Abstract
Background and purpose — Autologous conditioned serum (ACS) is a disease-modifying drug for treatment of knee osteoarthritis, and modest superiority over placebo was reported in an earlier randomized controlled trial (RCT). We hypothesized that when given the opportunity, placebo-treated patients from that RCT would now opt for ACS treatment, which would result in a greater clinical improvement than placebo.
Methods — Of 74 patients treated with placebo in the previous trial, 20 opted for ACS treatment. Patients who did not choose further treatment were interviewed about their reasons. Clinical improvement of the 20 ACS-treated patients was measured using knee-specific clinical scores, as was “response shift” at 3 and 12 months.
Results — In the 20 patients who did opt for ACS, the visual analog scale (VAS) score for pain improved; but after 12 months, clinical results were similar to those after placebo treatment. Response shift measurement demonstrated that the 20 patients had adapted to their disabilities during treatment.
Interpretation — Placebo-treated patients from an earlier trial were reluctant to undergo ACS treatment, in part due to the laborious nature of the therapy. In a subset of patients who opted for treatment, ACS treatment after placebo did not result in greater clinical improvement than placebo treatment only. However, due to the limited power of the current study and possible selection bias, definite advice on using or refraining from ACS cannot be given.
Oral, topical, and intra-articular disease-modifying therapies (DMOADs) for treatment of OA have been studied in several randomized clinical trials (CitationCibere et al. 2004, CitationClegg et al. 2006, CitationPetrella and Petrella 2006, CitationJuni et al. 2007, CitationMazieres et al. 2007). Autologous conditioned serum (ACS; Orthokine) is one of these DMOADs. ACS therapy consists of 6 consecutive intra-articular injections with autologous serum, after incubation of whole blood in the presence of glass beads for 6 hours. The proposed working mechanism of the resulting product is intra-articular inhibition of the proinflammatory cytokine interleukin-1 (IL-1) through the injection of autologous incubated serum containing increased levels of IL-1 receptor antagonist (IL-1ra) (CitationMeijer et al. 2003). ACS has been studied in 2 human randomized controlled trials (RCTs) (CitationBaltzer et al. 2009, CitationYang et al. 2008) and 1 equine RCT (CitationFrisbie et al. 2007). The results of these trials are in favor of ACS; however, several remarks should be made. In the study by CitationYang et al. (2008), the only scores that demonstrated superiority of ACS over placebo were the subscores of KOOS (Knee Injury and Osteoarthritis Outcome Score (CitationRoos and Toksvig-Larsen 2003)) for symptoms and sports. Most of the scores examined improved from baseline, but with a statistically insignificant difference between placebo and ACS. In the study by CitationBaltzer et al. (2009), the 6-fold improvement from ACS treatment was significant but comparison was with a control group receiving 3 injections instead of 6. Nevertheless, since in both RCTs (CitationYang et al. 2008, CitationBaltzer et al. 2009) the absolute clinical scores of the ACS-treated patients improved significantly from baseline and were higher (albeit limited) than during placebo treatment, it appears that the cytokines in ACS have some sort of chondro-protective action.
During inclusion of patients for the earlier RCT of CitationYang et al. (2008) comparing ACS and placebo, the patients were informed that if they were randomized to placebo treatment, and in the event that an advantage of ACS was demonstrated, they would be given the opportunity to undergo additional ACS treatment. As an advantage of ACS was indeed demonstrated, albeit limited, all placebo-treated patients were asked if they would like to undergo ACS treatment. The same questionnaires were used as in the previous trial. In addition, “response shift” was measured—a method to evaluate the adaptation of patients to OA symptoms and to treatment effects over time (CitationKorfage et al. 2007).
We hypothesized that patients who had received placebo treatment in an earlier RCT comparing placebo with ACS (CitationYang et al. 2008), and who were now offered ACS treatment, would opt to undergo ACS treatment and would achieve an even greater improvement in OA clinical scores.
