhile.
All non-revised patients achieved satisfactory mobility of the wrist, grip strength, and pain relief. The majority of patients gained wrist motion, and all have a functional ROM (that is, motion around the neutral position). Overall the decrease in DASH in the fourth study was substantial. A change in DASH of less than 10 points is considered a minor change, and a change of over 19 is considered a major disability alteration. It is important to separate group and individual analysis of DASH scores. The internal consistency is strong for group as well as for individual comparisons, although for individual patient assessment with DASH the magnitude of the score change has to be studied on an individual level (Gummesson et al. Citation2003). Also, a higher DASH score is expected with increasing age, and with a longer observation period for our patients, this general increase has to be taken into consideration when comparing with preoperative measurements. In a study by Jester et al. (Citation2005) a DASH of 14 was found in a healthy working population between 30 and 49 years, and 19 in the population between 50 and 69 years. A Norwegian translation was first introduced in Norway late in 2001 (CitationFinsen 2001). The DASH scoring has a special strength when it is done both before and after intervention due to the belief that concomitant problems on the same side will have a similar influence. The DASH results in the third study should be interpreted with caution due to a low number of patients and a lack of a preoperative score (Gummesson et al. Citation2003).
The increase in grip strength in our patients in the fourth study, from 63% of the contralateral side preoperatively to almost 80% at 2 to 3 years is encouraging. Although preoperative grip strength was not performed in the third study, the follow-up reached 72%, mainly due to one patient (nr 4) giving a very large contribution to the inferior result. Grip strength has been considered an objective tool for measurement not only of strength but also as a global evaluation of the hand function, because it eliminates the “eager to please” effect of subjective scoring schemes (Goodson et al. Citation2007). Reports indicating high satisfaction with subjective increase in the hand function without any increase in grip strength should be interpreted with caution.
It seems that arthrodesis of the wrist with a graft from the iliac crest can be performed as a salvage procedure without specific complications, and with a healing time similar to that of a primary arthrodesis. We have performed 4 arthrodesis in the last two studies, and although 3 were complicated procedures due to infection or inflammation, only one needed an extra distal regrafting and fixation. It could therefore be advised to employ the prosthetic replacement as the first option of treatment for a ruined wrist. The third study has however indicated that a fused wrist can be rearticulated and a good functional result obtained. The arthroplasty used in the rearticulating procedure was the Motec wrist implant. Only primary procedures were included in the fourth arthroplasty paper, and this patient was not included the final paper.
Total wrist arthroplasty has mostly been limited to low demand patients due to problems with loosening, instability and implant breakage (Jolly et al. Citation1992, Gellman et al. Citation1997, Schill et al. Citation2001). Few publications report wrist replacement in non-rheumatoid patients with a normal life and workload. The Destot prosthesis was used in 25 posttraumatic arthritis (SNAC and SLAC) patients. The clinical results were good and similar to ours regarding motion and strength although six carpal components migrated, three metacarpal stems loosened and two fractured (Levadoux and Legre Citation2003). The arthroplasty was made of rough blasted 316-L steel and poor bone conductive properties may explain the distal fixation issues. A long-term follow-up of the initially promising results will not be performed because the patients are spread throughout Europe (M Levadoux, personal communication). Furthermore, the arthroplasty has been withdrawn from the market. Kretschmer et al. implanted the Biax arthroplasty predominantly in posttraumatic wrists (Kretschmer and Fansa Citation2007). One-year results were promising, but within 2.6 years dorsal polyethylene wear, foreign body reaction, synovitis, loosening and permanent luxation necessitated revision in 11 patients. Four postoperative dislocations stabilized after reposition, and nine wrists demonstrated osteolysis. The authors recommended abandoning the prosthesis (Kretschmer and Fansa Citation2007). Radmer reported encouraging 1.5 years results in 30 rheumatoid patients with a newly developed cobalt-chrome (APH) wrist arthroplasty with a titanium coated articulation (Radmer et al. Citation1999). After 2–6 years all wrists where revised or scheduled for revision (Radmer et al. Citation2003). The metal articulation was too soft and could not bear the wrist strain and stress.
Few reports exist on contemporary arthroplasties. After initial promising results with the Universal wrist, a recent 5–10 year follow-up of 20 rheumatoid patients demonstrated 10 revisions due to polyethylene wear, metallosis, carpal component loosening or persistent dislocation. Two radiologically loose carpal components were not revised (Ward et al. Citation2011). Two 3.8 and 5.5 year reports on a total of 38 modified Universal 2 prostheses, mainly in rheumatoids, demonstrated increased motion and patient satisfaction (strength not reported), although there were two distal loosenings and one dislocation (van Winterswijk and Bakx Citation2010, Ferreres et al. Citation2011). The 2.7 year results with the Remotion wrist were good in 13 rheumatoids (ROM = 70°, grip strength increased from 7 to 11 kg), and acceptable in 7 non-rheumatoids (ROM = 50°, grip strength increased from 13 to 14 kg). One distal and one proximal component (in rheumatoids) were loose (Herzberg Citation2011). These patients were also part of a newly published prospective multicentre study including 215 cases, 112 had more than 2 years follow up (average 4 years) and approximately 30% were non-rheumatoid (Herzberg et al. Citation2012). The clinical result was good, with pain and QDASH reduction and gained grip strength. The patients experienced a minor decrease in motion. 4% of the non-rheumatoid patients were revised due to loosening, another 18% were radiologically loose. The Maestro wrist arthroplasty system (Biomet, Warsaw, IN, USA) was reported upon by Nydick et al. (Citation2012) in a retrospective short term (4–55 months) follow-up study of 22 wrists in 21 patients with a mixed aetiology. The clinical results were satisfactory with a slight increase in motion when compared with preoperative measurements. At follow-up the grip strength averaged 60% of the contralateral side, and the DASH score was 31. They experienced one deep infection (converted to arthrodesis) and one dislocation (stable after closed reduction). They recommended wrist arthroplasty as an alternative to arthrodesis. The Norwegian Arthroplasty Register (NAR) published the results after wrist arthroplasty including the Biax and Motec wrist arthroplasties( Krukhaug et al. Citation2011). They found a survival rate of approximately 80% at 5 and 4 years respectively, and a higher revision rate in females as compared to males. The Biax patients had a longer follow-up but consisted almost exclusively of inflammatory wrists. The few non-inflammatory wrists where excluded from the comparison. The Motec arthroplasty demonstrated a similar revision rate in the non-inflammatory and inflammatory patients, but the findings must be interpreted with caution due to the incompleteness of registration of wrist arthroplasties (52%) in the NAR.
Conclusion
HA- and Bonit®-coated screws gave a stronger bone-implant fixation than gritblasted titanium-alloy.
Bonit® represents a true resorbable calcium phosphate coating, transforming the interface into direct bone-implant contact.
Bonit®-coated screws exhibit a predictable, but slower bone ingrowth pattern than HA.