Patients and methods
Study design
All 74 placebo-treated patients who had completed follow-up in a previous ACS trial (CitationYang et al. 2008) were informed by mail about their placebo treatment and about the results of the trial, explaining that a “moderate” effect of ACS treatment was seen as compared to placebo. These patients were given the option to participate in a new trial, in which they would now be treated with ACS, and patients who decided not to participate were asked to explain their decision. A power analysis was performed on the participating patients. The study was performed at the University Medical Center, Utrecht, according to the guidelines set out in the Helsinki declaration, and it was approved by the local medical ethics committee (extension to public trial register ISRCTN44912979). Written informed consent was obtained from all participants before inclusion in the trial.
Patients
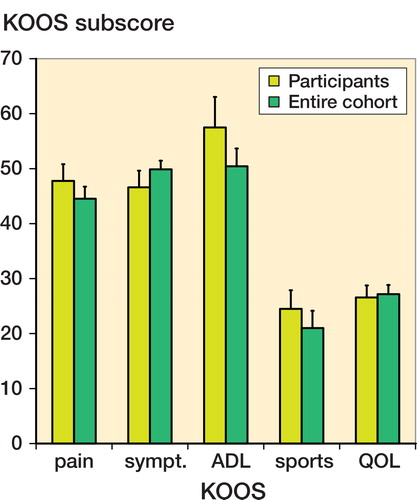
All the patients who participated were older than 18 years and had evidence of OA, as defined by clinical criteria (pain, stiffness, disability) and radiological criteria (Kellgren-Lawrence index grade I–III). Exclusion criteria were poor general health as judged by the orthopedic surgeon, physical conditions that would interfere with evaluation of the affected knee, OA grade IV, or participation in other trials within 3 months of inclusion. Of the 74 placebo-treated patients who had completed the former study, 60 patients responded to the follow-up request. Of these patients, 20 opted for treatment with ACS and 40 decided not to be treated with ACS. Baseline clinical scores of the 20 participants were similar to the scores of the 40 patients who decided not to undergo treatment ().
Intervention
For preparation of the ACS serum, see CitationYang et al. (2008). The ACS syringes (Orthogen, Düsseldorf, Germany) were incubated in the local Good Medical Practice (GMP) laboratory during 6 hours. The 6 intra-articular injections with ACS were performed within 3 weeks (on days 0, 3, 7, 10, 14, and 21). Remaining synovial fluid was aspirated from the knee joint to minimize dilution. Through the same needle, 2 mL of ACS was injected into the knee joint using a sterile 0.22-µm pore size anti-bacterial filter.
Study outcomes and follow-up
At baseline (before ACS treatment), and at 3, 6, 9, and 12 months after treatment, patients completed VAS for pain (0 = no pain, 100 = extreme pain), the KOOS (sub-domains Pain, Symptoms, Function, Sports, and Quality of Life (QOL)) and the Knee Society clinical rating scale (KSCRS) (0 = severe disability and 100 = no disability). The 24-item WOMAC score was deduced from the separate KOOS items. The scores at 3 and 12 months were used to distinguish “short-term” and “extended” treatment effects, respectively. Questionnaires were sent to the patients by mail or filled out during clinical follow-up, without any assistance from the independent study physician to ensure that responses were based entirely on self-assessment.