The Bonit®-coated screws demonstrate increased fixation from 6 to 52 weeks.
No increase in HA fixation is seen between 6 and 52 weeks.
HA coating yields a quicker and more powerful fixation than Bonit® after 6 and 12 weeks, at 52 weeks Bonit® was non-significantly stronger than HA.
The screw fixed prototype arthroplasty is stable and provide bone implant fixation and good clinical performance in 4 out of 8 non-rheumatoid patients. However, the prototypes of the wrist prosthesis and instrumentation had inherent design features that jeopardized the fixation of the distal screw and increased the risk of cortical perforation.
The fully developed wrist arthroplasty demonstrates good clinical results in high demand post-traumatic patients after 3.2 (1.1–6.1) years.
The spherical MOM articulation is stable and provides good functional range of motion.
The threaded uncemented conical implants demonstrate stable bone fixation and radiologically intimate bone-implant contact.
Conversion from failed arthroplasty to arthrodesis was uncomplicated.
The fully developed wrist arthroplasty is an alternative to wrist arthrodesis, and can be recommended to post-traumatic wrist osteoarthritis.
References
- Adams BD. Total wrist arthroplasty. Orthopedics 2004;27:3): 278-84.
- Adams BD. Total wrist arthroplasty. Tech Hand Up Extrem Surg 2004;8:3): 130-7.
- Adey L, Ring D, Jupiter JB. Health status after total wrist arthrodesis for posttraumatic arthritis. J Hand Surg Am 2005;30:5): 932-6.
- Albrektsson T, Wennerberg A. Oral implant surfaces: Part 1--review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int J Prosthodont 2004;17:5): 536-43.
- Anderson MC, Adams BD. Total wrist arthroplasty. Hand Clin 2005;21:4): 621-30.
- Beaton DE, Katz JN, Fossel AH, et al. Measuring the whole or the parts? Validity, reliability, and responsiveness of the Disabilities of the Arm, Shoulder and Hand outcome measure in different regions of the upper extremity. J Hand Ther 2001;14:2): 128-46.
- Becker W, Becker BE, Ricci A, et al. A prospective multicenter clinical trial comparing one- and two-stage titanium screw-shaped fixtures with one-stage plasma-sprayed solid-screw fixtures. Clin Implant Dent Relat Res 2000;2:3): 159-65.
- Becker P, Neumann HG, Nebe B, et al. Cellular investigations on electrochemically deposited calcium phosphate composites. J Mater Sci Mater Med 2004;15:4): 437-40.
- Biemond JE, Eufrasio TS, Hannink G, et al. Assessment of bone ingrowth potential of biomimetic hydroxyapatite and brushite coated porous E-beam structures. J Mater Sci Mater Med 2011;22:4): 917-25.
- Bloebaum RD, Bachus KN, Rubman MH, Dorr LD. Postmortem comparative analysis of titanium and hydroxyapatite porous-coated femoral implants retrieved from the same patient. A case study. J Arthroplasty 1993;8:2): 203-11.
- Boe BG, Rohrl SM, Heier T, et al. A prospective randomized study comparing electrochemically deposited hydroxyapatite and plasma-sprayed hydroxyapatite on titanium stems. Acta Orthop 2011;82:1): 13-9.
- Bornstein MM, Schmid B, Belser UC, et al. Early loading of non-submerged titanium implants with a sandblasted and acid-etched surface. 5-year results of a prospective study in partially edentulous patients. Clin Oral Implants Res 2005;16:6): 631-8.
- Bosco JA3rd, Bynum DK, Bowers WH. Long-term outcome of Volz total wrist arthroplasties. J Arthroplasty 1994;9:1): 25-31.
- Burr DB, Martin RB, Schaffler MB, Radin EL. Bone remodeling in response to in vivo fatigue microdamage. J Biomech 1985;18:3): 189-200.
- Buser D, Broggini N, Wieland M, et al. Enhanced bone apposition to a chemically modified SLA titanium surface. J Dent Res 2004;83:7): 529-33.
- Bystrom S, Espehaug B, Furnes O, Havelin LI. Femoral head size is a risk factor for total hip luxation: a study of 42,987 primary hip arthroplasties from the Norwegian Arthroplasty Register. Acta Orthop Scand 2003;74:5): 514-24.
- Carlsson L, Rostlund T, Albrektsson B, Albrektsson T. Removal torques for polished and rough titanium implants. Int J Oral Maxillofac Implants 1988;3:1): 21-4.
- Catalano LW3rd, Cole RJ, Gelberman RH, Evanoff BA, Gilula LA, Borrelli J, Jr. Displaced intra-articular fractures of the distal aspect of the radius. Long-term results in young adults after open reduction and internal fixation. J Bone Joint Surg Am 1997;79:9): 1290-302.
- Catelas I, Wimmer MA. New insights into wear and biological effects of metal-on-metal bearings. J Bone Joint Surg Am 2011;93:Suppl 2: 76-83.
- Cavaliere CM, Chung KC. A systematic review of total wrist arthroplasty compared with total wrist arthrodesis for rheumatoid arthritis. Plast Reconstr Surg 2008;122:3): 813-25.
- Chang YL, Lew D, Park JB, Keller JC. Biomechanical and morphometric analysis of hydroxyapatite-coated implants with varying crystallinity. J Oral Maxillofac Surg 1999;57:9): 1096-108; discussion 108-9.
- Chanlalit C, Fitzsimmons JS, Shukla DR, et al. Micromotion of plasma spray versus grit-blasted radial head prosthetic stem surfaces. J Shoulder Elbow Surg 2011;20:5): 717-22.
- Chung KC, Watt AJ, Kotsis SV. A prospective outcomes study of four-corner wrist arthrodesis using a circular limited wrist fusion plate for stage II scapholunate advanced collapse wrist deformity. Plast Reconstr Surg 2006;118:2): 433-42.
- Clayton ML. Surgical Treatment at the Wrist in Rheumatoid Arthritis: A Review of Thirty-Seven Patients. J Bone Joint Surg Am 1965;47:741-50
- Cobb TK, Beckenbaugh RD. Biaxial total-wrist arthroplasty. J Hand Surg Am 1996;21:6): 1011-21
- Cobb AG, Schmalzreid TP. The clinical significance of metal ion release from cobalt-chromium metal-on-metal hip joint arthroplasty. Proc Inst Mech Eng H 2006;220:2): 385-98.
- Cooney WP3rd, Beckenbaugh RD, Linscheid RL. Total wrist arthroplasty. Problems with implant failures. Clin Orthop Relat Res 1984;187:10): 121-8.
- Cooney WP, Linscheid RL, Dobyns JH. Scaphoid fractures. Problems associated with nonunion and avascular necrosis. Orthop Clin North Am 1984;15:2): 381-91.
- Courtman NH, Sochart DH, Trail IA, Stanley JK. Biaxial wrist replacement. Initial results in the rheumatoid patient. J Hand Surg Br 1999;24:1): 32-4.