Response shift
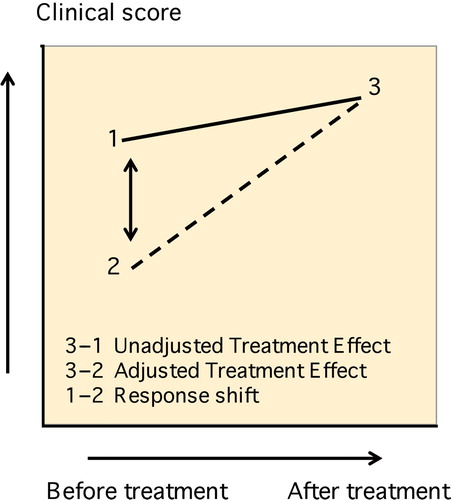
During treatment with a “proven effective” substance, patients are inevitably susceptible to placebo bias. From the “response shift theory” (CitationSchwartz and Sprangers 2002), a simple test has been developed which may distinguish placebo effects from “real” treatment effects. The test is called the “then-test” and has been used in orthopedic research settings (CitationRazmjou et al. 2006, CitationZhang et al. 2012). Treatment effects, which are otherwise obscured by the patient’s adaptation towards their “disease-related disability”, may now be revealed (CitationRazmjou et al. 2006, CitationKorfage et al. 2007). 12 months after ACS treatment, the patients were asked to retrospectively assess their health situation at the start of the treatment (“then-test”). A distinction could then be made between “unadjusted treatment effect” (post-test – pre-test) and “adjusted treatment effect” (post-test – then-test). We hypothesized that the “adjusted treatment effect” would be greater than the “unadjusted treatment effect” (). Apart from the “response shift” analysis, follow-up procedures were similar to follow-up during earlier placebo treatment. Patients undergoing additional interventions due to knee OA during follow-up were considered treatment failures. Data sets of these patients, as well as data sets of patients undergoing (non-) surgical procedures of the knee not related to knee OA during follow-up, were completed using the “last observation carried forward” method. For example, if a patient experienced a trauma to the knee requiring arthroscopic intervention at 10 months follow-up, the scores observed at 9 months were transferred to 12 months.
Statistics
The power of the study was 73% (20 patients, delta 20%, SD 33%). Data were tested for normality using the Kolmogorov-Smirnov test and for homogeneity of variances using Levene’s test of equality of variances. Assuming normality and equal variances, continuous variables were analyzed using paired-samples t-tests at 3 months (“short-term effects”) and at 12 months (“extended effects”), followed by a Bonferroni correction. The Bonferroni correction was performed to correct for multiple comparisons to the baseline measurement. To this extent, the p-value obtained from the pairwise comparison of 3 and 12 months to baseline was multiplied by 2, and only considered significant when remaining below 0.05. When both the placebo treatment in the previous trial and the ACS treatment in the current study gave a significant improvement, the size of the effect was compared using paired-samples t-tests. 12-month clinical scores after ACS treatment were compared to baseline clinical scores before placebo treatment. All p-values less than 0.05 were considered statistically significant. Graphs show mean values ± 95% confidence-interval (CI). SPSS version 15.0 for Windows was used for data analysis.
Results
Patients
60 of the 74 placebo-treated patients responded to the follow-up request. Of these patients, 20 accepted the ACS treatment and were followed for 12 months (14 men). Of the patients who declined further treatment, 10 had achieved an acceptable level of pain after the placebo treatment, 8 had undergone either a hemi-knee or total knee arthroplasty and did not require further intra-articular injections, 7 had experienced substantial pain from earlier injections and declined additional injections, 5 were discouraged by the laborious nature of the treatment (frequency/ logistics), 5 were not convinced by the earlier study results, and 5 had undergone another nonoperative treatment or gave another reason for not participating. The male-to-female ratio was lower in the cohort that declined further treatment (1.2:1.0) than in the cohort that opted for ACS treatment (2.3:1.0), but mean age and baseline clinical scores were not significantly different (Supplementary data).
The mean age of the 20 patients who opted for ACS treatment was 50 (34–70) years. The left knee was treated in 5 patients, the right knee in 12, and both knees in 3. For the 3 patients who received ACS in both knees, the VAS pain score of the knee that was first included was recorded. No treatment failures occurred. 2 of the 20 patients experienced a knee trauma during the 12 months of follow-up: 1 patient had an ACL reconstruction and 1 underwent an arthroscopy in which microfracturing was combined with nettoyage of the lateral meniscus. Their scores were completed using the “last follow-up carried forward” method. Treatment with ACS started within an average of 14 (6–25) months after completion of the ACS trial.