- Craigen MA, Stanley JK. Wrist kinematics. Row, column or both?. J Hand Surg Br 1995;20:2): 165-70.
- Crisco JJ, Heard WM, Rich RR, et al. The mechanical axes of the wrist are oriented obliquely to the anatomical axes. J Bone Joint Surg Am 2011;93:2): 169-77.
- Cui FZ, Zhang Y, Wen HB, Zhu XD. Microstructural evolution in external callus of human long bone. Materials Science and Engineering C 2000;11:1): 27-33.
- Dagnall H. Exploring surface textures. Exploring surface textures. Rank Taylor Hobsen Limited: Leicester; 1986.
- De Smet L, Truyen J. Arthrodesis of the wrist for osteoarthritis: outcome with a minimum follow-up of 4 years. J Hand Surg Br 2003;28:6): 575-7.
- Delaunay CP, Bonnomet F, Clavert P, et al. THA using metal-on-metal articulation in active patients younger than 50 years. Clin Orthop Relat Res 2008;466:2): 340-6.
- Delaunay C, Petit I, Learmonth ID, et al. Metal-on-metal bearings total hip arthroplasty: the cobalt and chromium ions release concern. Orthop Traumatol Surg Res 2010;96:8): 894-904.
- Dellacqua D. Total Wrist Arthroplasty. Techniques in Orthopaedics 2009;24:1): 49-57.
- Derezende M, Johansson C. Quantitative bone tissue response to commercially pure titanium implants. J Mater Sci-Mater-Med 1993;4:3): 233-9.
- DiDonna ML, Kiefhaber TR, Stern PJ. Proximal row carpectomy: study with a minimum of ten years of follow-up. J Bone Joint Surg Am 2004; 86-A (11): 2359-65.
- Divelbiss BJ, Sollerman C, Adams BD. Early results of the Universal total wrist arthroplasty in rheumatoid arthritis. J Hand Surg Am 2002;27:2): 195-204.
- Donath K. Die Trenn-Dünnschliff-Technik zur Herstellung histologischer von nicht schneidbaren Gewebe und Materialen. Der Präparator 1988;34:197-206
- Dowson D, Hardaker C, Flett M, Isaac GH. A hip joint simulator study of the performance of metal-on-metal joints: Part II: design. J Arthroplasty 2004;19:8 Suppl 3): 124-30.
- Ducheyne P, Healy KE. The effect of plasma-sprayed calcium phosphate ceramic coatings on the metal ion release from porous titanium and cobalt-chromium alloys. J Biomed Mater Res 1988;22:12): 1137-63.
- Ducheyne P, Van Raemdonck W, Heughebaert JC, Heughebaert M. Structural analysis of hydroxyapatite coatings on titanium. Biomaterials 1986;7:2): 97-103.
- Dujovne AR, Bobyn JD, Krygier JJ, et al. Mechanical compatibility of noncemented hip prostheses with the human femur. J Arthroplasty 1993;8:1): 7-22.
- Dumbleton JH, D’Antonio JA, Manley MT, et al. The basis for a second-generation highly cross-linked UHMWPE. Clin Orthop Relat Res 2006;453:12): 265-71.
- Duppe H, Johnell O, Lundborg G, et al. Long-term results of fracture of the scaphoid. A follow-up study of more than thirty years. J Bone Joint Surg Am 1994;76:2): 249-52.
- Ferreres A, Lluch A, Del Valle M. Universal total wrist arthroplasty: midterm follow-up study. J Hand Surg Am 2011;36:6): 967-73.
- Figgie MP, Ranawat CS, Inglis AE 3rd, et al. Trispherical total wrist arthroplasty in rheumatoid arthritis. J Hand Surg Am 1990;15:2): 217-23.
- Filiaggi MJ, Coombs NA, Pilliar RM. Characterization of the interface in the plasma-sprayed HA coating/Ti-6Al-4V implant system. J Biomed Mater Res 1991;25:10): 1211-29.
- Finsen V. Norwegian translation of the DASH questionnaire/Norsk oversettelse av skjemaet “Dysfunksjon i arm, skulder og hånd” (DASH). In: Norwegian Surgical Society Meeting/Kirurgisk Høstmøte Norwegian translation of the DASH questionnaire/Norsk oversettelse av skjemaet “Dysfunksjon i arm, skulder og hånd” (DASH). 2001; 214.
- Finsen V. [Norwegian version of the DASH questionnaire for examination of the arm shoulders and hand]. Tidsskr Nor Laegeforen 2008;128:9): 1070.
- Fleury C, Petit A, Mwale F, et al. Effect of cobalt and chromium ions on human MG-63 osteoblasts in vitro: morphology, cytotoxicity, and oxidative stress. Biomaterials 2006;27:18): 3351-60.
- Fourastier J, Le Breton L, Alnot YPidhorz L. [Guepar’s total radio-carpal prosthesis in the surgery of the rheumatoid wrist. Apropos of 72 cases reviewed]. Rev Chir Orthop Reparatrice Appar Mot 1996;82:2): 108-15.
- Franke-Stenport V. On Growth Factors and Titanium Implant Integration in Bone. In: Dep of Biomaterials/Handicap Research, Inst for Surgical Sciences On Growth Factors and Titanium Implant Integration in Bone. University of Gothenburg: Gothenburg; 2002.
- Franko OI, Zurakowski D, Day CS. Functional disability of the wrist: direct correlation with decreased wrist motion. J Hand Surg Am 2008;33:4): 485-92.
- Gaisne E, Dap F, Bour C, Merle M. [Arthrodesis of the wrist in manual workers. Apropos of 36 cases]. Rev Chir Orthop Reparatrice Appar Mot 1991;77:8): 537-44.
- Gelberman RH, Bauman TD, Menon J, Akeson WH. The vascularity of the lunate bone and Kienbock’s disease. J Hand Surg Am 1980;5:3): 272-8.
- Gellman H, Hontas R, Brumfield RH Jr., Tozzi J, Conaty JP. Total wrist arthroplasty in rheumatoid arthritis. A long-term clinical review. Clin Orthop Relat Res 1997;342:12): 71-6.
- Germain MA, Hatton A, Williams S, et al. Comparison of the cytotoxicity of clinically relevant cobalt-chromium and alumina ceramic wear particles in vitro. Biomaterials 2003;24:3): 469-79.
- Goldberg VM, Stevenson S, Feighan J, Davy D. Biology of grit-blasted titanium alloy implants. Clin Orthop 1995;319:12): 122-9.
- Goldstein JN, Ecklin D., Fiori P., Urshin C. ,E.. Scanning electron microscopy and X-ray Microanalysis. Plenum Press, New York 1988.
- Goodson A, McGregor AH, Douglas J, Taylor P. Direct, quantitative clinical assessment of hand function: usefulness and reproducibility. Man Ther 2007;12:2): 144-52.