Placebo treatment: improvement of all clinical scores at 3 and 12 months
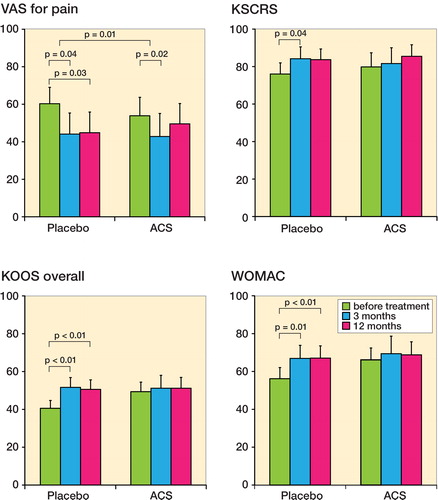
During treatment in the previous trial, the 20 placebo-treated patients reported improvement in all clinical outcome scores at 3 months and 12 months ( and Supplementary data).
Figure 3. Clinical scores before and 3 and 12 months after placebo and ACS treatment (n = 20). A. VAS for pain. B. KSCRS. C. KOOS overall score. D. WOMAC. Patients reported improvement for all scores during placebo treatment, with short-term follow-up (3 months) and extended follow-up (12 months). For ACS treatment, significant short-term improvement is only reported for VAS (pain) after 3 months of treatment. The KSCRS (B) was the only score that showed improvement after the combination of placebo and ACS treatment. Bars represent mean ± 95% CI.
ACS treatment: improvement of VAS pain at 3 months
During ACS treatment in the current trial, VAS pain improved after 3 months but not after 12 months. The improvement was similar to that with previous placebo treatment. In contrast to the response to earlier placebo treatment, no improvement was seen after ACS treatment at 3 or 12 months for KSCRS, KOOS overall, KOOS individual, and WOMAC scores ( and Supplementary data).
Overall improvement
Clinical scores after 12 months of ACS treatment were similar to those after 12 months of placebo treatment. Before ACS treatment of the 20 patients, the clinical scores had decreased slightly. This indicates that some sort of relapse took place after placebo treatment, followed by a “renewed” improvement (significant for VAS pain (p = 0.02) at 3 months).
Response shift: improvement in KOOS 12 months after ACS treatment
When asked to rate their health in retrospect using the then-test, the patients reported an improvement in KOOS overall score 12 months after ACS treatment (p = 0.02). It is interesting that retrospectively, they actually rated their initial health (all outcomes) as being lower than how they had rated it before the actual ACS treatment, but this difference was not statistically significant.
Discussion
The low number of patients who opted for ACS treatment surprised us. One-fifth of the patients who did not participate reported a satisfactory level of reduction of symptoms after placebo treatment. Some sort of lasting placebo effect had indeed occurred, as overall KOOS scores and WOMAC scores of the 20 patients who did opt for ACS treatment were higher than the baseline scores before placebo treatment. Due to the higher baseline, but also to lower patient expectations, ACS was challenged to achieve an even higher improvement. Use of pain medication or other OA therapies between the trials may have added to this effect, and the VAS pain score was the only score that showed temporary improvement after ACS.
Overall, placebo effects seem to play an important role during intra-articular treatment of OA. Long-lasting placebo effects (CitationZeidler 2011) may be responsible for four-fifths of the treatment effect (CitationLo et al. 2003), in part due to the “invasiveness” experienced from the use of subsequent injections (CitationZhang et al. 2008). From a more biological point of view, pure “flushing” of the joint was hypothesized to be effective, but in a comparison of arthroscopic lavage with debridement or placebo surgery (CitationMoseley et al. 2002), no greater reduction in OA symptoms was observed after lavage.
CitationBaltzer et al. (2009) found an improvement in clinical symptoms lasting up to 2 years when they compared 6 intra-articular ACS injections with 3 injections of hyaluronan or saline. The authors concluded that it remained to be determined how these “disease-modifying” effects resulted from in vivo chondro-protective or chondro-regenerative mechanisms. In parallel with the current trial, ACS composition was investigated and apart from chondro-protective cytokines, chondro-degradative cytokines were also found in ACS (CitationRutgers et al. 2010). Moreover, it is questionable whether IL-1 should be the main target at all during OA treatment. In an earlier RCT comparing injection of various concentrations of IL-1RA to placebo, no advantage of IL-1RA was found (CitationChevalier et al. 2009). The lack of effectiveness in vitro in combination with unknown effects of IL-1RA on pain and inflammation that have already been described (CitationChevalier et al. 2005) demands further investigation of ACS—both in vitro and in vivo.