- Gottlander M, Albrektsson T. Histomorphometric studies of hydroxylapatite-coated and uncoated CP titanium threaded implants in bone. Int J Oral Maxillofac Implants 1991;6:4): 399-404.
- Gottlander M, Albrektsson T. Histomorphometric analyses of hydroxyapatite-coated and uncoated titanium implants. The importance of the implant design. Clin Oral Implants Res 1992;3:2): 71-6.
- Gottlander M, Johansson CB, Albrektsson T. Short- and long-term animal studies with a plasma-sprayed calcium phosphate-coated implant. Clin Oral Implants Res 1997;8:5): 345-51.
- Green TR, Fisher J, Stone M, et al. Polyethylene particles of a ‘critical size’ are necessary for the induction of cytokines by macrophages in vitro. Biomaterials 1998;19:24): 2297-302.
- Groot D, Gosens T, Leeuwen NC, et al. Wear-induced osteolysis and synovial swelling in a patient with a metal-polyethylene wrist prosthesis. J Hand Surg Am 2006;31:10): 1615-8.
- Grosland NM, Rogge RD, Adams BD. Influence of articular geometry on prosthetic wrist stability. Clin Orthop Relat Res 2004;421:13): 134-42.
- Gummesson C, Atroshi I, Ekdahl C. The disabilities of the arm, shoulder and hand (DASH) outcome questionnaire: longitudinal construct validity and measuring self-rated health change after surgery. BMC Musculoskelet Disord 2003;4:11
- Gupta A. Total wrist arthroplasty. Am J Orthop 2008;37:8 Suppl 1): 12-6.
- Haddad RJ, Jr., Riordan DC. Arthrodesis of the wrist. A surgical technique. J Bone Joint Surg Am 1967;49:5): 950-4.
- Haddad FS, Thakrar RR, Hart AJ, et al. Metal-on-metal bearings: the evidence so far. J Bone Joint Surg Br 2011;93:5): 572-9.
- Hallab NJ, Vermes C, Messina C, et al. Concentration- and composition-dependent effects of metal ions on human MG-63 osteoblasts. J Biomed Mater Res 2002;60:3): 420-33.
- Hamilton WG, McAuley JP, Dennis DA, et al. THA with Delta ceramic on ceramic: results of a multicenter investigational device exemption trial. Clin Orthop Relat Res 2010;468:2): 358-66.
- Harlingen D, Heesterbeek PJ, de Vos MJ. High rate of complications and radiographic loosening of the biaxial total wrist arthroplasty in rheumatoid arthritis: 32 wrists followed for 6 (5-8) years. Acta Orthop 2011;82 (6): 721-6.
- Hayashi K, Mashima T, Uenoyama K. The effect of hydroxyapatite coating on bony ingrowth into grooved titanium implants. Biomaterials 1999;20:2): 111-9.
- Hayden RJ, Jebson PJ. Wrist arthrodesis. Hand Clin 2005;21:4): 631-40.
- Heath JC, Freeman MA, Swanson SA. Carcinogenic properties of wear particles from prostheses made in cobalt-chromium alloy. Lancet 1971;1:7699): 564-6.
- Herzberg G. Prospective study of a new total wrist arthroplasty: short term results. Chir Main 2011;30:1): 20-5.
- Herzberg G, Boeckstyns M, Sorensen AI, et al. “Remotion” Total Wrist Arthroplasty: Preliminary Results of a Prospective International Multicenter Study of 215 Cases. Journal of Wrist Surgery 2012;01:01): 17-23.
- Hove LM. Epidemiology of scaphoid fractures in Bergen, Norway. Scand J Plast Reconstr Surg Hand Surg 1999;33:4): 423-6.
- Hove LM, Fjeldsgaard K, Reitan R, et al. Fractures of the distal radius in a Norwegian city. Scand J Plast Reconstr Surg Hand Surg 1995;29:3): 263-7.
- Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am J Ind Med 1996;29:6): 602-8.
- Imam S, Aldridge C, Lyall H. Bilateral idiopathic avascular necrosis of the scaphoid: a rare case of Preiser’s disease. J Bone Joint Surg Br 2009;91:10): 1400-2.
- Inoue G, Sakuma M. The natural history of scaphoid non-union. Radiographical and clinical analysis in 102 cases. Arch Orthop Trauma Surg 1996;115:1): 1-4.
- Ivanoff C-J. On Surgical and Implant Relatet Factors Influencing Integration and Function of Titanium Implants. Experimental and Clinical Aspects. In: Dep of Biomaterials/Handicap Research, Inst for Surgical Sciences On Surgical and Implant Relatet Factors Influencing Integration and Function of Titanium Implants. Experimental and Clinical Aspects. University of Gothenburg: Gothenburg; 1999.
- Jarcho M. Biomaterial aspects of calcium phosphates. Properties and applications. Dent Clin North Am 1986;30:1): 25-47.
- Jester A, Harth A, Germann G. Measuring levels of upper-extremity disability in employed adults using the DASH Questionnaire. J Hand Surg Am 2005;30:5): 1074 e1- e10.
- Jinno T, Goldberg VM, Davy D, Stevenson S. Osseointegration of surface-blasted implants made of titanium alloy and cobalt-chromium alloy in a rabbit intramedullary model. J Biomed Mater Res 1998;42:1): 20-9.
- Johanssen C. On tissue reactions to metal implants. In: Dep of Biomaterials/Handicap Research, Inst for Surgical Sciences and Dep of Prosthetic Dentistry On Tissue Reactions to Metal Implants. University of Gothenburg: Gothenburg; 1991; 128.
- Johansson CB, Morberg P. Cutting directions of bone with biomaterials in situ does influence the outcome of histomorphometrical quantifications. Biomaterials 1995;16:13): 1037-9.
- Johansson CB, Han CH, Wennerberg A, Albrektsson T. A quantitative comparison of machined commercially pure titanium and titanium-aluminum-vanadium implants in rabbit bone. Int J Oral Maxillofac Implants 1998;13:3): 315-21.
- Johansson CB, Hansson S, Wennerberg A and Stefenson P. Comparison of data from commercially pure titanium implant integration in rabbit tibia. In: European Society for Biomaterials (ESB) Conference 25th ESB Anniversary Conference Comparison of data from commercially pure titanium implant integration in rabbit tibia. London, UK; 2001.
- Johansson CB, Jimbo R, Stefenson P. Ex Vivo and In Vivo Biomechanical Test of Implant Attachment to Various Materials: Introduction of a New User-Friendly Removal Torque Equipment. Clin Implant Dent Relat Res 2010;14:4): 603-11.
- Jolly SL, Ferlic DC, Clayton ML, et al. Swanson silicone arthroplasty of the wrist in rheumatoid arthritis: a long-term follow-up. J Hand Surg Am 1992;17:1): 142-9.
- Karachalios T, Tsatsaronis C, Efraimis G, et al. The long-term clinical relevance of calcar atrophy caused by stress shielding in total hip arthroplasty: a 10-year, prospective, randomized study. J Arthroplasty 2004;19:4): 469-75.
- Kaufmann R, Pfaeffle J, Blankenhorn B, et al. Kinematics of the midcarpal and radiocarpal joints in radioulnar deviation: an in vitro study. J Hand Surg Am 2005;30:5): 937-42.
- Kijima Y, Viegas SF. Wrist anatomy and biomechanics. J Hand Surg Am 2009;34:8): 1555-63.
- Kistler U, Weiss AP, Simmen BR, Herren DB. Long-term results of silicone wrist arthroplasty in patients with rheumatoid arthritis. J Hand Surg Am 2005;30:6): 1282-7.
- Klaassen MA, Martinez-Villalobos M, Pietrzak WS, et al. Midterm survivorship of a press-fit, plasma-sprayed, tri-spike acetabular component. J Arthroplasty 2009;24:3): 391-9.
- Krakauer JD, Bishop AT, Cooney WP. Surgical treatment of scapholunate advanced collapse. J Hand Surg Am 1994;19:5): 751-9.
- Kretschmer F, Fansa H. [BIAX total wrist arthroplasty: management and results after 42 patients]. Handchir Mikrochir Plast Chir 2007;39:4): 238-48.
- Kretschmer F, Wannske M. [The BIAX total wrist prosthesis as an alternative to arthrodesis in degenerative and posttraumatic arthritis--early results in twenty-one patients]. Handchir Mikrochir Plast Chir 2003;35:1): 31-42.
- Krukhaug Y, Lie SA, Havelin LI, et al. Results of 189 wrist replacements. Acta Orthop 2011;82:4): 405-9.
- Kuzyk PR, Saccone M, Sprague S, et al. Cross-linked versus conventional polyethylene for total hip replacement: a meta-analysis of randomised controlled trials. J Bone Joint Surg Br 2011;93:5): 593-600.
- Langton DJ, Jameson SS, Joyce TJ, et al. Accelerating failure rate of the ASR total hip replacement. J Bone Joint Surg Br 2011;93:8): 1011-6.
- Learmonth ID, Young C, Rorabeck C. The operation of the century: total hip replacement. Lancet 2007;370:9597): 1508-19.
- Lee TM, Wang BC, Yang YC, et al. Comparison of plasma-sprayed hydroxyapatite coatings and hydroxyapatite/tricalcium phosphate composite coatings: in vivo study. J Biomed Mater Res 2001;55:3): 360-7.
- LeGeros RZ, Lin S, Rohanizadeh R, et al. Biphasic calcium phosphate bioceramics: preparation, properties and applications. J Mater Sci Mater Med 2003;14:3): 201-9.
- Levadoux M, Legre R. Total wrist arthroplasty with Destot prostheses in patients with posttraumatic arthritis. J Hand Surg Am 2003;28:3): 405-13.
- Lewis PM, Al-Belooshi A, Olsen M, et al. Prospective randomized trial comparing alumina ceramic-on-ceramic with ceramic-on-conventional polyethylene bearings in total hip arthroplasty. J Arthroplasty 2010;25:3): 392-7.
- Lichtman DM, Mack GR, MacDonald RI, et al. Kienbock’s disease: the role of silicone replacement arthroplasty. J Bone Joint Surg Am 1977;59:7): 899-908.
- Lind M, Overgaard S, Bunger C, Soballe K. Improved bone anchorage of hydroxypatite coated implants compared with tricalcium-phosphate coated implants in trabecular bone in dogs. Biomaterials 1999;20:9): 803-8.
- Lintner F, Zweymuller K, Brand G. Tissue reactions to titanium endoprostheses. Autopsy studies in four cases. J Arthroplasty 1986;1:3): 183-95.
- Lintner F, Zweymuller K, Bohm G, Brand G. Reactions of surrounding tissue to the cementless hip implant Ti-6Al-4V after an implantation period of several years. Autopsy studies in three cases. Arch Orthop Trauma Surg 1988;107:6): 357-63.
- Locardi B, Pazzaglia UE, Gabbi C, Profilo B. Thermal behaviour of hydroxyapatite intended for medical applications. Biomaterials 1993;14:6): 437-41.
- Lombardi AV Jr., Berend KR, Mallory TH, Skeels MD, Adams JB. Survivorship of 2000 tapered titanium porous plasma-sprayed femoral components. Clin Orthop Relat Res 2009;467:1): 146-54.
- Lorei MP, Figgie MP, Ranawat CS, Inglis AE. Failed total wrist arthroplasty. Analysis of failures and results of operative management. Clin Orthop Relat Res 1997;342:12): 84-93.
- Louie GH, Ward MM. Changes in the rates of joint surgery among patients with rheumatoid arthritis in California, 1983-2007. Ann Rheum Dis 2010;69:5): 868-71.
- Luetzner J, Krummenauer F, Lengel AM, et al. Serum metal ion exposure after total knee arthroplasty. Clin Orthop Relat Res 2007;461:13): 136-42.
- Mabilleau G, Kwon YM, Pandit H, et al. Metal-on-metal hip resurfacing arthroplasty: a review of periprosthetic biological reactions. Acta Orthop 2008;79:6): 734-47.
- MacDonald SJ, McCalden RW, Chess DG, et al. Metal-on-metal versus polyethylene in hip arthroplasty: a randomized clinical trial. Clin Orthop Relat Res 2003;406:13): 282-96.
- Mahendra G, Pandit H, Kliskey K, et al. Necrotic and inflammatory changes in metal-on-metal resurfacing hip arthroplasties. Acta Orthop 2009;80:6): 653-9.
- Mai K, Verioti C, Ezzet KA Jr. Incidence of ‘squeaking’ after ceramic-on-ceramic total hip arthroplasty. Clin Orthop Relat Res 2010;468:2): 413-7.
- Makela KT, Visuri T, Pulkkinen P, et al. Risk of cancer with metal-on-metal hip replacements: population based study. BMJ 2012;345:e4646
- Mannerfelt L, Malmsten M. Arthrodesis of the wrist in rheumatoid arthritis. A technique without external fixation. Scand J Plast Reconstr Surg 1971;5:2): 124-30.
- Massei G TP, Malchiodi L, Szmukler-Moncler S. Immediately loaded FBR-coated Pitt-Easy Bio-Oss implants. A histologic evaluation in 3 patients after 8-12 weeks of function. In: European Association for Osseointegration Immediately loaded FBR-coated Pitt-Easy Bio-Oss implants. A histologic evaluation in 3 patients after 8-12 weeks of function. (Ed. M S): Milan, Italy; 2001; 409.
- McKee GK, Watson-Farrar J. Replacement of arthritic hips by the McKee-Farrar prosthesis. J Bone Joint Surg Br 1966;48:2): 245-59.