Patients had scored their baseline health status higher “in real-time” than when scoring their baseline health status in retrospect, after ACS treatment. Over time, their perception of the disabilities caused by OA had changed. Considering the variety of treatments for OA and the equal amounts of placebo effects that can result, an understanding of patient adaptation during treatment may improve our knowledge of the long-term implications of OA as a chronic disease, in addition to “real-time” recording of KOOS, VAS, and WOMAC. To date, this has only been performed in patients undergoing total knee arthroplasty (CitationRazmjou et al. 2006, CitationZhang et al. 2012).
Even though response shift measurement is helpful, it should be used as a supplement—as the “then-test” methodology is subject to the possible influence of recall bias (i.e. recall of a previous state being influenced by the patient’s current state), and this influence should be considered when drawing conclusions (CitationRobling and Hood 2002, CitationVisser et al. 2005, CitationRiddle and Lingard 2007).
Several remarks can be made about the current study. Most importantly, selection bias probably occurred as patients were free to opt for ACS treatment. Responder-non-responder analysis revealed that more males chose ACS treatment, although baseline clinical scores did not differ significantly. The 20 study patients should nevertheless be considered as a separate cohort rather than being representative of the entire cohort of earlier placebo-treated patients. Furthermore, the power of the study was lower than commonly used, due to the smaller number of participants than expected. In 3 of 20 patients, treating the knee that was not included may have influenced some of the function-related questionnaires (KOOS ADL, QOL, and sports) and the KSCRS and WOMAC.
In conclusion, we found that in a self-selected group of 20 placebo-treated patients from an earlier randomized controlled trial, ACS treatment did not improve OA symptoms further relative to previous placebo treatment. Placebo-treated patients who chose not to undergo ACS treatment were in part discouraged by the laborious nature of the therapy.
Supplementary data
The Table is available at Acta’s website (www.actaorthop.org), identification number 7009.
IORT_A_950467_SM8437.pdf
Download PDF (34.8 KB)MR: conception and design, analysis and interpretation of the data, drafting of the article, final approval of the article, statistical expertise, and collection and assembly of data. LC, KGAY, NR, WD, and DS: conception and design, analysis and interpretation of the data, administrative, technical, or logistic support, and critical revision and final approval of the article. MR and DS take responsibility for the integrity of the work as a whole.
We thank Miss NE Buisman for assistance during treatment and follow-up of the patients and Dr P Westers for statistical help. We also thank the Anna Foundation for Musculoskeletal Research in the Netherlands, the Netherlands Organisation for Health Research and Development (NWO), and the Dutch Arthritis Association (Reumafonds) for their continuous support.
The authors state that they have no competing interests. DBFS is supported by the Netherlands Organisation for Health Research and Development (NWO). LBC is supported by the Dutch Arthritis Society (Reumafonds). The sponsors had no influence on this publication.
- Baltzer AW , Moser C , Jansen SA , Krauspe R . Autologous conditioned serum (Orthokine) is an effective treatment for knee osteoarthritis. Osteoarthritis Cartilage 2009; 17 (2): 152-60.
- Chevalier X , Goupille P , Beaulieu AD , et al. Results from a double blind, placebo-controlled, multicenter trial of a single intra-articular injection of anakinra (kineret (R)) in patients with osteoarthritis of the knee. Arthritis Rheum 2005; 52 (9): S507.
- Chevalier X , Goupille P , Beaulieu AD , et al. Intraarticular injection of anakinra in osteoarthritis of the knee: a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum 2009; 61 (3): 344-52.
- Cibere J , Kopec JA , Thorne A , et al. Randomized, double-blind, placebo-controlled glucosamine discontinuation trial in knee osteoarthritis. Arthritis Rheum 2004; 51 (5): 738-45.
- Clegg DO , Reda DJ , Harris CL , et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med 2006; 354 (8): 795-808.