- McKellop H, Park SH, Chiesa R, et al. In vivo wear of three types of metal on metal hip prostheses during two decades of use. Clin Orthop Relat Res 1996; 329 Suppl (13): S128-40.
- Menon J. Total wrist replacement using the modified Volz prosthesis. J Bone Joint Surg Am 1987;69:7): 998-1006.
- Menon J. Universal Total Wrist Implant: experience with a carpal component fixed with three screws. J Arthroplasty 1998;13:5): 515-23.
- Merle C, Streit MR, Volz C, et al. Bone remodeling around stable uncemented titanium stems during the second decade after total hip arthroplasty: a DXA study at 12 and 17 years. Osteoporos Int 2011;22:11): 2879-86.
- Meuli HC. Arthroplasty of the wrist. Clin Orthop Relat Res 1980;149:8): 118-25.
- Meuli H C. Meuli total wrist arthroplasty. Clin Orthop Relat Res 1984;187:10): 107-11.
- Meuli H C. Total wrist arthroplasty. Experience with a noncemented wrist prosthesis. Clin Orthop Relat Res 1997;342:12): 77-83.
- Meuli HC, Fernandez DL. Uncemented total wrist arthroplasty. J Hand Surg Am 1995;20:1): 115-22.
- Moritomo H, Apergis EP, Herzberg G, et al. 2007;IFSSH committee report of wrist biomechanics committee: biomechanics of the so-called dart-throwing motion of the wrist. J Hand Surg Am 2007; 32 (9): 1447-53
- Morscher EW, Hefti A, Aebi U. Severe osteolysis after third-body wear due to hydroxyapatite particles from acetabular cup coating. J Bone Joint Surg Br 1998;80:2): 267-72.
- Mueller WD, Gross U, Fritz T, et al. Evaluation of the interface between bone and titanium surfaces being blasted by aluminium oxide or bioceramic particles. Clin Oral Implants Res 2003;14:3): 349-56.
- Mulford JS, Ceulemans LJ, Nam D, Axelrod TS. Proximal row carpectomy vs four corner fusion for scapholunate (Slac) or scaphoid nonunion advanced collapse (Snac) wrists: a systematic review of outcomes. J Hand Surg Eur Vol 2009;34:2): 256-63.
- Murphy DM, Khoury JG, Imbriglia JE, Adams BD. Comparison of arthroplasty and arthrodesis for the rheumatoid wrist. J Hand Surg Am 2003;28:4): 570-6.
- NAR.The Norwegian Arthroplasty Register. In: http://nrlwebihelsenet/eng/defaulthtmTheNorwegianArthroplastyRegister. 2010.
- Narayanan R, Seshadri SK, Kwon TY, Kim KH. Calcium phosphate-based coatings on titanium and its alloys. J Biomed Mater Res B Appl Biomater 2008;85:1): 279-99.
- Nelson DL. Functional wrist motion. Hand Clin 1997;13:1): 83-92.
- Nikolaou VS, Edwards MR, Bogoch E, et al. A prospective randomised controlled trial comparing three alternative bearing surfaces in primary total hip replacement. J Bone Joint Surg Br 2012;94:4): 459-65.
- Norton MR, Yarlagadda R, Anderson GH. Catastrophic failure of the Elite Plus total hip replacement, with a Hylamer acetabulum and Zirconia ceramic femoral head. J Bone Joint Surg Br 2002;84:5): 631-5.
- Nydick JA, Greenberg SM, Stone JD, et al. Clinical outcomes of total wrist arthroplasty. J Hand Surg Am 2012;37:8): 1580-4.
- O’Flynn HM, Rosen A, Weiland AJ. Failure of the hinge mechanism of a trispherical total wrist arthroplasty: a case report and review of the literature. J Hand Surg Am 1999;24:1): 156-60.
- O’Leary JF, Mallory TH, Kraus TJ, Lombardi AV Jr., Lye CL. Mittelmeier ceramic total hip arthroplasty. A retrospective study. J Arthroplasty 1988;3:1): 87-96.
- Palarie V, Bicer C, Lehmann KM, et al. Early outcome of an implant system with a resorbable adhesive calcium-phosphate coating-a prospective clinical study in partially dentate patients. Clin Oral Investig 2012;16:4): 1039-48.
- Palmer AK, Werner FW, Murphy D, Glisson R. Functional wrist motion: a biomechanical study. J Hand Surg Am 1985;10:1): 39-46.
- Palmquist A, Jarmar T, Hermansson L, et al. Calcium aluminate coated and uncoated free form fabricated CoCr implants: a comparative study in rabbit. J Biomed Mater Res B Appl Biomater 2009;91:1): 122-7.
- Pandit H, Glyn-Jones S, McLardy-Smith P, et al. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br 2008;90:7): 847-51.
- Pomerance J. Outcome after repair of the scapholunate interosseous ligament and dorsal capsulodesis for dynamic scapholunate instability due to trauma. J Hand Surg Am 2006;31:8): 1380-6.
- Predecki P, Auslaender BA, Stephan JE, et al. Attachment of bone to threaded implants by ingrowth and mechanical interlocking. J Biomed Mater Res 1972;6:5): 401-12.
- Preiser G. Eine typische posttraumatische und zur spontanfraktur fuhrende ostitits des naviculare carpi. Fortschr Geb Roentgenstrahlen 1910;1:15): 189-97.
- Prudhommeaux F, Hamadouche M, Nevelos J, et al. Wear of alumina-on-alumina total hip arthroplasties at a mean 11-year followup. Clin Orthop Relat Res 2000;379:13): 113-22.
- Radmer S, Andresen R, Sparmann M. Wrist arthroplasty with a new generation of prostheses in patients with rheumatoid arthritis. J Hand Surg Am 1999;24:5): 935-43.
- Radmer S, Andresen R, Sparmann M. Total wrist arthroplasty in patients with rheumatoid arthritis. J Hand Surg Am 2003;28:5): 789-94.
- Rahimtoola ZO, Hubach P. Total modular wrist prosthesis: a new design. Scand J Plast Reconstr Surg Hand Surg 2004;38:3): 160-5.
- Raju P, Kini SG. Loss of correction in unstable comminuted distal radius fractures with external fixation and bone grafting--a long term followup study. J Orthop Surg Res 2011; 6 (May): 23.
- Reigstad O, Siewers P, Rokkum M, Espehaug B. Excellent long-term survival of an uncemented press-fit stem and screw cup in young patients: follow-up of 75 hips for 15-18 years. Acta Orthop 2008;79:2): 194-202.
- Reigstad O, Thorkildsen R, Grimsgaard C, et al. Is revision bone grafting worthwhile after failed surgery for scaphoid nonunion? Minimum 8 year follow-up of 18 patients. J Hand Surg Eur Vol 2009;34:6): 772-7.
- Reigstad O, Grimsgaard C, Thorkildsen R, et al. Long-term results of scaphoid nonunion surgery: 50 patients reviewed after 8 to 18 years. J Orthop Trauma 2012;26:4): 241-5.
- Rikli DA, Honigmann P, Babst R, et al. Intra-articular pressure measurement in the radioulnocarpal joint using a novel sensor: in vitro and in vivo results. J Hand Surg Am 2007;32:1): 67-75.
- Ritt MJ, Stuart PR, Naggar L, Beckenbaugh RD. The early history of arthroplasty of the wrist. From amputation to total wrist implant. J Hand Surg Br 1994;19:6): 778-82.
- Rizzo M, Beckenbaugh RD. Results of biaxial total wrist arthroplasty with a modified (long) metacarpal stem. J Hand Surg Am 2003;28:4): 577-84.
- Rizzo M, Ackerman DB, Rodrigues RL, Beckenbaugh RD. Wrist arthrodesis as a salvage procedure for failed implant arthroplasty. J Hand Surg Eur Vol 2011;36:1): 29-33.
- Rohde RS, Crisco JJ, Wolfe SW. The advantage of throwing the first stone: how understanding the evolutionary demands of Homo sapiens is helping us understand carpal motion. J Am Acad Orthop Surg 2010;18:1): 51-8.
- Rokkum M, Reigstad A. Polyethylene wear with an entirely HA-coated total hip replacement: 79 hips followed for 5 years. Acta Orthop Scand 1998;69:3): 253-8.
- Rokkum M, Brandt M, Bye K, et al. Polyethylene wear, osteolysis and acetabular loosening with an HA-coated hip prosthesis. A follow-up of 94 consecutive arthroplasties. J Bone Joint Surg Br 1999;81:4): 582-9.
- Rokkum M, Reigstad A, Johansson CB. HA particles can be released from well-fixed HA-coated stems: histopathology of biopsies from 20 hips 2-8 years after implantation. Acta Orthop Scand 2002;73:3): 298-306.
- Rossler S, Sewing A, Stolzel M, et al. Electrochemically assisted deposition of thin calcium phosphate coatings at near-physiological pH and temperature. J Biomed Mater Res A 2003;64:4): 655-63.
- Ryu JY, Cooney WP 3rd, Askew LJ, An KN, Chao EY. Functional ranges of motion of the wrist joint. J Hand Surg Am 1991;16:3): 409-19.
- Sahu A, Charalambous CP, Mills SP, et al. Reoperation for metalwork complications following the use of volar locking plates for distal radius fractures: a United Kingdom experience. Hand Surg 2011;16:2): 113-8.
- Sauerbier M, Kluge S, Bickert B, Germann G. Subjective and objective outcomes after total wrist arthrodesis in patients with radiocarpal arthrosis or Kienbock’s disease. Chir Main 2000;19:4): 223-31.
- Schill S, Thabe H, Mohr W. [Long-term outcome of Swanson prosthesis management of the rheumatic wrist joint]. Handchir Mikrochir Plast Chir 2001;33:3): 198-206.
- Schiltenwolf M, Martini AK, Mau HC, et al. Further investigations of the intraosseous pressure characteristics in necrotic lunates (Kienbock’s disease). J Hand Surg Am 1996;21:5): 754-8.
- Schmidmaier G, Wildemann B, Schwabe P, et al. A new electrochemically graded hydroxyapatite coating for osteosynthetic implants promotes implant osteointegration in a rat model. J Biomed Mater Res 2002;63:2): 168-72.
- Schuind F, Eslami S, Ledoux P. Kienbock’s disease. J Bone Joint Surg Br 2008;90:2): 133-9.
- Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet 2010;376:9746): 1094-108.
- Seppanen M, Makela K, Virolainen P, et al. Hip resurfacing arthroplasty: short-term survivorship of 4,401 hips from the Finnish Arthroplasty Register. Acta Orthop 2012;83:3): 207-13.
- Sieber HP, Rieker CB, Kottig P. Analysis of 118 second-generation metal-on-metal retrieved hip implants. J Bone Joint Surg Br 1999;81:1): 46-50.
- Smith AJ, Dieppe P, Porter M, Blom AW. Risk of cancer in first seven years after metal-on-metal hip replacement compared with other bearings and general population: linkage study between the National Joint Registry of England and Wales and hospital episode statistics. BMJ 2012;344:e2383
- Soballe K. Hydroxyapatite ceramic coating for bone implant fixation. Mechanical and histological studies in dogs. Acta Orthop Scand Suppl 1993;255:1-58
- Soballe K, Overgaard S. The current status of hydroxyapatite coating of prostheses. J Bone Joint Surg Br 1996;78:5): 689-91.
- Solem H, Berg NJ, Finsen V. Long term results of arthrodesis of the wrist: a 6-15 year follow up of 35 patients. Scand J Plast Reconstr Surg Hand Surg 2006;40:3): 175-8.
- Stanley JK, Tolat AR. Long-term results of Swanson silastic arthroplasty in the rheumatoid wrist. J Hand Surg Br 1993;18:3): 381-8.
- Stegeman M, Rijnberg WJ, van Loon CJ. Biaxial total wrist arthroplasty in rheumatoid arthritis. Satisfactory functional results. Rheumatol Int 2005;25:3): 191-4.
- Stenport VF, Johansson CB. Evaluations of bone tissue integration to pure and alloyed titanium implants. Clin Implant Dent Relat Res 2008;10:3): 191-9.
- Strunk S, Bracker W. [Wrist joint arthroplasty: results after 41 prostheses]. Handchir Mikrochir Plast Chir 2009;41-3): 141-7.
- Sul Y-T. On the Bone Response to Oxidised Titanium Implants: The role of microporous structure and chemical composition of the surface oxide in enhanced osseointegration. In: Dep of Biomaterials/Handicap Research, Inst for Surgical Sciences On the Bone Response to Oxidised Titanium Implants: The role of microporous structure and chemical composition of the surface oxide in enhanced osseointegration. University of Gothenburg: Gothenburg; 2002.
- Sumner DR, Galante JO. Determinants of stress shielding: design versus materials versus interface. Clin Orthop Relat Res 1992;274:12): 202-12.
- Sun L, Berndt CC, Gross KA, Kucuk A. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: a review. J Biomed Mater Res 2001;58:5): 570-92.
- Sundfeldt M. On the Aetiology of Aseptic Loosening in Joint Arthroplasties, and Routes to Improved cemented Fixation. In: Dep of Biomaterials/Handicap Research, Inst for Surgical Sciences On the Aetiology of Aseptic Loosening in Joint Arthroplasties, and Routes to Improved cemented Fixation. University of Gothenburg: Gothenburg, Sweden; 2002.
- Swanson AB, de Groot Swanson G, Maupin BK. Flexible implant arthroplasty of the radiocarpal joint. Surgical technique and long-term study. Clin Orthop Relat Res 1984;187:10): 94-106.