- Frisbie DD , Kawcak CE , Werpy NM , Park RD , McIlwraith CW . Clinical, biochemical, and histologic effects of intra-articular administration of autologous conditioned serum in horses with experimentally induced osteoarthritis. Am J Vet Res 2007; 68 (3): 290-6.
- Juni P , Reichenbach S , Trelle S , et al. Efficacy and safety of intraarticular hylan or hyaluronic acids for osteoarthritis of the knee: a randomized controlled trial. Arthritis Rheum 2007; 56 (11): 3610-9.
- Korfage IJ , de Koning HJ , Essink-Bot ML . Response shift due to diagnosis and primary treatment of localized prostate cancer: a then-test and a vignette study. Qual Life Res 2007; 16 (10): 1627-34.
- Lo GH , LaValley M , McAlindon T , Felson DT . Intra-articular hyaluronic acid in treatment of knee osteoarthritis: a meta-analysis. Jama 2003; 290 (23): 3115-21.
- Mazieres B , Hucher M , Zaim M , Garnero P . Effect of chondroitin sulphate in symptomatic knee osteoarthritis: a multicentre, randomised, double-blind, placebo-controlled study. Ann Rheum Dis 2007; 66 (5): 639-45.
- Meijer H , Reinecke J , Becker C , Tholen G , Wehling P . The production of anti-inflammatory cytokines in whole blood by physico-chemical induction. Inflamm Res 2003; 52 (10): 404-7.
- Moseley JB , O’Malley K , Petersen NJ , et al. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med 2002; 347 (2): 81-8.
- Petrella RJ , Petrella M . A prospective, randomized, double-blind, placebo controlled study to evaluate the efficacy of intraarticular hyaluronic acid for osteoarthritis of the knee. J Rheumatol 2006; 33 (5): 951-6.
- Razmjou H , Yee A , Ford M , Finkelstein JA . Response shift in outcome assessment in patients undergoing total knee arthroplasty. J Bone Joint Surg (Am) 2006; 88 (12): 2590-5.
- Riddle DL , Lingard EA . Re: Response shift in outcome assessment in patients undergoing total knee arthroplasty. J Bone Joint Surg (Am) 2007; 89 (8): 1865; author reply -6.
- Robling M , Hood K . Response shift, responsiveness or recall bias? Br J Gen Pract 2002; 52 (480): 585.
- Roos EM , Toksvig-Larsen S . Knee injury and Osteoarthritis Outcome Score (KOOS) - validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes 2003; 1: 17.
- Rutgers M , Saris DB , Dhert WJ , Creemers LB . Cytokine profile of autologous conditioned serum for treatment of osteoarthritis, in vitro effects on cartilage metabolism and intra-articular levels after injection. Arthritis Res Ther 2010; 12 (3): R114.
- Schwartz CE , Sprangers MA . An introduction to quality of life assessment in oncology: the value of measuring patient-reported outcomes. Am J Manag Care (18 Suppl) 2002; 8: S550-9.
- Visser MR , Oort FJ , Sprangers MA . Methods to detect response shift in quality of life data: a convergent validity study. Qual Life Res 2005; 14 (3): 629-39.
- Yang KG , Raijmakers NJ , van Arkel ER , et al. Autologous interleukin-1 receptor antagonist improves function and symptoms in osteoarthritis when compared to placebo in a prospective randomized controlled trial. Osteoarthritis Cartilage 2008; 16 (4): 498-505.
- Zeidler H . Paracetamol and the placebo effect in osteoarthritis trials: a missing link? Pain Res Treat 2011; 2011: 696791.
- Zhang W , Robertson J , Jones AC , Dieppe PA , Doherty M . The placebo effect and its determinants in osteoarthritis - meta-analysis of randomised controlled trials. Ann Rheum Dis 2008; 67 (12): 1716-23.
- Zhang XH , Li SC , Xie F , et al. An exploratory study of response shift in health-related quality of life and utility assessment among patients with osteoarthritis undergoing total knee replacement surgery in a tertiary hospital in Singapore. Value Health (1 Suppl) 2012; 15: S72-8.