- Szmukler-Moncler S PD, Ahossi V. Pointaire Ph. Evaluation of BONIT®, a fully resorbable CaP coating obtained by electrochemical deposition, after 6 weeks of healing: A pilot study in the pig maxilla. In: Proceedings of the 13th Int Symp on Ceramics in Medicine. Bologna, Italy; 2000; 395-8.
- Takwale VJ, Nuttall D, Trail IA, Stanley JK. Biaxial total wrist replacement in patients with rheumatoid arthritis. Clinical review, survivorship and radiological analysis. J Bone Joint Surg Br 2002;84:5): 692-9.
- Teurlings L, Miller GJ, Wright TW. Pressure mapping of the radioulnar carpal joint: effects of ulnar lengthening and wrist position. J Hand Surg Br 2000;25:4): 346-9.
- Thomas TR. Rough surfaces. 2 ed. World Scientific Publishing Company, London 1999.
- Trieb K. Treatment of the Wrist in Rheumatoid Arthritis. J Hand Surg Am 2008;33:1): 113-23.
- Tsuge S, Nakamura R. Anatomical risk factors for Kienbock’s disease. J Hand Surg Br 1993;18:1): 70-5.
- Underwood R, Matthies A, Cann P, et al. A comparison of explanted Articular Surface Replacement and Birmingham Hip Resurfacing components. J Bone Joint Surg Br 2011;93:9): 1169-77.
- Urban JA, Garvin KL, Boese CK, et al. Ceramic-on-polyethylene bearing surfaces in total hip arthroplasty. Seventeen to twenty-one-year results. J Bone Joint Surg Am 2001; 83-A (11): 1688-94.
- van Winterswijk PJ, Bakx PA. Promising clinical results of the universal total wrist prosthesis in rheumatoid arthritis. Open Orthop J 2010;4:67-70
- Velard F, Laurent-Maquin D, Guillaume C, et al. Polymorphonuclear neutrophil response to hydroxyapatite particles, implication in acute inflammatory reaction. Acta Biomater 2009;5:5): 1708-15.
- Vidalain JP. Twenty-year results of the cementless Corail stem. Int Orthop 2011;35:2): 189-94.
- Viegas SF, Patterson RM, Todd PD, McCarty P. Load mechanics of the midcarpal joint. J Hand Surg Am 1993;18:1): 14-8.
- Visuri T, Pulkkinen P, Paavolainen P, Pukkala E. Cancer risk is not increased after conventional hip arthroplasty. Acta Orthop 2010;81:1): 77-81
- Volz RG. The development of a total wrist arthroplasty. Clin Orthop Relat Res 1976;116:8): 209-14.
- Volz RG. Total wrist arthroplasty. A clinical review. Clin Orthop Relat Res 1984;187:10): 112-20.
- Wang BC, Lee TM, Chang E, Yang CY. The shear strength and the failure mode of plasma-sprayed hydroxyapatite coating to bone: the effect of coating thickness. J Biomed Mater Res 1993;27:10): 1315-27
- Ward CM, Kuhl T, Adams BD. Five to ten-year outcomes of the universal total wrist arthroplasty in patients with rheumatoid arthritis. J Bone Joint Surg Am 2011;93:10): 914-9.
- Watson HK, Ballet FL. The SLAC wrist: scapholunate advanced collapse pattern of degenerative arthritis. J Hand Surg Am 1984;9:3): 358-65.
- Wennerberg A. On surface roughness and implant incorporation. In: Dep of Biomaterials/Handicap Research, Inst for Surgical Sciences and Dep of Prosthetic Dentistry On surface roughness and implant incorporation. University of Gothenburg: Gothenburg; 1996; 125.
- Wennerberg A, Albrektsson T. Suggested guidelines for the topographic evaluation of implant surfaces. Int J Oral Maxillofac Implants 2000;15:3): 331-44.
- Wennerberg A, Albrektsson T. Effects of titanium surface topography on bone integration: a systematic review. Clin Oral Implants Res 2009;20:Suppl 4): 172-84.
- Wennerberg A, Albrektsson T, Andersson B, Krol JJ. A histomorphometric and removal torque study of screw-shaped titanium implants with three different surface topographies. Clin Oral Implants Res 1995;6:1): 24-30.
- Wennerberg A, Albrektsson T, Johansson C, Andersson B. Experimental study of turned and grit-blasted screw-shaped implants with special emphasis on effects of blasting material and surface topography. Biomaterials 1996;17:1): 15-22.
- Wennerberg A, Albrektsson T, Lausmaa J. Torque and histomorphometric evaluation of c.p. titanium screws blasted with 25- and 75-microns-sized particles of Al2O3. J Biomed Mater Res 1996;30:2): 251-60.
- Wennerberg A, Ektessabi A, Albrektsson T, et al. A 1-year follow-up of implants of differing surface roughness placed in rabbit bone. Int J Oral Maxillofac Implants 1997;12:4): 486-94.
- Wennerberg A, Hallgren C, Johansson C, Danelli S. A histomorphometric evaluation of screw-shaped implants each prepared with two surface roughnesses. Clin Oral Implants Res 1998;9:1): 11-9
- Wennerberg A, Ide-Ektessabi A, Hatkamata S, et al. Titanium release from implants prepared with different surface roughness. Clin Oral Implants Res 2004;15:5): 505-12.
- Willert HG, Buchhorn GH. Osseointegration of cemented and noncemented implants in artificial hip replacement: long-term findings in man. J Long Term Eff Med Implants 1999;9:1-2): 113-30.
- Williams D. The golden anniversary of titanium biomaterials. Med Device Technol 2001;12:7): 8-11.
- Williams S, Tipper JL, Ingham E, et al. In vitro analysis of the wear, wear debris and biological activity of surface-engineered coatings for use in metal-on-metal total hip replacements. Proc Inst Mech Eng H 2003;217:3): 155-63.
- Wolfe SW, Hotchkiss RN, Pederson WC, Kozin SH. Green’s Operative Hand Surgery. In: Green’s Operative Hand Surgery. (Eds. Wolfe SW, Hotchkiss RN, Pederson WC, Kozin S H). Elsevier Churchill Livingstone: Philadelphia; 2011; 431-46.
- Wright CS, McMurtry RY. AO arthrodesis in the hand. J Hand Surg Am 1983;8:6): 932-5.
- Youm Y, Flatt AE. Kinematics of the wrist. Clin Orthop Relat Res 1980;149:8): 21-32.
- Youm Y, McMurthy RY, Flatt AE, Gillespie TE. Kinematics of the wrist. I. An experimental study of radial-ulnar deviation and flexion-extension. J Bone Joint Surg Am 1978;60:4): 423-31.
- Zicat B, Engh CA, Gokcen E. Patterns of osteolysis around total hip components inserted with and without cement. J Bone Joint Surg Am 1995;77:3): 432-9.
- Zywiel MG, Sayeed SA, Johnson AJ, et al. State of the art in hard-on-hard bearings: how did we get here and what have we achieved?. Expert Rev Med Devices 2011;8:2): 187-207.